Abstract
Rationale: Noninvasive ventilation (NIV) is standard of care in amyotrophic lateral sclerosis (ALS), yet few data exist regarding its benefits.
Objectives: We sought to identify whether the use of NIV was associated with survival in ALS.
Methods: This was a single-center retrospective cohort study of 452 patients with ALS seen between 2006 and 2015. We matched one or more NIV subjects (prescribed NIV) to non-NIV subjects (never prescribed NIV) without replacement. The outcome was time from NIV prescription date (NIV subjects) or matched date (non-NIV subjects) until death. We performed a multivariable Cox proportional hazards model with NIV hourly usage as a time-varying covariate and stratified by matched groups.
Results: After creating 180 matched groups and adjusting for age, body mass index, ALS Functional Rating Scale Revised dyspnea score, and hourly NIV use, NIV was associated with a 26% reduction in the rate of death compared with non-NIV subjects (hazard ratio [HR], 0.74; 95% confidence interval [CI], 0.57–0.98; P = 0.04). Among those with limb-onset ALS, NIV subjects had a 37% lower rate of death compared with non-NIV subjects (HR, 0.63; 95% CI, 0.45–0.87; P = 0.006). Among NIV subjects, we found that NIV use for an average of ≥4 h/d was associated with improved survival.
Conclusions: NIV use was associated with significantly better survival in ALS after matching and adjusting for confounders. Increasing duration of daily NIV use was associated with longer survival. Randomized clinical trials should be performed to identify ideal thresholds for improving survival and optimizing adherence in ALS.
Keywords: amyotrophic lateral sclerosis, respiratory failure, noninvasive ventilation, survival analysis, matching
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease that commonly progresses to respiratory muscle weakness. It is the most frequent neurodegenerative disorder in middle age, with an average onset in the mid to late 50s (1). The most common cause of death is respiratory failure through a variety of mechanisms, including aspiration, diminished airway clearance because of ineffective cough, recurrent pulmonary infections, and chronic hypercapnic respiratory failure (2). The cornerstone of managing respiratory insufficiency in ALS involves noninvasive ventilation (NIV), which has been shown to improve quality of life and potentially survival (3–6).
To our knowledge, there has been only one randomized clinical trial (RCT) of NIV in ALS (3). This trial randomized 41 patients with ALS to NIV or standard care and demonstrated a median survival benefit of 205 days for those randomized to NIV, largely seen in those without severe bulbar disease. Other retrospective observational studies have described survival of aggregate groups according to static attributes (e.g., bulbar disease and diagnosis age) rather than characteristics that change over time (e.g., forced vital capacity [FVC], body mass index [BMI], and ALS Functional Rating Scale Revised [ALSFRS-R] score) (4, 7, 8). Guidelines from various international societies provide moderate to strong recommendations for NIV but acknowledge a paucity of high-quality evidence (9, 10). In addition, these guidelines suggest several FVC thresholds for NIV initiation (9, 10).
Multiple prior studies have been limited by lack of 1) a formal survival analysis (6), 2) adjustment for confounders (11), 3) accounting for immortal time bias (7), 4) a control group (8, 12), and 5) adjustment for varying NIV use over time. In the present study, we attempted to address each of these limitations with a survival analysis that stratified results by matched groups and adjusted for changes in hours of daily NIV use over time. The current study aimed to determine whether NIV was associated with better survival in ALS. We hypothesized that NIV would be associated with improved overall survival with heterogeneity of treatment effect by onset site of disease (13).
Methods
Study Design and Study Population
We performed a retrospective cohort study of subjects evaluated at the Penn Comprehensive ALS Center with a first visit between January 1, 2006 and December 31, 2015.
Data Collection
The study population included 1,061 prospectively entered subjects in a secure online data portal known as the Penn Integrated Neurodegenerative Disease Database. An attending neurologist diagnosed ALS in the subjects with reference to the World Federation of Neurology El Escorial Criteria (14). We reported El Escorial criteria that represented the attending neurologists’ initial impression.
After each clinic visit, an attending neurologist completed clinical summary data entry. For this study, we excluded subjects with few to no usable data or nonphysiologic values (n = 96), initiation of NIV before enrollment or after last visit date (n = 85), and ever receiving a tracheostomy (n = 16). The final dataset contained 864 individuals. Subjects were followed via outpatient neurology clinic visits at approximately 3-month intervals. All subjects were followed until September 1, 2016. All participating individuals provided informed consent for research data collection.
We defined the NIV group and non-NIV group by whether they ever received a prescription for NIV during follow-up. Time of NIV initiation was defined by the date of NIV prescription. Subjects were included in the NIV group for all follow-up subsequent to the NIV prescription date. Hours of daily NIV use were recorded at each clinical visit on the basis of patient-reported averages. For information on variables such as symptom onset site, ALSFRS-R score, and diagnosis delay, please see online supplement.
Outcomes
The outcome was survival since either the day of NIV prescription (for NIV subjects), or the day of matching (for non-NIV subjects). Follow-up time ended at the date of death or censoring at the last visit date. Death was determined by electronic medical record documentation, social security disability insurance data, or caregiver notification. Because we were unable to report survival time for each stratified group, we report unadjusted median survival in months.
Statistical Analysis
Data were summarized using mean ± SD or median (interquartile range [IQR]) for continuous variables, and number of subjects (percentage) for categorical variables. All subjects had visits chronologically numbered in sequential order (e.g. 1–10).
We matched subjects within the same visit number using Mahalanobis metric matching with a caliper distance of 0.25 Mahalanobis distance units (15, 16). We chose matching variables historically found to be associated with the outcome and to influence the need for NIV (17). We sought to avoid overmatching and maintain balance across matched groups (15). Based on prior literature of association with NIV use and ALS survival (18–27), we matched on the following variables: diagnosis delay, symptom onset site (limb or bulbar), ALSFRS-R orthopnea score (either >2 or ≤2), and FVC% predicted normal. To account for immortal time bias, we also matched by time since first visit to the day of matching. The aggregated difference of the respective matching variables was converted into a single distance measure.
The NIV group was then matched to the non-NIV group so as to minimize the sum of differences across all groups. For each non-NIV subject, we matched as many NIV subjects as possible within the 0.25 Mahalanobis distance. We matched non-NIV subjects without replacement across all visits (i.e., once a non-NIV subject was matched at a visit, that subject would not be eligible for matching in all subsequent visits). We aggregated matched datasets from each individual visit into one dataset for analysis.
After creating matched sets, we performed a multivariable Cox proportional hazards model. For the matched NIV subjects, the survival time started from the initiation of NIV to death or the last visit, whichever came last. For the matched non-NIV subjects, the survival time started from the time of matching to death or the last visit, whichever came last. We performed an unadjusted and adjusted survival analysis. We chose confounders that were both 1) not involved in matching and 2) associated with ALS disease progression, need for NIV, and overall survival (18–27). Available baseline characteristics included diagnosis age, sex, race, initial El Escorial criteria, and prior history of smoking, coronary artery disease, diabetes mellitus, or hypertension. We also adjusted for the following characteristics present at the time of matching: BMI, ALSFRS-R total score, and ALSFRS-R dyspnea score. Our final adjusted model used a purposeful variable selection method to adjust for diagnosis age, BMI, and ALSFRS-R dyspnea score, which may act as confounders based on historical literature (17, 19, 22, 26, 27).
At each clinic visit, subjects reported average hours of daily NIV use since last visit. To account for varying NIV use over time, we adjusted the Cox proportional hazards analyses for patient-reported daily NIV hours as a time-varying covariate when appropriate. All non-NIV subjects were coded as having 0 NIV hours. We stratified survival analyses results by matched groups, as appropriate. We examined the proportionality assumption using Schoenfeld residuals plots (28, 29).
We performed a secondary survival analysis to examine how FVC and symptom onset site were associated with survival. For further information on subgroup and secondary analyses, see online supplement.
We considered all P values of less than 0.05 as statistically significant. We performed all analyses using Stata version 15.0 (StataCorp LP).
Results
The study sample included 452 subjects with 180 matched groups. The matching algorithm grouped one to nine NIV subjects with each non-NIV subject. The mean age was 64 years, 54% of participants were self-reported male, and 82% were white (Table 1). Most people had a normal BMI or were overweight at baseline. The median diagnosis delay was 0.9 years. Seventy-four percent of the cohort had limb-onset disease, and average FVC at baseline was 69%. The mean baseline ALSFRS-R total score was 35, whereas the majority (78%) of patients had no significant dyspnea at baseline by ALSFRS-R. Fifty-two percent of the cohort classified themselves as never-smokers. The 452-subject cohort had a median follow-up time of 1.5 years (IQR, 0.8–2.4). Ninety-five percent of the cohort died during follow-up. Baseline characteristics of the full Penn cohort and comparison of unmatched subjects with matched subjects are shown in Tables E1 and E2 in the online supplement.
Table 1.
Baseline characteristics of Penn cohort (N = 452)
| Variable | Results |
|---|---|
| Age at diagnosis, yr | 64 ± 12 |
| Male sex, n (%) | 243 (54) |
| Race, n (%) | |
| White | 369 (82) |
| Black | 48 (10) |
| Other | 35 (8) |
| Body mass index class, n (%) | |
| <18.5 kg/m2 | 23 (5) |
| 18.5–24.9 kg/m2 | 200 (44) |
| 25–29.9 kg/m2 | 149 (33) |
| ≥30 kg/m2 | 79 (18) |
| Diagnosis delay, yr | 0.9 (0.5, 1.2) |
| El Escorial criteria, n (%) | |
| Definite ALS | 111 (25) |
| Possible ALS | 118 (26) |
| Probable ALS | 148 (33) |
| Suspected ALS | 75 (16) |
| Symptom onset site, n (%) | |
| Limb | 333 (74) |
| Bulbar | 119 (26) |
| Forced vital capacity% predicted | 69 ± 23 |
| ALSFRS-R total score | 35 ± 7 |
| ALSFRS-R dyspnea score, n (%) | |
| >2 | 354 (78) |
| ≤2 | 98 (22) |
| ALSFRS-R orthopnea score, n (%) | |
| >2 | 390 (86) |
| ≤2 | 62 (14) |
| Smoking history, n (%) | |
| Current | 45 (10) |
| Previous | 173 (38) |
| Never | 234 (52) |
| Coronary artery disease, n (%) | 36 (8) |
| Diabetes mellitus, n (%) | 53 (12) |
| Hypertension, n (%) | 190 (42) |
Definition of abbreviations: ALS = amyotrophic lateral sclerosis; ALSFRS-R = ALS Functional Rating Scale Revised; SD = standard deviation.
Data are mean ± SD or median (25th percentile, 75th percentile) unless otherwise indicated.
NIV subjects were more likely to be younger men and to have increased severity of dyspnea at the time of matching (ALSFRS-R dyspnea score ≤2) (Table 2). Median unadjusted survival time was 8.0 months from date of NIV prescription (IQR, 3.5–15.5) for NIV subjects and was 7.4 months from date of matching (IQR, 2.7–15.5) for non-NIV subjects (log-rank test of equality, P = 0.67) (Figure E1). In our unadjusted analysis, NIV subjects had a 20% reduction in rate of death compared with non-NIV subjects, with borderline statistical significance (hazard ratio [HR], 0.80; 95% confidence interval [CI], 0.63–1.01; P = 0.06). After adjusting for diagnosis age, BMI, ALSFRS-R dyspnea score, and NIV hourly usage, NIV subjects had a 26% reduction in rate of death compared with non-NIV subjects (HR, 0.74; 95% CI, 0.57–0.98; P = 0.04)(Table 3). The multivariable model did not violate the proportional hazards assumption.
Table 2.
Characteristics at time of NIV initiation (NIV subjects) or time of matching (non-NIV subjects) (N = 452)
| Non-NIV Subjects (n = 180) | NIV Subjects (n = 272) | |
|---|---|---|
| Age at diagnosis, yr | 66 ± 13 | 63 ± 11 |
| Male sex, n (%) | 79 (44) | 164 (60) |
| Race, n (%) | ||
| White | 141 (78) | 228 (84) |
| Black | 21 (12) | 27 (10) |
| Other | 18 (10) | 17 (6) |
| Body mass index class, n (%) | ||
| <18.5 kg/m2 | 18 (10) | 28 (10) |
| 18.5–24.9 kg/m2 | 92 (51) | 137 (50) |
| 25–29.9 kg/m2 | 50 (28) | 69 (25) |
| ≥30 kg/m2 | 20 (11) | 38 (14) |
| Diagnosis delay, yr | 0.8 (0.5, 1.2) | 0.9 (0.5, 1.2) |
| Follow-up days since first visit | 135 (52, 329) | 133 (60, 291) |
| El Escorial criteria, n (%) | ||
| Definite ALS | 50 (28) | 61 (22) |
| Possible ALS | 53 (29) | 65 (24) |
| Probable ALS | 53 (29) | 95 (35) |
| Suspected ALS | 24 (14) | 51 (19) |
| Symptom onset site, n (%) | ||
| Limb | 127 (71) | 206 (76) |
| Bulbar | 53 (29) | 66 (24) |
| Forced vital capacity% predicted, n (%) | ||
| <40 | 42 (23) | 80 (29) |
| 40–49 | 43 (23) | 70 (26) |
| 50–59 | 38 (21) | 63 (23) |
| ≥60 | 58 (33) | 59 (22) |
| ALSFRS-R total score | 28 ± 7 | 28 ± 7 |
| ALSFRS-R dyspnea score, n (%) | ||
| >2 | 128 (71) | 120 (44) |
| ≤2 | 52 (29) | 152 (56) |
| ALSFRS-R orthopnea score, n (%) | ||
| >2 | 133 (74) | 178 (65) |
| ≤2 | 47 (26) | 94 (35) |
| Smoking history, n (%) | ||
| Current | 17 (10) | 28 (10) |
| Previous | 67 (37) | 106 (39) |
| Never | 96 (53) | 138 (51) |
| Coronary artery disease, n (%) | 14 (8) | 22 (8) |
| Diabetes mellitus, n (%) | 22 (12) | 31 (11) |
| Hypertension, n (%) | 80 (44) | 110 (40) |
| Survival since matching, mo | 7.4 (2.7, 15.5) | 8.0 (3.5, 15.5) |
Definition of abbreviations: ALS = amyotrophic lateral sclerosis; ALSFRS-R = ALS Functional Rating Scale Revised; NIV = noninvasive ventilation; SD = standard deviation.
Data are mean ± SD or median (25th percentile, 75th percentile) unless otherwise indicated.
Table 3.
Results of survival regression analysis for noninvasive ventilation use, adjusted for time-varying daily hours of noninvasive ventilation use, stratified by matched groups (N = 452)
| Variable* | Univariate Analysis |
Multivariate Analysis |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Noninvasive ventilation | 0.80 | 0.63–1.01 | 0.06 | 0.74 | 0.57–0.98 | 0.04 |
| Age at diagnosis, per decade | 1.30 | 1.16–1.45 | <0.001 | 1.34 | 1.18–1.53 | <0.001 |
| Sex, M | 1.17 | 0.91–1.51 | 0.23 | — | — | — |
| Race | ||||||
| White | — | — | — | — | — | — |
| Black | 0.75 | 0.51–1.11 | 0.15 | — | — | — |
| Other | 1.11 | 0.66–1.87 | 0.70 | — | — | — |
| Body mass index class | ||||||
| <18.5 kg/m2 | 1.46 | 0.93–2.29 | 0.10 | 1.44 | 0.87–2.37 | 0.15 |
| 18.5–24.9 kg/m2 | — | — | — | — | — | — |
| 25–29.9 kg/m2 | 0.71 | 0.54–0.95 | 0.02 | 0.76 | 0.55–1.04 | 0.09 |
| ≥30 kg/m2 | 0.54 | 0.37–0.80 | 0.002 | 0.57 | 0.37–0.88 | 0.01 |
| El Escorial criteria | ||||||
| Definite ALS | — | — | — | — | — | — |
| Possible ALS | 0.81 | 0.57–1.15 | 0.24 | — | — | — |
| Probable ALS | 0.94 | 0.68–1.31 | 0.73 | — | — | — |
| Suspected ALS | 0.71 | 0.47–1.07 | 0.10 | — | — | — |
| ALSFRS-R total score per 6 decrease | 1.00 | 0.87–1.15 | 0.99 | — | — | — |
| ALSFRS-R dyspnea score | 0.02 | 0.005 | ||||
| >2 | — | — | — | — | ||
| ≤2 | 1.38 | 1.06–1.78 | 1.54 | 1.14–2.07 | ||
| Smoking history | ||||||
| Never | — | — | — | — | — | — |
| Previous | 1.74 | 1.33–2.29 | <0.001 | — | — | — |
| Current | 1.55 | 1.04–2.32 | 0.03 | — | — | — |
| Coronary artery disease | 1.53 | 0.97–2.43 | 0.07 | — | — | — |
| Diabetes mellitus | 0.83 | 0.59–1.18 | 0.31 | — | — | — |
| Hypertension | 1.28 | 1.00–1.64 | 0.051 | — | — | — |
| Time-varying covariate | ||||||
| Daily hours of noninvasive ventilation | — | — | — | 1.00 | 0.99–1.00 | 0.99–1.00 |
Definition of abbreviations: ALS = amyotrophic lateral sclerosis; ALSFRS-R = ALS Functional Rating Scale Revised; CI = confidence interval; HR = hazard ratio.
Variables used in matching not shown.
All variable estimates are adjusted for daily hours of noninvasive ventilation use and are stratified by matched groups.
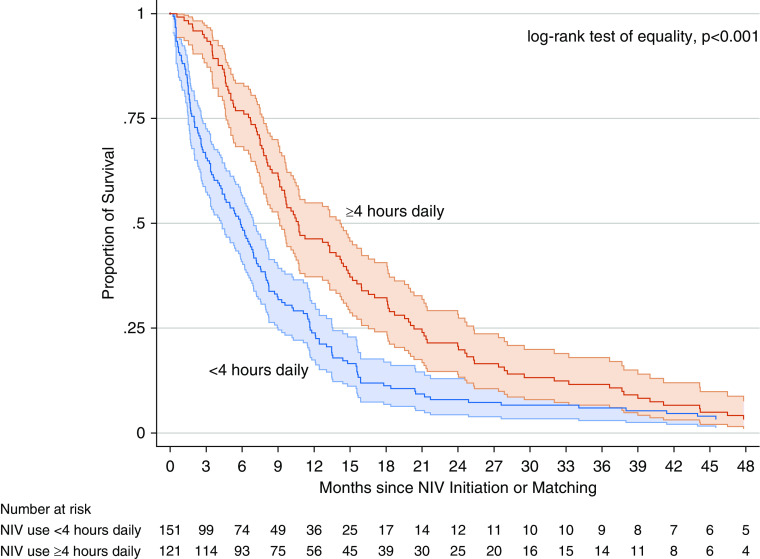
We investigated NIV subjects only (n = 272) to estimate whether average hours of NIV use was associated with survival. We found that median daily hourly NIV gradually increased early and plateaued throughout the remainder of illness (Figure E2). When adjusting for BMI and diagnosis age, we found that NIV use ≥an average of 4 h/d was associated with a 33% reduction in the rate of death (HR, 0.67; 95% CI, 0.52–0.86; P = 0.002). The median unadjusted survival time was 10.7 months (IQR, 6.7–20.3) for NIV use ≥4 h/d and was 5.9 months (IQR, 2–11.7) for NIV use <4 h/d (log-rank test of equality, P < 0.001) (Figure 1).
Figure 1.
Survival among noninvasive ventilation subjects only, stratified by average daily use ≥4 hours (n = 121) versus <4 hours (n = 151) with 95% confidence intervals. NIV = noninvasive ventilation.
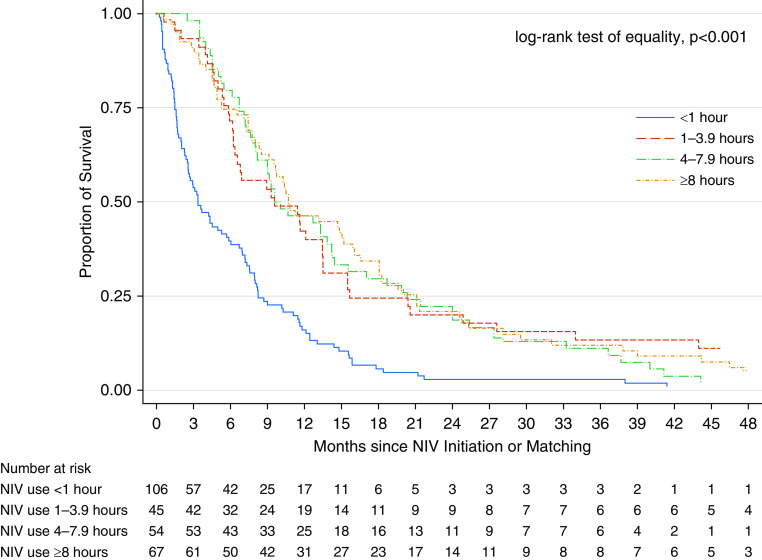
Among NIV subjects, we found that 25% (n = 67) reported average daily use of NIV for ≥8 hours, 20% (n = 54) reported use for 4–7.9 hours, 16% (n = 45) reported use for 1–3.9 hours, and 39% (n = 106) reported use for <1 hour (Table 4). When adjusting for BMI, diagnosis age, and dyspnea score among NIV subjects (n = 272), patients with shorter estimated use times had an increased rate of death compared with those with longer estimated use times (Table 4) (test for trend, P < 0.001). Specifically, patients with ALS with <1 hour of daily use had a higher rate of death compared with those patients who used NIV ≥8 hours daily. Median unadjusted survival time by subgroup was 3.7 months (IQR, 1.5–8.2) for <1 h/d, 9.6 months (IQR, 5.8–15.7) for 1–4 h/d, 9.6 months (IQR, 6.7–20.3) for 4–8 h/d, and 10.7 months (5.3–21) for >8 h/d (log-rank test of equality, P < 0.001) (Figure 2).
Table 4.
Results of survival regression analysis adjusted for average daily hours of noninvasive ventilation use among noninvasive ventilation subjects (n = 272)
| Variable | n (%) | Male, n (%) | Symptom onset site | Multivariate Analysis |
||
|---|---|---|---|---|---|---|
| Limb; Bulbar | HR | 95% CI | P Value | |||
| Average daily hours of NIV | ||||||
| ≥8 h | 67 (25) | 48 (72) | 55; 12 | — | — | — |
| 4–7.9 h | 54 (20) | 31 (57) | 45; 9 | 1.05 | 0.76–1.45 | 0.78 |
| 1–3.9 h | 45 (16) | 22 (49) | 30; 15 | 0.82 | 0.55–1.23 | 0.34 |
| <1 h | 106 (39) | 63 (59) | 76; 30 | 2.31 | 1.65–3.23 | <0.001 |
| Age at diagnosis per decade | 1.25 | 1.12–1.40 | <0.001 | |||
| Body mass index class | ||||||
| <18.5 kg/m2 | 2.29 | 1.53–3.43 | <0.001 | |||
| 18.5–24.9 kg/m2 | — | — | — | |||
| 25–29.9 kg/m2 | 0.87 | 0.64–1.17 | 0.35 | |||
| ≥30 kg/m2 | 0.69 | 0.48–0.99 | 0.049 | |||
| ALSFRS-R dyspnea score | 0.053 | |||||
| >2 | — | — | ||||
| ≤2 | 1.29 | 0.997–1.663 | ||||
Definition of abbreviations: ALSFRS-R = Amyotrophic Lateral Sclerosis Functional Rating Scale Revised; CI = confidence interval; HR = hazard ratio; NIV = noninvasive ventilation.
Figure 2.
Survival since noninvasive ventilation initiation for average daily use of noninvasive ventilation comparing <1 hour (n = 106), 1–3.9 hours (n = 45), 4–7.9 hours (n = 54), and ≥8 hours (n = 67). NIV = noninvasive ventilation.
Focusing only on limb-onset ALS (N = 333, with 127 non-NIV subjects and 206 NIV subjects), NIV use was associated with a 37% lower rate of death compared with that of non-NIV subjects after adjusting for diagnosis age, BMI, ALSFRS-R dyspnea score, and NIV hourly usage (HR, 0.63; 95% CI, 0.45–0.87; P = 0.006) (Table E3). In an adjusted analysis, we found no significant association between NIV use and survival in 119 subjects with bulbar-onset ALS (HR, 1.2; 95% CI, 0.71–2.08; P = 0.49).
In our secondary analysis of time-matched groups by diagnosis delay time and follow-up time since first visit date, we matched 263 non-NIV subjects with 357 NIV subjects (N = 620). In our survival analysis, we adjusted for diagnosis age, BMI, ALSFRS-R dyspnea score, FVC, and daily NIV usage as a time-varying covariate (Table 5). Compared with non-NIV subjects, we found a 39% decreased rate of death for NIV subjects (HR, 0.61; 95% CI, 0.46–0.82; P = 0.001). There was a 25% increased rate of death associated with a decrease in FVC (HR per 10% decrease in FVC, 1.25; 95% CI, 1.17–1.34; P < 0.001) (Table 5). When categorizing FVC, we found that a lower FVC was associated with an increased rate of death (test for trend, P < 0.001) (Table 6). There was no significant interaction between NIV use and categories of FVC. We found a nonsignificant association with symptom onset site in this multivariable model.
Table 5.
Results of secondary analysis of groups matched by diagnosis delay and follow-up time (N = 620)
| Variable | Multivariate Analysis |
||
|---|---|---|---|
| HR | 95% CI | P Value | |
| Noninvasive ventilation | 0.61 | 0.46–0.82 | 0.001 |
| Age at diagnosis per decade | 1.16 | 1.04–1.30 | 0.01 |
| Body mass index class | |||
| <18.5 kg/m2 | 1.85 | 1.14–2.99 | 0.01 |
| 18.5–24.9 kg/m2 | — | — | — |
| 25–29.9 kg/m2 | 0.76 | 0.55–1.04 | 0.09 |
| ≥30 kg/m2 | 0.61 | 0.40–0.94 | 0.03 |
| ALSFRS-R dyspnea score | |||
| >2 | — | — | — |
| ≤2 | 1.41 | 1.07–1.86 | 0.02 |
| Forced vital capacity, per 10% decrease | 1.25 | 1.17–1.34 | <0.001 |
| Time-varying covariate | |||
| Daily hours of noninvasive ventilation | 1.00 | 0.999–1.004 | 0.25 |
Definition of abbreviations: ALSFRS-R = Amyotrophic Lateral Sclerosis Functional Rating Scale Revised; CI = confidence interval; HR = hazard ratio.
Table 6.
Results of secondary analysis of groups matched by diagnosis delay and follow-up time (N = 620)
| Variable | Multivariate Analysis |
||
|---|---|---|---|
| HR | 95% CI | P Value | |
| Noninvasive ventilation | 0.68 | 0.51–0.91 | 0.01 |
| Age at diagnosis per decade | 1.18 | 1.06–1.32 | 0.004 |
| Body mass index class | |||
| <18.5 kg/m2 | 1.90 | 1.17–3.10 | 0.01 |
| 18.5–24.9 kg/m2 | — | — | — |
| 25–29.9 kg/m2 | 0.76 | 0.56–1.03 | 0.07 |
| ≥30 kg/m2 | 0.57 | 0.38–0.86 | 0.007 |
| ALSFRS-R dyspnea score | |||
| >2 | — | — | — |
| ≤2 | 1.44 | 1.09–1.89 | 0.009 |
| Forced vital capacity | |||
| <40% | 2.58 | 1.77–3.75 | <0.001 |
| 40–49% | 2.00 | 1.40–2.86 | <0.001 |
| 50–59% | 1.60 | 1.10–2.32 | 0.01 |
| ≥60% | — | — | — |
| Time-varying covariate | |||
| Daily hours of noninvasive ventilation | 1.002 | 0.999–1.005 | 0.16 |
Definition of abbreviations: ALSFRS-R = Amyotrophic Lateral Sclerosis Functional Rating Scale Revised; CI = confidence interval; HR = hazard ratio.
Discussion
We found that NIV use was associated with significantly improved survival in patients with ALS. This survival advantage appeared primarily in patients with limb-onset ALS. Higher self-reported hours of daily usage were associated with better survival in those who used NIV.
Our results are consistent with prior studies suggesting improved survival with NIV use in ALS (3, 7, 11). The RCT by Bourke and colleagues used bilevel ventilators in spontaneous/timed mode with fixed pressure settings and demonstrated a median survival of 219 days (IQR, 75–1,382) (3). Although observational, our study had a larger sample size, included most patients seen in our clinic, and used ventilators capable of adjusting inspiratory pressures to a target tidal volume. Kleopa and colleagues found improved survival with NIV use ≥4 h/d (14 mo) compared with <4 h/d (7 mo) versus no NIV use (3 mo) (11). However, this study did not use Cox proportional hazards modeling or adjustment for confounding in multivariate analyses. Berlowitz and colleagues performed a methodologically rigorous study that found a multivariate adjusted HR of 0.72 (95% CI, 0.60–0.88), which is very similar to the effect estimate in the current study (7). Berlowitz and colleagues also performed a secondary analysis with matched pairs and found an unadjusted HR similar to that of our matched study; however, it was not statistically significant (HR, 0.83; 95% CI, 0.62–1.11; P = 0.20). Our study builds on this literature with a larger cohort, matching by several possible confounding variables, and performing a multivariable-adjusted survival analysis of matched groups with time-varying adjustment for NIV hourly use.
Similar to the Berlowitz and Kleopa studies, we found that NIV subjects tended to be younger men (7, 11). Kleopa and colleagues found a higher proportion of women in the group using NIV <4 h/d. The reason for these differences remains unknown. Future studies should investigate how demographics affect NIV acceptance and clinician practice patterns.
We found that NIV was associated with a survival improvement specifically in patients with limb-onset ALS, similar to prior studies (3, 4, 8). There are several possible explanations for the lack of benefit in bulbar-onset ALS, including difficulty with oral mask fit, laryngospasm from upper motor neuron involvement, and inability to control secretions driven posteriorly under positive pressure ventilation. Patients may feel discomfort and thus become less tolerant to NIV use. Our study results support this theory, as 45% of bulbar patients used NIV on average <1 h/d, and 68% used NIV for <4 h/d. Although nasal masks may be easier to tolerate for patients with bulbar ALS, we did not record mask type for NIV subjects.
Our study findings are consistent with two prior studies that found an improved survival for patients with ALS who used NIV ≥4 h/d (11, 30). LoCoco and colleagues found that NIV use ≥4 h/d was an independent predictor of survival (relative risk, 0.32; 95% CI, 0.13–0.78). Our study also suggests a monotonic increase in survival with increasing hourly usage of NIV over time after adjustment for confounders.
The ideal threshold for NIV initiation remains controversial. In the United Sates, current medical insurance reimbursement criteria for a ventilator in neuromuscular disease (including ALS) follow the American Academy of Neurology recommendation of initiating NIV once FVC falls below 50% predicted normal (9). However, to our knowledge, this threshold has not been rigorously investigated. Lechtzin and colleagues demonstrated improved survival when starting NIV with FVC ≥65% compared with <65% if used ≥4 h/d (2.7 yr versus 1.8 yr) (31). Vitacca and colleagues found that NIV initiation at FVC ≥80% was associated with improved all-cause mortality regardless of tracheostomy use (32). The 2009 American Academy of Neurology guidelines on respiratory management of ALS recommended future research “evaluate the impact of early NIV initiation on survival and quality of life” (9). Prior work has demonstrated a clinical prediction tool that can be used at initial clinic presentation to predict 6-month risk of respiratory failure (26). In addition, FVC has been used to identify respiratory phenotypes by longitudinal FVC trajectory (27). The optimal timing and/or FVC threshold for NIV initiation remains unknown. Use of clinical prediction tools and respiratory phenotypes would facilitate the study of NIV in early ALS.
There are several mechanisms by which NIV might improve survival in ALS. Positive airway pressure during assisted ventilation may produce larger tidal volumes and increased minute ventilation. With improved minute ventilation, NIV likely improves carbon dioxide tensions while decreasing work of breathing by offloading the diaphragm. Nocturnal NIV possibly improves sleep quality, as prior polysomnographic studies have demonstrated improved gas exchange, partial pressure of carbon dioxide, and apnea–hypopnea index (33, 34). Increased lung expansion may reduce mucus plugging and improve airway atelectasis (31). The reduced respiratory fatigue, lowered carbon dioxide tensions, and improved airway clearance likely mediate a survival benefit by preventing (or at least delaying) acute respiratory failure and respiratory infections in the setting of increased aspiration risk.
Our study has several strengths. We used a large cohort with prospectively collected observational data. Such a study may represent more realistic conditions and provide generalizable real-world data, as opposed to the highly selected populations characteristic of RCTs. We performed a rigorous matching analysis to minimize confounding and verified these results across multiple analyses. As it would be unethical to randomize patients with ALS to forego NIV, our matching analysis may represent the most feasible method of studying NIV impact.
There were also several limitations to this study. An observational study comparing outcomes of those who were prescribed a therapeutic intervention and those who were not is subject to confounding by indication (confounding by severity of disease). At time of matching, the ALSFRS-R total scores were similar between the NIV and non-NIV groups (mean 28 ± 7), suggesting similar stage of disease. The NIV group had lower dyspnea scores (suggesting increased symptoms) at time of matching (Table 2). It is possible that the non-NIV group refused NIV because of fewer symptoms and that those prescribed NIV were sicker despite the matching. Given that we found a significantly better adjusted outcome with NIV, our findings might underestimate the actual effectiveness of NIV. Alternatively, NIV could be prescribed to those who were healthier, had better health literacy or socioeconomic status, or were more motivated in general, which could make NIV appear to be associated with better survival when it was not. We attempted to account for this by adjusting for patient-reported hours of daily NIV use, showing that NIV subjects with minimal adherence (average <1 h per day) had worse outcomes than those with better adherence. However, we have been cautious about implying causation, which would require a large, albeit unethical, RCT of NIV.
We did not account for FVC slope preceding NIV. For FVC decline to contribute to confounding by indication, physicians would have had to prescribe NIV to patients with more flat FVC slope and defer NIV in those with a rapidly declining FVC. However, such a prescribing pattern is counterintuitive and is not borne out in clinical practice. In addition, adjusting for FVC slope would require multiple clinical visits preceding NIV initiation, possibly leading to selection bias.
It is possible that non-NIV subjects were offered NIV at some point in their disease; however, our dataset did not record incidence or reason for NIV refusals. Measuring survival related to an intervention offered at various time points in disease is subject to immortal time bias. We attempted to control for this by matching patients both by time from symptom onset to evaluation at our clinic and follow-up time from presentation to matching. Although insurance data were not available, ALS is a Medicare-qualifying diagnosis, which likely mitigated differences in payer coverage for NIV.
NIV adherence rates were based on patient-reported averages of daily use, which is subject to recall and misclassification bias. Ventilator data monitoring was not routinely available or used for this cohort. The majority of misclassification bias is likely caused by patients overestimating hourly use in an attempt to please the physician. If NIV hourly usage rates are actually lower than recorded and patients therefore derived less benefit, then this would bias our results toward the null. NIV usage reporting may be more accurate among the extremely adherent and nonadherent. Misclassification of those with moderate adherence may mix individuals between groups and may explain the lack of survival trend with increasing hourly NIV use (Table 4). Current cloud-based ventilator data monitoring will facilitate future studies investigating NIV adherence.
Initiation of NIV and death could be misclassified. However, our follow-up was complete, and we were able to verify the date of death in 95% of patients in our cohort. Unmeasured confounding is possible; however, our variables are representative of most ALS clinical trial data. We enrolled patients from a single academic medical center, which may not be generalizable to other centers. However, our results do align with prior studies.
Conclusions
Our study suggests that NIV is associated with better survival in ALS, particularly in limb-onset disease and with increased NIV adherence. Obtaining patient and caregiver perspectives on respiratory care may improve introducing assisted ventilation in a patient-centered fashion. Given the variability in guidelines on thresholds for NIV initiation, future work should examine outcomes of starting NIV at various stages of respiratory muscle weakness. By further refining ideal candidates for NIV, we may improve outcomes through a personalized approach to respiratory care for ALS.
Supplementary Material
Footnotes
Supported by the U.S. National Institutes of Health (T32 HL-007891, K24 HL-103844, and F32 HL-144145).
Author Contributions: J.A., J.Y.H., J.H.-F., L.E., and S.M.K. designed the study and implemented the project, manuscript, and managed the submission. J.A. and J.Y.H. performed the statistical analysis. J.A. and L.E. collected the data. All authors revised the manuscript critically for important intellectual content and gave final approval of the version to be published.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Brown RH, Al-Chalabi A. Amyotrophic lateral sclerosis. N Engl J Med. 2017;377:162–172. doi: 10.1056/NEJMra1603471. [DOI] [PubMed] [Google Scholar]
- 2.Tsai C-P, Chang B-H, Lee CT-C. Underlying cause and place of death among patients with amyotrophic lateral sclerosis in Taiwan: a population-based study, 2003-2008. J Epidemiol. 2013;23:424–428. doi: 10.2188/jea.JE20130045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourke SC, Tomlinson M, Williams TL, Bullock RE, Shaw PJ, Gibson GJ. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurol. 2006;5:140–147. doi: 10.1016/S1474-4422(05)70326-4. [DOI] [PubMed] [Google Scholar]
- 4.Sancho J, Servera E, Morelot-Panzini C, Salachas F, Similowski T, Gonzalez-Bermejo J. Non-invasive ventilation effectiveness and the effect of ventilatory mode on survival in ALS patients. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:55–61. doi: 10.3109/21678421.2013.855790. [DOI] [PubMed] [Google Scholar]
- 5.Paipa AJ, Povedano M, Barcelo A, Domínguez R, Saez M, Turon J, et al. Survival benefit of multidisciplinary care in amyotrophic lateral sclerosis in Spain: association with noninvasive mechanical ventilation. J Multidiscip Healthc. 2019;12:465–470. doi: 10.2147/JMDH.S205313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bach JR. Amyotrophic lateral sclerosis: prolongation of life by noninvasive respiratory AIDS. Chest. 2002;122:92–98. doi: 10.1378/chest.122.1.92. [DOI] [PubMed] [Google Scholar]
- 7.Berlowitz DJ, Howard ME, Fiore JF, Jr, Vander Hoorn S, O’Donoghue FJ, Westlake J, et al. Identifying who will benefit from non-invasive ventilation in amyotrophic lateral sclerosis/motor neurone disease in a clinical cohort. J Neurol Neurosurg Psychiatry. 2016;87:280–286. doi: 10.1136/jnnp-2014-310055. [DOI] [PubMed] [Google Scholar]
- 8.Peysson S, Vandenberghe N, Philit F, Vial C, Petitjean T, Bouhour F, et al. Factors predicting survival following noninvasive ventilation in amyotrophic lateral sclerosis. Eur Neurol. 2008;59:164–171. doi: 10.1159/000114037. [DOI] [PubMed] [Google Scholar]
- 9.Miller RG, Jackson CE, Kasarskis EJ, England JD, Forshew D, Johnston W, et al. Quality Standards Subcommittee of the American Academy of Neurology. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: drug, nutritional, and respiratory therapies (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2009;73:1218–1226. doi: 10.1212/WNL.0b013e3181bc0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersen PM, Abrahams S, Borasio GD, de Carvalho M, Chio A, Van Damme P, et al. EFNS Task Force on Diagnosis and Management of Amyotrophic Lateral Sclerosis. EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS): revised report of an EFNS task force. Eur J Neurol. 2012;19:360–375. doi: 10.1111/j.1468-1331.2011.03501.x. [DOI] [PubMed] [Google Scholar]
- 11.Kleopa KA, Sherman M, Neal B, Romano GJ, Heiman-Patterson T. Bipap improves survival and rate of pulmonary function decline in patients with ALS. J Neurol Sci. 1999;164:82–88. doi: 10.1016/s0022-510x(99)00045-3. [DOI] [PubMed] [Google Scholar]
- 12.Sancho J, Martínez D, Bures E, Díaz JL, Ponz A, Servera E. Bulbar impairment score and survival of stable amyotrophic lateral sclerosis patients after noninvasive ventilation initiation. ERJ Open Res. 2018;4:1–10. doi: 10.1183/23120541.00159-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kent DM, van Klaveren D, Paulus JK, D’Agostino R, Goodman S, Hayward R, et al. The Predictive Approaches to Treatment effect Heterogeneity (PATH) statement: explanation and elaboration. Ann Intern Med. 2020;172:W1–W25. doi: 10.7326/M18-3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooks BR, Miller RG, Swash M, Munsat TL World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 15.Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25:1–21. doi: 10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubin DB. Bias reduction using Mahalanobis metric matching. Ets Res Bulletin Ser. 1978;1978:1–10. [Google Scholar]
- 17.Lederer DJ, Bell SC, Branson RD, Chalmers JD, Marshall R, Maslove DM, et al. Control of confounding and reporting of results in causal inference studies: guidance for authors from editors of Respiratory, Sleep, and Critical Care journals. Ann Am Thorac Soc. 2019;16:22–28. doi: 10.1513/AnnalsATS.201808-564PS. [DOI] [PubMed] [Google Scholar]
- 18.Czaplinski A, Yen AA, Simpson EP, Appel SH. Predictability of disease progression in amyotrophic lateral sclerosis. Muscle Nerve. 2006;34:702–708. doi: 10.1002/mus.20658. [DOI] [PubMed] [Google Scholar]
- 19.Czaplinski A, Yen AA, Appel SH. Amyotrophic lateral sclerosis: early predictors of prolonged survival. J Neurol. 2006;253:1428–1436. doi: 10.1007/s00415-006-0226-8. [DOI] [PubMed] [Google Scholar]
- 20.Czaplinski A, Yen AA, Appel SH. Forced vital capacity (FVC) as an indicator of survival and disease progression in an ALS clinic population. J Neurol Neurosurg Psychiatry. 2006;77:390–392. doi: 10.1136/jnnp.2005.072660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paganoni S, Deng J, Jaffa M, Cudkowicz ME, Wills AM. Body mass index, not dyslipidemia, is an independent predictor of survival in amyotrophic lateral sclerosis. Muscle Nerve. 2011;44:20–24. doi: 10.1002/mus.22114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calvo A, Moglia C, Lunetta C, Marinou K, Ticozzi N, Ferrante GD, et al. Factors predicting survival in ALS: a multicenter Italian study. J Neurol. 2017;264:54–63. doi: 10.1007/s00415-016-8313-y. [DOI] [PubMed] [Google Scholar]
- 23.Westeneng H-J, Debray TPA, Visser AE, van Eijk RPA, Rooney JPK, Calvo A, et al. Prognosis for patients with amyotrophic lateral sclerosis: development and validation of a personalised prediction model. Lancet Neurol. 2018;17:423–433. doi: 10.1016/S1474-4422(18)30089-9. [DOI] [PubMed] [Google Scholar]
- 24.Magnus T, Beck M, Giess R, Puls I, Naumann M, Toyka KV. Disease progression in amyotrophic lateral sclerosis: predictors of survival. Muscle Nerve. 2002;25:709–714. doi: 10.1002/mus.10090. [DOI] [PubMed] [Google Scholar]
- 25.Bourke SC, Bullock RE, Williams TL, Shaw PJ, Gibson GJ. Noninvasive ventilation in ALS: indications and effect on quality of life. Neurology. 2003;61:171–177. doi: 10.1212/01.wnl.0000076182.13137.38. [DOI] [PubMed] [Google Scholar]
- 26.Ackrivo J, Hansen-Flaschen J, Wileyto EP, Schwab RJ, Elman L, Kawut SM. Development of a prognostic model of respiratory insufficiency or death in amyotrophic lateral sclerosis. Eur Respir J. 2019;53:1802237. doi: 10.1183/13993003.02237-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ackrivo J, Hansen-Flaschen J, Jones BL, Wileyto EP, Schwab RJ, Elman L, et al. Classifying patients with amyotrophic lateral sclerosis by changes in FVC: a group-based trajectory analysis. Am J Respir Crit Care Med. 2019;200:1513–1521. doi: 10.1164/rccm.201902-0344OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boher J-M, Filleron T, Giorgi R, Kramar A, Cook RJ. Goodness-of-fit test for monotone proportional subdistribution hazards assumptions based on weighted residuals. Stat Med. 2017;36:362–377. doi: 10.1002/sim.7153. [DOI] [PubMed] [Google Scholar]
- 29.Schoenfeld D. Chi-squared goodness-of-fit tests for the proportional hazards regression model. Biometrika. 1980;67:145–153. [Google Scholar]
- 30.Lo Coco D, Marchese S, Pesco MC, La Bella V, Piccoli F, Lo Coco A. Noninvasive positive-pressure ventilation in ALS: predictors of tolerance and survival. Neurology. 2006;67:761–765. doi: 10.1212/01.wnl.0000227785.73714.64. [DOI] [PubMed] [Google Scholar]
- 31.Lechtzin N, Scott Y, Busse AM, Clawson LL, Kimball R, Wiener CM. Early use of non-invasive ventilation prolongs survival in subjects with ALS. Amyotroph Lateral Scler. 2007;8:185–188. doi: 10.1080/17482960701262392. [DOI] [PubMed] [Google Scholar]
- 32.Vitacca M, Montini A, Lunetta C, Banfi P, Bertella E, De Mattia E, et al. ALS RESPILOM Study Group. Impact of an early respiratory care programme with non-invasive ventilation adaptation in patients with amyotrophic lateral sclerosis. Eur J Neurol. 2018;25:556–e33. doi: 10.1111/ene.13547. [DOI] [PubMed] [Google Scholar]
- 33.Boentert M, Brenscheidt I, Glatz C, Young P. Effects of non-invasive ventilation on objective sleep and nocturnal respiration in patients with amyotrophic lateral sclerosis. J Neurol. 2015;262:2073–2082. doi: 10.1007/s00415-015-7822-4. [DOI] [PubMed] [Google Scholar]
- 34.Vrijsen B, Buyse B, Belge C, Robberecht W, Van Damme P, Decramer M, et al. Noninvasive ventilation improves sleep in amyotrophic lateral sclerosis: a prospective polysomnographic study. J Clin Sleep Med. 2015;11:559–566. doi: 10.5664/jcsm.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.