Abstract
To minimize transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the novel coronavirus responsible for coronavirus disease (COVID-19), the U.S. Centers for Disease Control and Prevention and the World Health Organization recommend wearing face masks in public. Some have expressed concern that these may affect the cardiopulmonary system by increasing the work of breathing, altering pulmonary gas exchange and increasing dyspnea, especially during physical activity. These concerns have been derived largely from studies evaluating devices intentionally designed to severely affect respiratory mechanics and gas exchange. We review the literature on the effects of various face masks and respirators on the respiratory system during physical activity using data from several models: cloth face coverings and surgical masks, N95 respirators, industrial respirators, and applied highly resistive or high–dead space respiratory loads. Overall, the available data suggest that although dyspnea may be increased and alter perceived effort with activity, the effects on work of breathing, blood gases, and other physiological parameters imposed by face masks during physical activity are small, often too small to be detected, even during very heavy exercise. There is no current evidence to support sex-based or age-based differences in the physiological responses to exercise while wearing a face mask. Although the available data suggest that negative effects of using cloth or surgical face masks during physical activity in healthy individuals are negligible and unlikely to impact exercise tolerance significantly, for some individuals with severe cardiopulmonary disease, any added resistance and/or minor changes in blood gases may evoke considerably more dyspnea and, thus, affect exercise capacity.
Keywords: face mask, SARS-CoV-2, pulmonary limitations to exercise
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the novel coronavirus responsible for coronavirus disease (COVID-19), has infected millions of individuals worldwide, resulting in over two million deaths. There is evidence for airborne transmission via both droplets and aerosols that contact mucosal surfaces and are inhaled directly into the upper airway (1), potentially infecting many people (2).
To minimize the risk of transmission of SARS-CoV-2, both the U.S. Centers for Disease Control and Prevention (3) and the World Health Organization (4) recommend wearing masks or face coverings in public, especially when physical distancing is impossible. Because any potentially negative effects of face masks are believed to be exacerbated by exercise, face masks are not universally required during exercise, even in indoor environments such as gyms and fitness centers, where the risk of a superspreading event increases (5). Purported reasons for not wearing a face mask include concerns about increased dyspnea and work of breathing (Wb) as well as about alterations in pulmonary gas exchange associated with reduced ventilation and rebreathing of exhaled carbon dioxide (4).
The purpose of this review is to synthesize the available literature on the effects of various masks and face coverings on the cardiorespiratory system during physical activity/exercise. Although more high-quality data from well-designed studies are needed, there is a substantial body of literature evaluating various effects on the cardiopulmonary system caused by the following types of face masks: low-resistance face coverings (i.e., cloth and surgical masks); N95 respirators; industrial respirators, such as self-contained breathing apparatuses (SCBAs); and applied external resistors, which generate high resistive loads or added dead space and are used in research studies.
Exercise and the Cardiopulmonary System
The healthy cardiopulmonary system is overbuilt for sedentary life but is challenged by physical activity. As exercise intensity increases, ventilation rises through an increase in breathing frequency and tidal volume. The increase in ventilation is approximately linear until the ventilatory threshold at about 60–70% of maximal exercise capacity is reached, after which it rises at a faster rate as carbon dioxide (CO2) production increases and arterial pH falls. In contrast, oxygen consumption (o2) and cardiac output increase linearly with workload until maximal exercise (see Reference 6 for review). The arterial O2 partial pressure (PaO2) is unchanged in most healthy subjects but may decrease in some patients and some highly trained athletes (reviewed in Reference 7). In the discussion that follows, we categorize the intensity of physical activity/exercise as light (20–40% of maximal o2 [o2max]), including activities such as yoga, walking, or daily activities; moderate (40–60% of o2max), including activities such as brisk walking; vigorous (60–85% of o2max), including activities such as jogging; and high and/or maximal (>85% of o2max) (8).
Mask Filtration and Resistance
A wide range of face masks are available, including loose-fitting handkerchiefs, homemade fabric masks, surgical masks, tight-fitting industrial and healthcare-standard respirators (e.g., N95) (9), and SCBAs (e.g., for firefighting use). Factors influencing filtration ability include the material, structure (e.g., knit, woven or fused), number of layers, shape (surgical style, conical, or duckbill), and facial fit (10). Well-fitted respirators are required to achieve >95% filtration of aerosols under standardized testing conditions. Medical-type surgical masks with an adjustable nose wire attain 50–90% filtration when used as designed, with most of the variability resulting from the quality of fit (11). When made either commercially or at home from tightly woven cotton, cloth face masks provide variable particle filtration when properly worn, ranging from <30% to up to ∼90% (11). Thus, the filtering protection conferred by masks is variable, although typically stable, over time and across flow rates of 30–85 L · min−1 (12). Moisture exerts only minimal influence on filtration effectiveness, likely without practical consequence (13). The filtering effects of face masks appear to be less effective in children (11, 12), likely because of problems with achieving adequate fit.
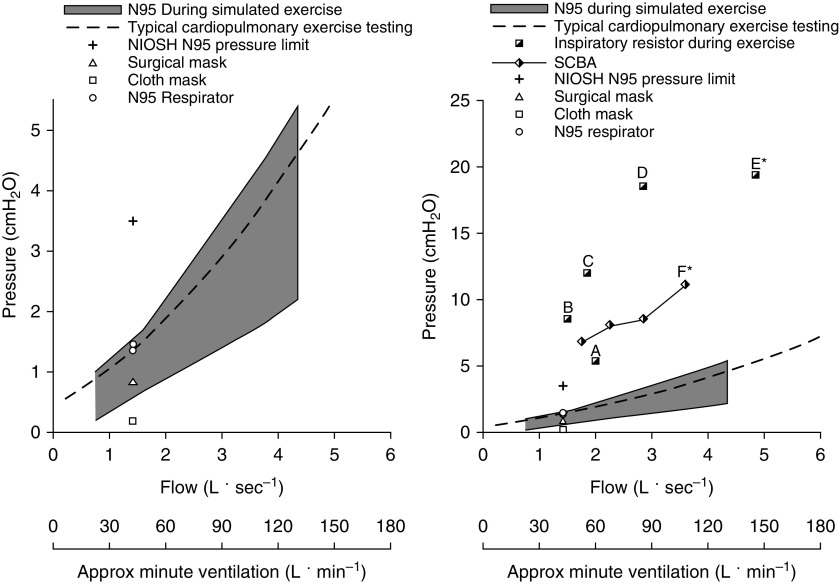
Resistance to airflow is a key element of face-mask function, as it reduces forward particle velocity and, potentially, the risk of infection among people in the vicinity of an infected individual (14). As shown in Figure 1, the National Institute for Occupational Safety and Health guidelines require that for standardized respirators (e.g., N95 respirators), the pressure drop across the mask cannot exceed 3.5 and 2.5 cm H2O for inspiration and expiration, respectively, at a standardized constant flow of 85 L · min−1 (9). Importantly, these limits represent maximal allowable values, and reported pressure drops are often significantly lower. For N95 respirators, the observed pressure drop is ∼0.4 cm H2O at a flow rate of at least 30 L · min−1 and no higher than 1.7 cm H2O at 85 L · min−1 (11, 15) (see Figure 1). Given that humans do not breathe at a constant flow rate, 85 L · min−1 of constant flow is comparable with an exercise ventilation of ∼30–50 L · min−1 (16), such as would occur during moderate-to-vigorous activity for healthy untrained individuals.
Figure 1.
Pressure difference across various masks, respirators, and resistors relative to flow (L · s−1) and measured or estimated minute ventilation (L · min−1) (16). Pressure difference/flow = resistance. The plot on the left displays data up to 5 cm H2O, whereas the graph on the right displays data up to 25 cm H2O. Minute ventilation was directly measured in human trials (16) or estimated on the basis of the reported flow in simulation trials (17) and extrapolated back to human data (16). The dashed line represents the reported pressure of a typical mouthpiece setup used in a cardiopulmonary exercise test (CPET) (19). The shaded area represents the reported pressure difference of an N95 respirator across various simulated flow rates (17). The + displays the peak inspiratory pressure allowed under National Institute for Occupational Safety and Health (NIOSH) guidelines at a standard flow of 1.4 L · s−1 (i.e., 85 L · min−1) (77). Surgical (triangle), cloth (square), and respirator (circle) data are reported resistances at 85 L · min−1 (11). The split square represents experimental resistors (17, 41), and the split diamond represents self-contained breathing apparatuses (SCBAs) (21). Surgical and cloth masks and respirators all have a mouth pressure/resistance that is well below NIOSH guidelines (9). When tested up to a minute ventilation of ∼120 L · min−1, N95 respirators have an airflow resistance that is similar to what is observed with a standard CPET mouthpiece setup (17, 19). External resistors provided a resistance that is 5–10 times the resistance of a typical mask. When these resistors are used, no change in dyspnea (points “A” and “B”) (19, 41) or metaboreflex (points “C” and “D”) (37) activation has been observed up to a ventilation of ∼90 L · min−1. It is only during intense exercise, when ventilating at ∼150 L · min−1 with a resistor, that the metaboreflex is initiated (point “E*”) (38). The SCBA provides a resistance that is 3–5 times greater than that of an N95 respirator, and only at a minute ventilation of >110 L · min−1 is the work of breathing greater than that observed with a standard a CPET mouthpiece (point “F*”) (21). *Indicates measurable changes. Approx = approximate.
Higher-intensity exercise necessitates higher ventilation. This results in greater airflow resistance, which does not necessarily increase linearly with increasing ventilation or flow rate. As expected, N95 respirators provide the greatest amount of protection but also have greater resistance than surgical masks/face masks. However, even at a ventilation >100 L · min−1, breathing simulation studies have shown that the resistance imposed by N95 respirators is <2 cm H2O · L−1 · s−1 (17) and remains low after prolonged simulated use (18). This resistance is similar to the resistance observed with the mouthpiece and tubing used during a standard cardiopulmonary exercise test (CPET) (19) (Figure 1). Surgical face masks have a mean pressure drop of <1 cm H2O at 85 L · min−1 of constant flow, with no difference observed when tested with inspired versus expired flow (11). The pressure drop with a handkerchief or two-layer cotton face mask at 85 L · min−1 has also been shown be <1 cm H2O (10), which is within the limit recommended by the World Health Organization for a nonmedical face mask (11). The testing described previously does not include extremely high minute ventilations and flow rates (e.g., >150 L · min−1) that can be achieved by exceptional aerobic athletes. The pressure drop across masks may be somewhat larger in such athletes at these high minute ventilations, and further research will be helpful to elucidate the precise effects of cloth and surgical masks on the cardiorespiratory system in highly trained athletes. However, it should be noted that the pressure drop across such masks would still be substantially less than that observed with applied external resisters as discussed below.
Wb
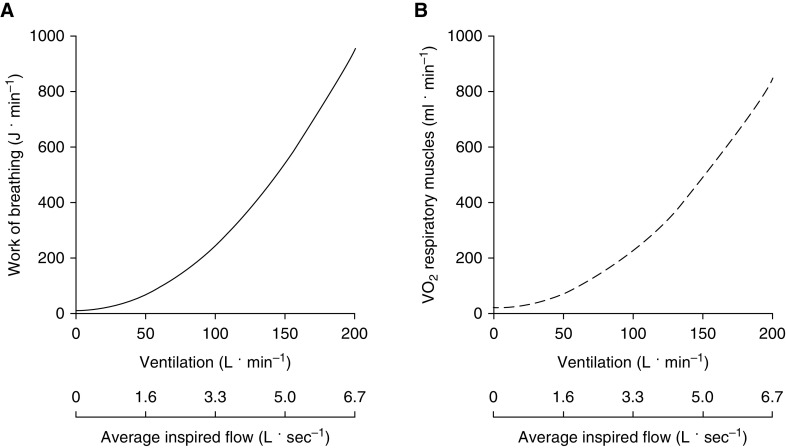
In healthy adults, the Wb at rest and during light exercise is minimal (1–3% of whole-body o2) and is almost exclusively the result of inspiratory elastic work (reviewed in Reference 20). As ventilation increases during exercise, the Wb rises in a curvilinear manner, primarily because of increased resistive work secondary to increased airflow, reaching 20–30 times resting levels during high-intensity exercise (Figure 2).
Figure 2.
Average (A) work of breathing and (B) o2 of the respiratory muscles across a range of minute ventilation and flow rates in healthy young males and females (16). The average inspired flow values were calculated on the basis of composite flow-volume loops from the same subjects. o2 = oxygen consumption.
Anything covering the mouth/nose has the potential to increase the resistive Wb. The majority of published data on Wb during physical activity have evaluated respirators such as N95 respirators and SCBAs used in industrial applications and firefighting. The SCBA provides ∼3 cm H2O · L−1 · s−1 of resistance (21) during exercise (see Figure 1), but the Wb is not greater during vigorous/high-intensity exercise when compared with a standard CPET system. It is not until exercise ventilation exceeds 110 L · min−1—which is very high and unlikely to be attained by most untrained individuals—that a significant increase in Wb with the SCBA is observed (21) (see Figure 1).
As mentioned previously, N95 respirators produce a pressure drop of <1.7 cm H2O at a minute ventilation of ∼30–50 L · min−1 (11). The added resistance at this ventilation is estimated to increase total Wb by ∼5 J · min−1 (i.e., 7–13%) and o2 by a trivial amount of ∼4 ml · min−1 (i.e., ∼0.25% of whole-body o2) (see Figure 2). As shown in Figure 1, the pressure drop from an N95 respirator is also similar to that of a CPET system and is well below the threshold at which increases in Wb are observed with a SCBA (Figure 1). With a mean pressure drop of <1 cm H2O at a constant flow of 85 L · min−1, the airflow resistance of surgical masks is less than that of a CPET system (Figure 1) (16, 20). In keeping with this, face masks with resistances similar to those of surgical and cloth masks have not been shown to significantly alter ventilation, breathing frequency, or tidal volume after 1 hour of light-to-moderate–intensity treadmill exercise (22). Importantly, healthy individuals have undertaken several weeks of high-intensity exercise training while wearing face masks that are specifically designed to cause a substantial load on the respiratory muscles (23) without reported adverse events, further suggesting that wearing a face mask/respirator during exercise is unlikely to cause harm in healthy individuals.
Arterial Blood Gases
Under normal unmasked conditions, inspired fresh air mixes with the previously exhaled air contained within anatomical dead space and is warmed and humidified before reaching the alveoli where gas exchange occurs, lowering O2 and increasing CO2 partial pressure. The net result is that the fractional concentration of O2 falls from 21% in ambient air (i.e., inspired partial pressure of O2 of ∼160 mm Hg at sea level) to a mean of ∼14–15% (PO2 of ∼100 mm Hg) in the alveolar space, whereas the fractional concentration of CO2 rises from essentially zero to ∼5–6% (alveolar CO2 partial pressure [PaCO2] of ∼40 mm Hg). In addition to the small added inspiratory and expiratory resistance to breathing discussed earlier, another potential issue with face masks is the inspiration of some fraction of the previously exhaled tidal volume that is partially depleted of O2 and enriched with CO2 (i.e., increased dead space). It is important to recognize that the concentrations of O2 and CO2 measured inside a face mask in published studies do not represent the gas concentrations delivered to the airways because these measurements represent the average of expired and inspired values. Thus, the true inspired fractions of O2 and CO2 will be higher and lower, respectively, and dependent on the metabolic rate and the amount of inspired fresh ambient air. The relative contributions of increased respiratory frequency and increased tidal volume to the increase in ventilation with exercise is also important: increasing tidal volume will result in the inspiration of more fresh ambient air (i.e., less dead space) than will increasing frequency. As both ventilation and inspiratory flow increase with exercise, there will be more entrainment of ambient air so that the effective inspired O2 concentration will rise, whereas the concentration of CO2 will fall (17, 24).
Generally at sea level, any fall in the inspired O2 fraction and the corresponding decrease in PaO2 does not stimulate increased ventilation via peripheral chemoreceptors until PaO2 is <60 mm Hg (25); this extent of hypoxemia is not expected with face masks (see below). With some degree of hypercapnia, the threshold for hypoxic stimulation moves to a higher PaO2. Nevertheless, it is the reinspiration of CO2 that would be the driving force for any increases in ventilation when breathing through a face mask. In normoxia, even a 1–mm Hg rise in PaCO2 will stimulate ventilation (26). Importantly, any changes in ventilation will be greater with exertion because the higher metabolic rate with exercise itself increases the ventilatory responsiveness to CO2 and O2 (27, 28).
There are limited data reporting arterial blood gases during exercise while wearing a face mask. Arterial saturation remains above 97% while wearing a surgical mask or N95 respirator while exercising at moderate intensity for 60 minutes (29, 30), indicating that changes in PaO2 sufficient to affect ventilation are unlikely. When breathing through a full-face industrial respiratory mask, the inspired fraction of CO2 was 1.5% at rest and decreased to 1.0% during heavy exercise (24). Of note, talking while exercising through a mask generally increased the inspired fraction of CO2 by ∼0.5% over not talking (24). A recent study examined the exercise responses with surgical masks and N95 respirators (31). Capillary PaO2, CO2, and pH at peak exercise did not differ among users of surgical masks, N95 respirators, and standard CPET face masks, suggesting that alveolar ventilation/gas exchange are not significantly impacted by face masks (31). Work using applied external dead-space loading as a means to stimulate the respiratory system generally shows little change in the end-tidal or arterial CO2 until the applied dead space is greater than 100–200 ml (32–34), a value that is larger than that expected with most face masks, other than some industrial respirators. However, studies measuring transcutaneous CO2 as a proxy for PaCO2 in young healthy adults show small increases of 1–2 mm Hg during moderate-intensity treadmill walking with an N95 respirator compared with being unmasked (29). The reason for the differences between these studies are unclear, but when viewed together, the studies suggest that these respirators may increase ventilation with exercise depending on an individual’s ventilatory response to CO2, with only limited effects on the PaO2.
Sympathetic Nervous System, Muscle Blood Flow, Cardiac Output, and Cerebral Blood Flow
During exercise, reflexes from limb skeletal muscle mediate increased sympathetic outflow to the systemic circulation to ensure adequate perfusion of a large active muscle mass and maintain arterial blood pressure. These reflexes originate in nerve endings (groups III–IV) in skeletal muscle and are activated by mechanical deformation, venous distention, and metabolite accumulation. Similar phenomena occur with the respiratory musculature (35).
Muscle Blood Flow and Fatigue
Studies designed to unload the respiratory system demonstrate that the normal work done by respiratory muscles affects vascular conductance, sympathetic vasomotor outflow, diaphragmatic fatigue, locomotor muscle fatigue, dyspnea, leg discomfort, and exercise performance during maximal exercise (see Reference 36 for review). These reflex effects are minor or absent during submaximal exercise (37).
The effect of increasing Wb during exercise has been studied by adding external resistors to markedly increase airflow resistance. For example, increasing inspiratory resistance by 3–10 cm H2O · L−1 · s−1 (see point “D” in Figure 1) during submaximal exercise elicits a 50–70% increase in the Wb, with no change to leg blood flow or sympathetic activity. Moreover, an increase in inspiratory resistance of this magnitude is not associated with changes in heart rate, blood pressure, arterial blood gases, lactate, or pH (37). Thus, given the low resistance of face coverings and surgical masks, they are unlikely to alter sympathetically mediated vascular control and limb fatigue.
Cardiac Output
Cardiac output during exercise is largely unaffected by increased Wb, even when Wb is experimentally increased by 50% during maximal exercise (38). At those high airflow resistances, there is a redistribution of blood flow from other working muscles toward the respiratory muscles to facilitate the increased Wb. This only occurs to a substantial degree, however, when the exercise intensity (>90% of o2max) and ventilation (∼150 L · min−1) are all very high and airway resistance is well in excess of resistance due to any mask or respirator (>3–7 cm H2O · L−1 · s−1) (38) (Figure 1). At lower exercise intensities and with lower airway resistance (i.e., face mask or N95 respirator), o2 (and thus cardiac output and/or oxygen extraction) increases minimally above values measured under conditions of normal airway resistance (37), whereas at maximal exercise, cardiac output is not changed by surgical masks or N95 respirators (31).
Cerebral Blood Flow
Cerebral blood flow is tightly regulated and remains relatively constant under a variety of physiological conditions. Changes in PaO2 and PaCO2 alter cerebral blood flow, with marked increases seen when the PaO2 falls below 50 mm Hg (39) or with slight increases in PaCO2 and accompanying decreases in brain-tissue pH (40). These are protective mechanisms that maintain constant cerebral blood flow and oxygen delivery under conditions far more abnormal than those experienced with the minimal alterations in PaO2 and PaCO2 when wearing a cloth mask or N95 respirator, as discussed above.
Dyspnea
Some individuals may be reluctant to exercise with masks because of increased dyspnea, a complex symptom defined as “a subjective experience of breathing discomfort that consists of qualitatively distinct sensations that vary in intensity” (28). Well-controlled laboratory experiments in healthy participants show that dyspnea-intensity ratings are not increased by low, externally imposed respiratory resistance (i.e., 2.7 cm H2O · L−1 · s−1) during high-intensity exercise (41). This was also true of higher applied resistances (i.e., 5.7 cm H2O · L−1 · s−1) during moderate-intensity exercise, despite a ∼40–50% increase in the Wb (19). Importantly, the amount of resistance in these studies far exceed resistance values in N95, cloth, and surgical face masks (see Figure 1).
It is possible that rebreathing a small volume of exhaled gas (i.e., ∼50–100 ml of added dead space) while wearing a face mask during exercise would increase dyspnea because of the effect of CO2 (42). During exercise with large applied additional dead space (i.e., 600 ml), healthy adults and those with chronic obstructive pulmonary disease (COPD) have higher end-tidal Pco2, higher minute ventilation, and more dyspnea than they have during exercise without additional dead space; however, the relationship between minute ventilation and dyspnea remains unaltered (43). Indeed, ventilatory stimulation with inhaled CO2 during incremental exercise has no effect on dyspnea at a given absolute ventilation in healthy adults (44). Thus, if wearing a face mask increases dyspnea during exercise as a result of CO2 rebreathing, this effect is attributable to the perception of increased ventilation rather than the increased PaCO2.
Although controlled laboratory experiments provide valuable insight into the relationship between externally imposed respiratory resistance and exertional dyspnea, they do not fully replicate the sensory experience of wearing face masks, which has resulted in conflicting findings. Several studies have been conducted to evaluate the effects of different face masks on dyspnea during light-to-moderate exercise intensities. Despite the varying experimental protocols, mask types, amount of resistance, and language used to evaluate dyspnea (e.g., “breathing resistance,” “breathing discomfort,” “inspiratory/expiratory effort,” etc.), most studies demonstrate increased dyspnea with face masks compared with controls (15, 45, 46), although this is not a universal finding (22). The discrepancy between studies on face masks (15, 45, 46) and studies adding external resistance to a breathing apparatus (41, 47) may be related, at least in part, to the type of resistance used (i.e., inspiratory vs. combined inspiratory + expiratory resistance), challenges associated with blinding participants, moisture- and temperature-related factors with face masks versus mouthpieces, and flexibility of soft face masks that may collapse and potentially increase dyspnea during exercise. The mechanisms of increased dyspnea with face masks are complicated by the fact that several studies fail to show changes in most physiological variables, despite increased dyspnea (15, 45). However, this also suggests that people may adapt to mask-wearing over time, as has been observed in patients who initially report symptoms of claustrophobia with continuous positive airway pressure devices (48).
Although this is speculative, some posit that increased facial skin temperature, face-mask moisture/heat, or temperature of the inhaled air could contribute to increased dyspnea when wearing a face mask (15). Of these possibilities, increased temperature of the ambient air has been shown to have a larger effect than humidity on participant-reported mask comfort, with increased humidity only affecting participant-reported face-mask comfort when the ambient air was above 25°C (49). Increasing facial airflow using a fan, which reduces the temperature and humidity of the air near the face, decreases dyspnea in healthy adults and those with COPD (50), suggesting that face masks may increase dyspnea by raising facial temperature/humidity.
Special Populations
Older Adults
The impacts of aging on the physiological and perceptual responses to exercise are well characterized (see Reference 51 for review). There is a need for further data on the effects of face masks on the cardiopulmonary response to exercise in this population. However, on the basis of current understanding of the effects of aging, it is unlikely that wearing a face mask during exercise would differentially affect younger and older adults for four main reasons. First, although aging increases the ventilatory cost of exercise at a given absolute intensity (47), older adults are likely to exercise at relative (rather than absolute) intensities similar to those of their younger counterparts. In this context, older and younger adults have a similar absolute ventilation for a given relative submaximal exercise intensity (47), meaning that any additional load on the respiratory muscles imposed by a face mask would also be similar. Second, the negative intrathoracic pressure swings associated with small elevations in the Wb while wearing a face mask during exercise are likely similar in older and younger adults and are too small to have a meaningful effect on stroke volume (52). Third, during work-related tasks, men over 45 years old are able to tolerate respiratory resistances well in excess of those caused by N95 respirators or cloth and surgical masks (i.e., ranging from 3.1 to 14.7 cm H2O · L−1 · s−1 at a constant flow of 1.67 L · s−1) to an extent similar to that of younger men (53). In fact, the addition of a respiratory resistance (i.e., 5.7 cm H2O · L−1 · s−1) does not affect dyspnea during moderate-intensity exercise in older men and women (19). Fourth, added ventilatory stimulation (via dead-space loading) has a similar effect on the mechanical ventilatory, gas-exchange, and perceptual responses to exercise in older and younger men, and the associated reduction in peak exercise capacity does not differ on the basis of age (54).
Pediatrics
There are important differences in respiratory physiology in infants and young children as compared with adults (see Reference 55 for review). Infants and young children have underdeveloped accessory muscles of respiration and thus rely more on the diaphragm for most of the Wb. An increase in respiratory muscle work is largely accomplished by an increase in the respiratory rate, and the diaphragm can become fatigued more quickly than in adults. Children under the age of 6 years have proportionally more extrathoracic anatomical dead space owing to the larger ratio of head size to body size (56). These anatomical differences combined with an inherently higher basal metabolic rate place infants and young children at greater risk of respiratory failure than adults from various significant health threats. These differences decrease as children age, and other than in children younger than 2 years and those with significant respiratory or neurological conditions, there are no significant differences in respiratory physiology for older children and adolescents that are expected to substantially alter the effects of masks as described above, but additional data are needed to clarify this issue.
Sex-based Differences
Compared with males, females have smaller lungs and rib cages and disproportionally smaller large conducting airways (57). These sex differences in respiratory system morphology affect the integrative response to exercise by influencing Wb, dyspnea, blood-gas homeostasis, and cardiovascular function (57). For example, narrower airways in females result in a greater resistive (∼50% greater) and total Wb (∼20% greater) during exercise when ventilation exceeds ∼60 L · min−1 (16, 58).
Males typically have a higher minute ventilation and generate greater air flow at a given relative, but not absolute, exercise intensity. Because the external resistance offered by a face mask is flow dependent, males may have a greater increase in Wb because of higher absolute flows while wearing a face mask. However, the additional Wb associated with a face mask during exercise is small (see Figure 1), and the associated physiological and perceptual consequences are likely correspondingly minor. The addition of external resistance (i.e., 5.7 cm H2O · L−1 · s−1) to increase Wb during moderate-intensity exercise in older (i.e., 60–80 yr) adults increases the absolute Wb to a greater extent in males than in females, but the relative increase in Wb is similar between sexes. Importantly, the external resistance used in this study had no effect on dyspnea in either sex (19). However, in one study of standardized simulated work tasks while wearing an N95 respirator, females reported higher symptom scores than males (59).
Patients with Cardiopulmonary Disease
On the surface, the addition of a small increase in the Wb and reinspiration of low concentrations of CO2 with any type of face mask would appear to pose more problems for individuals with underlying cardiopulmonary disease. Other drawbacks for such individuals with face-mask wearing may include anxiety and greater dyspnea (60, 61), reduced fine-motor performance (62), possible cognitive effects as a result of slight CO2 retention and mildly increased hypoxemia, and increased Wb (63).
Increased temperature around the face (64) and a 0.5°C body-temperature elevation with loss of normal respiratory heat dissipation (65) may also have effects. Patients with mild-to-moderate pulmonary disease will likely tolerate cloth/surgical masks with an acceptable extent of discomfort, but with advanced disease, this may become more burdensome because of the effects of mask wearing described above (66, 67). More efficient filtering masks will be difficult for almost anyone with severe nonasthmatic lung disease and may warrant closer monitoring of symptoms and arterial saturation with oximetry. Patients with altered ventilatory control and blunted drives to breathe, such as those with obesity hypoventilation syndrome, may also warrant monitoring for greater hypoxemia and increased CO2 retention, resulting from potential small increases in dead space with a face mask.
Data regarding face-mask use with exercise in cardiopulmonary disease are very limited. Patients with COPD and high dyspnea scores or markedly impaired pulmonary function (forced expiratory volume in 1 s [FEV1] < 30% predicted) may be less likely to tolerate moderate exercise, such as a that within a 6-minute walk test, while wearing an N95 respirator, with a 1.5–mm Hg greater rise in end-tidal CO2 and a 1% greater fall in O2 saturation as measured by pulse oximetry (SpO2) (68) when compared with performing the test without a mask. However, a recent study demonstrated no changes in SpO2 and end-tidal CO2 in patients with severe COPD (mean FEV1 = 44%) at rest while wearing a surgical mask for up to 30 minutes (69). Furthermore, when these patients performed a 6-minute walk test while wearing a surgical mask, PaCO2 increased by <1 mm Hg, indicating that significant alveolar hypoventilation and CO2 retention is unlikely to be induced by surgical masks during self-paced exercise.
The addition of 5 cm H2O · L−1 · s−1 of inspiratory resistance and 1.5 cm H2O · L−1 · s−1 of expiratory resistance during exercise at a o2 of 0.8 L · min−1 resulted in declines in respiratory rate and ventilation and increases in tidal volume, end-tidal CO2, and mouth-pressure swings in individuals with various forms of parenchymal restrictive lung disease (70). However, with the exception of the larger mouth-pressure swings, there were no significant differences in the magnitude of these changes when compared with healthy control subjects (70). Importantly, these external resistances are greater than would be expected from surgical or other face masks. Although expiratory loading improves the stroke volume index and cardiac index during semirecumbent exercise at 60% of maximal exercise capacity in individuals with heart failure (71), no studies have examined the specific effects of respirator masks on exercise in heart failure or other forms of cardiac disease. Given the lesser amounts of expiratory resistance with a more loosely fitting face mask, it is unlikely that patients with heart failure will experience these benefits.
For at least one particular form of lung disease, however—exercise-induced bronchoconstriction—face masks may have beneficial effects with exercise. Multiple studies (72–74) have demonstrated that wearing a face mask is associated with a smaller decline in FEV1 with exercise in cold and/or dry air compared with control conditions. Although most studies used face masks with heat and moisture exchangers—masks that would not likely be widely used as part of COVID-19–prevention protocols—similar benefits have also been demonstrated with standard surgical face masks (75) or woolen scarves (76), which have been used widely during the current pandemic.
Conclusions
This review has examined the effects of various face masks and on the physiological and perceptual responses to physical activity. Although the body of literature directly evaluating this issue is evolving, for healthy individuals, the available data suggest that face masks, including N95 respirators, surgical masks, and cloth face masks, may increase dyspnea but have small and often difficult-to-detect effects on Wb, blood gases, and other physiological parameters during physical activity, even with heavy/maximal exercise. There is currently no evidence to suggest that wearing a face mask during exercise disproportionally hinders younger or older individuals, and significant sex-based differences are not expected. Depending on the severity of their underlying illness, individuals with cardiopulmonary disease are more likely than healthy individuals to experience increased exertional dyspnea with a face mask because of small increases in resistance and reinspiration of warmer and slightly enriched CO2 air. Such problems may serve as a basis for seeking exemptions from mask regulations, but the benefits of decreased dyspnea will need to be weighed against the risks of contracting the SARS-CoV-2 infection.
Supplementary Material
Acknowledgment
The authors thank Andra Scott for assisting with the review of literature.
Footnotes
Supported by the U.S. National Institutes of Health (NIH) (R01HL129990 and R01HL119201), the Canadian Institutes of Health Research, and the Natural Sciences and Engineering Research Council of Canada. S.R.H. was supported by NIH grants HL-119201 and HL-129990. A.W.S. was supported by the Natural Sciences and Engineering Council of Canada. R.C.S. was supported by NIH grants HL-119201 and HL-129990. M.K.S. was supported by grants from the Canadian Institutes of Health Research and the Natural Sciences and Engineering Research Council of Canada.
Author Contributions: Data interpretation was performed by S.R.H., P.B.D., C.K.D., J.A.G., A.M.L., Y.M.-S., R.C.S., A.W.S., E.R.S., and M.K.S. The manuscript was drafted and revised by all authors. All authors provided final approval of the final version to be published.
CME will be available for this article at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Byambasuren O, Cardona M, Bell K, Clark J, McLaws ML, Glasziou P. Estimating the extent of true asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. JAMMI. doi: 10.3138/jammi-2020-0030. [online ahead of print] 9 Oct 2020; DOI: 10.3138/jammi-2020-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang R, Li Y, Zhang AL, Wang Y, Molina MJ. Identifying airborne transmission as the dominant route for the spread of COVID-19. Proc Natl Acad Sci USA. 2020;117:14857–14863. doi: 10.1073/pnas.2009637117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Division of Viral Diseases, National Center for Immunization and Respiratory Diseases (NCIRD), U.S. Centers for Disease Control and Prevention. Atlanta, GA: U.S. Centers for Disease Control and Prevention; 2020. Recommendation regarding the use of cloth face coverings, especially in areas of significant community-based transmission. [accessed 2020 Jul 23]. Available from: https://stacks.cdc.gov/view/cdc/86440. [Google Scholar]
- 4.World Health Organization. Geneva, Switzerland: World Health Organization; 2020. Advice on the use of masks in the context of COVID-19: interim guidance. [updated 2020 Jun 5; accessed 2020 Jul 23]. Available from: https://apps.who.int/iris/bitstream/handle/10665/332293/WHO-2019-nCov-IPC_Masks-2020.4-eng.pdf?sequence=1&isAllowed=y. [Google Scholar]
- 5.Hamner L, Dubbel P, Capron I, Ross A, Jordan A, Lee J, et al. High SARS-CoV-2 attack rate following exposure at a choir practice: Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:606–610. doi: 10.15585/mmwr.mm6919e6. [DOI] [PubMed] [Google Scholar]
- 6.American Thoracic Society; American College of Chest Physicians. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 7.Stickland MK, Lindinger MI, Olfert IM, Heigenhauser GJ, Hopkins SR. Pulmonary gas exchange and acid-base balance during exercise. Compr Physiol. 2013;3:693–739. doi: 10.1002/cphy.c110048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norton K, Norton L, Sadgrove D. Position statement on physical activity and exercise intensity terminology. J Sci Med Sport. 2010;13:496–502. doi: 10.1016/j.jsams.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 9.National Institute for Occupational Safety and Health. 42 CFR 84: respiratory protective devices—final rules and notice. Fed Regist. 1995;60:30336–30398. [Google Scholar]
- 10.Zangmeister CD, Radney JG, Vicenzi EP, Weaver JL. Filtration efficiencies of nanoscale aerosol by cloth mask materials used to slow the spread of SARS-CoV-2. ACS Nano. 2020;14:9188–9200. doi: 10.1021/acsnano.0c05025. [DOI] [PubMed] [Google Scholar]
- 11.Jung H, Kim JK, Lee S, Lee J, Kim J, Tsai P, et al. Comparison of filtration efficiency and pressure drop in anti-yellow sand masks, quarantine masks, medical masks, general masks, and handkerchiefs. Aerosol Air Qual Res. 2013;14:991–1002. [Google Scholar]
- 12.van der Sande M, Teunis P, Sabel R. Professional and home-made face masks reduce exposure to respiratory infections among the general population. PLoS One. 2008;3:e2618. doi: 10.1371/journal.pone.0002618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brosseau LM, McCullough NV, Vesley D. Mycobacterial aerosol collection efficiency of respirator and surgical mask filters under varying conditions of flow and humidity. Appl Occup Environ Hyg. 1997;12:435–445. doi: 10.1016/S0003-4878(97)00022-7. [DOI] [PubMed] [Google Scholar]
- 14.Kähler CJ, Hain R. Fundamental protective mechanisms of face masks against droplet infections. J Aerosol Sci. 2020;148:105617. doi: 10.1016/j.jaerosci.2020.105617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JH, Wu T, Powell JB, Roberge RJ. Physiologic and fit factor profiles of N95 and P100 filtering facepiece respirators for use in hot, humid environments. Am J Infect Control. 2016;44:194–198. doi: 10.1016/j.ajic.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dominelli PB, Render JN, Molgat-Seon Y, Foster GE, Romer LM, Sheel AW. Oxygen cost of exercise hyperpnoea is greater in women compared with men. J Physiol. 2015;593:1965–1979. doi: 10.1113/jphysiol.2014.285965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sinkule EJ, Powell JB, Goss FL. Evaluation of N95 respirator use with a surgical mask cover: effects on breathing resistance and inhaled carbon dioxide. Ann Occup Hyg. 2013;57:384–398. doi: 10.1093/annhyg/mes068. [DOI] [PubMed] [Google Scholar]
- 18.Roberge RJ, Bayer E, Powell JB, Coca A, Roberge MR, Benson SM. Effect of exhaled moisture on breathing resistance of N95 filtering facepiece respirators. Ann Occup Hyg. 2010;54:671–677. doi: 10.1093/annhyg/meq042. [DOI] [PubMed] [Google Scholar]
- 19.Molgat-Seon Y, Ramsook AH, Peters CM, Schaeffer MR, Dominelli PB, Romer LM, et al. Manipulation of mechanical ventilatory constraint during moderate intensity exercise does not influence dyspnoea in healthy older men and women. J Physiol. 2019;597:1383–1399. doi: 10.1113/JP277476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheel AW, Romer LM. Ventilation and respiratory mechanics. Compr Physiol. 2012;2:1093–1142. doi: 10.1002/cphy.c100046. [DOI] [PubMed] [Google Scholar]
- 21.Butcher SJ, Jones RL, Eves ND, Petersen SR. Work of breathing is increased during exercise with the self-contained breathing apparatus regulator. Appl Physiol Nutr Metab. 2006;31:693–701. doi: 10.1139/h06-073. [DOI] [PubMed] [Google Scholar]
- 22.Roberge RJ, Kim JH, Powell JB, Shaffer RE, Ylitalo CM, Sebastian JM. Impact of low filter resistances on subjective and physiological responses to filtering facepiece respirators. PLoS One. 2013;8:e84901. doi: 10.1371/journal.pone.0084901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porcari JP, Probst L, Forrester K, Doberstein S, Foster C, Cress ML, et al. Effect of wearing the elevation training mask on aerobic capacity, lung function, and hematological variables. J Sports Sci Med. 2016;15:379–386. [PMC free article] [PubMed] [Google Scholar]
- 24.Smith CL, Whitelaw JL, Davies B. Carbon dioxide rebreathing in respiratory protective devices: influence of speech and work rate in full-face masks. Ergonomics. 2013;56:781–790. doi: 10.1080/00140139.2013.777128. [DOI] [PubMed] [Google Scholar]
- 25.Weil JV, Byrne-Quinn E, Sodal IE, Friesen WO, Underhill B, Filley GF, et al. Hypoxic ventilatory drive in normal man. J Clin Invest. 1970;49:1061–1072. doi: 10.1172/JCI106322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellingsen I, Sydnes G, Hauge A, Zwart JA, Liestøl K, Nicolaysen G. CO2 sensitivity in humans breathing 1 or 2% CO2 in air. Acta Physiol Scand. 1987;129:195–202. doi: 10.1111/j.1748-1716.1987.tb08059.x. [DOI] [PubMed] [Google Scholar]
- 27.Weil JV, Byrne-Quinn E, Sodal IE, Kline JS, McCullough RE, Filley GF. Augmentation of chemosensitivity during mild exercise in normal man. J Appl Physiol. 1972;33:813–819. doi: 10.1152/jappl.1972.33.6.813. [DOI] [PubMed] [Google Scholar]
- 28.Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, et al. American Thoracic Society Committee on Dyspnea. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185:435–452. doi: 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim JH, Benson SM, Roberge RJ. Pulmonary and heart rate responses to wearing N95 filtering facepiece respirators. Am J Infect Control. 2013;41:24–27. doi: 10.1016/j.ajic.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 30.Roberge RJ, Kim JH, Benson SM. Absence of consequential changes in physiological, thermal and subjective responses from wearing a surgical mask. Respir Physiol Neurobiol. 2012;181:29–35. doi: 10.1016/j.resp.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 31.Fikenzer S, Uhe T, Lavall D, Rudolph U, Falz R, Busse M, et al. Effects of surgical and FFP2/N95 face masks on cardiopulmonary exercise capacity. Clin Res Cardiol. doi: 10.1007/s00392-020-01704-y. [online ahead of print] 6 Jul 2020; DOI: 10.1007/s00392-020-01704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones NL, Levine GB, Robertson DG, Epstein SW. The effect of added dead space on the pulmonary response to exercise. Respiration. 1971;28:389–398. doi: 10.1159/000192827. [DOI] [PubMed] [Google Scholar]
- 33.Ward SA, Whipp BJ. Ventilatory control during exercise with increased external dead space. J Appl Physiol. 1980;48:225–231. doi: 10.1152/jappl.1980.48.2.225. [DOI] [PubMed] [Google Scholar]
- 34.Stannard JN, Russ EM. Estimation of critical dead space in respiratory protective devices. J Appl Physiol. 1948;1:326–332. doi: 10.1152/jappl.1948.1.4.326. [DOI] [PubMed] [Google Scholar]
- 35.Dempsey JA, Romer L, Rodman J, Miller J, Smith C. Consequences of exercise-induced respiratory muscle work. Respir Physiol Neurobiol. 2006;151:242–250. doi: 10.1016/j.resp.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 36.Sheel AW, Boushel R, Dempsey JA. Competition for blood flow distribution between respiratory and locomotor muscles: implications for muscle fatigue. J Appl Physiol (1985) 2018;125:820–831. doi: 10.1152/japplphysiol.00189.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wetter TJ, Harms CA, Nelson WB, Pegelow DF, Dempsey JA. Influence of respiratory muscle work on VO(2) and leg blood flow during submaximal exercise. J Appl Physiol (1985) 1999;87:643–651. doi: 10.1152/jappl.1999.87.2.643. [DOI] [PubMed] [Google Scholar]
- 38.Harms CA, Wetter TJ, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, et al. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J Appl Physiol (1985) 1998;85:609–618. doi: 10.1152/jappl.1998.85.2.609. [DOI] [PubMed] [Google Scholar]
- 39.Masamoto K, Tanishita K. Oxygen transport in brain tissue. J Biomech Eng. 2009;131:074002. doi: 10.1115/1.3184694. [DOI] [PubMed] [Google Scholar]
- 40.Kety SS, Schmidt CF. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J Clin Invest. 1948;27:484–492. doi: 10.1172/JCI101995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lane R, Adams L, Guz A. Is low-level respiratory resistive loading during exercise perceived as breathlessness? Clin Sci (Lond) 1987;73:627–634. doi: 10.1042/cs0730627. [DOI] [PubMed] [Google Scholar]
- 42.Banzett RB, Lansing RW, Brown R, Topulos GP, Yager D, Steele SM, et al. ‘Air hunger’ from increased Pco2 persists after complete neuromuscular block in humans. Respir Physiol. 1990;81:1–17. doi: 10.1016/0034-5687(90)90065-7. [DOI] [PubMed] [Google Scholar]
- 43.Chin RC, Guenette JA, Cheng S, Raghavan N, Amornputtisathaporn N, Cortés-Télles A, et al. Does the respiratory system limit exercise in mild chronic obstructive pulmonary disease? Am J Respir Crit Care Med. 2013;187:1315–1323. doi: 10.1164/rccm.201211-1970OC. [DOI] [PubMed] [Google Scholar]
- 44.Lane R, Adams L, Guz A. The effects of hypoxia and hypercapnia on perceived breathlessness during exercise in humans. J Physiol. 1990;428:579–593. doi: 10.1113/jphysiol.1990.sp018229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Person E, Lemercier C, Royer A, Reychler G. Effect of a surgical mask on six minute walking distance [in French] Rev Mal Respir. 2018;35:264–268. doi: 10.1016/j.rmr.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 46.Chen Y, Yang Z, Wang J, Gong H. Physiological and subjective responses to breathing resistance of N95 filtering facepiece respirators in still-sitting and walking. Int J Ind Ergon. 2016;53:93–101. [Google Scholar]
- 47.Molgat-Seon Y, Dominelli PB, Ramsook AH, Schaeffer MR, Molgat Sereacki S, Foster GE, et al. The effects of age and sex on mechanical ventilatory constraint and dyspnea during exercise in healthy humans. J Appl Physiol (1985) 2018;124:1092–1106. doi: 10.1152/japplphysiol.00608.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chasens ER, Pack AI, Maislin G, Dinges DF, Weaver TE. Claustrophobia and adherence to CPAP treatment. West J Nurs Res. 2005;27:307–321. doi: 10.1177/0193945904273283. [DOI] [PubMed] [Google Scholar]
- 49.Nielsen R, Gwosdow AR, Berglund LG, DuBois AB. The effect of temperature and humidity levels in a protective mask on user acceptability during exercise. Am Ind Hyg Assoc J. 1987;48:639–645. doi: 10.1080/15298668791385336. [DOI] [PubMed] [Google Scholar]
- 50.Qian Y, Wu Y, Rozman de Moraes A, Yi X, Geng Y, Dibaj S, et al. Fan therapy for the treatment of dyspnea in adults: a systematic review. J Pain Symptom Manage. 2019;58:481–486. doi: 10.1016/j.jpainsymman.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 51.Harms CA, Cooper D, Tanaka H. Exercise physiology of normal development, sex differences, and aging. Compr Physiol. 2011;1:1649–1678. doi: 10.1002/cphy.c100065. [DOI] [PubMed] [Google Scholar]
- 52.Miller JD, Pegelow DF, Jacques AJ, Dempsey JA. Skeletal muscle pump versus respiratory muscle pump: modulation of venous return from the locomotor limb in humans. J Physiol. 2005;563:925–943. doi: 10.1113/jphysiol.2004.076422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Love RG, Muir DC, Sweetland KF, Bentley RA, Griffin OG. Acceptable levels for the breathing resistance of respiratory apparatus: results for men over the age of 45. Br J Ind Med. 1977;34:126–129. doi: 10.1136/oem.34.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Faisal A, Webb KA, Guenette JA, Jensen D, Neder JA, O’Donnell DE Canadian Respiratory Research Network. Effect of age-related ventilatory inefficiency on respiratory sensation during exercise. Respir Physiol Neurobiol. 2015;205:129–139. doi: 10.1016/j.resp.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 55.Polgar G, Weng TR. The functional development of the respiratory system from the period of gestation to adulthood. Am Rev Respir Dis. 1979;120:625–695. doi: 10.1164/arrd.1979.120.3.625. [DOI] [PubMed] [Google Scholar]
- 56.Numa AH, Newth CJ. Anatomic dead space in infants and children. J Appl Physiol (1985) 1996;80:1485–1489. doi: 10.1152/jappl.1996.80.5.1485. [DOI] [PubMed] [Google Scholar]
- 57.Dominelli PB, Molgat-Seon Y, Sheel AW. Sex differences in the pulmonary system influence the integrative response to exercise. Exerc Sport Sci Rev. 2019;47:142–150. doi: 10.1249/JES.0000000000000188. [DOI] [PubMed] [Google Scholar]
- 58.Guenette JA, Querido JS, Eves ND, Chua R, Sheel AW. Sex differences in the resistive and elastic work of breathing during exercise in endurance-trained athletes. Am J Physiol Regul Integr Comp Physiol. 2009;297:R166–R175. doi: 10.1152/ajpregu.00078.2009. [DOI] [PubMed] [Google Scholar]
- 59.Harber P, Santiago S, Wu S, Bansal S, Liu Y, Yun D. Subjective response to respirator type: effect of disease status and gender. J Occup Environ Med. 2010;52:150–154. doi: 10.1097/JOM.0b013e3181cfcf09. [DOI] [PubMed] [Google Scholar]
- 60.Wu S, Harber P, Yun D, Bansal S, Li Y, Santiago S. Anxiety during respirator use: comparison of two respirator types. J Occup Environ Hyg. 2011;8:123–128. doi: 10.1080/15459624.2011.549780. [DOI] [PubMed] [Google Scholar]
- 61.Kanezaki M, Terada K, Ebihara S. Effect of olfactory stimulation by L-menthol on laboratory-induced dyspnea in COPD. Chest. 2020;157:1455–1465. doi: 10.1016/j.chest.2019.12.028. [DOI] [PubMed] [Google Scholar]
- 62.Tabary A, Rassler B. Increased breathing resistance compromises the time course of rhythmical forearm movements-a pilot study. J Transl Int Med. 2015;3:161–166. doi: 10.1515/jtim-2015-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schönhofer B, Rosenblüh J, Kemper P, Voshaar T, Köhler D. Effect of a face mask on work of breathing in patients with chronic obstructive respiratory disease [in German] Pneumologie. 1995;49:209–211. [PubMed] [Google Scholar]
- 64.Nielsen R, Berglund LG, Gwosdow AR, DuBois AB. Thermal sensation of the body as influenced by the thermal microclimate in a face mask. Ergonomics. 1987;30:1689–1703. doi: 10.1080/00140138708966058. [DOI] [PubMed] [Google Scholar]
- 65.Roberge RJ, Kim JH, Coca A. Protective facemask impact on human thermoregulation: an overview. Ann Occup Hyg. 2012;56:102–112. doi: 10.1093/annhyg/mer069. [DOI] [PubMed] [Google Scholar]
- 66.Bansal S, Harber P, Yun D, Liu D, Liu Y, Wu S, et al. Respirator physiological effects under simulated work conditions. J Occup Environ Hyg. 2009;6:221–227. doi: 10.1080/15459620902729218. [DOI] [PubMed] [Google Scholar]
- 67.Harber P, Santiago S, Bansal S, Liu Y, Yun D, Wu S. Respirator physiologic impact in persons with mild respiratory disease. J Occup Environ Med. 2010;52:155–162. doi: 10.1097/JOM.0b013e3181ca0ec9. [DOI] [PubMed] [Google Scholar]
- 68.Kyung SY, Kim Y, Hwang H, Park JW, Jeong SH. Risks of N95 face mask use in subjects with COPD. Respir Care. 2020;65:658–664. doi: 10.4187/respcare.06713. [DOI] [PubMed] [Google Scholar]
- 69.Samannan R, Holt G, Calderon-Candelario R, Mirsaeidi M, Campos M. Effect of face masks on gas exchange in healthy persons and patients with COPD. Ann Am Thorac Soc. doi: 10.1513/AnnalsATS.202007-812RL. [online ahead of print] 2 Oct 2020; DOI: 10.1513/AnnalsATS.202007-812RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hodous TK, Boyles C, Hankinson J. Effects of industrial respirator wear during exercise in subjects with restrictive lung disease. Am Ind Hyg Assoc J. 1986;47:176–180. doi: 10.1080/15298668691389540. [DOI] [PubMed] [Google Scholar]
- 71.Lalande S, Luoma CE, Miller AD, Johnson BD. Expiratory loading improves cardiac output during exercise in heart failure. Med Sci Sports Exerc. 2012;44:2309–2314. doi: 10.1249/MSS.0b013e318267bb5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nisar M, Spence DP, West D, Haycock J, Jones Y, Walshaw MJ, et al. A mask to modify inspired air temperature and humidity and its effect on exercise induced asthma. Thorax. 1992;47:446–450. doi: 10.1136/thx.47.6.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beuther DA, Martin RJ. Efficacy of a heat exchanger mask in cold exercise-induced asthma. Chest. 2006;129:1188–1193. doi: 10.1378/chest.129.5.1188. [DOI] [PubMed] [Google Scholar]
- 74.Stewart EJ, Cinnamond MJ, Siddiqui R, Nicholls DP, Stanford CF. Effect of a heat and moisture retaining mask on exercise induced asthma. BMJ. 1992;304:479–480. doi: 10.1136/bmj.304.6825.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brenner AM, Weiser PC, Krogh LA, Loren ML. Effectiveness of a portable face mask in attenuating exercise-induced asthma. JAMA. 1980;244:2196–2198. [PubMed] [Google Scholar]
- 76.Millqvist E, Bake B, Bengtsson U, Löwhagen O. Prevention of asthma induced by cold air by cellulose-fabric face mask. Allergy. 1995;50:221–224. doi: 10.1111/j.1398-9995.1995.tb01137.x. [DOI] [PubMed] [Google Scholar]
- 77.Washington, DC: U.S. Government Publishing Office; 2007. Airflow resistance tests. 42 CFR 84.180. [updated 2007 Oct 1; accessed 2020 Jul 5]. Available from: http://www.gpo.gov/fdsys/pkg/CFR-2007-title42-vol1/pdf/CFR-2007-title42-vol1-sec84-180.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.