To the Editor:
Spontaneous pneumothorax (SP) has been estimated to occur at a frequency of 24 cases/100,000 men and 9.8 cases/100,000 women per year in the United Kingdom (1). The epidemiology of SP in the United States is not well established (2, 3). SP is a common manifestation in patients with diffuse cystic lung diseases (DCLDs) such as lymphangioleiomyomatosis (LAM), Birt-Hogg-Dubé syndrome (BHD), and pulmonary Langerhans cell histiocytosis (PLCH). The lifetime prevalence of SP has been estimated to be approximately 60–70% in LAM, 25–75% in BHD, and 15–20% in PLCH (4–9), making this a common cause of morbidity and healthcare utilization in these patients. SP is often the presenting manifestation of patients with DCLDs; it has been estimated that 5–10% of patients presenting with an apparent primary SP have underlying LAM, BHD, or PLCH (10–14). The objectives of our study were to better understand the epidemiology and healthcare burden of SP in the United States and to determine the real-world burden of SPs secondary to DCLDs.
Methods
Records were obtained from the 2015 National Inpatient Sample (NIS) and Nationwide Emergency Department Sample (NEDS) data files (15, 16), which were created for the Healthcare Cost and Utilization Project, sponsored by the Agency for Healthcare Research and Quality. The NIS is a large, publicly available all-payer inpatient database in the United States and is a nationally representative 20% stratified sample of the U.S. hospital discharges. The 2015 NIS contains data on more than 7 million annual inpatient hospitalizations, representing over 35 million weighted national discharges, and includes weights for calculating national estimates. The NEDS is a large, publicly available all-payer emergency department (ED) database in the United States and is a nationally representative 20% stratified sample of hospital-owned ED visits. The 2015 NEDS contains data from approximately 30 million ED visits, representing over 143 million weighted national ED visits. Stratification variables for the NIS include census division, urban or rural location, teaching status, ownership, and bed count, and for the NEDS, they include U.S. region, urban or rural location, teaching status, ownership, and trauma level. We queried the 2015 NIS and NEDS to identify patients with SP and DCLDs using the International Classification of Diseases (ICD) revision 9 codes during the first three quarters of the year and revision 10 codes during the last quarter of the year. NEDS visits that resulted in admission to the same hospital or transfer to another short-term hospital were excluded from the analyses to avoid potential overlap with NIS data. ICD-9 and -10 codes were used to distinguish between primary SP and secondary SP. As BHD does not have a specific ICD code, we were only able to ascertain the SP burden posed by LAM and PLCH in our analysis.
Descriptive statistics are reported as percentages for categorical variables and as median values and interquartile range for continuous variables. Rates are expressed as percentages of hospital visits. To account for the NIS and NEDS survey designs, survey-specific procedures in SAS (SAS Institute) were used to obtain estimates of descriptive statistics. Domain analyses were incorporated in analyses involving subpopulations to obtain precise estimates and their variances. All analyses were performed using SAS software version 9.4.
Results
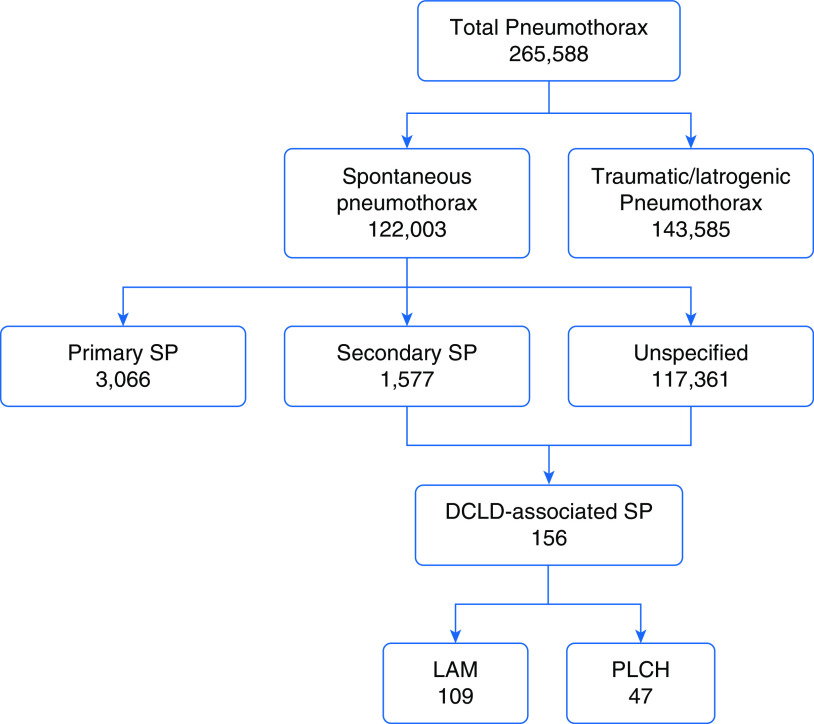
The weighted estimate of the total number of hospital visits in the United States in 2015 was 157,671,785 (35,769,942 inpatient admissions and 121,901,843 ED visits). Pneumothorax was listed as a discharge diagnosis in 265,588 cases, accounting for 0.17% of total hospital visits (231,675 inpatient admissions and 33,913 ED visits). Excluding the 143,585 traumatic and iatrogenic pneumothoraces, there were a total of 122,003 encounters related to SPs, accounting for 0.08% of the total hospital visits (Figure 1). The annual prevalence of SP in the United States was 77 cases/100,000 (111 cases/100,000 male and 51 cases/100,000 female) hospital visits.
Figure 1.
Flowchart depicting the distribution of pneumothoraces in our database. DCLD = diffuse cystic lung disease; LAM = lymphangioleiomyomatosis; PLCH = pulmonary Langerhans cell histiocytosis; SP = spontaneous pneumothorax.
There were a total of 1,461 hospital encounters among patients with DCLDs (LAM: 763/1461, 52%; PLCH: 698/1461, 48%). Of these visits, 156 (11%) were related to an SP, 109 for LAM and 47 for PLCH, accounting for 0.13% of all SPs. SP accounted for 14% (109/763) and 7% (47/698) of all hospital visits for LAM and PLCH, respectively. Details regarding patient demographics, hospital costs, and inpatient length of stay for patients with LAM and PLCH with SP compared with patients with primary SP are summarized in Table 1. SPs were managed by pleurodesis in 32% (35/109) of patient encounters with LAM and 40% (19/47) of patient encounters with PLCH.
Table 1.
Details of SP-related encounters in patients with LAM and PLCH compared with primary SP
| Variable | LAM | PLCH | Primary SP |
|---|---|---|---|
| Number of SP visits | 109 (90 inpatient, 19 ED) | 47 (40 inpatient, 7 ED) | 3,066 (2,550 inpatient, 516 ED) |
| Median age, yr (IQR) | 37 (26–42) | 30 (26–39) | 32 (20–60) |
| Sex | All women | 25 (53%) men, 22 (47%) women | 2,260 (74%) men, 806 (26%) women |
| Median length of inpatient hospital stay, d (IQR) | 4.3 (2.5–8.5) | 6.9 (2.0–8.9) | 3.9 (2.0–7.1) |
| Mean length of inpatient hospital stay, d (SE) | 7.2 (1.4) | 11.7 (4.3) | 6.3 (0.3) |
| Total annual length of inpatient hospital stay, d | 645 | 470 | 16,040 |
| Median charge of inpatient hospitalization, $ (IQR) | 42,179 (16,092–105,858) | 55,438 (31,057–94,874) | 29,416 (14,224–60,871) |
| Mean charge of inpatient hospitalization, $ (SE) | 91,052 (27,572) | 109,263 (39,643) | 53,931 (4,510) |
| Total annual charge of inpatient hospitalization, $ | 8,194,680 | 4,370,520 | 137,524,050 |
Definition of abbreviations: ED = emergency department; IQR = interquartile range; LAM = lymphangioleiomyomatosis; PLCH = pulmonary Langerhans cell histiocytosis; SE = standard error; SP = spontaneous pneumothorax.
Discussion
The major findings of our analysis are 1) the prevalence of SP in the United States is 77 cases per 100,000 hospital visits, 111/100,000 in men and 51/100,000 in women; 2) LAM and PLCH account for 0.13% of the total SPs; 3) the annual total days spent in the inpatient hospital stay for management of SP was 645 days, amounting to a total cost of $8,194,680 for patients with LAM and 470 days, amounting to a total cost of $4,370,520 for patients with PLCH; and 4) pleurodesis is performed in less than half of the patient encounters with LAM and PLCH admitted with an SP.
The prevalence of SP in our analysis is almost fivefold higher than the previous estimates derived from the United Kingdom (1). Although the exact reasons for these differences are unclear, putative reasons include regional differences, increased detection because of more frequent utilization of chest computed tomographic (CT) scans, or improved case-capture in the NIS and NEDS databases as compared with the UK databases.
A recently published analysis has shown that performing screening chest CT scans in patients presenting with an apparent primary SP to facilitate early detection of DCLDs (LAM, BHD, and PLCH) is cost effective (10). The calculations in this analysis were derived from reasonable assumptions about the estimates of DCLD prevalence in patients with an SP and are subject to error (10). We found a prevalence of 0.13% of LAM and PLCH in patients with SPs, an estimate that lies within the cost-effectiveness threshold of performing screening CTs and provides real-world data to support this strategy. In reality, the prevalence of DCLDs in patients presenting with an apparent primary SP is almost certainly higher than 0.13%, as we were unable to evaluate the contribution of BHD in our analysis, and a substantial proportion of the cases in our analysis represent known secondary SPs due to an underlying disease process rather than an apparent primary SP. For instance, the three most common diagnoses associated with SP in our analysis were sepsis, lung cancer, and pneumonia—situations in which an SP would not be considered as an apparent primary SP.
There is an extremely high rate of recurrence in SPs secondary to underlying DCLDs (∼70% in LAM and ∼60% in PLCH) if managed conservatively, and early pleurodesis following the first episode of SP is recommended in these patients as opposed to waiting for a recurrent episode (4, 5, 7, 8). In our analysis, pleurodesis following SP was employed in 32% of the patient encounters with LAM and 40% of the patient encounters with PLCH. The exact reasons for underutilization of pleurodesis are unclear, but it is worth mentioning that a number of variables factor into the decision whether to pursue pleurodesis or not, such as patient preferences and the perceived need for future lung transplantation, that might explain, at least in part, the seemingly low rate of utilization of pleurodesis in this patient population (17).
There are several noteworthy limitations of our analysis. The diagnosis of SP was ascertained based on ICD codes and is subject to error. Although unlikely, it is not possible to determine if an individual was admitted more than once during the time period in which data sampling took place. We were unable to include BHD in our analysis because of the lack of a specific ICD code, which almost certainly resulted in an underestimation of the true SP burden related to DCLDs.
Conclusions
The prevalence of SP in the United States is 77 cases per 100,000 hospital visits: 111/100,000 in men and 51/100,000 in women. SPs caused by DCLDs account for a small but significant proportion of morbidity and healthcare utilization in the United States. Increased emphasis on management strategies aimed at early detection of DCLDs such as screening chest CT scans in patients presenting with an apparent primary SP and reducing recurrences such as early pleurodesis might help reduce the healthcare burden from DCLD-related SPs.
Supplementary Material
Footnotes
Supported by U.S. National Institutes of Health/National Heart, Lung, and Blood Institute grant R34HL138235 (N.G.).
Author Contributions: A.C. and N.G. conceived the study idea and design. A.C. and K.M. obtained and collated the data. K.M. performed the data analysis. All listed authors made substantial contributions toward writing and editing the manuscript. All listed authors have access to all of the study data. A.C. and N.G. had final responsibility for the decision to submit for publication.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Gupta D, Hansell A, Nichols T, Duong T, Ayres JG, Strachan D. Epidemiology of pneumothorax in England. Thorax. 2000;55:666–671. doi: 10.1136/thorax.55.8.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumann MH, Strange C, Heffner JE, Light R, Kirby TJ, Klein J, et al. AACP Pneumothorax Consensus Group. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest. 2001;119:590–602. doi: 10.1378/chest.119.2.590. [DOI] [PubMed] [Google Scholar]
- 3.Melton LJ, III, Hepper NG, Offord KP. Incidence of spontaneous pneumothorax in Olmsted County, Minnesota: 1950 to 1974. Am Rev Respir Dis. 1979;120:1379–1382. doi: 10.1164/arrd.1979.120.6.1379. [DOI] [PubMed] [Google Scholar]
- 4.Almoosa KF, Ryu JH, Mendez J, Huggins JT, Young LR, Sullivan EJ, et al. Management of pneumothorax in lymphangioleiomyomatosis: effects on recurrence and lung transplantation complications. Chest. 2006;129:1274–1281. doi: 10.1378/chest.129.5.1274. [DOI] [PubMed] [Google Scholar]
- 5.Gupta N, Finlay GA, Kotloff RM, Strange C, Wilson KC, Young LR, et al. ATS Assembly on Clinical Problems. Lymphangioleiomyomatosis diagnosis and management: high-resolution chest computed tomography, transbronchial lung biopsy, and pleural disease management. An Official American Thoracic Society/Japanese Respiratory Society clinical practice guideline. Am J Respir Crit Care Med. 2017;196:1337–1348. doi: 10.1164/rccm.201709-1965ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta N, Kopras EJ, Henske EP, James LE, El-Chemaly S, Veeraraghavan S, et al. Spontaneous pneumothoraces in patients with Birt-Hogg-Dubé syndrome. Ann Am Thorac Soc. 2017;14:706–713. doi: 10.1513/AnnalsATS.201611-886OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mendez JL, Nadrous HF, Vassallo R, Decker PA, Ryu JH. Pneumothorax in pulmonary Langerhans cell histiocytosis. Chest. 2004;125:1028–1032. doi: 10.1378/chest.125.3.1028. [DOI] [PubMed] [Google Scholar]
- 8.Singla A, Kopras EJ, Gupta N. Spontaneous pneumothorax and air travel in Pulmonary Langerhans cell histiocytosis: a patient survey. Respir Investig. 2019;57:582–589. doi: 10.1016/j.resinv.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toro JR, Pautler SE, Stewart L, Glenn GM, Weinreich M, Toure O, et al. Lung cysts, spontaneous pneumothorax, and genetic associations in 89 families with Birt-Hogg-Dubé syndrome. Am J Respir Crit Care Med. 2007;175:1044–1053. doi: 10.1164/rccm.200610-1483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta N, Langenderfer D, McCormack FX, Schauer DP, Eckman MH. Chest computed tomographic image screening for cystic lung diseases in patients with spontaneous pneumothorax is cost effective. Ann Am Thorac Soc. 2017;14:17–25. doi: 10.1513/AnnalsATS.201606-459OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johannesma PC, Reinhard R, Kon Y, Sriram JD, Smit HJ, van Moorselaar RJ, et al. Amsterdam BHD working group. Prevalence of Birt-Hogg-Dubé syndrome in patients with apparently primary spontaneous pneumothorax. Eur Respir J. 2015;45:1191–1194. doi: 10.1183/09031936.00196914. [DOI] [PubMed] [Google Scholar]
- 12.Ren HZ, Zhu CC, Yang C, Chen SL, Xie J, Hou YY, et al. Mutation analysis of the FLCN gene in Chinese patients with sporadic and familial isolated primary spontaneous pneumothorax. Clin Genet. 2008;74:178–183. doi: 10.1111/j.1399-0004.2008.01030.x. [DOI] [PubMed] [Google Scholar]
- 13.Hagaman JT, Schauer DP, McCormack FX, Kinder BW. Screening for lymphangioleiomyomatosis by high-resolution computed tomography in young, nonsmoking women presenting with spontaneous pneumothorax is cost-effective. Am J Respir Crit Care Med. 2010;181:1376–1382. doi: 10.1164/rccm.200910-1553OC. [DOI] [PubMed] [Google Scholar]
- 14.Gupta N. Primary spontaneous pneumothorax: looking beyond the usual. Acad Emerg Med. 2018;25:470–472. doi: 10.1111/acem.13363. [DOI] [PubMed] [Google Scholar]
- 15.HCUP National Inpatient Sample (NIS) Rockville, MD: Agency for Healthcare Research and Quality; 2012. Healthcare Cost and Utilization Project (HCUP) [accessed 2020 Aug 27]. Available from: www.hcup-us.ahrq.gov/nisoverview.jsp. [Google Scholar]
- 16.HCUP Nationwide Emergency Department Sample (NEDS) Healthcare Cost and Utilization Project (HCUP) Rockville, MD: Agency for Healthcare Research and Quality; 2007–2009. [accessed 2020 Aug 27]Available from: www.hcup-us.ahrq.gov/nedsoverview.jsp [Google Scholar]
- 17.Young LR, Almoosa KF, Pollock-Barziv S, Coutinho M, McCormack FX, Sahn SA. Patient perspectives on management of pneumothorax in lymphangioleiomyomatosis. Chest. 2006;129:1267–1273. doi: 10.1378/chest.129.5.1267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.