Abstract
Background:
Rheumatoid arthritis (RA) is the most common chronic inflammatory joint disease. Complications of RA can cause low quality of life and disabilities. Nowadays, despite all the medical developments, etiology of RA is unclear. Both soft and hard tissue damages occur in RA and periodontitis due to chronic inflammation and also since tissue damage presentation and pathogenesis of RA and periodontitis are the same, this study was done for evaluating the relationship between clinical and laboratory findings in RA patients with their oral status and disease activity.
Methods:
This case-control study was performed on 236 patients; 118 RA patients and 118 cases of normal people. Gingivitis, dental caries and plaques, oral hygiene and severity of periodontitis were measured based on gingival index, plaque index, clinical attachment level, Decayed Missing Filled index and oral hygiene index-simplified. Disease activity was assessed according to Diseases Activity Score-28. Blood samples were taken to evaluate the level of anti-CCP, RF, ESR, CRP, and CBC. Data were analyzed by t-test and chi-square.
Results:
RA patients are more susceptible to periodontitis, plaque formation and dental caries. There is a relationship among RA disease and periodontitis, oral hygiene, gingivitis. There is also a reverse relationship between RF level and periodontitis severity likewise dental caries. There is no significant relationship between other laboratory findings and the oral status of patients.
Conclusion:
This is more likely for RA patients to experience periodontitis which can destruct alveoli bone and it can also cause early tooth loss. Regular examination and early treatment are highly recommended.
Key Words: Rheumatoid arthritis, Laboratories, Mouth diseases, Disease activity score
Rheumatoid arthritis (RA) is the most common chronic inflammatory joint disease which involves 1 % of population world-wide (1). RA disease can strongly affect quality of life of patients so that it provokes joint destruction, disability, movement disorders and limitations (2). Serious external joint injuries and systemic complications can also cause early death in RA patients (3).In accordance with mental and physical consequences of being diagnosed as an RA case, it is helpful to evaluate disease mechanisms (4,5). In spite of medical progresses and developments, still there is no precise etiology of RA, however, inflammatory processes, pathogenesis, genetic factors, autoimmune causes mostly in the site of inflammation and infection has been specified as causative factor during the recent decades (6). Anti ccp antibody is important in the diagnosis and prognosis of RA disease (7).
Due to the fact that antibodies against citrulinated proteins are mostly located in the site of inflammation and infection. Oral diseases are possibly considered as one of the environmental factors in RA incidence (5, 6). Periodontitis, the most common mouth disease, is an inflammatory disease which can destroy both soft and hard dental surrounding tissues (8). It is also one of the known reasons of tooth loss in adulthood (9). Bacterial plaques are the most common cause of periodontitis. These plaques are similar to a rigid and adhesive membrane that can be expanded over the area based on environmental factors, acquired diseases and genetic background of patients (10). Dental caries is as well one of the most common chronic diseases which people all around the world are involved with. According to the evidence, prevalence of dental caries due to alteration of lifestyle and diet has been increased (11). In spite of the fact that mouth infections are diagnosed via clinical examination and they are almost curable by observing oral hygiene besides daily diet, if people do not receive the proper treatment in an appropriate time, it can provoke tooth loss and consequently nutrition imbalance, aesthetic issues and quality of life disturbances (12).
Studies showed that there are similarities in pathobiology of RA and periodontitis (6, 8, 9). They resemble signs and symptoms such as pain, inflammation, sensitivity and bone destruction in both diseases. These findings are approximately ascribed to inflammatory cell aggregation in liquids around bones and high level of pre-inflammatory cytokines in the area (13,14). Some trials demonstrated that active and chronic RA patients are more susceptible to oral infections than normal population (6). In Ah Choi (2016) showed that periodontitis severity is inextricably intertwined to rheumatoid arthritis disease (15). Pablo et al (2009), also claims that periodontitis and tooth loss are more common in RA patients, so periodontitis could be a primer factor for autoimmune responses in these patients or it can continue and create an opportunity for auto immune responses to occur constantly (16). Pischon N et al. (2008) reported that depth of periodontal pockets; gingival bleeding and plaque index are more likely to be found in RA patients than their control group (17). Although Mobini M, et al. (2017) showed that 60 % of RA patients suffer from periodontitis they found no relationship between RA disease and periodontitis severity (18).Taheri M et al.(2011), reported that RA patients had a greater level of periodontal disease compared with the healthy control group. (19). According to the studies, there is a relationship of periodontitis and great spectrum of chronic diseases such as cardiovascular conditions, diabetes, RA and also premature delivery of newborns. (2, 11, 20). Thus, the evaluation of effects of chronic inflammatory pattern of these diseases on incidence of some systemic disorders can lead us to a better understanding of etiology and pathogenesis of diseases. It can also provide innovative and new treatment approaches for specific illnesses (7, 21).
Considering the high prevalence of RA disease and unclear etiology in addition to resembling pathobiology of periodontitis and RA, this study is implemented to investigate the relationship between clinical and laboratory findings of rheumatoid arthritis patients with their oral status and disease activity.
Methods
This is a case-control study in which 236 patients have been assigned into two groups including (118) RA patients and (118) healthy persons (without systemic disorders). This study received ethical approval from the Ethics Committee of Iran University of Medical Sciences, Tehran (IR.IUMS.REC.1393.105.6009). Our research was conducted in accordance with the ethical standards of the institutional and national research committee and the most recent version of Declaration of Helsinki. The aim and procedures were explained to all participants before participation. Both groups underwent clinical examinations and para clinical surveys in order to fill informational consent forms. Trial group has been selected from patients who are referred to Firoozgar rheumatology clinics and they were diagnosed as RA patients according to American Rheumatoid Association Criteria moreover, selection of patients was confirmed by rheumatologists (2, 22).
Control group includes healthy people (hospital personnel or patient’s companions) without any history of RA, any related medical history or any signs and symptoms attributed to RA. Exclusion criteria were as follows; age ≤ 18 years, systemic diseases such as diabetes, cardiovascular conditions, epilepsy, Sjogren disease, xerostomia, osteoporosis and immune system disorders. Antibiotic use in the last 3 months, pregnant women, breast feeding women, alcoholics, smokers, in addition to patients who experienced chemotherapy and radiotherapy or those who have used drugs which have orodental side effects related to periodontium or had manifestation of periodontitis such as phenytoin, cyclosporine, anti-hypertensive drugs, calcium channel blockers, sedatives and patients who have received treatments for periodontitis during the recent 6 months and small number of teeth (less than 15) and sensitivity to chlorhexidine digluonate,amoxiilli and metronidazole contraindications, were excluded from the study (15, 23). Following the consent of participants, demographic information, duration of their disease and medical history have been taken precisely. Generally, diagnosis of RA is possible with clinical symptoms but laboratory findings are nonspecific. So, there is not any laboratory test that can detect RA alone.
Blood tests presented the progress of the disease, and it can help the rheumatologist for RA diagnosis. After RA diagnosis, blood tests are performed to monitor the side effects of the drugs used during treatment. Rheumatoid factor (RF) may be positive during rheumatoid arthritis, but negative results cannot rule out the RA. About 80% of RA patients have a positive RF titer. Although the normal value of this factor is negative, few normal persons have low titers of RF. Initial laboratory evaluation should include complete blood cell count (CBC) which can indicate anemia and assess liver and kidney functions. Because these are useful for choice and alter treatment options. Therefore, all patients require a CBC test to evaluate disease activity and drug side effects (1-3).
So, then blood samples have been taken from patients to evaluate anti-CCP, C- reactive protein (CRP), erythrocyte sedimentation rate (ESR) (2, 7).
Immunoturbidimetry method used for serum RF measurement (Roche, Basel, Switzerland), and chemiluminescent microparticle immunoassay used for anti-CCP antibody measurement (Abbott, Lake Bluff, IL, USA) based on the manufacturer’s instructions. Antibody titer over 5 arbitrary units/mL of anti-CCP was considered as a positive result. In case group, clinical parameters were evaluated and laboratory findings (ESR, CRP) were measured and anti-CCP, serum rheumatoid factor (RF) were evaluated. Diseases activity score (DAS-28) has been used to distinguish disease activity since this is a standard and valid questionnaire in this regard. Formulation for calculation the DAS-28 was (0.56 × √ (28 TJC) + 0.28 × √ (28 SJC) + 0.70 × ln (ESR)) × 1.08 + 0.16. (15,24) Trial groups were categorized as follows; deteriorated, low activity, moderate activity and high activity which scored less than 2.6, between 2.6 and 3.2, between 3.2 and 5.1 and more than 5.1,respectively (25). All the samples have been referred to dental clinics of the hospital. Likewise, oral hygiene has been categorized according to oral health index-simplified (OHI-s); 0-1.2 good, 1.3-3 moderate, 3.1-6 weak (17).
Orodental examination was performed by one oral medicine specialist with dental explorer, mouth mirror and (Williams and WHO) periodontal probe (8, 11). Dental plaques, gingivitis and periodontitis severity were assessed based on Plaque index (PI) (Silness & Loe) (14, 26), gingival index (GI) (Loe) (8), and Clinical Attachment level (CAL) parameters (8, 27). Decayed, missing, filled (DMF) index was used for evaluating the prevalence of dental caries for 28 permanent dentitions. Dental caries were detected based on visual-tactile method (26). Measurements were taken at four locations which include 1 lingual point and 3 buccal points of each tooth. Then the average values (from maximum 112 sites in 32 teeth) were used for computing the PI (27-29).
Periodontitis severity was evaluated based on average CAL and attachment loss (distance of CEJ and pocket depth). CAL parameter was categorized as below; CAL ≤ 3mm (normal), 3 < CAL ≤ 4mm (mild), 4 < CAL ≤ 5mm (moderate) and CAL > 5mm (severe) (17, 30) Analysis was done by SPSS Version 20 and information, following t- test and chi-square analysis was extracted.
Results
The outcome of the analysis showed that trial group has age range of 20 to 73 years old with average of 51.49±10.91. 17.8% of trial group were males and 82.2 % were females. Duration of disease was also 9.15±7.88. PI evaluation demonstrated that dental plaques in RA patients are significantly more than the others (p<0.001). Likewise, dental caries were more prevalent in RA patients (P=0.001). However, severe dental caries occur more in control group. Oral hygiene was not significantly different between two groups (P=0.563).
Oral hygiene in both groups was as follows; 27.1% (32 patients) in trial group and 29.7% (35 persons) in control group have good mouth hygiene. 50 % (59 patients) in trial group and 43.2% (51 persons) in control group have moderate mouth hygiene, 45.8% (27 patients) and 27.1 (32 persons) have low oral hygiene. GI parameter assessment revealed that 55.4% (63 patients) in trial group and 64.4% (76 persons) in control group have gingivitis and there was no significant difference between two groups in this regard (P=0.16). gingivitis and dental caries were found more in males than females. (p<0.05) (Table 1).
Table 1.
Relation between gingivitis and gender in RA patients
| P Value | Gingivitis | Frequency (Percent) | ||
|---|---|---|---|---|
| negative | positive | |||
| <0.001 | 2(9.5) | 19(90.5) | Male 21(100) | Gender |
| 52(53.6) | 45(46.4) | Female97(100) | ||
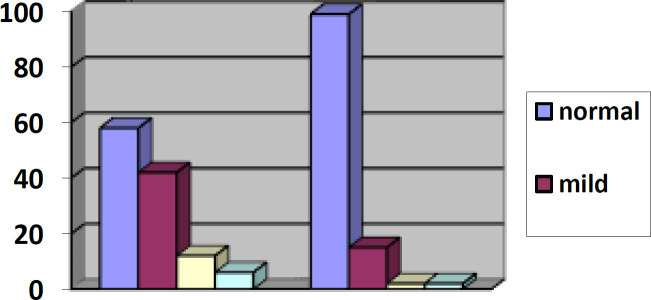
Average attachment loss and CAL appraisal showed that prevalence and severity of periodontitis are substantially higher in RA patients than control group (p<0.001) (Figure 1).
Figure 1.
Comparison of periodontal severity status between control and case group
RF test result is as below; 27.1% (32 persons), 45.7% (54 persons) and 27.1% (32 persons) result were 1+,2+ and 3+ respectively in RA patients (trial group). Therefore, there was a reverse relationship between RF and periodontitis (P=0.042, R= -0.25) and also dental caries (P= 0.005, R= -0.33). Disease activity based on DAS-28 was 2.880± 0.99 in RA patients and 42.4% (50 patients) were in disease amelioration phase, 25.4% (30 patients), 28% (33 patients) and 4.2% (5 patients) have low, moderate and high disease activity, respectively (Table 2).
Table 2.
Relation between RA disease and periodontitis
| Pvalue | Periodontitis | Frequency(percent) Disease Activity | |||
|---|---|---|---|---|---|
| negative | positive | ||||
| 0.002 | 35(70) | 15(30) | 50(100) | Amelioration Low | DAS- 28 |
| 23(76.6) | 7(23.3) | 30(100) | |||
| 10(30.3) | 23(69.7) | 33(100) | Moderate Severe |
||
| 1(20) | 4(80) | 5(100) | |||
Findings showed that the mean±SD of ESR (mm/h) of RA patients was 27.40±15.64. According to statistical analysis, there is relationship among disease activity and periodontitis (P=0.002), oral hygiene (P=0.001), gingivitis (PV=0.01) and dental caries (P=0.02), whereas, there was no relationship of laboratory findings such as anti CCP, ESR, CRP, WBC, PLT in patients and gingivitis, periodontitis, oral hygiene, dental caries and plaque formation. (p>0.05) (Table 2).
Discussion
The outcome of this study reveals that there is significant relation between RA and periodontitis due to the similarity of these two diseases since they are chronic inflammatory diseases which can destruct soft and hard tissues. Etiology of these illnesses are not completely clear, however there are theories which consider them as a multifactorial disease. Causative factors such as environment, host and genetic are mentioned (6, 7). Periodontitis is the most common oral disease that can be caused by opportunistic microorganism (Porphyromonase Gingivalis). On the other hand, gingivitis is more common and severe in patients who consume immunosuppressive drugs to control chronic inflammatory diseases such as RA (8, 29). Also, the periodontal mucosa is a common site for presentation of benign and malignant tumors. Some investigators reported cases which manifested in gingival tissue. So, it seems that evaluation of periodontal status is critical (24, 31).
Bacterial and viral infections could be considered as the leading cause of periodontitis and RA since these microorganisms can pose autoimmune disorders. Although the etiology of both periodontitis and RA is distinct, it seems that they have the same pathobiology. They both showed increased osteoclasts and some vessel injuries (7, 30, 32). In addition, the disease deterioration has been accompanied by the constant presence of a large number of inflammatory cytokines, matrix metalloproteinase and prostaglandin E2 secreted from macrophages, fibroblasts and other settled or migratory inflammatory cells, low amounts of tissue inhibitors and metalloproteinase, which are active in both diseases (33,34). In this regard, result of this study is similar to a study performed by Ulvestad E et al. (2001) which evaluated IgM – RF in 171 periodontitis patients and 10 RA patients. 9.4% (16 persons) of patients in their study have positive RF and it has been mentioned that antibodies in systemic circulation can be produced by symptomatic and asymptomatic microorganisms (35). High titer of antibodies against present bacteria in the serum of RA patients and their resemblance to antibodies available in the periodontal pockets was considered as a reason that explains the presence of periodontitis in RA patients more than normal population. Increased level of serum antibodies could be an indicator of oral microorganism prevalence in periodontal pockets, also, antibodies in patient’s serum can describe susceptibility of patients to RA disease (32). Since the pathogenesis of these two diseases are similar, there is a theory introducing RA as a possible reason to pose periodontitis in addition to considering periodontitis as a cause for RA (2, 6, 11).
In the present study, in parallel with the Ah Choi research, the prevalence of moderate and severe periodontitis was significantly higher in RA patients than control group (p<0.001). Although the statistical data in this study (50.8% vs 16.1%) were not exactly similar to the Choi et al.’s study (63.6% vs 34.1%). (15) Also, Berthelot et al. (2010), reported a relationship between RA and periodontitis which is consistent with the result of the present study (13). In addition, Dissick et al. (2010) claimed that periodontitis is found in RA patients more than the normal population (36). Mercado and Araujo et al. have done a review study that demonstrated that periodontitis prevalence and severity are more common in RA patients (9, 20). It seems that RA patients are more prone to gingivitis due to immunologic changes related to RA pathobiology and imbalance of host responses and also the high prevalence of periodontal microorganisms can make patients more susceptible (8, 34).
In this study, the incidence of RA was higher in females (82%) than in males similar to other researches such as: Mobini et al. (91.9%), Choi et al. (87.5%), Taheri et al. (88.6%), Alebooyeh (80%). (15,18,19,23) Furthermore, in the present study, the average age of RA patients was 51.49± 10.91 years, which was consistent with other studies of Mobini (47.01±8.1), Choi (58.2±12.0) (15,18). However, the average age in some studies was lower like in the studies of Taheri et al. (45±12.9), Kiani Yazdi et al. (35/95± 9/87) (19, 37). In this study, dental plaques appear in RA patients more than the control group (PV<0.001). Pischon et al. (2008) studied 57 patients and Hashimoto H et al. studied 89 RA patients with regard dental plaques. Result of both studies are compatible with this study (17, 39).
It could also be the consequence of joint pain and limitation of motion. In RA patients so that they cannot clean dental plaques regularly. Mercado et al. studied 65 patients and Mobini et al. also evaluated 74 (P=0.18) patients but unlike the outcome of the present study, none of them could find significant relationship between periodontitis and plaque index (9, 18). The paradox in the findings could be the result of low sample size and racial or environmental differences while this study has been implemented on huge number of patients and also assessing the oral status of patients by clinical parameters is considered the strength of our study. According to the result of the present study, Mercado et al. (2001) and Pablo et al. (2008) (40); RA patients have more dental caries in comparison to the control group which could be caused by higher plaque index and papillary bleeding (9,35). Tooth loss and periodontal tissue involvement can also be the consequence of high plaque index and papillary bleeding.
In the present study, the same as Pischon et al. (2008) who evaluated 57 RA patients, there was no significant difference in terms of oral hygiene and gingivitis (17). In this study, disease activity was significantly associated with periods of periodontitis, oral hygiene, gingivitis and dental caries. In addition, there was relationship between RA activity and periodontal disease severity which is consistent with the result of our study. Disease activity in our study (2.88± .99) was similar to other researches of Alebooyeh et al. (3.84±0.92), Choi et al. (3.3±1.4). For instance in Ah Choi et al. (2016) and Alebooye et al. (2019) performed a study in Korea and Iran on 164 and 30 RA patients (p<0.001) (15, 23). Also, the results of the current study (P=0.002) similar to Choi’s research (P=0.041), showed that gingivitis was significantly correlated with disease activity score 28, although both were slightly different in terms of significance level (15). Which may be due to differences in sample size and population. Besides, de Smith MJ et al. performed a review study in 2015 and Monsarrat et al. (2014) (41, 42) also showed the direct effects of RA disease activity on the severity of periodontitis. Alebooye in 2019 (23) showed that on the contrary Joseph (2013) (38), Khantisopon (2014) (43) and specially Mobini et al. (2017) (15) who studied 74 RA patients in the North of Iran demonstrated no relationship among RA severity, PI, GI, oral hygiene, periodontitis, and gingivitis (PV=0.22) (18). It seems that the contrast between results of different studies is attributed to the numbers of samples and difference among the patients of various studies (44, 45).
The limitation of the present study that can be noted is that there have been few case-control studies comparing laboratory findings and oral status of RA patients, thus limited us to writing the discussion part of our study in this session. Comparison of the results of laboratory tests was hence similar to other researches; seropositive for RF and anti-CCP, in our study was (72.9%, 73.2%) consistent with Mobini (76.4%, 76.9%) and Choi (68.5%, 69.1%). (15) In our study, the mean±SD of ESR (mm/h) of RA patients (27.40 ±15.64) was similar to Alebooyeh N et al.’s (20.57 ±11.01) in 2019. The overall survey showed that both groups were different in ESR, DAS-28 as a result of disease activity (23). Previous studies showed inconsistencies in the oral hygiene status of RA patients. It seems that this can be due to the environmental and racial differences of the samples, the standardization of the evaluation criteria, and the lack of attention to some confounding factors (46). Since most studies have assessed some of the parameters in a low sample size, it is suggested that other researchers investigate expanded cohort studies with histopathologic approaches and other periodontal parameters associated with bone destruction with large number of cases.
In conclusion to sum it up, this study indicates a relationship between RA and periodontitis, which could reflect a common pathogenesis as dysfunction of immune/inflammatory responses. On the other hand, according to greater prevalence of periodontitis in RA patients which can ultimately lead to early tooth loss, periodic examinations and regular oral health monitoring of these patients and early dental treatments are highly recommended.
Acknowledgments
The authors wish to thank the patient participants and the Research Vice President of Firoozgar Hospital Research Center.
Financial issues:
Nil
Conflicts of interest:
The authors have no conflicts of interest to disclose.
Ethics Approval:
The Ethics Committee of Iran University of Medical Sciences, Tehran (IUMS) approved the protocol of this study. All the participants signed informed consent forms.
References
- 1.Bax M, Van Heemst J, Huizinga TW, Toes REM. Genetics of rheumatoid arthritis: what have we learned? Immunogenetics. 2011;63:459–66. doi: 10.1007/s00251-011-0528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carsons S, Firestein G, Budd R, et al. Kelley and Firestein's textbook of rheumatology. 10th ed. Philadelphia: Elsevier. 2016;pp:1105–24. [Google Scholar]
- 3.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 4.Norton S, Koduri G, Nikiphorou E, et al. A study of baseline prevalence and cumulative incidence of co-morbidity and extra-articular manifestations in RA and their impact on outcome. Rheumatology (Oxford) . 2013;52:99–110. doi: 10.1093/rheumatology/kes262. [DOI] [PubMed] [Google Scholar]
- 5.Joseph R, Raj MJ, Sundareswaran S, et al. Does a biological link exist between periodontitis and rheumatoid arthritis? World J Rheumatol. 2014;4:80–7. [Google Scholar]
- 6.Guo Q, Wang Y, Xu D, et al. Rheumatoid arthritis: pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018;6:15. doi: 10.1038/s41413-018-0016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derksen VFAM, Huizinga TWJ, Van der Woude D. The role of autoantibodies in the pathophysiology of rheumatoid arthritis. Semin Immunopathol . 2017;39:437–46. doi: 10.1007/s00281-017-0627-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newman M , Takei H , Klokkevold P , et al. Newman and Carranza's Clinical Periodontology. 13th ed. Philadelphia: Saunders. 2019;pp:208–14. [Google Scholar]
- 9.Mercado FB, Marshall RI, Klestov AC, Bartold PM. Relationship between rheumatoid arthritis and periodontitis. J Periodontol. 2001;72:779–87. doi: 10.1902/jop.2001.72.6.779. [DOI] [PubMed] [Google Scholar]
- 10.Wankhede AN, Wankhede SA, Wasu SP. Role of genetic in periodontal disease. J Int Clin Dent Res Organ. 2017;9:53–8. [Google Scholar]
- 11.Glick M, William M. Burket’s oral medicine. 12th ed. London: People’s Medical Publishing House. 2015;pp:509–20. [Google Scholar]
- 12.Singh S, Shivhare UD, Sakarka SN. Oral infections, a mirror of our overall health condition: An overview. Int J Pharm Life Sci. 2013;4:2976–82. [Google Scholar]
- 13.Berthelot JM, Le Goff B. Rheumatoid arthritis and periodontal disease. Joint Bone Spine. 2010;77:537–41. doi: 10.1016/j.jbspin.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 14.Neville BW, Damm DD, Allen CM, et al. Oral and maxillofacial pathology. 4th ed. Elsevier Health Sciences. Missouri: St. Louis. 2016;pp:331–621. [Google Scholar]
- 15.Choi IA, Kim JH, Kim YM, et al. Periodontitis is associated with rheumatoid arthritis: a study with longstanding rheumatoid arthritis patients in Korea. Korean J Intern Med. 2016;31:977–86. doi: 10.3904/kjim.2015.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Pablo P, Chapple IL, Buckley CD, Dietrich T. Periodontitis in systemic rheumatic diseases. Nat Rev Rheumatol. 2009;5:218–24. doi: 10.1038/nrrheum.2009.28. [DOI] [PubMed] [Google Scholar]
- 17.Pischon N, Pischon T, Kröger J, et al. Association among rheumatoid arthritis, oral hygiene, and periodontitis. J Periodontol. 2008;79:979–86. doi: 10.1902/jop.2008.070501. [DOI] [PubMed] [Google Scholar]
- 18.Mobini M, Maboudi A, Mohammadpour RA. Periodontitis in rheumatoid arthritis patients, abundance and association with disease activity. Med J Islam Repub Iran. 2017;31 doi: 10.14196/mjiri.31.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taheri M, Saghafi M, Najafi MH, et al. Investigation of periodontal conditions in patients with rheumatoid arthritis. J Mash Dent Sch. 2011;350:283–8. [Google Scholar]
- 20.Araújo VMA, Melo IM, Lima V. Relationship between periodontitis and rheumatoid arthritis: review of the literature. Mediator Inflammat . 2015;2015:259074. doi: 10.1155/2015/259074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schackert HK, Agha-Hosseini F, Gorgens H, et al. Complete homozygous deletion of CTSC in an Iranian family with Papillon-Lefevre syndrome. Int J Dermatol. 2014;53:885–7. doi: 10.1111/j.1365-4632.2012.05769.x. [DOI] [PubMed] [Google Scholar]
- 22.Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis . 2010;69:1580–8. doi: 10.1136/ard.2010.138461. [DOI] [PubMed] [Google Scholar]
- 23.Alebooyeh N, Moghimi J, Hasani A, Ghorbani R, Taheri G. Periodontitis and rheumatoid arthritis: A neglected association in developing countries. Romatol Res. 2019;4:9–16. [Google Scholar]
- 24.Shabestari SB, Shirinbak I, Agha-Hosseini F. Maxillary metastasis of a medullary thyroid carcinoma in a 21-year-old woman 7 years after thyroidectomy. J Oral Maxillofac Surg. 2012;70:1495–9. doi: 10.1016/j.joms.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 25.van der Heijde DM, van 't Hof M, van Riel PL, van de Putte LB. Development of a disease activity score based on judgment in clinical practice by rheumatologists. J Rheumatol. 1993;20:579–81. [PubMed] [Google Scholar]
- 26.Loe H. The gingival index, the plaque index and the retention index systems. J Periodontol. 1967;38:610–6. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- 27.Pihlstrom BL. Measurement of attachment level in clinical trials: probing methods. J Periodontol. 1992;63:1072–7. doi: 10.1902/jop.1992.63.12s.1072. [DOI] [PubMed] [Google Scholar]
- 29.Silness J, Loe H. Periodontal disease in pregnancy II Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 30.Kim YJ, Viana AC, Curtis KM, et al. Association of haplotypes in the IL8 gene with susceptibility to chronic periodontitis in a Brazilian population. Clin Chim Acta. 2010;411:1264–8. doi: 10.1016/j.cca.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Basir Shabestari S, Shirinbak I, Saghafi-Khadem S, et al. Schwannoma in the posterior hard palate and anterior mandibular gingiva: a report of two cases. J Kerman Univ Med Sci. 2018;25:375–81. [Google Scholar]
- 32.Mikuls TR, Payne JB, Yu F, et al. Periodontitis and Porphyromonas gingivalis in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014;66:1090–100. doi: 10.1002/art.38348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Detert J, Pischon N, Burmester GR, Buttgereit F. The association between rheumatoid arthritis and periodontal disease. Arthritis Res Ther. 2010;12:218. doi: 10.1186/ar3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bartold PM, Marshall RI, Haynes DR. Periodontitis and rheumatoid arthritis: A Review. J Periodontol. 2005;76:2066–74. doi: 10.1902/jop.2005.76.11-S.2066. [DOI] [PubMed] [Google Scholar]
- 35.Ulvestad E, Wilfred LL, Kristoffersen EK. Measurement of IgM rheumatoid factor by ELISA. Scand J Rheumatol . 2001;30:366. doi: 10.1080/030097401317148598. [DOI] [PubMed] [Google Scholar]
- 36.Dissick A, Redman RS, Jones M, et al. Association of periodontitis with rheumatoid arthritis: A pilot study. J Periodontol. 2010;81:223–30. doi: 10.1902/jop.2009.090309. [DOI] [PubMed] [Google Scholar]
- 37.Kiani F, Setoudeh Maram S, Masoumi S, Kamali sarvestani E, Aflaki E. Association between Periodontal Disease and serum levels of IL-17 in Patients with Rheumatoid Arthritis. J Dentistry. 2012;13:398–407. [Google Scholar]
- 38.Joseph R, Rajappan S, Nath SG, Paul BJ. Association between chronic periodontitis and rheumatoid arthritis: a hospital-based case-control study. Rheumatol Int. 2013;33:103–9. doi: 10.1007/s00296-011-2284-1. [DOI] [PubMed] [Google Scholar]
- 39.Hashimoto H, Hashimoto S, Muto A, Dewake N, Shimazaki Y. Influence of plaque control on the relationship between rheumatoid arthritis and periodontal health status among Japanese rheumatoid arthritis patients. J Periodontol. 2018;89:1033–42. doi: 10.1002/JPER.17-0575. [DOI] [PubMed] [Google Scholar]
- 40.Pablo DP, Dietrich T, Mcalindon TE. Association of periodontal disease and tooth loss with rheumatoid arthritis in the US population. J Rheumatol. 2008;35:70–6. [PubMed] [Google Scholar]
- 41.Monsarrat P, Vergnes J N, Blaizot A, et al. Oral health status in outpatients with rheumatoid arthritis: the OSARA study. Oral Health Dent Manag. 2014;13:113–19. [PubMed] [Google Scholar]
- 42.Smit DMJ, Westra J, Brouwer E, et al. Periodontitis and Rheumatoid Arthritis: What Do We Know? J Periodontol. 2015;86:1013–9. doi: 10.1902/jop.2015.150088. [DOI] [PubMed] [Google Scholar]
- 43.Khantisopon N, Louthrenoo W, Kasitanon N, et al. Periodontal disease in Thai patients with rheumatoid arthritis. Int J Rheum Dis. 2014;17:511–8. doi: 10.1111/1756-185X.12315. [DOI] [PubMed] [Google Scholar]
- 44.de Souza S, Bansal RK, Galloway J. Managing patients with rheumatoid arthritis. BDJ Team. 2017;4:19–26. [Google Scholar]
- 45.Shabestari SB, Shirinbak I, Azadarmaki RA. Comprehensive Look at Oromaxillofacial and Laryngopharyngeal Cancers. In: Mehdipour P, editor. Cancer Genetics and Psychotherapy. Springer: Cham; 2017. [Google Scholar]
- 46.Nazemi Salman B, Basir Shabestari S, Shaboyi Jam M, Alizadeh Tari S, Shirinbak I. Periodontal parameters and oral hygiene in diabetic and nondiabetic adolescents in Zanjan. Med J Islam Repub Iran. 2020;34:12. doi: 10.34171/mjiri.34.12. [DOI] [PMC free article] [PubMed] [Google Scholar]