Abstract
Background:
This study was designed to explore the effects of hsa-miR-9-5p on radiotherapy sensitivity of nasopharyngeal carcinoma (NPC) by targeting hexokinase 2 (HK2).
Methods:
The levels of hsa-miR-9-5 and HK2 in NPC patients and radiosensitive and resistant cells were determined using qRT-PCR. The dual luciferase reporter gene system was used to determine hsa-miR-9-5p targeting HK2. The level of HK2 expression in NPC were determined using qRT-PCR and western blotting after the administration of hsa-miR-9-5p agomir. The effects of hsa-miR-9-5p on proliferation and apoptosis with or without irradiation (IR) were examined using CCK-8, flow cytometry and colony formation assays. (18F)-Flourodeoxyglucose uptake was used to evaluate the growth of tumor with or without radiation therapy in vivo.
Results:
hsa-miR-9-5p target to inhibit HK2. Moreover, the cell proliferation was seen in a decreased trend while the cell apoptosis increased in the hsa-miR-9-5p group following radiation therapy hsa-miR-9-5p also showed a significant inhibitory effect on the growth of tumor in vivo with radiation therapy.
Conclusions:
hsa-miR-9-5p improved the radiosensitivity of NPC by targeting HK2.
Keywords: nasopharyngeal carcinoma, hsa-miR-9-5p, hexokinase 2, radiation
Introduction
Nasopharyngeal carcinoma (NPC) is one of the most common cancer in China and studies have shown the incidence in the rise, yearly.1 Most NPC have insidiously onset and metastasis, which lead to poor prognosis. Serine-threonine kinase (AKT) is a protein kinase that phosphorylates serine/threonine,2 and it plays an essential role in cell metabolism, proliferation and migration. Hexokinase 2 (HK2) is a subtype of the kinase, which is closely associated with the occurrence and development of various tumors such as thyroid, ovarian and cervical cancers.2-4 Cancer cells mainly depend on glycolysis instead of mitochondria oxidative phosphorylation for energy production and the high expression of HK2 promotes glycolytic activity in cancer cells.5 Radiotherapy is one of the local treatments for NPC. In recent years, HK2 has been confirmed to be associated with resistance of NPC to radiotherapy.6
MicroRNA is a class of non-coding RNA, which degrades the target gene and down-regulates the expression of gene by complementing the target gene mRNA 3’-end non-translation region (3’ UTR).7 With this, microRNA participates in the regulation of gene expression in the pathogenesis. Through bioinformatics, we found that 3’UTR of HK-2 has dense regulatory sites of miR-9-5p and verified the targeted matching relationship between hsa-miR-9-5p and HK2 genes using Dual-Luciferase® reporter technology. We used real-time PCR and Western blotting to detect the expression of HK2 genes after administration of hsa-miR-9-5p agomir into human radioresistance NPC cell lines. Through this, the regulation effect of hsa-miR-9-5p on the expression of HK2 genes was verified. The effects of hsa-miR-9-5p on radiation therapy effects were detected in vitro and in vivo, and the application value of hsa-miR-9-5p against NPC was revealed at the molecular level.
Materials and Methods
Clinic Samples and Cell Culture
Twenty8 NPC biopsies and 10 non-cancerous biopsies were provided by the Department of Pathology. Within 20 minutes after collection, total mRNA were extracted and kept in a freezer until the time of experiment. Specimen collection was performed through informed consent form signed by the patients.
NPC cell line 5-8F with low-differentiation and high-metastasis was obtained from Shanghai Cell Bank. NPC cell line in exponential growth period was irradiated with increasing radiotherapy doses of 1 Gy, 2 Gy, 4 Gy, 6 Gy and 8 Gy. After a single dose treatment, the cells were cultured until 80% confluency. The cells were treated again using the same dose and this was repeated for third time. Then, the next dose was used to treat in the same way for a period of 12 months to construct 5-8FR.The cell lines were grown in dulbecco’s modified eagle medium (DMEM) containing 12% inactivated fetal bovine serum (FBS) with 5% CO2 at 37° C. Logarithmic growth phase cells were used in all experiments.
Dual-Luciferase® Reporter Gene Detection
Target gene prediction softwareMiRBase, microRNA and Targetscan were used to predict the target matching relationship between hsa-miR-9-5p and HK2 genes. Then the 3’UTR of HK2 was cloned using forward primer 5’-CCGAGCTCAGAAAAGCAATAATGTCCAG-3’ and reverse primer 5’ -GCTCTAGATCCATTCACCAGGGAGCAG-3’. The PCR product was detected by electrophoresis and cloned into pMD 18-T vector (DaKaRa company), and then sequenced. The mutated 3’UTR (3’m-UTR) of HK2 was completed in strict accordance with the requirements of the site-directed mutagenesis kit (QuickChange site-directed mutagenesis kit, Stratagene, USA). Then after double digestion of pmirGLO plasmid (Beijing Promega Company) with Xba I and Sac I, 3′UTR and 3′m-UTR fragments of HK2 were ligated with the plasmid using DNA ligation kit (DaKaRa company). The plasmids were transformed into Escherichia coli DH5α competent cells (DaKaRa Company), amplified and recombinant plasmid was extracted using plasmid extraction kit (Hangzhou Axygen company). 5-8F cells were cultured in DMEM medium containing 12% FBS, trypsinized the cells and plated them with a density of 90%-95% for transfection. Liposome 2000 (Invitrogen, USA) was used to transfect 3’UTR-pmirGLO or 3’m-UTR-pmirGLO 200 ng and hsa-miR-9-5p agomir (Shanghai GenePharma) or negative control miRNA 30 nmol/L into 5-8F cells. After 24 hours, the fluorescence intensity was detected according to the instructions of the Dual-Luciferase® reporter gene detection system (Beijing Promega Company). hsa-miR-9-5p agomir and its corresponding control (NC) were purchased from GenePharma (Suzhou, China).The sequence of hsa-miR-9-5p agomir were 5’-CUCUGGAACCAUGUUUUGGUGA-3’ and the sequence of control was 5’-UUCUCCGAACGUGUCACGUTT-’3.
Quantitative Real-Time PCR (qRT-PCR)
Trizol (DaKaRa Company) was added to each group of cells to extract total mRNA. Obtained through reverse transcription, SYBR Premix Ex TaqTM II (DaKaRa Company) and primers were then used for PCR amplification. The specific sequence included are, U6:5’-CTCGCTTCGGCAGCACA-3’(forward), 5’-TGGTGTCGTGGAGTCG-3’(reverse); hsa-miR-9-5p:5’-AAAGTGCTTCCTTTTAGAGGG-3’(forward), 5’-GCGAGCACAGAATTAATACGAC-3’(reverse); GAPDH:5’-CCCCTTCATTGACCTCAACT-3’(forward), 5’-GATGAGTCCTTCCAC-3’(reverse); HK2: 5’-GAGGTCATGGAGCACAGGTT-3’(forward), 5’-CTGGTCCAGCTCCAGTAAGC-3’(reverse). 40 cycles of PCR reaction was performed on a real-time quantitative PCR instrument, and software was applied to quantitatively analyze the reaction results. The relative mRNA was analyzed by using 2-ΔΔCTmethods.9
Western Blot
Cells from the control group, hsa-miR-9-5p agomir group and NC group were lysed using RIPA buffer (Dingguo, Beijing, China), ultrasonically disrupted and centrifuged at 4 C for 30 min at 10,000 rpm. The protein content was determined by BCA (Dingguo, Beijing, China) method. 50 µg of protein were separated using 10% SDS-PAGE and then transferred to PVDF membranes (Dingguo, Beijing, China). After being blocked with 4% bovine serum albumin (BSA) for 1 h, the membranes were incubated with rabbit anti-human HK2 antibody (AF7080, 1:250, Beyotime, Shanghai, China) overnight at 4 C. Then, the secondary antibody (1:1 000, Santa Cruz, California, USA) was incubated at 4°C for 1 h and the gel imaging system ECL (Invitrogen, USA) was used for luminescence development.
X-ray Irradiation
X-ray irradiation was performed with Varian 23EX Linaclinear accelerator (VARIAN, USA). The radiation dose rate is 2Gy/min, and the radiation field is 15 cm×15 cm.
Cell Counting Kit (CCK-8) Assay
Cells from the control group, hsa-miR-9-5p agomir group and NC group were treated with different doses of X-rays (0, 2, 4, 6 and 8 Gy) and then dispensed into 96-well plates (1×104 cells/ well) in triplicates. The plates were incubated at 37°C, 5% CO2 for 48 hours. Then 10µl CCK-8 (Sangon, Shanghai, China) was added to each well for an additional 4 hours. The absorbance was obtained at 490 nm using a microplate reader (BioTek, Winooski, VT, USA).10
Flow Cytometry
The cells (106 per well) from the control group, hsa-miR-9-5p agomir group and NC group were seeded in 6-well plates. Following overnight culture, the cells were exposed to different doses of X-rays (0, 2, 4, 6, 8 Gy) and then continued to culture until 48 hours. The cells were then collected and the apoptosis was assayed using Annexin V-FICT and PI reagent (Bestbio, Shanghai, China) according to instructions.
Colony Formation Assay
Cells (approximately 200 cells/well), were cultured in a 24-well plate at 37°C, 5% CO2 and the medium was changed after every 2 to 3 days. With visible colonies in the wells, the supernatant was discarded, the cells were washed twice using PBS and fixed in methanol for 15 minutes, and then stained using Giemsa solution for 15 minutes. The number of colonies with more than 40 cells were counted under a light microscope.
Mouse Xenograft NPC Models and Treatment
Twelve BALB/C nude mice (4-6 weeks old, female and 18-22 g) were obtained from Experimental Animal Center of Jiujiang NO.1 People’s Hospital. All animal experiments were approved by the Institutional Animal Care and Use Committees of the NO.1 People’s Hospital of Jiangxi (JJJX-7-123). Mice were reared adaptively in sterile environment for one week. Then they were randomly divided into two groups, which were inoculated with 5-8FR cells transfected by NC or hsa-miR-9-5pagomir. Transfected cells were digested and adjusted to a density of 109 cells/ml and injected subcutaneously on the right side of the animal. The tumor volume was measured every 5 days. Starting from the 20th day, one and a half of each group was used as control, and the other one and a half of each group was irradiated with X-ray irradiation (2Gy/day for 5 consecutive days). The tumor volume was calculated according to the formula: Tumor volume = 1/2 long diameter × short diameter.11
Micro PET/CT Imaging of Mice
18F-FDG was presented by the Department of Nuclear Medicine of Jiujiang First People’s Hospital. 18F-FDG PET/CT imaging was performed using a Micro-PET/CT scanner (Siemens; Siemens, Munich, Germany). Mice were fasted at least 6 hours before imaging, and 5.5 MBq (150 uCi) of 18F-FDG were injected into tail vein exactly 1 h prior to scanning. Then each mouse was fixed in the prone position at the midpoint of the field of view on the scanning bed. First, the mice were scanned by CT, and then by PET.6
Immuno Histochemistry Examinations (IHC)
The xenograft tumor tissue was fixed in 10% neutral paraformaldehyde for 24 hours. The tissues were then embedded in paraffin wax, sliced (5μm) and stained with IHC. The Ki67antibody (Santa Cruz, California, USA) was used to observe tumor growth. And the photographs were recorded under a light microscope (Olympus, Tokyo, Japan, ×400).
Statistical Analysis
Statistical software SPSS 16.0 was used for data analysis. The obtained data is the result of three independent experiments and is expressed as mean ±SD. The differences were analyzed by one-way ANOVA. And p < 0.05 showed that the difference was statistically significant.
Results
hsa-miR-9-5p Down Regulates HK2 Expression
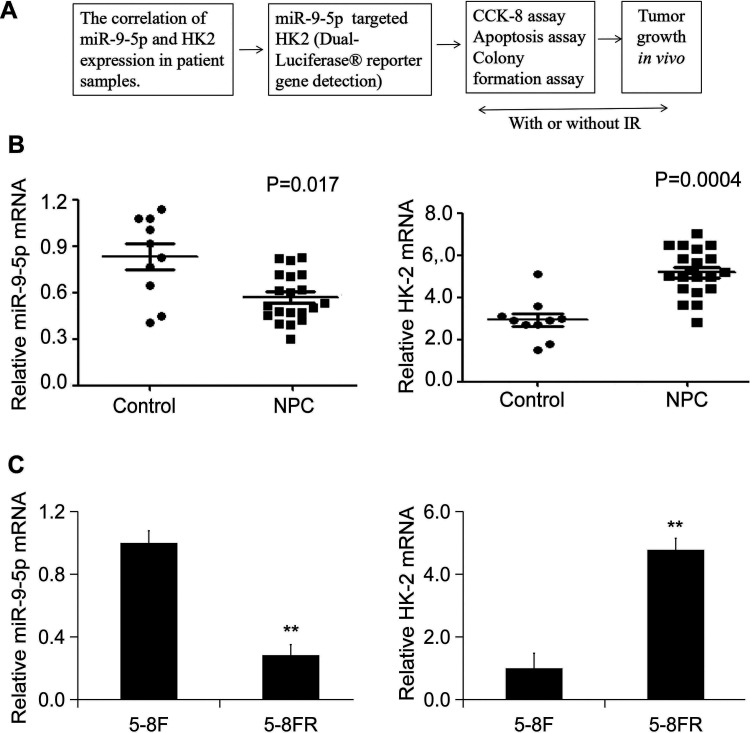
The flow chart of this experiment is summarized in Figure 1A.To validate the expression of hsa-miR-9-5p and HK2 in NPC patients, twenty NPC biopsies and 10 non-cancerous biopsies were collected and subjected to real-time PCR .The results showed that samples demonstrated a significant downregulation for hsa-miR-9-5p and high expression pattern for HK-2(Figure 1B). Moreover, hsa-miR-9-5p was significantly downregulated in 5-8FR and HK-2 showed high expression in 5-8FR cells (Figure 1C).
Figure 1.
The correlation of hsa-miR-9-5p and HK2 expression in patient samples and radio sensitive and resistant cells. (A) A schematic diagram for the study. (B) The mRNA expression levels of hsa-miR-9-5p and HK2 in NPC patients (n = 20). (C) The mRNA expression levels of hsa-miR-9-5p and HK2 in 5-8F and 58FR cells (n = 4) (** p < 0.01 vs. 5-8F).
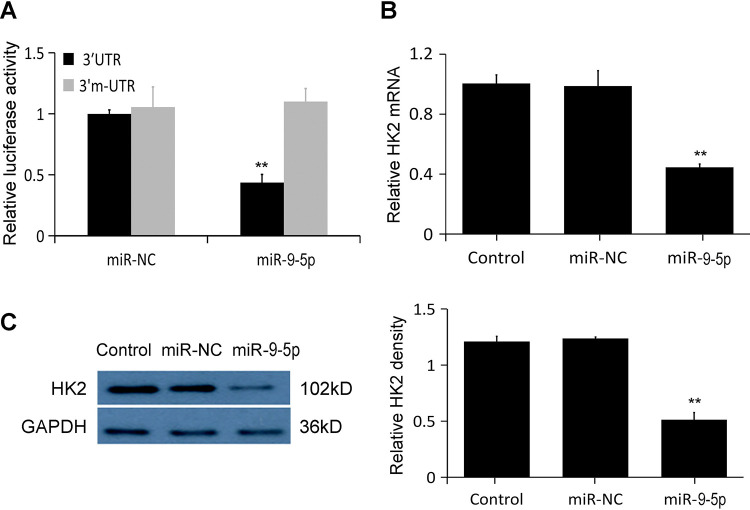
Going extra miles in order to find the target matching relationship between hsa-miR-9-5p and HK2 genes, TargetScan and miRanda were used to predict respectively. TargetScan showed that hsa-miR-9-5p has a conserved target site on HK2 mRNA 3′UTR. Its type is 8mer and its match score is 77. The miRanda results showed that the mirSVR score of hsa-miR-9-5p and the target site was -0.1028, both of which indicated that hsa-miR-9-5p and HK2 matched well. The test results of the dual luciferase reporter gene system showed that if the luciferase expression level of the control group HK2 3′UTR-pmirGLO was set to 1, hsa-miR-9-5p can make the luciferase expression decrease to (38.772 ± 5.162)% (Figure 2A); while the luciferase expression level of the mutated group was (1.043 ± 0.045).
Figure 2.
hsa-miR-9-5p down regulates HK2 expression. (A) The results of the dual luciferase reporter gene system showed that hsa-miR-9-5p directly targeted to HK2. (B) The effects of hsa-miR-9-5p on HK2 mRNA was determined using qRT-PCR. HK2 mRNA was significantly reduced by treatment with miR-9-5p agomir. (C) The effects of hsa-miR-9-5p on HK2 protein expression was determined using Western blot. HK2 protein was significantly inhibited by miR-9-5p agomir (** p < 0.01 vs. miR-NC).
Next, hsa-miR-9-5p agomir were transfected into 5-8FR cell lines to explore the effect of hsa-miR-9-5p agomiron HK2 expression. The results from qRT-RCR and Western blot showed that both mRNA and protein levels of HK2 were significantly reduced after treatment with miR-9-5p agomir (p < 0 .05, Figure 2B, C).
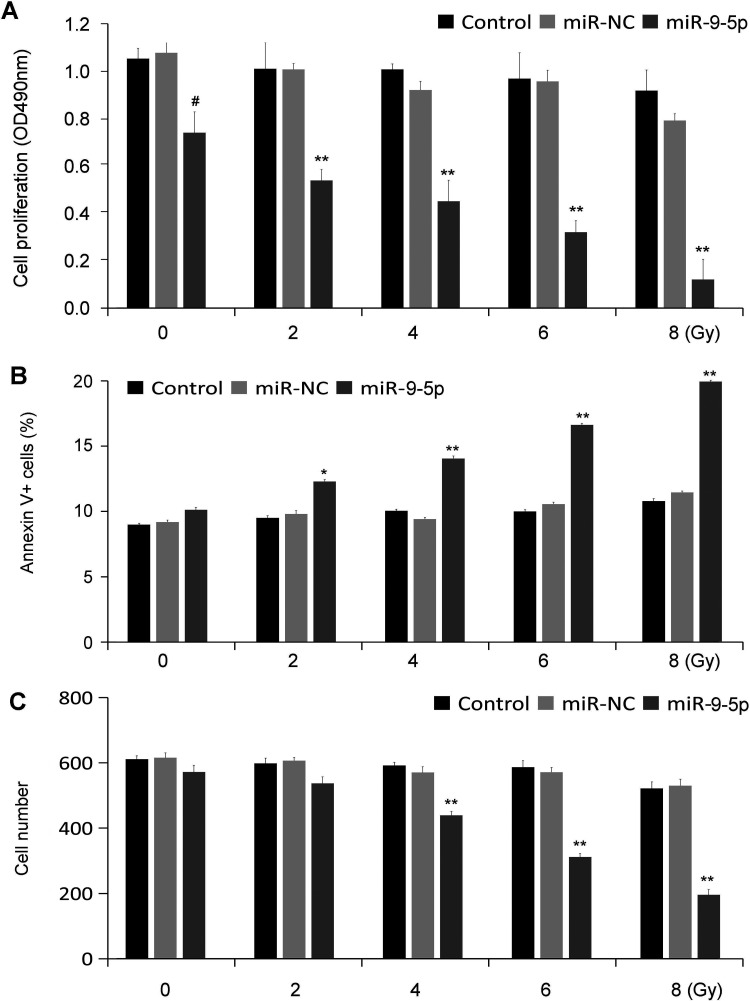
hsa-miR-9-5p Inhibited Cell Proliferation and Promoted Cell Apoptosis With Irradiation (IR)
The effect of hsa-miR-9-5p on cell proliferation was determined using CCK-8 assay. When exposed to different doses of X-rays (2, 4, 6 and 8 Gy), the cells viability in the hsa-miR-9-5p exhibited a more significant decrease (p < 0.01, Figure 3A). In addition, this study further detected the apoptosis rate in different groups exposed or not exposed to X-ray, and found that the apoptosis rate in hsa-miR-9-5p group was higher than that of the control group (Figure 3B). The ability of colony formation after exposure to X-ray was also significantly reduced (Figure 3C).
Figure 3.
hsa-miR-9-5p promoted cell proliferation and inhibited cell apoptosis after irradiation (IR) in vitro (n = 5). (A) The cell proliferation was determined using CCK-8. Compared with Control group, the cell proliferation in hsa-miR-9-5p group was significantly decreased with IR. (B) The cell apoptosis was determined using flow cytometry. Cell apoptosis in hsa-miR-9-5p group with exposure to IR were significantly higher than those in the control groups. (C) The colony formation ability with exposure to IR was significantly decreased in hsa-miR-9-5p group as compared with the control groups (# p < 0.05 vs. miR-NC; * p < 0.05 and ** p < 0.01 vs. corresponding miR-NC+IR).
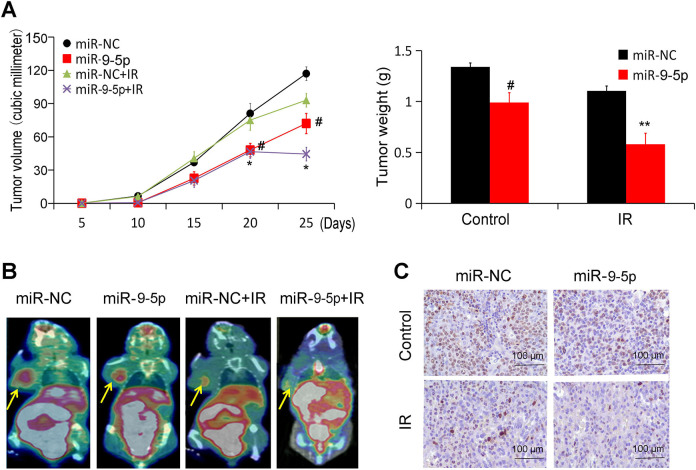
hsa-miR-9-5p Improves18f-FDG PET/CT Radiation Effect
NPC xenograft models were observed every 5 days starting from Day 5. Half of the animals in the two groups were treated with radiotherapy (RT) from Day 20 (2Gy/day for 5 consecutive days). The results showed that the tumor growth in hsa-miR-9-5p group was evidently slower than that of the control without radiotherapy (Figure 4A). After radiotherapy from Day 20, hsa-miR-9-5p showed more significant reduction in growth and tumor weight (Figure 4A).
Figure 4.
hsa-miR-9-5p improves 18F-FDG PET/CT radiation effect. (A) The tumor volume and weights were measured in vivo. (# p < 0.05 vs. miR-NC; * p < 0.05 and ** p < 0.01 vs. miR-NC+IR). (B) The tumor volume was assayed using 18F-FDG micro PET/CT image. (C) The tumor proliferation was determined using Ki67 immunohistochemical staining with or without IR (×400).
On the fifth day after radiotherapy, 18F-FDG microPET/CT was performed to observe the tumor growth. Compared with control group, the tumor volume of hsa-miR-9-5p group showed a decreased trend by 60% (Figure 4 B). Ki67 is closely related to the proliferation and invasion of NPC. After radiotherapy, the proportion of positive cells in hsa-miR-9-5p group was also significantly lower than that of control group (Figure 4C).
Discussion
The metabolism and proliferation of NPC are closely related to the expression and activity of HK2 protein.12,13 Yu found that there is a positive expression of HK2 protein in the cancer tissue of patients with NPC, and the high expression of HK2 protein is significantly related to the malignancy of NPC, lymph node metastasis and clinical stage.12 It has been shown that NPC possess higher levels of glycolytic activity. HK2 catalyzes the first step of glycolysis, which is overexpressed in many types of cancers and promotes the proliferation and growth of cancer cells. However, in most normal tissues, the expression is low or absent. So, HK2 seems to be an ideal target for cancer therapy.14
NPC is particularly sensitive to radiotherapy. However, radiation resistance is the main obstacle that often contributes to the failure of clinical treatment. There are many factors affecting radiation resistance. Hypoxia is a common factor that limits the effect of radiotherapy, because hypoxia helps to recover damaged double-stranded DNA.15 Secondly, the lactic acid content in tumor cells was also positively correlated with radiation sensitivity.17 HK2 was activated during hypoxia and lactic acid overproduction, indicating that downregulation of HK2 can improve radiation sensitivity.16
Many microRNAs play important roles in the occurrence and development of NPC, and are closely related to the diagnosis and prognosis of NPC. HK2 is also regulated by numerous microRNAs. For example, Fang reported that miR-143 regulated cancer glycolysis via targeting HK2gene.17 Jiang reported a miR-155/miR-143 cascade controls glycolysis by regulating HK2 in breast cancercells.18 Studies have shown that hsa-miR-9-5p has strong tumor suppressor function.19,20 The results of this study show that hsa-miR-9-5p can directly target HK2 to inhibit tumor growth, so it has a good application value in the process of anti-NPC.
Malignant tumor is not only related to the infinite proliferation of cells, but also to the inhibition of apoptosis. It has been reported that the binding of HK2 to mitochondria can block the interaction between pro-apoptotic factors VDAC and Bax, thus inhibiting apoptosis.19 In this study, we studied the relationship between hsa-miR-9-5p and radiosensitivity of NPC from the point of view of proliferation and apoptosis. The results showed that hsa-miR-9-5p could inhibit cell proliferation and promote apoptosis during radiotherapy. Moreover, the tumor uptake of 18F-FDG and the proportion of Ki67 positive cells decreased significantly, indicating that hsa-miR-9-5p improved radiosensitivity of NPC. While we speculated that this study is significant to species or experimental conditions including human biology, we recommend the needs for further study.
In summary, it is evident that hsa-miR-9-5p improves radiosensitivity of NPC via targeting HK2 and effectively overcome the radio-resistance.
Footnotes
Author Contributions: SZ and BN performed the experiment. BN did the Data analysis. SZ wrote the manuscript. Both authors approved its submission.
Authors’ Note: Baoliang Ni is also affiliated with Depatment of Otorhinolaryngology, Shenzhen Maternal and Child Health Hospital.
Availability of Data and Materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics Approval: All experiments were approved by the Ethics Committee of the NO.1 People’s Hospital of Jiangxi(JJJX-7-123).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Shanqiang Zhan, PhD  https://orcid.org/0000-0003-3968-9696
https://orcid.org/0000-0003-3968-9696
References
- 1. Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet. 2019;394(10192):64–80. [DOI] [PubMed] [Google Scholar]
- 2. Lyu X, Wang J, Guo X, et al. EBV-miR-BART1-5P activates AMPK/mTOR/HIF1 pathway via a PTEN independent manner to promote glycolysis and angiogenesis in nasopharyngeal carcinoma. PLoS Pathog. 2018;14(12):e1007484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coelho RG, Fortunato RS, Carvalho DP. Metabolic reprogramming in thyroid carcinoma. Front Oncol. 2018;8:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zeng Q, Chen J, Li Y, et al. LKB1 inhibits HPV-associated cancer progression by targeting cellular metabolism. Oncogene. 2017;36(9):1245–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jiao L, Zhang HL, Li DD, et al. Regulation of glycolytic metabolism by autophagy in liver cancer involves selective autophagic degradation of HK2 (hexokinase 2). Autophagy. 2018;14(4):671–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang D, Wang H, Yu W, Qiao F, Su X, Xu H. Downregulation of hexokinase 2 improves radiosensitivity of breast cancer. Transl Cancer Res. 2019;8(1):290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Caixia L, Yang X, Yurong T, Xiaoqun Q. Involvement of epigenetic modification in epithelial immune responses during respiratory syncytial virus infection. Microb Pathog. 2019;130:186–189. [DOI] [PubMed] [Google Scholar]
- 8. Xu D, Jin J, Yu H, et al. Chrysin inhibited tumor glycolysis and induced apoptosis in hepatocellular carcinoma by targeting hexokinase-2. J Exp Clin Cancer Res. 2017;36(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Qin L, Qiu K, Hu C, Wang L, Wu G, Tan Y. Respiratory syncytial virus promoted the differentiation of Th17 cells in airway microenvironment through activation of Notch-1/Delta3. J Med Microbiol. 2019;68(4):649–656. [DOI] [PubMed] [Google Scholar]
- 10. Li L, Liu M, Kang L, et al. HHEX: a crosstalker between HCMV infection and proliferation of VSMCs. Front Cell Infect Microbiol. 2016;6:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang P, He Q, Lei Y, et al. m6A-mediated ZNF750 repression facilitates nasopharyngeal carcinoma progression. Cell Death Dis. 2018;9(12):1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yu X, Wang R, Zhang Y, et al. Skp2-mediated ubiquitination and mitochondrial localization of Akt drive tumor growth and chemoresistance to cisplatin. Oncogene. 2019;38(50):7457–7472. [DOI] [PubMed] [Google Scholar]
- 13. Xiao L, Hu ZY, Dong X, et al. Targeting Epstein-Barr virus oncoprotein LMP1-mediated glycolysis sensitizes nasopharyngeal carcinoma to radiation therapy. Oncogene. 2014;33(37):4568–4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DeWaal D, Nogueira V, Terry AR, et al. Hexokinase-2 depletion inhibits glycolysis and induces oxidative phosphorylation in hepatocellular carcinoma and sensitizes to metformin. Nat Commun. 2018;9(1):446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lu YC, Cheng AJ, Lee LY, et al. MiR-520b as a novel molecular target for suppressing stemness phenotype of head-neck cancer by inhibiting CD44. Sci Rep. 2017;7(1):2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou W, Wu Y, Pan M, Liu D, Liu B. Proliferation and migration of lung cancer could be inhibited by oxymatrine through the regulation for miR-520/VEGF. Am J Chin Med. 2019;47(4):865–878. [DOI] [PubMed] [Google Scholar]
- 17. Fang R, Xiao T, Fang Z, et al. MicroRNA-143 (miR-143) regulates cancer glycolysis via targeting hexokinase 2 gene. J Biol Chem. 2012;287(27):23227–23235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jiang S, Zhang LF, Zhang HW, et al. A novel miR-155/miR-143 cascade controls glycolysis by regulating hexokinase 2 in breast cancer cells. EMBO J. 2012;31(8):1985–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harada H. How can we overcome tumor hypoxia in radiation therapy? J Radiat Res. 2011;52(5):545–556. [DOI] [PubMed] [Google Scholar]
- 20. Liu Y, Murray-Stewart T, Casero RA, Jr, et al. Targeting hexokinase 2 inhibition promotes radiosensitization in HPV16 E7-induced cervical cancer and suppresses tumor growth. Int J Oncol. 2017;50(6):2011–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]