Abstract
Objective:
The aim of this study was to assess the association between sodium-glucose cotransporter-2 (SGLT2) inhibitors and the risk of orthostatic hypotension (OH) in patients with type 2 diabetes mellitus (T2DM).
Method:
A systematic literature retrieval was performed using PubMed, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL) from inception up to 16 October 2019. Data for study characteristics and outcomes of interest were extracted from each eligible study. Pooled risk ratios (RRs) with 95% confidence intervals (CI) for OH were calculated using a random-effects model.
Result:
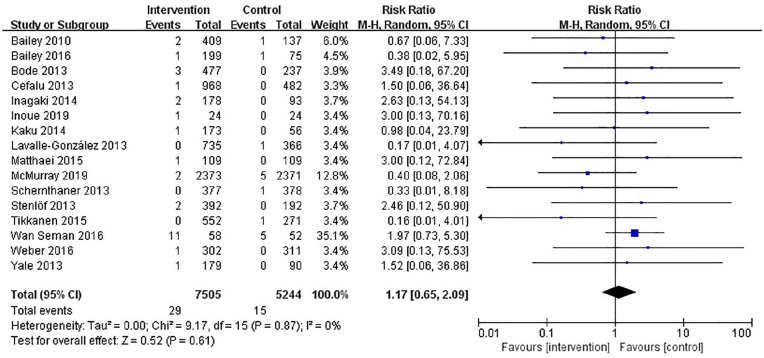
A total of 16 studies (n = 12,749) were included in our meta-analysis, with a result of 44 incident OH cases (29 in the SGLT2 inhibitor group, and 15 in the control group). The pooled RR was 1.17 (95% CI: 0.65–2.09). There was no evidence that receiving SGLT2 inhibitors increased the risk of OH, when stratified by age, duration of T2DM, or placebo-control or active-control and baseline blood pressure.
Conclusion:
This meta-analysis suggested that, in general, SGLPT2 inhibitors did not increase the risk of OH in patients with T2DM. The possibility of OH should be, therefore, considered on an individual basis, especially in patients with a history of OH, long duration of T2DM, or comorbidities.
Keywords: Sodium-glucose-cotransport 2 inhibitor, adverse event, orthostatic hypotension, diabetes
Bulleted novelty statement
What is known about this topic?
Sodium-glucose cotransporter-2 (SGLT2) inhibitors have an impact on blood pressure, as there have been reports of orthostatic hypotension relevant adverse events in RCTs. There has been a lack of analysis to assess the risk of orthostatic hypotension with use of SGLT2 inhibitors; thus, it was reasonable to provide this information for practitioners in clinical practice.
What does this study add?
There was no evidence of SGLT2 inhibitors increasing the risk of orthostatic hypotension in patients with T2DM (RR, 1.17 95% CI: 0.65–2.09), when stratified by category, age, duration of T2DM, or placebo-control or active-control and baseline blood pressure.
Introduction
Orthostatic hypotension (OH) is a frequent disorder among patients with diabetes.1 Blood pressure (BP) maintains the homeostasis ascribed to adaptive compensatory adjustments, which are regulated by the autonomic nervous system, occurring on the upright position in the normal population,2 whereas the functional impairment of the autonomic nervous system via diabetes shows an increased incidence of OH.3 The presence of OH independently predicts the mortality and incidence of myocardial infarction, stroke, heart failure, and atrial fibrillation.4–6 Not only is OH the second most common cause of syncope, it also predicts mortality from coronary events, congestive heart failure (CHF), and cardiovascular disease, and the concurrent development of OH in patients with diabetes mellitus (DM) and CHF predicts poorer outcomes for these disease states.7–10 OH management is not satisfactory due to asymptomatic patients or cases with minimal symptoms.11 Exact diagnosis and well management of OH can improve quality of life and minimize cardiovascular risk.
Sodium-glucose cotransporter-2 (SGLT2) inhibitors are a novel class of oral antidiabetic drugs that reduce plasma glucose concentration by enhancing glycosuria.12 They also lower blood pressure through osmotic diuresis and natriuresis.13 Given their particular insulin-independent modes of action, SGLT2 inhibitors have marked benefits on cardiovascular-renal outcomes in moderate-to-high risk patients with type 2 diabetes mellitus (T2DM).14,15 However, the potential adverse effects related to volume depletion, such as orthostatic hypotension (OH) and dehydration, should not be neglected. Moreover, orthostatic hypotension is associated with diabetes mellitus.16 Patients with T2DM and OH often suffer a higher incident rate of transient, posture-mediated cognitive deficits than those without OH.17
The present meta-analysis was performed to appraise OH risk in patients with T2DM treated with SGLT2 inhibitors compared with placebo or active control treated patients.
Method
Literature search
A systematic and comprehensive literature search was conducted using PubMed, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL) from the inception of this study up to 16 October 2019. The search strategy combined both the Medical Subject Heading and the text words canagliflozin, dapagliflozin, empagliflozin, ipragliflozin, remogliflozin, ertugliflozin, sergliflozin, luseogliflozin, sotagliflozin, tofogliflozin, Sodium glucose co-transporter, SGLT2, SGLT-2, and SGLT 2. These terms were adjusted to conform to the searching principals of each database, and we also conducted an additional literature search in each aforementioned database on 22 December 2019. Citations were limited to randomized controlled trials (RCTs) published in English and did not contain any studies with animals.
Study selection
Two authors (X.L. and Q.G.) independently reviewed all potentially relevant studies according to a prespecified criteria. Inclusion criteria were: (1) RCTs that included adult patients with T2DM; (2) SGLT2 inhibitors compared with placebo or active drugs regardless of back-ground treatments; (3) a duration of follow-up of at least 12 weeks; and (4) reports on data on orthostatic hypotension, which was identified using preferred terms from the Medical Dictionary for Regulatory Activities (MedDRA) or patient self-report defined by investigators. In specific, orthostatic hypotension was defined as a decrease from supine to standing blood pressure of >20 mmHg in systolic blood pressure or >10 mmHg in diastolic blood pressure. Lists of preferred terms as follow: Blood osmolarity increased, blood pressure ambulatory decreased, blood pressure decreased, blood pressure diastolic decreased, blood pressure immeasurable, blood pressure orthostatic abnormal, blood pressure orthostatic decreased, blood pressure systolic decreased, blood pressure systolic inspiratory decreased, central venous pressure decreased, circulatory collapse, decreased ventricular preload, diastolic hypotension, hypotension, mean arterial pressure decreased, orthostatic heart rate response increased, orthostatic hypotension. Data from completed published manuscripts were considered for inclusion in this analysis.
Data extraction and validity assessment
Two researchers (X.L. and Q.G.) independently screened and extracted the data using an a priori defined standardized Microsoft Excel sheet. The following information was extracted from each eligible trial: first author, year of publication, trial identifier, study duration, intervention drug, control drug, sample size, patient characteristics, duration of T2DM, and incident orthostatic hypotension events. These data were further examined by another investigator (X.R.), and any discrepancies resolved by discussion and consensus. If orthostatic hypotension events were not reported in the published paper, then these data were instead extracted from the trial register website. If the trial register website also did not provide the data of orthostatic hypotension events, then the incidence of such events was assumed to be zero. If the study did not design to observe any adverse events, this study was excluded. Two reviewers independently applied the Cochrane risk-of-bias tool18 to assess the quality of included RCTs based on the following domains: random sequence generation, allocation concealment, blinding of study participants and personnel, incomplete outcome data, selective reporting, and other biases. The quality of trials was evaluated in terms of low, unclear, or high risk of bias, with disagreements being resolved through discussion.
Data synthesis and statistical analysis
Most of analyses were performed using RevMan (version 5.3.5; Cochrane Collaboration). For dichotomous data, risk ratios (RRs) and 95% confidence intervals (CI) were calculated to appraise the risk of orthostatic hypotension with SGLT2 inhibitors. Furthermore, subgroup analyses were conducted on the category of SGLT2 inhibitors, patients’ age, duration of T2DM, placebo-control or active-control and baseline blood pressure, baseline HbA1c (%), and patients with or without established cardiovascular disease to evaluate whether the risk of OH could be modified by clinical variables. A chi-square test (χ2) and I2 statistics were used to assess heterogeneity. Heterogeneity was assessed as low, moderate, or high with I2 values of 25%, 50%, and 75%, respectively. A random-effects model was adopted if there was evidence of statistical heterogeneity or clinical diversity (p < 0.01, I2 > 50%); otherwise, a fixed-effects model was used if there was no statistically significant of heterogeneity (p > 0.01, I2 < 50%). The presence of publication bias was evaluated by visual inception for funnel plot asymmetry.19 Egger’s test also was performed on STATA (version 15.0; STATA software) to assess publication bias. We performed sensitivity analyses by omitting one study at a time and calculated the combined RR for the remaining studies to determine whether the result of the original analyses were robust.
Results
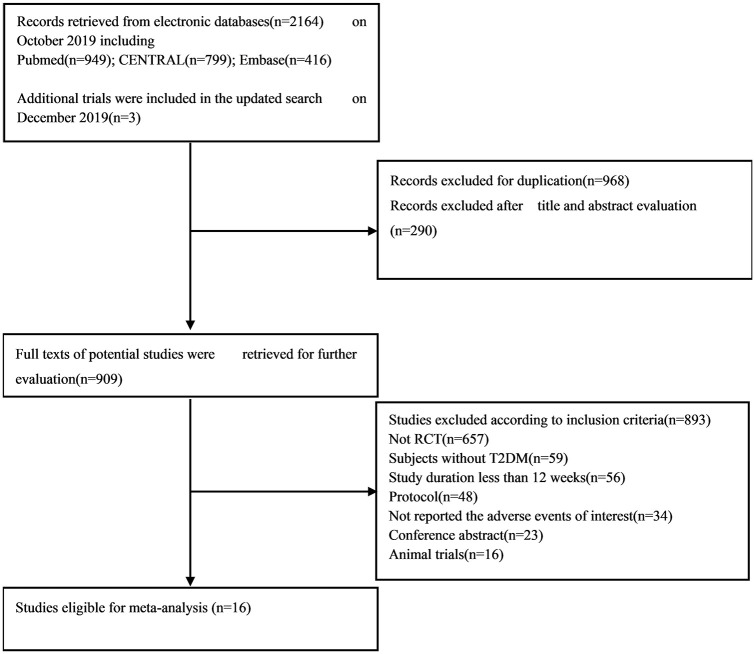
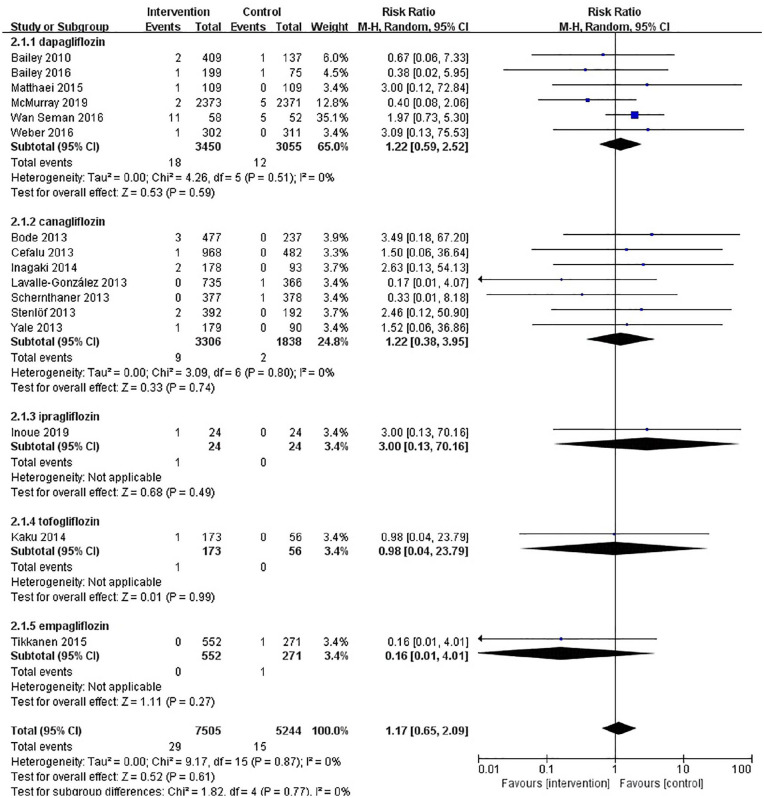
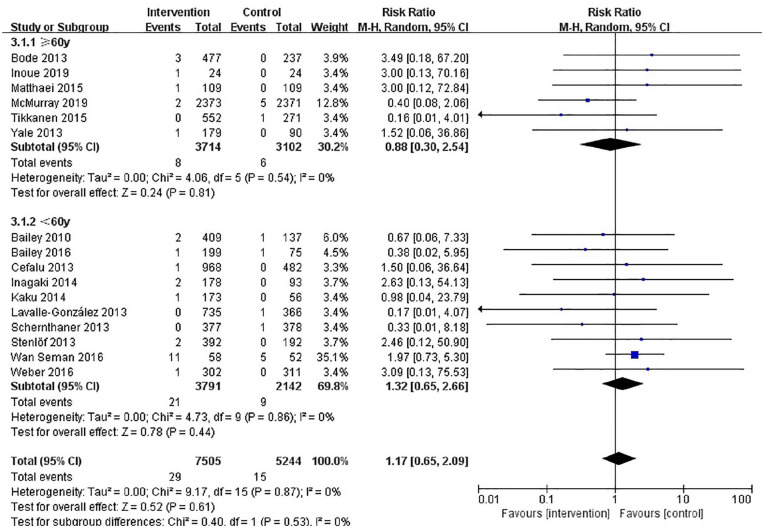
The results of our retrieve methodology and literature screening process are outlined in Figure 1. In brief, we initially screened 2163 citations, but after exclusion of 968 duplications and exclusion of 290 articles based on their titles and abstracts, 909 full-text articles were further assessed. Of these 909 papers, 553 were excluded for being non-randomized controlled trials, 59 were excluded for enrolling patient without T2DM, 56 were excluded for having a follow-up less than 12 weeks, 48 citations were excluded as they are trial protocols, 34 were excluded for not designed to observe any adverse events, and 23 and 16 were excluded for being conference abstracts and animal trials, respectively. Another three papers were included on 22 December 2019. Finally, a total of 16 eligible RCTs involving 12,749 patients with T2DM were included in our meta-analysis.20–35 Six RCTs (n = 6505) evaluated dapagliflozin, seven RCTs (n = 5144) evaluated canagliflozin, one RCT (n = 48) evaluated ipragliflozin, one RCT (n = 229) tofogliflozin, and one RCT (n = 547) evaluated empagliflozin. The characteristics of these included RCTs are summarized in Table 1. The results of our risk of bias assessment are shown in Supplemental Figure S1. Overall, the whole of the 16 RCTs were determined to have a high risk of other bias due to consideration for conflicts of interest. Low risk of reporting bias was evident for all RCTs. Random sequence generation was confirmed in all but one of these RCTs.32 Similarly, incomplete outcome data were not obtained in the same RCT. In addition, five studies did not offer enough information to evaluate allocation concealment.21,22,30,32,33 In total, the risk of bias in the included studies was low. A symmetrical funnel plot of SGLT2 inhibitors versus placebo for orthostatic hypotension indicated no evidence of publication bias Supplemental Figure S2. Egger’s test also confirmed there was no publication bias in the included studies (p = 0.48). There was no significant difference in the risk of orthostatic hypotension between SGLT2 inhibitors and control patients, and the number of orthostatic hypotension events was 29 in the SGLT2 inhibitors group and 15 in the control group. The pooled RR for orthostatic hypotension with the SGLT2 inhibitor group was 1.17 (95% CI: 0.65–2.09) compared with the control group. The heterogeneity, as assessed by I2, was 0% (p = 0.87), suggesting there was no significant heterogeneity between studies (Figure 2). The result of sensitivity analyses indicated that the combined RRs were all not statistically significant and similar to one another, with a range from 0.88 (95% CI: 0.42–1.82) to 1.36 (95% CI: 0.73–2.55). This implied that the results of our meta-analysis were robust. When SGLT2 inhibitors were analyzed by category, dapagliflozin (RR, 1.22; 95% CI: 0.59–2.52), or canagliflozin (RR, 1.22; 95% CI: 0.38–3.95), similar results were seen Figure 3. In a subgroup analysis based on age (<60 years old and ⩾60 years old), the pooled RRs were 1.32 (0.65–2.66) and 0.88 (0.30–2.54), respectively. Figure 4. SLGT2 inhibitors had a lower risk of orthostatic hypotension in placebo-control studies (RR, 1.00; 95% CI: 0.45–2.20) than in active-control studies (RR: 1.41; 95% CI: 0.59–3.37) Supplemental Figure S3. Additional subgroup analyses were performed to assess the effect of different durations of T2DM on the risk of orthostatic hypotension. There was a trend toward a higher risk in patients with a long duration of T2DM Supplemental Figure S4. The pooled RR (1.86, 95% CI: 0.87–3.97) was significantly higher in subgroups with a baseline blood pressure great than or equal to 130/80 mmHg than in the subgroup with a baseline blood pressure less than 130/80 mmHg (RR, 0.67; 95% CI: 0.24–1.86), but there was no statistical significance for blood pressure subgroup differences (p = 0.12) (Supplemental Figure S5). When assessing the different baseline HbA1c (%) impact on the risk of OH, the pooled RRs for HbA1c ⩾ 8% and <8% were 1.7 (95% CI: 0.47–6.15) and 1.28 (95% CI: 0.62–2.61), respectively (Supplemental Figure S6). In addition, in a subgroup analysis where patients were stratified into those with or without established cardiovascular diseases, the pooled RR for those with established diseases was 1.03 (95% CI: 0.36–2.97) versus 1.23 (95% CI: 0.61–2.49) for those without cardiovascular diseases. Tests for subgroup differences had no statistical significance (p = 0.79) (Supplemental Figure S7).
Figure 1.
Flow chart of study selection for analysis.
Table 1.
Characteristics of the randomized controlled studies (RCTs) included in the meta-analysis.
| Author | Trial identifier | Study |
Intervention | Control | Patients (n) |
Age (years) |
Duration of T2DM (years) |
HbA1c (%) |
Case of OH |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duration | Intervention | Controls | Intervention | Controls | Intervention | Controls | Intervention | Controls | Intervention | Controls | ||||
| Bailey et al.24 | NCT00528879 | 24 weeks | DAPA 2.5 mg,5 mg, | PLA | 137,137,135 | 137 | 55 ± 9.3, 54.3 ± 9.4, | 53.7 ± 10.3 | 6 ± 6.2, 6.4 ± 5.8, | 5.8 ± 5.1 | 7.99 ± 0.9, 8.17 ± 0.96, | 8.11 ± 0.96 | 0,2,0 | 1 |
| 10 mg | 52.7 ± 9.9 | 6.1 ± 5.4 | 7.92 ± 0.82 | |||||||||||
| Cefalu et al.16 | NCT00968812 | 52 weeks | CANA 100 mg,300 mg | GLIM 8 mg | 483,485 | 482 | 56.4 ± 9.5, 55.8 ± 9.2 | 56.3 ± 9.0 | 6.5 ± 5.5, 6.7 ± 5.5 | 6.6 ± 5.0 | 7.8 ± 0.8, 7.8 ± 0.8 | 7.8 ± 0.8 | 0,1 | 0 |
| Lavalle-Gonzalez et al.17 | NCT01106677 | 52 weeks | CANA 100 mg,300 mg | SITA | 368,367 | 366 | 55.5 ± 9.4, 55.3 ± 9.2 | 55.5 ± 9.6 | 6.7 ± 5.4, 7.1 ± 5.4 | 6.8 ± 5.2 | 7.9 ± 0.9, 7.9 ± 0.9 | 7.9 ± 0.9 | 0,0 | 1 |
| 100 mg | ||||||||||||||
| Schernthaner et al.25 | NCT01137812 | 52 weeks | CANA 300 mg | SITA | 377 | 378 | 56.6 ± 9.6 | 56.7 ± 9.3 | 9.4 ± 6.1 | 9.7 ± 6.3 | 8.1 ± 0.9 | 8.1 ± 0.9 | 0 | 1 |
| 100 mg | ||||||||||||||
| Stenlof et al.26 | NCT01081834 | 26 weeks | CANA 100 mg,300mg | PLA | 195,197 | 192 | 55.1 ± 10.8, 55.3 ± 10.2 | 55.7 ± 10.9 | 4.5 ± 4.4, 4.3 ± 4.7 | 4.2 ± 4.1 | 8.1 ± 1.0, 8.0 ± 1.0 | 8.0 ± 1.0 | 0,2 | 0 |
| Bode et al.27 | NCT01106651 | 26 weeks | CANA 100 mg,300 mg | PLA | 241,236 | 237 | 64.3 ± 6.5, 63.4 ± 6.0 | 63.2 ± 6.2 | 12.3 ± 7.8, 11.3 ± 7.2 | 11.4 ± 7.3 | 7.8 ± 0.8, 7.7 ± 0.8 | 7.8 ± 0.8 | 2,1 | 0 |
| Inagaki et al.28 | NCT01413204 | 24 weeks | CANA 100 mg,200 mg | PLA | 90,88 | 93 | 58.4 ± 10.4, 57.4 ± 11.1 | 58.2 ± 11.0 | 4.72 ± 4.59, 5.88 ± 5.93 | 5.63 ± 5.76 | 7.98 ± 0.73, 8.04 ± 0.77 | 8.04 ± 0.70 | 0,2 | 0 |
| Kaku et al.29 | JapicCTI-101349 | 24 weeks | TOFO 10 mg, 20 mg, | PLA | 57,58,58 | 56 | 58.6 ± 9.8, 56.6 ± 10.2, | 56.8 ± 9.9 | 6.3 ± 7.1, 6.4 ± 5.1, | 6.0 ± 6.1 | 8.45 ± 0.75, 8.34 ± 0.81, | 8.41 ± 0.78 | 0,0,1 | 0 |
| 40 mg | 57.0 ± 9.1 | 6.7 ± 5.5 | 8.37 ± 0.77 | |||||||||||
| Yale et al.30 | NCT01064414 | 26 weeks | CANA 100 mg,300 mg | PLA | 90,89 | 90 | 69.5 ± 8.2, 67.9 ± 8.2 | 68.2 ± 8.4 | 15.6 ± 7.4, 17.0 ± 7.8 | 16.4 ± 10.1 | 7.9 ± 0.9, 8.0 ± 0.8 | 8.0 ± 0.9 | 0,1 | 0 |
| Matthaei et al.31 | NCT01392677 | 24 weeks | DAPA 10 mg | PLA | 109 | 109 | 61.1 ± 9.7 | 60.9 ± 9.2 | 9.3 ± 6.5 | 9.6 ± 6.2 | 8.08 ± 0.91 | 8.24 ± 0.87 | 1 | 0 |
| Tikkanen et al.18 | NCT01370005 | 12 weeks | EMPA 10 mg, 25 mg | PLA | 276,276 | 271 | 60.6 ± 8.5, 59.9 ± 9.7 | 60.3 ± 8.8 | NR | NR | 7.87 ± 0.77, 7.92 ± 0.72 | 7.90 ± 0.72 | 0,0 | 1 |
| Bailey et al.32 | NCT00528372 | 102 weeks | DAPA 2.5 mg, 5 mg | PLA | 65,64,70 | 75 | 53.0 ± 11.7, 52.6 ± 10.9, | 52.7 ± 10.3 | 2.1 ± 3.2, 1.0 ± 1.6, | 2.1 ± 3.1 | 7.92 ± 0.9, 7.86 ± 0.94, | 7.84 ± 0.87 | 0,0,1 | 1 |
| 10 mg | 50.6 ± 10.0 | 2.3 ± 3.7 | 8.01 ± 0.96 | |||||||||||
| Wan Seman et al.15 | NR | 12 weeks | DAPA 10 mg | SU | 58 | 52 | 53 ± 9.1 | 56 ± 9.1 | 5.0 (3.0, 9.0)* | 6.0 (3.0, 10.3)* | 7.7 (7.08, 8.43)* | 7.6 (6.9, 8.1)* | 11 | 5 |
| Weber et al.19 | NCT01137474 | 12 weeks | DAPA 10 mg | PLA | 302 | 311 | 55.6 ± 8.4 | 56.2 ± 8.9 | 8.2 ± 6.4 | 7.6 ± 6.2 | 8.1 ± 1.0 | 8.0 ± 0.9 | 1 | 0 |
| Inoue et al.33 | UMIN000018839 | 24 weeks | IPRA 50 mg | PLA | 24 | 24 | 60.5 ± 9.8 | 60.8 ± 12.1 | 15.9 ± 7.7 | 19.1 ± 10.7 | 8.12 ± 0.93 | 8.30 ± 0.65 | 1 | 0 |
| McMurray et al.34 | NCT03036124 | 72.8 weeks | DAPA 10 mg | PLA | 2373 | 2371 | 66.2 ± 11.0 | 66.5 ± 10.8 | NR | NR | NR | NR | 2 | 5 |
BMI: body mass index; HbA1c: glycated hemoglobin; IQR: interquartile range; s.d.: standard deviation; DAPA: dapagliflozin; CANA: canagliflozin; IPRA: ipragliflozin; TOFO: tofogliflozin; EMPA: empagliflozin; PLA: placebo; GLIM: glimepiride; SITA: sitagliptin; SU: sulphonylurea; NR: not report.
Data are number of patients (n) or mean (sd) unless stated otherwise.
Median (IQR).
Figure 2.
Forest plot of SGLT2 inhibitors versus control for risk of orthostatic hypotension.
Figure 3.
Forest plot of different SGLT2 inhibitors versus control for orthostatic hypotension.
Figure 4.
Forest plot of the effect of different age for orthostatic hypotension.
Discussion
The present meta-analysis of 16 RCTs involving proximately 12,000 patients demonstrated that SGLT2 inhibitors did not increase the risk of orthostatic hypotension. There was no evidence that the specific category of SGLT2 inhibitors used was associated with a greater risk of orthostatic hypotension compared with each other. When stratified by age there was a slight increase in risk with ages less than 60 years, and the same result was observed when the duration of T2DM was greater or equal to 9 years. When stratified by baseline blood pressure, SGLT2 inhibitor treatment with a baseline BP < 130/80 mmHg appeared to have beneficial effects for preventing orthostatic hypotension. However, if the baseline BP was ⩾130/80 mmHg, there was an increasing risk of orthostatic hypotension with SGLT2 inhibitor treatment. Additionally, there was a higher RR for patients with a baseline HbA1c ⩾ 8%, as well as patients with established cardiovascular diseases.
As they have been strongly recommended by ESC/EASD36 and ADA,37 SGLT2 inhibitors have attracted much attention from researchers. There have been numerous meta-analyses of efficacy and safety.38 But to date there has been a lack of meta-analysis of the risk of orthostatic hypotension. As far as we know, the present meta-analysis is a novel review on the risk of orthostatic hypotension associated with SGLT2 inhibitors. Our conclusions, however, are consistent with the results of a previous meta-analysis that suggested that SGLT-2 inhibitors did not increase the risk of orthostatic hypotension.17 It should be noted, however, that it focused on assessing the BP lowering ability of SGLT2 inhibitors. Moreover, it did not distinguish the risk of orthostatic hypotension from hypotension. We further performed subgroup analysis to investigate the effect of category, age, duration of T2DM, placebo/active control, baseline blood pressure, baseline HbA1c (%), and patients with or without established cardiovascular disease on the risk of orthostatic hypotension. The increasing risk of orthostatic hypotension with less than 60 years of age, however, seemed to be contradictory to the pathophysiology of orthostatic hypotension. Comprehensively, there was no significant difference between ⩾60 years and <60 years. The finding that with a longer duration of T2DM came a greater risk of orthostatic hypotension can be explained by the pathophysiology of orthostatic hypotension.39 However, orthostatic hypotension is a major finding in patients with diabetic autonomic neuropathy, a complication that may be more frequent in older patients as well as in those with long-standing T2DM and poorly controlled diabetes. Such an assumption could explain why age >60 years and duration >9 years were predictors of OH. When taking into account the baseline blood pressure, patients with BP ⩾ 130/80 mmHg tended to have a higher risk compared with those with a BP < 130/80 mmHg. We speculate that a possible reason is that a larger proportion of concomitant therapy is conducted with antihypertensives among patients with BP ⩾ 130/80 mmHg and this was also observed in the ACCORD trial.16 This meta-analysis also suggested that SGLT2 inhibitor-treated patients with HbA1c ⩾ 8% or with established cardiovascular diseases may be predisposed to OH. Moreover, sympathetic hyperactivity is a characteristic of type2 diabetes.40 Abnormal sympathetic nervous activity plays a pivotal role in the pathogenesis of OH. Previous studies have indicated that SGLT2 inhibitors decrease BP without a compensatory increase or notable changes in heart rates,41 implying the sympathoinhibitory effects of SGLT2 inhibitors.42,43 SGLT2 inhibitor-induced reduction of sympathetic activity may be an important reason why SGLT2 inhibitors do not increase the risk of OH in patients with T2DM.
Study limitations
This meta-analysis had certain limitations. First, the assessment of orthostatic hypotension was based upon a predefined list of preferred terms. This strategy was conservative, as orthostatic hypotension can be asymptomatic. Second, we excluded RCTs that reported hypotension adverse events in consideration of the different definitions of hypotension. Third, there were insufficient synthesized data in order to detect the effect of estimated glomerular filtration rates (eGFR) and concomitant antihypertensives on the risk of orthostatic hypotension. Last, SGLT2 inhibitor reduction of sympathetic activity may be important for their cardioprotective effects. The sympathoinhibitory effect of SGLT2 inhibitors could have an impact on the risk of OH. Although our meta-analysis included 16 studies, there was not sufficient information to assess the influence on the circadian rhythms of BP or the sympathetic activity of SGLT2 inhibitors. Further studies are necessary to test the hypothesis of the sympathoinhibitory effects of SGLT2 inhibitors.
Conclusion
The present meta-analysis demonstrated that SGLT2 inhibitors do not increase the risk of orthostatic hypotension in patients with T2DM. However, these results need to be interpreted with caution, due to the possibility of underestimating risk of asymptomatic orthostatic hypotension. Large-scale RCTs with prespecified, well-measured orthostatic hypotension are now called for to assess the impact of SGLT2 inhibitors on orthostatic hypotension. Thus far, it is important to pay attention to the risk of orthostatic hypotension associated with SGLT2 inhibitors, especially in patients with a long duration of T2DM and comorbidities of hypertension.
Supplemental Material
Supplemental material, supplemental_File for Risk of orthostatic hypotension associated with sodium-glucose cotransporter-2 inhibitor treatment: A meta-analysis of randomized controlled trials by Xi Rong, Xinran Li, Qiling Gou, Kai Liu and Xiaoping Chen in Diabetes & Vascular Disease Research
Supplemental material, supplemental_File_2 for Risk of orthostatic hypotension associated with sodium-glucose cotransporter-2 inhibitor treatment: A meta-analysis of randomized controlled trials by Xi Rong, Xinran Li, Qiling Gou, Kai Liu and Xiaoping Chen in Diabetes & Vascular Disease Research
Footnotes
Author contributions: X.R. was involved in designing the study, as well as the data analysis and manuscript preparation. X.L., Q.G. were involved in extracting data, K.L. provided suggestion on carrying out the study and X.C. were involved in manuscript preparation.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental material: Supplemental material for this article is available online.
References
- 1. Wu J-S, Yang Y-C, Lu F-H, et al. Population-based study on the prevalence and risk factors of orthostatic hypotension in subjects with pre-diabetes and diabetes. Diabetes Care 2009; 32(1): 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chopra S, Baby C, Jacob JJ. Neuro-endocrine regulation of blood pressure. Indian J Endocrinol Metab 2011; 15(Suppl. 4): S281–S288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Robertson D. The pathophysiology and diagnosis of orthostatic hypotension. Clin Auton Res 2008; 18(Suppl. 1): 2–7. [DOI] [PubMed] [Google Scholar]
- 4. Verwoert GC, Mattace-Raso FUS, Hofman A, et al. Orthostatic hypotension and risk of cardiovascular disease in elderly people: the Rotterdam study. J Am Geriatr Soc 2008; 56(10): 1816–1820. [DOI] [PubMed] [Google Scholar]
- 5. Ricci F, Fedorowski A, Radico F, et al. Cardiovascular morbidity and mortality related to orthostatic hypotension: a meta-analysis of prospective observational studies. Eur Heart J 2015; 36(25): 1609–1617. [DOI] [PubMed] [Google Scholar]
- 6. Ko D, Preis SR, Lubitz SA, et al. Relation of orthostatic hypotension with new-onset atrial fibrillation (From the Framingham Heart Study). Am J Cardiol 2018; 121(5): 596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gaspar L, Kruzliak P, Komornikova A, et al. Orthostatic hypotension in diabetic patients-10-year follow-up study. J Diabetes Complications 2016; 30(1): 67–71. [DOI] [PubMed] [Google Scholar]
- 8. Chang J, Hou YP, Wu JL, et al. Blood pressure circadian rhythms and adverse outcomes in type 2 diabetes patients diagnosed with orthostatic hypotension. J Diabetes Investig 2018; 9(2): 383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fedorowski A, Gibbons C. Orthostatic hypotension and diabetes are dangerous companions. J Diabetes Complications 2016; 30(1): 5–6. [DOI] [PubMed] [Google Scholar]
- 10. Xin W, Mi S, Lin Z, et al. Orthostatic hypotension and the risk of incidental cardiovascular diseases: a meta-analysis of prospective cohort studies. Prev Med 2016; 85: 90–97. [DOI] [PubMed] [Google Scholar]
- 11. Fedorowski A, Melander O. Syndromes of orthostatic intolerance: a hidden danger. J Intern Med 2013; 273(4): 322–335. [DOI] [PubMed] [Google Scholar]
- 12. Ferrannini E. Sodium-Glucose co-transporters and their inhibition: clinical physiology. Cell Metab 2017; 26(1): 27–38. [DOI] [PubMed] [Google Scholar]
- 13. Heerspink HJL, Perkins BA, Fitchett DH, et al. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 2016; 134(10): 752–772. [DOI] [PubMed] [Google Scholar]
- 14. Packer M, Anker SD, Butler J, et al. Effects of sodium-glucose cotransporter 2 inhibitors for the treatment of patients with heart failure: proposal of a novel mechanism of action. JAMA 2017; 2(9): 1025–1029. [DOI] [PubMed] [Google Scholar]
- 15. Madaan T, Akhtar M, Najmi AK. Sodium glucose cotransporter 2 (SGLT2) inhibitors: current status and future perspective. Eur J Pharm 2016; 93: 244–252. [DOI] [PubMed] [Google Scholar]
- 16. Fleg JL, Evans GW, Margolis KL, et al. Orthostatic hypotension in the ACCORD (action to control cardiovascular risk in diabetes) blood pressure trial: prevalence, incidence, and prognostic significance. Hypertension 2016; 68(4): 888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baker WL, Smyth LR, Riche DM, et al. Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Soc Hypertens 2014; 8(4): 262–275.e269. [DOI] [PubMed] [Google Scholar]
- 18. Higgins JPT, Altman DG, Gøtzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928–d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 316(7129): 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bailey CJ, Gross JL, Pieters A, et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 375(9733): 2223–2233. [DOI] [PubMed] [Google Scholar]
- 21. Cefalu WT, Leiter LA, Yoon KH, et al. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet 2013; 382(9896): 941–950. [DOI] [PubMed] [Google Scholar]
- 22. Lavalle-Gonzalez FJ, Januszewicz A, Davidson J, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia 2013; 56(12): 2582–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schernthaner G, Gross JL, Rosenstock J, et al. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care 2013; 36(9): 2508–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stenlof K, Cefalu WT, Kim KA, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab 2013; 15(4): 372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bode B, Stenlöf K, Sullivan D, et al. Efficacy and safety of canagliflozin treatment in older subjects with type 2 diabetes mellitus: a randomized trial. Hosp Pract 2013; 41(2): 72–84. [DOI] [PubMed] [Google Scholar]
- 26. Inagaki N, Kondo K, Yoshinari T, et al. Efficacy and safety of canagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled with diet and exercise: a 24-week, randomized, double-blind, placebo-controlled, phase III study. Expert Opin Pharmacother 2014; 15(11): 1501–1515. [DOI] [PubMed] [Google Scholar]
- 27. Kaku K, Watada H, Iwamoto Y, et al. Efficacy and safety of monotherapy with the novel sodium/glucose cotransporter-2 inhibitor tofogliflozin in Japanese patients with type 2 diabetes mellitus: a combined phase 2 and 3 randomized, placebo-controlled, double-blind, parallel-group comparative study. Cardiovasc Diabetol 2014; 13: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yale JF, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab 2013; 15(5): 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matthaei S, Bowering K, Rohwedder K, et al. Dapagliflozin improves glycemic control and reduces body weight as add-on therapy to metformin plus sulfonylurea: a 24-Week randomized, double-blind clinical trial. Diabetes Care 2015; 38(3): 365–372. [DOI] [PubMed] [Google Scholar]
- 30. Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care 2015; 38(3): 420–428. [DOI] [PubMed] [Google Scholar]
- 31. Bailey CJ, Morales Villegas EC, et al. Efficacy and safety of dapagliflozin monotherapy in people with type 2 diabetes: a randomized double-blind placebo-controlled 102-week trial. Diabet Med 2015; 32(4): 382–383. [DOI] [PubMed] [Google Scholar]
- 32. Wan Seman WJ, Kori N, Rajoo S, et al. Switching from sulphonylurea to a sodium-glucose cotransporter2 inhibitor in the fasting month of Ramadan is associated with a reduction in hypoglycaemia. Diabetes Obes Metab 2016; 18(6): 628–632. [DOI] [PubMed] [Google Scholar]
- 33. Weber MA, Mansfield TA, Alessi F, et al. Effects of dapagliflozin on blood pressure in hypertensive diabetic patients on renin-angiotensin system blockade. Blood Press 2016; 25(2): 93–103. [DOI] [PubMed] [Google Scholar]
- 34. Inoue H, Morino K, Ugi S, et al. Ipragliflozin, a sodium-glucose cotransporter 2 inhibitor, reduces bodyweight and fat mass, but not muscle mass, in Japanese type 2 diabetes patients treated with insulin: a randomized clinical trial. J Diabetes Investig 2019; 10(4): 1012–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019; 381(21): 1995–2008. [DOI] [PubMed] [Google Scholar]
- 36. Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2019; 40(39): 3215–3217. [DOI] [PubMed] [Google Scholar]
- 37. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2019. Diabetes Care 2019; 42(Suppl. 1): S90–S102. [DOI] [PubMed] [Google Scholar]
- 38. Palmer SC, Mavridis D, Nicolucci A, et al. Comparison of clinical outcomes and adverse events associated with glucose-lowering drugs in patients with type 2 diabetes: a meta-analysis. JAMA 2016; 316(3): 313–324. [DOI] [PubMed] [Google Scholar]
- 39. Freeman R, Abuzinadah AR, Gibbons C, et al. Orthostatic hypotension: JACC state-of-the-art review. J Am Coll Cardiol 2018; 72(11): 1294–1309. [DOI] [PubMed] [Google Scholar]
- 40. Thorp AA, Schlaich MP. Relevance of sympathetic nervous system activation in obesity and metabolic syndrome. J Diabetes Res 2015; 2015: 341583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Reed JW. Impact of sodium-glucose cotransporter 2 inhibitors on blood pressure. Vasc Health Risk Manag 2016; 12: 393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Herat LY, Magno AL, Rudnicka C, et al. SGLT2 inhibitor-induced sympathoinhibition: a novel mechanism for cardiorenal protection. JACC Basic Transl Sci 2020; 5(2): 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wan N, Rahman A, Hitomi H, et al. The effects of sodium-glucose cotransporter 2 inhibitors on sympathetic nervous activity. Front Endocrinol 2018; 9: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, supplemental_File for Risk of orthostatic hypotension associated with sodium-glucose cotransporter-2 inhibitor treatment: A meta-analysis of randomized controlled trials by Xi Rong, Xinran Li, Qiling Gou, Kai Liu and Xiaoping Chen in Diabetes & Vascular Disease Research
Supplemental material, supplemental_File_2 for Risk of orthostatic hypotension associated with sodium-glucose cotransporter-2 inhibitor treatment: A meta-analysis of randomized controlled trials by Xi Rong, Xinran Li, Qiling Gou, Kai Liu and Xiaoping Chen in Diabetes & Vascular Disease Research