Abstract
Aims:
To investigate the association of metabolic parameters including hemoglobin glycation index (HGI, observed HbA1c – predicted HbA1c) with systemic arterial stiffness assessed by cardio-ankle vascular index (CAVI).
Subjects:
We analyzed the cross-sectional data from 22,696 subjects (mean age 48.0 years, mean FPG 88 mg/dL, mean HbA1c 5.5%) with or without past history of metabolic disorders including diabetes.
Results:
Men had higher body mass index (BMI), CAVI, blood pressure (BP), FPG, HbA1c, total cholesterol and triglyceride; and lower age, HGI and HDL-cholesterol. After stratifying subjects into HGI quartiles, the highest quartile (Q4) group showed higher age, female ratio, and frequencies of obesity, hypertension, diabetes, and dyslipidemia. Furthermore, bivariate logistic regression model revealed that the Q4 of HGI was a significant predictor of high CAVI (⩾9.0) independent of the presence of diabetes.
Conclusion:
High HGI is associated with systemic arterial stiffening independent of hyperglycemia. This index is therefore expected to be not only a predictor of hypoglycemia, but also a therapeutic guide for atherosclerosis.
Keywords: Glycemic parameters, hemoglobin glycation index, arterial stiffness, cardio-ankle vascular index
Introduction
Diabetes is associated with an increased risk of cardiovascular disease (CVD) by at least two- to three-fold compared with nondiabetic subjects.1 On the other hand, the susceptibility to developing macrovascular complications differs between patients, even when they have similar levels of hyperglycemia evaluated by glycated hemoglobin (HbA1c).2 HbA1c is a measure of mean glycemia over the preceding 1 to 2 months, and is considered the gold standard for assessing the compensation and treatment of diabetes.3 However, only 60% to 80% of the variance in HbA1c level could be explained by the mean blood glucose (MBG) level.4 The remaining variance in HbA1c is presumed to be affected by interindividual variations in biological factors involved in glucose metabolism, genetic factors and passive hemoglobin glycation rates, or in red cell survival among different ethnic groups.5 For this reason, the hemoglobin glycation index (HGI) was established to quantify the variance.6 HGI is defined as the measured HbA1c levels minus predicted HbA1c calculated from a linear regression of blood glucose versus HbA1c levels. Therefore, HGI indicates the degree of nonenzymatic glycation of hemoglobin. Since patient with high HGI has higher HbA1c value compared to blood glucose level, if the patient’s diabetes treatment is intensified (further blood glucose reduction) based on HbA1c, the risk of hypoglycemia is increased.7 Furthermore, high HGI has been reported to be associated with increased risks of CVDs,8 obesity-related metabolic disorders9 and carotid atherosclerosis in nondiabetic individuals.10
Arterial stiffness is a feature of vascular aging and a predictor of CVDs.11 Increased arterial stiffness corresponds to lower vascular distensibility, and may be a potential therapeutic target for cardiometabolic complications. Recently, the cardio-ankle vascular index (CAVI) has been developed as a blood pressure (BP)-independent parameter for arterial stiffness.12 CAVI has adequate reproducibility for clinical use, and is associated with diabetes13 and other CVD risk factors,14,15 severity of CVD16 and future CVD events.17 Furthermore, we have reported that improved diabetes control or insulin sensitivity may contribute to reduce CAVI in diabetic patients.18,19 In other words, CAVI reflects impaired glucose tolerance-induced arterial stiffening that can be improved by appropriate therapeutic interventions. However, the relationship between HGI and arterial stiffness is unclear.
On these premises, the primary aim of this cross-sectional study was to investigate in detail the relationship of the metabolic parameters including HGI with arterial stiffness assessed by CAVI in real-world Japanese population.
Materials and methods
Design
We performed a retrospective cross-sectional study in Japanese who underwent health screening between April 2010 and March 2019 in Japan. This study was approved by the Institutional Review Board and Ethics Committee of Sakura Hospital, School of Medicine, Toho University (No. S-19066). Written informed consent was obtained from the participants.
Data collection and laboratory assay methods
The population-based sample used in the present analysis comprised 76,720 Japanese subjects residing in major cities nationwide, who participated in the CVD and cancer screening program organized by the Japan Health Promotion Foundation. Duplicate subjects were weeded out. Participants were volunteers who were not paid and were not recruited for this study (unlike subjects of a clinical trial). We also recruited subjects taking any medication and/or having a history of heart disease, treatment for hypertension, stroke, diabetes or gout, and excluded those with insufficient data. Finally, 22,696 subjects were enrolled.
All parameters were evaluated using standardized methods. Height and body weight (BW) were measured, and body mass index (BMI) was calculated as follows: BW (kg) divided by square of height (m). Obesity was defined as BMI ⩾ 25 kg/m2, according to the Examination Committee of Criteria for Obesity Disease in Japan.20
Blood was collected from the antecubital vein in the morning after 12 h of fasting to measure fasting plasma glucose (FPG), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), and serum uric acid level. All the blood levels were determined according to standard procedures.
Dyslipidemia was defined as hyper-cholesterolemia (TC ⩾ 220 mg/dL), hypo-HDL cholesterolemia (HDL-C < 40 mg/dL) and/or hypertriglyceridemia (TG ⩾ 150 mg/dL), or treatment with lipid-lowering agents. Hyperuricemia was diagnosed by serum uric acid ⩾7.0 mg/dL, treatment with uric acid-lowering agents or past history of gout attack. HbA1c (%) measured by the Japan Diabetes Society (JDC) method was converted to NGSP value (%) using the following formula: HbA1c (NGSP) (%) = 1.019 × HbA1c (JDS) (%) + 0.30%.21 A combination of ⩾6.5% HbA1c with ⩾126 mg/dL FPG was adopted to detect diabetes.21 Participants receiving antidiabetic agents were also diagnosed as diabetes. To estimate the interindividual variance in HbA1c levels, the HGI was calculated using HbA1c and FPG levels.7,22 The linear relationship between HbA1c and FPG was estimated from linear regression analysis of the data of the subject population (regression equation: HbA1c = 0.0251 × FPG + 3.311, r = 0.718 and p < 0.001). Predicted HbA1c level was then calculated from the regression equation using each subject’s FPG value. HGI was defined as the difference between the measured HbA1c and the predicted HbA1c (HGI = measured HbA1c − predicted HbA1c). Besides, Non-HDL-C was defined as TC minus HDL-C. We also evaluated the triglycerides and glucose (TyG) index as a marker of insulin resistance, calculated as ln([TG (mg/dL) × FPG (mg/dL)]/2).23
BP was measured simultaneously with CAVI, and hypertension was diagnosed by systolic BP (SBP) ⩾ 140 mmHg and/or diastolic BP ⩾ 90 mmHg,24 or treatment with BP-lowering agents.
Measurement of CAVI
CAVI was measured automatically using VaSera VS-1500 (Fukuda Denshi Co Ltd, Tokyo, Japan). With the subject lying supine and the head held in midline position, cuffs were wrapped bilaterally around both upper arms and both ankles. Electrocardiography electrodes were placed on both wrists, and a microphone was attached over the sternum to detect heart sounds.
CAVI was calculated according to the following formula:
CAVI = a{(2ρ/ΔP)×ln(Ps/Pd)PWV2} + b, where Ps is systolic blood pressure; Pd is diastolic blood pressure; ΔP is Ps − Pd; ρ is blood density; PWV is cardio-ankle pulse wave velocity, and a and b are constants.
The details of CAVI have been described previously.25 Right and left CAVI were measured, and the higher value was used for analysis. Subjects with ankle-brachial indices lower than 0.90 were excluded, because patients with severe arterial occlusive diseases may give falsely low CAVI.
Statistical analysis
All data are expressed as mean ± standard deviation (SD). The SPSS software (version 11.5, Chicago, IL, USA) was used for statistical processing. Mann-Whitney U test or Fisher’s exact test was performed to examine gender difference in clinical variables or prevalence of major CVD risk factors. The relationship of CAVI or HGI with clinical variables was analyzed using multiple regression analysis. Analysis of variance (ANOVA) followed by Bonferroni multiple comparison test and Cochran-Armitage test followed by Cochran-Q test were used to compare clinical characteristics across quartiles of HGI. Finally, we arbitrarily defined “high CAVI” as equal to or higher than 9.0 in all participants, corresponding substantially to the cut-off value for the presence of coronary artery stenosis.12,16 High CAVI was adopted as the surrogate endpoint for coronary artery disease in this study. Bivariate logistic regression analysis was performed to identify contributors to high CAVI and expressed as odds ratio (OR) with 95% confidence interval (CI). Pearson’s product-moment correlation coefficient (r) given the scatter plot was used to study the relationship between HGI and HbA1c data. The p values were derived from interaction tests among the subgroup factors. In all comparisons, p values less than 0.05 were considered statistically significant.
Results
Clinical and biochemical characteristics of male and female participants
In this cross-sectional study, a total of 22,696 Japanese urban residents (10,586 men and 12,110 women, mean age 48.0 years, mean FPG 88 mg/dL, mean HbA1c 5.5%) were enrolled. Table 1 compares the clinical characteristics of male and female participants.
Table 1.
Clinical and biochemical characteristics of male and female participants.
| Variables | All | Male | Female | p value |
|---|---|---|---|---|
| Number of subjects | 22,696 | 10,586 | 12,110 | – |
| Age (years) | 48.0 ± 15.3 | 47.1 ± 14.6 | 48.7 ± 15.8 | <0.001 |
| Old age (⩾65 years) | 18.9% | 15.7% | 21.8% | <0.001* |
| Height (cm) | 1.63 ± 0.09 | 1.70 ± 0.06 | 1.57 ± 0.06 | <0.001 |
| Body weight (kg) | 59.4 ± 12.2 | 67.8 ± 10.5 | 52.0 ± 8.3 | <0.001 |
| BMI (kg/m2) | 22.2 ± 3.4 | 23.4 ± 3.2 | 21.2 ± 3.3 | <0.001 |
| Obesity (⩾25 kg/m2) | 18.2% | 25.8% | 11.5% | <0.001* |
| CAVI | 7.75 ± 1.03 | 7.80 ± 1.00 | 7.69 ± 1.01 | <0.001 |
| High CAVI (⩾9) | 14.2% | 15.2% | 13.4% | <0.001* |
| SBP (mmHg) | 123 ± 17 | 127 ± 15 | 120 ± 18 | <0.001 |
| DBP (mmHg) | 72 ± 11 | 75 ± 11 | 69 ± 11 | <0.001 |
| Hypertension | 22.5% | 25.9% | 19.6% | <0.001* |
| FPG (mg/dL) | 88 ± 16 | 90 ± 18 | 85 ± 13 | <0.001 |
| HbA1c (%) | 5.51 ± 0.53 | 5.55 ± 0.62 | 5.48 ± 0.44 | <0.001 |
| Diabetes | 4.8% | 6.8% | 3.1% | <0.001* |
| HGI (%) | 0.00 ± 0.37 | −0.03 ± 0.41 | 0.02 ± 0.33 | <0.001 |
| TC (mg/dL) | 209 ± 37 | 208 ± 36 | 210 ± 37 | <0.001 |
| TG (mg/dL) | 101 ± 83 | 122 ± 103 | 82 ± 54 | <0.001 |
| HDL-C (mg/dL) | 67 ± 17 | 61 ± 15 | 73 ± 17 | <0.001 |
| Non-HDL-C (mg/dL) | 142 ± 37 | 147 ± 37 | 137 ± 37 | <0.001 |
| TyG index | 8.19 ± 0.63 | 8.41 ± 0.63 | 8.01 ± 0.56 | <0.001 |
| Dyslipidemia | 44.9% | 48.6% | 41.7% | <0.001* |
| Uric acid (mg/dL) | 5.2 ± 1.4 | 6.1 ± 1.2 | 4.4 ± 1.0 | <0.001 |
| Hyperuricemia | 11.8% | 23.8% | 1.3% | <0.001* |
BMI: body mass index; CAVI: cardio-ankle vascular index; SBP: systolic blood pressure; DBP: diastolic blood pressure; FPG: fasting plasma glucose; HbA1c: glycated hemoglobin; HGI: hemoglobin glycation index; TC: total cholesterol; TG: triglyceride; HDL-C: high-density lipoprotein-cholesterol; TyG index: triglycerides and glucose index.
Data are presented as mean ± standard deviation. Mann-Whitney U test and *Fisher’s exact test were used to compare male and female subjects.
Compared with women, men had significantly higher BMI, CAVI, BP, FPG, HbA1c, Non-HDL-C, TG, TyG index, and uric acid level; and lower age, TC, HGI, and HDL-C. The frequencies of obesity, hypertension, diabetes, dyslipidemia, and hyperuricemia were higher in males.
Correlation of CAVI with clinical variables analyzed by multiple regression model
Next, we examined the factors associated with CAVI (Table 2). The multiple regression model for the correlation of CAVI with clinical variables (Model; r2 = 0.760, p < 0.001) revealed that age was the major independent predictor of CAVI (β coefficient = 0.813). Additionally, a weak correlation between CAVI and BMI (β = −0.140) or SBP (β = 0.106) was observed.
Table 2.
Correlation of CAVI with clinical variables analyzed by multiple regression model.
| Variable | β coefficient | SE | p value |
|---|---|---|---|
| Male gender | 0.089 | 0.009 | <0.001 |
| Age (years) | 0.813 | 0.000 | <0.001 |
| BMI (kg/m2) | −0.140 | 0.001 | <0.001 |
| SBP (mmHg) | 0.106 | 0.000 | <0.001 |
| HbA1c (%) | 0.052 | 0.007 | <0.001 |
| Non-HDL-C (mg/dL) | 0.007 | 0.000 | 0.064 |
| HDL-C (mg/dL) | −0.015 | 0.000 | <0.001 |
| Uric acid (mg/dL) | 0.039 | 0.003 | <0.001 |
CAVI: cardio-ankle vascular index; BMI: body mass index; SBP: systolic blood pressure; HbA1c: glycated hemoglobin; HDL-C: high-density lipoprotein-cholesterol; SE: standard error.
Model: r2 = 0.760, p < 0.001.
Correlation of HGI with clinical variables analyzed by multiple regression model
Factors related to HGI by gender were also examined (Table 3). The coefficient of determination of the HGI-related multiple regression model using clinical variables was low for both men and women (Model; r2 = 0.040 in men, 0.058 in women, p < 0.001). However, a weak correlation between HGI and BMI in both gender (β = 0.121 in men, 0.110 in women); or age (β = 0.118) or FPG (β = −0.195) in women.
Table 3.
Correlation of HGI with clinical variables analyzed by multiple regression model.(a) Male
| Variable | β coefficient | SE | p value |
|---|---|---|---|
| Age (years) | 0.089 | 0.000 | <0.001 |
| BMI (kg/m2) | 0.121 | 0.001 | <0.001 |
| SBP (mmHg) | −0.057 | 0.000 | <0.001 |
| FPG (mg/dL) | 0.010 | 0.000 | <0.001 |
| Non-HDL-C (mg/dL) | 0.058 | 0.000 | 0.064 |
| HDL-C (mg/dL) | −0.071 | 0.000 | <0.001 |
| Uric acid (mg/dL) | −0.057 | 0.003 | <0.001 |
Model: r2 = 0.040, p < 0.001.
(b) Female
| Variable | β coefficient | SE | p value |
|---|---|---|---|
| Age (years) | 0.118 | 0.000 | <0.001 |
| BMI (kg/m2) | 0.110 | 0.001 | <0.001 |
| SBP (mmHg) | −0.084 | 0.000 | <0.001 |
| FPG (mg/dL) | −0.195 | 0.000 | <0.001 |
| Non-HDL-C (mg/dL) | 0.086 | 0.000 | <0.001 |
| HDL-C (mg/dL) | −0.061 | 0.000 | <0.001 |
| Uric acid (mg/dL) | 0.001 | 0.003 | 0.937 |
HGI: hemoglobin glycation index; SE: standard error; CAVI: cardio-ankle vascular index; BMI: body mass index; SBP: systolic blood pressure; HbA1c: glycated hemoglobin; Non-HDL-C: non-high-density lipoprotein-cholesterol; HDL-C: high-density lipoprotein-cholesterol.
Model: r2 = 0.058, p < 0.001.
Characteristics of participants stratified by quartile of HGI
The subjects were stratified by HGI into four groups: lowest quartile (Q1), Q2, Q3, and highest quartile (Q4). Table 4 compares their clinical characteristics.
Table 5.
Bivariate logistic regression model for high CAVI (⩾9.0).
| Variables | Odds ratio | 95% CIs | p value |
|---|---|---|---|
| Male gender | 1.86 | 1.66–2.07 | <0.001 |
| Old age | 26.2 | 23.6–29.2 | <0.001 |
| Obesity | 0.499 | 0.438–0.569 | <0.001 |
| Dyslipidemia | 1.54 | 1.39–1.70 | <0.001 |
| Hypertension | 3.56 | 3.21–3.95 | <0.001 |
| Diabetes | 2.89 | 2.41–3.46 | <0.001 |
| Hyperuricemia | 1.29 | 1.11–1.51 | 0.001 |
| Highest HGI quartile (Q4) | 1.20 | 1.07–1.34 | 0.002 |
CI: confidence interval; HGI: hemoglobin glycation index.
Akaike’s Information Criterion: 10,825, residual deviance: 10,807, p < 0.001.
Table 4.
Characteristics of participants stratified by quartile of hemoglobin glycation index.
| Variables | Quartile of hemoglobin glycation index (HGI) | p value | |||
|---|---|---|---|---|---|
| Q1 (Lowest) | Q2 | Q3 | Q4 (Highest) | ||
| Number of subjects | 5673 | 5609 | 5675 | 5739 | – |
| Male | 55.2% | 48.0% | 42.1% | 41.3%* | <0.001a |
| Age (years) | 47.2 ± 15.2 | 47.3 ± 15.4 | 46.9 ± 15.3 | 50.4 ± 14.9† | <0.001b |
| Old age (⩾65 years) | 16.9% | 18.6% | 18.1% | 22.1%* | <0.001b |
| BMI (kg/m2) | 22.2 ± 3.1 | 22.0 ± 3.2 | 21.9 ± 3.3 | 22.7 ± 3.9† | <0.001b |
| Obesity (⩾25 kg/m2) | 16.9% | 15.7% | 16.6% | 23.4%* | <0.001a |
| CAVI | 7.72 ± 0.99 | 7.71 ± 1.00 | 7.68 ± 1.00 | 7.88 ± 1.03† | <0.001b |
| High CAVI (⩾9) | 12.9% | 13.2% | 13.1% | 17.7% | <0.001a |
| SBP (mmHg) | 125 ± 17 | 123 ± 17 | 121 ± 17 | 123 ± 18† | <0.001b |
| DBP (mmHg) | 74 ± 11 | 72 ± 11 | 71 ± 11 | 72 ± 12† | <0.001b |
| Hypertension | 24.5% | 20.8% | 19.5% | 25.2%* | 0.003a |
| FPG (mg/dL) | 92 ± 16 | 87 ± 10 | 85 ± 11 | 87 ± 22† | <0.001b |
| HbA1c (%) | 5.2 ± 0.3 | 5.4 ± 0.3 | 5.5 ± 0.3 | 5.9 ± 0.8† | <0.001b |
| Diabetes | 3.2% | 1.5% | 1.8% | 12.6%* | <0.001a |
| HGI (%) | −0.40 ± 0.23 | −0.11 ± 0.05 | 0.07 ± 0.06 | 0.43 ± 0.36† | <0.001b |
| TC (mg/dL) | 206 ± 36 | 209 ± 36 | 208 ± 36 | 212 ± 38† | <0.001 |
| TG (mg/dL) | 104 ± 92 | 98 ± 86 | 94 ± 68 | 106 ± 84 | <0.001b |
| HDL-C (mg/dL) | 68 ± 17 | 68 ± 17 | 67 ± 17 | 65 ± 17† | <0.001b |
| Non-HDL-C (mg/dL) | 138 ± 36 | 141 ± 36 | 141 ± 37 | 147 ± 39† | <0.001b |
| TyG index | 8.27 ± 0.63 | 8.17 ± 0.59 | 8.11 ± 0.59 | 8.22 ± 0.63 | <0.001b |
| Dyslipidemia | 42.9% | 43.4% | 42.7% | 50.6%* | <0.001a |
| Uric acid (mg/dL) | 5.3 ± 1.4 | 5.2 ± 1.4 | 5.1 ± 1.4 | 5.2 ± 1.4† | <0.001b |
| Hyperuricemia | 13.3% | 12.0% | 10.7% | 11.1%* | 0.002a |
BMI: body mass index; CAVI: cardio-ankle vascular index; SBP: systolic blood pressure; DBP: diastolic blood pressure; FPG: fasting plasma glucose; HbA1c: glycated hemoglobin; HGI: hemoglobin glycation index; TC: total cholesterol; TG: triglyceride; HDL-C: high-density lipoprotein-cholesterol; TyG index: triglycerides and glucose index.
Data are presented as mean ± standard deviation.
p < 0.005, Cochran-Q test, Q1 versus Q4.
p < 0.001, Bonferroni test, Q1 versus Q4.
Cochran-Armitage test.
ANOVA, p value for trend.
Compared with Q1 group, Q4 group had significantly higher age, BMI, CAVI, HbA1c, TC, TG, and Non-HDL-C; and significantly lower male ratio and HDL-C. Furthermore, the frequencies of obesity, hypertension, diabetes, and dyslipidemia were higher in Q4 group compared to Q1 group.
Bivariate logistic regression model for high CAVI (⩾9.0)
Furthermore, we examined the factors associated with high CAVI (⩾9.0) using bivariate logistic regression analysis of dichotomous variables (Table 5). In addition to the highest HGI quartile (Q4), gender and major cardiovascular risk factors were entered into the model. The analysis identified all of the variables including male gender, old age, obesity, dyslipidemia, hypertension, diabetes and, hyperuricemia to be associated with high CAVI, respectively.
Notably, highest HGI quartile (Q4) was a significant predictor for high CAVI independent of the presence of diabetes.
Correlation of HGI with HbA1c
Finally, the relationship between HGI and HbA1c was investigated (Figure 1). A highly significant statistical positive correlation was observed between HGI and HbA1c (r = 0.681, p < 0.001).
Figure 1.
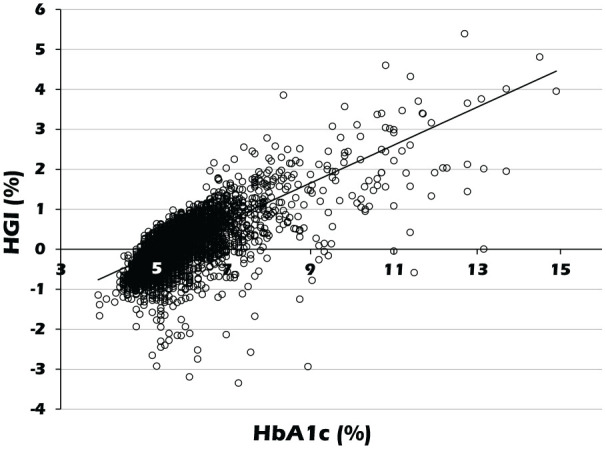
Correlation of HGI with HbA1c. The scatter plot shows the distribution of HGI with HbA1c.
HGI: hemoglobin glycation index; HbA1c: glycated hemoglobin.
Discussion
In the present study of a real-world Japanese population of 22,696 subjects, gender, age, BMI, and SBP were major independent confounders of CAVI. Additionally, the highest HGI quartile was associated with high CAVI independent of the presence of diabetes. This may be the first report to demonstrate the association of HGI with arterial stiffness.
Increased arterial stiffness along with increase in HGI is consistent with the finding in the Diabetes Control and Complications Trial (DCCT) that HGI is a predictor for progression of diabetic angiopathy.26 As for the possible mechanisms for the relationship between high HGI and systemic arterial stiffening, there are several hypotheses. The first assumed mechanism is increased formation of advanced glycation end products (AGEs), which cause both insulin resistance and atherosclerosis.27 A previous study has found an association between increased HGI and high levels of AGEs in diabetic patients.28 It is therefore possible that subjects with high HGI have a propensity to glycate other proteins and macromolecules leading to a higher risk of developing vascular complications. Furthermore, Marini et al.10 reported that high HGI was associated with obesity-related metabolic disorders including high triglycerides, uric acid, and fasting insulin, together with low insulin-stimulated glucose disposal, independent of confounders such as age and gender in non-diabetic individuals. Similar findings are confirmed in our study (Table 4), which may indicate that HGI is a useful tool to identify a subset of subjects with high risk of CVD. On the other hand, in the present study, the prevalence of hyperuricemia was almost equal between HGL quartile groups, which might be influenced by decreased male gender ratio with increasing HGI quartile.
Another explanation for the association of HGI with systemic arterial stiffening is the influence of postprandial hyperglycemia that could not be detected by HGI in our study. Unlike previous studies using MBG for the calculation of HGI, we used only FPG. While HbA1c is mainly contributed by FPG and postprandial glucose,7 calculation of HGI from FPG alone may omit the contribution of postprandial hyperglycemia. However, previous studies have shown that HGI calculated from MBG correlates highly with that obtained using FPG alone.6 Furthermore, previous studies have also revealed that HGI calculated from FPG is adequately useful to verify the clinical implications for diabetes, as well as to assess the risk of hypoglycemia and inflammatory status.7,22 Therefore, it seems reasonable to calculate HGI from FPG instead of MBG, considering convenience. In any case, HGI is a risk factor for atherosclerosis independent of hyperglycemia, and may be useful as a therapeutic target in addition to assessing the risk of hypoglycemia during intensive diabetes treatment.
HGI correlates closely with HbA1c, to the extent that differentiating the two as separate epidemiological risk factors can be difficult.29 In this study, there was a significant correlation between HbA1c and HGI levels (Figure 1), which may raise a concern about multicollinearity. Actually, relatively high value of CAVI observed in highest HGI quartile group disappeared after adjusting for HbA1c (data not shown). The association of HGI with diabetic complications may be therefore explained largely by HbA1c in the same patient. However, we think that it is invalid to assert that HGI should not be used to evaluate risk of complications or to guide therapy. It has been reported that the individual regression line of MBG versus HbA1c maintains approximately the same distance to the population regression line during consistent tracking.6 Furthermore, in the present study, high HGI was associated with arterial stiffening independent of the presence of diabetes. These indicate that individual HGI does not fluctuate easily despite changes in HbA1c. Therefore, HGI may be a glycemic parameter that reflects arterial stiffness independent of HbA1c.
Gender difference in HGI has not been studied in detail. In the present study, women showed higher HGI, and female ratio increased with increasing HGI quartile. Additionally, several studies have also suggested a stronger association of female with high HGI.7,8 On the contrary, gender difference of HGI was not observed in many studies.9,30 The effects of gender, race, and ethnic group on HGI remain controversial. Further investigations on the factors associated with HGI are required.
The limitations of this study include the lack of data on some potential confounders such as proteinuria, heart rate, alcohol consumption, menopause, and smoking status. From these viewpoints, longitudinal cohort studies are needed to clarify the changes in HGI and arterial stiffness during the evolution of cardiovascular risks.
A key strength of the work is the investigation of a large number of individuals and inclusion of a mixed population without and with diabetes. However, there are a number of weaknesses that should be acknowledged. First, this was a cross-sectional study and therefore a causal relationship between HGI and vascular risk cannot be proven. Second, the vast majority of the population studied did not have diabetes and therefore data appear to be limited to individuals with normal glucose metabolism. Third, the mechanistic pathways linking HGI with increased risk of vascular pathology are unclear. Finally, it remains to be established whether HGI can be used in routine clinical practice as a predictor of vascular pathology.
In conclusion, high HGI is associated with systemic arterial stiffening independent of hyperglycemia. This index is therefore expected to be not only a predictor of hypoglycemia, but also as a therapeutic target for atherosclerosis.
Acknowledgments
The authors are grateful to Dr Kenji Suzuki, Japan Health Promotion Foundation, for his enormous contribution to this study, and we gratefully acknowledge the investigators, coinvestigators, study coordinators, and patients who participated in this study.
Footnotes
Author contributions: All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Daiji Nagayama  https://orcid.org/0000-0001-6119-8012
https://orcid.org/0000-0001-6119-8012
References
- 1. Laakso M. Cardiovascular disease in type 2 diabetes: challenge for treatment and prevention. J Intern Med 2001; 249(3): 225–235. [DOI] [PubMed] [Google Scholar]
- 2. Nathan DM, Genuth S, Lachin J, et al.; Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329(14): 977–986. [DOI] [PubMed] [Google Scholar]
- 3. American Diabetes Association. 6. Glycemic targets. Diabetes Care 2015; 38(Suppl. 1): S33–S40. [DOI] [PubMed] [Google Scholar]
- 4. Rohlfing CL, Wiedmeyer H-M, Little RR, et al. Defining the relationship between plasma glucose and HbA1c: analysis of glucose profiles and HbA1c in the diabetes control and complications trial. Diabetes Care 2002; 25(2): 275–278. [DOI] [PubMed] [Google Scholar]
- 5. Cohen RM, Snieder H, Lindsell CJ, et al. Evidence for independent heritability of the glycation gap (glycosylation gap) fraction of HbA1c in nondiabetic twins. Diabetes Care 2006; 29(8): 1739–1743. [DOI] [PubMed] [Google Scholar]
- 6. Hempe JM, Gomez R, McCarter RJ, Jr, et al. High and low hemoglobin glycation phenotypes in type1 diabetes: a challenge for interpretation of glycemic control. J Diabetes Complications 2002; 16(5): 313–320. [DOI] [PubMed] [Google Scholar]
- 7. Hempe JM, Liu S, Myers L, et al. The hemoglobin glycation index identifies subpopulations with harms or benefits from intensive treatment in the ACCORD trial. Diabetes Care 2015; 38(6): 1067–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ahn CH, Min SH, Lee D-H, et al. Hemoglobin glycation index is associated with cardiovascular diseases in people with impaired glucose metabolism. J Clin Endocrinol Metab 2017; 102(8): 2905–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee B, Heo YJ, Lee YA, et al. Association between hemoglobin glycation index and cardiometabolic risk factors in Korean pediatric nondiabetic population. Ann Pediatr Endocrinol Metab 2018; 23(4): 196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marini MA, Fiorentino TV, Succurro E, et al. Association between hemoglobin glycation index with insulin resistance and carotid atherosclerosis in non-diabetic individuals. PLoS One 2017; 12(4): e0175547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mitchell GF, Hwang S-J, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham heart study. Circulation 2010; 121(4): 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saiki A, Ohira M, Yamaguchi T, et al. New horizons of arterial stiffness developed using cardio-ankle vascular index (CAVI). J Atheroscler Thromb. Epub ahead of print 26 June 2020. DOI: 10.5551/jat.RV17043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim ES, Moon S-D, Kim H-S, et al. Diabetic peripheral neuropathy is associated with increased arterial stiffness without changes in carotid intima-media thickness in type 2 diabetes. Diabetes Care 2011; 34(6): 1403–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nagayama D, Watanabe Y, Saiki A, et al. Lipid parameters are independently associated with cardio-ankle vascular index (CAVI) in healthy Japanese subjects. J Atheroscler Thromb 2018; 25(7): 621–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nagayama D, Watanabe Y, Saiki A, et al. Difference in positive relation between cardio-ankle vascular index (CAVI) and each of four blood pressure indices in real-world Japanese population. J Hum Hypertens 2019; 33(3): 210–217. [DOI] [PubMed] [Google Scholar]
- 16. Nakamura K, Tomaru T, Yamamura S, et al. Cardio-ankle vascular index is a candidate predictor of coronary atherosclerosis. Circ J 2008; 72(4): 598–604. [DOI] [PubMed] [Google Scholar]
- 17. Sato Y, Nagayama D, Saiki A, et al. Cardio-ankle vascular index is independently associated with future cardiovascular events in outpatients with metabolic disorders. J Atheroscler Thromb 2016; 23(5): 596–605. [DOI] [PubMed] [Google Scholar]
- 18. Nagayama D, Saiki A, Endo K, et al. Improvement of cardio-ankle vascular index by glimepiride in type 2 diabetic patients. Int J Clin Pract 2010; 64(13): 1796–1801. [DOI] [PubMed] [Google Scholar]
- 19. Nagayama D, Endo K, Ohira M, et al. Effects of body weight reduction on cardio-ankle vascular index (CAVI). Obes Res Clin Pract 2013; 7(2): e139–e145. [DOI] [PubMed] [Google Scholar]
- 20. Examination Committee of Criteria for ‘Obesity Disease’ in Japan and Japan Society for the Study of Obesity. New criteria for ‘obesity disease’ in Japan. Circ J 2002; 66(11): 987–992. [DOI] [PubMed] [Google Scholar]
- 21. Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus, Seino Y, Nanjo K, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig 2010; 1(5): 212–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu S, Hempe JM, McCarter RJ, et al. Association between inflammation and biological variation in hemoglobin A1c in U.S. nondiabetic adults. J Clin Endocrinol Metab 2015; 100(6): 2364–2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord 2008; 6(4): 299–304. [DOI] [PubMed] [Google Scholar]
- 24. Carretero OA, Oparil S. Essential hypertension. Part I: definition and etiology. Circulation 2000; 101(3): 329–335. [DOI] [PubMed] [Google Scholar]
- 25. Shirai K, Utino J, Otsuka K, et al. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J Atheroscler Thromb 2006; 13(2): 101–107. [DOI] [PubMed] [Google Scholar]
- 26. McCarter RJ, Hempe JM, Gomez R, et al. Biological variation in HbA1c predicts risk of retinopathy and nephropathy in type 1 diabetes. Diabetes Care 2004; 27(6): 1259–1264. [DOI] [PubMed] [Google Scholar]
- 27. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001; 414(6865): 813–820. [DOI] [PubMed] [Google Scholar]
- 28. Felipe DL, Hempe JM, Liu S, et al. Skin intrinsic fluorescence is associated with hemoglobin A1c and hemoglobin glycation index but not mean blood glucose in children with type 1 diabetes. Diabetes Care 2011; 34(8): 1816–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lachin JM, Genuth S, Nathan DM, et al. The hemoglobin glycation index is not an independent predictor of the risk of microvascular complications in the diabetes control and complications trial. Diabetes 2007; 56(7): 1913–1921. [DOI] [PubMed] [Google Scholar]
- 30. van Steen SC, Schrieks IC, Hoekstra JB, et al.; Ale Cardio study group. The haemoglobin glycation index as predictor of diabetes-related complications in the AleCardio trial. Eur J Prev Cardiol 2017; 24(8): 858–866. [DOI] [PubMed] [Google Scholar]


