Abstract
Background:
The evidence regarding triple oral combination therapy for patients with pulmonary arterial hypertension (PAH) is scarce. This study was performed to investigate the effectiveness and safety of triple oral combination therapy with macitentan, riociguat, and selexipag.
Methods:
Among consecutive patients with PAH who were referred to our hospital from 2009 to 2020, those who underwent triple oral combination therapy using macitentan, riociguat, and selexipag were retrospectively analyzed. Hemodynamic and echocardiographic assessments and Kaplan–Meier analyses of all-cause death and initiation of prostacyclin infusion were conducted.
Results:
Twenty-six patients underwent this combination therapy. These patients were predominantly female (73.1%) with a median age of 38 years at baseline and nine patients were taking some PAH medications at baseline. The median time from initiation of the first PAH drug to the third PAH drug in treatment naïve patients was 24 days (interquartile range, 12–47 days). Four patients (15.0%) discontinued taking any of the three vasodilators because of adverse events, and 17 patients (65.4%) reached the maximum dose of all three drugs. The mean pulmonary arterial pressure, pulmonary vascular resistance, and cardiac output improved by 29%, 65%, and 82%, respectively (median observation period: 441 days) and similar improvements were observed in treatment-naïve patients at baseline. The survival rate and prostacyclin infusion-free rate since administration of all three vasodilators was 93.3% and 74.6% at 3 years, respectively. When patients were divided by risk stratification, the prostacyclin-free rate at 3 years was 92.9% in low-/intermediate-risk patients and 55.0% in high-risk patients.
Conclusion:
Triple oral combination therapy with macitentan, riociguat, and selexipag sufficiently improved clinical parameters and was well tolerated in patients with PAH. This combination could be a particularly promising strategy in patients with low/intermediate risk and possibly even in half of patients with high risk. Further studies are needed to validate these findings.
The reviews of this paper are available via the supplemental material section.
Keywords: prognosis, pulmonary arterial hypertension, right ventricular function, risk stratification, triple oral combination therapy
Introduction
Pulmonary arterial hypertension (PAH) is a lethal disease characterized by elevated pulmonary arterial pressure (PAP) due to remodeling of the pulmonary arterial bed and right sided-heart failure. Three types of PAH-specific vasodilators targeting the endothelin pathway, nitric oxide pathway, and prostacyclin pathway have been developed. Combination therapy targeting these different pathways has recently been shown to improve hemodynamics, clinical functions, and even hard endpoints including survival or worsening of PAH in several recent randomized controlled trials and metanalyses.1–5 The effectiveness of triple combination therapy targeting all three of these pathways has also been reported.6–8 However, two such studies analyzed patients who were treated with two types of oral vasodilators and prostacyclin infusion,7,8 and another study was a subgroup analysis of a randomized controlled trial demonstrating a change in the symptom burden by the addition of selexipag, a prostacyclin receptor agonist, to background double combination therapy targeting other pathways.6 Therefore, evidence of the efficacy and safety of triple oral combination therapy remains scarce.
The most recently approved PAH-specific vasodilators targeting each of the three types of pathways are macitentan, which is an endothelin receptor antagonist (ERA); riociguat, which is a soluble guanylate cyclase stimulator targeting the nitric oxide pathway; and selexipag, which is an oral non-prostanoid prostacyclin receptor agonist. All three of these are orally available drugs. The effectiveness of these vasodilators for PAH has recently been proven;2,3,9 however, no clinical trials have been performed to investigate the effectiveness of the combination of these three vasodilators. Furthermore, although the current guideline recommends initial oral therapy for patients with low or intermediate risk,10 how many patients with low or intermediate risk can actually be treated with only oral combination therapy remains poorly documented to date.
Therefore, the purposes of this study were to clarify the clinical effectiveness and safety of triple oral combination therapy with macitentan, riociguat, and selexipag and to assess which risk groups can be adequately treated with this combination therapy.
Methods
Study design
This retrospective study was performed in Keio University Hospital in Japan. This study was approved by the Ethics Committee of Keio University Hospital, and written informed consent was obtained from all patients when they received genetic tests or were hospitalized. Patients with PAH who had received triple oral combination therapy with macitentan, riociguat, and selexipag were evaluated among all consecutive patients who were referred to Keio University Hospital from 2009 to 2020.
The diagnosis of PAH was made according to the current guideline.11 Patients who were lost to follow-up or underwent maintenance hemodialysis were excluded. Baseline data were measured at the time of referral to our hospital. If patients were treatment-naïve upon referral to our hospital, the combination of macitentan, riociguat, and selexipag was initiated after diagnosis. The method of up-titration and prioritization of the vasodilators as well as the selection of sequential therapy or upfront therapy was determined at the discretion of the specialized physicians. The doses of the vasodilators were titrated to the maximal tolerated doses. For patients who had already received other PAH-specific vasodilators at the time of enrollment, these drugs targeting the endothelin pathway, nitric oxide pathway, and prostacyclin pathway were switched to macitentan, riociguat, and selexipag, respectively, with the intention of further improvement of hemodynamics.
Patients received genetic counseling, and genetic tests were performed with informed consent. The methods of the genetic tests, including whole-exome sequencing, are described in our previous reports.12,13
Parameters and outcomes
Dedicated cardiologists performed right-heart catheterization (RHC) without sedation at baseline and follow-up. The right arterial pressure, mean PAP, and pulmonary arterial wedge pressure were measured by RHC. The zero pressure point was set at the level of the midthorax. Cardiac output (CO) was calculated by the Fick technique using oxygen consumption estimated by 125 times the body surface area according to a previous report.14 Pulmonary vascular resistance (PVR) was calculated as the difference between the mean PAP and pulmonary arterial wedge pressure divided by the CO.
The 6-minute walk distance (6MWD), blood concentration of B-type natriuretic peptide (BNP), and World Health Organization functional class (WHO-FC) were measured at the time of hospitalization for RHC.
Standard two-dimensional, M-mode, and Doppler images, including the tricuspid annular plane systolic excursion (TAPSE) and right ventricular systolic excursion velocity (RVS’), were obtained by echocardiographers in accordance with the current echocardiography guideline.15 Dedicated cardiologists analyzed the data and measured the right atrial area (RAA), right ventricular end-diastolic area (RVEDA), right ventricular end-systolic area, and right ventricular fractional area change (RVFAC).
Hemodynamic changes measured by RHC, echocardiographic changes, and changes in the 6MWD, BNP concentration, and WHO-FC were assessed from baseline to follow-up.
The events of all-cause death, hospitalization for heart failure, initiation of prostacyclin infusion, and discontinuation of any of the three vasodilators (macitentan, riociguat, and selexipag) because of adverse events since administration of all three vasodilators were collected. The observation period was terminated when patients died, underwent prostacyclin infusion, or stopped taking macitentan, riociguat, or selexipag for any reason.
All patients enrolled in this study were divided into two groups by risk assessment using the three-category REVEAL 2.0 risk score,16 and the prostacyclin infusion-free rate was analyzed in each group. If the value was unmeasured, the value of zero was assigned. According to the three-category REVEAL 2.0 risk score, the high-risk group was defined as patients with a predicted 1-year survival rate of <90% (REVEAL 2.0 risk score ⩾9), and the low-/intermediate-risk group was defined as patients with a predicted 1-year survival rate of ⩾90% (REVEAL 2.0 risk score ⩽8).
Subgroup analysis
Patients who were treatment-naïve at baseline and treated with only macitentan, riociguat, and selexipag for PAH were also independently assessed by subgroup analysis. Their hemodynamics, echocardiography, and clinical function were measured and clinical events were collected similarly to the overall patient analysis.
Statistical analysis
Data are described as median and interquartile range unless otherwise indicated. Wilcoxon’s signed rank test was used to compare the parameters of hemodynamics, echocardiography, 6MWD, and BNP concentration from baseline to the latest follow-up. The cases which had a missing value either at baseline or follow-up were excluded from each analysis. Differences with a p-value of <0.05 were considered statistically significant. All p-values were two-tailed. Event-free rates were analyzed using the Kaplan–Meier method. All analyses were performed using R software (version 3.6.3), and figures were obtained using GraphPad Prism (version 8.0 for Mac; GraphPad Software, La Jolla, CA, USA).
Results
Patient characteristics
The patients’ baseline characteristics and medications are presented in Table 1 and Supplemental material Table 1 online. One patient who was lost to follow-up and one patient who had been undergoing maintenance hemodialysis were excluded; thus, 26 patients were enrolled in this study. The patients were predominantly female (73.1%) with a median age of 38 years. The major etiology of PAH was idiopathic PAH (38.5%) followed by connective tissue disease-associated PAH (26.9%), heritable PAH (19.2%), and congenital heart disease-associated PAH (15.4%). Most patients were treatment-naïve (65.4%), and others were taking some PAH medications at baseline.
Table 1.
Demographics and characteristics at baseline.
| Variable | Triple oral combination
therapy N = 26 |
|---|---|
| Female | 19 (73.1) |
| Age, years | 38 (23–48) |
| WHO-FC | |
| I/II/III/IV | 0/9/16/1 (0.0/34.6/61.5/3.8) |
| BNP, pg/mL | 105.25 (36.7–285.5) |
| 6MWD, m | 397.5 (312.8–441.8) |
| PAH etiology | |
| IPAH | 10 (38.5) |
| HPAH | 5 (19.2) |
| CTD-PAH | 7 (26.9) |
| CHD-PAH | 4 (15.4) |
| Medication | |
| Treatment-naïve | 17 (65.4) |
| Single | 3 (11.5) |
| Double | 2 (7.7) |
| Triple | 4 (15.4) |
| Risk assessment* | |
| High risk | 12 (46.2) |
| Intermediate risk | 4 (15.4) |
| Low risk | 10 (38.5) |
| Genetic mutation** | |
| BMPR2 | 5 (23.8) |
| ACVRL | 1 (4.8) |
| RNF213 | 1 (4.8) |
Data are expressed as number (%) or median (interquartile range).
Risk assessment was calculated using the three-category REVEAL 2.0 risk score.16 The high-risk group was defined as patients with a predicted 1-year survival rate of <90% (REVEAL 2.0 risk score ⩾9), the intermediate-risk group was defined as patients with a predicted 1-year survival rate of 90–<95% (REVEAL 2.0 risk score = 7 or 8), and the low-risk group was defined as patients with a predicted 1-year survival rate of ⩾95% (REVEAL 2.0 risk score ⩽6).
Among 26 patients, 21 were genetically tested and 15 had no genetic mutations related to PAH.
6MWD, 6-minute walk distance; ACVRL1, activin A receptor-like kinase 1; BMPR2, bone morphogenetic protein receptor type 2; BNP, B-type natriuretic peptide; CHD, congenital heart disease; CTD, connective tissue disease; HPAH, heritable pulmonary arterial hypertension; IPAH, idiopathic pulmonary arterial hypertension; PAH, pulmonary arterial hypertension; RNF213, ring finger protein 213; WHO-FC, World Health Organization functional class.
Ten patients with idiopathic PAH, five with heritable PAH, three with connective tissue disease-associated PAH, and three with congenital heart disease-associated PAH were genetically tested. All five patients with heritable PAH had mutations in known PAH-related genes such as bone morphogenetic protein receptor type 2 (BMPR2) and one patient with idiopathic PAH had a mutation in ring finger protein 213 (RNF213), which we recently reported as a novel PAH-related gene.13,17
Safety and tolerability
Discontinuation of macitentan, riociguat, or selexipag because of adverse events occurred in four of 26 patients (15%). Among these four patients, one had diarrhea with selexipag, one had suspected myelosuppression with macitentan, one had nausea with riociguat, and one had hypotension with riociguat. These patients changed their drug to another drug targeting the same pathway. No adverse events, including death, hospitalization for heart failure, or prostacyclin infusion, were observed after discontinuation of each drug.
The maximum tolerated doses of the vasodilators are summarized in Supplemental Table 2. In detail, 25 (96.2%), 24 (92.3%), and 20 (76.9%) patients were taking the maximum dose of macitentan, riociguat, and selexipag, respectively. Seventeen (65.4%) patients reached the maximum dose of all three vasodilators.
Changes and outcomes after treatment
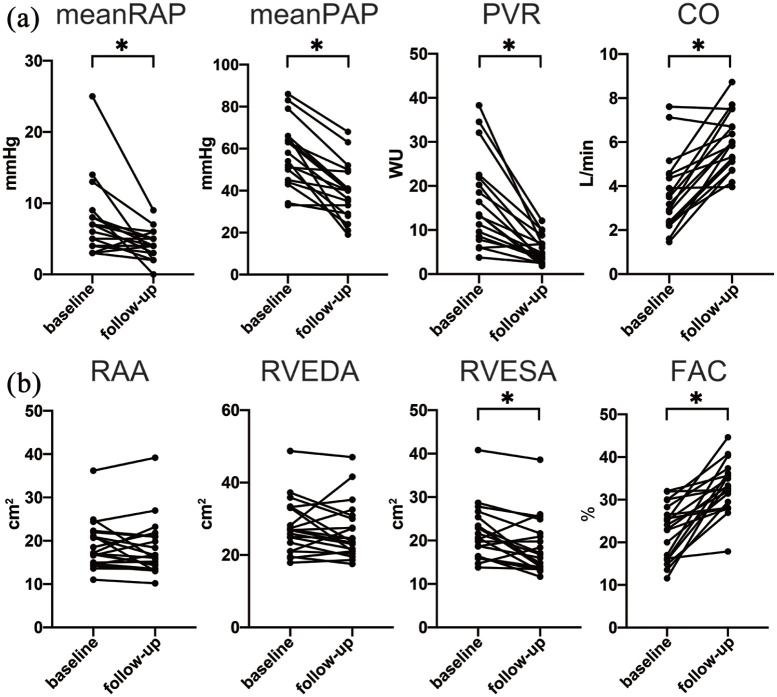
Hemodynamic data were available for 18 patients. The detailed study flow chart is shown in Supplemental Figure 1. The hemodynamics, WHO-FC, BNP concentration, and 6MWD at baseline and follow-up are presented in Table 2. The median observation period was 441 (229–1103) days. Hemodynamic parameters were significantly improved with a 29% decrease in the mean PAP and 65% decrease in the mean PVR. The CO significantly increased by 82% from baseline to follow-up. Although >70% of patients had a WHO-FC of III or IV at baseline, 89% of patients had a WHO-FC of I or II at follow-up. The 6MWD and BNP concentration were also significantly improved [Figure 1(a)].
Table 2.
Changes in clinical parameters.
| Variable | Baseline | Follow-up | Change (%) | p-value* |
|---|---|---|---|---|
| Hemodynamics | ||||
| Mean RAP, mmHg | 6.5 (4.0–8.0) | 4.0 (3.0–5.0) | −2.5 (−38) | 0.005 |
| Mean PAP, mmHg | 56.0 (46.3–65.5) | 40.0 (30.0–47.0) | −16.0 (−29) | <0.001 |
| PAWP, mmHg | 7.5 (6.0–9.8) | 9.0 (8.0–11.0) | 1.5 (20) | 0.68 |
| CO, L/min | 3.3 (2.4–4.5) | 6.0 (5.1–6.7) | 2.7 (82) | <0.001 |
| CI, L/min per m2 | 2.2 (1.7–2.7) | 3.8 (3.3–4.5) | 1.6 (73) | <0.001 |
| PVR, WU | 13.5 (8.8–21.5) | 4.7 (2.8–6.9) | −8.8 (−65) | <0.001 |
| Echocardiography | ||||
| TAPSE, cm | 1.5 (1.3–1.8) | 2.3 (1.9–2.6) | 0.8 (53) | 0.001 |
| RVS’, cm | 9.7 (7.8–11.8) | 13.3 (12.4–14.4) | 3.6 (37) | 0.001 |
| RAA, cm2 | 17.2 (14.9–21.3) | 16.4 (14.1–21.0) | −0.8 (−5) | 0.49 |
| RVEDA, cm2 | 26.9 (22.8–33.1) | 24.1 (21.0–30.3) | −2.8 (−10) | 0.21 |
| RVESA, cm2 | 20.4 (16.3–23.9) | 16.4 (13.8–21.1) | −4.0 (−20) | 0.006 |
| FAC, % | 23.6 (16.2–26.9) | 32.9 (29.1–35.8) | 9.3 (39) | <0.001 |
| WHO-FC | ||||
| I or II | 4 (22) | 16 (89) | – | – |
| III or IV | 14 (78) | 2 (11) | – | – |
| 6MWD, m | 405.0 (303.0–441.8) | 472.0 (439.5–493.5) | 67.0 (17) | 0.046 |
| BNP, pg/mL | 187.8 (36.7–379.5) | 17.5 (10.3–27.2) | −170.3 (−91) | <0.001 |
Data are expressed as number (%) or median (interquartile range). The number of subjects at baseline and follow-up was the same equal number in each parameter. Eighteen patients were analyzed in hemodynamics, and 20 patients in echocardiography.
p-value was calculated using a Wilcoxon’s signed rank test for comparison of baseline and follow-up.
6MWD, 6-minute walk distance; BNP, B-type natriuretic peptide; CI, cardiac index; CO, cardiac output; FAC, fractional area change; PAP, pulmonary arterial pressure; PAWP, pulmonary arterial wedge pressure; PVR, pulmonary vascular resistance; RAA, right atrial area; RAP, mean right atrial pressure; RVEDA, right ventricular end-diastolic area; RVESA, right ventricular end-systolic area; RVS’, right ventricular systolic excursion velocity; TAPSE, tricuspid annular plane systolic excursion; WHO-FC, World Health Organization functional class; WU, Wood units.
Figure 1.
Hemodynamic and echocardiographic changes.
The patients’ (a) hemodynamic parameters and (b) echocardiographic parameters at baseline and follow-up are compared.
*p < 0.05.
CO, cardiac output; FAC, fractional area change; PAP, pulmonary arterial pressure; PVR, pulmonary vascular resistance; RAA, right atrial area; RAP, right atrial pressure; RVEDA, right ventricular end-diastolic area; RVESA, right ventricular end-systolic area; WU, Wood units.
Echocardiographic data were available for 20 patients (Supplemental Figure 1). The echocardiographic data are presented in Table 2. The median follow-up period was 737 (256–1179) days. Right ventricular function based on the TAPSE, RVS’, and RVFAC was significantly improved from baseline to follow-up, although the RAA and RVEDA were not [Figure 1(b)].
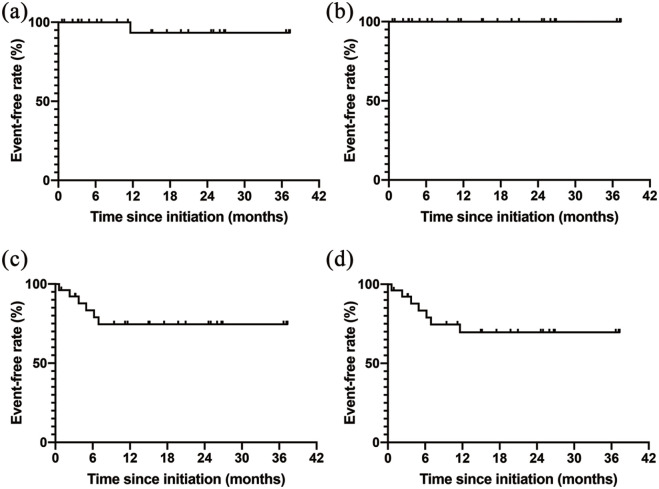
The Kaplan–Meier curves since administration of all three vasodilators are shown in Figure 2. The 1- and 2-year survival rates were both 93.3%. One patient died of multiple organ failure after femoral neck fracture during the observation period. The overall survival rate was 92.3% with median follow-up period of 33 (24–43) months. No patients were hospitalized for heart failure during the observation period. Prostacyclin infusion was initiated in six patients (25.4%) within 1 year. Among these six patients, selexipag was switched to subcutaneous infusion of treprostinil in one patient, an intravenous infusion of epoprostenol was added to the triple oral combination therapy in another patient, and a subcutaneous infusion of treprostinil was added to the triple oral combination therapy in the remaining four patients. Selexipag was not discontinued in some patients to reduce maintenance dose of the prostacyclin for infusion. No other patients underwent prostacyclin infusion after 1 year. Among six patients who underwent prostacyclin infusion within 1 year, three patients had a BMPR2 mutation, one patient had an RNF213 mutation, and one patient had Eisenmenger syndrome due to an atrial septal defect. Composite events of all-cause death, hospitalization for heart failure, and initiation of prostacyclin infusion occurred in 16.6% of the patients within 6 months and 30.4% of the patients in 1 and 2 years.
Figure 2.
Event-free rates in all patients with triple oral combination therapy.
The Kaplan–Meier curves for event-free rates since initiation of triple combination therapy are shown (n = 26). The events are defined as (a) all-cause death, (b) hospitalization for heart failure, (c) prostacyclin infusion, and (d) the composite of all-cause death, hospitalization, and heart failure.
HF, heart failure.
Among 14 patients who were classified as low-/intermediate-risk by the three-category REVEAL 2.0 risk score,16 the prostacyclin-free rate was 92.9% at 3 years, while the rate was 55.0% at 2 years in 12 high-risk patients (Figure 3). The difference was not statistically significant (p = 0.058). Hemodynamic changes from baseline to follow-up in each low/intermediate and high-risk group are summarized in Supplemental Table 3. Among five patients who were classified as high-risk based on the three-category REVEAL 2.0 risk score and needed prostacyclin infusion, three patients had BMPR2 mutations and one patient had a RNF213 mutation.
Figure 3.
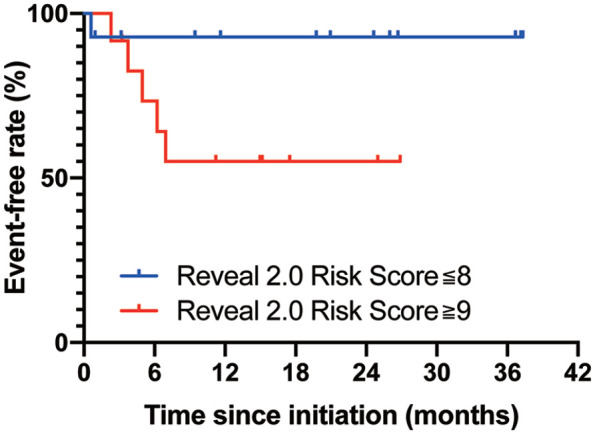
Kaplan–Meier curve for prostacyclin-free rate in each risk category.
The prostacyclin infusion-free rates in each risk category are shown. The patients were divided into two risk categories by the REVEAL 2.0 risk score.16 The p-values were calculated by the log-rank test.
Subgroup analysis in treatment-naïve patients
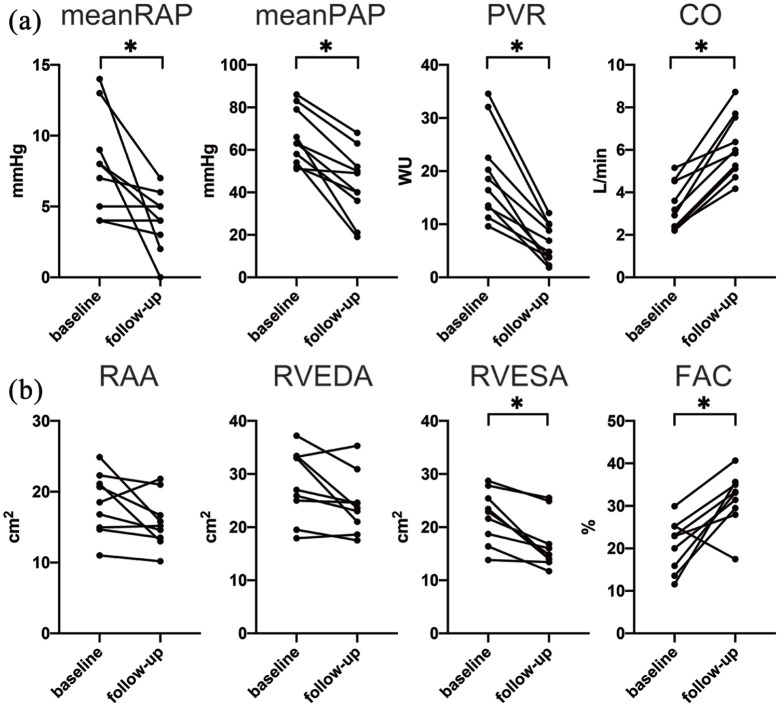
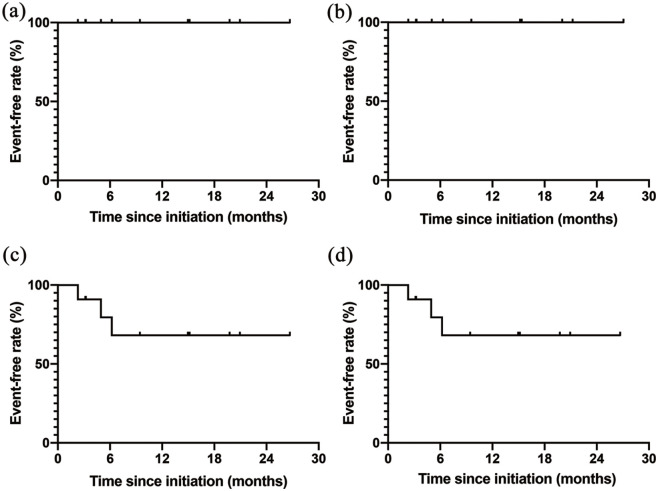
Seventeen patients were treatment-naïve at baseline. Six patients were treatment naïve at baseline, but treated with other pulmonary vasodilators. In five out of these six patients, oral medication was switched from beraprost to selexipag, and in one patient, it was switched from ambrisentan and tadalafil to macitentan and riociguat. Thus, these six patients were excluded from the subgroup analysis. Among the remaining 11 patients for the subgroup analysis, the median time from initiation of the first PAH drug to the start of the third PAH drug was 24 (12–47) days. The way of up-titration in treatment naïve patients was as follows. Macitentan 10 mg was initiated at first and followed by riociguat 3.0 mg within 2 weeks. The dose of riociguat was up-titrated by 1.5 mg every 2 weeks with attention to adverse effects. In 36% of treatment naïve patients, selexipag was initiated after riociguat was up-titrated to maximum tolerated dose, whereas selexipag was started within 2 weeks from initiating macitentan in 64% of patients. Selexipag was increased by 0.4 mg every 2 weeks to maximum tolerated dose with attention to side effects. One patient (9.1%) discontinued macitentan because of suspected myelosuppression, and another patient (9.1%) discontinued riociguat because of nausea. RHC data in 10 patients and echocardiographic data in nine patients were available at baseline and at follow-up during the triple oral combination therapy. The patients’ hemodynamic and right ventricular function parameters are presented in Table 3 and Figure 4. The median follow-up period of the hemodynamic analysis was 293 (211–473) days. The hemodynamic parameters were significantly improved with a 29% decrease in the mean PAP and 66% decrease in the PVR. The CO increased by 90% from baseline to follow-up. The median follow-up period of echocardiography was 385 (230–594) days. The TAPSE, RVS’, and RVFAC, indicating right ventricular function, increased significantly. The WHO-FC, 6MWD, and BNP concentration in this subgroup also improved significantly. In the Kaplan–Meier analysis, no patients died or were hospitalized for heart failure (Figure 5). Prostacyclin infusion was initiated in 31.8% of patients within 7 months, and no patients underwent prostacyclin infusion after 7 months.
Table 3.
Changes in clinical parameters among treatment-naïve patients at baseline.
| Variable | Baseline | Follow-up | Change (%) | p-value* |
|---|---|---|---|---|
| Hemodynamics | ||||
| Mean RAP, mmHg | 7.5 (4.3–8.8) | 4.0 (3.3–5.0) | −3.5 (−47) | 0.022 |
| Mean PAP, mmHg | 63.0 (55.0–75.8) | 44.5 (37.0–51.5) | −18.5 (−29) | 0.006 |
| PAWP, mmHg | 7.5 (6.3–9.8) | 8.0 (6.5–10.3) | 0.5 (7) | 0.61 |
| CO, L/min | 3.1 (2.4–4.3) | 5.9 (5.1–7.2) | 2.8 (90) | 0.006 |
| CI, L/min per m2 | 2.1 (1.7–2.5) | 4.2 (3.5–4.6) | 2.1 (100) | 0.006 |
| PVR, WU | 17.5 (13.2–21.9) | 5.9 (3.8–9.6) | −11.6 (−66) | 0.006 |
| Echocardiography | ||||
| TAPSE, cm | 1.4 (1.3–1.5) | 2.3 (2.1–2.7) | 0.9 (64) | 0.009 |
| RVS’, cm | 9.3 (7.9–10.8) | 13.5 (13.0–14.3) | 4.2 (45) | 0.035 |
| RAA, cm2 | 18.5 (15.0–21.1) | 15.2 (13.5–16.7) | −3.3 (−18) | 0.076 |
| RVEDA, cm2 | 27.0 (25.0–33.2) | 23.6 (21.0–24.6) | −3.4 (−13) | 0.058 |
| RVESA, cm2 | 22.9 (18.7–25.4) | 14.8 (14.0–16.8) | −8.1 (−35) | 0.009 |
| FAC, % | 22.9 (15.9–25.2) | 33.1 (29.5–35.0) | 10.2 (45) | 0.018 |
| WHO-FC | ||||
| I or II | 1 (10) | 8 (80) | ||
| III or IV | 9 (90) | 2 (20) | ||
| 6MWD, m | 400.0 (326.5–432.0) | 471.5 (451.5–479.2) | 71.5 (18) | 0.030 |
| BNP, pg/mL | 200.8 (41.9–379.5) | 20.2 (12.6–24.5) | −180.6 (−90) | 0.006 |
Data are expressed as number (%) or median (interquartile range). The number of subjects at baseline and follow-up was the same equal number in each parameter. Ten patients were analyzed in hemodynamics, and nine patients in echocardiography.
p-value was calculated using a Wilcoxon’s signed rank test for comparison of baseline and follow-up.
6MWD, 6-minute walk distance; BNP, B-type natriuretic peptide; CI, cardiac index; CO, cardiac output; FAC, fractional area change; PAP, mean pulmonary arterial pressure; PAWP, pulmonary arterial wedge pressure; PVR, pulmonary vascular resistance; RAA, right atrial area; RAP, mean right atrial pressure; RVEDA, right ventricular end-diastolic area; RVESA, right ventricular end-systolic area; RVS’, right ventricular systolic excursion velocity; TAPSE, tricuspid annular plane systolic excursion; WHO-FC, World Health Organization functional class; WU, Wood units.
Figure 4.
Hemodynamic and echocardiographic changes in treatment-naïve patients.
The patients’ (a) hemodynamic parameters and (b) echocardiographic parameters at baseline and follow-up are compared.
*p < 0.05.
CO, cardiac output; FAC, fractional area change; PAP, pulmonary arterial pressure; PVR, pulmonary vascular resistance; RAA, right atrial area; RAP, right atrial pressure; RVEDA, right ventricular end-diastolic area; RVESA, right ventricular end-systolic area; WU, Wood units.
Figure 5.
Event-free rates in treatment-naïve patients.
The Kaplan–Meier curves for event-free rates since initiation of triple combination therapy in treatment-naïve patients are shown. The events are defined as (a) all-cause death, (b) hospitalization for heart failure, (c) prostacyclin infusion, and (d) the composite endpoint of all-cause death, hospitalization, and heart failure.
HF, heart failure.
Discussion
In the present study, triple oral combination therapy with macitentan, riociguat, and selexipag improved the hemodynamics, right ventricular function, and clinical function with good tolerability in patients with PAH. Most of the low-/intermediate-risk patients and around half of the high-risk patients could be treated sufficiently with this triple oral combination therapy although it is difficult to generalize these findings.
Two previous studies investigated the efficacy of the combination of prostacyclin infusion, an ERA, and a phosphodiesterase-5 inhibitor (PDE-5i) for PAH,7,8 but data regarding triple oral combination therapy are limited. One study related to triple oral combination therapy is the subgroup analysis of the GRIPHON study, which demonstrated a change in the symptom burden after administration of selexipag in addition to an ERA and PDE-5i.6 Recently, a randomized control study (TRITON study) is also under analysis which investigated the efficacy and safety of triple oral combination therapy with macitentan, tadalafil, and selexipag versus dual oral combination therapy with macitentan and tadalafil.18 The present study is the first to investigate the effectiveness and safety of triple oral combination therapy with macitentan, riociguat, and selexipag using real-world data.
Our results of improved hemodynamics are consistent with previous observational studies that investigated the effectiveness of triple combination therapy including prostacyclin infusion.7 Sitbon et al.7 reported that with intravenous administration of epoprostenol and oral administration of bosentan and sildenafil, the mean PAP, PVR, and cardiac index improved by 33%, 71%, and 119%, respectively. Another observational study involving a combination of ambrisentan, tadalafil, and subcutaneous treprostinil also demonstrated significant improvement of the mean PAP, PVR, and cardiac index by 30%, 66%, and 94%, respectively.8 There are similarities between the results of these previous studies and the results of the present study, suggesting that triple oral combination therapy with macitentan, riociguat, and selexipag can powerfully improve the hemodynamics in patients with PAH. In the TRITON study, triple oral combination therapy improved PVR by 54%, although it was almost the same degree as dual oral combination therapy.18 Improvement of hemodynamic in this study was greater than that of the TRITON study, partly because of the difference of the study design. Although findings from observational study, results in this study provided the possibility that triple oral combination therapy could also improve hemodynamics in the real-world population as well as the randomized controlled trial.
Our study also demonstrated a favorable long-term prognosis as well as improvement of the RVFAC, 6MWD, WHO-FC, and BNP concentration with triple oral combination therapy. In a past retrospective study of the dual combination of an ERA and PDE-5i, the 3-year overall survival rate was nearly 80%.19 In another French registry in which most patients were treated with monotherapy, the 3-year survival rate was <70%.20 The prognosis in the present study appears to be superior to that in previous reports. The low rate of mortality and hospitalization for heart failure was consistent with the TRITON study, in which the risk of first disease progression events was decreased about 41% in the triple oral therapy group.18 These results in this study could verify the efficacy of triple oral combination therapy in preventing disease progression of triple oral combination therapy found in the randomized controlled study.
In the present study, the treatment response in most of the low-/intermediate-risk patients was favorable with administration of the triple oral combination therapy, and around half of the high-risk patients did not need prostacyclin infusion. The current PAH guideline recommends initial oral therapy for low-/intermediate-risk patients, while high-risk patients are recommended to receive initial combination therapy including prostacyclin infusion.10 Patients in this study did not present decompensated heart failure and the urgent initiation of prostacyclin infusion was not necessary. Therefore, the triple oral combination therapy was initiated first and its efficacy was assessed within 6 months. Our data support recommendations in guidelines in low-/intermediate-risk patients and provide a novel possibility that around half of high-risk patients can be treated without prostacyclin infusion. Moreover, 66.7% of the patients who needed prostacyclin infusion had gene mutations, suggesting that the need for prostacyclin infusion can be largely affected by their genetic background. Therefore, we should be especially careful when we use triple oral combination therapy to treat patients with specific etiologies of PAH such as gene mutations.
With respect to safety, a past study investigating the combination of tadalafil as a PDE-5i and ambrisentan as an ERA showed that the discontinuation rate of dual combination therapy because of adverse events was 12% within 2 years of the mean duration of drug use.1 In the subgroup analysis of the GRIPHON study, which investigated the effectiveness of selexipag for the patients of PAH, a triple oral combination therapy with selexipag, ERA, and PDE-5i was discontinued in 19.0% of patients due to adverse events.6 The rate of 15% in the present study was lower than that of the GRIPHON study, suggesting good tolerability in real world data. With regard to the maximum tolerated dose, a past subgroup analysis that investigated the change in the symptom burden after taking selexipag in addition to an ERA and PDE-5i showed that only 27.9% of patients who received selexipag reached the maximum dose of selexipag.6 Another study of the combination of riociguat and macitentan showed that about 70% of patients reached the maximum maintenance dose of riociguat. In the present study, 76.9% and 92.3% of patients could be up-titrated to the maximum dose with selexipag and riociguat, respectively. Thus, this study demonstrated that it is quite possible to up-titrate each drug to the maximum dose, even in patients treated with triple oral combination therapy.
The subgroup analysis showed that the hemodynamics, right ventricular function, and clinical function powerfully improved, and the degree of improvement was similar to those in the overall patient analysis. Moreover, no patients died or were hospitalized for heart failure during the observation period. These results suggest that triple oral combination therapy with macitentan, riociguat, and selexipag can be a promising treatment strategy in treatment-naïve patients.
This study has some limitations. This was a small, single-center, retrospective study that included various types of PAH and some patients were not treatment naïve at baseline. Each etiology of PAH might have affected the results of the study differently. Prostacyclin infusion was initiated within 7 months, and no patients underwent prostacyclin infusion after 7 months, which is towards a bias of an initial high-risk population of patients in which initial triple oral combination treatment was chosen. The drug regimens were decided by the physicians. Furthermore, indirect Fick method was adopted as the measurement of CO. This was not recommended in the European Society of Cardiology/European Respiratory Society Guidelines 2015, but was admitted for use in the practical situation in the Japanese PAH guideline.11 As the results could be affected by the measurement methods of CO, further validation would be warranted.
Conclusion
This study demonstrated the effectiveness of triple oral combination therapy with macitentan, riociguat, and selexipag in patients with PAH. Our data suggest that this triple oral combination therapy is associated with improvement of hemodynamics, right ventricular function, and clinical function with good tolerability. In particular, this triple oral combination therapy can be a promising therapeutic strategy in patients with low/intermediate risk and possibly even in half of patients with high risk. Further investigation is required to validate these findings.
Supplemental Material
Supplemental material, sj-pdf-1-tar-10.1177_1753466621995048 for Triple oral combination therapy with macitentan, riociguat, and selexipag for pulmonary arterial hypertension by Mizuki Momoi, Takahiro Hiraide, Yoshiki Shinya, Hiromi Momota, Shogo Fukui, Michiyuki Kawakami, Yuji Itabashi, Keiichi Fukuda and Masaharu Kataoka in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-2-tar-10.1177_1753466621995048 for Triple oral combination therapy with macitentan, riociguat, and selexipag for pulmonary arterial hypertension by Mizuki Momoi, Takahiro Hiraide, Yoshiki Shinya, Hiromi Momota, Shogo Fukui, Michiyuki Kawakami, Yuji Itabashi, Keiichi Fukuda and Masaharu Kataoka in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-3-tar-10.1177_1753466621995048 for Triple oral combination therapy with macitentan, riociguat, and selexipag for pulmonary arterial hypertension by Mizuki Momoi, Takahiro Hiraide, Yoshiki Shinya, Hiromi Momota, Shogo Fukui, Michiyuki Kawakami, Yuji Itabashi, Keiichi Fukuda and Masaharu Kataoka in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-4-tar-10.1177_1753466621995048 for Triple oral combination therapy with macitentan, riociguat, and selexipag for pulmonary arterial hypertension by Mizuki Momoi, Takahiro Hiraide, Yoshiki Shinya, Hiromi Momota, Shogo Fukui, Michiyuki Kawakami, Yuji Itabashi, Keiichi Fukuda and Masaharu Kataoka in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-5-tar-10.1177_1753466621995048 for Triple oral combination therapy with macitentan, riociguat, and selexipag for pulmonary arterial hypertension by Mizuki Momoi, Takahiro Hiraide, Yoshiki Shinya, Hiromi Momota, Shogo Fukui, Michiyuki Kawakami, Yuji Itabashi, Keiichi Fukuda and Masaharu Kataoka in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-7-tar-10.1177_1753466621995048 for Triple oral combination therapy with macitentan, riociguat, and selexipag for pulmonary arterial hypertension by Mizuki Momoi, Takahiro Hiraide, Yoshiki Shinya, Hiromi Momota, Shogo Fukui, Michiyuki Kawakami, Yuji Itabashi, Keiichi Fukuda and Masaharu Kataoka in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-tif-6-tar-10.1177_1753466621995048 for Triple oral combination therapy with macitentan, riociguat, and selexipag for pulmonary arterial hypertension by Mizuki Momoi, Takahiro Hiraide, Yoshiki Shinya, Hiromi Momota, Shogo Fukui, Michiyuki Kawakami, Yuji Itabashi, Keiichi Fukuda and Masaharu Kataoka in Therapeutic Advances in Respiratory Disease
Acknowledgments
We thank all the doctors, nurses, and supporting medical staff members who were involved in the care of the patients enrolled in this study. We also thank Angela Morben, DVM, ELS, from Edanz Group (https://en-author-services.edanzgroup.com/ac), for editing a draft of this manuscript.
Footnotes
Author contribution(s): Mizuki Momoi: Data curation; Formal analysis; Investigation; Writing-original draft.
Takahiro Hiraide: Conceptualization; Methodology; Writing-review & editing.
Yoshiki Shinya: Conceptualization; Validation; Writing-review & editing.
Hiromi Momota: Data curation; Validation; Methodology; Writing-review & editing.
Shogo Fukui: Data curation; Validation; Formal analysis; Writing-review & editing.
Michiyuki Kawakami: Data curation; Validation; Formal analysis; Writing-review & editing.
Yuji Itabashi: Methodology; Data curation; Resources, Writing-review & editing.
Keiichi Fukuda: Supervision; Investigation; Writing-review & editing.
Masaharu Kataoka: Conceptualization; Project administration; Writing-review & editing.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Masaharu Kataoka  https://orcid.org/0000-0001-8034-1005
https://orcid.org/0000-0001-8034-1005
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Mizuki Momoi, Department of Cardiology, Keio University School of Medicine, Tokyo, Japan.
Takahiro Hiraide, Department of Cardiology, Keio University School of Medicine, Tokyo, Japan.
Yoshiki Shinya, Department of Cardiology, Keio University School of Medicine, Tokyo, Japan.
Hiromi Momota, Department of Cardiology, Keio University School of Medicine, Tokyo, Japan.
Shogo Fukui, Department of Rehabilitation, Keio University Hospital, Tokyo, Japan.
Michiyuki Kawakami, Department of Rehabilitation Medicine, Keio University Hospital, Tokyo, Japan.
Yuji Itabashi, Department of Laboratory Medicine, Keio University School of Medicine, Tokyo, Japan.
Keiichi Fukuda, Department of Cardiology, Keio University School of Medicine, Tokyo, Japan.
Masaharu Kataoka, Department of Cardiology, Keio University School of Medicine, Shinanomachi 35, Shinjuku-ku, Tokyo 160-8582, Japan; Second Department of Internal Medicine, University of Occupational and Environmental Health, Kitakyushu, Japan.
References
- 1. Galie N, Barbera JA, Frost AE, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 2015; 373: 834–844. [DOI] [PubMed] [Google Scholar]
- 2. Pulido T, Adzerikho I, Channick RN, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 2013; 369: 809–818. [DOI] [PubMed] [Google Scholar]
- 3. Sitbon O, Channick R, Chin KM, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med 2015; 373: 2522–2533. [DOI] [PubMed] [Google Scholar]
- 4. Lajoie AC, Lauzière G, Lega JC, et al. Combination therapy versus monotherapy for pulmonary arterial hypertension: a meta-analysis. Lancet Respir Med 2016; 4: 291–305. [DOI] [PubMed] [Google Scholar]
- 5. Fox BD, Shtraichman O, Langleben D, et al. Combination therapy for pulmonary arterial hypertension: a systematic review and meta-analysis. Can J Cardiol 2016; 32: 1520–1530. [DOI] [PubMed] [Google Scholar]
- 6. Coghlan JG, Channick R, Chin K, et al. Targeting the prostacyclin pathway with selexipag in patients with pulmonary arterial hypertension receiving double combination therapy: insights from the randomized controlled GRIPHON study. Am J Cardiovasc Drugs 2018; 18: 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sitbon O, Jaïs X, Savale L, et al. Upfront triple combination therapy in pulmonary arterial hypertension: a pilot study. Eur Respir J 2014; 43: 1691–1697. [DOI] [PubMed] [Google Scholar]
- 8. D’Alto M, Badagliacca R, Argiento P, et al. Risk reduction and right heart reverse remodeling by upfront triple combination therapy in pulmonary arterial hypertension. Chest 2020; 157: 376–383. [DOI] [PubMed] [Google Scholar]
- 9. Ghofrani HA, Galiè N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 2013; 369: 330–340. [DOI] [PubMed] [Google Scholar]
- 10. Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 11. Fukuda K, Date H, Doi S, et al. Guidelines for the treatment of pulmonary hypertension (JCS 2017/JPCPHS 2017). Circ J 2019; 83: 842–945. [DOI] [PubMed] [Google Scholar]
- 12. Kataoka M, Aimi Y, Yanagisawa R, et al. Alu-mediated nonallelic homologous and nonhomologous recombination in the BMPR2 gene in heritable pulmonary arterial hypertension. Genet Med 2013; 15: 941–947. [DOI] [PubMed] [Google Scholar]
- 13. Suzuki H, Kataoka M, Hiraide T, et al. Genomic comparison with supercentenarians identifies RNF213 as a risk gene for pulmonary arterial hypertension. Circ Genomic Precis Med 2018; 11: 70–80. [DOI] [PubMed] [Google Scholar]
- 14. Dehmer GJ, Firth BG, Hillis LD. Oxygen consumption in adult patients during cardiac catheterization. Clin Cardiol 1982; 5: 436–440. [DOI] [PubMed] [Google Scholar]
- 15. Lang RM, Badano LP, Victor M-A, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 28: 1–39.e14. [DOI] [PubMed] [Google Scholar]
- 16. Benza RL, Gomberg-Maitland M, Elliott CG, et al. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest 2019; 156: 323–337. [DOI] [PubMed] [Google Scholar]
- 17. Hiraide T, Kataoka M, Suzuki H, et al. Poor outcomes in carriers of the RNF213 variant (p.Arg4810Lys) with pulmonary arterial hypertension. J Hear Lung Transplant 2020; 39: 103–112. [DOI] [PubMed] [Google Scholar]
- 18. Galié N, Sitbom O, Doelberg M, et al. Long-term outcomes in newly diagnosed pulmonary arterial hypertension (PAH) patients receiving initial triple oral combination therapy: insights from the randomised controlled TRITON study. Presented at the European Society of Cardiology Congress, Virtual, 31 August–1 September 2020. [Google Scholar]
- 19. Sitbon O, Sattler C, Bertoletti L, et al. Initial dual oral combination therapy in pulmonary arterial hypertension. Eur Respir J 2016; 47: 1727–1736. [DOI] [PubMed] [Google Scholar]
- 20. Humbert M, Sitbon O, Yaïci A, et al. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J 2010; 36: 549–555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tar-10.1177_1753466621995048 for Triple oral combination therapy with macitentan, riociguat, and selexipag for pulmonary arterial hypertension by Mizuki Momoi, Takahiro Hiraide, Yoshiki Shinya, Hiromi Momota, Shogo Fukui, Michiyuki Kawakami, Yuji Itabashi, Keiichi Fukuda and Masaharu Kataoka in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-2-tar-10.1177_1753466621995048 for Triple oral combination therapy with macitentan, riociguat, and selexipag for pulmonary arterial hypertension by Mizuki Momoi, Takahiro Hiraide, Yoshiki Shinya, Hiromi Momota, Shogo Fukui, Michiyuki Kawakami, Yuji Itabashi, Keiichi Fukuda and Masaharu Kataoka in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-3-tar-10.1177_1753466621995048 for Triple oral combination therapy with macitentan, riociguat, and selexipag for pulmonary arterial hypertension by Mizuki Momoi, Takahiro Hiraide, Yoshiki Shinya, Hiromi Momota, Shogo Fukui, Michiyuki Kawakami, Yuji Itabashi, Keiichi Fukuda and Masaharu Kataoka in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-4-tar-10.1177_1753466621995048 for Triple oral combination therapy with macitentan, riociguat, and selexipag for pulmonary arterial hypertension by Mizuki Momoi, Takahiro Hiraide, Yoshiki Shinya, Hiromi Momota, Shogo Fukui, Michiyuki Kawakami, Yuji Itabashi, Keiichi Fukuda and Masaharu Kataoka in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-5-tar-10.1177_1753466621995048 for Triple oral combination therapy with macitentan, riociguat, and selexipag for pulmonary arterial hypertension by Mizuki Momoi, Takahiro Hiraide, Yoshiki Shinya, Hiromi Momota, Shogo Fukui, Michiyuki Kawakami, Yuji Itabashi, Keiichi Fukuda and Masaharu Kataoka in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-pdf-7-tar-10.1177_1753466621995048 for Triple oral combination therapy with macitentan, riociguat, and selexipag for pulmonary arterial hypertension by Mizuki Momoi, Takahiro Hiraide, Yoshiki Shinya, Hiromi Momota, Shogo Fukui, Michiyuki Kawakami, Yuji Itabashi, Keiichi Fukuda and Masaharu Kataoka in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-tif-6-tar-10.1177_1753466621995048 for Triple oral combination therapy with macitentan, riociguat, and selexipag for pulmonary arterial hypertension by Mizuki Momoi, Takahiro Hiraide, Yoshiki Shinya, Hiromi Momota, Shogo Fukui, Michiyuki Kawakami, Yuji Itabashi, Keiichi Fukuda and Masaharu Kataoka in Therapeutic Advances in Respiratory Disease