Abstract
Objective:
To explore real-life use of glucose lowering drugs and prognosis after acute myocardial infarction (AMI) with a special focus on metformin.
Methods:
Patients (n = 70270) admitted for AMI 2012–2017 were stratified by diabetes status and glucose lowering treatment and followed for mortality and MACE+ (AMI, heart failure (HF), stroke, mortality) until end of 2017 (mean follow-up time 3.4 ± 1.4 years) through linkage with national registries and SWEDEHEART. Hazard ratios (HR) were calculated in adjusted Cox proportional hazard regression models.
Results:
Mean age was 68 ± 11 years and 70% were male. Of patients with diabetes (n = 16356; 23%), a majority had at least one glucose lowering drug (81%) of whom 51% had metformin (24% monotherapy), 43% insulin and a minority any SGLT2i/GLP-1 RA (5%). Adjusted HR for patients with versus without diabetes was 1.31 (95% CI 1.27–1.36) for MACE+ and 1.48 (1.41–1.56) for mortality. Adjusted HR for MACE+ for diabetes patients on metformin was 0.92 (0.85–0.997), p = 0.042 compared to diet treated diabetes.
Conclusion:
Diabetes still implies a high complication risk after AMI. Metformin and insulin were the most common treatment used in almost half of the diabetes population. Furthermore, patients treated with metformin had a lower cardiovascular risk after AMI and needs to be confirmed in prospective controlled trials.
Keywords: Diabetes, outcome, myocardial infarction, glucose lowering treatment, metformin
Introduction
Diabetes is associated with an increased risk of cardiovascular (CV) complications after acute myocardial infarction (AMI).1–3 In established diabetes, newer glucose lowering agents, such as Sodium Glucose Lowering Transport 2 receptor inhibitors (SGLT2i) and Glucagon Like Peptide Receptor Agonists (GLP-1 RA), on top of standard treatment, have shown CV preventive effects which might change this scenario.4–8 In prediabetes, which is common in AMI patients,2,9 there is no glucose lowering treatment that has been shown to be protective of CV diseases after AMI. Lifestyle modification and treatment with metformin prevents diabetes development in obese prediabetes individuals10,11 and metformin in addition to lifestyle prevents CV complications in overweight type 2 diabetes in a primary preventive setting.12 These studies have for long been the rational for metformin being the first line therapy in diabetes. There is, however, an ongoing debate on the role of metformin as first or second line therapy, especially if CV disease is present, where the latest European guidelines on diabetes and prediabetes recommend newer glucose lowering drugs (GLDs) before metformin in high risk patients and in patients with established CV disease.13,14 This is due to the fact that there is no randomised trial on metformin investigating possible CV benefit, while several large randomised trials have shown cardio-protective effects with the newer drugs.4–8,14 However, this does not imply the non-existence of CV effects by metformin. Furthermore, in some countries, metformin is used in prediabetes, gestational diabetes, obesity with prediabetes and in addition in patients with polycystic ovarian syndrome and also sometimes off-label.15,16
The aim of this study was to explore prognosis after AMI in patients with diabetes and patterns of real-life use of glucose lowering drugs in a contemporary setting with a special focus on metformin.
Methods
Data sources and patient cohort
Consecutive patients undergoing a coronary angiography in Sweden with the indication non-ST-segment elevation myocardial infarction (NSTEMI) or ST-segment elevation MI (STEMI) during 2012–2017 reported in the Swedish angiography and angioplasty registry (SCAAR) and with a physician judged myocardial infarction in the Swedish Register of Cardiac Intensive Care (RIKS-HIA) were included in the study. The RIKS-HIA registry and the SCAAR-registry (http://www.ucr.uu.se) are part of the SWEDEHEART registry where SCAAR includes monitored and verified information on every patient undergoing coronary angiography at any centre undergoing coronary angiography and RIKS-HIA contains information from all cardiac intensive care units in Sweden. Information on the index-AMI was collected from RIKS-HIA. Patients were prospectively followed for major CV events until December, 31 2017 and all-cause death until June, 30 2018. Mortality the first 90 days were excluded from analysis in order to limit healthy selection bias of dispensed GLDs. To obtain information on mortality, the SWEDEHEART data was cross referenced with the Swedish Population Register and for complete information on comorbidities, the National Patient Registry (NPR) was used. The SWEDEHEART holds no specific information on classes of glucose lowering drugs, thus data on dispensed glucose lowering drugs up to 6 months after discharge from the hospitalisation for AMI was collected from the Prescribed Drug Registry (PDR) and has been analysed as a categorical variable rather than a time-dependent variable. Information on diabetes diagnosis was derived from SCAAR and the NPR (International statistical classification of diseases and related health problems (ICD)-10 codes E10-E14). Events during follow-up was obtained by cross referencing the SWEDEHEART data with the NPR. No patient was lost to follow-up of those still residents in Sweden.
Definitions
Established Diabetes Mellitus at the index AMI was defined as either registered in SWEDEHEART as having diabetes or having a diabetes diagnosis in the NPR. Accordingly, patients were classified as non-diabetes if no diagnosis of diabetes in any register. Patients were not possible to be categorised by type of diabetes.
Glucose lowering therapies and treatments within 6 months after the AMI hospitalisation was classified into the nine diabetes groups listed below (i.e. based on the most common clinical used combinations) and into three non-diabetes groups. Of note group V comprises SGLT2i/GLP-1 RA either as monotherapy or in combination with other GLDs. Information on insulin treatment was derived both from the SWEDEHART and the PDR while information on other glucose lowering therapies was derived from the PDR only. For instance, patients without reported diabetes but discharged with newly prescribed diabetes drugs was categorised as not having diabetes if no recorded diabetes diagnosis in any register was present.
Established diabetes:
I. Diet (defined as no GLD but with recorded diabetes diagnosis in SWEDEHEART or NPR)
II. Monotherapy metformin
III. Monotherapy sulfonylurea (SU)
IV. Monotherapy insulin
V. Any sodium-glucose co-transporter-2 inhibitor (SGLT2i) or glucagon-like peptide-1 receptor agonist (GLP-1 RA) with or without combination of other GLD
VI. Monotherapy dipeptidyl peptidase-4 inhibitor (DPP4i)
VII. Insulin + metformin
VIII. Insulin + SU
IX. Unspecified combination of GLD
No diabetes:
I. No diabetes, no GLD
II. No diabetes + unspecified combination of GLD
III. No diabetes + monotherapy metformin
Outcome
The following diagnoses were considered: all-cause death, was collected until June, 30 2018 from the Swedish Population Register. First heart failure hospitalisation (ICD-10 code I50), stroke (ICD-10 code I63) and myocardial infarction (ICD-10 code I21-I22) until December, 31, 2017 was collected from the NPR. Major adverse cardiovascular event (MACE) consists of first of MI, stroke, all-cause death while MACE+ consists of MI, stroke, all-cause death and heart failure.
Statistical analysis
Baseline characteristics and outcome were compared across diabetes status and glucose lowering treatment. Baseline characteristics are presented as median and interquartile range (IQR) for continuous variables and numbers and percentages for categorical variables. The group including SGLT2i/GLP-1 RA was selected to be prioritised when stratifying for GLD and thus SGLT2i/GLP-1 RA therefore include either monotherapy or a combination of other GLD, since monotherapy with SGLT2i or GLP-1 RA was rare. To compare baseline characteristics between the different groups, χ2 or Fisher exact test was used. Cumulative event rates for the clinical outcomes were calculated using the Kaplan-Meier method and graphically displayed. In adjusted Cox proportional hazards model, the association between diabetes status, glucose lowering treatment groups and future events were assessed. Hazard ratios (HR, 95% confidence interval (CI)) were adjusted for age, sex, smoking, creatinine, previous diagnosis of MI/ heart failure/CABG/cancer/dementia/dialysis/hypertension/chronic obstructive pulmonary disease/renal failure/stroke/peripheral artery disease, year, indication, hospital, angiographic findings, primary decision after angiography, cardiac shock and medications at discharge (ACE inhibitor, angiotensin II receptor antagonist, lipid lowering agents, aspirin, beta blockade, oral anticoagulant, other antiplatelet therapy). A two-sided p-value of <0.05 was accepted as statistically significant. All analyses were conducted using the SPSS statistical program (SPSS, version 26) software from SPSS Inc, Chicago, IL.
Ethical consideration
The study complies with the declaration of Helsinki and has been approved by the local ethical boards at the Karolinska Institutet (DNR 2017/432-32) and Uppsala University (DNR 2011/333/5). Individual patient consent for entering the SWEDEHEART registry was not obtained but patients were informed about permission to opt out.
Results
Baseline characteristics
Baseline characteristics in all groups (nine diabetes and three non-diabetes) are depicted in Supplementary Table 1. In the total cohort (n = 70 270), mean age was 68 (SD ± 11) years and 70% were male. The indication for coronary angiography was STEMI in 37% and NSTEMI in 63% of the study population where revascularisation (PCI or CABG within 3 months after index AMI) was performed in 85%. Proportion of GLDs use in patients with and without diabetes is depicted in Supplemental Figure 1. Of patients with diabetes diagnosis at hospitalisation (n = 16 356; 23%), a majority had at least one GLD (81%) of whom 51% had metformin (24% monotherapy), and 43% were on insulin either alone or in combination with another GLD (Supplemental Table 2). A minority of the patients were on a SGLT2i or GLP-1 RA (5%) and in a majority combined with other GLD. Eleven percent were on SU of which potentially 8% could be in combination with metformin. In patients discharged without a diabetes diagnosis, n = 871 (2% of the non-diabetes group) were on metformin monotherapy and n = 156 (0.3% of the non-diabetes group) were on an unspecified combination of GLDs during the first 6 months after the AMI hospitalisation. Non-diabetes patients on metformin were younger (65 vs 69 years), more often male (76% vs 70%) and had more often left ventricular ejection fraction (LVEF) ⩾50% (66% vs 64%) when compared to non-diabetes patients without a GLD. Patients with established diabetes were heterogeneous in terms of baseline characteristics where those treated with insulin had the most severe comorbidity profile with more often a history of previous MI, previous heart failure, previous renal failure and more widespread coronary artery disease. Secondary prevention was extensive regardless of diabetes status and glucose lowering therapy (Supplemental Table 1).
Outcome
Diabetes versus no diabetes
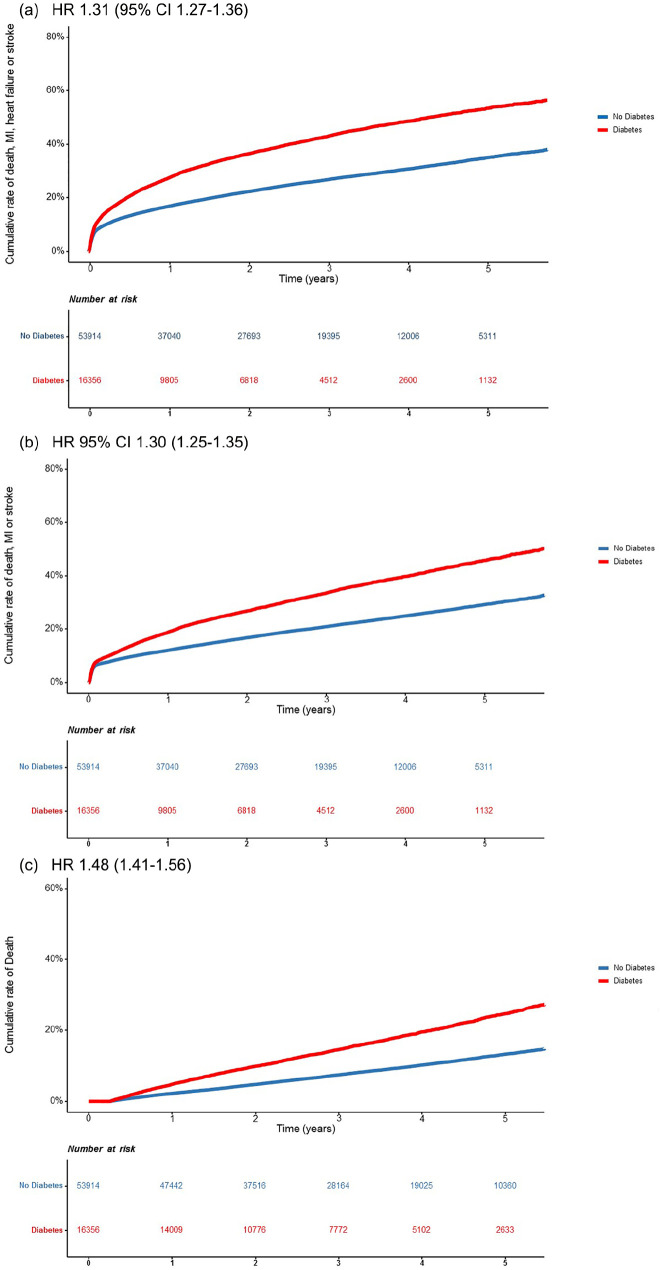
Outcome stratified for diabetes status is depicted in Figure 1(a)–(c). During a mean follow-up time of 3.4 (SD 1.4) years, MACE+ occurred in 29% (n = 20 485) of the patients (40% of those with diabetes and 26% of those without diabetes) and MACE in 23% (n = 16 319; 32% of those with diabetes and 21% of those without diabetes). Death occured in 7354 (10%) of the patients (16% in those with diabetes vs. 9% in patients without diabetes). Diabetes was associated with higher risk for MACE+; adjusted HR 1.31 (95% CI 1.27–1.36), for MACE 1.30 (1.25–1.35) and for mortality 1.48 (1.41–1.56; Figure 1).
Figure 1.
Time to hospitalisation for (a) MACE+ (first of myocardial infarction, heart failure, stroke or death), (b) MACE (first of myocardial infarction, stroke or death) and (c) mortality after index myocardial infarction by diabetes status. Mortality the first 90 days were excluded. Adjusted Hazard Ratios with 95% CI are presented in the figure.
Glucose lowering treatment versus diabetes with diet treatment only
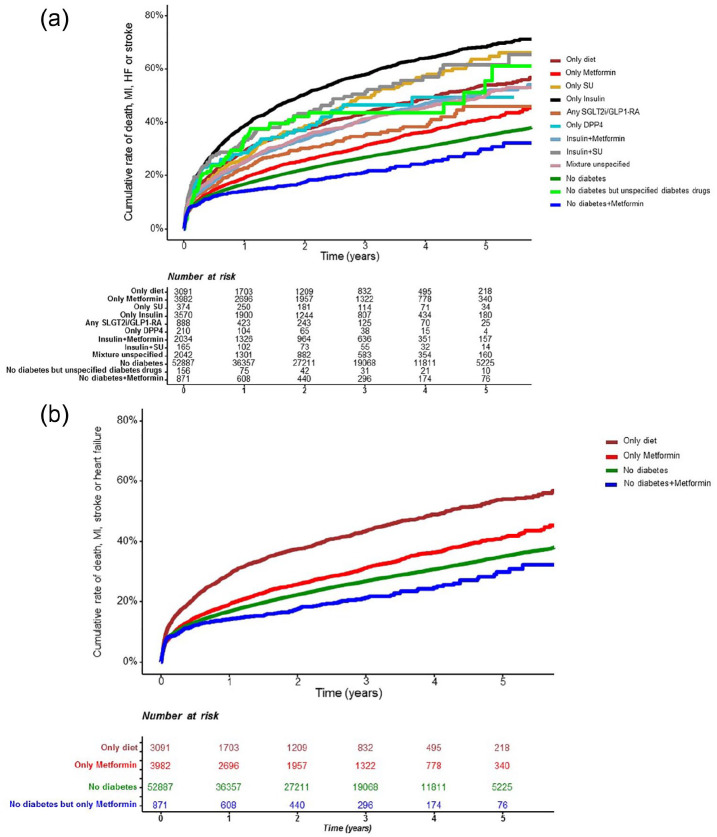
Details on cumulative MACE+ rate stratified by diabetes status and GLDs are presented in Figure 2(a) (all 12 classes) and (b) (for clarity, information only on those with and without metformin) where MACE and mortality are depicted in Supplemental Figures 2 and 3.
Figure 2.
Time to hospitalisation for MACE+ (first of myocardial infarction, heart failure, stroke or death) after index myocardial infarction by: (a) diabetes status and glucose lowering treatment and for clarity and (b) by diabetes status and treatment with metformin. Mortality the first 90 days were excluded.
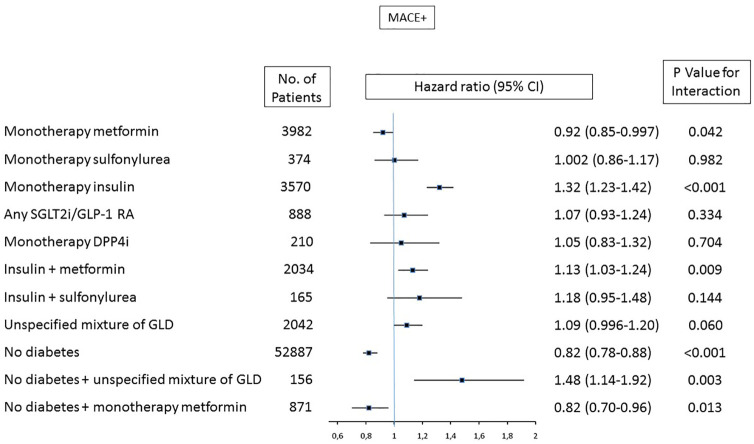
In models stratified by glucose lowering treatment and diabetes status, where established diabetes with diet treatment served as a reference group, patients treated with insulin in monotherapy had the highest event rate and risk (adjusted HR for MACE+ 1.32 [1.23–1.42], p < 0.001; Figures 2 and 3). Patients treated with metformin had lower risk of MACE+ compared with diet treated diabetes, this finding was present both in patients with diabetes on metformin (0.92 [0.85–0.997], p = 0.042) and in patients without diabetes but on metformin (0.82 [0.70–0.96], p = 0.013) where the latter was similar to the HR seen in patients without diabetes (HR 0.82 [95% CI 0.78–0.88], p < 0.001). Corresponding figures for patients with diabetes on insulin in monotherapy was 1.32 (1.23–1.42) and 1.13 (1.03–1.24) in patients on insulin plus metformin. Resembling associated risk pattern was observed for MACE (Supplemental Table 3). Corresponding figures for non-diabetes patients without GLD were (HR 0.69 [0.63–0.76], p < 0.001). Patients treated with metformin had lower risk of mortality compared with diet treated diabetes, both in patients with diabetes on metformin (0.81 [0.71–0.92], p = 0.002) and in patients without diabetes but on metformin (0.57 [0.41–0.80], p = 0.001; Supplemental Table 3).
Figure 3.
Adjusted associated HR (95% CI) for MACE + (mortality, myocardial infarction, stroke or heart failure) by diabetes status and treatment groups. Diet treated diabetes patients were used as reference with HR 1.0.
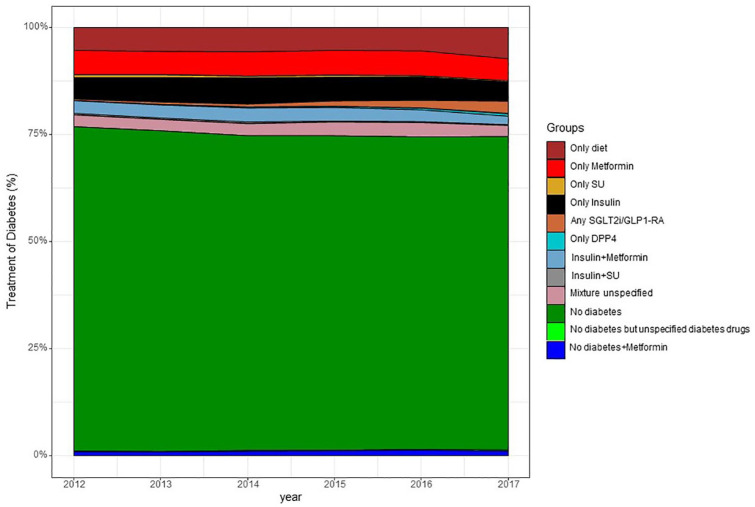
Trends in diabetes diagnosis and dispensed GLD between 2012 and 2017 are depicted in Figure 4 demonstrating a stable pattern with only a discrete increase in proportion of patients with diabetes diagnosis and diabetes treated with diet only. From 2015 there is a minor uptake of dispensed GLD with cardio protective effects (SGLT2i or GLP-1 RA).
Figure 4.
Glucose lowering treatment over time 2012–2017 in patients discharged after myocardial infarction. Relative proportion of patients per group by year. Individual patients could only belong to one group.
Discussion
In this large comprehensive study analysing impact of diabetes and diabetes treatment in an unselected cohort of patients undergoing coronary angiography and surviving AMI there are three major findings. First, diabetes is still, despite modern coronary care, associated with a 30% to 50% increased risk of CV events and mortality. Second, patients treated with metformin in monotherapy had lower event rates compared to patients without GLD. Third, newer classes of GLDs with cardio-protective effects were used in a very low proportion post-MI and often in mixed combinations, while the most commonly used GLDs are metformin and insulin, used in almost half of the diabetes population, while only 18% had no GLD treatment at all. As expected in this post-MI cohort the combination of SU and metformin was less common than in a cohort without CV disease.
Disappointingly, despite dramatic improvements in survival rates after AMI and secondary preventive care,17 the over-risk for diabetes still remains and with a similar magnitude as was seen more than a decade ago.18 This remaining almost unchanged gap between those with and without diabetes, indicate a failure of introducing novel therapeutic approaches for the diabetes group and highlight the urgent need to faster take up the recently introduced cardio-protective drug classes, where monitoring of improvements should be demanded in order to better reach success.13,14 Importantly, there is a heterogeneity within the diabetes group even post-MI, with higher CV complication rates in patients already burdened with CV complications.19 For instance the risk for heart failure and death is doubled if there is a previous reported myocardial infarction and even higher risk if discharged with signs of depressed myocardial function as LVEF less than 50%.19 The present study further extends this information, identifying insulin and multiple GLD-treated patients as a high risk group in accordance with several earlier reports.1,20 Patients on insulin had the highest cumulative event rate with a fast onset of recurrent CV events already the first year indicating an urgent need for preventive efforts. It is highly probable that insulin in this context is a proxy for a more long standing diabetes with increased prevalence of comorbidities rather than insulin being the sole driver of the adverse outcome, although risk associated with insulin use has been suggested.21 A high event rate in type 2 diabetes patients on insulin and known atherosclerotic CV disease was also demonstrated in a recent post hoc analysis of the EMPA-REG Outcome trial.22 Importantly, the evidence for empagliflozin and a reduction of CV events was not limited to patients at low risk, but was seen across the full range of underlying CV risk. This finding, together with the present data that identifies patients treated with insulin as a very high risk group, strengthens that future strategies should not exclude those or other severely affected diabetes patients, since even in this group there are novel effective treatments and if left untreated will be at very high risk.22,23 In this study patients on insulin plus metformin had a better outcome in terms of CV events and mortality compared to insulin in monotherapy, a similar finding as was shown by Holden et al. although in a primary preventive setting.24 However, in the present observational study patients on insulin alone had more co-morbidities as previous MI, PAD, heart failure and renal failure why it cannot be excluded that, despite extensive adjustments, the difference can be due to patients on concomitant metformin being healthier.
Even in patients with established diabetes but without any GLD (i.e. diet group), constituting around one fifth of this post-MI diabetes population, there was an increased risk (+18%) of CV complications when compared to patients without diabetes (HR 95% CI 0.82; 0.77–0.87). In the large CV outcome trials (EMPA-REG OUTCOME, CANVAS, DECLARE) only a minority where completely GLD naïve at randomisation with 2% in EMPA-REG OUTCOME4 and 3.6% in LEADER,5 and CV effects on sub-analyses on completely drug-naïve is to the best of our knowledge not yet reported. However, in the EMPA-REG the CV effects of SGLT2i seemed to be present across all levels of HbA1c and regardless of level of HbA1c reduction implying CV protective effects might also be present in drug naïve diabetes patients with lower range of HbA1c.25 In a recent meta-analysis on treatment with SGLT2i the reduction in CV and renal complications was consistent regardless of concomitant metformin or not.26
Interestingly, in the present observational study, although marginal and could be due to unmeasured confounding, we found lower rates of CV complication in diabetes patients treated with metformin (mono-therapy) than with diet alone. Furthermore, non-diabetes patients treated with metformin had a similar risk for future CV event than non-diabetes patients. The reason why non-diabetes had been prescribed metformin is not known but one could speculate that it can be due to that diabetes has been revealed in the cardiac rehabilitation phase or by the GP in the post-MI phase. Whatever the reason, the similar risk for future CV event as the non-diabetes patients without GLD is indeed interesting. For long, metformin has globally been recommended as the first line therapy in patients with type 2 diabetes,15 primarily based on the DPP-study and of a subgroup population of the UKPDS study.10,12 In the long-term follow-up of the DPP study preventive effects of metformin on diabetes development where established.10 In UKPDS, obese patients (n = 342) with newly detected diabetes and on metformin, experienced a reduction in mortality and CV events after a decade of follow-up that persisted also during long-term follow-up.12,27 The present study supports the UKPDS long-term data indicating that metformin could be CV protective, although causality in an observational study cannot be established.27 After nearly 60 years of clinical use, apart from the UKPDS, there is a lack of evidence and RCTs evaluating the effect of metformin on CV outcomes. In patients with established CV disease there is no large RCT at all evaluating the secondary preventive effect of metformin. The Glucose Lowering In Non-diabetic hyperglycaemia Trial (GLINT) aimed to explore CV preventive effects of metformin, but ended with a pilot study in the primary setting.28 In USA the ongoing Investigation of Metformin in Pre-Diabetes on Atherosclerotic Cardiovascular OuTcomes (VA-IMPACT) study is randomising metformin or placebo to estimated 7868 patients with prediabetes with the primary aim to study CV protective effects of metformin.29 One recent meta-analyses based on 40 clinical trials (RCTs, retrospective cohort studies and case-control studies) and 1,066,408 patients with coronary artery disease, metformin was associated with lower CV risk in type 2 diabetes but no significant risk reduction was seen in non-diabetes patients.30 The recent MET-REMODEL trial showed reduced left ventricular hypertrophy, evaluated by MRI, in patients (n = 68) with coronary artery disease and insulin resistance but without diabetes when randomised to metformin or placebo for 12 months (p = 0.033).31 Thus, so far clinical evidence on CV effect of metformin is limited. On the contrary, mechanistic evidence for CV favourable effects are abundant where novel data are almost monthly reported. Metformin’s mechanisms of actions are not fully known, but some proposed favourable mechanisms include reducing hepatic gluconeogenesis/the metabolic syndrome,32 altering the glucose metabolism by the microbiota in the intestine,33 decreasing cardiac fibrosis34 and immunomodulatory and anti-aging effects.35 Recently a novel mechanism associated with increase in GDF-15 and weight loss36–38 was described. On the contrary, proposed non-favourable effects include decrease of lean mass gain in healthy elderly (⩾65 years) impeding the benefit of physical activity.39
In the recently updated European guidelines for diabetes and CV disease SGT2i and GLP-1 RA are first line treatment if CV disease is present while the ADA and EASD consensus report still recommend metformin as first line therapy.13,14 Anyhow, regardless of guidelines recommendations, for sure newer glucose lowering drugs should be used to a greater extent than 5% reported in the current and other studies and in real life practice several alternatives and combinations of GLD are needed to accomplish glucose control.40 In most CV outcome trials, novel GLD have been used on top of metformin (used in 74%, 77%, 79%, respectively in EMPA-REG OUTCOME,4 CANVAS,7 DECLARE8) where benefit seems to be there regardless if metformin is used or not, at least with no interaction reported with metformin in the EMPA-REG OUTOMCE post-hoc analyses.25
There clearly is a need for trials assessing possible CV protective effects of metformin, but due to lack of an economic profit it is unlikely that such trials will be conducted unless initiatives are taken by national governments.
There are several limitations on the present study; the lack of variables related to diabetes status as type of diabetes (where the proportion of type 1 diabetes is estimated to be very low), duration and glucose control (HbA1c reported in 22%) where the non-diabetes group could have undiagnosed diabetes (since OGTT is not routinely performed). In addition, there is no information about medications during long-term follow-up. Although of interest as CV outcome trials on the newer glucose lowering drugs are published from 2016 and forward, access to data on newer glucose lowering drugs after the end of 2017 was not possible to obtain due to logistical reasons. Furthermore propensity matched metformin analyses to limit indication bias was not performed and judged to be unjustified due to lack of important variables to match for as metabolic measurement (glucose, central obesity, insulin levels) in both non-diabetes and diabetes cohorts. Lastly, the observational study design comprises a possibility of unknown residual confounders not possible to control for despite the extensive adjustments that have been performed to control for potential confounding variables as the different prevalence of concomitant disease as heart failure and renal failure among the glucose lowering groups. On the other hand we have a large unselected nationwide cohort with a complete follow-up where healthy selection bias has been limited as mortality the first 90 days were excluded from analysis. Notably, individuals with AMI who did not undergo coronary angiography were excluded.
Conclusion
Diabetes is still associated with a compromised outcome after AMI supporting an urgent need of implementing optimised diabetes care post-MI including an increased use of glucose lowering drugs with CV benefit. Furthermore, both diabetes patients treated with metformin, and a group with previously unreported diabetes treated with metformin, had less CV complications after AMI in this observational analyses but needs to be confirmed in a prospective controlled trial.
Supplemental Material
Supplemental material, sj-pdf-1-dvr-10.1177_1479164120973676 for Diabetes, metformin and glucose lowering therapies after myocardial infarction: Insights from the SWEDEHEART registry by Viveca Ritsinger, Bo Lagerqvist, Pia Lundman, Emil Hagström and Anna Norhammar in Diabetes & Vascular Disease Research
Footnotes
Author contribution: The study design was developed by V.R., B.L. and A.N. V.R. and A.N. finalised the manuscript after adjustments by B.L., E.H. and P.L. B.L. performed the statistical analyses and managed the database. All authors, V.R., B.L., P.L., E.H. and A.N, made substantial contributions to this article and took part in the interpretation of the results.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: V.R. has received honoraria on expert group participation from Astra Zeneca, Novo Nordisk and Boehringer Ingelheim. A.N. has received honoraria from Astra Zeneca, Merck Sharp & Dohme, Eli Lilly and Company, Novo Nordisk and Boehringer Ingelheim on expert group participation. B.L. reports no conflicts of interest. E.H. reports participation in expert committees, lecturing fees from Bayer, AstraZeneca and NovoNordisk and institutional grants from Sanofi and Amgen. P.L. has received honoraria for expert group participation and speaker’s fees from Astra Zeneca, Amgen, Boehringer-Ingelheim, Novo-Nordisk and Sanofi.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by the Swedish Heart-Lung Foundation, the Department of Research and Development Region Kronoberg and the Family Kamprad Foundation.
ORCID iD: Viveca Ritsinger  https://orcid.org/0000-0003-3385-4777
https://orcid.org/0000-0003-3385-4777
Supplemental material: Supplemental material for this article is available online.
References
- 1. Ritsinger V, Saleh N, Lagerqvist B, et al. High event rate after a first percutaneous coronary intervention (PCI) in patients with diabetes results from the Swedish coronary angiography and angioplasty registry (SCAAR). Circ Cardiovasc Interv 2015; 8: e00232. [DOI] [PubMed] [Google Scholar]
- 2. Ritsinger V, Tanoglidi E, Malmberg K, et al. Sustained prognostic implications of newly detected glucose abnormalities in patients with: long-term follow-up of the glucose tolerance in patients with acute myocardial infarction cohort. Diab Vasc Dis Res 2015; 12(1): 23−32. [DOI] [PubMed] [Google Scholar]
- 3. Rossello X, Ferreira JP, McMurray JJ, et al. High-risk myocardial infarction database initiative. Impact of insulin-treated diabetes on cardiovascular outcomes following high-risk myocardial infarction. Eur Heart J Acute Cardiovasc Care 2019; 8(3): 231−241. [DOI] [PubMed] [Google Scholar]
- 4. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015; 373: 2117−2128. [DOI] [PubMed] [Google Scholar]
- 5. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016; 375(4): 311−322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019; 394(10193): 121−130. [DOI] [PubMed] [Google Scholar]
- 7. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017; 377(7): 644−657. [DOI] [PubMed] [Google Scholar]
- 8. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019; 380: 347−357. [DOI] [PubMed] [Google Scholar]
- 9. Bartnik M, Rydén L, Ferrari R, et al. The prevalence of abnormal glucose regulation in patients with coronary artery disease across Europe. The euro heart survey on diabetes and the heart. Eur Heart J 2004; 25(21): 1880−1890. [DOI] [PubMed] [Google Scholar]
- 10. Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the diabetes prevention program outcomes study. Lancet Diabetes Endocrinol 2015; (11): 866−875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gong Q, Zhang P, Wang J, et al. Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30-year results of the Da Qing diabetes prevention outcome study. Lancet Diabetes Endocrinol 2019; 7(6): 452−461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998; 352(9131): 854−865. [PubMed] [Google Scholar]
- 13. Buse JB, Wexler DJ, Tsapas A, et al. 2019 Update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American diabetes association (ADA) and the European association for the study of diabetes (EASD). Diabetes Care 2020; 43(2): 487−493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2019; pii: ehz486. [DOI] [PubMed] [Google Scholar]
- 15. American Diabetes Association. Standards of Medical Care in Diabetes-2019. Diabetes Care 2019; 42(Suppl 1): S1−S186.30559224 [Google Scholar]
- 16. Goodman NF, Cobin RH, Futterweit W, et al. American association of clinical endocrinologists, American college of endocrinology, and androgen excess and PCOS society disease state clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome–part 1. Endocr Pract 2015; 21(11): 1291−1300. [DOI] [PubMed] [Google Scholar]
- 17. Szummer K, Wallentin L, Lindhagen L, et al. Improved outcomes in patients with ST-elevation myocardial infarction during the last 20 years are related to implementation of evidence-based treatments: experiences from the SWEDEHEART registry 1995–2014. Eur Heart J 2017; 38: 3056−3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Norhammar A, Lindbäck J, Rydén L, et al. Improved but still high short- and long-term mortality rates after myocardial infarction in patients with diabetes mellitus: a time-trend report from the Swedish register of information and knowledge about Swedish heart intensive care admission. Heart 2007; 93(12): 1577−1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ritsinger V, Nyström T, Saleh N, et al. Heart failure is a common complication after acute myocardial infarction in patients with diabetes: a nationwide study in the SWEDEHEART registry. Eur J Prev Cardiol. Epub ahead of print 2020. DOI: 10.1177/2047487319901063. PMID: 32019365. [DOI] [PubMed] [Google Scholar]
- 20. The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545−2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Packer M. Potentiation of insulin signaling contributes to heart failure in type 2 diabetes. JACC Basic Transl Sci 2018; 3(3): 415−419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fitchett D, Inzucchi SE, Cannon CP, et al. Empagliflozin reduced mortality and hospitalization for heart failure across the spectrum of cardiovascular risk in the EMPA-REG OUTCOME trial. Circulation 2019; 139(11): 1384−1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Petrie MC, Verma S, Docherty KF, et al. Effect of dapagliflozin on worsening heart failure and cardiovascular death in patients with heart failure with and without diabetes. JAMA. Epub ahead of print 2020. DOI: 10.1001/jama.2020.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Holden SE, Jenkins-Jones S, Currie CJ. Association between insulin monotherapy versus insulin plus metformin and the risk of all-cause mortality and other serious outcomes: a retrospective cohort study. PLoS ONE 2016; 11(5): e0153594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Inzucchi SE, Fitchett D, Jurišić-Eržen D, et al. Are the cardiovascular and kidney benefits of empagliflozin influenced by baseline glucose-lowering therapy? Diabetes Obes Metab. Epub ahead of print 2019. DOI: 10.1111/dom.13938. [DOI] [PubMed] [Google Scholar]
- 26. Neuen BL, Arnott C, Perkovic V, et al. SGLT2 inhibitors with and without metformin: a meta-analysis of cardiovascular, kidney and mortality outcomes. Diabetes Obes Metab. Epub ahead of print 2020. DOI: 10.1111/dom.14226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359(15): 1577−1589. [DOI] [PubMed] [Google Scholar]
- 28. Griffin SJ, Bethel MA, Holman RR, et al. Metformin in non-diabetic hyperglycaemia: the GLINT feasibility RCT. Health Technol Assess 2018; 22(18): 1−64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schwartz GG. National Library of Medicine (US). Identifier NCT02915198, Investigation of Metformin in Pre-Diabetes on Atherosclerotic Cardiovascular OuTcomes (VA-IMPACT); 2016 Feb 26 [cited 2020 Nov 10], https://clinicaltrials.gov/ct2/show/NCT02915198
- 30. Han Y, Xie H, Liu Y, et al. Effect of metformin on all-cause and cardiovascular mortality in patients with coronary artery diseases: a systematic review and an updated meta-analysis. Cardiovasc Diabetol 2019; 18(1): 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mohan M, Al-Talabany S, McKinnie A, et al. A randomized controlled trial of metformin on left ventricular hypertrophy in patients with coronary artery disease without diabetes: the MET-REMODEL trial. Eur Heart J 2019; 40(41): 3409−3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia 2017; 60(9): 1577−1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pollak M. The effects of metformin on gut microbiota and the immune system as research frontiers. Diabetologia 2017; 60(9): 1662−1667. [DOI] [PubMed] [Google Scholar]
- 34. Xiao H, Ma X, Feng W, et al. Metformin attenuates cardiac fibrosis by inhibiting the TGFbeta1-Smad3 signalling pathway. Cardiovasc Res 2010; 87(3): 504−513. [DOI] [PubMed] [Google Scholar]
- 35. Valencia WM, Palacio A, Tamariz L, et al. Metformin and ageing: improving ageing outcomes beyond glycaemic control. Diabetologia 2017; 60(9): 1630−1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coll AP, Chen M, Taskar P, et al. GDF15 mediates the effects of metformin on body weight and energy balance. Nature 2020; 578(7795): 444−448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Day EA, Ford RJ, Smith BK, et al. Metformin-induced increases in GDF15 are important for suppressing appetite and promoting weight loss. Nat Metab 2019; 1: 1202−1208. [DOI] [PubMed] [Google Scholar]
- 38. Natali A, Nesti L, Venturi E, et al. Metformin is the key factor in elevated plasma growth differentiation factor-15 levels in type 2 diabetes: a nested, case-control study. Diabetes Obes Metab 2019; 21(2): 412−416. [DOI] [PubMed] [Google Scholar]
- 39. Walton RG, Dungan CM, Long DE, et al. Metformin blunts muscle hypertrophy in response to progressive resistance exercise training in older adults: a randomized, double-blind, placebo-controlled, multicenter trial: the MASTERS trial. Aging Cell 2019; 18(6): e13039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Arnold SV, Inzucchi SE, Tang F, et al. Real-world use and modeled impact of glucose-lowering therapies evaluated in recent cardiovascular outcomes trials: an NCDR® research to practice project. Eur J Prev Cardiol 2017; 24(15): 1637−1645. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-dvr-10.1177_1479164120973676 for Diabetes, metformin and glucose lowering therapies after myocardial infarction: Insights from the SWEDEHEART registry by Viveca Ritsinger, Bo Lagerqvist, Pia Lundman, Emil Hagström and Anna Norhammar in Diabetes & Vascular Disease Research