Abstract
Background:
NLR family pyrin domain containing 3 (NLRP3) inflammasome has been implicated in the development of atherosclerosis and several studies have suggested that inhibiting NLRP3 inflammasome could be a potential therapeutic approach to treat atherosclerosis. Baicalin is a flavone glycoside with anti-inflammation, anti-oxidative activities. The inhibition of NLRP3 inflammasome activation by baicalin has also been described. Therefore, the effects of baicalin on NLRP3 inflammasome activation and atherosclerosis were evaluated in present study.
Methods:
We established the apolipoprotein E-deficient atherosclerosis mice model. After baicalin treatment, the IL-1, IL-18, and reactive oxygen species (ROS) production, and the plaque area was monitored. We also measured the NLRP3, ASC, caspase-1, ICAM-1, and VCAM-1 expression in atherosclerosis mice after baicalin treatment. We silenced NLRP3 by administration of lentivirus expressing NLRP3 shRNA to atherosclerosis mice and monitored the IL-1, IL-18, and ROS production, and NLRP3 inflammasome activation.
Results:
Baicalin remarkably inhibited the production of IL-1, IL-18, mitochondria ROS, total ROS, ICAM-1, and VCAM-1. Baicalin reduced the expression of NLRP3 inflammasome and suppressed its activation. Baicalin significantly reduced the plaque area. Silencing NLRP3 resulted in decreased production of IL-1, IL-18, mitochondria ROS, total ROS, ICAM-1, and VCAM-1, and inhibition of NLRP3 inflammasome activation.
Conclusion:
Baicalin ameliorated atherosclerosis by inhibiting NLRP3 inflammasome.
Keywords: Baicalin, atherosclerosis, NLRP3, inflammasome, inflammatory disease, ROS production
Introduction
Atherosclerosis is an inflammatory disease with high mortality.1 Atherosclerosis is the key factor of most cardiovascular diseases and is responsible for more than 50% of death over the world.2 In atherosclerosis, the atherosclerotic plaques are formed, which involves multiple mechanism including inflammation, oxidative stress, and vascular proliferation.3 Lipid deposition, adhesion molecules expression, and leukocyte infiltration have been identified in atherosclerosis.3
Increasing evidences have suggested the significant role of NLR family pyrin domain containing 3 (NLPR3) inflammasome in atherosclerosis development. NLRP3 inflammasome is composed of NLRP3, apoptosis-associated speck-like protein containing a C terminal caspase recruitment domain (ASC), and caspase-1. Once activated by stimuli, caspase-1 is activated by the NLRP3 inflammasome, resulting in cleaving inactive pro-IL-1β into mature IL-1β. Elevated IL-1β level has been identified in atherosclerotic coronaries and the disease severity has a positive correlation with NLRP3 expression.4,5 Emerging evidences indicates that inhibiting NLRP3 inflammasome activation ameliorates the progress of atherosclerosis.6,7
Baicalin (5,6-dihydroxy-7-O-glucuronide flavone) is a flavone glucuronide which is found in several species in the genus Scutellari. Baicalin is with various activities including anti-inflammation,8 anti-oxidation,9 anti-apoptosis,10 and anti-viral.11,12 Interestingly, the suppression of NLRP3 inflammasome activation by baicalin has been also described.13–15 These previous studies suggest potential protective effects of baicalin on atherosclerosis. In present study, the effects of baicalin on atherosclerosis were evaluated.
Materials and methods
Animals and treatment
Apolipoprotein E-deficient (ApoE−/−) mice (8 weeks old), the most popular murine model for atherosclerotic study, were used in present study.16 Male ApoE−/− mice were obtained from Charles River (Beijing, China) and fed with a diet containing 15% fat and 0.25% cholesterol (Beijing Hufukang Bioscience Co. INC) for 4 weeks with free access to water. For baicalin treatment, baicalin (purity >97%) (Sigma, St. Louis, MO, USA) was dissolved in saline and intragastrically injected into mice once a day. The doses of baicalin used in present study were based on previous report.17,18 ApoE−/− mice were divided into following groups: Group 1, mice with atherosclerosis (AS group) received the same volume of saline. Group 2, atherosclerosis mice intragastrically injected with 20 mg/kg baicalin. Group 3, atherosclerosis mice intragastrically injected with 50 mg/kg baicalin. Group 4, atherosclerosis mice intragastrically injected with 100 mg/kg baicalin. C57BL/6J wild-type (WT) mice with matched age received the same volume of saline were used as control. Each group had 14 mice. In the control group, mice were maintained with a standard laboratory chow diet. 8 weeks later, mice were sacrificed for analysis. The study was supported by the Ethics Commitment of Qilu Hospital of Shandong University (#2018-0392).
Lentiviral vector expressing Nlrp3 shRNA or control shRNA was purchased from Origene (Beijing, China). Lentivirus were made by co-transfection of lentiviral vector and lenti-vpak packaging plasmids (Origene, China) following manufacturer’s instructions. After 4 weeks fed with Western type diet, concentrated lentivirus (2 × 108 Tfu) was injected via tail vein into atherosclerosis mice. The injection of lentivirus was repeated once 2 weeks after the first injection. 8 weeks after first injection, mice were sacrificed for analysis.
ELISA
IL-1β and IL-18 levels in protein extraction from aortas were measured using commercial ELISA kits (cat# PMLB00C & DY122-05, R&D Systems, Minneapolis, MN, USA) following manufacturer’s instructions.
Tissue staining
After anesthetization, mice aortic root was collected and embedded for cryosections. Oil Red O stain kit (Abcam, Beijing, China) was used for lipid staining and the plaque sizes were quantitated by a trained observer blinded to the mice groups via delineating plaque areas in 5–10 sections per mouse.
ROS production measurement
For mitochondrial ROS production measurement, the fresh frozen aortic sections were inoculated with 5 µM MitoSOX Red (Thermo Fisher, Waltham, MA, USA) for 10 min at 37°C. To measure the total ROS production, frozen sections were stained with dihydroethidium (DHE) (Thermo Fisher, USA) for 30 min at 37°C. Fluorescence intensities of total ROS and mitochondrial ROS were measured using ImageJ software.
Western blot
Total proteins of aortas were extracted by RIPA buffer (Thermo Fisher, USA) and then subjected to SDS PAGE. After transfer, the PVDF membrane was blocked with 5% blotting grade blocker (Bio-rad, Hercules, CA, USA). Then, primary antibodies including anti-NLRP3 (1:1000 dilution, ab270449, Abcam, China), anti-caspase-1 p20 (1:1000 dilution, ab138483, Abcam, China), anti-β-actin (1:5000 dilution, ab179467, Abcam, China), anti-ICAM-1 (1:1000 dilution, ab222736, Abcam, China), anti-VCAM-1 (1:2000 dilution, ab134047, Abcam, China) was added and incubated for overnight at 4°C. Next day, secondary antibodies were incubated for 1 h at room temperature. The ECL Substrate (Thermo Fisher, USA) was added to visualize the bands. The band intensity was quantitated and analyzed using ImageJ.
RT-PCR
The aortas were homogenized in Trizol reagent (Thermo Fisher, USA) for total RNA extraction. Then cDNA was synthesized using cDNA Synthesis Kit (Takara, Beijing, China). TB Green® Advantage® qPCR Premix (Takara, China) was used to set up the real-time PCR. The primers used in present study included: Caspase-1 Forward: 5′-TGGAAATGTGCCATCTTCTTT-3′, Reverse: 5′-TCAGCTCCATCAGCTGAAAC-3′. NLRP3 Forward: 5′-GAGTTCTTCGCTGCTATGT-3′, Reverse: 5′-ACCTTCACGTCTCGGTTC-3′. ICAM-1 Forward: 5′-CACTACCATCGCCTTCGCCTG-3′, Reverse: 5′-CCGTCGTTCATCCGTTCCTGG-3′. VCAM-1 Forward: 5′-GAAGAATTAACACTCGGTTGAAGTCAGA-3′, Reverse: 5′-GGGCTTGAGGAACGTGAGATGA-3′. β-actin primer Forward: 5′-GTCCCTCACCCTCCCAAAAG-3′, Reverse: 5′-GCTGCCTCAACACCTCAACCC-3′.
Statistical analysis
Data were presented as mean ± SD. One-way ANOVA analysis followed by Dennett’s post hoc test was used to calculate the difference. When p < 0.05, the statistical difference was considered as significant.
Results
Baicalin reduced inflammatory cytokines in arteries of ApoE−/− mice
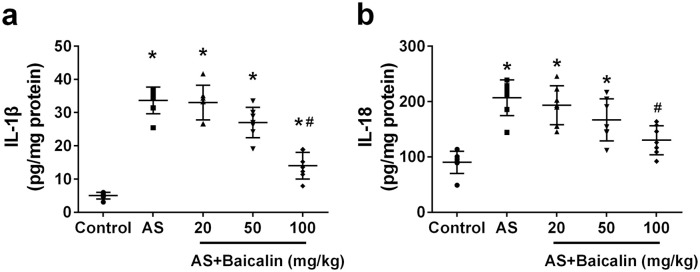
First, we evaluated the effects of baicalin on IL-1β and IL-18 production in arteries of atherosclerosis mice. Consistent to previous report,19 we detected significantly elevated level of IL-1β (Figure 1(a)) and IL-18 (Figure 1(b)) in arteries of atherosclerosis mice when compared to that in control mice, Administration of 20 mg/kg or 50 mg/kg baicalin did not obviously affect the level of IL-1β or IL-18 in arteries of atherosclerosis mice. In contrast, administration of 100 mg/kg baicalin atherosclerosis mice significantly decreased IL-1β (Figure 1(a)) and IL-18 level in arteries. Collectively, baicalin reduced inflammatory cytokines production in arteries of atherosclerosis mice.
Figure 1.
Baicalin reduced inflammatory cytokines in aortas of ApoE−/− mice: IL-1β (a) and IL-18 (b) levels were measured by the ELISA assay within aortas.
Data were shown as mean ± SD. n = 7 for each group. *p < 0.05 compared with the control group; #p < 0.05 compared with the AS group.
Baicalin prevented the progression of atherosclerotic lesion in atherosclerosis mice
Next, we evaluated the effects of baicalin on progression of atherosclerotic plaque in atherosclerosis mice. Compared to control mice, clear aortic root plaques were observed in atherosclerosis mice after oil red O staining (Figure 2(a)). Atherosclerosis mice administrated with 20 mg/kg or 50 mg/kg still got obvious aortic plaques while atherosclerosis mice treated with 100 mg/kg had obviously decreased aortic root plaques. After quantitation, significantly increased plaque area was detected in Atherosclerosis mice when compared to control mice (Figure 2(b)). 20 mg/kg baicalin treatment did not significantly affect plaque area while 50 mg/kg and 100 mg/kg baicalin treatment significantly decreased plaque area. Collectively, our data demonstrated that baicalin prevented the progression of atherosclerotic lesion.
Figure 2.
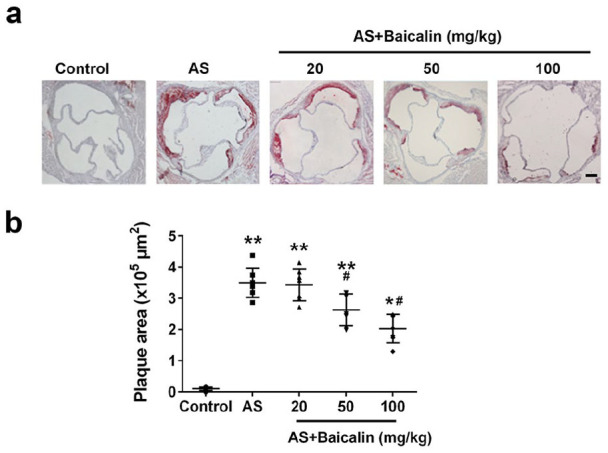
Baicalin treatment significantly reduced atherosclerotic plaques of aortic root: (a) representative images of aortic root plaques stained with Oil Red O, and (b) plaques area quantification. Scale bar = 50 μm.
Data were shown as mean ± SD. n = 7 for each group. *p < 0.05, **p < 0.01 compared with the control group; #p < 0.05 compared with the AS group.
Baicalin attenuated ROS production in arteries of atherosclerosis mice
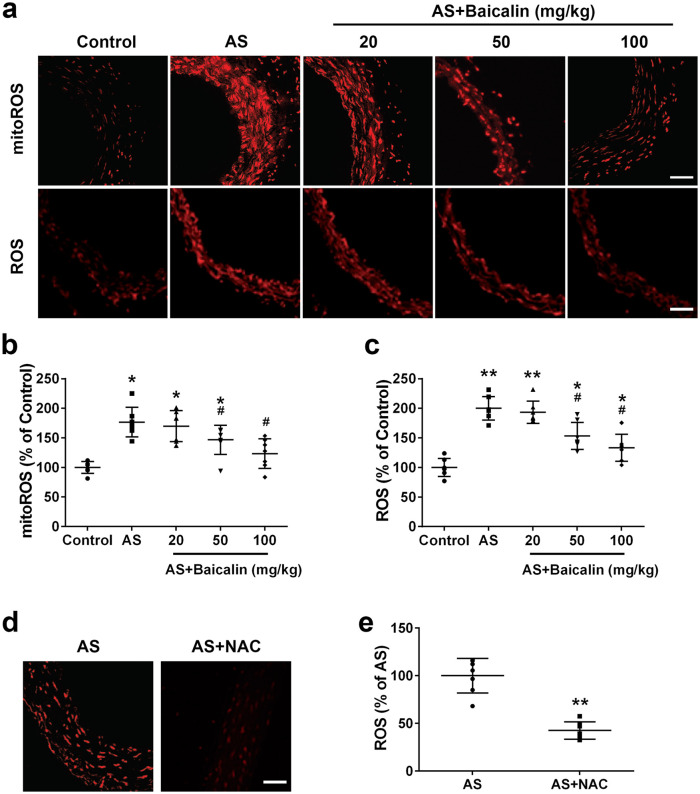
We continued to evaluate the effects of baicalin on ROS, a key factor in atherosclerosis.20 We detected obviously increased fluorescence signal of both mitochondria ROS and total ROS in atherosclerosis mice. In contrast, baicalin treatment decreased the signals (Figure 3(a)). After quantification, significantly increased mitochondria ROS (Figure 3(b)) and total ROS (Figure 3(c)) were detected in arteries of atherosclerosis mice. 20 mg/kg baicalin treatment did not affect mitochondria ROS and total ROS production while 50 and 100 mg/kg baicalin treatment significantly suppressed both mitochondria ROS and total ROS production in arteries of atherosclerosis mice. Interestingly, the mitochondria ROS and total ROS levels in atherosclerosis mice treated with 50 and 100 mg/kg baicalin were still significantly higher than those in control mice, indicating that baicalin only attenuated but did not totally abolish ROS production in atherosclerosis mice. Pre-incubation of artery sections from Atherosclerosis mice with NAC (10 mM), a ROS scavenger, resulted in significantly decreased DHE fluorescence, indicating the DHE fluorescence reflected ROS levels (Figure 3(d) and (e)). Taken together, these results indicated that baicalin suppressed ROS production in arteries of Atherosclerosis mice.
Figure 3.
Baicalin treatment attenuated ROS production in aortas of ApoE−/− mice: (a) representative MitoSOX Red and DHE fluorescent images of aortas, (b) mtROS levels were detected with MitoSOX Red, (c) ROS levels were detected with DHE, and (d, e) DHE fluorescence signal can be blocked by a ROS scavenger, NAC. Scale bar = 50 μm.
Data were shown as mean ± SD. n = 7 for each group. *p < 0.05, **p < 0.01 compared with the control group; #p < 0.05 compared with the AS group.
Baicalin inhibited activation of NLRP3 inflammasome in arteries
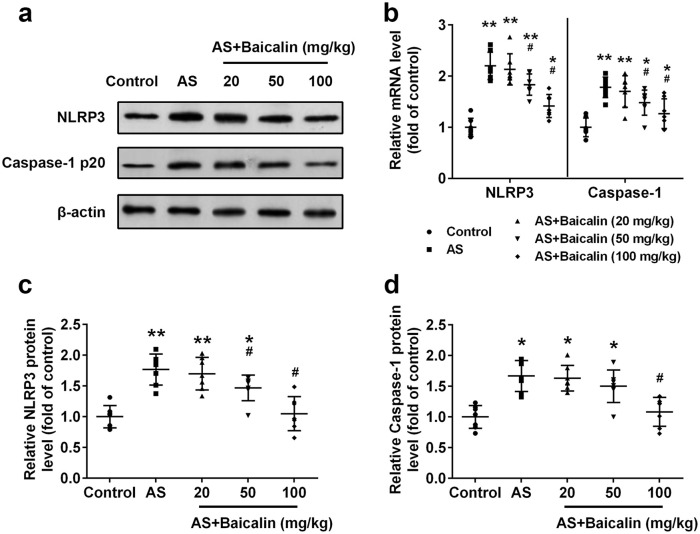
NLRP3 inflammasome has been implicated in atherosclerosis.21,22 We continued to evaluate the effects of baicalin on NLRP3 inflammasome activation in atherosclerosis mice. Significantly increased protein (Figure 4(a) and (c)) and mRNA (Figure 4(b)) levels of NLRP3 were detected in arteries of atherosclerosis mice. Baicalin suppressed NLRP3 expression. 20 mg/kg baicalin treatment did not affect NLRP3 expression while 50 and 100 mg/kg baicalin treatment significantly decreased both protein and mRNA level of NLRP3. Similarly, baicalin decreased protein level of caspase-1 p20 (Figure 4(a) and (d)) and mRNA level of caspase-1 (Figure 4(b)). Significantly decreased caspase-1 p20 level was detected in 100 mg/kg baicalin-treated atherosclerosis mice. 50 and 100 mg/kg baicalin treatment significantly decreased the caspase-1 mRNA level. Collectively, our data demonstrated that baicalin suppressed activation of NLRP3 inflammasome in atherosclerosis mice.
Figure 4.
Baicalin inhibited NLRP3 inflammasome activation in aortas: (a) Western blot analysis of NLRP3 and Caspase-1 p20, (b) relative mRNA levels of NLRP3 and caspase-1 were detected by qRT-PCR. Relative protein levels of NLRP3 (c), and caspase-1 (d) from western blot were quantified.
Data were shown as mean ± SD. n = 7 for each group. *p < 0.05, **p < 0.01 compared with the control group; #p < 0.05 compared with the AS group.
Baicalin reduced the expression of the adhesion molecules ICAM-1 and VCAM-1 in arteries
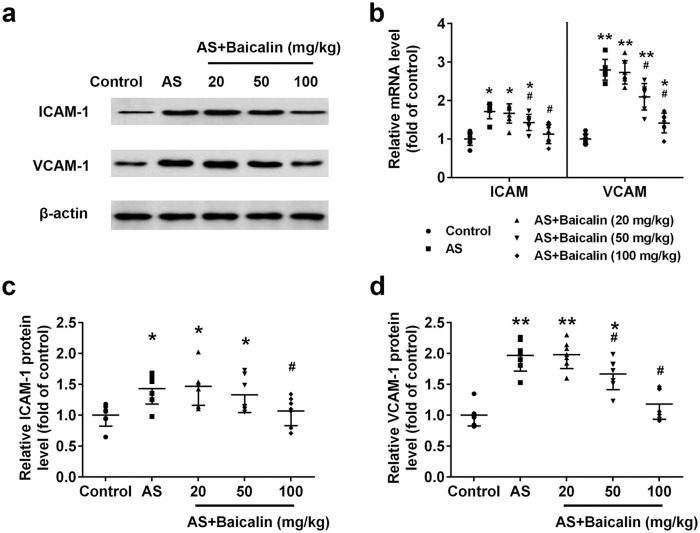
Elevated expression of cell adhesion molecules contributed to development of atherosclerosis.23 We evaluated whether baicalin treatment could affect the expression of adhesion molecules ICAM-1 and VCAM-1. Remarkably increased ICAM-1 protein (Figure 5(a) and (c)) as well as mRNA expression (Figure 5(b)) were detected in atherosclerosis of atherosclerosis mice. Baicalin decreased both protein and mRNA level of ICAM-1. 50 mg/kg baicalin treatment significantly decreased mRNA expression of ICAM-1 and 100 mg/kg baicalin significantly decreased both protein and mRNA level of ICAM-1. Similarly, baicalin prevented both protein (Figure 5(a) and (d)) and mRNA (Figure 5(b)) expression of VCAM-1. 50 and 100 mg/kg baicalin treatment significantly decreased both protein and mRNA level of VCAM-1.
Figure 5.
Baicalin reduced the expression of the adhesion molecules ICAM-1 and VCAM-1 in aortas: (a) Western blot analysis of ICAM-1 and VCAM-1, (b) relative mRNA levels of ICAM-1 and VCAM-1 were detected by qRT-PCR. Relative protein levels of ICAM-1 (c), and VCAM-1 (d) from western blot were quantified. n = 7 for each group.
Data were shown as mean ± SD. *p < 0.05, ** p < 0.01 compared with the control group; #p < 0.05 compared with the AS group.
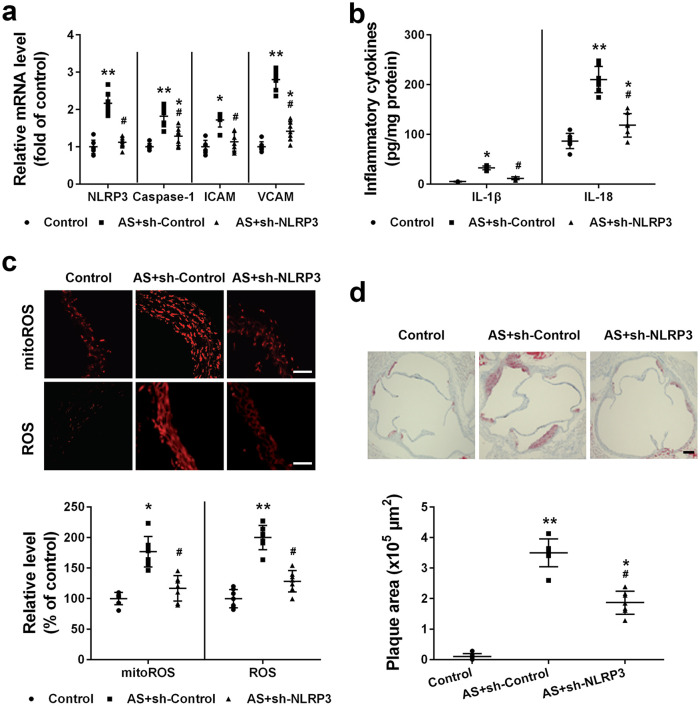
Silencing NLRP3 prevented the progression of atherosclerosis in atherosclerosis mice
Finally, we evaluated the effects of knocking down NLRP3 on atherosclerosis progression. Compared to control and atherosclerosis mice treated with lentivirus expressing control shRNA, atherosclerosis mice administrated with lentivirus expressing NLRP3 shRNA had significantly decreased NLRP3 mRNA level (Figure 6(a)). Correspondingly, silencing NLRP3 also resulted in significantly decreased mRNA level of caspase-1, ICAM, and VCAM (Figure 6(a)). Atherosclerosis mice had significantly increased IL-1β and IL-18 production while silencing NLRP3 resulted in significantly decreased IL-1β and IL-18 level (Figure 6(b)). Silencing NLRP3 also prevented ROS production as in atherosclerosis mice treated with lentivirus expressing NLRP3 shRNA, significantly decreased mitochondria ROS and total ROS were detected when compared to atherosclerosis mice (Figure 6(c)). Eventually, silencing NLRP3 resulted in significantly decreased plaque area in atherosclerosis mice (Figure 6(d)). Taken together, our data demonstrate that silencing NLRP3 attenuated atherosclerosis.
Figure 6.
Effects of NLRP3 silencing on the progression of atherosclerosis in ApoE−/− mice: (a) relative mRNA levels of NLRP3, caspase-1, ICAM-1, and VCAM-1 were detected by qRT-PCR, (b) IL-1β and IL-18 levels were measured by the ELISA assay, (c) mtROS and ROS levels were detected, and (d) atherosclerotic plaques area of aortic root was quantified. Scale bar = 50 μm.
Data were shown as mean ± SD. n = 7 for each group. *p < 0.05, **p < 0.01 compared with the control group; #p < 0.05 compared with the AS+sh-Control group. Mice of Control and AS+sh-Control group were treated with lentivirus expressing control shRNA, and mice of AS+sh-NLRP3 group were treated with lentivirus expressing NLRP3 shRNA.
Discussion
Using ApoE−/− atherosclerosis mice model, we demonstrated that baicalin prevented atherosclerosis development by inhibiting the activation of NLRP3 inflammasome. In baicalin-treated atherosclerosis mice, the level of pro-inflammatory cytokines IL-1β and IL-18 was significantly decreased. Baicalin also reduced the plaque area of atherosclerosis mice, and inhibited cell adhesion molecules expression and ROS production. More importantly, Baicalin decreased both NLRP3 and caspase-1 expression, and inhibited the activation of NLRP3 inflammasome. We further confirmed that silencing NLRP3 could reduce inflammation and oxidative stress, and attenuate atherosclerosis progression. Taken together, our data revealed that baicalin protected atherosclerosis by inhibiting NLRP3 inflammasome, suggesting baicalin and targeting NLPR3 could be used as potential therapeutic treatment for AS.
Inflammation is the key factor in atherosclerosis pathogenesis.24 Increasing experimental and clinical evidence have implicated inflammation in atherosclerosis. As a pro-inflammatory cytokine, IL-1 could induce the expression of cytokines, chemokines, and cell adhesion molecules, which resulted in inflammatory cells recruitment.25 ApoE−/− mice administrated with IL-1 antagonist has decreased atherosclerotic lesions size, indicating IL-1 could be a potential target for atherosclerosis therapy. Using ApoE−/− IL-1β−/− mice, Kirii et al.26 reported that IL-1β deficiency resulted in decreased severity of atherosclerosis in atherosclerosis mice. Significant increased plasma IL-18 was detected in atherosclerosis mice and blockage of IL-18 signaling reduced the inflammation and restored the plaque instability in atherosclerosis mice.27 In present study, we also observed elevated expression of IL-1β and IL-18 in atherosclerotic lesions of atherosclerosis mice.
The anti-inflammatory activities of baicalin have been widely reported. Ye et al.15 reported that baicalin suppressed lipopolysaccharides (LPS)-induced production of IL-1β and IL-18 in mononuclear phagocytes. Fu et al.28 described that baicalin inhibited the production of IL-1β, IL-6, TNF-α, and IL-18 in Haemophilus parasuis infection. In present study, the anti-inflammatory activity of baicalin was also detected. In baicalin-treated atherosclerosis mice, the level of IL-1β and IL-18 in atherosclerotic lesions was significantly decreased. IL-1β could induce the expression of cell adhesion molecules ICAM-1 and VCAM-1,29 it was not surprised that baicalin also repressed the expression of ICAM-1 and VCAM-1 in atherosclerotic lesions of atherosclerosis mice.
Both IL-1β and IL-18 need to be proteolytic cleaved for function, which was mediated by inflammasome.30 Recent studies have implicated that NLRP3 inflammasome was activated in atherosclerosis and played essential role in the development of atherosclerosis. Using bone marrow transplantation approach, Duewell et al.22 found that Ldlr−/− mice transplanted with bone marrow deficient in NLRP3 had reduced atherosclerotic lesion when compared to mice transplanted with wild type bone marrow. By analyzing transcriptional profile of atherosclerotic plaques, Paramel Varghese et al.31 found that the expressions of NLRP3, ASC, and caspase-1 were significantly increased in atherosclerotic plaques when compared to normal arteries. Our present study confirmed these findings as we detected significantly increased mRNA and protein level of NLRP3, ASC, caspase-1 in atherosclerotic lesion. The activation of NLRP3 inflammasome resulted in increased production of IL-1β and IL-18 in atherosclerotic lesion. Impressively, baicalin inhibited the expression and activation of NLRP3 inflammasome, thus inhibited the progress of atherosclerosis. The inhibition of NLRP3 inflammasome by baicalin has been described previously. Zhang et al.32 described that baicalin inhibited Mycobacterium-induced activation of NLRP3, which contributed to the increased bacterial killing in macrophage. Li et al.13 reported that baicalin inhibited ATP-induced NLRP3 activation in LPS-primed bone marrow-derived macrophages. Interestingly, in pig primary aortic vascular endothelial cells, Fu et al.33 found baicalin reduced Haemphilus parasius-induced inflammatory response and NLRP3 inflammasome activation, resulting in controlling H. parasuis infection. Therefore, the inhibitory activity of baicalin on activation of NLRP3 inflammasome contributes to the protective effects of baicalin on atherosclerosis.
Besides the anti-inflammatory activities, the anti-oxidative function of baicalin are also described. Chang et al.34 demonstrated that baicalin prevented UVB-induced production of ROS and protected keratinocytes against apoptosis. Kang et al.35 reported that baicalin alleviated oxidative stress-induced cell damage by inhibiting ROS production. Baicalin still functioned as anti-oxidative in present study. Administration of baicalin to atherosclerosis mice significantly decreased both mitochondria and total ROS production in atherosclerotic lesions. Interestingly, mitochondrial ROS has been shown to be a stimulus for NLRP3 activation.36,37 Therefore, the decreased ROS generation after baicalin treatment could contribute to the decreased NLRP3 inflammasome activation. Besides the anti-oxidative, anti-inflammation activities of baicalin, other mechanisms about baicalin-mediated amelioration of atherosclerosis have been described. For example, Wang et al.38 demonstrated that baicalin inhibited the development of atherosclerosis by increasing Wnt1 and inhibiting dickopf-related protein-1 expression. Liu et al.39 reported that baicalin induced regression of atherosclerosis by inhibiting dendritic cells maturation in bone marrow and infiltration into lesions. Baicalin also inactivated both NF-κB and p39 MAPK signaling pathways, resulting in relieving oxidative stress and inflammation, and alleviating atherosclerosis.17 Liao et al.40 demonstrated that baicalin up-regulated Foxp3 expression, promoted the number and function of Treg cells, resulting in amelioration of atherosclerotic lesions. Collectively, previous and present studies strongly suggested that baicalin could be used as a potential therapeutic reagent to treat atherosclerosis.
Conclusion
In the present study, we demonstrated that baicalin ameliorated the progress of atherosclerosis by inhibiting inflammation, ROS production, and NLRP3 inflammasome activation. IN present study we only evaluated the effects of baicalin on inflammation and oxidation using apolipoprotein E-deficient mice mode. Baicalin has diverse activities and have been shown to ameliorate atherosclerosis through several mechanisms. It is still worth exploring other potential underlying mechanisms.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Qingbo Su  https://orcid.org/0000-0002-6630-4176
https://orcid.org/0000-0002-6630-4176
References
- 1. Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med 1999; 340: 115–126. [DOI] [PubMed] [Google Scholar]
- 2. Lusis AJ. Atherosclerosis. Nature 2000; 407: 233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352: 1685–1695. [DOI] [PubMed] [Google Scholar]
- 4. Galea J, Armstrong J, Gadsdon P, et al. Interleukin-1 beta in coronary arteries of patients with ischemic heart disease. Arterioscler Thromb Vasc Biol 1996; 16: 1000–1006. [DOI] [PubMed] [Google Scholar]
- 5. Zheng F, Xing S, Gong Z, et al. NLRP3 inflammasomes show high expression in aorta of patients with atherosclerosis. Heart Lung Circ 2013; 22: 746–750. [DOI] [PubMed] [Google Scholar]
- 6. Ma S, Chen J, Feng J, et al. Melatonin ameliorates the progression of atherosclerosis via mitophagy activation and NLRP3 inflammasome inhibition. Oxid Med Cell Longev 2018; 2018: 9286458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zheng F, Xing S, Gong Z, et al. Silence of NLRP3 suppresses atherosclerosis and stabilizes plaques in apolipoprotein E-deficient mice. Mediators Inflamm 2014; 2014: 507208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee W, Ku SK, Bae JS. Anti-inflammatory effects of Baicalin, Baicalein, and Wogonin in vitro and in vivo. Inflammation 2015; 38: 110–125. [DOI] [PubMed] [Google Scholar]
- 9. Shi L, Hao Z, Zhang S, et al. Baicalein and baicalin alleviate acetaminophen-induced liver injury by activating Nrf2 antioxidative pathway: the involvement of ERK1/2 and PKC. Biochem Pharmacol 2018; 150: 9–23. [DOI] [PubMed] [Google Scholar]
- 10. Wang Y, Jia Y, Yang X, et al. A potential role of Baicalin to inhibit apoptosis and protect against acute liver and kidney injury in rat preeclampsia model. Biomed Pharmacother 2018; 108: 1546–1552. [DOI] [PubMed] [Google Scholar]
- 11. Ding Y, Dou J, Teng Z, et al. Antiviral activity of baicalin against influenza A (H1N1/H3N2) virus in cell culture and in mice and its inhibition of neuraminidase. Arch Virol 2014; 159: 3269–3278. [DOI] [PubMed] [Google Scholar]
- 12. Nayak MK, Agrawal AS, Bose S, et al. Antiviral activity of baicalin against influenza virus H1N1-pdm09 is due to modulation of NS1-mediated cellular innate immune responses. J Antimicrob Chemother 2014; 69: 1298–1310. [DOI] [PubMed] [Google Scholar]
- 13. Li CG, Yan L, Mai FY, et al. Baicalin inhibits NOD-like receptor family, pyrin containing domain 3 inflammasome activation in murine macrophages by augmenting protein kinase A signaling. Front Immunol 2017; 8: 1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang J, Zhang H, Deng X, et al. Baicalin protects AML-12 cells from lipotoxicity via the suppression of ER stress and TXNIP/NLRP3 inflammasome activation. Chem Biol Interact 2017; 278: 189–196. [DOI] [PubMed] [Google Scholar]
- 15. Ye C, Li S, Yao W, et al. The anti-inflammatory effects of baicalin through suppression of NLRP3 inflammasome pathway in LPS-challenged piglet mononuclear phagocytes. Innate Immun 2016; 22: 196–204. [DOI] [PubMed] [Google Scholar]
- 16. Meir KS, Leitersdorf E. Atherosclerosis in the apolipoprotein-E-deficient mouse: a decade of progress. Arterioscler Thromb Vasc Biol 2004; 24: 1006–1014. [DOI] [PubMed] [Google Scholar]
- 17. Wu Y, Wang F, Fan L, et al. Baicalin alleviates atherosclerosis by relieving oxidative stress and inflammatory responses via inactivating the NF-κB and p38 MAPK signaling pathways. Biomed Pharmacother 2018; 97: 1673–1679. [DOI] [PubMed] [Google Scholar]
- 18. Jin X, Liu MY, Zhang DF, et al. Baicalin mitigates cognitive impairment and protects neurons from microglia-mediated neuroinflammation via suppressing NLRP3 inflammasomes and TLR4/NF-κB signaling pathway. CNS Neuroscience & Therapeutics 2019; 25: 575–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alexander MR, Moehle CW, Johnson JL, et al. Genetic inactivation of IL-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice. J Clin Invest 2012; 122: 70–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mugge A. The role of reactive oxygen species in atherosclerosis. Z Kardiol 1998; 87: 851–864. [DOI] [PubMed] [Google Scholar]
- 21. Karasawa T, Takahashi M. Role of NLRP3 inflammasomes in atherosclerosis. J Atheroscler Thromb 2017; 24: 443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Duewell P, Kono H, Rayner KJ, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010; 464: 1357–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Davies MJ, Gordon JL, Gearing AJ, et al. The expression of the adhesion molecules ICAM-1, VCAM-1, PECAM, and E-selectin in human atherosclerosis. J Pathol 1993; 171: 223–229. [DOI] [PubMed] [Google Scholar]
- 24. Hansson GK, Robertson AK, Soderberg-Naucler C. Inflammation and atherosclerosis. Annu Rev Pathol 2006; 1: 297–329. [DOI] [PubMed] [Google Scholar]
- 25. Dinarello CA. Biologic basis for interleukin-1 in disease. Blood 1996; 87: 2095–2147. [PubMed] [Google Scholar]
- 26. Kirii H, Niwa T, Yamada Y, et al. Lack of interleukin-1beta decreases the severity of atherosclerosis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol 2003; 23: 656–660. [DOI] [PubMed] [Google Scholar]
- 27. Bhat OM, Kumar PU, Giridharan NV, et al. Interleukin-18-induced atherosclerosis involves CD36 and NF-kappaB crosstalk in Apo E−/− mice. J Cardiol 2015; 66: 28–35. [DOI] [PubMed] [Google Scholar]
- 28. Fu S, Liu H, Xu L, et al. Baicalin modulates NF-kappaB and NLRP3 inflammasome signaling in porcine aortic vascular endothelial cells infected by Haemophilus parasuis Causing Glasser’s disease. Sci Rep 2018; 8: 807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang X, Feuerstein GZ, Gu JL, et al. Interleukin-1 beta induces expression of adhesion molecules in human vascular smooth muscle cells and enhances adhesion of leukocytes to smooth muscle cells. Atherosclerosis 1995; 115: 89–98. [DOI] [PubMed] [Google Scholar]
- 30. Satoh T, Otsuka A, Contassot E, et al. The inflammasome and IL-1beta: implications for the treatment of inflammatory diseases. Immunotherapy 2015; 7: 243–254. [DOI] [PubMed] [Google Scholar]
- 31. Paramel Varghese G, Folkersen L, Strawbridge RJ, et al. NLRP3 inflammasome expression and activation in human atherosclerosis. J Am Heart Assoc 2016; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang Q, Sun J, Wang Y, et al. Antimycobacterial and anti-inflammatory mechanisms of baicalin via induced autophagy in macrophages infected with mycobacterium tuberculosis. Front Microbiol 2017; 8: 2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fu S, Liu H, Xu L, et al. Baicalin modulates NF-κB and NLRP3 inflammasome signaling in porcine aortic vascular endothelial cells Infected by Haemophilus parasuis Causing Glässer’s disease. Sci Rep 2018; 8: 807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chang WS, Lin EY, Hsu SW, et al. Baicalin scavenged reactive oxygen species and protected human keratinocytes against UVB-induced cytotoxicity. In Vivo 2016; 30: 605–610. [PubMed] [Google Scholar]
- 35. Kang KA, Zhang R, Piao MJ, et al. Baicalein inhibits oxidative stress-induced cellular damage via antioxidant effects. Toxicol Ind Health 2012; 28: 412–421. [DOI] [PubMed] [Google Scholar]
- 36. Hoyt LR, Randall MJ, Ather JL, et al. Mitochondrial ROS induced by chronic ethanol exposure promote hyper-activation of the NLRP3 inflammasome. Redox Biol 2017; 12: 883–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kim SR, Kim DI, Kim SH, et al. NLRP3 inflammasome activation by mitochondrial ROS in bronchial epithelial cells is required for allergic inflammation. Cell Death Dis 2014; 5: e1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang B, Liao PP, Liu LH, et al. Baicalin and geniposide inhibit the development of atherosclerosis by increasing Wnt1 and inhibiting dickkopf-related protein-1 expression. Journal of Geriatric Cardiology: JGC 2016; 13: 846–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu L, Liao P, Wang B, et al. Oral administration of baicalin and geniposide induces regression of atherosclerosis via inhibiting dendritic cells in ApoE-knockout mice. International Immunopharmacology 2014; 20: 197–204. [DOI] [PubMed] [Google Scholar]
- 40. Liao P, Liu L, Wang B, et al. Baicalin and geniposide attenuate atherosclerosis involving lipids regulation and immunoregulation in ApoE−/− mice. Eur J Pharmacol 2014; 740: 488–495. [DOI] [PubMed] [Google Scholar]