Abstract
Background: In patients with synchronous colorectal cancer (SCRC), understanding the underlying molecular behavior of such cases is mandatory for designing individualized therapy. The aim of this paper is to highlight the importance of transdisciplinary evaluation of the pre- and post-operative assessment of patients with SCRCs, from imaging to molecular investigations. Methods: Six patients with SCRCs presented with two carcinomas each. In addition to the microsatellite status (MSS), the epithelial mesenchymal transition was checked in each tumor using the biomarkers β-catenin and E-cadherin, same as KRAS and BRAF mutations. Results: In two of the patients, the second tumor was missed at endoscopy, but diagnosed by a subsequent computed-tomography-scan (CT-scan). From the six patients, a total of 11 adenocarcinomas (ADKs) and one squamous cell carcinoma (SCC) were analyzed. All the examined carcinomas were BRAF-wildtype microsatellite stable tumors with an epithelial histological subtype. In two of the six cases, KRAS gene status showed discordance between the two synchronous tumors, with mutations in the index tumors and wildtype status in the companion ones. Conclusions: Preoperative CT-scans can be useful for detection of synchronous tumors which may be missed by colonoscopy. Where synchronous tumors are identified, therapy should be based on the molecular profile of the indexed tumors.
Keywords: synchronous tumors, colorectal cancer, microsatellite status, KRAS, BRAF, E-cadherin
1. Introduction
Colorectal cancer (CRC) is a heterogenous tumor and the third most common type of malignancy worldwide [1,2,3]. It accounts for 10% of new cancer diagnoses each year, and it is the second leading cause of cancer deaths in males and the third leading cause of cancer death in females [2,3,4]. Despite the high incidence of CRC, it is one of the more curable malignancies if it is diagnosed in its early stages [5].
One challenge in diagnosing CRC is the possibility of a second, concurrent malignant lesion—a synchronous tumor—that is overlooked by sub-optimal diagnostic methods [6]. According to Warren and Gates, synchronous tumors are defined as two or more neoplasms in the same patient [7]. Diagnosis of a synchronous tumor must satisfy the following criteria: each tumor has to be malignant; each lesion has to be distinct; the likelihood that one tumor is the metastasis of the other must be excluded; and the synchronous lesions must be diagnosed at the same time or within six months from the first diagnosis [6,7]. The most pathologically advanced lesion is considered the index tumor, while the others are designated companion tumors [4].
The reported incidence of synchronous colorectal cancer (SCRC) ranges between 2–12% [4,8]. The development of SCRCs is presumed to be a stochastic or non-random process [9]. Exogenous factors such as diet, lifestyle, environment, and microbiome influence immune system cells and aberrant differentiation, and they can also contribute to the heterogeneity of the neoplastic process [10].
Surgical treatment is the primary therapeutic approach, followed by chemotherapy, which is mainly based on 5-Fluorouracil (5-FU) derivates [7,11,12]. In metastatic cases, targeted therapy focusing on the SCRCs with distinct molecular profiles requires molecular profile examination, knowing that BRAF and KRAS mutant tumors show resistance to anti-EGFR (epidermal growth factor receptor) therapy [13]. However, this has not yet become a therapeutic guideline.
After a review of the literature, the aim of our study was to propose better practices for the management of SCRCs, from diagnosis to molecular assessment of tumor heterogeneity.
2. Materials and Methods
2.1. Pre- and Intraoperative Assessments
This prospective study includes six individual patients diagnosed with SCRCs, each of them presenting with two carcinomas that benefited from surgical removal of the tumors between December 2018 and October 2020. The Ethical Approval of Clinical County Emergency Hospital of Targu-Mures, Romania, was obtained to include these patients in the prospective study. From each of the six patients, signed informed consent was obtained prior surgery for both permissions to perform the colectomy and the subsequent use of patient information in the publication of scientific data.
Preoperative diagnoses were based on colonoscopies and biopsies, except Case No. 6, where the patient was admitted to urgent care for abdominal pain and rectorrhagy, and a CT scan was the first to detect the synchronous lesions. In the cases with rectal tumors, MRI exams completed thoracic and abdominal CT-scans for preoperative staging (Figure 1, Figure 2, Figure 3, Figure 4 and Figure 5). For rectal cancer, a GE 1,5T MRI machine was used for pelvic MRI protocol (sequences used: Cor STIR, Ax T2—perpendicular on the tumor, Sag T2—parallel with the anal canal, Ax DWI, Ax T1). For the other tumors, thoraco-abdominal contrast-enhanced CT exams were performed using a Siemens Somaton 64 channels CT scanner with administration of intravenous iodinated contrast media (Optiray 350, Ioversol 74%, 350 mg I/mL) in a dose of 1 mL/kg of body weight with a flow rate ranging from 2 to 3 mL/s). In Case No. 3, due to technical issues, imaging data was not obtainable. Assessment of locoregional invasion was based on the following parameters: thickening of the intestinal wall, depth of tumor infiltration, and infiltration of the mesenteric fat (for rectal cancer). The diagnosis of an SCRC was based on the Warren and Gates criteria [7]. Additional colorectal lesions such polyps were also checked, along with LN status and the presence or absence of distant metastases. The imaging investigations sought to identify local and distant suspect LNs. The short axis diameter of each visible LN was registered. Malignancy suspicions were based on the size, shape, and heterogeneity of the examined LNs (Table 1).
Figure 1.
Case No. 1: synchronous cecal and rectal tumor. Circumferential thickening with inhomogenous enhancement and showing infiltration of the mesenteric fat at the level of the caecum (white arrow). Suspected lymph nodes (LNs) are visible on pelvic MRI scan, T2 weigthed FRFSE axial (A) and sagital (B) view. They are also marked on contrast-enhanced abdominal CT scan, venous phase, axial view (C). (D)—Based on imagistic aspects, the map of nodal stations is edited—adapted with permission from Yamamoto S et al. [14]—black circle—visible LN stations, without suspicious criteria; red circle- suspect LN stations. Table: imagistic-histopathological correlation of node stations. The two suspicious LN in 201 nodal station were negative by pathologic report. The perirectal tumor deposit was imagistically detected as suspected for LN metastasis.
Figure 2.
Case No. 2: synchronous cecal and rectal tumor. LNs can be seen on an abdominopelvic contrast-enhanced CT scan, venous phase, axial (A,B) and coronal view (C). No suspected LNs are detected. (D)—The map of LN stations—black circles emphasize LN stations, without suspicious features. Table: correlation of LN stations, based on imaging studies and pathological reports. Imaging did not detect any suspicious LNs that was confirmed by pathology report.
Figure 3.
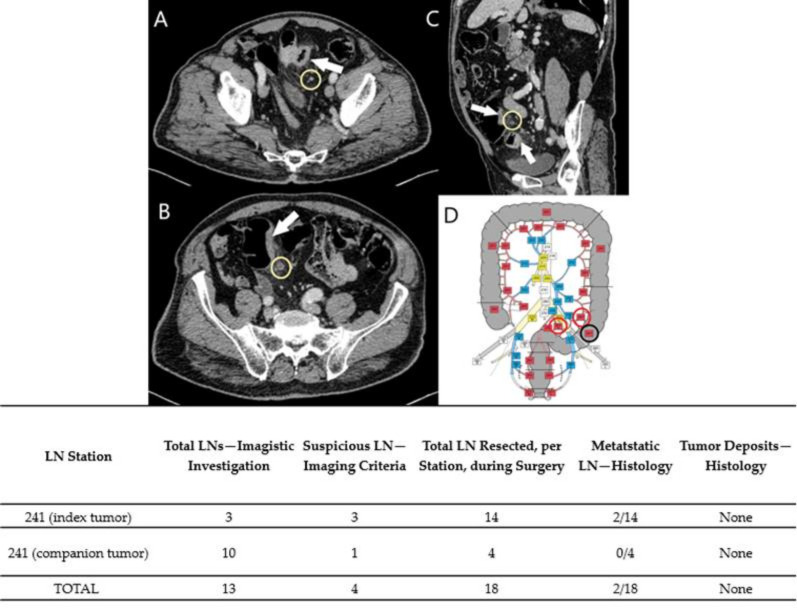
Case No. 4: synchronous sigmoid ADK and SCC of the anal canal. Circumferential thickening with inhomogeneous enhancement at the level of sigmoid, proves infiltration of the mesenteric fat (white arrow). Perisigmoid and inferior mesentery LN stations were seen on contrast-enhanced CT, venous phase, axial views (A,C). The tumor mass in the anal region shows heterogenous enhancement in the arterial phase (B), with no visible LNs. (D) A map of nodal stations involved was made: black circle—visible LN, without suspicious features; red circle—suspicious LN for malignancy. Table: map correlation showed that positive LN was successfully detected on CT scan.
Figure 4.
Case No. 5. Two synchronous sigmoid tumors. Parietal thickening of the colic wall with contrast-enhancement (white arrows), infiltration of pericolic fat. Association of regional LNs (yellow circle), some with suspicious criteria, seen on contrast-enhancement CT scan, venous-phase, axial-views (A,B) and sagittal view (C). A map of nodal stations was made: black circle: visible, non-suspicious LNs, red circle: suspicious LNs (D). Table: correlation of LNs stations - two of the four suspect LNs were positive, both of them found in the index tumor.
Figure 5.
Case No. 6. Synchronous sigmoid and cecal tumors (white arrow), seen on contrast-enhanced CT scan, venous phase, axial views (A,B) and coronal view (C), with infiltration of pericolic fat, regional LNs (yellow circle) and left fallopian tube metastasis (white arrow-head). Map of nodal stations shows nodal stations with suspect LNs (rec circle) and nodal stations with visible LNs, without suspicious criteria (D). Table: correlation of suspect nodal stations with LN metastasis.
Table 1.
Suspicious LNs based on imaging malignant features (size and morphologic characteristics).
| Malignant Characteristics of LN | Roundness | Heterogeneity | Irregular Contour |
|---|---|---|---|
| <5 mm | All three characteristics | ||
| 5–10 mm | Association of any two characteristics | ||
| >10 mm | Always suspicious | ||
Based on the imaging information adapted from Yamamoto et al. [14], with permission obtained from the authors, a map of abdominopelvic nodal stations was generated (Figure 1, Figure 2, Figure 3, Figure 4 and Figure 5).
All patients benefited from surgical excision of the SCRCs through total or partial colectomy with LN removal based on the imaged abdominopelvic map. All palpable locoregional LNs were prelevated according to current guidelines in order to correlate imaging and histopathological aspects. If suspect LNs were located outside the colectomy specimen anatomic area, the surgeon removed those LNs as well and sent them in separately for histopathological assessment.
2.2. Histopathological Assessment
Gross assessment of the surgical specimens was done according to the current guidelines and imaging map. All the palpable LNs were included for histological examination and comparison of imaging and microscopic features. Tumor deposits (TDs), number of LN metastases, and lymph node ratio (LNR) were included in the histopathological report for TNM staging.
2.3. Immunohistochemical and Molecular Examination
Immunohistochemical (IHC) stains were performed on formalin-fixed paraffin-embedded tissue (FFPE) blocks. Microsatellite status was checked based on the four IHC markers: MLH1 (clone ES05, Leica Microsystems GmbH; dilution 1:100, high retrieval solution—pH 10), MSH2 (clone 25D12, Leica Microsystems GmbH; dilution 1:50, high retrieval solution—pH 10), PMS2 (clone M0R4G, Leica Microsystems GmbH; dilution 1:50, high retrieval solution—pH 10), and MSH6 (clone PU29, Leica Microsystems GmbH; dilution 1:100, high retrieval solution—pH 10). Cases with nuclear positivity for all the four biomarkers were given microsatellite stable status (MSS).
For assessment of tumor molecular subtype, E-cadherin (clone NCH-38, Dako Agilent Technologies, Inc.; dilution 1:50, high retrieval solution—pH 10) and β-catenin (clone β-catenin-1, Dako Agilent Technologies, Inc.; dilution 1:150, high retrieval solution—pH 10) were used. For these markers, cellular localization was evaluated in both tumor cores and invasion fronts (tumor buds). Cases with membrane expression in both core and buds for E-cadherin and β-catenin were designated as epithelial subtypes; cases with loss of E-cadherin expression in the cell membrane with nuclear translocation of β-catenin were designated as mesenchymal subtypes.
To check the molecular status of the eight tumors, we extracted DNA from fresh tumor tissues using the Qiagen (Hilden-Germany) QIAmp DNA mini kit, according to manufacturer protocol. CE IVD kits (Quiagen therascreen KRAS and BRAF RGQ PCR kits) and the Rotor-Gene Q-MDx instrument from Qiagen were used for molecular analysis of KRAS and BRAF genes according to manufacturer protocols. In the case of KRAS genes, seven somatic mutations in codons 12 and 13 can be detected, while in the case of BRAF genes, five mutations in codon 600 of the gene were checked.
2.4. Follow-Up
One patient (Case No. 1) died three days after surgery. For the other cases, follow-up was performed between 6 and 18 months after surgery.
3. Results
3.1. Clinicopathological, Imaging and Histological Aspects
Cases 1 through 6 consisted of three males and three females with SCRCs, median age 70.16 years, and a total of 12 malignant tumors (two tumors per case). One patient (Case No. 2) declared that her sister had a rectal cancer, too. Additionally, in the same case, polyposis of the colon was found.
In two of the cases (Case Nos. 1 and 2), the preoperative CT-scan detected secondary tumors that were missed during endoscopy due to improper bowel preparation. In both cases, the tumors were in the cecum and were considered index tumors. In Case No. 6, the CT scan was the first performed and successfully detected both tumors and the right ovary metastasis. In one patient (Case No. 3), the imaging data was not available for pathological correlation, due to technical issues.
Five tumors were localized in the proximal colon, four in the distal colon, two in the rectum, and one at the level of the anal canal. All tumors were MSS ADKs (Figure 6), except the one from the anal canal (Case No. 4), which was an MSS squamous cell carcinoma (SCC) (Table 2). In Case No. 4, the index tumor was the ADK of the sigmoid colon, while the SCC with invasion limited to the muscularis propria was considered the companion tumor. Ten tumors were included in the epithelial subtype, with two (Case No. 4 and No. 5) hybrid subtypes: membrane expression for E-cadherin and β-catenin in the tumor core, with loss of E-cadherin and nuclear translocation of β-catenin in buds (Figure 7).
Figure 6.

The microsatellite stable status is immunohistochemically proved by nuclear expression of MLH1 (A), MSH2 (B), PMS2 (C) and MSH6 (D).
Table 2.
Clinicopathological characteristics of the patients.
| Case No. 1 | Case No. 2 | Case No. 3 | Case No. 4 | Case No. 5 | Case No. 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Male | Female | Female | Male | Male | Female | ||||||
| Age (years) | 63 | 60 | 73 | 79 | 78 | 68 | ||||||
| I | C | I | C | I | C | I | C | I | C | I | C | |
| Localization | Cecal | Inferior rectum | Cecal | Superior rectum | Ascending colon | Ascending colon | Sigma | Anal canal | Sigma | Sigma | Cecal | Descending colon |
| Method of detection | CT | E | CT | E | E | E | E | E | E | E | CT | CT |
| Histological type and grade | G2-ADK | G2-ADK with mucinous component | G2-ADK with mucinous component | G2-ADK | G2-ADK with mucinous component | G2-ADK with mucinous component | G2-ADK | NK SCC | G2-ADK with mucinous component | Sigilocelular carcinoma | ADK, micropapillary variant | ADK, micropapillary variant |
| Grade of tumor budding | Low | Low | Low | Low | High | Moderate | Low | Low | High | Low | High | High |
| No. Of positive LN/total LN resected | 0/16 and one perirectal tumor deposit | 0/15 | 1/17 = 0.05 | 1/27 = 0.03 | 2/14 = 0.14 | 12/46 = 0.26 | ||||||
| Distant metastasis | Peritoneal metastasis | None | None | None | None | Right ovary and fallopian tubes bilateral | ||||||
| TNM staging | pT3N1cM1a; pT3N1cM1a | pT2N0N0M0; pT1N0M0 |
pT4aN1M0; pT3N0M0 |
pT4N1M0; pT3N0M0 |
pT3N1bM0; pT4aN0M0 |
pT4bN2bM1; pT4bN0M1 |
||||||
| AJCC Stage | IVA | I | IIIB | IIIB | IIIB | IVB | ||||||
| Other colorectal lesions | - | Colic polyposis | - | rectal polyp | sigmoidian polyps | - | ||||||
| Surgical procedure | For index tumor—right hemicolectomy with ileotransverse anastomosis and ileal resection For companion tumor-rectal amputation |
For both tumors- total colectomy with ileorectal anastomosis | For both tumors—right hemicolectomy | For sigmoid tumor -rectosigmoid ectomy (Hartmann I) For anorectal junction tumor—excision |
For both tumors- left hemicolectomy | For both tumors—subtotal colectomy with segmentary ileal resection Histerectomy with bilateral anexectomy |
||||||
| Early postoperative complications | Metabolical imbalance—death at three days after surgery | None | None | None | None | None | ||||||
| Chemotherapy/Radiotherapy | - | Not indicated | Oxaliplatin perfusion + oral capecitabine | Oxaliplatin perfusion + oral capecitabine Delayed due to hip surgery and COVID period, initiated at 9 months after surgery |
Preoperative radiotherapy (50 Gy/25fr/42 days/pelvis + oral capecitabine | Oxaliplatin perfusion + oral capecitabine | ||||||
| Follow-up | - | No recurrence (18 months follow-up) | No recurrence (12 months follow-up) | No recurrence (at 12 months follow-up) | No recurrence (at 12 months follow-up) | Under chemotherapy | ||||||
I—index tumor, C—companion tumor, CT—computed tomography, E—endoscopy, ADK—adenocarcinoma, G2—moderately differentiated SCC—squamous cell carcinoma, NK—non-keratinized.
Figure 7.

Histological aspect, correlated with the molecular subtype determined with E-cadherin and β-catenin. All tumors expressed membranar E-cadherin and β-catenin in the tumor core (A–F). In the epithelial subtype, the membrane expression of E-cadherin (B) and beta-catenin (C) can be seen in both tumor core and invasion area (buds). The hybrid molecular subtype is characterized by membranar expression of E-cadherin and beta-catenin in the tumor core (E,F) with nuclear positivity of β-catenin in the buds (F).
Regarding the LNs, only one of the cases (Case No. 2) did not show TDs or LN metastases (Table 2). Positive LNs were considered suspect during the imaging investigation (Case Nos. 4–6) and TD was identified preoperatively in Case No. 1 as a LN metastasis. The LNs that showed no suspicious imaging features correlated with negative LNs (Figure 1, Figure 2 and Figure 3).
Intraoperatively, one peritoneal metastasis (ileal) and a perirectal tumoral deposit (TD) were detected in Case No. 1 (Table 2).
3.2. KRAS and BRAF Gene Profiles
All tumors presented wildtype (non-mutated) BRAF gene status. For the KRAS gene, no mutations were identified in Case Nos. 3, 4 and 6. In three cases, we found KRAS mutations (Case Nos. 1, 2 and 5). In Case Nos. 1 and 2, there was discordance in the status between the lesions in each patient. In both patients, the index tumor presented KRAS mutations (12Val and 12Cys) and the companion one showed wildtype KRAS. In Case No. 5, both tumors showed codon 12 KRAS mutation (12Ala in the index tumor and 12Val in the companion tumor) (Table 3).
Table 3.
Molecular subtyping, grade of tumor budding and status of KRAS and BRAF genes.
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synchronous tumors | I | C | I | C | I | C | I | C | I | C | I | C |
| Molecular subtype | E | E | E | E | E | E | H | E | H | E | E | E |
| Grade of tumor budding | G1 | G1 | G1 | G1 | G3 | G2 | G1 | G1 | G1 | G3 | G1 | G1 |
| Microsatellitar status | MSS | MSS | MSS | MSS | MSS | MSS | MSS | MSS | MSS | MSS | MSS | MSS |
| KRAS gene (codon 12 and 13) | Mutated 12Val | Wild-type | Mutated 12Cys | Wild-type | Wild-type | Wild-type | Wild-type | Wild-type | Mutated 12Ala | Mutated 12Val | Wild-type | Wild-type |
| BRAF gene (V600E) | Wild-type | Wild-type | Wild-type | Wild-type | Wild-type | Wild-type | Wild-type | Wild-type | Wild-type | Wild-type | Wild-type | Wild-type |
I—index tumor, C—companion tumor; E—epithelial, H—hybrid. the bold and italic: the mutated cases.
3.3. Postoperative Treatment and Follow-Up of the Patients
As the patient in Case No. 1 died and the patient in Case No. 2 was in an early stage, no chemotherapy was necessary for either. CT-scans and carcinoembryonic antigen (CEA) serum level monitoring showed that, in Case No. 2, there were no recurrences at 6-, 12-, and 18-month follow-ups.
In Case No. 3, the patient was diagnosed with stage IIIB adenocarcinomas of the ascending colon with MSS status; surgery was followed by adjuvant chemotherapy consisting of six 21-day cycles of intravenous oxaliplatin and oral capecitabine. The follow-up CT-scan showed no recurrence at 12 months post-surgery.
In Case No. 4, the index tumor that showed lymph node metastases was the adenocarcinoma of the sigmoid colon, and adjuvant chemotherapy was indicated using the same regimen as for Case No. 3. Due to patient’s underlying condition (anemia), COVID-era concerns, and other non-tumor-related comorbidities (hip surgery for coxarthrosis), chemotherapy was delayed, being initiated at 9 months after surgery, with no recurrence at 12-month follow-up.
In Case No. 5, both tumors having KRAS mutation, preoperatory radiotherapy (50 GY/23fr/42 days/pelvis) and oral capecitabine was initiated. No recurrence at 12-month follow-up.
Case No. 6 presented lymph nodes and systemic metastases being classified as stage IVB. Chemotherapy with oxaliplatin and oral capecitabine was indicated, same as cases No. 3 and 4. The patient is currently under chemotherapy.
4. Discussion
In the presented cases, the preoperative imaging assessment was done according to the Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma (JCCRC), developed and updated in 2019 by the Japanese Society of Cancer of the Colon and Rectum (JSCCR) guidelines [14]. This assessment proved extremely useful considering the strong concordance between imaging and histological assessments of the LNs.
Also, particularly valuable to the study was the complex evaluation of the cases by a transdisciplinary team using a variety of approaches including imaging and molecular assessment. We chose to evaluate the epithelial to mesenchymal transition (EMT), which is known to induce aggressive tumor behavior and higher rates of chemoresistance [14]. The hybrid phenotype, which is characterized by loss of E-cadherin and membrane-to-nuclear translocation of β-catenin in tumor buds [15] was observed in two of the cases.
Evaluating the MSS status was necessary for predictive purposes. It is known that over 10% of CRCs might present microsatellite instability (MSI) and do not benefit from 5-FU-based chemotherapy [12,13,16]. In this study, all of the cases were MSS tumors.
Examination of KRAS and BRAF mutation statuses is indicated in metastatic cases since wildtype KRAS and BRAF carcinomas are eligible for anti-EGFR therapy [13]. There are no guidelines regarding the discordant cases and their postoperative oncologic management [17,18,19,20]. Previous studies have reported that the presence of a mucinous component and BRAF mutation rate appear at a higher frequency in SCRCs than in solitary CRCs, but none of our cases had BRAF mutations. [13,19,20]. In the literature, the reported concordance rates for MSI, KRAS mutation, and BRAF mutation between tumors in the same patient varied from 9–30%, 11–40% and 14%, respectively [9,13,21]. In a study on 59 patients with SCRCs, Arakawa et al. found that, although low, there was significant correlation between the subtypes of MSI, BRAF mutation, and β-catenin between the lesions in each patient, while KRAS mutation had poor correlation [13]. Although one of our patients presented with a familial history of CRC the connection between genetic background and the incidence of SCRCs is not confirmed [17,18].
Although most of the SCRCs include two concomitant lesions, there have been reported cases with three, four, and even seven simultaneous tumors in the same patient [19,20]. Barium enemas and colonoscopies remain the primary modalities for detecting SCRCs [19,22]. While intra-operatory palpation of entire colon can help detect a concurrent CRC in cases of an acute onset of complete or partial obstruction, the sensitivity of SCRC diagnoses has a 50% rate of failure, especially in early staged tumors [6]. None of these methods are optimal, and tumors can be missed for various reasons, like poor bowel preparation and retained feces or inability to pass the endoscope through the lumen in cases of stenosing type tumors [6]. A complete colonoscopy implies visualization of the ileocecal valve and appendiceal orifice or terminal ileum [5].
Virtual colonoscopy and CT colonography are more accurate diagnostic methods, able to detect synchronous lesions in the colon proximal to an obstructing lesion; with a correct CRC staging rate of 81%, virtual colonoscopy and CT colonography demonstrate similar accuracy to conventional colonoscopies in identifying lesions greater than 1 cm [23]. Other useful methods are MRI colonography or CT colonography combined with PET [6]. Ultimately, a routine CT-scan could identify suspect concomitant lesions if they present with a narrowed intestinal lumen and visible pericolic LNs, as well as detect distant metastases. However, for preoperative staging of colorectal cancer, the gold standard remains the MRI [24].
Regarding LNs, size alone was not correlated with the presence of metastatic cells. Other morphological features, such as irregular borders or heterogenous signal intensity, display better sensitivity and specificity (85% and 97%, respectively) as indicators of positive LNs [25,26].
One issue that deserves attention is the presence of tumor deposits (TDs), which are usually identified under microscope by the pathologist. TDs are defined as nodules within the mesorectum that contain carcinoma cells without the obvious structure of a lymph node [27]. In one of our cases, TDs were observed on imaging exams and included in the group of suspect LNs (Case No. 1). This underscored the accuracy of CT-scan evaluations in such cases. Although LNs and deposits might be differentiated on MRI based on their relationship with vascular structures, confusion associated with the use of the CT-scan can easily be made [27].
The standard initial treatment for SCRCs is surgical resection. Some authors recommend a more extensive resection for patients with lesions in adjacent segments [11], while others suggest that a subtotal or a total colectomy should be performed even when synchronous lesions are in separate segments in order to reduce the possibility that other concomitant lesions may be overlooked leading to repeated surgery and an overall poorer prognosis [6]. One of our cases was treated with a total colectomy despite the tumors being situated in different segments of the bowel (caecum and rectum), but an association of colic polyposis may predispose the patient to new malignancies, making the more extensive procedure the better option. Of course, postoperative chemotherapy is still indicated for stages III and above [12,28,29].
In SCRCs with colon and rectal tumors, management becomes even more challenging, and decisions such as the extent of surgery or whether to initiate neoadjuvant RCT depend very much on the preoperative findings. None of our cases with rectal cancer were eligible for neoadjuvant RCT, and the stage I case was treated solely with a total colectomy with no sign of recurrence at 18 months.
A point of interest among these cases was the combination of an ADK in the sigmoid colon with a SCC of anal canal in Case No. 4. Anal carcinoma is a rare tumor, responsible for just 2–4% of reported incidents of lower digestive tract malignancies [29], and the synchronicity of this type of tumor appearing with a colorectal ADK is very rare [30,31]. This case underscored the importance of establishing the histologic type of concomitant tumors when planning the surgical resection and implementing postoperative RCT treatment [30].
The prognosis of patients with SCRCs is not well documented; various studies suggest the same, better, or worse outcomes than solitary CRC [9,20]. Life-long clinical follow-up is recommended, as local or distal recurrence is reported in 30–50% of the cases [32]. One of our patients, Case No. 1, died soon after the surgery due to metabolic imbalance, preventing us from following the progression of his disease and evaluating the efficacy of treatment decisions; however, no recurrence was noted in the other patients during their follow-up evaluations.
Our study had its limitations. Although the incidence of SCRCs is rising, it is still a rare pathology, explaining the small number of cases available for study. Additionally, due to lack of imaging studies, in Case No. 3 we failed to correlate the preoperative LNs status with the pathological findings. For more statistically significant results, further research for longer periods on larger cohorts of patients using a standardized preoperative evaluation scheme that includes colonoscopies and imaging studies in all cases and molecular analysis of each tumor is needed.
5. Conclusions
Due to the molecular heterogeneity of SCRCs, they should be carefully checked before any colorectal tumor surgery. CT and MRI scans can be successfully used to identify suspect LNs, but an in-house protocol should be standardized. This paper highlights, in the era of molecular pathology, the importance of molecular analysis in any SCRC cases.
Acknowledgments
The authors would like to express their sincere gratitude for Genoveva Rigmanyi for her technical help.
Author Contributions
P.S. drafted the article and contributed to the imaging methods and map correlation of lymph nodes; I.J. and L.B. contributed to the diagnosis and immunohistochemical assessment; Z.K. performed the molecular assessment; T.B. and Z.Z.F. performed the surgical interventions and contributed to the interpretation of the maps; I.S. contributed to the imaging examinations; S.G. designed the research and confer the final agreement for publication. All authors have equal contribution to the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Romanian National Authority of Research, ANCS-UEFISCDI, project no PCCF 20/2018.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Clinical County Emergency Hospital of Targu-Mures, Romania. (no. 17358/21 July 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The database can be obtained from the authors, upon request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nojadeh J.N., Behrouz Sharif S., Sakhinia E. Microsatellite instability in colorectal cancer. EXCLI J. 2018;17:159–168. doi: 10.17179/excli2017-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fitzmaurice C., Dicker D., Pain A., Hamavid H., Moradi-Lakeh M., Macintyre M.F., Allen C., Hansen G., Woodbrook R., Wolfe C., et al. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1:505–527. doi: 10.1001/jamaoncol.2015.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.García-Figueiras R., Baleato-González Sandra Padhani A.R., Luna-Alcalá A., Marhuenda A., Vilanova J.C., Osorio-Vázquez I., Martínez-de-Alegría A., Gómez-Caamaño A. Advanced Imaging Techniques in Evaluation of Colorectal Cancer. RadioGraphics. 2018;38:740–765. doi: 10.1148/rg.2018170044. [DOI] [PubMed] [Google Scholar]
- 4.Kato T., Alonso S., Muto Y., Noda H., Miyakura Y., Suzuki K., Tsujinaka S., Saito M., Perucho M., Rikiyama T. Clinical characteristics of synchronous colorectal cancers in Japan. World J. Surg. Oncol. 2016;14:272. doi: 10.1186/s12957-016-1027-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agnew J.L., Abbadessa B., Leitman I.M. Strategies to Evaluate Synchronous Carcinomas of the Colon and Rectum in Patients That Present for Emergent Surgery. Int. J. Surg. Oncol. 2013;2013:309439. doi: 10.1155/2013/309439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang J., Peng J.Y., Chen W. Synchronous Colorectal Cancers: A Review of Clinical Features, Diagnosis, Treatment, and Prognosis. Dig. Surg. 2011;28:379–385. doi: 10.1159/000334073. [DOI] [PubMed] [Google Scholar]
- 7.Warren S., Gates O. Multiple primary malignant tumors, a survey of the literature and statistical study. Am. J. Cancer. 1932;16:1358–1414. [Google Scholar]
- 8.Lee B.C., Yu C.S., Kim J., Lee J.L., Kim C.W., Yoon Y.S., Park I.J., Lim S.-B., Kim J.C. Clinicopathological features and surgical options for synchronous colorectal cancer. Medicine. 2017;96:e6224. doi: 10.1097/MD.0000000000006224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nosho K., Kure S., Irahara N., Shima K., Baba Y., Spiegelman D., Meyerhardt J.A., Giovannucci E.L., Fuchs C.S., Ogino S. A prospective cohort study shows unique epigenetic, genetic, and prognostic features of synchronous colorectal cancers. Gastroenterology. 2009;137:1609–1620.e203. doi: 10.1053/j.gastro.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molinari C., Marisi G., Passardi A., Matteucci L., De Maio G., Ulivi P. Heterogeneity in Colorectal Cancer: A Challenge for Personalized Medicine? Int. J. Mol. Sci. 2018;19:3733. doi: 10.3390/ijms19123733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Passman M.A., Pommier R.F., Vetto J.T. Synchronous colon primaries have the same prognosis as solitary colon cancers. Dis. Colon. Rectum. 1996;39:329–334. doi: 10.1007/BF02049477. [DOI] [PubMed] [Google Scholar]
- 12.Chan G.H.J., Chee C.E. Making sense of adjuvant chemotherapy in colorectal cancer. World J. Gastroenterol. Oncol. 2019;10:1183–1192. doi: 10.21037/jgo.2019.06.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arakawa K., Hata K., Nozawa H., Kawai K., Tanaka T., Nishikawa T., Sasaki K., Shuno Y., Kaneko M., Hiyoshi M., et al. Molecular Subtypes Are Frequently Discordant Between Lesions in Patients with Synchronous Colorectal Cancer: Molecular Analysis of 59 Patients. Anticancer Res. 2019;39:1425–1432. doi: 10.21873/anticanres.13258. [DOI] [PubMed] [Google Scholar]
- 14.Japanese Society for Cancer of the Colon and Rectum Japanese Classification of Colorectal, Appendiceal, and Anal Carcinoma: The 3d English Edition [Secondary Publication] J. Anus Rectum Colon. 2019;3:175–195. doi: 10.23922/jarc.2019-018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banias L., Jung I., Bara T., Fulop Z., Simu P., Simu I., Satala C., Gurzu S. Immunohistochemical-based molecular subtyping of colorectal carcinoma using maspin and markers of epithelial-mesenchymal transition. Oncol. Lett. 2020;19:1487–1495. doi: 10.3892/ol.2019.11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sargent D.J., Marsoni S., Monges G., Thibodeau S.N., Labianca R., Hamilton S.R., French A.J., Kabat B., Foster N., Torri V., et al. Defective Mismatch Repair as a Predictive Marker for Lack of Efficacy of Fluorouracil-Based Adjuvant Therapy in Colon Cancer. J. Clin. Oncol. 2010;28:3219–3226. doi: 10.1200/JCO.2009.27.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura T., Iwagaki H., Fuchimoto S., Hizuta A., Orita K. Synchronous colorectal carcinomas. Hepatogastroenterology. 1994;41:409–412. [PubMed] [Google Scholar]
- 18.Strashilov S., Yordanov A. A rare clinical case of synchronous colorectal cancer, affecting the transverse colon. AMJ. 2019;12:34–37. doi: 10.21767/AMJ.2018.3552. [DOI] [Google Scholar]
- 19.Kaibara N., Koga S., Jinnai D. Synchronous and metachronous malignancies of the colon and rectum in Japan with special reference to a coexisting early cancer. Cancer. 1984;54:1870–1874. doi: 10.1002/1097-0142(19841101)54:9<1870::AID-CNCR2820540917>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Lam A.K., Chan S.S., Leung M. Synchronous colorectal cancer: Clinical, pathological and molecular implications. World J. Gastroenterol. 2014;20:6815–6820. doi: 10.3748/wjg.v20.i22.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giannini R., Lupi C., Loupakis F., Servadio A., Cremolini C., Sensi E., Chiarugi M., Antoniotti C., Basolo F., Falcone A., et al. KRAS and BRAF genotyping of synchronous colorectal carcinomas. Oncol. Lett. 2014;7:1532–1536. doi: 10.3892/ol.2014.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeuchi H., Toda T., Nagasaki S., Kawano T., Minamisono Y., Maehara Y., Sugimachi K. Synchronous multiple colorectal adenocarcinomas. J. Surg. Oncol. 1997;64:304–307. doi: 10.1002/(SICI)1096-9098(199704)64:4<304::AID-JSO10>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 23.Morrin M.M., Farrell F.J., Raptopoulos V., McGee J.B., Bleday R., Kruskal J.B. Role of virtual computed tomographic colonography in patients with colorectal cancer and obstructing colorectal lesions. Dis. Colon. Rectum. 2000;43:303–311. doi: 10.1007/BF02258293. [DOI] [PubMed] [Google Scholar]
- 24.Bauer F. The importance of preoperative staging of rectal cancer usic multiparametric MRI. A systematic Review—Part 1. Chirurgia. 2016;111:379–392. doi: 10.21614/chirurgia.111.5.379. [DOI] [PubMed] [Google Scholar]
- 25.Märkl B., Schaller T., Kokot Y., Endhardt K., Kretsinger H., Hirschbühl K., Aumann G., Schenkirsch G. Lymph nod size as a simple prognostic factor in node negative colon cancer and an alternative thesis to stage migration. Am. J. Surg. 2016;212:775–780. doi: 10.1016/j.amjsurg.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 26.Brown G., Richards C.J., Bourne M.W., Newcombe R.G., Radcliffe A.G., Dallimore N.S., Williams J.T. Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolutionMR imaging with histopathologic comparison. Radiology. 2003;227:371–377. doi: 10.1148/radiol.2272011747. [DOI] [PubMed] [Google Scholar]
- 27.Lord A.G., D’Souza N., Shaw A., Day N., Brown G. The Current Status of Nodal Staging in Rectal Cancer. Curr. Colorectal Cancer Rep. 2019;15:143–148. doi: 10.1007/s11888-019-00441-3. [DOI] [Google Scholar]
- 28.Moertel C.G., Fleming T.R., Macdonald J.S., Haller D.G., Laurie J.A., Goodman P.J., Ungerleider J.S., Emerson W.A., Tormey D.C., Glick J.H., et al. Levamisole and Fluorouracil for Adjuvant Therapy of Resected Colon Carcinoma. N. Engl. J. Med. 1990;322:352–358. doi: 10.1056/NEJM199002083220602. [DOI] [PubMed] [Google Scholar]
- 29.Haller D.G., Tabernero J., Maroun J., de Braud F., Price T., Van Cutsem E., Hill M., Gilberg F., Rittweger K., Schmoll H.-J. Capecitabine Plus Oxaliplatin Compared with Fluorouracil and Folinic Acid As Adjuvant Therapy for Stage III Colon Cancer. J. Clin. Oncol. 2011;29:1465–1471. doi: 10.1200/JCO.2010.33.6297. [DOI] [PubMed] [Google Scholar]
- 30.ElGendy K., AlSuwailem M., Salem A. Synchronous anal squamous carcinoma and sigmoid adenocarcinoma. BMJ Case Rep. 2014;2014:bcr2014206364. doi: 10.1136/bcr-2014-206364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weston S.A., Wheeler W.E. Synchronous squamous carcinoma of the anus and adenocarcinoma of the rectum. West Va. Med. J. 1992;88:9–10. [PubMed] [Google Scholar]
- 32.Buechter K.J., Boustany C., Caillouette R., Cohn I., Jr. Surgical management of the acutely obstructed colon. A review of 127 cases. Am. J. Surg. 1988;156:163–168. doi: 10.1016/S0002-9610(88)80056-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The database can be obtained from the authors, upon request.