Abstract
BACKGROUND
Cancer stem-like cells are a major cause of resistance to therapy in patients with glioblastoma (GBM) as well as other cancers. Tumor cells are maintained in a stem-like proliferative state in large part through the Notch signaling pathway. The function of this pathway in turn depends on gamma secretase activity. Inhibition of this enzyme therefore inhibits the Notch pathway and tumor growth as measured by a reduction in the formation of brain tumor neurospheres in murine models. RO4929097 is an oral gamma secretase inhibitor.
OBJECTIVE
To estimate the 6-mo progression-free survival rate (PFS6) in patients with progressive GBM and to inhibit by 50% the generation of neurospheres in fresh tissue resected from patients treated with RO4929097.
METHODS
In this phase II and pharmacodynamic study, patients with recurrent GBM received RO4929097 in a study of 2 groups. Group A patients had unresectable disease and received drug in a standard phase II design. Group B patients had resectable disease and received drug before and after surgical resection. Endpoints included PFS6 and the inhibition of neurosphere formation in the resected tumor samples.
RESULTS
A total of 47 patients received treatment, 7 of whom had tumor resection. The PFS6 was 4%, and the inhibition of neurosphere formation occurred in 1 of 7 patient samples.
CONCLUSION
RO4929097 was inactive in recurrent GBM patients and demonstrated minimal inhibition of neurosphere formation in fresh tissue samples.
Keywords: Gamma secretase, Notch, Pharmacodynamic, Phase 2, Recurrent glioblastoma, RO4929097, Stem cell
Graphical Abstract
Graphical Abstract.
ABBREVIATIONS
- ABTC
Adult Brain Tumor Consortium
- CI
confidence interval
- CSC
cancer stem-like cell
- GBM
glioblastoma
- MRI
magnetic resonance imaging
- PFS
progression-free survival
- RANO
Response Assessment in Neuro-Oncology
- WHO
World Health Organization
Most patients with glioblastoma (GBM) suffer disease recurrence within 8 mo of diagnosis.1 Patients with recurrent GBM treated on phase 2 trials have a median survival of 25 wk and a 6 mo progression-free survival rate (PFS6) of 15%.2 Similar pooled data for patients with recurrent GBM showed a PFS6 of approximately 10% to 15%.3 With the exception of bevacizumab, the PFS6 has not improved with standard chemotherapy regimens or experimental therapies. Examples of causes of treatment resistance include insufficient target modulation, redundant pathways of signal transduction, selective pressure in favor of resistant tumor clones, hypoxic tumor environments, and inadequate drug delivery. New agents are needed to improve the PFS and overall survival (OS) and quality of life for these patients.
Cancer stem-like cells (CSCs) comprise a small subset of cells that are capable of self-renewal within a tumor. They typically divide slowly and are resistant to radiation and chemotherapy. Thus, CSCs represent a major cause of treatment resistance.4 Furthermore, CSCs promote tumor angiogenesis.5 Therefore, CSCs are an attractive target for therapy of GBM.
Proliferation of CSCs is driven by several developmental pathways including the Notch pathway. This pathway consists of 4 receptors (Notch 1-4) and 5 ligands (DLL 1, 3, and 4, and JAG 1 and 2).6,7 Activation of Notch signaling imparts a tumor growth advantage by keeping tumor cells in a stem cell-like proliferative state.8 Because Notch signaling has been associated with an oncogenic role in multiple cancers, the Notch pathway is a logical therapeutic target.8
Overexpression of Notch-3 appears to have a role in the development of certain primary brain tumors.9 Expression of Notch-1, DLL1, and Jagged-1 is critical for cell survival and proliferation of human glioma cell lines and primary human gliomas.10 Gamma secretase (γ-secretase) cleaves the Notch receptor to form an Notch intracellular domain (N-ICD), which then moves to the nucleus.7,11 N-ICD becomes part of a larger transcriptional complex that regulates the transcription of various target genes. Inhibition of Notch signaling by the γ-secretase inhibitor LLNIe CHO suppresses growth of cells with dysregulation of the Notch pathway.12 Furthermore, NOTCH pathway inhibition appears to deplete CD133+ glioma stem cells (GSCs) in culture through reduced proliferation and prolonged survival in nude mice bearing GBM xenografts.13 Thus, disruption of the Notch pathway by inhibition of γ-secretase is a logical treatment strategy for patients with GBM.
RO4929097 is a selective oral inhibitor of γ-secretase, producing inhibitory activity of Notch signaling in tumor cells.14 A phase I trial of RO4929097 determined the most common toxicities to be nausea, vomiting, diarrhea, fatigue, hypophosphatemia, and rash.15 The authors observed activity in 1 patient each with colorectal cancer with neuroendocrine features, sarcoma, and melanoma.15 RO4929097 has variable blood-brain barrier penetration (tissue concentrations 0.33-1.3 μmol/L) and target modulation in patients with newly diagnosed high-grade gliomas.16 That trial demonstrated intratumoral concentrations of the drug comparable with its IC50 values against human brain cancer cell lines.14,17 Target modulation included decreases in Notch intracellular domain expression by tumor cells and blood vessels as well as a decrease in the population of cancer-initiating cells.16
Based on these data, we conducted a phase II and pharmacodynamic study to assess the efficacy of RO4929097 against recurrent GBM and to conduct biomarker assays on freshly resected GBM samples from patients receiving this agent. The overall goal was to provide proof of principle that CSC-targeted drug therapy can cross the blood tumor barrier and alter the molecular and functional aspects of CSCs.
METHODS
Study Design
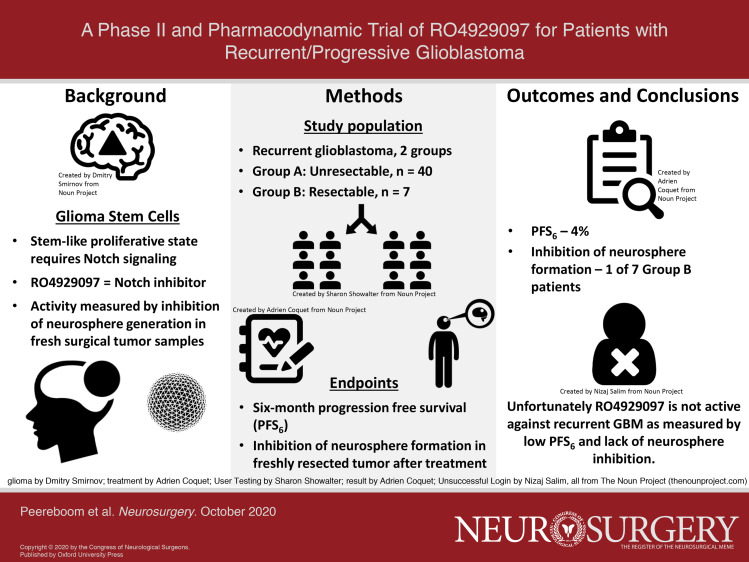
This study was a phase II, open label, nonrandomized trial of single agent RO4929097. The trial was conducted at 6 sites in the Adult Brain Tumor Consortium (ABTC). Two cohorts of adults with recurrent GBM enrolled concurrently (Figure 1). Group A included 40 patients with recurrent GBM not amenable to surgery or radiation therapy who received RO4929097, 20 mg days 1 to 3 every week (3 d on/4 d off weekly) with a primary endpoint of PFS6 on a standard phase II design.
FIGURE 1.
Schema.
Group B was an exploratory cohort of up to 20 patients for whom resection of a recurrent/progressive GBM was clinically indicated. Patients in this cohort received RO4929097 for 6 d prior to surgical resection of tumor and then resumed drug upon postoperative recovery. In addition to clinical endpoints such as PFS and OS, the primary endpoint for this cohort was the inhibition of neurosphere generation as well as proliferation of CD133+ neurosphere cells in the tumors of patients exposed to RO4929097. As a prespecified reference data set for assessment of the in Vivo effect of RO4929097, the neurosphere proliferation in resected tumor of Group B patients was modeled after and compared to those in a contemporaneous ABTC trial of vismodegib (NCT00980343) with identical entry criteria. In that trial, 20 patients with recurrent GBM who had not received any preoperative treatment underwent resection. Those specimens had the identical neurosphere assays performed in the same lab as the Group B patients in this trial. We therefore felt that those specimens provided meaningful reference data on the ability of untreated recurrent GBMs to generate neurospheres. Postoperatively, Group B patients received RO4929097 on the same schedule as Group A patients, and were followed for the same clinical parameters as the Group A patients. Accrual to Group B was to be suspended if Group A did not reach its primary endpoint.
Patient Eligibility
Eligible patients had histologically proven World Health Organization (WHO) grade IV glioma (2000 WHO classification) that had progressed or recurred by Response Assessment in Neuro-Oncology (RANO) criteria after radiation therapy with or without chemotherapy. Patients were allowed to have received an unlimited number of prior regimens or resections but no prior γ-secretase inhibitors. Additional standard eligibility criteria are detailed in Text, Supplemental Digital Content 1. Patients in Group B were required to have a tumor size >2.5 cm in diameter with the expectation that at least 50% of the enhancing portion would be resectable.
Treatment
Group A patients received RO4929097, 20 mg daily on days 1 to 3 every week, with a primary endpoint of PFS6 on a standard phase II design. Group B patients received RO4929097, 10 mg daily for 6 d preoperatively with up to 4 additional days of dosing permitted for unexpected delays in surgery. The dose scheduling for the preoperative therapy was devised empirically with the intent to reach a steady state serum concentration of drug and to minimize possible toxicity of giving more than a cumulative total of 60 mg, the total dose administered in the normal dose schedule (20 mg/d × 3 d). Patients received the last preoperative dose the evening before surgery. Within 30 d after surgery, patients resumed drug at the standard dose of 20 mg daily on days 1 to 3 every week until tumor progression. Fresh tumor from Group B patients was assayed for neurosphere generation.
Dose Modifications
Standard dose modifications were applied as detailed in Text, Supplemental Digital Content 2.
Response Assessment
Response to therapy was assessed by standard RANO criteria.18 Magnetic resonance imaging (MRI) was performed every 8 wk, just prior to every odd-numbered cycle of RO4929097. Complete and partial responses were to be confirmed by MRI prior to the next cycle, with the patient returning to the original schedule of having an MRI every odd-numbered cycle. This schedule was mandated to confirm the duration of response. Patients were classified as responders if they had a minimum duration of response for 4 wk at any time after the first cycle of RO4929097. MRI scans of patients showing tumor response were reviewed centrally by a neuroradiologist who assessed tumor size independently and computed the percent tumor regression. Adverse events were graded according to CTCAE version 4.0.
Neurosphere Generation Assays
Functional analysis of tumor stem cells was performed on fresh tumor samples from Group B patients after evaluation for viability and the relative presence of necrosis vs tumor. Specimens were cultured in serum-free medium in well-defined stem cell conditions and assessed for their ability to form neurospheres in serum-free medium as detailed in Text, Supplemental Digital Content 3.
Statistical Considerations
The study design was intended to determine if PFS6 could be improved from 10% to 25% in Group A patients. The PFS6 benchmark of 10% was derived from the combined results of phase II trials in patients with recurrent GBMs performed in a cooperative group (North American Brain Tumor Consortium) very similar to the ABTC.3 To detect this improvement with 90% power, 1-tailed with alpha = 0.1, 40 evaluable patients were required. The study would have been considered a success if 7 or more patients were progression-free at 6 mo. Secondary endpoints included radiographic response rate, toxicity and OS.
In Group B, the primary endpoint was suppression of neurosphere generation after treatment with RO4929097. A 50% reduction in neurosphere generation was considered significant. This degree of reduction was decided upon arbitrarily since no comparable data existed to describe a clinically important magnitude of reduction. Approximately 70% of tumor samples from pretreated GBM patients appear to generate neurospheres.19 The control arm from a contemporaneous randomized trial of vismodegib, a hedgehog inhibitor, in which 20 patients with recurrent GBM underwent resection before drug treatment with assays of neurosphere generation performed in the same laboratory as the samples from this trial served as a reference cohort. The importance of this reference cohort is that the tumor samples obtained for neurosphere generation in that trial were from patients who had not received any preoperative treatment. The trials were performed at approximately the same time, and the neurosphere formation assays were performed in the same laboratory for both studies. For this trial, the sample size of Group B was arbitrarily set at a maximum of 20 to enroll for as long as Group A was still accruing patients. Further accrual to Group B was to be suspended if Group A did not reach its primary endpoint. With 20 patients in each of the 2 surgery groups and doing a direct comparison with Fisher's exact test (alpha = 0.05, 1-sided), there would be 90% power to detect a reduction in development of neurospheres from 70% to 20%.
Time of PFS was calculated from date of treatment started to date of disease progression or censored at the time of analysis if patient was free of progressive disease. Time of OS was calculated from date of treatment started to date of death or censored at the time of analysis if patients were alive. Baseline patient and disease characteristics were summarized using standard descriptive summaries. Proportion of PFS6 was estimated using binomial distribution along with 95% CI. OS and PFS were estimated using the Kaplan-Meier method, and the CIs of median OS and PFS were constructed by the method of Brookmeyer-Crowley.20,21 All adverse events with relationship of possible, or probable, or definite attribution to RO4929097 were summarized using descriptive statistics. All analyses were performed using the SAS software, version 9.4 (SAS Institute).
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Cancer Therapy Evaluation Program, National Cancer Institute) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This trial was reviewed and approved by the scientific review committees and institutional review boards at each of the participating institutions. All patients provided written informed consent before any study procedures were performed.
RESULTS
Patient Characteristics
A total of 47 patients – 40 in Group A and 7 in Group B – were enrolled from 6 ABTC participating centers between February 2011 and May 2012. Manuscript submission was delayed by several changes in first authorship as well as data acquisition from the ongoing reference trial. All 47 patients were eligible and evaluable for toxicity, with 40 patients evaluable for response. Seven patients enrolled in Group B from 5 sites. Because Group B was an exploratory cohort, it was halted after the drug was determined to be ineffective based on clinical outcomes of patients in Group A. Table 1 summarizes the pretreatment patient and disease characteristics. All patients are off study.
TABLE 1.
Patient and Disease Characteristics
| Group A (n = 40) | Group B (n = 7) | Total (n = 47) | |
|---|---|---|---|
| Agea | 58 (35-75) | 56 (34-75) | 57 (34-74) |
| Gender maleb | 68% (27) | 71% (5) | 68% (32) |
| KPSa | 80 (60-90) | 80 (60-90) | 80 (60-90) |
| Mini Mental Score | 28 (17-30) | 27 (23-30) | 28 (17-30) |
| Number of relapsesa | 2 (1-8) | 1 (1-4) | 2 (1-8) |
| Number of prior surgeries | 1.5 (1-3) | 1 (1-2) | 1 (1-3) |
| Steroid use at enrollmentb | 35 (14) | 71 (5) | 40 (19) |
aMedian (range).
bPercent (N).
KPS = Karnofsky Performance Status.
Safety
Therapy was overall well tolerated with no unexpected toxicities and no treatment related grade 4 or 5 toxicities. In Group A, grade 3 treatment-related toxicities included 4 patients with hypophosphatemia and 1 each with elevated alanine aminotransferase, cognitive disturbance, fatigue, and skin infection. Group B patients had no grade 3 treatment-related toxicities. A full list of toxicities with a possible, probable, or definite relationship to RO4929097 is included in Table, Supplemental Digital Content 4.
Efficacy
The primary endpoint for Group A (PFS6) was 5% (95% CI: 0.06%-17%), well below the goal of 25%. Among all 47 patients, the PFS6 was 4% (0.05%-14%). One patient in Group A achieved a complete response but no other patients had a radiographic response. Efficacy data are summarized in Table 2, with the PFS illustrated in Figure 2.
TABLE 2.
Outcomes
| Group A (n = 40) | Group B (n = 7) | Total (n = 47) | |
|---|---|---|---|
| Cyclesa | 1 (1-4) | ||
| PFS6%c | 5 (0.1-17) | 0 | 4 (0-14) |
| PFS monthsc | 1.7 (1.2-1.8) | 1.6 (0.8-2.3) | 1.7 (1.2-1.8) |
| OS monthsc | 7.0 (5.4-9.1) | 6.9 (0.8-11) | 7.0 (5.4-9.1) |
| Responsesb | |||
| CR | 2% (1) | 2(1) | |
| PR | 0 | 0 | |
| SD | 6 (3) | ||
| PD | 81 (38) | ||
| Inevaluable | 11 (5) |
aMedian (range).
bBest response, percent (N).
cMedian (95% CI).
FIGURE 2.
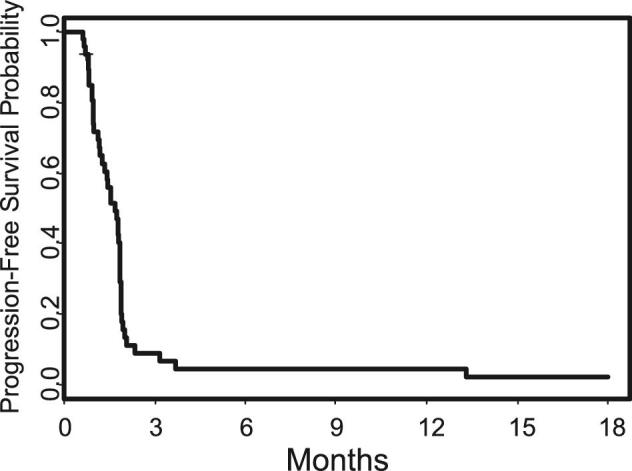
Progression-free survival.
Neurosphere Generation
Specimens from 6 of the 7 (86%) patients generated viable neurospheres and CD133+ neurospheres.
DISCUSSION
Key Results
This trial did not achieve its primary goal of producing a PFS6 of 25% with RO4929097 in patients with recurrent GBM. RO4929097 allowed the formation of neurospheres in 6 of 7 (86%) tumor samples. The number of samples was small as the trial was halted after Group A accrual was completed, with RO4929097 having failed to meet its efficacy endpoint. At that time, 7 patients had been accrued in Group B.
Despite treatment with RO4929097, tissue from 6 out of 7 patients generated neurospheres in Group B. This rate compares unfavorably to both arms of the sister study ABTC-0904, in which 11 of 19 (58%) of the untreated patients in Arm II and 3 of 20 (15%) of the patients pretreated with vismodegib (Arm I) generated viable CD133+ neurospheres (unpublished data).
Limitations
The failure of RO4929097 to inhibit neurosphere generation relative to controls from ABTC-0904 could have resulted from several causes. One possibility is that the drug did not reach the tumor. A clinical trial of RO4929097 in high-grade gliomas demonstrated tumor tissue concentrations of approximately 0.7 to 0.8 μmol/L in both enhancing and nonenhancing tissues.16 These values in the low micromolar range were similar to IC50 values against human glioma tumor-initiating cells.14,17 While the tumor tissue concentration data (0.7-0.8 μmol/L) were generated in newly diagnosed high-grade gliomas, the patients in this trial had recurrent disease after radiation therapy. Prior radiation therapy can decrease blood brain barrier permeability, thus decreasing drug delivery to the brain tumor.22
While additional biomarker assays were not performed in this trial due to the lack of neurosphere inhibition, RO4929097 appears to inhibit γ-secretase as suggested by reductions in the expression of Notch intracellular domain, the immediate product of γ-secretase-mediated cleavage of the inner domain of the Notch receptor.16,23 It is not clear, however, that the degree of γ-secretase inhibition was sufficient to affect GSC proliferation in Vivo in the setting of recurrent GBM. Alternatively, it is also possible that γ-secretase inhibition by itself is insufficient to inhibit GBM CSC proliferation. It is also possible that the preoperative regimen of 10 mg/d continuously was insufficient to reach the tumor. This dose was chosen so that patients would have drug exposure continuously leading up to the day before surgery. The risk of the trial schedule of 20 mg/d for 3 of 7 d was that unforeseen delays in surgery by 1 or more days could substantially decrease the amount of drug available for blood-brain barrier penetration or target inhibition or both.
Interpretation and Generalizability
These results are disappointing in light of xenograft data that demonstrate that Notch pathway blockade depletes stem-like cells in GBMs, suggesting that γ-secretase inhibitors may be useful as chemotherapeutic agents to target CSCs in high-grade gliomas.13 One of the strengths of this study is that the assays of neurosphere generation were performed in the same lab and at the same time as those from a contemporaneous control population. This study does have some weaknesses. The sample size of Arm B was small, as a result of the study being halted by design once the accrual goal for Arm A had been reached. The small number in group B was not surprising given the difficulty of enrolling surgical candidates for the studies in recurrent GBM. Biomarkers of Notch activity were not repeated for this report. This decision was made by the correlative laboratory once it was determined that the clinical and neurosphere endpoints were not reached. Confounding factors for the interpretation of these data include the small sample size, lack of randomization, and comparison of PFS6 to historic controls.
CONCLUSION
RO9729097 is not an active agent against recurrent GBM as assessed in this and other trials. This agent demonstrated minimal inhibition of neurosphere formation in fresh tissue samples. CSCs remain an attractive target for the therapy of GBM. As with most therapies in GBM as well as other cancers, CSC inhibition may contribute to clinical activity in the setting of combination therapies.24
Funding
This study was funded by the Adult Brain Tumor Consortium (1 U01 CA137433) and Scott Hamilton CARES Initiative.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplementary Material
Notes
This work was previously presented as a poster presentation at the European Association of Neuro-Oncology Annual Meeting, September 8, 2012, in Marseille, France and at the Society for NeuroOncology Annual Meeting, November 16, 2012, in Washington, District of Columbia.
Contributor Information
David M Peereboom, Burkhardt Brain Tumor Center, Cleveland Clinic, Cleveland, Ohio.
Xiaobu Ye, Department of Neurosurgery, Johns Hopkins University, Baltimore, Maryland.
Tom Mikkelsen, Department of Neurosurgery, Henry Ford Hospital, Detroit, Michigan.
Glenn J Lesser, Hematology and Oncology, Comprehensive Cancer Center of Wake Forest University, Winston-Salem, North Carolina.
Frank S Lieberman, Department of Neurology, Hillman Cancer Center of University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania.
H Ian Robins, Department of Human Oncology, University of Wisconsin Carbone Cancer Center, Madison, Wisconsin.
Manmeet S Ahluwalia, Burkhardt Brain Tumor Center, Cleveland Clinic, Cleveland, Ohio.
Andrew E Sloan, Department of Neurological Surgery, Seidman Cancer Center, University Hospitals & Case Comprehensive Cancer Center, Cleveland, Ohio.
Stuart A Grossman, The Sidney Kimmel Comprehensive Cancer Center, The Johns Hopkins University School of Medicine, Baltimore, Maryland.
Supplemental Digital Content 1. Text document. Standard eligibility criteria.
Supplemental Digital Content 2. Text document. Dose modifications.
Supplemental Digital Content 3. Text document. Correlative studies methods.
Supplemental Digital Content 4. Table. Toxicities. Toxicities possibly, probably, or definitely related to RO4929097.
COMMENT
The stem-like proliferative state of glioblastoma cells may be maintained by the Notch signaling pathway whose function depends on gamma secretase activity. This phase II, open label, non-randomized multicenter clinical trial tested the efficacy of RO4929097 (a gamma secretase inhibitor) in patients with recurrent glioblastoma. One group was treated with drug at recurrence, while others were treated with drug prior to resection. The trial was based on results of a Phase 0/1 trial of the same agent in newly diagnosed patients. The study endpoints were to measure progression-free survival at 6 months and inhibition of neurosphere formation in resected tumor samples. In the end, this was a negative study; data analysis suggested R04929097 did not affect tumor recurrence or significantly inhibit neurosphere formation. The reasons for this lack of efficacy are likely myriad; the study design, although innovative, was not pure and the number of patients small; the drug may have lacked penetration into the central nervous system or the ability to modulate glioblastoma cells or post-radiation hypoxia may have limited it's efficacy. Nonetheless, novel therapeutic approaches to glioblastoma are an important aspect of the crusade against this disease. Mature and well-equipped clinical and basic science groups such as this are the best avenue for progress. An age-old axiom is that we learn as much from our failures as our successes. Negative trials provide valuable benefit in directing increasingly scarce research money in new directions that may hold more promise. Although not effective in this study, the promise of gamma secretase inhibition remains viable; the goal is to find the right combination of anti-proliferative, anti-angiogenic, and anti-invasive therapies for glioblastoma.
Jigisha P. Thakkar
Kevin P. Barton
Vikram C. Prabhu
Maywood, Illinois
REFERENCES
- 1. Stupp R, Mason WP, van den Bent MJ et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996. [DOI] [PubMed] [Google Scholar]
- 2. Wong ET, Hess KR, Gleason MJ et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17(8):2572-2578. [DOI] [PubMed] [Google Scholar]
- 3. Lamborn KR, Yung WK, Chang SM et al. Progression-free survival: an important end point in evaluating therapy for recurrent high-grade gliomas. Neuro Oncol. 2008;10(2):162-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bao S, Wu Q, McLendon RE et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756-760. [DOI] [PubMed] [Google Scholar]
- 5. Bao S, Wu Q, Sathornsumetee S et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66(16):7843-7848. [DOI] [PubMed] [Google Scholar]
- 6. Bolos V, Grego-Bessa J, de la Pompa JL. Notch signaling in development and cancer. Endocr Rev. 2007;28(3):339-363. [DOI] [PubMed] [Google Scholar]
- 7. Bray SJ. Notch signalling: a simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7(9):678-689. [DOI] [PubMed] [Google Scholar]
- 8. Wang J, Sullenger BA, Rich JN. Notch signaling in cancer stem cells. Adv Exp Med Biol. 2012;727:174-185. [DOI] [PubMed] [Google Scholar]
- 9. Dang L, Fan X, Chaudhry A, Wang M, Gaiano N, Eberhart CG. Notch3 signaling initiates choroid plexus tumor formation. Oncogene. 2006;25(3):487-491. [DOI] [PubMed] [Google Scholar]
- 10. Purow BW, Haque RM, Noel MW et al. Expression of notch-1 and its ligands, delta-like-1 and jagged-1, is critical for glioma cell survival and proliferation. Cancer Res. 2005;65(6):2353-2363. [DOI] [PubMed] [Google Scholar]
- 11. Shi W, Harris AL. Notch signaling in breast cancer and tumor angiogenesis: cross-talk and therapeutic potentials. J Mammary Gland Biol Neoplasia. 2006;11(1):41-52. [DOI] [PubMed] [Google Scholar]
- 12. Kanamori M, Kawaguchi T, Nigro JM et al. Contribution of notch signaling activation to human glioblastoma multiforme. J Neurosurg. 2007;106(3):417-427. [DOI] [PubMed] [Google Scholar]
- 13. Fan X, Khaki L, Zhu TS et al. Notch pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells. 2010;28(1):5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Luistro L, He W, Smith M et al. Preclinical profile of a potent gamma-secretase inhibitor targeting notch signaling with in vivo efficacy and pharmacodynamic properties. Cancer Res. 2009;69(19):7672-7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tolcher AW, Messersmith WA, Mikulski SM et al. Phase I study of RO4929097, a gamma secretase inhibitor of notch signaling, in patients with refractory metastatic or locally advanced solid tumors. J Clin Oncol. 2012;30(19):2348-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu R, Shimizu F, Hovinga K et al. Molecular and clinical effects of notch inhibition in glioma patients: a phase 0/I trial. Clin Cancer Res. 2016;22(19):4786-4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saito N, Fu J, Zheng S et al. A high notch pathway activation predicts response to gamma secretase inhibitors in proneural subtype of glioma tumor-initiating cells. Stem Cells. 2014;32(1):301-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wen PY, Macdonald DR, Reardon DA et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963-1972. [DOI] [PubMed] [Google Scholar]
- 19. Laks DR, Masterman-Smith M, Visnyei K et al. Neurosphere formation is an independent predictor of clinical outcome in malignant glioma. Stem Cells. 2009;27(4):980-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assoc. 1958;53(282):457-481. [Google Scholar]
- 21. Brookmeyer R, Crowley J. A confidence-interval for the median survival-time. Biometrics. 1982;38(1):29-41. [Google Scholar]
- 22. Remsen LG, McCormick CI, Sexton G, Pearse HD, Garcia R, Neuwelt EA. Decreased delivery and acute toxicity of cranial irradiation and chemotherapy given with osmotic blood-brain barrier disruption in a rodent model: the issue of sequence. Clin Cancer Res. 1995;1(7):731-739. [PubMed] [Google Scholar]
- 23. Gordon WR, Vardar-Ulu D, Histen G, Sanchez-Irizarry C, Aster JC, Blacklow SC. Structural basis for autoinhibition of notch. Nat Struct Mol Biol. 2007;14(4):295-300. [DOI] [PubMed] [Google Scholar]
- 24. Dantas-Barbosa C, Bergthold G, Daudigeos-Dubus E et al. Inhibition of the Notch pathway using gamma-secretase inhibitor RO4929097 has limited antitumor activity in established glial tumors. Anticancer Drugs. 2015;26(3):272-283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.