Abstract
Necrotizing enterocolitis (NEC) is an intestinal inflammatory disease with high morbidity and mortality that affects almost exclusively premature infants. Breast milk feeding is known to substantially lower NEC incidence, and specific components of breast milk, such as immunoglobulin (Ig) A, have been identified as mediating this protective effect. On the other hand, accumulating evidence suggests dysbiosis of the neonatal intestinal microbiome contributes to NEC pathogenesis. In mice, neonates can inherit a dysbiotic microbiome from dams that experience stress during pregnancy. Here we show that while prenatal stress lowers fecal IgA levels in pregnant mice, it does not result in lower levels of IgA in the breast milk. Nevertheless, coating of female, but not male, offspring microbiota by IgA is increased by prenatal stress. Accordingly, prenatal stress was found to alter the bacterial community composition in female neonates but not male neonates. Furthermore, female, but not male, offspring of prenatally stressed mothers exhibited more severe colonic tissue damage in a NEC-like injury model compared to offspring with non-stressed mothers. Our results point to prenatal stress as a possible novel risk factor for NEC and potentially reveal new avenues in NEC prevention and therapy.
Keywords: Stress, Corticosterone, Microbiome, IgA, Necrotizing enterocolitis
1. Introduction
Psychological stress is defined as emotional tension or strain resulting from adverse circumstances (Butler, 1993). Examples of stress that affect pregnant women are extremes of reproductive age, emotional and physical abuse, financial hardship, and/or lack of quality prenatal care (Coussons-Read, 2013). During pregnancy there is a 4-fold increase in the stress hormone cortisol, which is needed for fetal growth and development (Harville, 2007). The amount of circulating cortisol that can cross the placenta is tightly regulated (Benediktsson, 1997). When pregnant mothers are exposed to external stressors these protective mechanisms may not have the capacity to regulate the fetal environment, resulting in exposure of the intrauterine environment and developing fetus to high levels of stress hormones. This excessive cortisol exposure results in reduced blood flow, and therefore reduced delivery of oxygen and vital nutrients, to the fetus (Guardino, 2016). Intriguingly, maternal cortisol levels are inversely proportional to gestational age and birth weight (Wilson and Thayer, 2017).
In a murine model of psychological stress, we have shown that mouse pups born to dams that were subjected to restraint stress during pregnancy have increased levels of serum corticosterone and decreased intestinal epithelial surface area (Shah, 2019). The effects of gestational stress on other aspects of the neonatal immune system are not fully known. Newborn immune development is shaped by environmental and maternal factors, such as microbial colonization and breast milk components. Seminal work from Warner et al. demonstrated that at birth, infants that go on to develop the acute gastrointestinal inflammatory disease necrotizing enterocolitis (NEC) have a relative increase in abundance of Gammaproteobacteria and a decrease in Negativicutes (Warner, 2016), underscoring the contribution of dysbiosis in NEC pathogenesis. On the other hand, immunoglobulin (Ig) A in the maternal breast milk is associated with protection from NEC (Gopalakrishna, 2019). Therefore, not only do postnatal factors influence the susceptibility of infants to NEC, but prenatal factors such as maternal stress merit attention as potential contributors to disease risk as well. Better understanding of the maternal-to-fetal interface is needed in developing therapeutic and preventative strategies for these at-risk infants.
Colonization of commensal bacteria is critical for proper early immunity, including epithelial barrier function. The development and maintenance of protective mechanisms along epithelial surfaces are needed to guard against injury from external pathogens. The gut represents the largest interface between the external and internal environments, with a dysfunctional epithelial barrier allowing for invasion of harmful microorganisms, resulting in sepsis (Arrieta et al., 2015; Azad, 2013). IgA is a major component of intestinal barrier defenses. After secretion by plasma cells in the intestinal lamina propria, IgA is transcytosed across the epithelium by binding to the polymeric immunoglobulin receptor (pIgR) and is finally released into the intestinal lumen, where it facilitates host/microbial symbiosis by neutralizing not only harmful luminal pathogens but also commensal microorganisms and microbial byproducts (Bridgman, 2016; McElroy and Weitkamp, 2011). This dynamic process of microbial colonization and IgA transport and function is needed for early life immune protection. In the present study we hypothesized that IgA-microbiota interactions are disrupted in mice offspring of stressed mothers, and that psychological stress during pregnancy predisposes these offspring to NEC-like injury.
2. Methods
2.1. Experimental animals
Timed pregnant C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME) to arrive in our animal facility on embryonic day 5 (E5). Dams were housed in specific pathogen free conditions under a 12 h light/dark cycle with standard chow diet and ad libitum access to drinking water. Gastric contents and fecal samples from one male and one female per litter (n = 8 per group) were used for offspring analyses. All experiments were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee.
2.2. Restraint stress model
On E7, pregnant dams were randomly assigned to either a control non-stressed group (n = 14) or a restraint stress group (n = 12). Mice assigned to the stress group were placed in a 50 mL conical tube with air holes for 2 h a day, twice a day through E20 and were exposed to bright light during the restraint stress procedure. Stressing occurred at the same time each day, and stressed dams were handled by the same investigator throughout the study. Control dams were left undisturbed in their cages.
2.3. Colonic spheroid and Transwell monolayer culture
Primary colonic epithelial stem cells from a 14-week-old C57BL/6J female mouse were isolated, grown, and maintained as spheroids as previously described (Miyoshi, Nature Protocols, 2013). Briefly, mouse colon was harvested, washed with PBS, minced, and digested in a 2 mg/mL solution of collagenase type 1 (Gibco) for 50 min at 37 °C. Minced tissue in this solution was then filtered through a 70 μm cell strainer and filtered cells were centrifuged at 930 RPM for 5 min. Pelleted cells were then resuspended in Matrigel (BD Biosciences), plated onto a 24-well plate, and cultured in 50% L-WRN (L cells expressing Wnt3a, Rspondin3, and Noggin) conditioned medium. The L-WRN medium was supplemented with 10 μM of the Rho-associated protein kinase (ROCK) inhibitor Y27632 and 10 μM of the transforming growth factor (TGF)-β type I receptor inhibitor SB431542, both from R&D Systems (Minneapolis, MN, USA). L-WRN medium was replaced every 2 days, and spheroids were passaged every 3 days.
Epithelial cell monolayers from these spheroids were generated as previously described (Moon, 2014). Briefly, 3-day-old spheroids were washed in a solution of 0.5 mM EDTA in PBS, dissociated into single cells by incubation in 0.05% trypsin/0.5 mM EDTA solution (Gibco) for 3.5 min at 37 °C, washed in DMEM/F12 media (Sigma, St. Louis, MO, USA) containing 10% FBS (Atlanta Biologicals, Flowery Branch, GA, USA), and filtered through a 40 μm cell strainer. Pelleted cells were then resuspended in 5% L-WRN conditioned medium supplemented with 10 μM Y27632 inhibitor and counted. 50% L-WRN medium was diluted to 5% using Advanced DMEM/F12 (Gibco) supplemented with 20% FBS (Atlanta Biologicals), 100 U/mL of penicillin, 100 μg/mL of streptomycin, and 2 mM L-glutamine. Transwell inserts (Corning) were coated with Matrigel diluted 1:30 in PBS. After incubation at 37 °C for 20–30 min, the Matrigel/PBS solution was removed and the cells immediately seeded in the apical Transwell compartment at a density of 100,000 cells per insert. This was considered day 0.
2.4. Cell treatments and IgA transcytosis assay
Cells in Transwell inserts were given fresh 5% L-WRN medium supplemented with 1 μg/mL lipopolysaccharide (LPS), 10 μM N-[N-(3,5-Difluorophenacetyl-L-alanyl)]-S-phenylglycine t-Butyl Ester (DAPT) γ-secretase inhibitor (Millipore, Burlington, MA, USA), and 10 μM Y27632 inhibitor on days 1 and 2 of the monolayer culture. Some monolayers were also treated with the indicated concentration of corticosterone (Sigma) on days 1 and 2. Concentrations were chosen based on previously reported physiological concentrations of corticosterone in the mouse gut (Cima, 2004). All treatments on days 1 and 2 were administered in both the basolateral and apical compartments of the Transwell insert.
IgA transcytosis assays were performed on day 3, as previously described (Moon, 2014). Briefly, monolayers were removed from their treatment conditions and switched to fresh 5% L-WRN medium with 10 μM Y27632 inhibitor. 5 μg/mL IgA (BD Pharmingen) was added to the basolateral compartment. After incubating for 6 h at 37 °C, apical supernatants were removed for IgA ELISA and RNA was collected from the cells (described below).
2.5. ELISA
Mouse fecal and gastric contents samples were processed for ELISA as we have previously described (Culbreath, 2015). IgA levels in day 3 apical supernatants, mouse fecal samples, and mouse gastric contents were measured by ELISA, as we have previously described (Culbreath, 2015).
Corticosterone in sera from pregnant dams (n = 6 control and 5 stressed) was measured with the Corticosterone ELISA Kit from Enzo (Farmingdale, NY, USA).
2.6. Gene expression analysis
Immediately following collection of apical supernatants on day 3, cells in Transwell inserts were lysed in TRIzol Reagent (Invitrogen) and RNA was isolated according to the manufacturer’s instructions. Complementary DNA synthesis was performed using 200 ng of RNA and the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Quantitative polymerase chain reactions were performed with TaqMan Fast Advanced Master Mix (Applied Biosystems). Both reverse transcription and qPCR were performed in a QuantStudio 3 thermal cycler (ThermoFisher, Waltham, MA, USA). Expression levels for each sample were determined in duplicate and normalized to expression of glyceraldehyde 3-phosphate dehydrogenase (Gapdh) by the ΔΔCT method. The primers used were: Gapdh, assay ID Mm99999915_g1; and Pigr, assay ID Mm00465049_m1, both from ThermoFisher.
2.7. Immunostainng
Epithelial cell monolayers were created as described above and seeded in Lab-Tek II 8-well glass chamber slides (ThermoFisher) at a density of 100,000 cells per well. Cells were treated on days 1 and 2 as described above. On day 3, cells were fixed in 2% paraformaldehyde on ice for 30 min. The cells were then washed three times in PBST (PBS with 0.1% Tween), permeabilized with 0.5% Triton-X100 for 20 min, washed again three times with PBST, and blocked with 2% bovine serum albumin in PBST for 1 h. Cells were then incubated with rat anti-mouse Pigr primary antibody (Abcam, Cambridge, MA, USA), diluted to 10 μg/mL in blocking buffer, at 4 °C overnight. The next day, slides were washed three times with PBST and incubated with Alexa Fluor 488-conjugated goat anti-rat IgG secondary antibody (Abcam), diluted 1:200, for 1 h at room temperature. Slides were then washed twice with PBS and stained with DAPI ((4′, 6-diamidino-2-phenylindole, dihydrochloride), Invitrogen) for 3 min in order to visualize nuclei. After two more washes in PBS, the walls of the chamber slide were detached, a drop of ProLong Glass Antifade Mountant (Invitrogen) was applied to each monolayer, and a coverslip was added to the slide. Slides were visualized with a Nikon (Tokyo, Japan) Eclipse TE2000-U microscope.
2.8. Bacterial flow cytometry
Mouse fecal pellets weighing ~5 mg wet weight, frozen at −80 °C immediately after collection, were resuspended in 1 mL of sterile PBS by vortexing for 5 min, placed on ice, and large debris allowed to settle by gravity for 10 min. The resulting supernatant was filtered through a 70 μm sterile nylon cell strainer into new sterile tubes. Tubes were centrifuged at 10,000g for 5 min to pellet bacteria. After removal of supernatant, bacteria were resuspended in 500 μl blocking buffer (PBS with 5% heat inactivated goat serum) containing a 1:300 dilution of SYTO BC (Life Technologies) and incubated on ice at 4 °C for 15 min in the dark. After this incubation, 50 μl of sample was transferred to a new, sterile tube, centrifuged at 10,000g for 5 min, and supernatant discarded. Bacteria were resuspended in 50 μl staining buffer (PBS with 1% bovine serum albumin) containing a 1:40 dilution of goat anti-mouse IgA conjugated to phycoerythrin (Southern Biotech) and incubated on ice at 4 °C for 20 min in the dark. After 2 washes with ice cold staining buffer, bacteria were resuspended in 200 μl staining buffer and analyzed using a LSR II instrument (BD Biosciences).
2.9. 16S rRNA gene sequencing and bioinformatics analysis
DNA was isolated from fecal pellets using the ZR Fecal DNA MiniPrep Kit (Zymo Research, Irvine, CA, USA). PCR, sequencing, and bioinformatics analysis were performed as we have described previously (Brawner, 2017).
2.10. Chemically-induced NEC-like injury model
Colitis was induced in 2-week-old pups born from control (n = 13 males and 22 females) and stressed (n = 21 males and 14 females) dams as previously described (MohanKumar, 2012; MohanKumar, 2016; Namachivayam, 2017). Briefly, mice were anesthetized in an isoflurane chamber and subsequently injected rectally with trinitrobenzene sulfonic acid (TNBS) using a 3.5 gauge French catheter. The TNBS was dissolved 30% weight/volume in ethanol and administered at a dose of 50 mg/kg body weight. After 24 h, mice were sacrificed by CO2 inhalation and distal colons were harvested for histological analysis.
2.11. Histology
Colon sections were stained with hematoxylin and eosin (H&E) and scored for injury by a blinded investigator. Intestinal injury was graded by the following scoring system: 0-no injury, 1-mild separation of the lamina propria from the muscularis mucosa, 2-moderate separation, 3-severe separation and/or edema in submucosa, 4-transmural injury.
2.12. Statistical analysis
Normality of data was determined using the D’Agostino-Pearson test or the Shapiro-Wilk test when the n was insufficient for D’Agostino-Pearson. For data with a normal distribution, statistical significance between two groups was determined by two-tailed unpaired Student’s t test or by a paired t test as indicated. Comparison of more than two groups was performed with one-way ANOVA followed by Tukey’s multiple comparisons test. For non-parametric data, statistical significance between two groups was determined by two-tailed Mann-Whitney U test. Calculations were performed using GraphPad Prism software version 8.4.2. A P value of < 0.05 was considered statistically significant.
3. Results
3.1. Prenatal stress disrupts increases in fecal IgA later in pregnancy without corticosterone affecting IgA transcytosis
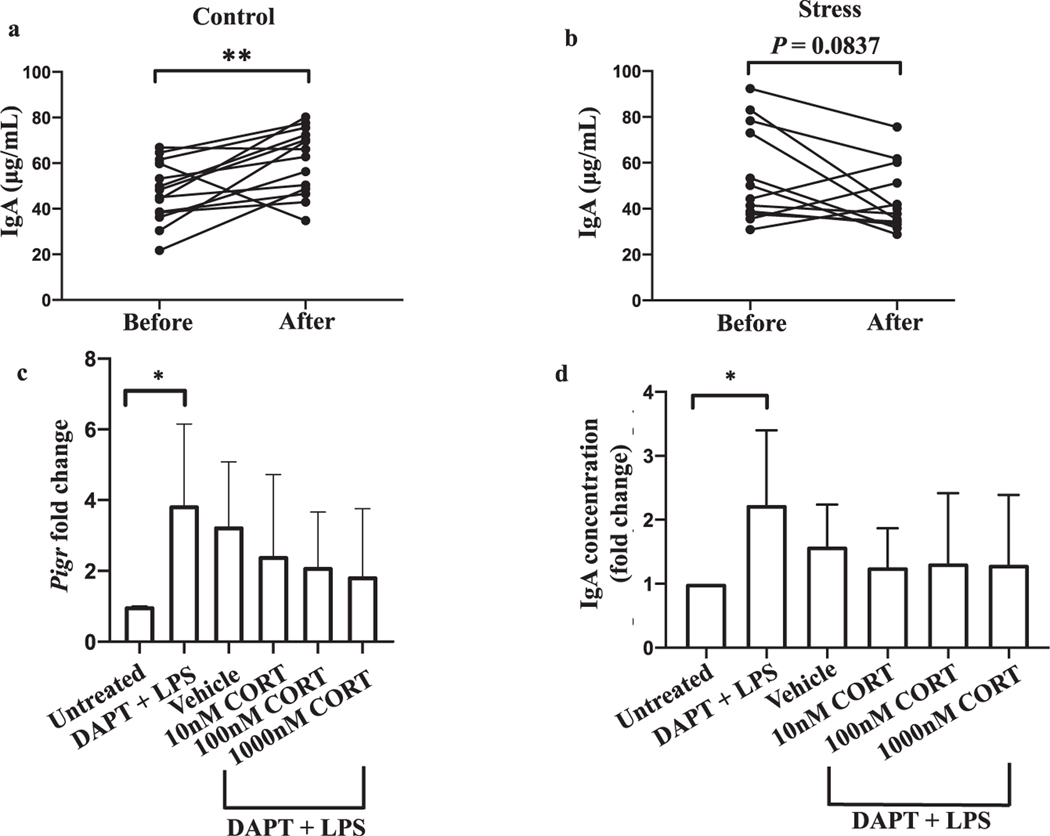
Restraint stress in non-pregnant mice has been shown to reduce IgA levels in small intestinal fluid (Jarillo-Luna, 2007) and IgA+ plasma cell numbers in small intestinal Peyer’s patches (Martinez-Carrillo, 2011). We first sought to determine if pregnant dams experiencing restraint stress exhibit decreased concentrations of fecal IgA. We found that in the absence of stress, fecal IgA concentrations increased from the second to third trimester (Fig. 1a, P = 0.0067). However, this pattern was disrupted in pregnant dams subjected to restraint stress (Fig. 1b, P = 0.0837). Specifically, the concentration of fecal IgA rose in 12 out of 14 (85.7%) control dams by an average of 48.8%. In contrast, fecal IgA levels declined in 9 out of 12 (75%) stressed dams by an average of 28.6%. Importantly, the beginning fecal IgA levels between the control and stress groups were similar (Supplementary Fig. 1, P = 0.2613).
Fig. 1.
Restraint stress in pregnant dams disrupts increases in fecal IgA later in pregnancy without corticosterone affecting IgA transcytosis. Stool samples were collected from pregnant dams on embryonic day 7 (E7) before stressing (Before) and again on E20 immediately after stressing (After). Concentrations of IgA in fecal supernatants from non-stressed (a) and stressed (b) dams were measured by ELISA. **p < 0.01, Student’s paired t test. n = 14 control and 12 stressed dams. Data are pooled from three independent experiments. An outlier (178 μg/mL, identified using a ROUT test with Q = 1%) was removed from the Before group in panel b and its paired data point in the After group (48.6 μg/mL) consequently also removed. With this data pair included, P = 0.0842. (c) Expression of Pigr by day 3 monolayers with the indicated treatments on days 1 and 2 was measured by PCR and is displayed as a fold change relative to the untreated condition. Except for the untreated group, monolayers were treated with DAPT and LPS to promote stem cell differentiation and induce Pigr expression, respectively. Chloroform served as the corticosterone vehicle. CORT, corticosterone. Error bars show mean + SD. (d) Concentrations of IgA in the apical compartment of Transwell inserts following transcytosis were measured by ELISA and are expressed as a fold change relative to the untreated group. Error bars show mean + SD. For c and d, *p < 0.05, Student’s t test, and comparisons among groups treated with vehicle or CORT were performed by one-way ANOVA with no significant differences found. For c and d, n = 3–6 per group.
We next sought to address a potential mechanism to explain the stress-induced disruption in rising fecal IgA. In response to a stressful stimulus, the neuroendocrine pathway known as the hypothalamic-pituitary adrenal (HPA) axis is activated, with the end result of cortisol in humans and corticosterone in rodents being secreted from the adrenal glands (Bailey, 2014). We have shown previously that serum corticosterone levels are increased in pregnant dams subjected to our restraint stress model (Shah, 2019). Furthermore, adrenalectomy and treatment with a glucocorticoid receptor antagonist partially rescued restraint stress-induced decreases in small intestinal IgA concentrations (Jarillo-Luna, 2007), indicating a role for adrenal gland-derived glucocorticoids like corticosterone in modulating intestinal IgA levels. We therefore sought to directly test if corticosterone is capable of interfering in the process of IgA transcytosis using an in vitro IgA transcytosis model with primary colonic mouse epithelial cells (Moon, 2014). We found no significant effect of corticosterone treatment on epithelial cell expression of Pigr (Fig. 1c, P = 0.5818, F = 0.6705). Furthermore, immunostaining revealed no effect of 100 nM corticosterone on Pigr protein levels (Supplementary Fig. 2). Consistent with no change in Pigr expression, we also found no effect of corticosterone on the concentration of IgA that transcytosed into the apical Transwell compartment (Fig. 1d, P = 0.9373, F = 0.1356). Surprisingly, however, we did observe a positive correlation between serum corticosterone levels and fecal IgA levels in both stressed (r2 = 0.8823, P = 0.0178), and non-stressed (r2 = 0.7974, P = 0.0166) dams at the conclusion of the stress protocol in a subset of animals (Supplementary Fig. 3). Overall, the data suggest that corticosterone does not directly influence IgA transcytosis in the colon and stress-related factors other than corticosterone might play a role in the disruption in fecal IgA we observed in stressed dams.
3.2. Prenatal stress does not interfere with vertical transmission of IgA after birth
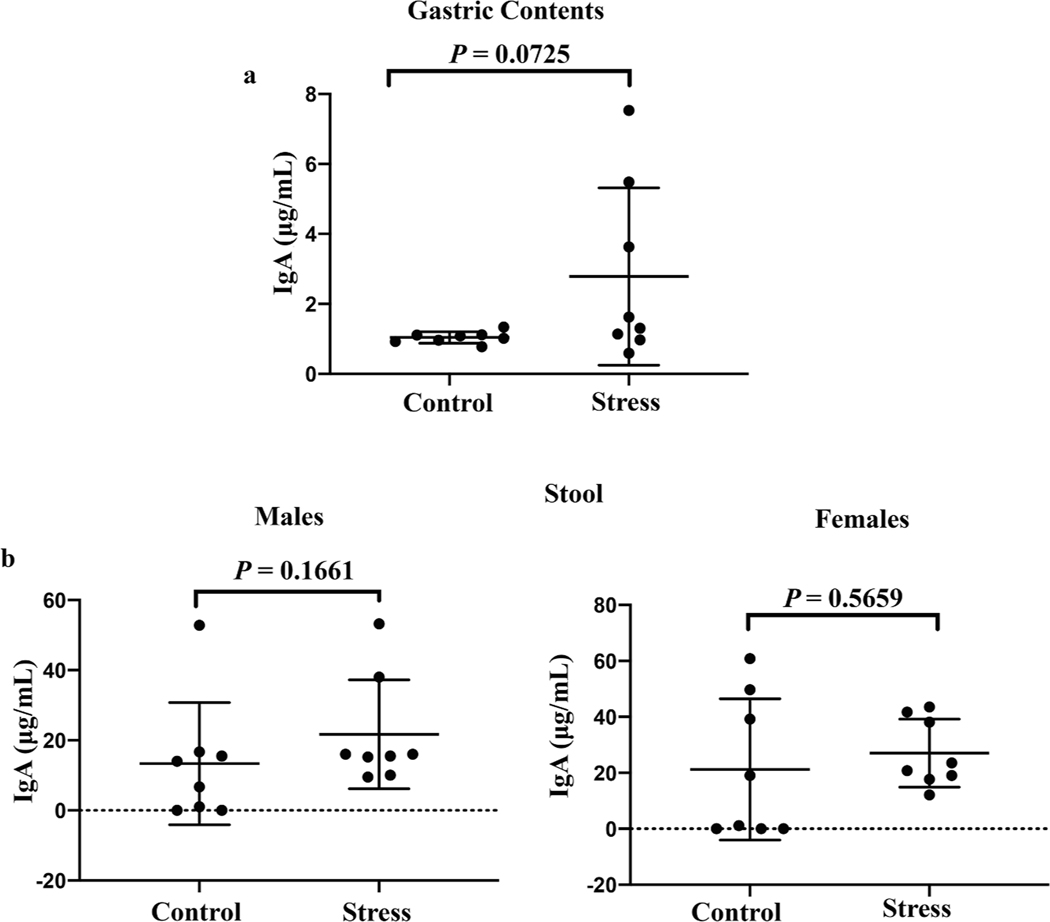
Our finding of prenatal stress disrupting increases in fecal IgA levels in pregnant dams raised the possibility that restraint stress leads to diminished secretion of IgA in mucosal compartments outside the gut. Given our interest in maternal-offspring interactions, we next focused on levels of IgA in the breast milk of stressed dams after they gave birth. As a proxy for breast milk, we evaluated the IgA concentration in gastric contents from breast milk-fed pups at 1 week of age, as we (Culbreath, 2015) and others (Nakayama et al., 2009) have done previously. Because mice do not begin to produce endogenous IgA until approximately 3 weeks of age (Harris, 2006), any IgA found in the gastric contents of the suckling pups should be derived from the maternal breast milk. Restraint stress during pregnancy did not result in lactating dams containing less IgA in their breast milk compared to dams that were not stressed during pregnancy (Fig. 2a, P = 0.0725). We also measured the fecal IgA concentration in 2-week-old offspring of stressed and non-stressed dams. We saw no difference in fecal IgA levels between pups that were born from stressed or non-stressed dams, regardless of the sex of the offspring (Fig. 2b, P = 0.1661 and 0.5659 for male and female offspring, respectively). Taken together, our results suggest that the disruption of rising IgA levels in stressed dams is at least partially gut-specific and that transmission of IgA from mother to offspring through the breast milk is not disrupted by maternal restraint stress.
Fig. 2.
Restraint stress during pregnancy does not interfere with vertical transmission of IgA after birth. The concentrations of IgA in the gastric contents (a) and fecal supernatants (b) of 1-week-old and 2-week-old pups, respectively, were measured by ELISA. Pups were born either from non-stressed dams (Control) or dams that experienced restraint stress while pregnant (Stress). Data are pooled from two independent experiments. n = 8 pups per group per sample type. Horizontal bars show mean + SD. P values were calculated using Student’s t test (gastric contents and female offspring stool) or Mann-Whitney U test (male offspring stool).
3.3. Prenatal stress alters IgA binding to offspring fecal microbiota in a sex-specific manner
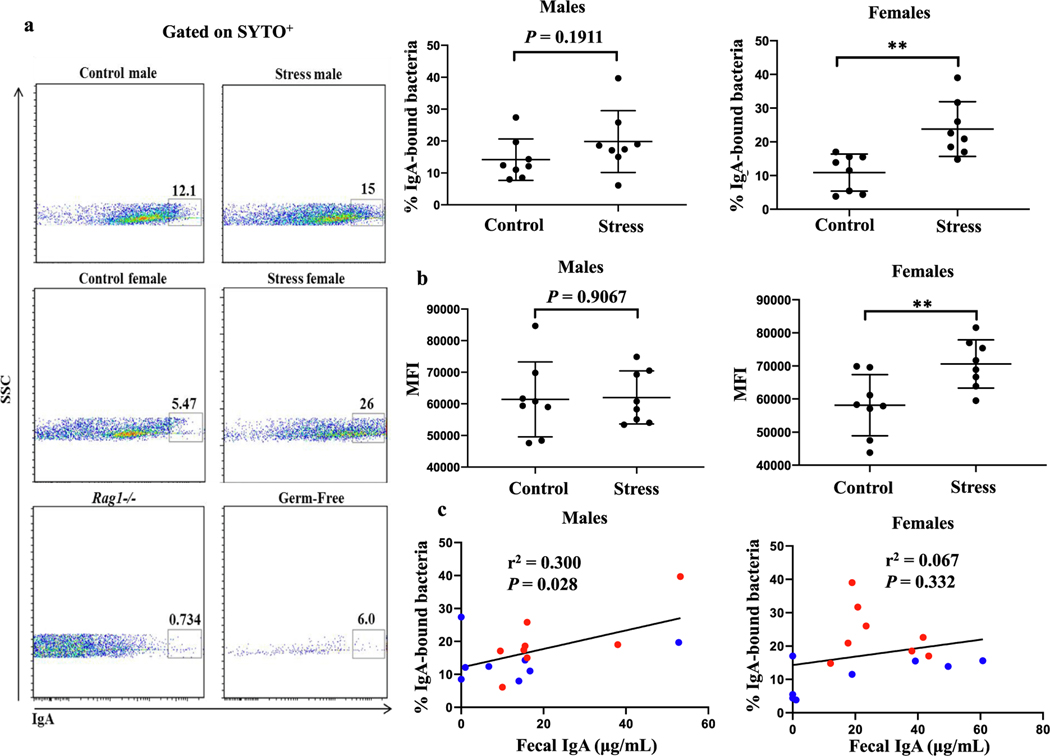
Even though concentrations of total IgA in the gastric contents and stool between pups born from stressed and non-stressed dams were similar, it remained possible that IgA coating of commensal microbes varied between offspring with stressed and non-stressed mothers. To test this hypothesis, we stained 2-week-old pup fecal samples with anti-IgA antibodies and measured the IgA+ populations using flow cytometry. We found that the proportion of IgA-bound bacteria from female (P = 0.0023), but not male (P = 0.1911), pups was increased following maternal restraint stress (Fig. 3a). Furthermore, the number of IgA molecules bound on a per cell basis was increased in female (P = 0.0099), but not male (P = 0.9067), pups with stressed mothers (Fig. 3b). In male (r2 = 0.300, P = 0.028), but not female (r2 = 0.067, P = 0.332), pups the concentration of fecal IgA was positively correlated with the proportion of IgA-bound bacteria (Fig. 3c). This finding suggests that in males, a simple relationship exists wherein ingestion of increasingly higher levels of IgA through the breast milk results in increasingly greater amounts of IgA available to bind to luminal gut bacteria. The lack of this relationship in females indicates that the increase in IgA+ bacteria seen in female offspring from stressed dams compared with non-stressed dams is independent of the amount of IgA ingested and is rather responding to another variable.
Fig. 3.
Maternal restraint stress alters IgA binding to offspring fecal microbiota in a sex-specific manner. (a) Stool from 2-week-old pups of the indicated sex born from control or stressed dams was stained with anti-IgA antibodies and analyzed by flow cytometry. Stool samples from Rag1−/− and germ-free mice were included to assess the degree of anti-IgA antibody nonspecific binding and bacterial contamination, respectively. Only populations positive for SYTO BC, a nucleic acid stain, were included in the analysis. Cumulative data is shown at top right. **p < 0.01, Student’s t test. n = 8 pups per group. Data are pooled from two independent experiments. Horizontal bars show mean + SD. (b) Mean fluorescent intensity (MFI) of IgA+ bacteria. **p < 0.01, Student’s t test. n = 8 pups per group. Data are pooled from two independent experiments. Horizontal bars show mean + SD. (c) Linear regression modeling of fecal supernatant IgA levels correlating with percentage of IgA+ fecal bacteria. Blue and red dots denote pups from non-stressed and stressed dams, respectively. r2 and P values were calculated with Pearson’s r correlation test. Data are pooled from two independent experiments. n = 8 pups per group. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. The microbiome composition of female, but not male, offspring is altered by prenatal stress
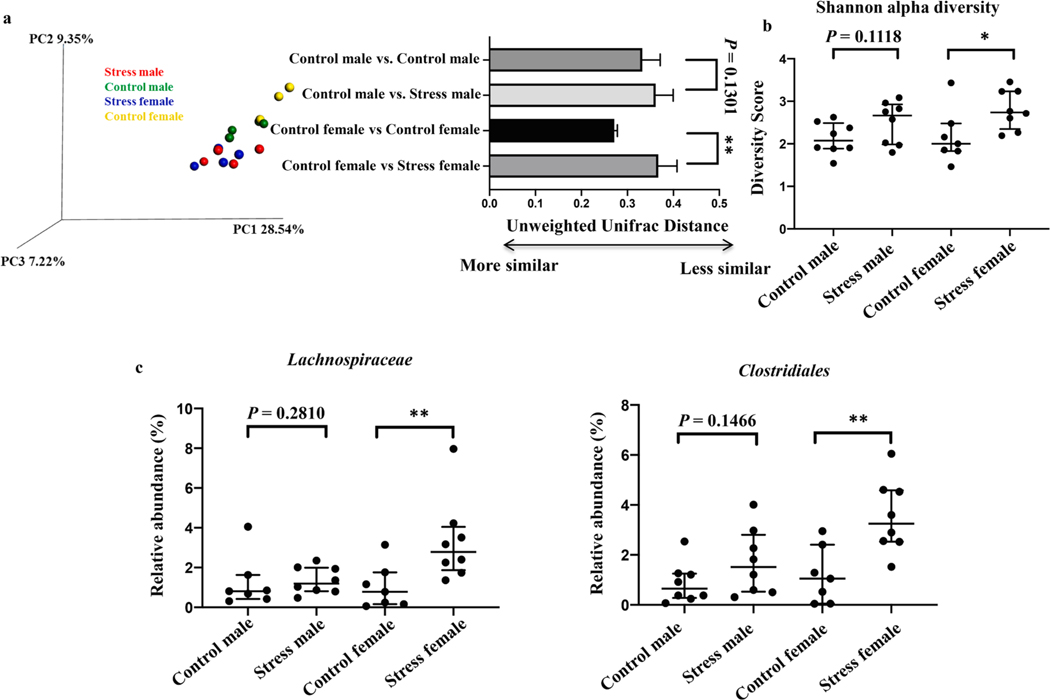
We reasoned that an altered microbiome composition in females from stressed dams compared with females from non-stressed dams could account for the differences we observed in IgA binding. We performed DNA sequencing of the 16S rRNA gene on fecal samples from 2-week-old male and female pups born from stressed and non-stressed dams. As expected, we observed global differences (beta diversity) in the bacterial communities in females (P = 0.0016), but not males (P = 0.1301), born from stressed and non-stressed dams (Fig. 4a). Furthermore, the alpha diversity of the microbiome of females from stressed dams was greater than that of females from non-stressed dams (Fig. 4b, P = 0.0443). On the other hand, no such difference in alpha diversity was observed in males from stressed and non-stressed dams (Fig. 4b, P = 0.1118).
Fig. 4.
The microbiome composition of female, but not male, offspring is altered by maternal stress. (a) Unweighted principal component analysis of the microbiome from 2-week-old pups of the indicated sex born from non-stressed or stressed dams. Quantification of distances shown at right. **p < 0.01, Student’s t test. n = 3–4 pups per group. Data are representative of two independent experiments. Error bars show mean + SD. (b) Alpha diversity scores for the indicated groups.*p < 0.05, Student’s t test. n = 7–8 pups per group. Data are pooled from two independent experiments. Horizontal bars show median with interquartile range. (c) Taxa that were differentially abundant between female pups from non-stressed dams compared with female pups from stressed dams. *p < 0.05**p < 0.01, Student’s t test for Clostridiales and Mann-Whitney test for Lachnospiraceae. n = 7–8 pups per group. Data are pooled from two independent experiments. Horizontal bars show median with interquartile range.
We next evaluated if the relative abundances of specific bacterial taxa were altered in pups born from stressed and non-stressed dams. We found that the relative abundances of Lachnospiraceae (P = 0.0093) and an unidentified Clostridiales family (P = 0.0044) were increased in females from stressed dams compared with females from non-stressed dams (Fig. 4c). These differences were not observed in males (Fig. 4c, P = 0.2810 and 0.1466 for Lachnospiraceae and Clostridiales, respectively), nor were the relative abundances of any taxa altered between males from stressed dams compared with males from non-stressed dams.
3.5. Prenatal stress predisposes female offspring to more severe NEC-like injury
Increased binding of IgA to intestinal bacteria and an increase in intestinal bacterial diversity are negatively correlated with the development in NEC in humans (Gopalakrishna, 2019). Furthermore, Lachnospiraceae species are known producers of short-chain fatty acids, metabolites with anti-inflammatory effects in the gut (Parada Venegas, 2019). We therefore hypothesized that the IgA binding and microbiome changes we observed in female pups from stressed dams relative to female pups from non-stressed dams were protective mechanisms against development of NEC-like injury, whereas male pups from stressed dams would display more severe NEC-like injury compared with males from non-stressed dams.
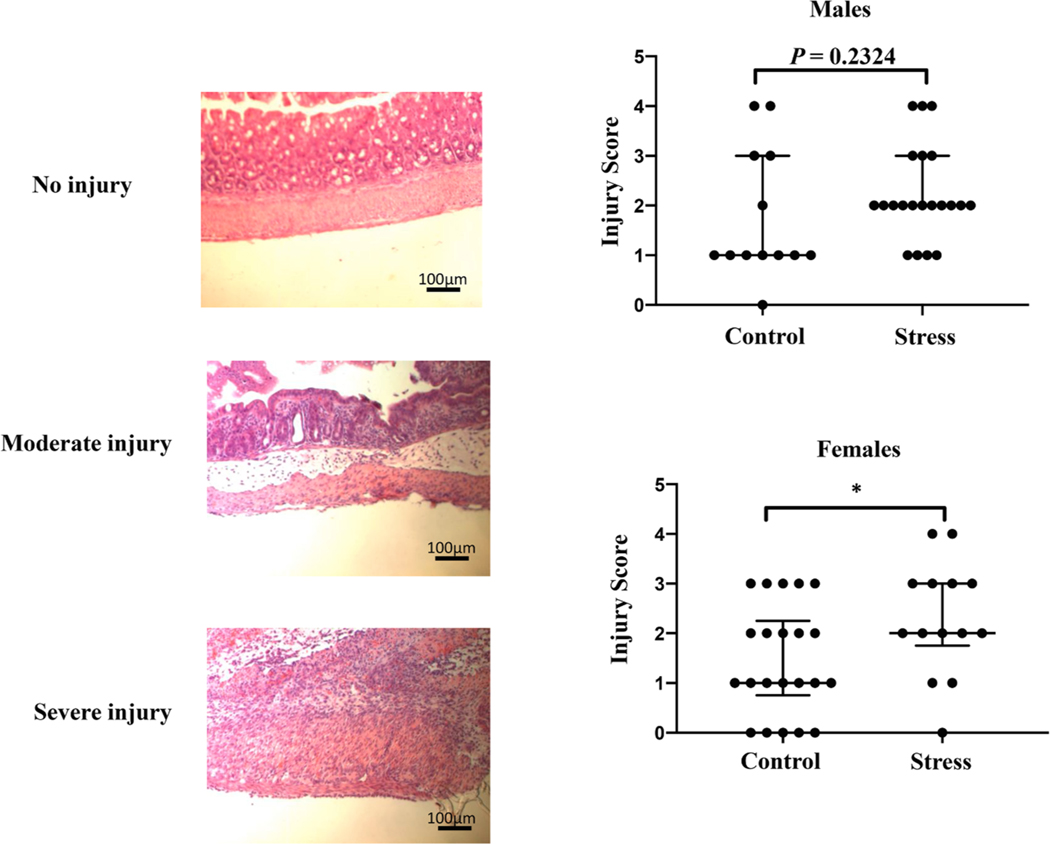
To test this hypothesis, we induced NEC-like injury in 2-week-old pups via rectal injection of TNBS (MohanKumar, 2012; MohanKumar, 2016; Namachivayam, 2017). After a 24-hour period, we harvested the colons for histological analysis. Contrary to our predictions, female pups from stressed dams had more severe colonic tissue damage compared to female pups from non-stressed dams (P = 0.0363), while no significant difference was observed in male pups (Fig. 5, P = 0.2324).
Fig. 5.
Prenatal stress predisposes female offspring to more severe NEC-like injury. Left: representative H& E images of an uninjured, moderately injured, and severely injured colon. The uninjured colon is from a mouse treated with only ethanol, the TNBS vehicle. Right: cumulative injury grade data of TNBS-treated male offspring from non-stressed control (n = 13) and stressed (n = 21) dams and TNBS-treated female offspring from non-stressed control (n = 22) and stressed (n = 14) dams. *p < 0.05, Student’s t test. Data are pooled from 3 independent experiments. Horizontal bars show median with interquartile range.
4. Discussion
NEC is the most common and most lethal gastrointestinal disease among premature infants, and unfortunately the mortality rate has remained unchanged for decades (Hodzic et al., 2017). Most investigations into possible causes of NEC have focused on perinatal and postnatal factors, including premature birth (Samuels, 2017), formula feeding (Chen, 2019), excessive antibiotic treatment (Cotten, 2009; Alexander et al., 2011), and caesarean section delivery (Samuels, 2017; Rose and Patel, 2018), although the effect of delivery method on NEC incidence is not as clear as these other factors. Given the ability of maternal prenatal stress to reprogram the fetal intestine to an inflammatory phenotype in mice (Jasarevic, 2018), in the present study we sought to determine if stress during pregnancy predisposes neonates to NEC-like injury. Because IgA in the maternal breast milk is protective in an animal model of NEC (Gopalakrishna, 2019), we chose to investigate the potential link between prenatal stress and NEC-like injury in the context of disrupted vertical transmission of IgA and/or altered binding of IgA to intestinal bacteria in the offspring. Even though we focused on breast milk IgA here, we should note that other molecules in breast milk have also been shown to be protective in animal models of NEC, including epidermal growth factor (EGF) (Dvorak, 2002; Good, 2015), heparin-binding EGF-like growth factor (Radulescu, 2010; Radulescu, 2010), and human milk oligosaccharides (Good, 2016; Jantscher-Krenn, 2012).
We began by first assessing the effect of restraint stress on the levels of fecal IgA in pregnant dams. Relatively few studies have focused on the role of stress on intestinal IgA levels. While restraint stress has been shown to reduce the concentration of IgA in the mouse small intestine (Jarillo-Luna, 2007), mouse colonic tissue (Zoppi, 2012) and rat colonic tissue (Ponferrada, 2007), we are unaware of any studies addressing the effect of restraint stress on fecal IgA levels in pregnant mice. We found that in the absence of stress, fecal IgA levels increase from the second to third trimester, whereas stress disrupts this effect. Our data showing increases in fecal IgA during pregnancy are consistent with previous findings of increased serum IgA levels in pregnant mice (Muzzio, 2014) and women (Ziegler, 2018) compared to their non-pregnant counterparts. Because of the known ability of maternal antibodies to promote healthy pregnancies (Muzzio, 2016; Clark et al., 2006; Chaouat, 1985), the disruption in IgA we observed in stressed pregnant dams may have implications for the health of the offspring even after birth. Mechanistically, the ability of corticosterone to act on epithelial cells to reduce Pigr expression and thus block IgA transcytosis would explain our findings, but we did not observe any effect of corticosterone on our in vitro IgA transcytosis model or on in vitro Pigr transcript or protein levels, consistent with the previously reported lack of correlation between corticosterone levels and Pigr expression in vivo (Viloria, 2011). However, we did observe a slight albeit insignificant effect of corticosterone on Pigr expression that possibly would achieve significance with additional trials. Another potential explanation of our findings could be corticosterone binding to B cells and limiting their ability to secrete IgA, given it has long been known that corticosterone can induce apoptosis (Garvy, 1993) and halt proliferation (Cupps, 1985) of B cells in vitro. Additionally, given that stressed pregnant dams harbor an altered fecal microbiome compared to non-stressed pregnant dams (Jasarevic, 2017), and given the ability of the microbiota to degrade IgA (Moon, 2015), it is also possible that the microbiome in the stressed dams in our study was reconfigured in such a way as to degrade luminal IgA after the transcytosis process.
We next asked if stress during pregnancy leads to reduced IgA levels in the breast milk. Due to the technical challenges in collecting breast milk from mice, we used the gastric contents of pups as a proxy. We found no significant difference in the concentration of gastric IgA between pups from non-stressed and stressed dams. We are unaware of any previous report on the effect of stress on maternal IgA in the breast milk of mice, but there is conflicting data in humans. A high level of perceived stress among postpartum mothers has been reported to be both positively (O’Connor, 1998) and negatively (Kawano and Emori, 2015; Groer, 2005) associated with IgA concentrations in the breast milk. Furthermore, using cortisol as a more objective measurement of stress, Groer et al. (1994) found that levels of cortisol and breast milk IgA were inversely related. On the other hand, another study (Thibeau, 2016) found no relationship between cortisol and breast milk IgA. These seemingly contradictory results might partially be explained by variations in the intensity and duration of the stressors present among the different cohorts, factors known to influence whether or not stress augments or suppresses immunity (Campos-Rodriguez, 2013).
The IgA we measured in the stool of the pups is free IgA, or IgA that is not bound to bacteria. However, the amount of free IgA present in the intestinal lumen is not necessarily a sufficient predictor of the proportion of luminal bacteria that will be coated with IgA (Tsuruta, 2009). We therefore sought to determine the proportion of IgA-bound bacteria in pups born from stressed or non-stressed dams. We found that the percentage of IgA-bound bacteria from female pups born from stressed dams was increased compared with female pups born from non-stressed dams, despite similar levels of free IgA in the stool from these groups. In contrast, no difference in IgA coating was observed in male pups. In order to explain these findings, we performed sequencing of the bacterial 16S ribosomal rRNA gene to elucidate the bacterial composition of pups born from stressed and non-stressed dams. We found that the fecal microbiome composition was significantly altered in female pups from stressed as opposed to non-stressed dams, while no difference in microbial composition was found in male pups. The ability of prenatal stress to alter the fecal microbiome of mice offspring has been previously reported (Jasarevic, 2017). That study revealed that the relative abundance of certain taxa is altered by prenatal stress in both male and female offspring, in contrast to our findings of no taxa being altered in males. The previous study stressed dams during the first trimester and employed a chronic variable stress model, whereas we stressed dams during the second and third trimesters and employed restraint stress exclusively. Furthermore, the authors in the previous study sampled offspring stool for microbiome analysis 2, 6, and 28 days after birth, whereas we collected samples only at 14 days after birth. Finally, the previous study employed mice on a mixed C57BL/6:129 background whereas the mice in our study were C57BL/6J. Therefore, the exact effect prenatal stress exerts on the composition of the offspring microbiome likely depends on a complex interaction between the timing and nature of the stressor, genetic background, and the postnatal age of the offspring. The appearance or expansion of taxa with epitopes recognized by maternal-derived IgA provides a ready explanation for why we observed increased IgA coating in females from stressed dams compared with non-stressed dams. However, we cannot rule out the possibility that prenatal stress leads to changes in bacterial genomes and/or bacterial gene expression that resulted in the production of epitopes recognized by IgA.
Abundant evidence exists to suggest a role of the microbiota in NEC pathogenesis. The onset of NEC at 2–6 weeks after birth correlates with the timing of microbial colonization of the gut (Coggins et al., 2015). Similarly, germ-free or antibiotic-treated animals are protected from experimental NEC (MohanKumar, 2012; Sodhi, 2012; Sangild, 2006). Dysbiosis patterns prior to NEC diagnosis have been described (Stewart, 2016; Morrow, 2013). Finally, intestinal tissue of patients with active NEC is characterized by an increased bacterial load and decreased microbial diversity (Brower-Sinning, 2014). Because IgA has been shown to limit the ability of bacteria to gain access to the intestinal tissue (Peterson, 2007; Cullender, 2013), we hypothesized that the increase in IgA coating we observed in female pups from stressed dams compared with non-stressed dams is a protective mechanism against NEC-like injury. Contrary to this prediction, we observed more severe NEC-like injury in stress-exposed female pups compared to female pups from non-stressed dams while seeing no difference between stress-exposed and non-exposed male pups. The seeming inability of increased IgA coating in females to protect against TNBS-induced NEC-like injury while being protective in human NEC underscores that even though the TNBS model shares similarities to human NEC, there are important differences as well. Future work will determine if the microbiome changes and increased IgA coating we observed play a causative role in this model. Although NEC is not known to have a gender bias (Wojkowska-Mach, 2014; Carter and Holditch-Davis, 2008), given the ability of prenatal stress to induce pro-inflammatory gene expression and immune cell recruitment in the fetal intestine in a sex-specific manner (Jasarevic, 2018), it would be of interest to evaluate a potential gender bias among NEC cases in which the mothers experienced above average prenatal stress.
In conclusion, we present what is to our knowledge the first evidence linking prenatal stress to exacerbated NEC-like injury, which is associated with a sex-dependent shift in microbiome composition and altered IgA coating of the microbiota. Although more validation is needed, our data suggest a potential preventative strategy for NEC by reducing stress levels in pregnant women. Such an approach would be a clear improvement over treating NEC only when symptoms develop after birth.
Supplementary Material
Acknowledgements
We thank Marissa Menard of the UAB Molecular Detection Core for assistance in preparing histology slides and the UAB Microbiome Resource for microbiome DNA sequencing assistance. The UAB Organogenesis Unit provided the reagents necessary for the spheroid work in this manuscript.
Funding
This work was funded by a grant to CAM by the American Surgical Association.
Footnotes
Research Data
All research data will be made available upon reasonable request.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbi.2020.07.008.
References
- Alexander VN, Northrup V, Bizzarro MJ, 2011. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J. Pediatr. 159 (3), 392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrieta MC, et al. , 2015. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 7(307), 307ra152. [DOI] [PubMed] [Google Scholar]
- Azad MB, et al. , 2013. Infant gut microbiota and the hygiene hypothesis of allergic disease: impact of household pets and siblings on microbiota composition and diversity. Allergy Asthma Clin. Immunol. 9 (1), 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MT, 2014. Influence of stressor-induced nervous system activation on the intestinal microbiota and the importance for immunomodulation. Adv. Exp. Med. Biol. 817, 255–276. [DOI] [PubMed] [Google Scholar]
- Benediktsson R, et al. , 1997. Placental 11 beta-hydroxysteroid dehydrogenase: a key regulator of fetal glucocorticoid exposure. Clin. Endocrinol. (Oxf.) 46 (2), 161–166. [DOI] [PubMed] [Google Scholar]
- Brawner KM, et al. , 2017. Helicobacter pylori infection is associated with an altered gastric microbiota in children. Mucosal Immunol. 10 (5), 1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgman SL, et al. , 2016. Infant gut immunity: a preliminary study of IgA associations with breastfeeding. J. Dev. Orig. Health Dis. 7 (1), 68–72. [DOI] [PubMed] [Google Scholar]
- Brower-Sinning R, et al. , 2014. Mucosa-associated bacterial diversity in necrotizing enterocolitis. PLoS One 9 (9), e105046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler G, 1993. Definitions of stress. Occas. Pap. R. Coll. Gen. Pract. 61, 1–5. [Google Scholar]
- Campos-Rodriguez R, et al. , 2013. Stress modulates intestinal secretory immunoglobulin A. Front. Integr. Neurosci. 7, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BM, Holditch-Davis D, 2008. Risk factors for necrotizing enterocolitis in preterm infants: how race, gender, and health status contribute. Adv. Neonatal. Care 8 (5), 285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaouat G, et al. , 1985. Immunologic consequences of vaccination against abortion in mice. J. Immunol. 134 (3), 1594–1598. [PubMed] [Google Scholar]
- Chen Y, et al. , 2019. Formula feeding and immature gut microcirculation promote intestinal hypoxia, leading to necrotizing enterocolitis. Dis. Model Mech. 12 (12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cima I, et al. , 2004. Intestinal epithelial cells synthesize glucocorticoids and regulate T cell activation. J. Exp. Med. 200 (12), 1635–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DA, Coulam CB, Stricker RB, 2006. Is intravenous immunoglobulins (IVIG) efficacious in early pregnancy failure? A critical review and meta-analysis for patients who fail in vitro fertilization and embryo transfer (IVF). J. Assist. Reprod. Genet. 23 (1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggins SA, Wynn JL, Weitkamp JH, 2015. Infectious causes of necrotizing enterocolitis. Clin. Perinatol. 42(1), 133–54, ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotten CM, et al. , 2009. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics 123 (1), 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussons-Read ME, 2013. Effects of prenatal stress on pregnancy and human development: mechanisms and pathways. Obstet. Med. 6 (2), 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbreath C, et al. , 2015. Environmental-mediated intestinal homeostasis in neonatal mice. J. Surg. Res. 198 (2), 494–501. [DOI] [PubMed] [Google Scholar]
- Cullender TC, et al. , 2013. Innate and adaptive immunity interact to quench microbiome flagellar motility in the gut. Cell Host Microbe 14 (5), 571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupps TR, et al. , 1985. Effects of in vitro corticosteroids on B cell activation, proliferation, and differentiation. J. Clin. Invest. 75 (2), 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak B, et al. , 2002. Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model. Am. J. Physiol. Gastrointest. Liver Physiol. 282 (1), G156–G164. [DOI] [PubMed] [Google Scholar]
- Garvy BA, et al. , 1993. Chronic elevation of plasma corticosterone causes reductions in the number of cycling cells of the B lineage in murine bone marrow and induces apoptosis. Immunology 80 (4), 587–592. [PMC free article] [PubMed] [Google Scholar]
- Good M, et al. , 2015. Breast milk protects against the development of necrotizing enterocolitis through inhibition of Toll-like receptor 4 in the intestinal epithelium via activation of the epidermal growth factor receptor. Mucosal Immunol. 8 (5), 1166–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good M, et al. , 2016. The human milk oligosaccharide 2’-fucosyllactose attenuates the severity of experimental necrotising enterocolitis by enhancing mesenteric perfusion in the neonatal intestine. Br. J. Nutr. 116 (7), 1175–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishna KP, et al. , 2019. Maternal IgA protects against the development of necrotizing enterocolitis in preterm infants. Nat. Med. 25 (7), 1110–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groer M, et al. , 2005. Neuroendocrine and immune relationships in postpartum fatigue. MCN Am. J. Matern. Child Nurs. 30 (2), 133–138. [DOI] [PubMed] [Google Scholar]
- Groer MW, Humenick S, Hill PD, 1994. Characterizations and psychoneuroimmunologic implications of secretory immunoglobulin A and cortisol in preterm and term breast milk. J. Perinat. Neonatal. Nurs. 7 (4), 42–51. [DOI] [PubMed] [Google Scholar]
- Guardino CM, et al. , 2016. Diurnal salivary cortisol patterns prior to pregnancy predict infant birth weight. Health Psychol. 35 (6), 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris NL, et al. , 2006. Mechanisms of neonatal mucosal antibody protection. J. Immunol. 177 (9), 6256–6262. [DOI] [PubMed] [Google Scholar]
- Harville EW, et al. , 2007. Patterns of salivary cortisol secretion in pregnancy and implications for assessment protocols. Biol. Psychol. 74 (1), 85–91. [DOI] [PubMed] [Google Scholar]
- Hodzic Z, Bolock AM, Good M, 2017. The role of mucosal immunity in the pathogenesis of necrotizing enterocolitis. Front. Pediatr. 5, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantscher-Krenn E, et al. , 2012. The human milk oligosaccharide disialyllacto-N-tetraose prevents necrotising enterocolitis in neonatal rats. Gut 61 (10), 1417–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarillo-Luna A, et al. , 2007. Effect of repeated restraint stress on the levels of intestinal IgA in mice. Psychoneuroendocrinology 32 (6), 681–692. [DOI] [PubMed] [Google Scholar]
- Jasarevic E, et al. , 2017. Stress during pregnancy alters temporal and spatial dynamics of the maternal and offspring microbiome in a sex-specific manner. Sci. Rep. 7, 44182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasarevic E, et al. , 2018. The maternal vaginal microbiome partially mediates the effects of prenatal stress on offspring gut and hypothalamus. Nat. Neurosci. 21 (8), 1061–1071. [DOI] [PubMed] [Google Scholar]
- Kawano A, Emori Y, 2015. The relationship between maternal postpartum psychological state and breast milk secretory immunoglobulin A level. J. Am. Psychiatr. Nurses Assoc. 21 (1), 23–30. [DOI] [PubMed] [Google Scholar]
- Martinez-Carrillo BE, et al. , 2011. Repeated restraint stress reduces the number of IgA-producing cells in Peyer’s patches. NeuroImmunoModulation 18 (3), 131–141. [DOI] [PubMed] [Google Scholar]
- McElroy SJ, Weitkamp JH, 2011. Innate immunity in the small intestine of the preterm infant. Neoreviews 12 (9), e517–e526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MohanKumar K, et al. , 2012. Gut mucosal injury in neonates is marked by macrophage infiltration in contrast to pleomorphic infiltrates in adult: evidence from an animal model. Am. J. Physiol. Gastrointest. Liver Physiol. 303 (1), G93–G102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MohanKumar K, et al. , 2016. Smad7 interrupts TGF-beta signaling in intestinal macrophages and promotes inflammatory activation of these cells during necrotizing enterocolitis. Pediatr. Res. 79 (6), 951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon C, et al. , 2014. Development of a primary mouse intestinal epithelial cell monolayer culture system to evaluate factors that modulate IgA transcytosis. Mucosal Immunol. 7 (4), 818–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon C, et al. , 2015. Vertically transmitted faecal IgA levels determine extra-chromosomal phenotypic variation. Nature 521 (7550), 90–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, et al. , 2013. Early microbial and metabolomic signatures predict later onset of necrotizing enterocolitis in preterm infants. Microbiome 1 (1), 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzzio DO, et al. , 2014. B cell development undergoes profound modifications and adaptations during pregnancy in mice. Biol. Reprod. 91 (5), 115. [DOI] [PubMed] [Google Scholar]
- Muzzio DO, et al. , 2016. Marginal zone B cells emerge as a critical component of pregnancy well-being. Reproduction 151 (1), 29–37. [DOI] [PubMed] [Google Scholar]
- Nakayama DK, Burd RS, Newman KD, 2009. Pediatric surgery workforce: supply and demand. J. Pediatr. Surg. 44 (9), 1677–1682. [DOI] [PubMed] [Google Scholar]
- Namachivayam K, et al. , 2017. Neonatal mice with necrotizing enterocolitis-like injury develop thrombocytopenia despite increased megakaryopoiesis. Pediatr. Res. 81 (5), 817–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor ME, et al. , 1998. Relaxation training and breast milk secretory IgA. Arch. Pediatr. Adolesc. Med. 152 (11), 1065–1070. [DOI] [PubMed] [Google Scholar]
- Parada Venegas D, et al. , 2019. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 10, 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson DA, et al. , 2007. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe 2 (5), 328–339. [DOI] [PubMed] [Google Scholar]
- Ponferrada A, et al. , 2007. The role of PPARgamma on restoration of colonic homeostasis after experimental stress-induced inflammation and dysfunction. Gastroenterology 132 (5), 1791–1803. [DOI] [PubMed] [Google Scholar]
- Radulescu A, et al. , 2010. Heparin-binding epidermal growth factor-like growth factor overexpression in transgenic mice increases resistance to necrotizing enterocolitis. J. Pediatr. Surg. 45 (10), 1933–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulescu A, et al. , 2010. Deletion of the heparin-binding epidermal growth factor-like growth factor gene increases susceptibility to necrotizing enterocolitis. J. Pediatr. Surg. 45 (4), 729–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AT, Patel RM, 2018. A critical analysis of risk factors for necrotizing enterocolitis. Semin. Fetal Neonatal Med. 23 (6), 374–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels N, et al. , 2017. Risk factors for necrotizing enterocolitis in neonates: a systematic review of prognostic studies. BMC Pediatr. 17 (1), 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangild PT, et al. , 2006. Diet- and colonization-dependent intestinal dysfunction predisposes to necrotizing enterocolitis in preterm pigs. Gastroenterology 130 (6), 1776–1792. [DOI] [PubMed] [Google Scholar]
- Shah J, et al. , 2019. The effects of gestational psychological stress on neonatal mouse intestinal development. J. Surg. Res. 235, 621–628. [DOI] [PubMed] [Google Scholar]
- Sodhi CP, et al. , 2012. Intestinal epithelial Toll-like receptor 4 regulates goblet cell development and is required for necrotizing enterocolitis in mice. Gastroenterology 143 (3), 708–718 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CJ, et al. , 2016. Temporal bacterial and metabolic development of the preterm gut reveals specific signatures in health and disease. Microbiome 4 (1), 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibeau S, et al. , 2016. Relationships of maternal stress with milk immune components in African American mothers of healthy term infants. Breastfeed Med. 11 (1), 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruta T, et al. , 2009. The amount of secreted IgA may not determine the secretory IgA coating ratio of gastrointestinal bacteria. FEMS Immunol. Med. Microbiol. 56 (2), 185–189. [DOI] [PubMed] [Google Scholar]
- Viloria M, et al. , 2011. Effect of moderate exercise on IgA levels and lymphocyte count in mouse intestine. Immunol. Invest. 40 (6), 640–656. [DOI] [PubMed] [Google Scholar]
- Warner BB, et al. , 2016. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: a prospective case-control study. Lancet 387 (10031), 1928–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M, Thayer Z, 2017. Maternal salivary cortisone to cortisol ratio in late pregnancy: an improved method for predicting offspring birth weight. Psychoneuroendocrinology 78, 10–13. [DOI] [PubMed] [Google Scholar]
- Wojkowska-Mach J, et al. , 2014. Necrotising enterocolitis in preterm infants: epidemiology and antibiotic consumption in the Polish neonatology network neonatal intensive care units in 2009. PLoS One 9 (3), e92865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler SM, et al. , 2018. Innate immune responses to toll-like receptor stimulation are altered during the course of pregnancy. J. Reprod. Immunol. 128, 30–37. [DOI] [PubMed] [Google Scholar]
- Zoppi S, et al. , 2012. Endogenous cannabinoid system regulates intestinal barrier function in vivo through cannabinoid type 1 receptor activation. Am. J. Physiol. Gastrointest. Liver Physiol. 302 (5), G565–G571. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.