In his rollicking 2005 book, Power, Sex and Suicide: Mitochondria and the Meaning of Life, Nick Lane illustrates how mitochondria rule the biological world, something we mitochondrial biologists have always suspected. The title of the book references what mitochondria can do—they generate ATP (power), their DNA is maternally inherited (sex), and they invoke programmed cell death (suicide). The “meaning of life” alludes to the evolution of the first eukaryote. A current leading hypothesis, the endosymbiont theory, suggests that ∼2 billion years ago two prokaryotes, an archaeon and an α-proteobacterium, developed a biological relationship in which both were mutually dependent on one another for nutritional requirements. Eventually, the archaeon, the host cell, acquired the α-proteobacterium, which became the primordial mitochondria. It is often assumed that the original symbiosis was based on the α-proteobacterium detoxifying oxygen and providing copious amount of ATP for the host cell. However, current data indicate that the symbiosis occurred before the presence of oxygen in the atmosphere, and it was the exchange of certain metabolites between the host archaeon and α-proteobacterium that was the metabolic basis of symbiosis. In fact, present-day mitochondria continue to constantly exchange metabolites with the cell. Interestingly, it is not clear whether the archaeon developed a nucleus before acquiring the α-proteobacterium. Furthermore, how the bacterium got inside the archaeon, the host cell, is still a mystery. But, like in many endosymbiotic relationships found in nature, the α-proteobacterium, over time, passed most of its DNA to the host nucleus, keeping just some of its genes. In fact, to date, all eukaryotic cells have mitochondria, or once had them and later lost them. Rickettsia prowazekii, the cause of typhus, has the most mitochondrial-like bacterial genome and bacteria, such as Paracoccus denitrificans, have similar biochemistry to a modern mitochondrion.
Today, we think of mitochondria as essential to maintenance of homeostasis in eukaryotes. If they fail to perform their critical functions, then pathologies result. A current leading hypothesis underlying the causes of diabetes, neurodegeneration, and aging is decline of mitochondria function (see the section Mitochondria and Disease). The biochemistry of mitochondria is well understood, but how this interfaces with the rest of the cell is not fully understood. Mitochondrial biologists hope to understand how these “living bacteria” within us work to shed insight into normal physiology and pathology.
ESSENTIAL FEATURES OF EUKARYOTIC MITOCHONDRIA
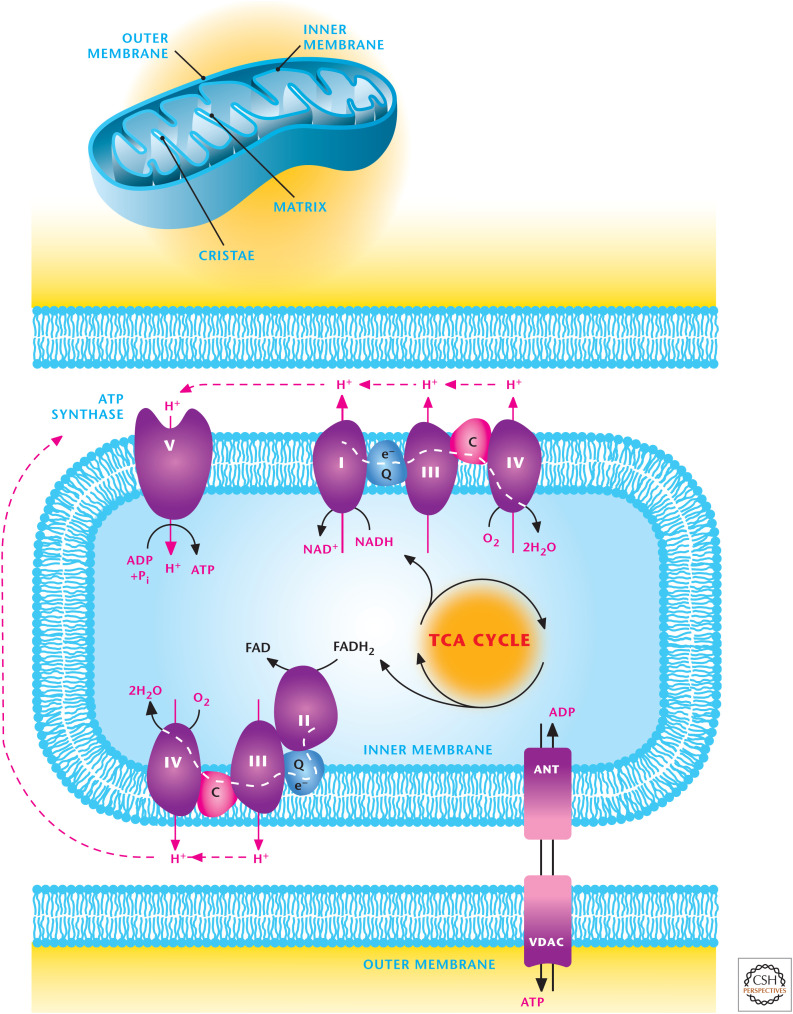
Mitochondria are oval-shaped organelles that have five distinct parts. They contain an outer and inner membrane with a space between the membranes called the intermembrane space. The cristae are formed by the infoldings of the inner membrane, and the matrix is the space within the inner membrane. Mitochondria are dynamic organelles that undergo fusion and fission to form constantly changing tubular networks.
Mitochondria can use glycolysis-derived pyruvate, fatty acids, and amino acids to generate ATP through a process known as oxidative phosphorylation.
Mitochondria are biosynthetic hubs that produce metabolites, which can enter anabolic pathways to generate glycogen, lipids, nucleotides, and proteins. Mitochondria also produce heme and iron–sulfur clusters for certain proteins.
The nuclear DNA encodes most mitochondrial proteins. However, mitochondria have their own circular DNA, resembling bacterial plasmids, that encode for a subset of critical proteins. A mitochondrion can have multiple copies of its DNA. Mitochondrial DNA (mtDNA) is maternally inherited. mtDNA encodes 37 genes, including 13 critical for oxidative phosphorylation. The remaining approximately 3000 genes affecting mitochondria are encoded in the cell nucleus, and the resultant proteins are transported to the mitochondria.
Mitochondria are signaling organelles that regulate multiple biological processes, including proliferation, cell death, and metabolic adaptation. Mitochondria regulate these biological processes through multiple mechanisms including, but not limited to, release of ROS and metabolites, such as acetyl-CoA, calcium, cytochrome c, and mtDNA. Furthermore, the outer mitochondrial membrane serves as a platform for signaling by serving a scaffold for many critical proteins involved in cellular signaling.
QUICK GUIDE TO THE ENERGY-GENERATING CAPACITY OF MITOCHONDRIA
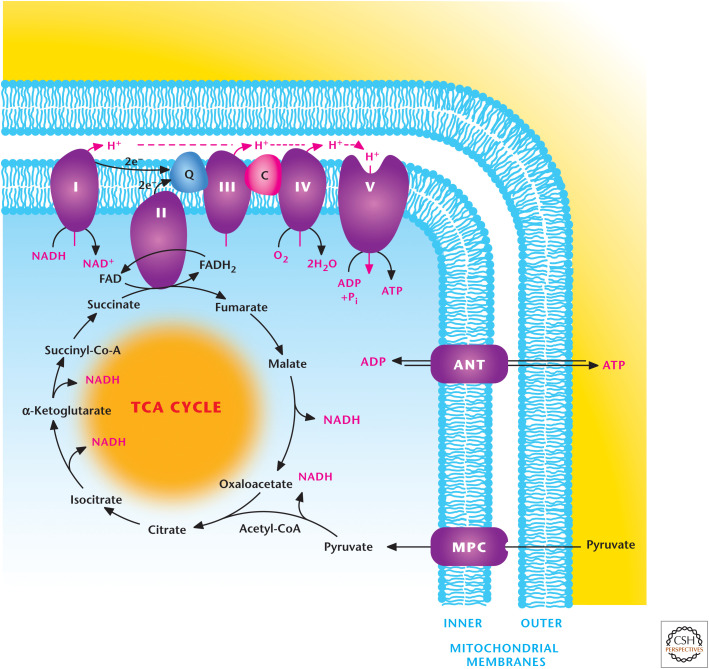
Most mitochondrial metabolic activity occurs in the mitochondrial matrix either by soluble enzymes or protein parts of complexes embedded in the inner mitochondrial membrane.
The inner mitochondrial membrane is impermeable to most ions and metabolites and has numerous transporters to shuttle ATP, pyruvate, and citrate. ATP and ADP are shuttled by the adenine nucleotide transporter (ANT). In contrast, the outer mitochondrial membrane is quite permeable to small metabolites and ions because of the presence of multiple copies of a porin, called a voltage-dependent anion channel (VDAC) (Fig. 1).
The TCA cycle begins with the two-carbon acetyl-CoA and combines with four-carbon oxaloacetate (OAA) to generate citrate. The completion of the TCA cycle generates 3 NADH and 1 FADH2.
Acetyl-CoA can be generated from pyruvate by pyruvate dehydrogenase (PDH) or through fatty acid oxidation.
Multiple amino acids can feed into the TCA cycle. Notably, glutamine can be converted into glutamate, which, subsequently, can generate the TCA cycle metabolite α-ketoglutarate (2-oxoglutarate) in a process known as glutaminolysis.
NADH and FADH2 can feed the electron transport chain (ETC) complex I (NADH dehydrogenase) and complex II (succinate dehydrogenase, SDH), respectively, which pass their electrons to ubiquinone (Q). Complex III (ubiquinol–cytochrome c reductase) transfers electrons from Q to cytochrome c. Complex IV (cytochrome c oxidase) transfers electrons from cytochrome c to oxygen, forming H2O. Complex I–III–IV and Complex II–III–IV form two different supercomplexes.
Complexes I, III, and IV extrude H+ from the matrix into the mitochondrial intermembrane space, thus, functioning as proton pumps. This generates proton motive force (pmf) composed of a small chemical component, ΔpH, and a large electrical component, membrane potential Δψ. Complex V (F1F0-ATP synthase) allows reentry of H+ back into the matrix, allowing the phosphorylation of ADP to generate ATP by using the pmf. This process is coupled to the use of oxygen and is referred to as oxidative phosphorylation.
Complex II participates in both the TCA cycle and ETC but does not conduct proton pumping.
Figure 1.
Overview of mitochondrial metabolism. Mitochondria contain an outer and inner membrane. The mitochondrial matrix enclosed by the inner membrane contains the TCA cycle that generates reducing equivalents NADH and FADH2, which donate electrons to the electron transport chain (ETC), resulting in the generation of a proton motive force to drive ATP synthesis. The ETC is composed of two supercomplexes consisting of either complex I–III–IV or II–III–IV. (Modified from http://en.wikipedia.org/wiki/Electron_transport_chain#mediaviewer/File:Mitochondrial_electron_transport_chain%E2%80%94Etc4.svg.)
THE TCA CYCLE IS AN ENERGY-GENERATING PATHWAY
In 1937, Hans Krebs and his colleague William Johnson published a groundbreaking paper, “The role of citric acid in intermediate metabolism in animal tissues” in Enzymologia, outlining the citric acid cycle, also known as TCA cycle or Krebs cycle. Initially, Krebs submitted his results to Nature; however, the editors at the journal cited a backlog of papers and thus could not publish it without significant delay. In his memoir, Krebs wrote, “This was the first time in my career, after having published more than fifty papers, that I experienced a rejection or semi-rejection.”
In 1988, seven years after Krebs's death, an anonymous editor published a letter in Nature acknowledging their mistake by stating “rejection of Hans Krebs' discovery of the tricarboxylic (Krebs') cycle, a pivot of biochemical metabolism, remains Nature's most egregious error (as far as we know).”
The TCA cycle lies at the core of eukaryotic cell metabolism because multiple substrates can feed into the cycle, including fatty acids, amino acids, and pyruvate generated from glucose via glycolysis. The cycle is distinct from linear pathways, like glycolysis, because OAA is the substrate for the first reaction catalyzed by citrate synthase and the product of the last reaction catalyzed by malate dehydrogenase (MDH), thus renewing the cycle. The TCA cycle is identified as an amphibolic pathway because it can provide intermediates for macromolecules (e.g., lipids) and synthesis (i.e., anabolism) and can generate the reducing equivalents NADH and FADH2 to ultimately produce ATP through oxidative phosphorylation (i.e., catabolism).
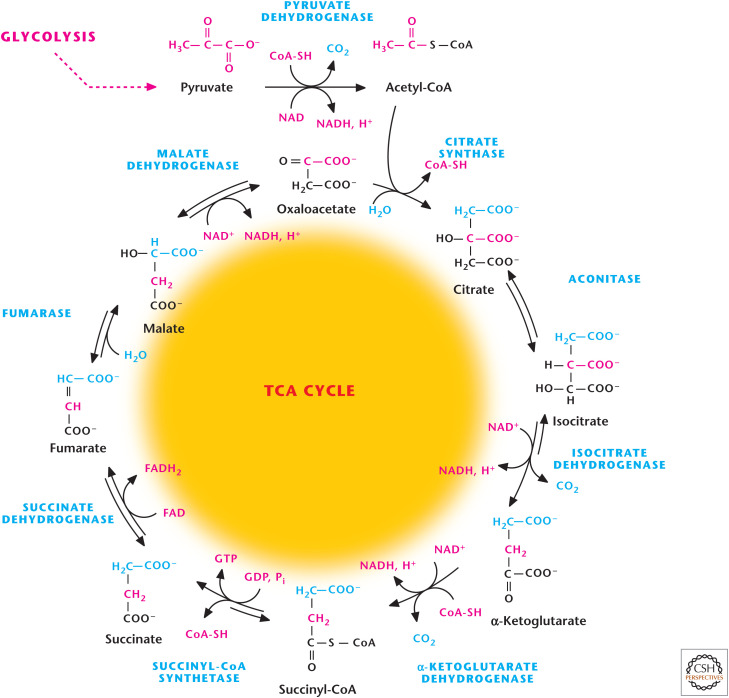
The TCA cycle begins when acetyl-CoA is generated by oxidation of pyruvate (e.g., from glycolysis; see Chandel 2020a) or other compounds, including fatty acids (Chandel 2020b) and amino acids (Chandel 2020c). The massive PDH complex converts three-carbon pyruvate to a two-carbon acetyl-CoA. The primary function of one turn of the TCA cycle from an energy-generating perspective is to oxidize acetyl-CoA to two CO2 molecules. The released electrons are transferred to the coenzymes NAD+ and FAD to form three NADH and one FADH2 molecules, which are reoxidized and used by the ETC to generate ATP through oxidative phosphorylation, discussed subsequently in this review.
In the first of eight reactions of the TCA cycle, the two-carbon acetyl-CoA transfers the acetyl group to the four-carbon OAA to generate a six-carbon citrate (Fig. 2). The cycle proceeds with two oxidative decarboxylation in steps 3 and 4, resulting in release of two molecules of CO2 and generation of two NADH molecules and a four-carbon succinyl-CoA. The fifth step of the TCA cycle converts succinyl-CoA into succinate, coupling it to the generation of GTP, which can be converted into ATP. The remaining three steps of the TCA cycle convert four-carbon succinate into a four-carbon OAA that can combine with another acetyl-CoA molecule to continue the TCA cycle. Most of the reactions of the TCA cycle are reversible except for the reactions catalyzed by citrate synthase, isocitrate dehydrogenase, and α-ketoglutarate dehydrogenase, which have the largest negative ΔG°′. In contrast, the last reaction catalyzed by MDH is the most unfavorable reaction and thus requires energy (ΔG°′ = +29.7 kJ/mole). Keeping the product of a reaction very low compared with reactant (law of mass action) can make an unfavorable reaction favorable (Chandel 2020d). Thus, the OAA concentration in the matrix of the mitochondria is kept extremely low, allowing the last reaction to have a favorable negative actual Gibbs free energy.
Figure 2.
Overview of TCA cycle. The TCA cycle starts when two-carbon acetyl-CoA combine with four-carbon OAA to generate citrate. The TCA cycle produces three NADH, one FADH2, and one GTP.
The overall reaction of the TCA cycle is
THE TCA CYCLE IS A BIOSYNTHETIC HUB
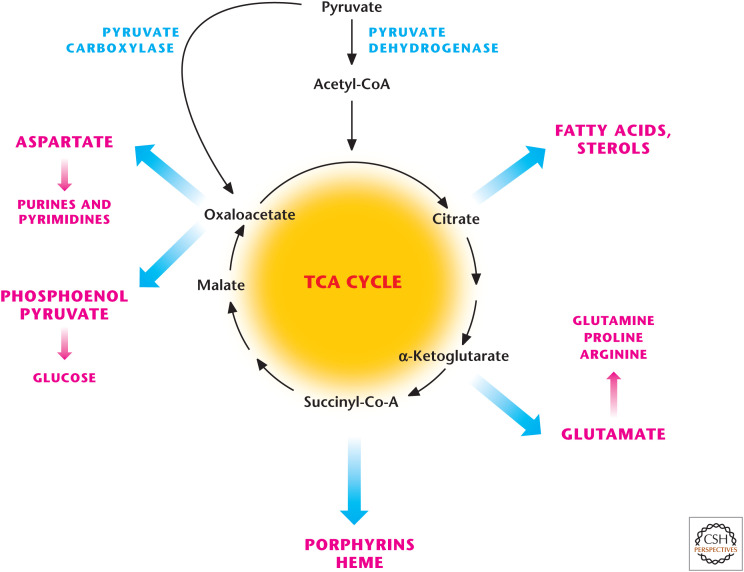
In his Nobel lecture in 1953, Hans Krebs noted “in some micro-organisms the cycle primarily supplies intermediates rather than energy, whilst in the animal and most other organisms it supplies both energy and intermediates.” Many of the enzymes involved in the TCA cycle were abundant well before the presence of oxygen in the Earth's environment. The likely function of the earliest components of the TCA cycle was to provide metabolites that could be used for biosynthesis. In eukaryotes, notable biosynthetic reactions in the TCA cycle are as follows (Fig. 3).
OAA is converted to phosphoenolpyruvate, which is a substrate for gluconeogenesis.
α-Ketoglutarate, also known as 2-oxoglutarate, is converted to glutamate, which can generate glutamine for use as a precursor to generate purine nucleotides (adenosine and guanosine).
Succinyl-CoA is a precursor of prophyrins, such as heme.
Citrate is exported to the cytosol where it is converted into OAA and acetyl-CoA. OAA can generate aspartate, which is used for purine and pyrimidine synthesis. Acetyl-CoA is used for lipid synthesis.
Figure 3.
The TCA cycle is a biosynthetic hub. TCA-cycle intermediates provided the building blocks for macromolecules including fatty acids, nucleotides, hemes, and porphyrins. Oxaloacetate provides the carbons for the generation for glucose (i.e., gluconeogenesis).
An important consideration is that when TCA-cycle intermediates are being siphoned off for biosynthetic purposes, the cycle has to be replenished or else it would cease. The replenishment of the TCA cycle is referred to as anaplerosis, which means “filling up.” The initial steps of gluconeogenesis involve the conversion of OAA to phosphoenolypyruvate, which goes through a series of reactions to make glucose (Chandel 2020e). Thus, if OAA is being depleted, it has to be replenished to maintain a minimal level to allow the cycle to function. There are multiple inputs into the TCA cycle, but two important anaplerotic mechanisms are the conversion of pyruvate to mitochondrial OAA by pyruvate carboxylase and the conversion of glutamine to glutamate and subsequently to α-ketoglutarate, referred to as glutaminolysis (Fig. 4). The latter mechanism is often used when citrate is exported from the mitochondria into the cytosol for de novo lipid synthesis, thus preventing the formation of subsequent TCA-cycle intermediates, such as α-ketoglutarate. Glutaminolysis feeds α-ketoglutarate into the TCA cycle, thereby allowing the cycle to continue functioning.
Figure 4.
Glutaminolysis. The conversion of glutamine into glutamate and, subsequently, into α-ketoglutarate is referred to as glutaminolysis. This is an important pathway to replenish TCA-cycle intermediates.
REGULATION OF THE TCA CYCLE
If we examine the changes in free energy of the reactions of the TCA cycle, it is apparent that there are three irreversible steps (indicated with large negative Gibbs free-energy values) that are catalyzed by citrate synthase, isocitrate dehydrogenase, and α-ketoglutarate dehydrogenase, but it should be noted that isocitrate dehydrogenase can be reversible under certain conditions. A fourth irreversible reaction that is not technically part of the TCA cycle, but is an important regulator, is PDH, which generates acetyl-CoA from pyruvate. The TCA cycle is regulated by substrate availability, such as the levels of OAA and acetyl-CoA that are required to initiate the TCA cycle. There are multiple positive and negative allosteric regulators that control the metabolic flux of the TCA cycle, including acetyl-CoA, succinyl-CoA, ATP, ADP, AMP, NAD+, and NADH (Fig. 5). NADH inhibits all of the regulatory enzymes in the TCA cycle. Because NADH generates ATP through the ETC and oxidative phosphorylation, ATP also is an allosteric inhibitor of PDH and isocitrate dehydrogenase. Thus, when cells have ample NADH and ATP, the cycle slows down. In contrast, high demand for ATP increases the ADP/ATP ratio and AMP levels, resulting in stimulation of the regulatory enzymes of the TCA cycle (Fig. 5).
Figure 5.
Regulation of the TCA cycle. Citrate synthase, as well as the three dehydrogenases, isocitrate, α-ketoglutarate, and PDH, are stimulated when ADP levels increase and dampened when NADH levels increase. The three dehydrogenases are positively regulated by calcium.
Three metabolites that are important regulators of the TCA cycle are acetyl-CoA, succinyl-CoA, and OAA. Abundant acetyl-CoA inhibits PDH but activates pyruvate carboxylase to shunt pyruvate to OAA, thus balancing the entry of OAA with acetyl-CoA. Succinyl-CoA inhibits both citrate synthase and α-ketoglutarate dehydrogenase to slow the cycle down. Citrate generated from acetyl-CoA and OAA is diminished and α-ketoglutarate generated by glutaminolysis is also decreased. An increase in OAA can slow the cycle down by inhibiting SDH. Finally, calcium is a positive regulator of the cycle by activating pyruvate, isocitrate, and α-ketoglutarate dehydrogenase. This is an important mechanism in muscle cells that use calcium for contraction, an energy-demanding process. The increase in cytoplasmic calcium triggers uptake of calcium in the mitochondria, and this stimulates these dehydrogenases to couple muscle contraction to the energy machinery. An exciting new area involves the multiple cellular signaling mechanisms uncovered recently that regulate the TCA cycle (see Chandel 2020f).
BASIC ASPECTS OF OXIDATIVE PHOSPHORYLATION
The reducing equivalents NADH and FADH2 generated by the TCA cycle have to be oxidized to NAD+ and FAD so that the cycle can continue to function. The ETC, also called the respiratory chain, consists of complexes I, II, III, and IV embedded in the inner membrane of the mitochondria (Fig. 6). Complex I, also referred to as NADH dehydrogenase, is composed of 46 subunits in human cells. It oxidizes NADH to NAD+ and passes two electrons to Q. Subsequently, Q donates two electrons to complex III and becomes ubiquinol (QH2). Complex III is also called the bc1 complex, which is composed of 11 subunits. Q then becomes ubiquinol. Complex III passes one electron at a time to cytochrome c, which donates electrons to complex IV, also known as cytochrome c oxidase, which is composed of 13 subunits. Complex IV takes four electrons from cytochrome c and donates them to the final electron acceptor molecular oxygen to generate two molecules of water (O2 + 4e + 4H → 2H2O). A molecule with a high- (most-positive) reduction potential is likely to accept electrons (Chandel 2020d). Thus, oxygen is the terminal acceptor of electrons in the ETC because it has the most-positive reduction potential. The driving force of the ETC is that each electron carrier has a higher standard reduction potential than the one from which it accepts electrons. Complex II, which also participates in the TCA cycle as SDH, has four subunits (SDHA–D). In the TCA cycle, SDH subunit A oxidizes succinate to fumarate, thereby reducing FAD to FADH2. The electrons from FADH2 are passed to subunits SDHB, -C, and -D and delivered to Q and the downstream complexes III and IV. The transfer of electrons is coupled to pumping of hydrogen ions from the matrix into the intermembrane space across the inner membrane by complexes I, III, and IV. There is no proton pumping associated with complex II, Q, and cytochrome c. This protein pumping generates the pmf, composed of a small chemical component (ΔpH) and a large electrical component (membrane potential Δψ). Complex V, the ATP synthase, uses the proton gradient established across the inner membrane to generate ATP from ADP and Pi, a process referred as oxidative phosphorylation.
Figure 6.
Oxidative phosphorylation. The electron transport chain complex elements I, III, and IV pump protons across the inner mitochondrial membrane as electrons are transferred through the chain to oxygen, the final electron acceptor. This protein pumping generates the pmf, composed of a small chemical component (ΔpH) and a large electrical component (membrane potential, Δψ). Complex V, the ATP synthase, uses the proton gradient established across the inner membrane to generate ATP from ADP and Pi, a process referred as oxidative phosphorylation.
The origins of the discovery of oxidative phosphorylation begin in the late 1950s. The British biochemist Peter Mitchell (see Box 1) was working on how bacteria take up certain molecules, such as sugar. He realized that proton gradients across the bacterial membranes were critical for nutrient uptake, and this led to his incredible insight that ATP synthesis is powered by proton gradients. Proton gradients are ubiquitous in nature and are essential for sustaining life. Proton power drives photosynthesis, active transport of molecules in an out of cells, and ATP generation. Even the earliest forms of life, such as α-proteobacteria and archaea, had proton pumps to generate ATP. This coupling of ATP synthesis to proton pumping, known as the “chemiosmotic theory,” is one of the key discoveries of the 20th century.
BOX 1.
CELEBRATING THE GENIUS OF PETER MITCHELL
The 20th century is full of iconoclastic scientists that are household names: Albert Einstein in physics, Linus Pauling in chemistry, and James Watson and Francis Crick in biology. Perhaps an outlier in this category is Peter Mitchell (1920–1992), the man who discovered the process by which living organisms generate energy. His career was unique compared with most scientists. His ideas stemmed from inductive reasoning, based on his findings on the essential role of proton pumping in nutrient uptake in bacteria during the years he spent at Cambridge University (1939–1955) and Edinburgh University (1955–1963). During these years, he was greatly influenced by Frederick Gowland Hopkins, James Danielli, David Keilin, and Malcolm Dixon, as well as the ancient Greek philosophers. The theoretical ideas that became his chemiosmotic theory were introduced in a series of papers published during the 1950s and consolidated in a 1961 Nature paper, “Coupling of phosphorylation to electron and hydrogen transfer by a chemiosmotic type of mechanism.” The prevailing theory at the time was the chemical intermediate theory, and Mitchell's vectorial approach with ideas of pmf, symport, antiport, and uniport were considered by most in the field to be retrograde, strange, and wrong. The result was years of skepticism, fierce controversy, and heated exchanges—the “ox-phos” wars. Initially Mitchell did not have extensive experimental evidence to support his revolutionary ideas. But oddly, severe health problems in the form of gastric ulcers resulted in a solution. Mitchell, using family resources, bought an estate in Cornwall and during 1962–1964 restored the estate with the aim of establishing a small, private research institute—the Glynn Research Institute. Mitchell credits several months of milking cows as helping to heal his ulcers. His research partner at Cambridge and Edinburgh, Jennifer Moyle, was a cofounder of the Institute. With Mitchell supplying the theoretical analysis, Moyle and research assistants performed the specific experiments providing the evidence for the chemiosmotic approach to energy generation in the cell. Glynn also provided a “quiet haven” for visiting scientists working in this burgeoning bioenergetics area. Soon other laboratories began to confirm Mitchell's ideas, and during the 1970s the chemiosmotic theory gained reluctant acceptance, although controversy remained for many years over proton/electron ratios. Mitchell's paradigm-changing ideas resulted in the Nobel Prize in Chemistry in 1978 for his “contribution to the understanding of biological energy transfer through the formulation of the chemiosmotic theory.” During the 1970s and 1980s, Mitchell extended his theories to propose the Q cycle, a mechanism by which complex III pumps protons to generate ATP, and to develop a model for the mechanism of ATPase. As evidenced by the trajectory of his career, he believed that scientists should be free of bureaucratic interference, as well as social responsibility; it was up to society to determine whether scientific results were to be used for good or ill. The Glynn Research Institute continued its work after Mitchell's death, but it was difficult to raise sufficient funds to continue its high level of investigation. In 1996, the Institute was absorbed into the University College of London as the Glynn Laboratory of Bioenergetics.
The salient feature of the chemiosmotic theory is that two systems, the transfer of electrons through the ETC and generation of ATP through complex V, are related through a pmf. We are used to thinking that when one particular process exerts influence on another, there are usually protein–protein interactions. But here the two systems are not in close contact or proximity and they communicate through the pmf generated by pumping of protons from the mitochondrial matrix into the intermembrane space through the inner membrane by complexes I, III, and IV. The vectorial H+ pumping by complexes I, III, and IV generates both a chemical gradient, measured as a ΔpH, and an electrical gradient based on separation of charge, measured as a membrane potential (ψ). The major contributor of the pmf is ψ, whereas the ΔpH across the mitochondrial inner membrane is only 1 pH unit. The stoichiometry of proton pumping is 4H+ for complexes I and III but only 2H+ in complex IV. This results in 10H+ for one molecule of NADH oxidized and 6H+ for FADH2 oxidized by the ETC. FADH2 oxidation does not use complex I; thus, it only has 6H+ protons pumped through complexes III and IV. The protons flow back down the gradient through the inner membrane-bound complex V in response to the different chemical (H+ concentration) and electrical (separation of charge) potentials across the membrane. We can think of this as a proton circuit.
Complex V is composed of two distinct multisubunit components, an ATP-hydrolyzing catalytic subunit known as the spherical “head” (F1-ATPase), which is hydrophilic and protrudes into the aqueous mitochondrial matrix, and the hydrophobic proton-pumping component (F0), embedded in the inner mitochondrial membrane. An isolated F1-ATPase catalyzes the hydrolysis of ATP to ADP and Pi, which is why it is called the F1-ATPase. However, in an intact mitochondrion, the protons that have accumulated in the mitochondrial intermembrane space as a result of ETC proton pumping enter the F0 complex and exit into the matrix. The energy dissipated as the protons travel down their concentration gradient rotates F0 in a clockwise direction, inducing repeated conformational changes in the F1-ATPase and enabling the conversion of ADP and Pi into ATP. This makes the complex V or F0F1-ATPase the smallest rotary machine ever known!
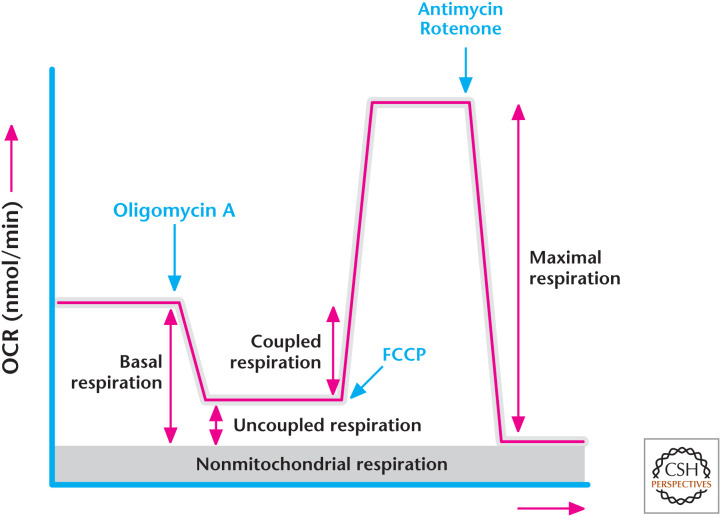
Mitochondrial oxygen consumption is a way to assess ETC and complex V function (see Box 2). Oxygen consumption is reflective of the reduction of oxygen to water by complex IV. The transfer of electrons through the ETC is coupled to pumping protons to generate the pmf. At some point, this force is high enough that the ETC cannot pump against this gradient anymore; when this happens, the transfer of electrons ceases and so does oxygen consumption. In the presence of ADP, the proton gradient can be dissipated through complex V, allowing the transfer of electrons through the ETC to complex IV, and oxygen consumption resumes. The rate of oxygen consumption by complex IV coupled to the generation of ATP synthesis by complex V is called coupled respiration. However, a portion of the protons can leak back across the inner membrane rather than moving to complex V. In the absence of ADP, this proton leak allows some dissipation of the proton gradient to permit electron transfer through the ETC, resulting in a small amount of oxygen consumption. The rate of oxygen consumption by complex IV that is not coupled to the generation of ATP, but is caused by the proton leak, is called uncoupled respiration. Thus, mitochondrial oxygen consumption is a combination of coupled respiration and uncoupled respiration. Most cells display high levels of coupled respiration. Brown fat cells are a notable exception. They show uncoupled respiration due to an abundance of uncoupling proteins, which increase proton leak by allowing protons to flow back into mitochondria without driving the generation of ATP. There are protonophores, such as carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP) and 2,4-dinitrophenol (DNP), which allow protons to equilibrate across the inner mitochondrial membrane by increasing the proton leak. Thus, the proton gradient never builds up to slow the ETC. This allows transfer of electrons through the ETC to complex IV at a maximal rate, a process called maximal respiration. This rate will be dependent on how quickly NADH and FADH2 can feed electrons to the ETC, as well as the efficiency of individual complexes to transfer electrons. Interestingly, in the 1930s DNP was used as an agent to combat obesity. However, it was quite toxic and quickly removed from the market because of an excessive increase in body temperature as a result of uncoupling. A recent study used a modified version of DNP targeted to the liver to decrease features of type II diabetes in rats, such as hyperglycemia, fatty liver, and insulin resistance. It will be of interest to see if this finding translates into humans.
BOX 2.
MEASURING THE CELLULAR OXYGEN CONSUMPTION RATE
A tool to assess mitochondrial ETC function is to measure the cellular oxygen consumption rate (OCR) in the presence of various inhibitors. Typically, cellular OCR is measured by adding (1) the ATP synthase inhibitor oligomycin, (2) uncoupler FCCP, and (3) complex I and III inhibitors rotenone and antimycin, respectively. Basal respiration defined as mitochondrial OCR is calculated by subtracting the residual OCR after administering ETC inhibitors rotenone and antimycin from the cellular oxygen consumption (Box 2, Fig. 1). The residual OCR is referred to as nonmitochondrial respiration. Coupled respiration is calculated by subtracting the residual respiration upon the addition of oligomycin, referred to as uncoupled respiration, from basal respiration. Uncoupled respiration is a measurement of the proton leak. Maximal respiration can be calculated by the addition of FCCP, a potent protonophore, which uncouples mitochondrial ATP generation from oxygen consumption (Box 2, Fig. 1).
Box 2, Figure 1.
Determining the oxygen consumption rate. (Modified from Anso et al. 2013.)
It is important to note when the ETC is not pumping protons and no membrane potential or pH gradient is generated, for example, in the absence of oxygen, or if proteins of the ETC are damaged or mutated, then glycolysis-generated ATP is imported into mitochondria where the F1-ATPase hydrolyzes this ATP to ADP and Pi, and protons flow from the matrix into the intermembrane space through F0. The maintenance of a membrane potential is vital for protein import and export from the mitochondrial matrix. Important sets of proteins synthesized in the mitochondria are those that contain iron–sulfur clusters and are exported into the cytosol; here these proteins serve functions, such as maintenance of nuclear genomic integrity. If mitochondria lose their inner mitochondrial membrane potential, then they are tagged for degradation through a process known as mitophagy. There are proteins, such as PTEN-induced putative kinase 1, that recognize depolarized mitochondria and recruit Parkin, which coats mitochondria that have low-mitochondrial-membrane potential with ubiquitin, thereby targeting them for degradation. If this mechanism to clear dysfunctional mitochondria fails, then there is a potential to accumulate these low-membrane-potential mitochondria that do not import or export protein efficiently and have diminished biosynthetic capacity and ATP generation. Thus, Mitchell's chemiosmotic theory of proton pumping is essential for mitochondria to maintain their function.
Let us crunch the numbers to figure out how much ATP is generated by NADH and FADH2 oxidation by the ETC. This is referred to as currency exchange. Three H+ are required to synthesize 1ATP when they flow back down the electrochemical proton gradient through complex V, and 1H+ is required to transport each negatively charged Pi molecule into the matrix. Based on these numbers, 1NADH oxidation by complex I results in 10H+ pumped out of the matrix by complexes I, III, and IV, and 4H+ pumped in by complex V for ATP synthesis, resulting in 10H+/4H+ = 2.5ATP/NADH. FADH2 oxidation by complex II generates only 6H+ through complexes III and IV proton pumping, resulting in 6H+/4H+ = 1.5ATP/FADH2.
THE ETC GENERATES ROS
An unfortunate consequence of electron transfer through the ETC is the generation of O2 −. The ETC is not a perfect system in which all the electrons from NADH or FADH2 are transferred eventually to complex IV and oxygen, which is the final acceptor of these electrons. There is small leakage (estimated to be <0.1%) through the system in which the electrons react in a nonenzymatic manner with oxygen to generate O2−, which is produced by a one-electron reduction of O2. O2− is rapidly converted into H2O2 by the enzyme SOD2 in the mitochondrial matrix. There are multiple proteins, such as GSH peroxidases and peroxiredoxins, which detoxify H2O2 to H2O in the mitochondrial matrix (discussed in Chandel 2020g). If the ETC complexes are not functioning properly, the levels of O2− and peroxide generation reach levels that can incur damage to mitochondrial proteins and mtDNA. Furthermore, H2O2 can leak from mitochondria, and at high levels this release can damage proteins in the cytosol, as well as oxidize guanine in the nuclear DNA. Abundant antioxidant protein levels limit the levels of ROS in the mitochondrial matrix. Based on isolated mitochondria studies, O2− production increases when the mitochondria are not making ATP and, consequently, have a high pmf as a result of limiting ADP availability in the matrix. Superoxide production also increases when there is a high NADH/NAD+ ratio in the mitochondrial matrix because of ETC inhibition. If mitochondria are actively generating ATP, then they have a lower pmf and NADH/NAD+ ratio, resulting in lower production of O2−. A paradoxical observation in cells is that decreasing or increasing oxygen levels both stimulate O2− production.
There are two unresolved questions regarding the efficiency of electron transfer through the ETC: How is it so efficient to begin with (i.e., there is only <0.1% leakage that generates O2−)? And, why has not nature selected to get rid of these “toxic molecules”? The answer to the first question might be that complexes I, III, and IV are found in distinct supercomplexes to allow for electrons to be channeled over short distances. This close proximity would increase efficiency, allowing tight coupling of electron transfer to proton pumping for the generation of membrane potential, as well as limiting electron leakage that would generate O2−. Recently, proteins such as hypoxia-inducible gene-1 (HIG1) have been identified as critical to the formation of these supercomplexes. The two mobile carriers Q and cytochrome c, which are not imbedded in the membranes, also seem to have distinct pools that are either freely mobile in the intermembrane space or bound close to the inner membrane in the intermembrane space. The answer to the second question could be that the low-level generation and release of ROS by mitochondria have a beneficial function. An emerging hypothesis is that the low levels of ROS emerging from mitochondria could serve as a mode of communication to the rest of the cell to convey its fitness. Thus, as healthy mitochondria generate low levels of ROS, their release in the cytosol targets specific ROS-sensitive proteins that are critical in maintaining homeostasis, as well as for adapting to stressful conditions. Recent studies indicate that mitochondrial-generated ROS leaking into the cytosol are necessary for optimal activation of hypoxic induction of genes for metabolic adaptation, autophagy, and innate and adaptive immunity. Furthermore, mitochondrial ROS have been shown to be beneficial in extending longevity in model organisms—a radical idea! (See Box 2.)
TRANSPORTERS THAT MOVE METABOLITES IN AND OUT OF MITOCHONDRIA
So far, we have covered how coupling of complexes of the ETC to complex V generates ATP by pmf. An important consideration recognized in Mitchell's original paper as an essential component of the proton circuit is the transport of metabolites, such as ADP and Pi into the mitochondria and transport of ATP into the cytosol. The outer mitochondrial membrane contains VDACs that allow these metabolites to be transported across this membrane. However, the inner mitochondrial membrane is impermeable to these metabolites and ions; otherwise, protons pumped by the ETC would flow right back and not go through complex V to generate ATP. The inner mitochondrial membrane contains an ANT, which catalyzes the exchange of ATP for ADP. ATP has four negative charges, whereas ADP has three negative charges at neutral pH, resulting in the loss of a net charge of –1 in the matrix. This transport uses some of the membrane potential generated by the ETC and favors the entry of ADP and exit of ATP. The phosphate transporter exchanges H2PO4− at neutral pH for OH− or cotransports with H+. The phosphate enters via the pH gradient and not by the membrane potential generated by ETC. Thus, ATP synthesis requires four protons, three for ATP synthase and one for Pi transport.
Chandel (2020a) covered how the glucose molecule becomes two pyruvate molecules through a series of metabolic reactions known as glycolysis. The two main products of glycolysis are NADH and pyruvate. If oxygen is present, then pyruvate and NADH are shuttled into the mitochondria. Both can pass through the outer mitochondrial membrane, which is permeable to small metabolites, but they need specialized mechanisms to cross the impermeable inner mitochondrial membrane. The elusive nature of the mitochondrial pyruvate carrier was deciphered recently. This carrier is composed of two proteins, MPC1 and MPC2, each ∼15 kDa in size, that form a large heterocomplex. An important detail of this transporter is that it costs one proton to transport a molecule of pyruvate into the matrix. The molecular details of how this heterocomplex functions are currently being unraveled.
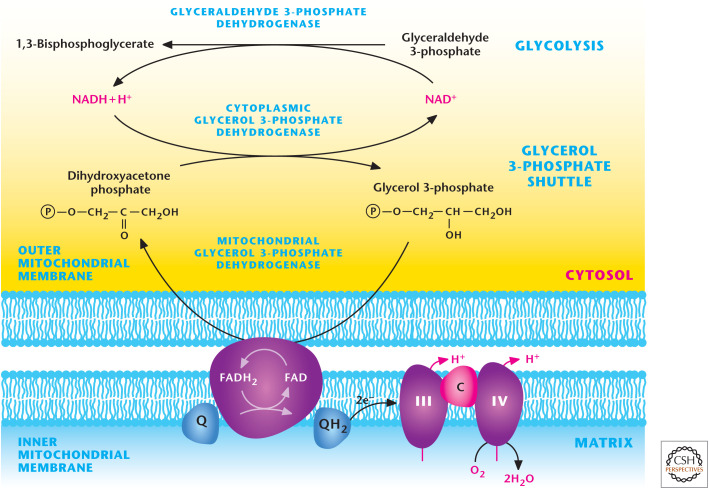
The NADH generated by glycolysis uses two shuttle mechanisms for transport into the mitochondrial matrix: the malate–aspartate and the glycerol 3-phosphate shuttles. The key enzyme in the malate–aspartate shuttle is MDH, located in the cytosol that oxidizes NADH to NAD+ to allow glycolysis to function, whereas the mitochondrial matrix MDH reduces NAD+ to NADH (Fig. 7). The glycerol 3-phosphate shuttle depends on the activity of two different glycerol 3-phosphate dehydrogenase enzymes, one using a NAD/NADH couple and the other a FAD-linked membrane-bound enzyme, which generates FADH2 that donates electrons to Q of the ETC (Fig. 8).
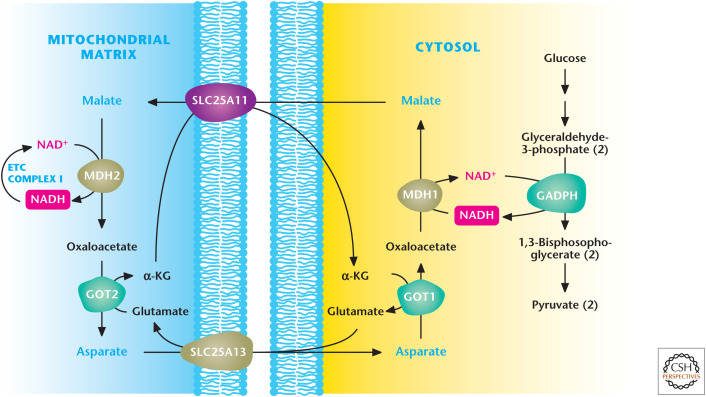
Figure 7.
Malate–aspartate shuttle. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) generates NADH during glycolysis. NADH can be regenerated to NAD+ to allow glycolysis to continue by the conversion of oxaloacetate (OAA) to malate by cytosolic malate dehydrogenase 1 (MDH1). Subsequently, malate is transported by the SLC25A1 transporter into the mitochondrial matrix, in which it is converted back to OAA coupled with NAD+ conversion into NADH. The ETC complex I converts NADH into NAD+ to keep malate dehydrogenase 2 functioning continuously. The mitochondrial OAA is converted into aspartate by aspartate aminotransferase 2 (GOT2) and, subsequently, transported into the cytosol, where aspartate can be converted back into cytosolic OAA by aspartate aminotransferase 1 (GOT1) for the shuttle to continue. α-KG, α-ketoglutarate. (Adapted with permission of themedicalbiochemistrypage, LLC.)
Figure 8.
Glycerol–phosphate shuttle. GAPDH generates NADH during glycolysis. NADH can be regenerated to NAD+ to allow glycolysis to continue by the conversion of dihydroxyacetone phosphate into glycerol 3-phosphate by cytosolic glycerol 3-phosphate dehydrogenase. Subsequently, glycerol 3-phosphate is converted back into dihydroxyacetone phosphate by coupling FAD into FADH2. FADH2 donates electrons to ubiquinone, which feeds electrons into complex III of the ETC.
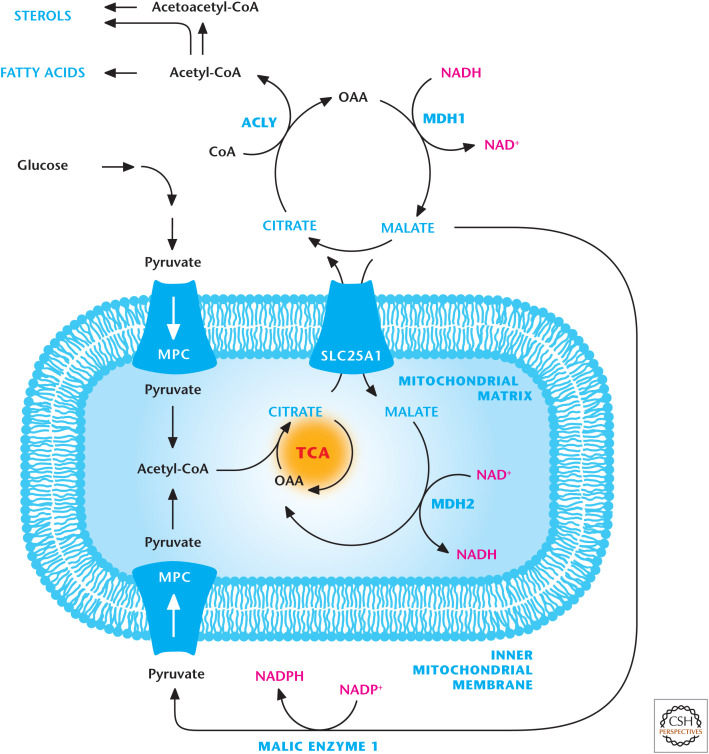
The other major transporters for citrate, isocitrate, malate, succinate, and α-ketoglutarate were quickly demonstrated (Fig. 9). These are all important transporters, but let us pay close attention to the citrate transporter (SLC25A1), which allows TCA-cycle-generated citrate to cross the inner mitochondrial membrane into the intermembrane space, where citrate goes through the VDACs located in the outer mitochondrial membrane. Citrate2– is exchanged for malate2–. Once in the cytosol, the six-carbon citrate can become two-carbon acetyl-CoA and four-carbon OAA by ATP citrate lyase (ACLY). As we discussed earlier, acetyl-CoA is an important metabolite that can be used for protein acetylation and fatty acid and sterol synthesis. The production of acetyl-CoA occurs predominantly in the mitochondria matrix. The three main sources of acetyl-CoA are pyruvate oxidation by PDH, the breakdown of fatty acids (Chandel 2020b), and catabolism of amino acids. The mitochondrial membrane is impermeable to acetyl-CoA molecules, and so the citrate transporter is a major mechanism for delivering acetyl-CoA into the cytosol.
Figure 9.
Citrate transporter. Citrate is transported into the cytosol by the SLC25A1 transporter in exchange for malate. Citrate in the cytosol is converted into acetyl-CoA and oxaloacetate (OAA) by ACLY. Acetyl-CoA is a precursor for sterol and fatty acid production. MDH1 converts the OAA into malate, which is transported into the mitochondrial matrix in exchange for citrate. Malate can also be converted into pyruvate by malic enzyme 1 (ME1).
COMPLETE OXIDATION OF GLUCOSE BY GLYCOLYSIS AND MITOCHONDRIA GENERATES 32 ATP
Using information from Chandel (2020a) on glycolysis and this review on the TCA and oxidative phosphorylation, we can now calculate how much ATP one molecule of glucose can generate (Fig. 10). From the combination of glycolysis, PDH reaction, and reducing equivalents generated by the TCA cycle, we obtain the net reaction
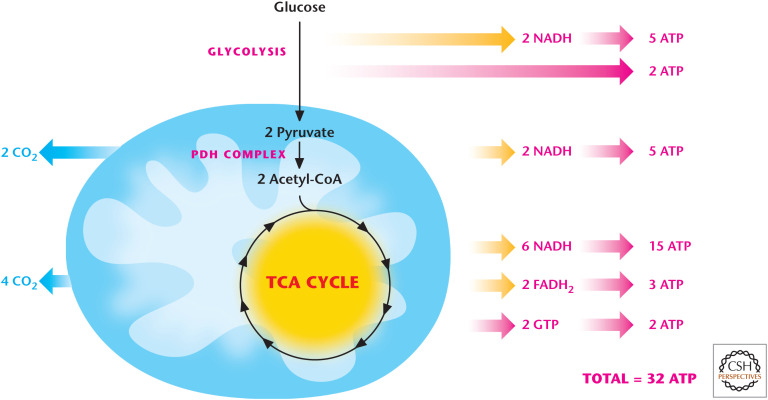
Figure 10.
Glucose oxidation generates 32 ATP. NADH generates 2.5 ATP, whereas FADH2 generates 1.5 ATP. Glucose oxidation through glycolysis and TCA cycle generates 10 NADH (25 ATP) and 2 FADH2 (3 ATP). Glycolysis and TCA cycle generate 2 ATP each through substrate level phosphorylation.
Remember that one molecule of NADH and FADH2 generates ∼2.5 ATP and ∼1.5 ATP, respectively. Therefore, glycolysis generates 2 ATP and 2 NADH (5 ATP); pyruvate oxidation to two molecules of acetyl-CoA generates 2 H (5 ATP) for a total of 12 ATP molecules. Each acetyl-CoA through the TCA cycle generates 3 NADH (7.5 ATP), 1 FADH2 (1.5 per each cycle), and 1 GTP (can be converted to ATP). Thus, one molecule of acetyl-CoA through the TCA cycle generates 10 ATP molecules and 20 ATP molecules from entry of two acetyl-CoA into the TCA cycle. The complete oxidation of glucose by the PDH complex and TCA cycle leads to the production of 30 molecules of ATP and six CO2 molecules as waste.
MULTIPLE FACTORS CONTROL CELLULAR RESPIRATION
Initial work in the 1950s by Britton Chance and G.R. Williams proposed that the respiratory rate in cells is controlled by cellular ATP use. In this model, the increase in cellular ATP use decreases cytosolic ATP levels and increases cytosolic ADP and Pi levels. The increase in cytosolic ADP levels leads to an increase in mitochondrial ADP via the increased activity of the adenine nucleotide translocase (ANT). The increased mitochondrial ADP concentration stimulates complex V to augment the rate of ATP synthesis, which results in a decrease in the mitochondrial membrane potential, thus stimulating the respiratory chain to consume oxygen.
In the ensuing decades, it has become clear that other factors also control the respiratory rate, such as the availability of reducing equivalents provided by the TCA cycle, electron flux through the ETC, the availability of ADP provided by cellular ATPases, ANT, and the magnitude of the proton leak. How to quantitatively measure the relative control exerted by these processes on the respiratory rate remained elusive until the early 1970s, when metabolic control analysis was developed. This helped determine control coefficients of a particular protein over metabolic flux through a pathway. The control coefficient is the percent change in the respiratory rate divided by the percent change in the protein or complex causing the change in the respiratory rate. For example, if a 10% change in the ANT results in a 10% change in the respiratory rate, then the control coefficient of the proton leak would be 1. However, if the 10% change resulted in 1% change in the respiratory rate, then the control coefficient would be 0.1. In the late 1980s, metabolic control analysis on isolated rat hepatocytes determined that 15%–30% of respiration is controlled by the NADH supply (this includes the pyruvate supply to the mitochondria, the TCA cycle, and any other NADH-supplying reaction), 20% is controlled by proton leakage, and 0%–15% is controlled by the ETC. The remaining 50% is controlled by ATP synthesis, transport, and use, of which, in most cells, the dominant factor is the rate of ATP use by cellular processes.
MITOCHONDRIA REGULATE CALCIUM HOMEOSTASIS
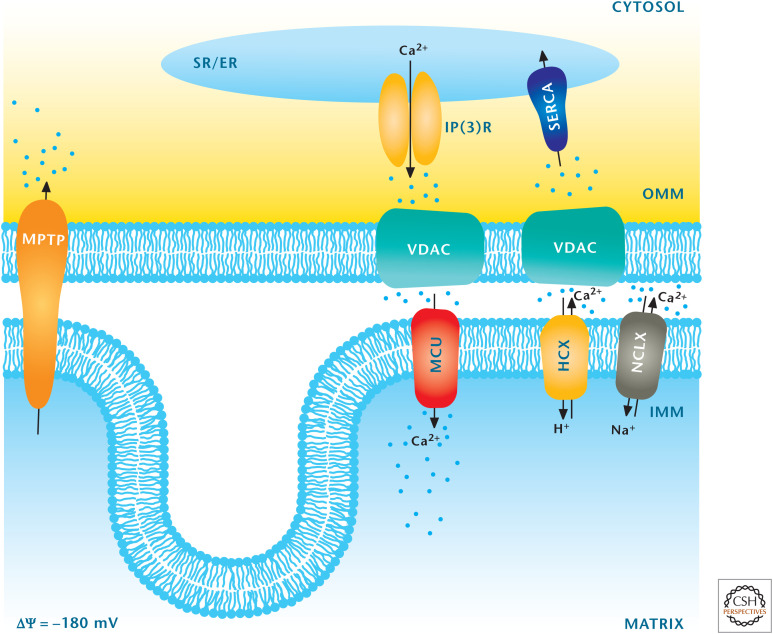
Calcium is an essential ion for multiple processes in the cell, and maintenance of calcium homeostasis is crucial for cells to function properly. An increase in cytosolic calcium has to be quickly restored to normal levels. Most of us think that the endoplasmic reticulum (ER) is the major organelle that rapidly takes up calcium to maintain calcium homeostasis. An underappreciated fact is that mitochondria have the capacity to take up calcium. The proton gradient generated by the ETC allows uptake of Ca2+ through a recently identified mitochondrial calcium uniporter (MCU) into the mitochondrial matrix. The MCU is located on the inner mitochondrial membrane. If the proton gradient dissipates (e.g., as when the protonophore FCCP is administered), then calcium is released from the mitochondrial matrix. There are normal mechanisms to release calcium from the matrix through antiporters that drive Ca2+ out of the mitochondrial matrix in exchange for either Na+ or H+ (Fig. 11). A key feature of these antiporters is that they saturate at low calcium and their kinetics are slow. Thus, the mitochondrial calcium levels are regulated by a high Vmax uptake uniporter (MCU) coupled to a slow and saturable system for efflux. This increases the risk of calcium overload in mitochondria, which then leads to a large increase in permeability of the inner mitochondrial membrane. The result is a loss of solutes having a molecular weight <1.5 kDa and cell death (apoptosis). This nonselective, large-conductance channel is called the mitochondrial permeability transition pore (MPTP). Mitochondrial calcium overload that triggers MPTP formation has been implicated in heart and brain ischemic injury, as well as in several neurodegenerative disorders, including Parkinson's disease. However, calcium uptake in the physiological range stimulates oxidative phosphorylation through allosteric activation of PDH, isocitrate dehydrogenase, and α-ketoglutarate dehydrogenase, as well as stimulation of the ATP synthase (complex V) and ANT. This allows for a coordinated up-regulation of oxidative phosphorylation caused by elevated mitochondrial calcium. Many cells increase their metabolic demand as a result of an increase in calcium. For example, the increase in cytosolic calcium triggers muscle contractility. This increased cytosolic calcium is sequestered in the mitochondrial matrix in which concomitant up-regulation of oxidative phosphorylation provides sufficient ATP to sustain contractility.
Figure 11.
Mitochondria regulate calcium homeostasis. The major organelle that regulates calcium is the ER, which takes up calcium into the ER by sarco/ER Ca2+-ATPase (SERCA) and releases calcium from the ER into the cytosol by inositol trisphosphate receptor (InsP3R). Mitochondria can also regulate calcium by sequestering cytosolic calcium through the MCU. Calcium is transported from mitochondria into the cytosol through the mitochondrial H+–Ca2+ exchanger (HCX) and mitochondrial Na+–Ca2+ exchanger (NCLX) transporters. (Adapted from Raffaello et al. 2012, with permission from Elsevier.)
MITOCHONDRIAL BIOSYNTHETIC ACTIVITY CAN BE UNCOUPLED FROM ATP-GENERATING CAPACITY
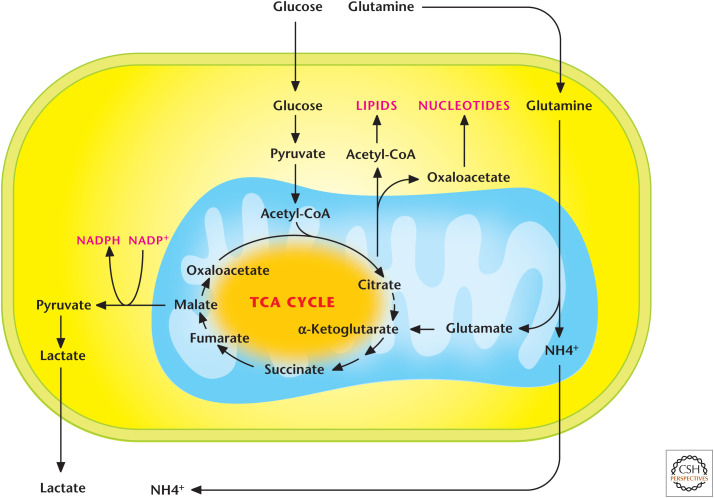
The earliest TCA cycle evolved in the absence of oxygen, thus it was not an oxidative clockwise cycle but rather a reductive counterclockwise cycle in which NADH or NADPH was consumed to drive the cycle and generate the intermediates that served as precursors for biosynthesis. Can mammalian cells generate TCA-cycle intermediates without generating ATP? There are instances in which the ETC is inhibited when cells are exposed to low-oxygen conditions or proteins of the ETC are mutated in certain diseases. In these scenarios, glycolysis has a remarkable capacity to generate the necessary ATP to sustain cell survival, and this ATP is imported into the matrix to generate a mitochondrial inner membrane potential by complex V hydrolysis of ATP. But, how are TCA-cycle intermediates generated in this case? As we learned earlier in this review, citrate is an essential TCA-cycle intermediate because it can be exported through the inner and outer mitochondrial membranes into the cytosol, where it is converted into acetyl-CoA and OAA by the enzyme ACLY. Cells with functional mitochondria generate acetyl-CoA from pyruvate and combine it with OAA to generate citrate, which is exported out to the cytosol, resulting in depletion of TCA-cycle intermediates, including OAA (Fig. 12). Glutaminolysis provides α-ketoglutarate, which enters the TCA cycle to generate OAA, which, in turn, condenses with pyruvate-generated acetyl-CoA to produce citrate.
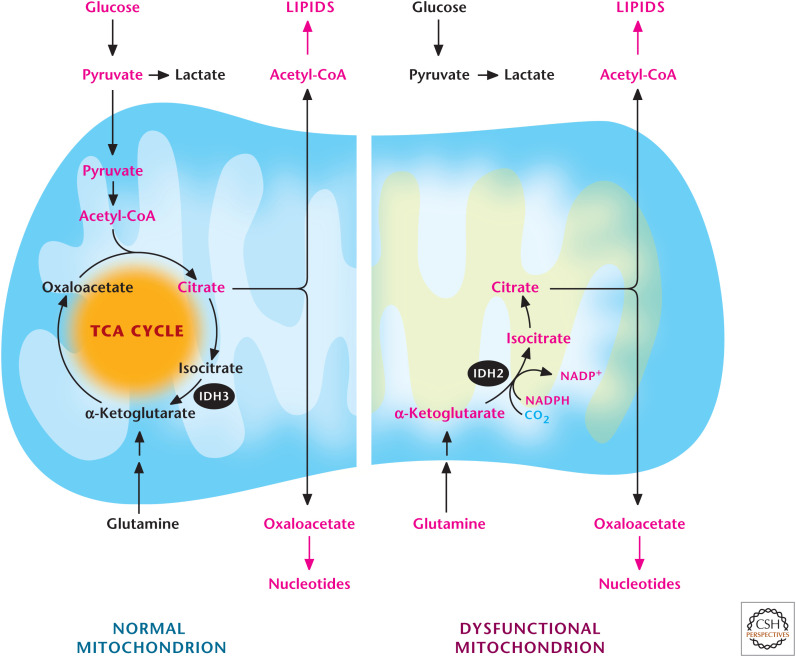
Figure 12.
Glutamine-dependent reductive carboxylation. Cells that have functional mitochondria can use glutamine to generate α-ketoglutarate to produce OAA and use pyruvate to generate acetyl-CoA. OAA and acetyl-CoA generate citrate, which can be exported into the cytosol to produce de novo lipids and nucleotides. Cells that have dysfunctional mitochondria due to loss-of-function mutations of proteins in the TCA cycle after the α-ketoglutarate dehydrogenase step or in the ETC cannot oxidize pyruvate into acetyl-CoA. They convert glutamine into α-ketoglutarate, which subsequently becomes citrate through a reverse IDH2-dependent reaction.
In mammalian cells with dysfunctional mitochondria, the TCA cycle can partly reverse itself to generate citrate through a process called glutamine-dependent reductive carboxylation. This recently discovered mechanism begins with conversion of glutamine to glutamate, which is converted to α-ketoglutarate and enters the TCA cycle. The five-carbon α-ketoglutarate is converted by two subsequent reactions catalyzed by NADPH-dependent isocitrate dehydrogenase 2 (IDH2) and aconitase to generate six-carbon citrate molecules through a carboxylation reaction using the reductive power of NADPH (Fig. 12). This finding illustrates that modern mitochondria also display robust metabolic plasticity just as their bacterial evolutionary predecessors have.
MITOCHONDRIA ARE SIGNALING ORGANELLES
The two major roles of mitochondria—the production of energy and support of biosynthesis—make them central to diverse biological outcomes, including proliferation, differentiation, and adaptation to stress. The classical conception of biological outcomes is that they are driven by commands from the nucleus, and changes in mitochondrial metabolism occur simply as a consequence of these commands. Mitochondria are rarely considered to dictate commands or provide signals themselves to change biological outcomes. However, should the cell commit to a process like proliferation or differentiation without adequate functioning mitochondria, it would likely undergo a metabolic crisis resulting in cell death or senescence. For optimal cell function, a health-status-feedback mechanism should exist in mitochondria to act as a checkpoint before cellular action. This feedback is analogous to the fuel gauge on your car, which predicts the distance you may drive. Thus, a mitochondrial checkpoint is required before cells commit to distinct biological outcomes, such as proliferation or differentiation. This reasoning suggests that mitochondria play a causal role in determining biological outcomes.
Over the past 20 years, there have been many studies showing that mitochondria play a vital role in signaling by releasing (1) metabolites, such as acetyl-CoA for protein acetylation, (2) proteins, such as cytochrome c, that induce caspase-dependent cell death, and (3) ROS, which can either inhibit or activate certain signaling pathways. Changes in mitochondrial ATP generation can also be transmitted to cytosol through the activation of AMP-activated protein kinase (AMPK). This kinase responds to a decrease in ATP levels concomitant with an increase in AMP levels. AMPK causes cells to decrease anabolic functions and go into a catabolic state to generate energy.
Mitochondria also control signaling by serving as a scaffold for signaling complexes through the tethering of signaling proteins to the outer mitochondrial membrane. One example is mitochondrial antiviral-signaling protein, which is important for appropriate responses to viral infection. Why this protein has to be on the outer mitochondrial membrane remains a mystery. But, there is a growing number of proteins that participate in signaling pathways that are in close proximity to mitochondria.
An exciting new area in mitochondrial signaling is the field of mitochondrial dynamics. Mitochondria are constantly undergoing fission and fusion, as well as moving around the cell. Depending on the environmental or internal signals, mitochondria can move around the cell to appropriate sites to participate in signaling, as well as undergo fission or fusion. Typically, fission-produced mitochondria tend to be less robust bioenergetically than fused mitochondria.
Finally, cells that have undergone necrosis and spilled their contents into the blood can invoke inflammation. Recent studies indicate that mtDNA can activate Toll-like receptor signaling on monocytes, resulting in activation of inflammatory cytokines. Going forward, one of the most exciting aspects of mitochondrial biology will be to understand how mitochondria participate in regulating signaling events in the cytosol to dictate biological outcomes.
MITOCHONDRIA AND DISEASE
One of the most devastating classes of diseases are those linked to mutations that impair oxidative phosphorylation; these affect at least 1 in 5000 live births. Mitochondrial diseases are caused by either inherited or spontaneous mutations in mitochondrial or nuclear DNA that change the function of mitochondrial proteins or RNA molecules. As mentioned earlier, mtDNA encodes for only 37 of the 3000 genes that create a mitochondrion. Many of the mtDNA-encoded genes make proteins that are critical subunits of the ETC. Several of these mitochondrial diseases occur early in infancy. They present with lactic acidosis, blindness, peripheral neuropathy, skeletal myopathy, deafness, and neurodegeneration, and cause tremendous suffering in the affected patient. Many of these diseases cause extensive damage to cells of the skeletal muscles, brain, heart, and liver.
One common early-onset mitochondrial disorder is MELAS (mitochondrial encephalomyopahty, lactic acidosis, and stroke-like episodes). MELAS is mainly caused by mutations in genes encoded by mDNA for mitochondrial-specific transfer RNAs, and it is maternally inherited. Interestingly, different mutations in mitochondrial and nuclear DNA can result in the same diseases. An example is Leigh syndrome, which is characterized by abnormalities in the brain stem, cerebellum, and basal ganglia and often displays elevated lactic acidosis. Mitochondrial disease symptoms can also by mimicked by drugs that have off-target toxicity. Fialuridine, an antiviral drug for hepatitis B, caused lactic acidosis, neuropathy, hepatic failure, and myopathy in a subset of patients in a phase II clinical trial because of an off-target effect on mitochondrial function, and the trial was halted. Interestingly, many widely prescribed therapeutics, including statins, antibiotics, and the HIV drug, zidovudine, are known to have off-target effects on mitochondria that impair function.
An exciting area of growth in the mitochondrial-linked diseases is the accumulating evidence that mitochondrial dysfunction is connected to several common diseases, such as diabetes, obesity, cancer, and neurodegenerative disorders. Progressive decline in the expression of mitochondrial genes is also a prominent feature of normal human aging, whereas caloric restriction, which may increase fitness and life span in humans, causes a robust increase in mitochondrial biogenesis. The big unanswered question is whether the decline in mitochondrial function during normal aging or many of the common diseases is causal or a consequence. In fact, it is not fully understood whether slight mitochondrial impairment observed in some diseases or in aging is beneficial or harmful. Recent data in model organisms ranging from yeast to mice indicate that diminishing mitochondrial function can promote metabolic health and longevity.
Footnotes
From the recent volume Navigating Metabolism by Navdeep S. Chandel
Additional Perspectives on Metabolism available at www.cshperspectives.org
SUGGESTED READING
*Reference is also in this collection.
- Anso E, Mullen AR, Felsher DW, Matés JM, Deberardinis RJ, Chandel NS. 2013. Metabolic changes in cancer cells upon suppression of MYC. Cancer Metab 1: 7. 10.1186/2049-3002-1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD, Kesseler A. 1995. Control analysis of energy metabolism in mitochondria. Biochem Soc Trans 23: 371–376. 10.1042/bst0230371 [DOI] [PubMed] [Google Scholar]
- Brand MD, Nicholls DG. 2011. Assessing mitochondrial dysfunction in cells. Biochem J 435: 297–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B, Williams GR. 1955. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem 217: 409–427. [PubMed] [Google Scholar]
- Chandel NS. 2014. Mitochondria as signaling organelles. BMC Biol 12: 34. doi:10.1186/s12915-014-0085-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Chandel NS. 2020a. Glycolysis. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a040535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Chandel NS. 2020b. Lipid metabolism. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a040576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Chandel NS. 2020c. Amino acid metabolism. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a040584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Chandel NS. 2020d. Basics of metabolic reactions. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a040527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Chandel NS. 2020e. Carbohydrate metabolism. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a040568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Chandel NS. 2020f. Signaling and metabolism. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a040600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Chandel NS. 2020g. NADPH—the forgotten reducing equivalent. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a040550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefani D, Rizzuto R. 2014. Molecular control of mitochondrial calcium uptake. Biochem Biophys Res Commun 449: 373–376. doi:10.1016/j.bbrc.2014.04.142 [DOI] [PubMed] [Google Scholar]
- Gray MW. 2012. Mitochondrial evolution. Cold Spring Harb Perspect Biol 4: a011403. doi:10.1101/cshperspect.a011403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs HA. 1937. The intermediate metabolism of carbohydrates. Lancet 230: 736–738. [Google Scholar]
- Krebs HA, Johnson WA. 1937. The role of citric acid in intermediate metabolism in animal tissues. Enzymologia 4: 148–156. [DOI] [PubMed] [Google Scholar]
- Lane N. 2014. Bioenergetic constraints on the evolution of complex life. Cold Spring Harb Perspect Biol 6: a015982. doi:10.1011/cshperspect.a015982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapuente-Brun E, Moreno-Loshuertos R, Acín-Pérez R, Latorre-Pellicer A, Colás C, Balsa E, Perales-Clemente E, Quirós PM, Calvo E, Rodríguez-Hernández MA, et al. 2013. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science 340: 1567–1570. doi:10.1126/science.1230381 [DOI] [PubMed] [Google Scholar]
- Lindley D, Clarke M. 1988. Nobel prizes announced for physics and for chemistry. Nature 335: 752–753. doi:10.1038/335752a0 [DOI] [PubMed] [Google Scholar]
- Liu X, Kim CN, Yang J, Jemmerson R, Wang X. 1996. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86: 147–157. doi: 10.1016/s0092-8674(00)80085-9 [DOI] [PubMed] [Google Scholar]
- Martin W, Müller M. 1998. The hydrogen hypothesis for the first eukaryote. Nature 392: 37–41. doi:10.1038/25040 [DOI] [PubMed] [Google Scholar]
- McKenzie R, Fried MW, Sallie R, Conjeevaram H, Di Bisceglie AM, Park Y, Savarese B, Kleiner D, Tsokos M, Luciano C, et al. 1995. Hepatic failure and lactic acidosis due to fialuridine (FIAU), an investigational nucleoside analogue for chronic hepatitis B. N Engl J Med 333: 1099–1105. doi:10.1056/NEJM199510263331702 [DOI] [PubMed] [Google Scholar]
- Mitchell P. 1961. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191: 144–148. doi:10.1038/191144a0 [DOI] [PubMed] [Google Scholar]
- Murphy MP. 2009. How mitochondria produce reactive oxygen species. Biochem J 417: 1–13. doi:10.1042/BJ20081386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunnari J, Suomalainen A. 2012. Mitochondria: in sickness and in health. Cell 148: 1145–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliarini DJ, Rutter J. 2013. Hallmarks of a new era in mitochondrial biochemistry. Genes Dev 27: 2615–2627. doi:10.1011/gad.229724.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaello A, De Stefani D, Rizzuto R. 2012. The mitochondrial Ca2+ uniporter. Cell Calcium 52: 16–21. doi: 10.1061/j.ceca.2012.04.006 [DOI] [PubMed] [Google Scholar]
- Sena LA, Chandel NS. 2012. Physiological roles of mitochondrial reactive oxygen species. Mol Cell 48: 158–167. doi:10.1016/j.molcel.2012.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vafai SB, Mootha VK. 2012. Mitochondrial disorders as windows into an ancient organelle. Nature 491: 374–383. doi:10.1038/nature11707 [DOI] [PubMed] [Google Scholar]
- Weber BH. 1991. Glynn and the conceptual development of the chemiosmotic theory: a retrospective and prospective view. Biosci Rep 11: 577–617. doi:10.1007/BF01130219 [DOI] [PubMed] [Google Scholar]
- West AP, Shadel GS, Ghosh S. 2011. Mitochondria in innate immune responses. Nat Rev Immunol 11: 389–402. doi:10.1038/nri2975 [DOI] [PMC free article] [PubMed] [Google Scholar]