Abstract
Bryophytes, including liverworts, mosses, and hornworts, are gametophyte-dominant land plants that are derived from a common ancestor and underwent independent evolution from the sporophyte-dominant vascular plants since their divergence. The plant hormone auxin has been shown to play pleiotropic roles in the haploid bodies of bryophytes. Pharmacological and chemical studies identified conserved auxin molecules, their inactivated forms, and auxin transport in bryophyte tissues. Recent genomic and molecular biological studies show deep conservation of components and their functions in auxin biosynthesis, inactivation, transport, and signaling in land plants. Low genetic redundancy in model bryophytes enable unique assays, which are elucidating the design principles of the auxin signaling pathway. In this article, the physiological roles of auxin and regulatory mechanisms of gene expression and development by auxin in Bryophyta are reviewed.
Land plants evolved from ancestral charophycean algae approximately 500 million years ago (Morris et al. 2018). Among the extant land plants, bryophytes, including liverworts, mosses, and hornworts, are the basalmost lineages that diverged from other land plants. Recent phylogenetic analyses support the monophyly of bryophytes (Nishiyama et al. 2004; Cox et al. 2014; Wickett et al. 2014; Puttick et al. 2018), and the synapomorphy of “Bryophyta” is the haploid gametophyte-dominant life cycle. Germinated spores develop filamentous protonemata or globular sporelings, which in turn give rise to three-dimensionally (3D) organized gametophores (in leafy liverworts and mosses) or thalli (in thalloid liverworts and hornworts), from which reproductive organs that contain germ cells develop. After fertilization, diploid sporophytes, consisting of a foot, seta or intercalary meristem, and sporogenous tissue, develop on the haploid bodies (Ligrone et al. 2012) and finally generate haploid spores via meiosis.
The plant hormone auxin is known to be involved in almost all aspects of development through regulation of cell division, expansion, and differentiation in sporophyte-dominant angiosperms (Demeulenaere and Beeckman 2014; Dresselhaus and Schneitz 2014; Landrein and Vernoux 2014; Rios et al. 2014). Classic auxin- and inhibitor-treatment experiments showed that in gametophyte-dominant bryophytes, auxin plays diverse roles in both gametophyte and sporophyte generations. Additionally, recent molecular approaches in model bryophytes and omics data revealed that the core components in auxin biology, including biosynthesis, metabolism, transport, and signaling, are highly conserved among land plants. At present, two model bryophytes, the moss Physcomitrium (Physcomitrella) patens and the liverwort Marchantia polymorpha, are widely used in part because of the ease of transformation and gene disruption, low genetic redundancy compared with angiosperms, and simple developmental traits (Rensing et al. 2008, 2020; Ishizaki et al. 2016; Bowman et al. 2017; Lang et al. 2018; Montgomery et al. 2020). These model bryophytes provide unique platforms for investigating auxin biology.
This article provides an overview of auxin biology in Bryophyta, including developmental roles revealed by pharmacological assays, homeostatic regulation revealed by chemical approaches, design principles of the nuclear auxin signaling (NAS) pathway to which genetic studies in model bryophytes contributed, and mechanisms underlying developmental regulation revealed by molecular biological studies. See also Bowman (2016), Kato et al. (2018), and Thelander et al. (2018) for reviews of classical auxin studies in gemma and gemmalings of M. polymorpha, recent auxin studies in M. polymorpha, and recent auxin studies in P. patens, respectively.
PHARMACOLOGICAL EFFECTS OF AUXINS AND THEIR INHIBITORS ON BRYOPHYTE DEVELOPMENT
Mosses spend a long time as protonemata, which consist of functionally distinct, interconvertible feeding (chloronema) and foraging (caulonema) filaments (Cove 2005). Some lateral branches on caulonema give rise to buds through several unique divisions, which in turn form 3D gametophores with stem-leaf structures (Harrison et al. 2009; Kofuji and Hasebe 2014). Exogenously supplied auxin was shown to inhibit protonema branching, induce caulonema differentiation, and promote bud initiation in caulonema together with cytokinin (Johri and Desai 1973; Ashton et al. 1979; Sood and Hackenberg 1979; Thelander et al. 2018). Because auxin transport inhibitors, N-1-naphthylphthalamic acid (NPA) and 3,4,5-triiodobenzoic acid, inhibit auxin-mediated caulonema differentiation, active transport seems necessary to accumulate sufficient auxin (Cove and Ashton 1984).
Moss gametophores, which harbor stem cell regions at their apices where leaves and reproductive organs arise, develop multicellular rhizoids from the basal epidermis (Sakakibara et al. 2003; Harrison et al. 2009; Kofuji and Hasebe 2014) and show various responses to exogenous auxin depending on the type and concentration of auxin used. Generally, low auxin enhances shoot development by elongating stems and increasing leaves, whereas high auxin arrests growth by stunting stalks and inhibiting leaf formation (Ashton et al. 1979; Bennett et al. 2014b; Lavy et al. 2016). Exogenous auxin generally elongates leaves because of longitudinal hyperelongation of cells (Barker 2011). Rhizoid development is also strikingly stimulated by auxin application (Ashton et al. 1979; Nyman and Cutter 1981; Chopra and Vashistha 1990; Sakakibara et al. 2003).
Various liverworts also show enhanced and/or ectopic rhizoid development in response to exogenous auxin, while excessive auxin sometimes suppresses rhizoid formation (LaRue and Narayanaswami 1957; Ilahi and Allsopp 1969; Allsopp and Ilahi 1970a,b). In the case of M. polymorpha (for its life cycle, see Fig. 1), auxin application enhances rhizoid development in sporelings and gemmalings (Rousseau 1950; Tarén 1958; Maravolo and Voth 1966; Allsopp et al. 1968; Ishizaki et al. 2012; Flores-Sandoval et al. 2015), whereas l-kynurenine (l-Kyn), an indole-3-acetic acid (IAA)-biosynthesis inhibitor, decreases rhizoids in growing thalli (Fig. 1; He et al. 2011; Flores-Sandoval et al. 2015). Under normal growth conditions, gemmae give rise to rhizoids from their ventral side after detaching from gemma cups as a germination process, which is regulated by light via phytochromes (Otto and Halbsguth 1976; Inoue et al. 2016). However, in the presence of moderate concentrations of auxin, rhizoids also emerge from the dorsal sides of the gemmae, even in the dark, suggesting that exogenous auxin compensates for light signals during gemma germination (Fig. 1; Rousseau 1954; Otto and Halbsguth 1976).
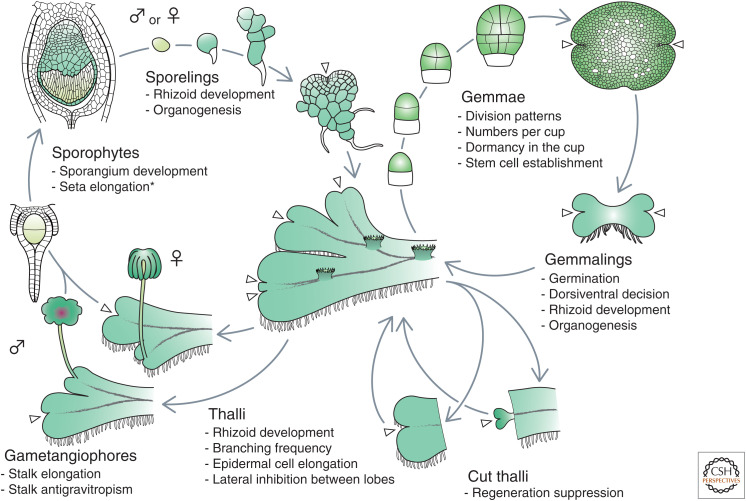
Figure 1.
Auxin-regulated processes in thalloid liverwort throughout the life cycle. Marchantia polymorpha is depicted as a representative thalloid liverwort. Sporelings of M. polymorpha quickly establish 3D thalli, which have meristematic stem cell regions at the apical notches (arrowheads) and outgrow through repetitive bifurcations of the notches (Inoue 1960; Shimamura 2016; Solly et al. 2017). Thalli produce single-celled rhizoids on the ventral surface for substrate anchoring and develop the assimilatory organ air chamber with a single air pore and the asexual reproductive organ gemma cup on the dorsal surface, in which multicellular elliptic discoid propagules, gemmae, with notches at both longitudinal tips are produced and kept dormant (Barnes and Land 1908; Apostolakos et al. 1982; Shimamura 2016; Kato et al. 2020b; Suzuki et al. 2020). Outside the cup, gemmae germinate and develop into thalli via gemmalings. Upon thallus excision, new thalli regenerate only from the pieces without apical notches. Male and female gametangiophores, consisting of stalks and heads, emerge from notches of thalli, which in turn develop sporophytes through fertilization (Shimamura 2016). Auxin-regulated processes are listed below each developmental stage. Illustrations of the largest, the second-largest sporelings, and the mature gemma are modified from Kny (1890). See also Thelander et al. (2018) for auxin responses in the moss Physcomitrella patens. *Auxin responses not examined in M. polymorpha.
Auxin is also involved in gemma dormancy within gemma cups. In the thalloid liverwort Lunularia cruciata, surgical excision of apical notches caused gemma germination within gemma cups on the remaining thalli, which was suppressed by IAA applied to the cut faces (LaRue and Narayanaswami 1957), suggesting that mobile signals from apical notches keep gemmae dormant within gemma cups and that IAA is a potential candidate. M. polymorpha grown in the presence of l-Kyn showed an elevated ratio of gemma cups containing nondormant gemmae, supporting the concept that IAA facilitates gemma dormancy within gemma cups (Fig. 1; Eklund et al. 2015). Ectopic regeneration is also suppressed by apical notch–derived mobile signals, probably IAA, because excision of apical notches caused regeneration from the cutting faces of the remaining thalli, which was suppressed by IAA application in L. cruciata and M. polymorpha (Fig. 1; Vöchting 1885; LaRue and Narayanaswami 1957; Rota and Maravolo 1975; Nishihama et al. 2015). Suppression of regeneration by auxin has also been reported in several phylogenetically distant liverworts (Allsopp and Ilahi 1970c; Ilahi and Allsopp 1970b). Thus, in general, asexual propagation is prevented by auxin.
Inhibitory effects of IAA on apical notches are also observed in intact thalli. Davidonis and Munroe (1972) showed that in M. polymorpha, notch bifurcations often resulted in two lobes with different growth rates and that surgical separation at an early stage after bifurcation released the smaller lobe from inhibition, which was suppressed by IAA application. Lateral inhibition seems to occur between the bifurcated lobes mediated by IAA (Fig. 1).
Moss gametophores also show inhibition by the apex: lateral branch primordia and basal stem regeneration were reactivated by removing apices, both of which were suppressed by IAA application to the decapitated stem tips (MacQuarrie and von Maltzahn 1959; Coudert et al. 2015). Auxin is also involved in regeneration in the moss Splachnum ampullaceum because regeneration of isolated leaves was promoted or inhibited by IAA application at low or high concentrations, respectively (MacQuarrie and von Maltzahn 1959).
Thallus shapes are also affected by auxin as l-Kyn caused a dose-dependent reduction of thallus growth in M. polymorpha (Solly et al. 2017). In general, exogenous auxin strongly inhibits growth at high concentrations, but slightly enhances growth at low concentrations in liverworts (Ilahi and Allsopp 1969, 1970a; Allsopp and Ilahi 1970b; Ishizaki et al. 2012), although the detailed effects on thallus growth vary according to plant species and auxin types. In Riccardia multifida, various auxins promoted and inhibited thallus elongation and branching at low and high concentrations, respectively. In Moerckia flotowiana, Pellia epiphylla, and Makinoa crispata, high auxin suppressed wing development and causes cylindrical thalli (Ilahi and Allsopp 1969). These results suggest that auxin greatly contributes to overall plant shapes.
In vascular plants, auxin is known to be a major player in tropic responses to suit environmental cues (Retzer et al. 2014). In M. polymorpha, the dorsiventrality of gemmalings is determined by sensing gravity soon after gemma germination, and exogenous auxin disturbs proper dorsiventral determination (Fig. 1; for review, see Bowman 2016). Auxin was also reported to elongate and bend stalks of gametangiophores, although the correlation with gravitropic or phototropic responses was not discussed (Fig. 1; Rousseau 1953). In P. patens, auxin-mediated negative gravitropism in the dark and positive phototropism were observed in gametophores (Cove et al. 1978; Bennett et al. 2014a; Bao et al. 2015). In the moss Ceratodon purpureus, the auxin efflux inhibitors NPA and pyrenoylbenzoic acid (PBA) inhibited the gravitropic curvature of protonemal cells (Schwuchow et al. 2001).
Auxin functions in sporophyte generation. Auxin promoted seta elongation in the liverwort P. epiphylla and the moss Polytrichum ohioense (Fig. 1; Schnepf et al. 1979; Thomas 1980; Poli et al. 2003). In the hornworts Anthoceros laevis and Anaxyrus punctatus, 2,4-dichlorophenoxyacetic acid (2,4-D) inhibited the elongation of sporophytes but increased their diameter (Rousseau 1958). In the hornwort Phaeoceros personii, although the application of IAA failed to promote or impede sporophyte elongation, an auxin antagonist, p-chlorophenoxyisobutyric acid (PCIB), reduced sporophyte elongation (Poli et al. 2003). In P. patens, NPA has been reported to cause branching of sporangia (Fujita et al. 2008).
AUXIN HOMEOSTASIS: BIOSYNTHESIS, METABOLISM, AND TRANSPORT
Auxin levels are controlled by biosynthesis, temporary inactivation, permanent degradation, intercellular transport, and isolation into several intracellular compartments (not mentioned here). In vascular plants, IAA is a major intrinsic auxin, which is also detected in bryophyte species and more basal algal sisters (Löbenberg 1959; Fries 1964; Schneider et al. 1967; Ashton et al. 1985; Atzorn et al. 1990; Cooke et al. 2002). In Arabidopsis thaliana, the indole-3-pyruvic acid (IPyA) pathway is known to be a major IAA biosynthesis route, where tryptophan is converted to IPyA and then to IAA. Each reaction is catalyzed by TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS 1/TRYPTOPHAN AMINOTRANSFERASE-RELATED (TAA1/TAR) and YUCCA (YUC), respectively (for review, see Kasahara 2016), both of which are conserved in all three taxa of Bryophyta (Rensing et al. 2008; Eklund et al. 2015; Bowman et al. 2017; Li et al. 2020; Zhang et al. 2020). TAA1/TAR is inactivated by phosphorylation at an evolutionarily conserved Thr residue in an auxin-dependent manner. MpTAA, the sole TAA1 homolog in M. polymorpha, loses its enzymatic activity by a phosphorylation-mimicking mutation at the corresponding residue (Wang et al. 2020). The knockout of MpTAA at the sporeling stage results in a cell mass phenotype without any organ differentiation, which is partially rescued by exogenous IAA (Eklund et al. 2015). Likewise, the IPyA pathway mediated by PpTARs and PpYUCs is the main IAA biosynthesis route in P. patens (Landberg et al. 2020), which is consistent with competitive effects on caulonema differentiation between a YUC inhibitor and exogenous auxin (Tsugafune et al. 2017). These results suggest that the IPyA pathway and its regulatory systems exist in the common ancestor of land plants and that the IPyA pathway is a major IAA source and plays pivotal roles in the development and environmental responses of bryophytes. Indeed, MpTAA was reported to be regulated under the control of the circadian clock to regulate the nyctinastic movement of M. polymorpha thalli (Lagercrantz et al. 2020). However, other IAA biosynthetic pathways have not yet been the focus in bryophytes, although their existence is implied by the finding that Mptaa plants still contain detectable levels of IAA (Eklund et al. 2015).
In addition to IAA, phenylacetic acid (PAA) is detected in a broad range of plants, including angiosperms, P. patens, and M. polymorpha (Sugawara et al. 2015). In Arabidopsis, IAA and PAA show different biochemical features, such as affinity to coreceptors and transport profiles. Although PAA shows auxin activity in P. patens, physiological differences between IAA and PAA remain unclear in bryophytes (Sugawara et al. 2015).
In Arabidopsis, IAA is temporarily inactivated by GRETCHEN HAGEN 3 (GH3)-mediated conjugation with amino acids (Staswick et al. 2005) and by UDP-glycosyltransferase-mediated ester linking with glucose (Jackson et al. 2001), or permanently inactivated by DIOXYGENASE FOR AUXIN OXIDATION 1 (DAO1)-mediated conversion into 2-oxindole-3-acetic acid (ox[IAA]; Porco et al. 2016; Zhang et al. 2016). Both amide- and ester-linked IAA conjugates were detected in the three Bryophyta taxa. oxIAA has been detected in some liverwort and moss species (Sztein et al. 1995, 1999, 2000; Ludwig-Müller et al. 2009b; Záveská Drábková et al. 2015; Landberg et al. 2020). Sztein et al. proposed that free-IAA levels are primarily regulated via the balance between biosynthesis and degradation in liverworts, whereas they are regulated by competitive conjugation versus hydrolysis of conjugates in hornworts and mosses, based on their observation that hornwort and moss species exhibited higher conjugation rates than liverwort species (Sztein et al. 1995, 1999, 2000; for review, see Cooke et al. 2002). However, Záveská Drábková et al. (2015) reached the opposite conclusion that liverworts preferred conjugation, whereas mosses favored degradation to maintain IAA homeostasis because liverwort species exhibited higher amino acid conjugation rates than moss species in their analysis. Although these studies could not be simply compared because of the differences in plant species and growth conditions, the conservation of both conjugation and degradation strategies to sustain IAA homeostasis in Bryophyta could be determined. Indeed, two GH3 homologs in P. patens have been shown to mediate IAA conjugation to amino acids (Ludwig-Müller et al. 2009a,b) and are thought to regulate free IAA levels under unfavorable environmental conditions (Mittag et al. 2015). Further molecular studies of GH3 and other IAA metabolic enzymes, which are conserved in the genomes of bryophytes (Rensing et al. 2008; Bowman et al. 2017; Li et al. 2020; Zhang et al. 2020), are needed to clarify the strategies for IAA homeostasis.
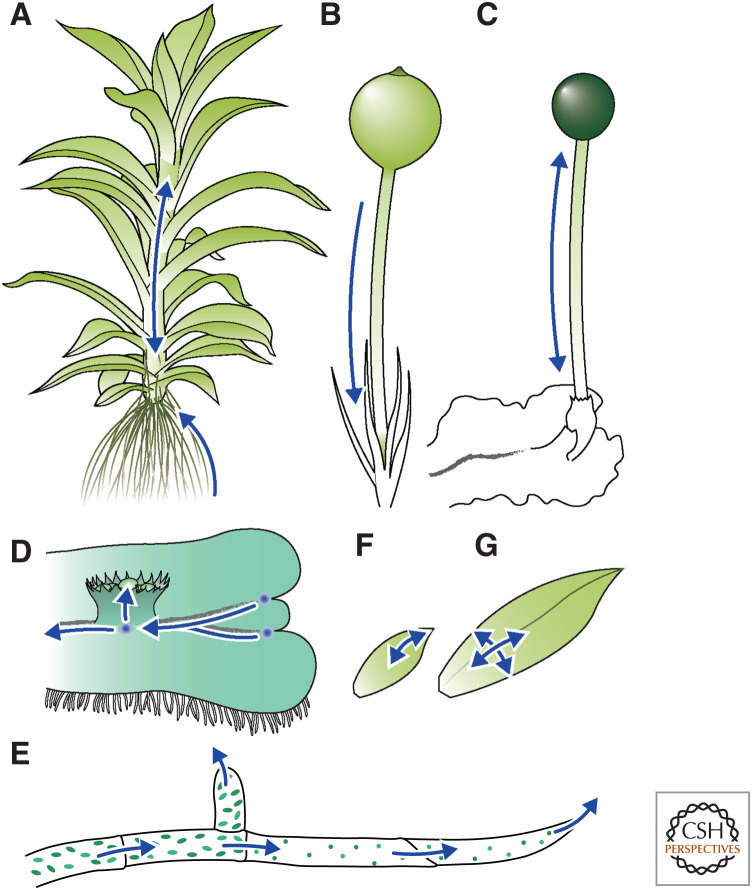
In Arabidopsis, in addition to several active auxin transporters, including PIN-FORMED (PIN) (for review, see Grones and Friml 2015), a plasmodesmatal auxin transport controlled by callose deposition has been suggested, which occurs in P. patens (Han et al. 2014; Coudert et al. 2015). P. patens encodes polarly membrane-localized long-PIN homologs (PpPINA/B/C) and an endoplasmic reticulum-localized short-PIN homolog ([PpPIND]; Viaene et al. 2013, 2014; Bennett et al. 2014a,b). Generally, mutations of the former result in an auxin-excessive phenotype, whereas their overexpression results in auxin-repressive phenotypes, which are probably caused by trapping and overdrainage of IAA, respectively (Bennett et al. 2014b; Viaene et al. 2014). These facts suggest that the long PpPINs act as efflux carriers. Indeed, basipetal polar transport could be detected by tracer assays of radiolabeled IAA within multicellular rhizoids of the moss Funaria hygrometrica (Fig. 2A; Rose and Bopp 1983). Tracer assays did not demonstrate polar transport in stalks of gametophores along the longitudinal axis but did show basipetal polar transport in setae of several mosses (Fig. 2B; Poli et al. 2003; Fujita et al. 2008). Meanwhile, based on the velocity and direction of transport, IAA is thought to be carried by nonpolar active transport or simple diffusion in setae of liverworts or young sporophytes of hornworts, respectively (Fig. 2C), suggesting that auxin transport strategies in sporophytes were independently evolved in bryophytes. Radiolabeled IAA was transported basipetally in thalli of M. polymorpha (Binns and Maravolo 1972; Maravolo 1976; Gaal et al. 1982), which was inhibited by 2,3,5-triiodobenzoic acid, a transport inhibitor, suggesting that an active transporter(s) basipetally carries IAA (Fig. 2D; Maravolo 1976).
Figure 2.
Auxin transport in bryophytes. Estimated auxin transport (blue arrows) in stalks (A), rhizoids (A), setae (B), protonemata (E), young leaves (F), and larger leaves (G) in mosses, and setae (C) and thalli (D) of liverworts.
PRINCIPLE OF AUXIN SIGNALING
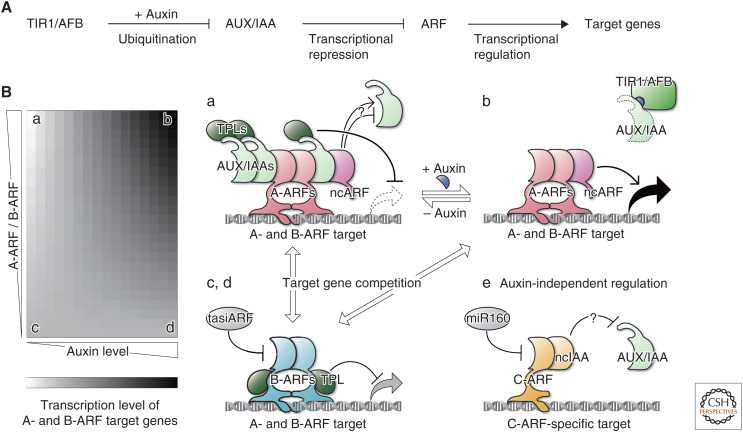
Auxin regulates gene expression through the NAS pathway, comprising TRANSPORT INHIBITOR RESPONSE 1/AUXIN SIGNALING F-BOX (TIR1/AFB) receptors, AUXIN/INDOLE-3-ACETIC ACID (AUX/IAA) transcriptional repressors, and AUXIN RESPONSE FACTOR (ARF) transcription factors (Fig. 3A; Table 1). TIR1/AFBs, an F-box-containing subunit of an SCF-type E3 ubiquitin ligase complex, form coreceptor complexes with AUX/IAAs via auxin, which in turn facilitate ubiquitination and subsequent degradation of AUX/IAAs (Gray et al. 1999, 2001; Dharmasiri et al. 2005; Kepinski and Leyser 2005; Tan et al. 2007). Canonical AUX/IAAs have three conserved domains: domain I (DI), domain II (DII), and the Phox and Bem1 domain ([PB1], also called domain III/IV). Each of these interacts with TOPLESS (TPL)-type corepressors, TIR1/AFBs, and PB1 domains of ARFs and AUX/IAAs, respectively (Kim et al. 1997; Dharmasiri et al. 2005; Kepinski and Leyser 2005; Szemenyei et al. 2008). DII is known as the degron region. ARFs consist of a DNA-binding domain (DBD), a middle region (MR), and a PB1 domain. Each of these binds to cis-elements, regulates transcriptional activity, and homo- and heterotypically interacts with ARFs and AUX/IAAs, respectively (Kim et al. 1997; Ulmasov et al. 1997, 1999a,b; Tiwari et al. 2003). Auxin releases ARFs from AUX/IAAs via TIR1/AFB-mediated degradation and hence facilitates ARF-mediated transcriptional regulation. Phylogenetic analyses demonstrated NAS establishment in the common ancestor of land plants (Bowman et al. 2017; Flores-Sandoval et al. 2018a; Mutte et al. 2018).
Figure 3.
Deduced design principles of nuclear auxin signaling (NAS) from studies with bryophytes. (A) Components and basic scheme of the NAS pathway. (B) Heat map showing putative expression levels of A- and B-ARFs’ common target genes (left) and functional models of the NAS components (right) at different A-ARF/B-ARF ratios and auxin levels (a–d). A-ARFs (and noncanonical ARFs [ncARFs]) recruit AUX/IAAs and thus repress target gene transcription at low auxin levels (a). A-ARFs (and ncARFs) activate target gene transcription at high auxin levels as TIR1/AFBs interact with AUX/IAAs via auxin and promote AUX/IAA degradation (b). B-ARFs buffer AUX/IAA-mediated intense repression at low auxin levels (c) and A-ARF-mediated activation at high auxin levels (d) by competing with A-ARFs for binding to their target DNA sites, which fine-tunes auxin responses. C-ARFs neither share target DNA sites with A- or B-ARFs, nor interact with AUX/IAAs, and thus regulate distinct target genes in an auxin-independent manner (e). ncIAA may prevent canonical AUX/IAAs from binding to C-ARFs. Note that the functions of ncARFs and ncIAAs are hypothetical. These models are based on the following papers and our deduction, in addition to the well-known NAS model established in angiosperms (A): Flores-Sandoval et al. (2018a,b), Kato et al. (2017, 2020a), Lavy et al. (2016), Lv et al. (2020), Mutte et al. (2018), and Tao and Estelle (2018).
Table 1.
Copy numbers and IDs of the nuclear auxin signaling (NAS) component homologs in bryophytes
| Marchantia polymorpha | Physcomitrium patens | Anthoceros angustus | Anthoceros agrestis (Bonn) | Anthoceros agrestis (Oxford) | Anthoceros punctatus | Arabidopsis thaliana | |
|---|---|---|---|---|---|---|---|
| TIR1/AFB | 1 (MpTIR1; Mp6g02750) | 4 (PpAFB1; Pp3c23_5870, PpAFB2; Pp3c20_17150, PpAFB3; Pp3c23_11300, PpAFB4; Pp3c24_9800/Pp3c24_9814) | 1 (AANG003364) | 1 (AagrBONN344.691) | 1 (AagrOXF000012l.465) | 1 (Apun000117l.230) | 6 |
| AUX/IAA | 1 (MpIAA; Mp6g05000) | 3 (PpIAA1A; BAB71765.1, PpIAA1B; BAB71766.1/Pp3c8_14720, PpIAA2; EDQ70046/Pp3c24_6610) | 1 (AANG009773) | 1 (AagrBONN117.2250) | 1 (AagrOXF000103l.142) | 1 (Apun000023l.749) | 28 |
| ncIAA | 1 (MpNCIAA/MpAXI2; Mp8g07850) | 0 | 1 (AANG012370) | 1 (AagrBONN117.1187) | 1 (AagrOXF000083l.132) | 1 (Apun000058l.129) | 1 |
| TPL/TPL-RELATED | 1 (MpTPL; Mp7g17410) | 2 (PpTPL1; Pp3c15_9880, PpTPL2; Pp3c9_21250) | 1 (AANG014214) | 1 (AagrBONN340.1726) | 1 (AagrOXF000065l.67) | 1 (Apun000149l.87) | 4 |
| A-ARF | 1 (MpARF1; Mp1g12750) | 8 (PpARFa1; Pp3c1_14480, PpARFa2; Pp3c1_14440, PpARFa3; Pp3c2_25890, PpARFa4; Pp3c13_4720, PpARFa5; Pp3c26_11550, PpARFa6; Pp3c17_19900, PpARFa7; Pp3c14_16990, PpARFa8; Pp3c1_40270) | 1 (AANG012813) | 1 (AagrBONN340.233) | 1 (AagrOXF000126l.206) | 1 (Apun000045l.202) | 5 |
| B-ARF | 1 (MpARF2; Mp4g11820) | 4 (PpARFb1; Pp3c27_60, PpARFb2; Pp3c16_6100, PpARFb3; Pp3c5_9420, PpARFb4; Pp3c6_21370) | 0 | 0 | 0 | 0 | 15 |
| C-ARF | 1 (MpARF3; Mp1g07070) | 3 (PpARFc1A; Pp3c4_12970, PpARFc1B; Pp3c4_13010, PpARFc2; Pp3c6_26890) | 3 (AANG000742, AANG000743, AANG012642) | 4 (AagrBONN340.199, AagrBONN344.1784, AagrBONN369.337, AagrBONN369.338) | 4 (AagrOXF000006l.365, AagrOXF000069l.94, AagrOXF000069l.95, AagrOXF000126l.173) | 4 (Apun000045l.242, Apun000089l.87, Apun000089l.88, Apun000144l.74) | 3 |
| Noncanonical (nc)ARF | 1 (MpNCARF/MpAXI1; Mp2g02890) | 2 (PpARFd1; Pp3c6_26890, PpARFd2; Pp3c15_9710) | 0 | 0 | 0 | 0 | 0 |
Homologs were obtained from MarpolBase (www.marchantia.info) for the liverwort M. polymorpha, and from Phytozome v13 (www . phytozome-next.jgi.doe.gov) and NCBI (www.ncbi.nlm.nih.gov) for the moss P. patens from DRYAD (www.datadryad.org/stash/dataset/doi:10.5061/dryad.msbcc2ftv) for hornwort A. angustus, from the University of Zurich (www.hornworts.uzh.ch/en.html) for the hornwort A. agrestis (Bonn), A. agrestis (Oxford), and A. punctatus. AagrOXF_evm.model.utg000008l.194.1 and Apun_evm.model.utg000039l.220.1 are omitted from TIR1/AFB candidates here, since they are closer to PpXFBs (Pp3c22_6020 and Pp3c18_16630), which are F-box/Leucine Rich Repeat proteins absent in angiosperms (Prigge et al. 2010) and are more homologous to jasmonate/oxo-phytodienoic acid receptors than TIR1/AFBs (see a phylogenetic tree in Mutte et al. 2018). Names: “AagrBONN_evm.model.Sc2ySwM_XXX.XXXX,” “AagrOXF_evm .model.utgXXXXl.XX,” and “Apun_evm.model.utgXXXXXXl.XXX” are modified as “AagrBONNXXX.XXXX,” “AagrOXFXXXXl.XX,” and “ApunXXXXXXl.XXX,” respectively, to save space.
In P. patens, four TIR1/AFB and three AUX/IAA homologs interact with each other in an auxin-dependent manner (Prigge et al. 2010). P. patens expressing stabilized mutants of PpIAA, which had been originally forward-genetically isolated as auxin-insensitive mutants, and PpAFB knockdown plants showed auxin depletion phenotypes, confirming that PpAFB–PpIAA pairs act as auxin coreceptors (Ashton et al. 1979; Prigge et al. 2010). M. polymorpha encodes a single canonical AUX/IAA homolog, MpIAA. Overexpression of a DII-mutated MpIAA protein conferred auxin tolerance, suggesting a conserved role of MpIAA in auxin perception (Kato et al. 2015).
Although several studies have used stabilized, dominant mutants of AUX/IAAs for functional analyses, reports of their loss-of-function phenotypes were limited. Lavy et al. (2016) performed a prominent mutational study with AUX/IAA-null mutants in P. patens in which all three PpIAA homologs were knocked out. The Ppiaa triple mutants showed no responses to exogenous auxin or l-Kyn, leading to an excessive auxin-response phenotype. Expression of a PpIAA1a mutant incapable of binding to TPL, which lacked an ethylene-responsive element binding factor-associated amphiphilic repression (EAR) motif (Causier et al. 2012) within DI and an EAR-like motif between DI and DII, still caused an excessive auxin phenotype, but slightly restored growth and auxin responsiveness, suggesting EAR-independent repressive functions of AUX/IAAs (Tao and Estelle 2018). Considering that Arabidopsis ARF5/MONOPTEROS interacts with chromatin remodeling ATPases to exert transcriptional regulation (Wu et al. 2015), simple binding of AUX/IAAs to ARFs could inhibit recruitment of other required factors, thus repressing ARF functions (Tao and Estelle 2018). A PB1 mutant of PpIAA1a, incapable of binding to itself nor PpARFs, failed to complement the null Ppiaa mutant. However, another PB1 mutant of PpIAA1a, incapable of self-binding but capable of binding to PpARFs, slightly rescued the mutant. Taken together, the PB1-mediated heterotypic interaction with ARFs is critical for the function of AUX/IAA, and their homotypic interactions and subsequent oligomerization contribute to, but are not essential for, their repressive functions (Tao and Estelle 2018).
ARF transcription factors are phylogenetically classified as A, B, and C (Finet et al. 2013). Comprehensive phylogenetic analysis in green plants showed that ancestral C-ARFs diverged from ancestral A/B-ARFs before the TIR1/AFB–AUX/IAA auxin coreceptor system (Flores-Sandoval et al. 2018a; Mutte et al. 2018). Based on sequence traits in the MR and results of transactivation assays, A- and B-ARFs are generally thought to be transcriptional activators and repressors, respectively (Ulmasov et al. 1999a; Tiwari et al. 2003; Kato et al. 2015). Overexpression of A- and/or B-ARF homologs in the Ppiaa triple mutant revealed that A- and B-ARFs share common target genes and that B-ARFs exert repressive functions in an AUX/IAA-independent manner (Lavy et al. 2016). Counterintuitively, knockout of B-ARFs reduced auxin sensitivity, and overexpression of A-ARFs down-regulated the transcription of some auxin-responsive genes. Based on these conflicting results, Lavy et al. (2016) proposed a model in which B-ARFs compete for target gene sites with A-ARFs, and affect the occupancy of A-ARFs and AUX/IAAs, providing a buffering capacity for fine-scale regulation of target genes.
M. polymorpha encodes three canonical ARFs, each belonging to class A, B, or C (Flores-Sandoval et al. 2015; Kato et al. 2015; Bowman et al. 2017). Knockout of MpARF1, the sole A-ARF, conferred auxin insensitivity and caused various developmental defects, including narrow twisted thalli, small tubular gemma cups, decreased gemmae per cups, nondormant gemmae, and disrupted patterning in gemma development (Kato et al. 2017). Interestingly, inhibition of NAS via DII-mutated nondegradable MpIAA resulted in very severe growth defects, such as formation of cell masses, ectopic air pores, ectopic serrated structures, and/or adventitious buds, and failure to develop thalli (Kato et al. 2015). Furthermore, the aforementioned Mptaa auxin biosynthesis mutant or overexpressors of a bacterial auxin-conjugating enzyme formed cell masses (Eklund et al. 2015). Mparf1 phenotypes are much milder than those in that they develop thalloid organs. One possible explanation is that AUX/IAA proteins are not recruited to target sites in the absence of the A-ARF, allowing its target genes to be transcribed at basal levels (Kato et al. 2017, 2018).
Kato et al. (2020a) investigated whether the conserved domains of MpARFs could be interchangeable by expressing various domain-swapped constructs in the Mparf1 mutant background. Chimeric MpARF1, which exchanged the DBD with MpARF2 but not MpARF3, restored development and auxin responsiveness, suggesting that MpARF1 and MpARF2 share target genes, while MpARF3 targets another group of genes. The MR of MpARF1 was interchangeable with that of neither MpARF2 nor MpARF3. Interestingly, MpARF2 was shown to interact directly with MpTPL through a conserved “LFG motif,” and small fragments containing the LFG motif but not a mutated motif from all MpARFs exhibited repressor activities upon coexpression with MpTPL. Thus, the MR of MpARF2 (and probably that of MpARF3) exerts auxin-independent transcriptional repression via direct recruitment of MpTPL (Kato et al. 2020a).
All combinations of homo- and heterotypic interactions among the PB1 domains of MpARFs and MpIAA were demonstrated except for MpARF3–MpARF3 homotypic interactions (Kato et al. 2015). In the domain-swapping assay, the PB1 domain of MpARF1 was not interchangeable with that of MpARF3. Chimeric MpARF1 with its PB1 domain swapped with that of MpARF2 partially rescued developmental defects of Mparf1 but did not rescue responsiveness to exogenous auxin. In general, PB1 domains have positive and negative faces and oligomerize through consecutive interactions between the two faces (Korasick et al. 2014). Docking of structural models, however, predicted that both the positive and negative faces of MpARF2 and MpARF3 PB1 domains interact with one face of the MpIAA PB1 domain, while one face of the MpARF1 PB1 domain does so, allowing further oligomeric interactions, which seems critical for establishing sufficient auxin responsiveness. Chimeric MpARF1, which has a distinct oligomerization domain in place of the PB1 domain, rescued Mparf1 growth defects but not auxin insensitivity, suggesting that MpARF1–MpARF1 homotypic interactions through the PB1 domain are critical for transcriptional activation by MpARF1 and that auxin response requires MpARF1–MpIAA oligomerization (Kato et al. 2020).
These results offer design principles of the auxin response system: in the absence of auxin, A-ARFs oligomerize with AUX/IAAs causing transcriptional repression, while in the presence of auxin, A-ARFs interact with each other and promote target transcription. B-ARFs compete for target sites with A-ARFs and A-ARF–AUX/IAA complexes to fine-tune auxin responsiveness (Kato et al. 2020a). Even though B-ARFs recruit TPLs, B-ARFs are speculated to exert weaker repression than A-ARF–AUX/IAA complexes, as the latter is capable of recruiting many more TPLs via oligomerized AUX/IAAs. Thus, B-ARFs could buffer both transcriptional activation and repression at high and low levels of auxin, respectively (Fig. 3B).
B-ARFs are known to be posttranscriptionally regulated by trans-acting small interfering RNA (tasiRNA) generated by miR390 from TAS3 gene products in angiosperms (Allen et al. 2005; Williams et al. 2005). P. patens and M. polymorpha also have conserved miR390, suggesting the existence of a similar regulatory system (Axtell et al. 2007; Arif et al. 2012; Lin et al. 2016; Plavskin et al. 2016; Xia et al. 2017). In P. patens, it is demonstrated that TAS3-derived tasiRNAs target transcripts of B-ARF genes to restrict their expression at a random subset of protonemal tip cells, which increases sensitivity and robustness of auxin responses. The tasiARFs thus allow stochastic modulation of cell differentiation and developmental plasticity in response to environmental cues (Plavskin et al. 2016). In M. polymorpha, conditional knockout of MpARF2 in gemmalings or thalli caused severe growth arrest, suggesting that B-ARF plays critical roles in development (Kato et al. 2020a). Interestingly, hornworts do not encode a B-ARF in their genome, which was probably lost after divergence from a liverwort/moss common ancestor (Flores-Sandoval et al. 2018a; Mutte et al. 2018; Li et al. 2020; Zhang et al. 2020), suggesting the acquisition of a distinct buffering mechanism for A-ARFs.
C-ARFs are posttranscriptionally regulated by miR160 (Mallory et al. 2005; Wang et al. 2005), whose target sites are conserved among land plants (Axtell et al. 2007; Lin et al. 2016; Tsuzuki et al. 2016; Flores-Sandoval et al. 2018a). In contrast to MpARF1 and MpARF2, neither knockout nor overexpression of MpARF3 affected auxin sensitivity, unless its miR160-resistant form is overexpressed, leading to auxin-insensitive cell masses (Flores-Sandoval et al. 2018a). Additionally, MpARF3 is not a part of the auxin-related coexpression module (Flores-Sandoval et al. 2018b). Together with the phylogenetic position, these studies suggest that C-ARFs are not under the control of auxin; rather, it is proposed that the TIR1/AFB–AUX/IAA- and miR160-mediated pathways evolved independently to regulate preexisting A/B- and C-ARF transcriptional networks, respectively (Flores-Sandoval et al. 2018a,b). Indeed, as the DBDs of MpARF1 and MpARF3 were unexchangeable, many, if not all, A- and B-ARFs seem to regulate gene sets distinct from target genes of C-ARFs (Kato et al. 2020a).
Phylogenetic analysis indicated the existence of conserved noncanonical AUX/IAAs (ncIAAs) lacking DI and DII (Flores-Sandoval et al. 2018a; Mutte et al. 2018), although ncIAA homologs are absent from P. patens. Liverworts, mosses, and lycophytes, but not hornworts and other vascular plants, have noncanonical ARFs (ncARFs) lacking DBD, which diverged from A-ARFs (Paponov et al. 2009; Mutte et al. 2018). M. polymorpha has one ncIAA, MpNCIAA/MpAXI2 and one ncARF, MpNCARF/MpAXI1 (Bowman et al. 2017). Although Mpncarf mutants developed relatively normal thalli, they showed tolerance to exogenous auxin and reduced fold changes of some auxin-up-regulated genes (Mutte et al. 2018). Based on these results, Mutte et al. (2018) proposed two hypothetical roles of ncARFs: ncARFs protect canonical ARFs from AUX/IAAs and/or recruit cofactors to ARF-target loci via interaction with canonical ARFs. In contrast to the first model, given that oligomeric interactions of AUX/IAAs to A-ARFs are required for sufficient auxin responsiveness (Kato et al. 2020a; see above), ncARFs may assist the A-ARF–AUX/IAA interaction as spacers (Fig. 3B). Meanwhile, the roles of MpNCIAA in NAS are unclear, as Mpnciaa mutants showed comparable auxin responses to wild-type (Mutte et al. 2018). AtIAA33, the sole ncIAA ortholog in Arabidopsis, has recently been demonstrated to interact with C-ARFs, AtARF10/16, which protects AtARF10/16 from canonical AUX/IAA-mediated repression (Lv et al. 2020). Additionally, like MpARF3, MpNCIAA is expressed independently of the auxin coexpression group (Flores-Sandoval et al. 2018b). Although further experiments are needed, ncIAAs may play a role in sequestering C-ARFs from NAS (Fig. 3B).
MOLECULAR MECHANISMS UNDERLYING DEVELOPMENTAL REGULATION
As seen in the previous sections, auxins and their inhibitors cause multiple responses in bryophytes. For these context-dependent, diverse auxin responses, its localization and responsiveness should be spatiotemporally controlled. Accordingly, reporters of the soybean GmGH3 promoter (Hagen et al. 1991), which operate under the control of NAS also in a bryophyte (Kato et al. 2015, 2017), exhibit high activities in multiple tissues or organs throughout the life cycle in both P. patens (Bierfreund et al. 2003; Fujita et al. 2008; Landberg et al. 2013) and M. polymorpha (Ishizaki et al. 2012). Additionally, the synthetic auxin response reporter DR5revV2 shows correlated expression patterns with those of GmGH3 (Thelander et al. 2019). As an ARF-independent auxin-sensing reporter, a moss-optimized “ratiometric version of two D2s,” PpR2D2, was recently developed. PpR2D2 semiquantitatively visualizes auxin sensitivities as AUX/IAA degradation rates and enables further fine monitoring in P. patens (Liao et al. 2015; Thelander et al. 2019).
In protonema colonies of P. patens, PpR2D2 shows a gradient along filaments where auxin-sensing levels are high in proximal cells and low in tip cells (Thelander et al. 2019). Both the GmGH3 and DR5revV2 reporters show stronger activity in proximal cells than in tip cells (Thelander et al. 2019). Thus, apical stem cells show relatively low auxin sensing and response levels. Polar localization of long PINs on the plasma membrane toward tips suggests acropetal auxin transport and its accumulation in and drainage from tip cells, which is needed for proper caulonema differentiation (Fig. 2E; Viaene et al. 2014). Although the auxin source is still unclear, PpSHI, a positive regulator of auxin biosynthesis homologous to Arabidopsis SHORT INTERNODES/STYLISH ([SHI/STY]; Sohlberg et al. 2006; Ståldal et al. 2008; Eklund et al. 2010a), is specifically expressed in caulonema (Eklund et al. 2010b). The above-mentioned B-ARF gradient generated by tasiARFs adds another tier of auxin response regulation for caulonema differentiation and bud formation (Plavskin et al. 2016). Under the control of auxin, homologs of ROOT HAIR DEFECTIVE 6-LIKEs and Lotus japonicus ROOTHAIRLESS LIKE (PpRSL1/2 and PpLRL1/2, respectively) function in caulonema differentiation (Jang and Dolan 2011; Tam et al. 2015). For gametophore development, auxin-responsive transcription factors, AINTEGUMENTA, PLETHORA, and BABY BOOM homologs (PpAPBs), act as master regulators of bud formation (Aoyama et al. 2012).
Consistent with the undetectable polar transport with radiolabeled IAA in gametophore stems (Fujita et al. 2008), Pppina/b double mutants, and NPA treatment have only a minor effect on branching patterns; rather, plasmodesmata are supposed to be a major transport route (Coudert et al. 2015). Nevertheless, since long PpPIN mutants exhibit a multifaceted phenotype in gametophores, such as stem elongation, leaf abnormalities, and disruption of tropic responses, PIN-mediated transport also plays pleiotropic roles in gametophores (Bennett et al. 2014b). During leaf development, PpPINA/B are expressed in apical regions and show bipolar localization on both apical and basal plasma membranes at the early stages. The expression zones move toward the basal region as leaves develop, where PpPINA/B localize on all sides. Such a shift in expression zones correlates with a similar shift in cell elongation (Viaene et al. 2014). The PpR2D2 reporter indicated that high auxin-sensing zones also expand from apex to base during leaf development (Fig. 2F,G; Thelander et al. 2019). Auxin application at a certain concentration transforms leaves into narrow, elongated shapes (Barker 2011; Bennett et al. 2014b). Knockout of long PINs also caused long narrow leaves because of enhanced longitudinal cell elongation and reduced mediolateral cell division, probably caused by auxin trapping (Bennett et al. 2014b). Taken together, long PINs affect auxin responsiveness via transport, which regulates the transition from cell division to expansion in leaf development (Bennett et al. 2014b; Viaene et al. 2014; Thelander et al. 2019).
Auxin promotes rhizoid development in both liverworts and mosses. In P. patens, the PpR2D2 reporter revealed that auxin-sensing levels are low in rhizoid precursors and show a gradient from proximal to tip after the three-cell stage (Thelander et al. 2019). Consistently, GmGH3 and DR5revV2 reporters show gradient patterns in multicelled stages, although they are also active in precursors (Thelander et al. 2019). On the other hand, PpSHI is highly expressed in tip cells (Eklund et al. 2010b). Considering the basipetal transport of radiolabeled IAA in F. hygrometrica rhizoids (Rose and Bopp 1983), it is suggested that IAA is biosynthesized in tip cells, is transported toward proximal regions, and then evokes signaling in rhizoids of mosses (Fig. 2A). In P. patens, mutations in the auxin-inducible PpRSL1/2 or PpLRL1/2 genes (see above) result in loss of rhizoid development regardless of auxin treatment, suggesting that these basic helix-loop-helix (bHLH) transcription factors regulate rhizoid development downstream of auxin (Menand et al. 2007; Jang et al. 2011; Tam et al. 2015). Their angiosperm homologs regulate root hair development (Masucci and Schiefelbein 1994; Menand et al. 2007; Ding et al. 2009; Karas et al. 2009). In particular, the class II RSL genes are regulated by auxin in Arabidopsis (Yi et al. 2010; Pires et al. 2013). It is known that their M. polymorpha homologs, MpRSL1 and MpLRL, also regulate rhizoid development, but their association with auxin is unclear (Breuninger et al. 2016; Proust et al. 2016).
In the thalli of M. polymorpha, both MpTAA and MpYUC2 promoters show strong activity at apical notches and the bottom of gemma cups (Eklund et al. 2015). In line with the observations that removal of apical notches broke gemma dormancy in the cup (LaRue and Narayanaswami 1957; Eklund et al. 2015), IAA biosynthesized at the bottom of gemma cups but also that supplied from apical notches (Maravolo 1976; Gaal et al. 1982) are suggested to play roles in maintaining gemmae dormancy. An increase in nondormant gemmae caused by MpYUC2 knockdown, overexpression of iaaL, and MpARF1 knockout also supports the notion that auxin promotes gemma dormancy (Eklund et al. 2015; Kato et al. 2017). Regulation of dormancy, however, seems to be a complex process as direct IAA application broke gemma dormancy within gemma cups (Tarén 1958). Additionally, the question remains as to how auxin affects mature gemmae detached from mother plants but still in the cup. Recently, abscisic acid (ABA) was shown to function autonomously in gemmae to initiate and/or maintain dormancy within gemma cups (Eklund et al. 2018), but the underlying mechanisms bridging the two signaling pathways remain unclear.
Auxin also mediates cell elongation in M. polymorpha. Gametangiophore stem elongation was suppressed by high doses of nondegradable MpIAA and dominant-negative TPL mutants (Flores-Sandoval et al. 2015). The former also caused defects in the antigravitropic bending of stalks, supporting that the NAS pathway regulates tropic responses (Kato et al. 2015). Additionally, it suppressed auxin-induced protrusion of air pores and elongation of gemma cup cells, suggesting that the NAS pathway regulates cell elongation. Several EXPANSIN genes shown to be up-regulated in response to auxin treatment might be potential targets (Kato et al. 2017; Mutte et al. 2018).
Analysis of the NAS components revealed that auxin also plays a significant role in apical stem cell functions. Consistent with the fact that auxins promote branching in R. multifida (Ilahi and Allsopp 1969), overexpression of MpARF1 and the dominant-negative MpTPL mutant enhanced thallus branching (Flores-Sandoval et al. 2015). Conditional knockout of MpARF2 terminated thallus growth, suggesting that coordinated regulation between A- and B-ARFs is essential for stem cell maintenance (Kato et al. 2020a). Mparf1 mutation causes abnormalities in division patterns and deviation of position and numbers of stem cell regions during gemma formation, suggesting that the establishment of developmental axes through formative divisions is a common trait of A-ARFs in land plants (Yoshida et al. 2014; Kato et al. 2017, 2018). A bHLH transcription factor, M. polymorpha TARGET OF MONOPTEROS 5 (MpTMO5), is also needed for proper patterning in gemma development (Lu et al. 2020). Given that Arabidopsis TMO5 is a direct target of A-ARF (Schlereth et al. 2010), it would be worthwhile to test whether MpARF1 targets MpTMO5 in gemma development.
In sporophytes, strong GmGH3 activities were observed in the whole or apical part of M. polymorpha and P. patens, respectively, at the immature stage, but limited to the basal side as they mature (Fujita et al. 2008; Ishizaki et al. 2012). In M. polymorpha, nondegradable AUX/IAA induction halts sporophyte development (Kato et al. 2015). In P. patens, transport disruption by NPA application or knockout of PpPINB occasionally caused the branching of sporangia, and knockout of both PpPINA and PpPINB caused severe or lethal growth arrest (Fujita et al. 2008; Bennett et al. 2014b). These studies highlight the importance of spatiotemporal auxin regulation in the sporophyte bodies of bryophytes. Although auxin response in sporophytic tissues plays an essential role in angiosperms, as shown by the embryonic lethal phenotype of AtTIR1/AFB sextuple mutants (Prigge et al. 2020), little is known about the molecular mechanisms underlying sporophyte development of bryophytes. Future analyses are needed to determine whether conserved regulatory systems are used for sporophytes.
CONCLUDING REMARKS
Studies with bryophytes have contributed to our understanding of how the auxin system evolved in green plants. As described above, bryophytes share with vascular plants, not only the core mechanisms of auxin biosynthesis, polar transport, inactivation, and signaling, but also various auxin-regulated physiological processes, such as cell division, cell elongation, cell differentiation, tissue and organ development, and tropic responses. Thus, most of the auxin system was established early in land plant lineages, which reemphasizes the utility of bryophytes as models for auxin biology. In particular, the design principle of the functions of the NAS components has been determined effectively using simple platforms.
Auxin plays a role in reproductive organ differentiation in bryophytes. In P. patens, PpTARs regulate gamete precursor development by local auxin biosynthesis (Landberg et al. 2020); PpSHI regulates egg cell differentiation, sexual organ opening, and programmed canal cell death (Landberg et al. 2013). In M. polymorpha, MpIAA is expressed in gametangia (Kato et al. 2015). In bryophytes, germline cells are formed directly on vegetative somatic tissues, distinct from seed plants where they are specified in meiosis-derived cells within several rounds of divisions (Hisanaga et al. 2019). Elucidation of the roles of auxin in reproductive development should provide insights into the reduction of gametophyte generation during land plant evolution. Further analyses are needed on how auxin biosynthesis, transport, signaling, and inactivation are used in various contexts in bryophytes.
Auxin is regarded as a signal that fuels programs predetermined by other mechanisms (Bennett and Leyser 2014) most likely via epigenetic regulation. However, its underlying biochemical basis has not been fully elucidated. For example, little is known regarding how A-ARFs reverse the deacetylated histone status. Additionally, there are still many unanswered questions on the functions of the NAS components, such as whether all or only a subset of A-ARF-target genes are coregulated by B-ARFs and whether and how the ratio of A- and B-ARFs varies for individual target genes. Simple auxin systems in bryophytes can make significant contributions to these central issues in auxin biology. So far, the majority of molecular studies on bryophytes have been reverse genetically performed. Given the low genetic redundancy, forward genetics would provide clues to explore novel molecular mechanisms for auxin biology.
ACKNOWLEDGMENTS
We thank Eduardo Flores-Sandoval for helpful information. Research in the authors’ laboratory was/is funded by MEXT/JSPS KAKENHI, Grant Nos.: JP18J12698 to H.S., JP17H07424 to T.K., and JP20H04884 to R.N. R.N. was also supported by SPIRITS 2017 of Kyoto University.
Footnotes
Editors: Dolf Weijers, Karin Ljung, Mark Estelle, and Ottoline Leyser
Additional Perspectives on Auxin Signaling available at www.cshperspectives.org
REFERENCES
- Allen E, Xie Z, Gustafson AM, Carrington JC. 2005. microRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 121: 207–221. 10.1016/j.cell.2005.04.004 [DOI] [PubMed] [Google Scholar]
- Allsopp A, Ilahi I. 1970a. Studies in the Metzgeriales (Hepaticae). III: The effects of sugars and auxins on Noteroclada confluens. Phytomorphology 20: 9–16. [Google Scholar]
- Allsopp A, Ilahi I. 1970b. Studies in the Metzgeriales (Hepaticae). V: Investigation on Blasia pusilla. Phytomorphology 20: 118–125. [Google Scholar]
- Allsopp A, Ilahi I. 1970c. Studies in the Metzgeriales (Hepaticae). VII: Regeneration in Noteroclada confluens and Blasia pusilla. Phytomorphology 20: 173–182. [Google Scholar]
- Allsopp A, Pearman C, Rao AN. 1968. The effects of some growth substances and inhibitors on the development of Marchantia gemmae. Phytomorphology 18: 84–94. [Google Scholar]
- Aoyama T, Hiwatashi Y, Shigyo M, Kofuji R, Kubo M, Ito M, Hasebe M. 2012. AP2-type transcription factors determine stem cell identity in the moss Physcomitrella patens. Development 139: 3120–3129. 10.1242/dev.076091 [DOI] [PubMed] [Google Scholar]
- Apostolakos P, Galatis B, Mitrakos K. 1982. Studies on the development of the air pores and air chambers of Marchantia paleacea 1. Light microscopy. Ann Bot 49: 377–396. 10.1093/oxfordjournals.aob.a086262 [DOI] [Google Scholar]
- Arif MA, Fattash I, Ma Z, Cho SH, Beike AK, Reski R, Axtell MJ, Frank W. 2012. DICER-LIKE3 activity in Physcomitrella patens DICER-LIKE4 mutants causes severe developmental dysfunction and sterility. Mol Plant 5: 1281–1294. 10.1093/mp/sss036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton NW, Grimsley NH, Cove DJ. 1979. Analysis of gametophytic development in the moss, Physcomitrella patens, using auxin and cytokinin resistant mutants. Planta 144: 427–435. 10.1007/BF00380118 [DOI] [PubMed] [Google Scholar]
- Ashton NW, Schulze A, Hall P, Bandurski RS. 1985. Estimation of indole-3-acetic acid in gametophytes of the moss, Physcomitrella patens. Planta 164: 142–144. 10.1007/BF00391040 [DOI] [PubMed] [Google Scholar]
- Atzorn R, Geier U, Sandberg G. 1990. The physiological role of indole acetic acid in the moss Funaria hygrometrica Hedw. I: Quantification of indole-3-acetic acid in tissue and protoplasts by enzyme immunoassay and gas chromatography-mass spectrometry. J Plant Physiol 135: 522–525. 10.1016/S0176-1617(11)80628-0 [DOI] [Google Scholar]
- Axtell MJ, Snyder JA, Bartel DP. 2007. Common functions for diverse small RNAs of land plants. Plant Cell 19: 1750–1769. 10.1105/tpc.107.051706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L, Yamamoto KT, Fujita T. 2015. Phototropism in gametophytic shoots of the moss Physcomitrella patens. Plant Signal Behav 10: e1010900. 10.1080/15592324.2015.1010900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker EI. 2011. “An examination of leaf morphogenesis in the moss, Physcomitrella patens.” PhD thesis, University of Regina, Regina, SK, Canada. [Google Scholar]
- Barnes CR, Land WJG. 1908. Bryological papers. II: The origin of the cupule of Marchantia. Bot Gaz 46: 401–409. 10.1086/329782 [DOI] [Google Scholar]
- Bennett T, Leyser O. 2014. The auxin question: a philosophical overview. In Auxin and its role in plant development (ed. Zažímalová E, Petrasek J, Benková E), pp. 3–19. Springer, Vienna, Austria. [Google Scholar]
- Bennett T, Brockington SF, Rothfels C, Graham SW, Stevenson D, Kutchan T, Rolf M, Thomas P, Wong GKS, Leyser O, et al. 2014a. Paralogous radiations of PIN proteins with multiple origins of noncanonical PIN structure. Mol Biol Evol 31: 2042–2060. 10.1093/molbev/msu147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett TA, Liu MM, Aoyama T, Bierfreund NM, Braun M, Coudert Y, Dennis RJ, O'Connor D, Wang XY, White CD, et al. 2014b. Plasma membrane-targeted PIN proteins drive shoot development in a moss. Curr Biol 24: 2776–2785. 10.1016/j.cub.2014.09.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierfreund NM, Reski R, Decker EL. 2003. Use of an inducible reporter gene system for the analysis of auxin distribution in the moss Physcomitrella patens. Plant Cell Rep 21: 1143–1152. 10.1007/s00299-003-0646-1 [DOI] [PubMed] [Google Scholar]
- Binns AN, Maravolo NC. 1972. Apical dominance, polarity, and adventitious growth in Marchantia polymorpha. Am J Bot 59: 691–696. 10.1002/j.1537-2197.1972.tb10141.x [DOI] [Google Scholar]
- Bowman JL. 2016. A brief history of Marchantia from Greece to genomics. Plant Cell Physiol 57: 210–229. 10.1093/pcp/pcv044 [DOI] [PubMed] [Google Scholar]
- Bowman JL, Kohchi T, Yamato KT, Jenkins J, Shu S, Ishizaki K, Yamaoka S, Nishihama R, Nakamura Y, Berger F, et al. 2017. Insights into land plant evolution garnered from the Marchantia polymorpha genome. Cell 171: 287–304.e15. 10.1016/j.cell.2017.09.030 [DOI] [PubMed] [Google Scholar]
- Breuninger H, Thamm A, Streubel S, Sakayama H, Nishiyama T, Dolan L. 2016. Diversification of a transcription factor family led to the evolution of antagonistically acting genetic regulators of root hair growth. Curr Biol 26: 1622–1628. 10.1016/j.cub.2016.04.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causier B, Ashworth M, Guo W, Davies B. 2012. The TOPLESS interactome: a framework for gene repression in Arabidopsis. Plant Physiol 158: 423–438. 10.1104/pp.111.186999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra RN, Vashistha BD. 1990. The effect of auxins and antiauxins on shoot-bud induction and morphology in the moss, Bryum atrovirens Will ex Brid. Aust J Bot 38: 177–184. 10.1071/BT9900177 [DOI] [Google Scholar]
- Cooke TJ, Poli D, Sztein AE, Cohen JD. 2002. Evolutionary patterns in auxin action. Plant Mol Biol 49: 319–338. 10.1023/A:1015242627321 [DOI] [PubMed] [Google Scholar]
- Coudert Y, Palubicki W, Ljung K, Novak O, Leyser O, Jill Harrison C. 2015. Three ancient hormonal cues co-ordinate shoot branching in a moss. eLife 4: e06808. 10.7554/eLife.06808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cove D. 2005. The moss Physcomitrella patens. Annu Rev Genet 39: 339–358. 10.1146/annurev.genet.39.073003.110214 [DOI] [PubMed] [Google Scholar]
- Cove DJ, Ashton WN. 1984. The hormonal regulation of gametophytic development in bryophytes. In The experimental biology of bryophytes (ed. Dyer AF, Duckett JG), pp. 177–201. Academic, London. [Google Scholar]
- Cove DJ, Schild A, Ashton NW, Hartmann E. 1978. Genetic and physiological studies of the effect of light on the development of the moss, Physcomitrella patens. Photochem Photobiol 27: 249–254. 10.1111/j.1751-1097.1978.tb07596.x [DOI] [Google Scholar]
- Cox CJ, Li B, Foster PG, Embley TM, Civáň P. 2014. Conflicting phylogenies for early land plants are caused by composition biases among synonymous substitutions. Syst Biol 63: 272–279. 10.1093/sysbio/syt109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidonis GH, Munroe MH. 1972. Apical dominance in Marchantia: correlative inhibition of neighbor lobe growth. Bot Gaz 133: 177–184. 10.1086/336631 [DOI] [Google Scholar]
- Demeulenaere MJF, Beeckman T. 2014. The interplay between auxin and the cell cycle during plant development. In Auxin and its role in plant development (ed. Zažímalová E, Petrasek J, Benková E), pp. 119–141. Springer, Vienna, Austria. [Google Scholar]
- Dharmasiri N, Dharmasiri S, Estelle M. 2005. The F-box protein TIR1 is an auxin receptor. Nature 435: 441–445. 10.1038/nature03543 [DOI] [PubMed] [Google Scholar]
- Ding W, Yu Z, Tong Y, Huang W, Chen H, Wu P. 2009. A transcription factor with a bHLH domain regulates root hair development in rice. Cell Res 19: 1309–1311. 10.1038/cr.2009.109 [DOI] [PubMed] [Google Scholar]
- Dresselhaus T, Schneitz K. 2014. The role of auxin for reproductive organ patterning and development. In Auxin and its role in plant development (ed. Zažímalová E, Petrasek J, Benková E), pp. 213–243. Springer, Vienna, Austria. [Google Scholar]
- Eklund DM, Ståldal V, Valsecchi I, Cierlik I, Eriksson C, Hiratsu K, Ohme-Takagi M, Sundström JF, Thelander M, Ezcurra I, et al. 2010a. The Arabidopsis thaliana STYLISH1 protein acts as a transcriptional activator regulating auxin biosynthesis. Plant Cell 22: 349–363. 10.1105/tpc.108.064816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund DM, Thelander M, Landberg K, Ståldal V, Nilsson A, Johansson M, Valsecchi I, Pederson ERA, Kowalczyk M, Ljung K, et al. 2010b. Homologues of the Arabidopsis thaliana SHI/STY/LRP1 genes control auxin biosynthesis and affect growth and development in the moss Physcomitrella patens. Development 137: 1275–1284. 10.1242/dev.039594 [DOI] [PubMed] [Google Scholar]
- Eklund DM, Ishizaki K, Flores-Sandoval E, Kikuchi S, Takebayashi Y, Tsukamoto S, Hirakawa Y, Nonomura M, Kato H, Kouno M, et al. 2015. Auxin produced by the indole-3-pyruvic acid pathway regulates development and gemmae dormancy in the liverwort Marchantia polymorpha. Plant Cell 27: 1650–1669. 10.1105/tpc.15.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund DM, Kanei M, Flores-Sandoval E, Ishizaki K, Nishihama R, Kohchi T, Lagercrantz U, Bhalerao RP, Sakata Y, Bowman JL. 2018. An evolutionarily conserved abscisic acid signaling pathway regulates dormancy in the liverwort Marchantia polymorpha. Curr Biol 28: 3691–3699.e3. 10.1016/j.cub.2018.10.018 [DOI] [PubMed] [Google Scholar]
- Finet C, Berne-Dedieu A, Scutt CP, Marlétaz F. 2013. Evolution of the ARF gene family in land plants: old domains, new tricks. Mol Biol Evol 30: 45–56. 10.1093/molbev/mss220 [DOI] [PubMed] [Google Scholar]
- Flores-Sandoval E, Eklund DM, Bowman JL. 2015. A simple auxin transcriptional response system regulates multiple morphogenetic processes in the liverwort Marchantia polymorpha. PLoS Genet 11: e1005207. 10.1371/journal.pgen.1005207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Sandoval E, Eklund DM, Hong SF, Alvarez JP, Fisher TJ, Lampugnani ER, Golz JF, Vázquez-Lobo A, Dierschke T, Lin SS, et al. 2018a. Class C ARFs evolved before the origin of land plants and antagonize differentiation and developmental transitions in Marchantia polymorpha. New Phytol 218: 1612–1630. 10.1111/nph.15090 [DOI] [PubMed] [Google Scholar]
- Flores-Sandoval E, Romani F, Bowman JL. 2018b. Co-expression and transcriptome analysis of Marchantia polymorpha transcription factors supports class C ARFs as independent actors of an ancient auxin regulatory module. Front Plant Sci 9: 1345. 10.3389/fpls.2018.01345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries K. 1964. Uber einen genuinen keimungs und streckungswachstumsaktiven hemmstoff bei Marchantia polymorpha L. [About genuine germination and extension growth inhibitor in Marchantia polymorpha L.]. Beitr Biol Pflanz 40: 177–235. [Google Scholar]
- Fujita T, Sakaguchi H, Hiwatashi Y, Wagstaff SJ, Ito M, Deguchi H, Sato T, Hasebe M. 2008. Convergent evolution of shoots in land plants: lack of auxin polar transport in moss shoots. Evol Dev 10: 176–186. 10.1111/j.1525-142X.2008.00225.x [DOI] [PubMed] [Google Scholar]
- Gaal DJ, Dufresne SJ, Maravolo NC. 1982. Transport of 14C-indoleacetic acid in the hepatic Marchantia polymorpha. Bryologist 85: 410–418. 10.2307/3242908 [DOI] [Google Scholar]
- Gray WM, del Pozo JC, Walker L, Hobbie L, Risseeuw E, Banks T, Crosby WL, Yang M, Ma H, Estelle M. 1999. Identification of an SCF ubiquitin-ligase complex required for auxin response in Arabidopsis thaliana. Genes Dev 13: 1678–1691. 10.1101/gad.13.13.1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M. 2001. Auxin regulates SCFTIR1-dependent degradation of AUX/IAA proteins. Nature 414: 271–276. 10.1038/35104500 [DOI] [PubMed] [Google Scholar]
- Grones P, Friml J. 2015. Auxin transporters and binding proteins at a glance. J Cell Sci 128: 1–7. 10.1242/jcs.159418 [DOI] [PubMed] [Google Scholar]
- Hagen G, Martin G, Li Y, Guilfoyle TJ. 1991. Auxin-induced expression of the soybean GH3 promoter in transgenic tobacco plants. Plant Mol Biol 17: 567–579. 10.1007/BF00040658 [DOI] [PubMed] [Google Scholar]
- Han X, Hyun TK, Zhang M, Kumar R, Koh E, Kang BH, Lucas WJ, Kim JY. 2014. Auxin-callose-mediated plasmodesmal gating is essential for tropic auxin gradient formation and signaling. Dev Cell 28: 132–146. 10.1016/j.devcel.2013.12.008 [DOI] [PubMed] [Google Scholar]
- Harrison CJ, Roeder AHK, Meyerowitz EM, Langdale JA. 2009. Local cues and asymmetric cell divisions underpin body plan transitions in the moss Physcomitrella patens. Curr Biol 19: 461–471. 10.1016/j.cub.2009.02.050 [DOI] [PubMed] [Google Scholar]
- He W, Brumos J, Li H, Ji Y, Ke M, Gong X, Zeng Q, Li W, Zhang X, An F, et al. 2011. A small-molecule screen identifies l-kynurenine as a competitive inhibitor of TAA1/TAR activity in ethylene-directed auxin biosynthesis and root growth in Arabidopsis. Plant Cell 23: 3944–3960. 10.1105/tpc.111.089029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisanaga T, Yamaoka S, Kawashima T, Higo A, Nakajima K, Araki T, Kohchi T, Berger F. 2019. Building new insights in plant gametogenesis from an evolutionary perspective. Nat Plants 5: 663–669. 10.1038/s41477-019-0466-0 [DOI] [PubMed] [Google Scholar]
- Ilahi I, Allsopp A. 1969. Studies in the Metzgeriales (Hepaticae). II: The effects of certain auxins on the growth and morphology of some thalloid species. Phytomorphology 19: 381–389. [Google Scholar]
- Ilahi I, Allsopp A. 1970a. Studies in the Metzgeriales (Hepaticae). IV: Further investigations on the effects of various physiologically active substances on Noteroclada confluens and on some thalloid species. Phytomorphology 20: 68–77. [Google Scholar]
- Ilahi I, Allsopp A. 1970b. Studies in the Metzgeriales (Hepaticae). VI: Regeneration and callus formation in some thalloid species. Phytomorphology 20: 126–136. [Google Scholar]
- Inoue H. 1960. Studies in spore germination and the earlier stages of gametophyte development in the Marchantiales. J Hattori Bot Lab 23: 148–191. [Google Scholar]
- Inoue K, Nishihama R, Kataoka H, Hosaka M, Manabe R, Nomoto M, Tada Y, Ishizaki K, Kohchi T. 2016. Phytochrome signaling is mediated by PHYTOCHROME INTERACTING FACTOR in the liverwort Marchantia polymorpha. Plant Cell 28: 1406–1421. 10.1105/tpc.15.01063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki K, Nonomura M, Kato H, Yamato KT, Kohchi T. 2012. Visualization of auxin-mediated transcriptional activation using a common auxin-responsive reporter system in the liverwort Marchantia polymorpha. J Plant Res 125: 643–651. 10.1007/s10265-012-0477-7 [DOI] [PubMed] [Google Scholar]
- Ishizaki K, Nishihama R, Yamato KT, Kohchi T. 2016. Molecular genetic tools and techniques for Marchantia polymorpha research. Plant Cell Physiol 57: 262–270. 10.1093/pcp/pcv097 [DOI] [PubMed] [Google Scholar]
- Jackson RG, Lim EK, Li Y, Kowalczyk M, Sandberg G, Hogget J, Ashford DA, Bowles DJ. 2001. Identification and biochemical characterization of an Arabidopsis indole-3-acetic acid glucosyltransferase. J Biol Chem 276: 4350–4356. 10.1074/jbc.M006185200 [DOI] [PubMed] [Google Scholar]
- Jang G, Dolan L. 2011. Auxin promotes the transition from chloronema to caulonema in moss protonema by positively regulating PpRSL1 and PpRSL2 in Physcomitrella patens. New Phytol 192: 319–327. 10.1111/j.1469-8137.2011.03805.x [DOI] [PubMed] [Google Scholar]
- Jang G, Yi K, Pires ND, Menand B, Dolan L. 2011. RSL genes are sufficient for rhizoid system development in early diverging land plants. Development 138: 2273–2281. 10.1242/dev.060582 [DOI] [PubMed] [Google Scholar]
- Johri MM, Desai S. 1973. Auxin regulation of caulonema formation in moss protonema. Nat New Biol 245: 223–224. 10.1038/newbio245223a0 [DOI] [PubMed] [Google Scholar]
- Karas B, Amyot L, Johansen C, Sato S, Tabata S, Kawaguchi M, Szczyglowski K. 2009. Conservation of Lotus and Arabidopsis basic helix-loop-helix proteins reveals new players in root hair development. Plant Physiol 151: 1175–1185. 10.1104/pp.109.143867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara H. 2016. Current aspects of auxin biosynthesis in plants. Biosci Biotechnol Biochem 80: 34–42. 10.1080/09168451.2015.1086259 [DOI] [PubMed] [Google Scholar]
- Kato H, Ishizaki K, Kouno M, Shirakawa M, Bowman JL, Nishihama R, Kohchi T. 2015. Auxin-mediated transcriptional system with a minimal set of components is critical for morphogenesis through the life cycle in Marchantia polymorpha. PLoS Genet 11: e1005084. 10.1371/journal.pgen.1005084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Kouno M, Takeda M, Suzuki H, Ishizaki K, Nishihama R, Kohchi T. 2017. The roles of the sole activator-type auxin response factor in pattern formation of Marchantia polymorpha. Plant Cell Physiol 58: 1642–1651. 10.1093/pcp/pcx095 [DOI] [PubMed] [Google Scholar]
- Kato H, Nishihama R, Weijers D, Kohchi T. 2018. Evolution of nuclear auxin signaling: lessons from genetic studies with basal land plants. J Exp Bot 69: 291–301. 10.1093/jxb/erx267 [DOI] [PubMed] [Google Scholar]
- Kato H, Mutte SK, Suzuki H, Crespo I, Das S, Radoeva T, Fontana M, Yoshitake Y, Hainiwa E, van den Berg W, et al. 2020a. Design principles of a minimal auxin response system. Nat Plants 6: 473–482. 10.1038/s41477-020-0662-y [DOI] [PubMed] [Google Scholar]
- Kato H, Yasui Y, Ishizaki K. 2020b. Gemma cup and gemma development in Marchantia polymorpha. New Phytol 228: 459–465. 10.1111/nph.16655 [DOI] [PubMed] [Google Scholar]
- Kepinski S, Leyser O. 2005. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435: 446–451. 10.1038/nature03542 [DOI] [PubMed] [Google Scholar]
- Kim J, Harter K, Theologis A. 1997. Protein–protein interactions among the Aux/IAA proteins. Proc Natl Acad Sci 94: 11786–11791. 10.1073/pnas.94.22.11786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kny L. 1890. Bau und Entwickelung von Marchantia polymorpha L. In Botanische Wandtafeln, pp. 364-401. VIII Abtheilung, Paul Parey, Berlin. [Google Scholar]
- Kofuji R, Hasebe M. 2014. Eight types of stem cells in the life cycle of the moss Physcomitrella patens. Curr Opin Plant Biol 17: 13–21. 10.1016/j.pbi.2013.10.007 [DOI] [PubMed] [Google Scholar]
- Korasick DA, Westfall CS, Lee SG, Nanao MH, Dumas R, Hagen G, Guilfoyle TJ, Jez JM, Strader LC. 2014. Molecular basis for AUXIN RESPONSE FACTOR protein interaction and the control of auxin response repression. Proc Natl Acad Sci 111: 5427–5432. 10.1073/pnas.1400074111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagercrantz U, Billhardt A, Rousku SN, Ljung K, Eklund DM. 2020. Nyctinastic thallus movement in the liverwort Marchantia polymorpha is regulated by a circadian clock. Sci Rep 10: 8658. 10.1038/s41598-020-65372-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landberg K, Pederson ERA, Viaene T, Bozorg B, Friml J, Jönsson H, Thelander M, Sundberg E. 2013. The moss Physcomitrella patens reproductive organ development is highly organized, affected by the two SHI/STY genes and by the level of active auxin in the SHI/STY expression domain. Plant Physiol 162: 1406–1419. 10.1104/pp.113.214023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landberg K, Šimura J, Ljung K, Sundberg E, Thelander M. 2020. Studies of moss reproductive development indicate that auxin biosynthesis in apical stem cells may constitute an ancestral function for focal growth control. New Phytol 10.1111/nph.16914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrein B, Vernoux T. 2014. Auxin, chief architect of the shoot apex. In Auxin and its role in plant development (ed. Zažímalová E, Petrasek J, Benková E), pp. 191–212. Springer, Vienna, Austria. [Google Scholar]
- Lang D, Ullrich KK, Murat F, Fuchs J, Jenkins J, Haas FB, Piednoel M, Gundlach H, Van Bel M, Meyberg R, et al. 2018. The Physcomitrella patens chromosome-scale assembly reveals moss genome structure and evolution. Plant J 93: 515–533. 10.1111/tpj.13801 [DOI] [PubMed] [Google Scholar]
- LaRue CD, Narayanaswami S. 1957. Auxin inhibition in the liverwort Lunularia. New Phytologist 56: 61–70. 10.1111/j.1469-8137.1957.tb07449.x [DOI] [Google Scholar]
- Lavy M, Prigge MJ, Tao S, Shain S, Kuo A, Kirchsteiger K, Estelle M. 2016. Constitutive auxin response in Physcomitrella reveals complex interactions between Aux/IAA and ARF proteins. eLife 5: e13325. 10.7554/eLife.13325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li FW, Nishiyama T, Waller M, Frangedakis E, Keller J, Li Z, Fernandez-Pozo N, Barker MS, Bennett T, Blázquez MA, et al. 2020. Anthoceros genomes illuminate the origin of land plants and the unique biology of hornworts. Nat Plants 6: 259–272. 10.1038/s41477-020-0618-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C-Y, Smet W, Brunoud G, Yoshida S, Vernoux T, Weijers D. 2015. Reporters for sensitive and quantitative measurement of auxin response. Nat Methods 12: 207–210. 10.1038/nmeth.3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligrone R, Duckett JG, Renzaglia KS. 2012. The origin of the sporophyte shoot in land plants: a bryological perspective. Ann Bot 110: 935–941. 10.1093/aob/mcs176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PC, Lu CW, Shen BN, Lee GZ, Bowman JL, Arteaga-Vazquez MA, Liu LYD, Hong SF, Lo CF, Su GM, et al. 2016. Identification of miRNAs and their targets in the liverwort Marchantia polymorpha by integrating RNA-Seq and degradome analyses. Plant Cell Physiol 57: 339–358. 10.1093/pcp/pcw020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löbenberg E. 1959. “Über genuine wuchsstoffe bei Marchantia polymorpha L.” [About genuine growth substances in Marchantia polymorpha L.]. PhD thesis, University of Frankfurt, Frankfurt, Germany. [Google Scholar]
- Lu KJ, van't Wout Hofland N, Mor E, Mutte S, Abrahams P, Kato H, Vandepoele K, Weijers D, de Rybel B. 2020. Evolution of vascular plants through redeployment of ancient developmental regulators. Proc Natl Acad Sci 117: 733–740. 10.1073/pnas.1912470117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig-Müller J, Decker EL, Reski R. 2009a. Dead end for auxin conjugates in Physcomitrella? Plant Signal Behav 4: 116–118. 10.4161/psb.4.2.7536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig-Müller J, Jülke S, Bierfreund NM, Decker EL, Reski R. 2009b. Moss (Physcomitrella patens) GH3 proteins act in auxin homeostasis. New Phytol 181: 323–338. 10.1111/j.1469-8137.2008.02677.x [DOI] [PubMed] [Google Scholar]
- Lv B, Yu Q, Liu J, Wen X, Yan Z, Hu K, Li H, Kong X, Li C, Tian H, et al. 2020. Non-canonical AUX/IAA protein IAA33 competes with canonical AUX/IAA repressor IAA5 to negatively regulate auxin signaling. EMBO J 39: e101515. 10.15252/embj.2019101515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQuarrie IG, von Maltzahn KE. 1959. Correlations affecting regeneration and reactivation in Splachnum ampullaceum (L.) Hedw. Can J Bot 37: 121–134. 10.1139/b59-011 [DOI] [Google Scholar]
- Mallory AC, Bartel DP, Bartel B. 2005. MicroRNA-directed regulation of Arabidopsis AUXIN RESPONSE FACTOR17 is essential for proper development and modulates expression of early auxin response genes. Plant Cell 17: 1360–1375. 10.1105/tpc.105.031716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maravolo NC. 1976. Polarity and localization of auxin movement in the hepatic, Marchantia polymorpha. Am J Bot 63: 526–531. 10.1002/j.1537-2197.1976.tb11841.x [DOI] [Google Scholar]
- Maravolo NC, Voth PD. 1966. Morphogenic effects of three growth substances on Marchantia gemmalings. Bot Gaz 127: 79–86. 10.1086/336346 [DOI] [Google Scholar]
- Masucci JD, Schiefelbein JW. 1994. The rhd6 mutation of Arabidopsis thaliana alters root-hair initiation through an auxin- and ethylene-associated process. Plant Physiol 106: 1335–1346. 10.1104/pp.106.4.1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menand B, Keke Yi SJ, Hoffmann L, Ryan E, Linstead P, Schaefer DG, Dolan L. 2007. An ancient mechanism controls the development of cells with a rooting function in land plants. Science 316: 1477–1480. 10.1126/science.1142618 [DOI] [PubMed] [Google Scholar]
- Mittag J, Gabrielyan A, Ludwig-Müller J. 2015. Knockout of GH3 genes in the moss Physcomitrella patens leads to increased IAA levels at elevated temperature and in darkness. Plant Physiol Biochem 97: 339–349. 10.1016/j.plaphy.2015.10.013 [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Tanizawa Y, Galik B, Wang N, Ito T, Mochizuki T, Akimcheva S, Bowman JL, Cognat V, Maréchal-Drouard L, et al. 2020. Chromatin organization in early land plants reveals an ancestral association between H3K27me3, transposons, and constitutive heterochromatin. Curr Biol 30: 573–588.e7. 10.1016/j.cub.2019.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JL, Puttick MN, Clark JW, Edwards D, Kenrick P, Pressel S, Wellman CH, Yang Z, Schneider H, Donoghue PCJ. 2018. The timescale of early land plant evolution. Proc Natl Acad Sci 115: E2274–E2283. 10.1073/pnas.1719588115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutte SK, Kato H, Rothfels C, Melkonian M, Wong GKS, Weijers D. 2018. Origin and evolution of the nuclear auxin response system. eLife 7: e33399. 10.7554/eLife.33399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishihama R, Ishizaki K, Hosaka M, Matsuda Y, Kubota A, Kohchi T. 2015. Phytochrome-mediated regulation of cell division and growth during regeneration and sporeling development in the liverwort Marchantia polymorpha. J Plant Res 128: 407–421. 10.1007/s10265-015-0724-9 [DOI] [PubMed] [Google Scholar]
- Nishiyama T, Wolf PG, Kugita M, Sinclair RB, Sugita M, Sugiura C, Wakasugi T, Yamada K, Yoshinaga K, Yamaguchi K, et al. 2004. Chloroplast phylogeny indicates that bryophytes are monophyletic. Mol Biol Evol 21: 1813–1819. 10.1093/molbev/msh203 [DOI] [PubMed] [Google Scholar]
- Nyman LP, Cutter EG. 1981. Auxin–cytokinin interaction in the inhibition, release, and morphology of gametophore buds of Plagiomnium cuspidatum from apical dominance. Can J Bot 59: 750–762. 10.1139/b81-106 [DOI] [Google Scholar]
- Otto KR, Halbsguth W. 1976. Die förderung der bildung von primärrhizoiden an brutkörpern von Marchantia polymorpha L. durch licht und IES [The promotion of the formation of primary rhizoids on brood bodies of Marchantia polymorpha L.]. Z Pflanzenphysiol 80: 197–205. 10.1016/S0044-328X(76)80021-9 [DOI] [Google Scholar]
- Paponov IA, Teale W, Lang D, Paponov M, Reski R, Rensing SA, Palme K. 2009. The evolution of nuclear auxin signalling. BMC Evol Biol 9: 126. 10.1186/1471-2148-9-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires ND, Yi K, Breuninger H, Catarino B, Menand B, Dolan L. 2013. Recruitment and remodeling of an ancient gene regulatory network during land plant evolution. Proc Natl Acad Sci 110: 9571–9576. 10.1073/pnas.1305457110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plavskin Y, Nagashima A, Perroud PF, Hasebe M, Quatrano RS, Atwal GS, Timmermans MCP. 2016. Ancient trans-acting siRNAs confer robustness and sensitivity onto the auxin response. Dev Cell 36: 276–289. 10.1016/j.devcel.2016.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poli D, Jacobs M, Cooke TJ. 2003. Auxin regulation of axial growth in bryophyte sporophytes: its potential significance for the evolution of early land plants. Am J Bot 90: 1405–1415. 10.3732/ajb.90.10.1405 [DOI] [PubMed] [Google Scholar]
- Porco S, Pěnčík A, Rashed A, Voß U, Casanova-Sáez R, Bishopp A, Golebiowska A, Bhosale R, Swarup R, Swarup K, et al. 2016. Dioxygenase-encoding AtDAO1 gene controls IAA oxidation and homeostasis in Arabidopsis. Proc Natl Acad Sci 113: 11016–11021. 10.1073/pnas.1604375113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge MJ, Lavy M, Ashton NW, Estelle M. 2010. Physcomitrella patens auxin-resistant mutants affect conserved elements of an auxin-signaling pathway. Curr Biol 20: 1907–1912. 10.1016/j.cub.2010.08.050 [DOI] [PubMed] [Google Scholar]
- Prigge MJ, Platre M, Kadakia N, Zhang Y, Greenham K, Szutu W, Pandey BK, Bhosale RA, Bennett MJ, Busch W, et al. 2020. Genetic analysis of the Arabidopsis TIR1/AFB auxin receptors reveals both overlapping and specialized functions. eLife 9: e54740. 10.7554/eLife.54740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proust H, Honkanen S, Jones VAS, Morieri G, Prescott H, Kelly S, Ishizaki K, Kohchi T, Dolan L. 2016. RSL class I genes controlled the development of epidermal structures in the common ancestor of land plants. Curr Biol 26: 93–99. 10.1016/j.cub.2015.11.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puttick MN, Morris JL, Williams TA, Cox CJ, Edwards D, Kenrick P, Pressel S, Wellman CH, Schneider H, Pisani D, et al. 2018. The interrelationships of land plants and the nature of the ancestral embryophyte. Curr Biol 28: 733–745.e2. 10.1016/j.cub.2018.01.063 [DOI] [PubMed] [Google Scholar]
- Rensing SA, Lang D, Zimmer AD, Terry A, Salamov A, Shapiro H, Nishiyama T, Perroud PF, Lindquist EA, Kamisugi Y, et al. 2008. The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science 319: 64–69. 10.1126/science.1150646 [DOI] [PubMed] [Google Scholar]
- Rensing SA, Goffinet B, Meyberg R, Wu SZ, Bezanilla M. 2020. The moss Physcomitrium (Physcomitrella) patens: a model organism for non-seed plants. Plant Cell 32: 1361–1376. 10.1105/tpc.19.00828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retzer K, Korbei B, Luschnig C. 2014. Auxin and tropisms. In Auxin and its role in plant development (ed. Zažímalová E, et al. ), pp. 361–387. Springer, Vienna, Austria. [Google Scholar]
- Rios AF, Yoshida S, Weijers D. 2014. Auxin regulation of embryo development. In Auxin and its role in plant development (ed. Zažímalová E, Petrasek J, Benková E), pp. 171–189. Springer, Vienna, Austria. [Google Scholar]
- Rose S, Bopp M. 1983. Uptake and polar transport of indoleacetic acid in moss rhizoids. Physiol Plant 58: 57–61. 10.1111/j.1399-3054.1983.tb04143.x [DOI] [Google Scholar]
- Rota JA, Maravolo NC. 1975. Transport and mobilization of 14C-sucrose during regeneration in the hepatic, Marchantia polymorpha. Bot Gaz 136: 184–188. 10.1086/336800 [DOI] [Google Scholar]
- Rousseau J. 1950. Action de l'acide indol beta-acétique sur les propagules de Marchantia polymorpha et Lunularia cruciata [Action of indole beta-acetic acid on the propagules of Marchantia polymorpha and Lunularia cruciata]. C R Hebd Seances Acad Sci 230: 675–676. [Google Scholar]
- Rousseau J. 1953. Action des hétéro-auxines sur les chapeaux du Marchantia polymorpha L. [Action of heteroauxins on the caps of Marchantia polymorpha L.]. Bull Soc Bot Fr 100: 179–180. 10.1080/00378941.1953.10833187 [DOI] [Google Scholar]
- Rousseau J. 1954. Action des hétéroauxines á l'obscurité sur les propagules de Marchantia polymorpha L. [Action of heteroauxins in the dark on the propagules of Marchantia polymorpha L.]. C R Hebd Seances Acad Sci 238: 2111–2112. [Google Scholar]
- Rousseau J. 1958. Morphoses du sporogone de deux Anthoceros par action de l'acide dichlorophénoxyacétique [Morphoses of the sporogonium of two Anthoceros by the action of dichlorophenoxyacetic acid]. Bull Soc Bot Fr 105: 234–236. 10.1080/00378941.1958.10837870 [DOI] [Google Scholar]
- Sakakibara K, Nishiyama T, Sumikawa N, Kofuji R, Murata T, Hasebe M. 2003. Involvement of auxin and a homeodomain-leucine zipper I gene in rhizoid development of the moss Physcomitrella patens. Development 130: 4835–4846. 10.1242/dev.00644 [DOI] [PubMed] [Google Scholar]
- Schlereth A, Möller B, Liu W, Kientz M, Flipse J, Rademacher EH, Schmid M, Jürgens G, Weijers D. 2010. MONOPTEROS controls embryonic root initiation by regulating a mobile transcription factor. Nature 464: 913–916. 10.1038/nature08836 [DOI] [PubMed] [Google Scholar]
- Schneider MJ, Voth PD, Troxler RF. 1967. Methods of propagating bryophyte plants, tissues, and propagules. Bot Gaz 128: 169–174. 10.1086/336394 [DOI] [Google Scholar]
- Schnepf E, Herth W, Morré DJ. 1979. Elongation growth of setae of Pellia (Bryophyta): effects of auxin and inhibitors. Z Pflanzenphysiol 94: 211–217. 10.1016/S0044-328X(79)80160-9 [DOI] [Google Scholar]
- Schwuchow J, Michalke W, Hertel R. 2001. An auxin transport inhibitor interferes with unicellular gravitropism in protonemata of the moss Ceratodon purpureus. Plant Biol 3: 357–363. 10.1055/s-2001-16459 [DOI] [Google Scholar]
- Shimamura M. 2016. Marchantia polymorpha: taxonomy, phylogeny and morphology of a model system. Plant Cell Physiol 57: 230–256. 10.1093/pcp/pcv192 [DOI] [PubMed] [Google Scholar]
- Sohlberg JJ, Myrenås M, Kuusk S, Lagercrantz U, Kowalczyk M, Sandberg G, Sundberg E. 2006. STY1 regulates auxin homeostasis and affects apical–basal patterning of the Arabidopsis gynoecium. Plant J 47: 112–123. 10.1111/j.1365-313X.2006.02775.x [DOI] [PubMed] [Google Scholar]
- Solly JE, Cunniffe NJ, Harrison CJ. 2017. Regional growth rate differences specified by apical notch activities regulate liverwort thallus shape. Curr Biol 27: 16–26. 10.1016/j.cub.2016.10.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood S, Hackenberg D. 1979. Interaction of auxin, antiauxin and cytokinin in relation to the formation of buds in moss protonema. Z Pflanzenphysiol 91: 385–397. 10.1016/S0044-328X(79)80253-6 [DOI] [Google Scholar]
- Ståldal V, Sohlberg JJ, Eklund DM, Ljung K, Sundberg E. 2008. Auxin can act independently of CRC, LUG, SEU, SPT and STY1 in style development but not apical-basal patterning of the Arabidopsis gynoecium. New Phytol 180: 798–808. 10.1111/j.1469-8137.2008.02625.x [DOI] [PubMed] [Google Scholar]
- Staswick PE, Serban B, Rowe M, Tiryaki I, Maldonado MT, Maldonado MC, Suza W. 2005. Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 17: 616–627. 10.1105/tpc.104.026690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara S, Mashiguchi K, Tanaka K, Hishiyama S, Sakai T, Hanada K, Kinoshita-Tsujimura K, Yu H, Dai X, Takebayashi Y, et al. 2015. Distinct characteristics of indole-3-acetic acid and phenylacetic acid, two common auxins in plants. Plant Cell Physiol 56: 1641–1654. 10.1093/pcp/pcv088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Harrison CJ, Shimamura M, Kohchi T, Nishihama R. 2020. Positional cues regulate dorsal organ formation in the liverwort Marchantia polymorpha. J Plant Res 133: 311–321. 10.1007/s10265-020-01180-5 [DOI] [PubMed] [Google Scholar]
- Szemenyei H, Hannon M, Long JA. 2008. TOPLESS mediates auxin-dependent transcriptional repression during Arabidopsis embryogenesis. Science 319: 1384–1386. 10.1126/science.1151461 [DOI] [PubMed] [Google Scholar]
- Sztein AE, Cohen JD, Slovin JP, Cooke TJ. 1995. Auxin metabolism in representative land plants. Am J Bot 82: 1514–1521. 10.1002/j.1537-2197.1995.tb13853.x [DOI] [Google Scholar]
- Sztein AE, Cohen JD, García De La Fuente I, Cooke TJ. 1999. Auxin metabolism in mosses and liverworts. Am J Bot 86: 1544–1555. 10.2307/2656792 [DOI] [PubMed] [Google Scholar]
- Sztein AE, Cohen JD, Cooke TJ. 2000. Evolutionary patterns in the auxin metabolism of green plants. Int J Plant Sci 161: 849–859. 10.1086/317566 [DOI] [Google Scholar]
- Tam THY, Catarino B, Dolan L. 2015. Conserved regulatory mechanism controls the development of cells with rooting functions in land plants. Proc Natl Acad Sci 112: E3959–E3968. 10.1073/pnas.1416324112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Calderon-Villalobos LIA, Sharon M, Zheng C, Robinson CV, Estelle M, Zheng N. 2007. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature 446: 640–645. 10.1038/nature05731 [DOI] [PubMed] [Google Scholar]
- Tao S, Estelle M. 2018. Mutational studies of the Aux/IAA proteins in Physcomitrella reveal novel insights into their function. New Phytol 218: 1534–1542. 10.1111/nph.15039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarén N. 1958. Factors regulating the initial development of gemmae in Marchantia polymorpha. Bryologist 61: 191–204. 10.1639/0007-2745(1958)61[191:FRTIDO]2.0.CO;2 [DOI] [Google Scholar]
- Thelander M, Landberg K, Sundberg E. 2018. Auxin-mediated developmental control in the moss Physcomitrella patens. J Exp Bot 69: 277–290. 10.1093/jxb/erx255 [DOI] [PubMed] [Google Scholar]
- Thelander M, Landberg K, Sundberg E. 2019. Minimal auxin sensing levels in vegetative moss stem cells revealed by a ratiometric reporter. New Phytol 224: 775–788. 10.1111/nph.16068 [DOI] [PubMed] [Google Scholar]
- Thomas RJ. 1980. Cell elongation in hepatics: the seta system. Bot Gaz 107: 229–245. [Google Scholar]
- Tiwari SB, Hagen G, Guilfoyle T. 2003. The roles of auxin response factor domains in auxin-responsive transcription. Plant Cell 15: 533–543. 10.1105/tpc.008417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugafune S, Mashiguchi K, Fukui K, Takebayashi Y, Nishimura T, Sakai T, Shimada Y, Kasahara H, Koshiba T, Hayashi K. 2017. Yucasin DF, a potent and persistent inhibitor of auxin biosynthesis in plants. Sci Rep 7: 13992. 10.1038/s41598-017-14332-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzuki M, Nishihama R, Ishizaki K, Kurihara Y, Matsui M, Bowman JL, Kohchi T, Hamada T, Watanabe Y. 2016. Profiling and characterization of small RNAs in the liverwort, Marchantia polymorpha, belonging to the first diverged land plants. Plant Cell Physiol 57: 359–372. 10.1093/pcp/pcv182 [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Hagen G, Guilfoyle TJ. 1997. ARF1, a transcription factor that binds to auxin response elements. Science 276: 1865–1868. 10.1126/science.276.5320.1865 [DOI] [PubMed] [Google Scholar]
- Ulmasov T, Hagen G, Guilfoyle TJ. 1999a. Activation and repression of transcription by auxin-response factors. Proc Natl Acad Sci 96: 5844–5849. 10.1073/pnas.96.10.5844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmasov T, Hagen G, Guilfoyle TJ. 1999b. Dimerization and DNA binding of auxin response factors. Plant J 19: 309–319. 10.1046/j.1365-313X.1999.00538.x [DOI] [PubMed] [Google Scholar]
- Viaene T, Delwiche CF, Rensing SA, Friml J. 2013. Origin and evolution of PIN auxin transporters in the green lineage. Trends Plant Sci 18: 5–10. 10.1016/j.tplants.2012.08.009 [DOI] [PubMed] [Google Scholar]
- Viaene T, Landberg K, Thelander M, Medvecka E, Pederson E, Feraru E, Cooper ED, Karimi M, Delwiche CF, Ljung K, et al. 2014. Directional auxin transport mechanisms in early diverging land plants. Curr Biol 24: 2786–2791. 10.1016/j.cub.2014.09.056 [DOI] [PubMed] [Google Scholar]
- Vöchting H. 1885. Ueber die regeneration der Marchantieen [About the regeneration of Marchantieen]. Jahrb Wiss Bot 16: 367–414. [Google Scholar]
- Wang JW, Wang LJ, Mao YB, Cai WJ, Xue HW, Chen XY. 2005. Control of root cap formation by microRNA-targeted auxin response factors in Arabidopsis. Plant Cell 17: 2204–2216. 10.1105/tpc.105.033076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Qin G, Cao M, Chen R, He Y, Yang L, Zeng Z, Yu Y, Gu Y, Xing W, et al. 2020. A phosphorylation-based switch controls TAA1-mediated auxin biosynthesis in plants. Nat Commun 11: 679. 10.1038/s41467-020-14395-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickett NJ, Mirarab S, Nguyen N, Warnow T, Carpenter E, Matasci N, Ayyampalayam S, Barker MS, Burleigh JG, Gitzendanner MA, et al. 2014. Phylotranscriptomic analysis of the origin and early diversification of land plants. Proc Natl Acad Sci 111: E4859–E4868. 10.1073/pnas.1323926111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L, Carles CC, Osmont KS, Fletcher JC. 2005. A database analysis method identifies an endogenous trans-acting short-interfering RNA that targets the Arabidopsis ARF2, ARF3, and ARF4 genes. Proc Natl Acad Sci 102: 9703–9708. 10.1073/pnas.0504029102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MF, Yamaguchi N, Xiao J, Bargmann B, Estelle M, Sang Y, Wagner D. 2015. Auxin-regulated chromatin switch directs acquisition of flower primordium founder fate. eLife 4: e09269. 10.7554/eLife.09269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia R, Xu J, Meyers BC. 2017. The emergence, evolution, and diversification of the miR390-TAS3-ARF pathway in land plants. Plant Cell 29: 1232–1247. 10.1105/tpc.17.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi K, Menand B, Bell E, Dolan L. 2010. A basic helix-loop-helix transcription factor controls cell growth and size in root hairs. Nat Genet 42: 264–267. 10.1038/ng.529 [DOI] [PubMed] [Google Scholar]
- Yoshida S, BarbierdeReuille P, Lane B, Bassel GW, Prusinkiewicz P, Smith RS, Weijers D. 2014. Genetic control of plant development by overriding a geometric division rule. Dev Cell 29: 75–87. 10.1016/j.devcel.2014.02.002 [DOI] [PubMed] [Google Scholar]
- Záveská Drábková L, Dobrev PI, Motyka V. 2015. Phytohormone profiling across the bryophytes. PLoS ONE 10: e0125411. 10.1371/journal.pone.0125411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Lin JE, Harris C, Campos Mastrotti Pereira F, Wu F, Blakeslee JJ, Peer WA. 2016. DAO1 catalyzes temporal and tissue-specific oxidative inactivation of auxin in Arabidopsis thaliana. Proc Natl Acad Sci 113: 11010–11015. 10.1073/pnas.1604769113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Fu XX, Li RQ, Zhao X, Liu Y, Li MH, Zwaenepoel A, Ma H, Goffinet B, Guan YL, et al. 2020. The hornwort genome and early land plant evolution. Nat Plants 6: 107–118. 10.1038/s41477-019-0588-4 [DOI] [PMC free article] [PubMed] [Google Scholar]