Abstract
Identifying environmental risk and protective exposures that have causal effects on health is an important scientific goal. Many environmental exposures are nonrandomly allocated and influenced by dispositional factors including inherited ones. We review family-based designs that can separate the influence of environmental exposures from inherited influences shared between parent and offspring. We focus on prenatal exposures. We highlight that the family-based designs that can separate the prenatal environment from inherited confounds are different to those that are able to pull apart later-life environmental exposures from inherited confounds. We provide a brief review of the literature on maternal smoking during pregnancy and offspring attention-deficit/hyperactivity disorder (ADHD) and conduct problems; these inconsistencies in the literature make a review useful and this illustrates that results of family-based genetically informed studies are inconsistent with a causal interpretation for this exposure and these two offspring outcomes.
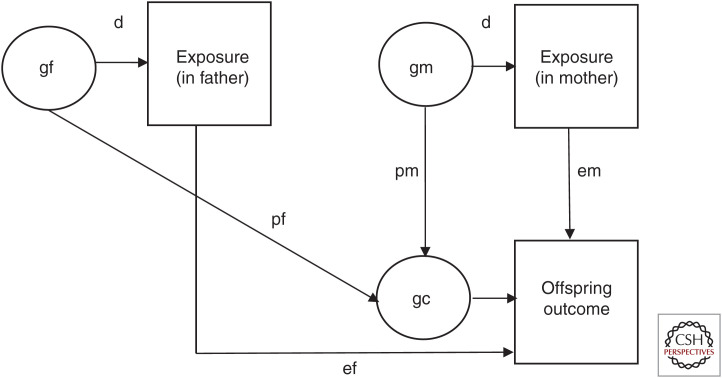
Identifying environmental risk and protective exposures that have causal effects on health and development is an important scientific and public health goal. However, as discussed in the literature, it is challenging to assess causality for reasons including confounding, reverse causation, and selection bias (Academy of Medical Sciences 2007; Gage et al. 2016; Pingault et al. 2018; Thapar and Rutter 2019). Environmental exposures are nonrandomly allocated and many are person-dependent—referred to as person–environment correlation—which means that people behave in ways that shape their environments and these environments have important implications for health, social, and economic outcomes (Rutter et al. 2001). Person effects on the environment are driven by dispositional factors including those that are inherited, a phenomenon known as gene–environment correlation (Rutter et al. 2001; Kong et al. 2018). Three types of gene–environment correlation have been described (e.g., Rutter et al. 2006): (1) passive gene–environment correlation, which comes about because parents provide both genes and the rearing environment for their child(ren) and alleles are correlated between parent(s) and child(ren); (2) evocative gene–environment correlation, which comes about because people's inherited behaviors evoke particular reactions from others, which may further exacerbate that behavior in the individual; (3) active gene–environment correlation that occurs when people seek out or “create” environments consistent with their inherited dispositional characteristics. The phenomenon of gene–environment correlation applies to prenatal exposures including health and lifestyle behaviors during pregnancy such as maternal cigarette smoking and alcohol consumption as well as postnatal and later exposures, including factors such as parenting style, stressful life events, social adversities, and lifestyle and health behaviors (Kendler and Baker 2007; Jaffee and Price 2008; Rice et al. 2018). Medical complications during pregnancy are also consistent with person effects on the prenatal environment. Pregnancy may reveal biological vulnerabilities for chronic disease as women who develop preeclampsia, gestational hypertension, and abruption or infarction of the placenta are at heightened risk of developing cardiovascular disease and diabetes after pregnancy (Kaaja and Greer 2005). Thus, it is unsurprising that many of the environmental factors hypothesized as contributors to health and disease have been observed to be genetically influenced (Plomin 2018). This raises the issue that observed associations between exposure and disease outcome could be explained by familial or genetic confounds that would not be identified in observational studies (Fig. 1). Passive gene–environment correlation is a special instance of a person–environment correlation, which creates challenges for causal interference because environmental exposures are indexed in part by parental characteristics including genetic factors that are transmitted to offspring (Fig. 1). Thus, parents create rearing environments that are correlated with their genotype (shown in “d” in Fig. 1). Features of the rearing environment can directly influence offspring development (“E”) or can in fact be markers of shared genetic liability in the parent and offspring generations (“P”) (i.e., correlation may come about because the parent passes on genetic material to the child, which is associated with exposure in the parent generation and outcome in the offspring generation (passive gene–environmental correlation). A final possibility is that of dynastic effects where parental alleles influence environmental exposure and the effect on the child does not depend on shared parent–offspring alleles but instead impacts directly on the child (Kong et al. 2018; Davies et al. 2019).
Figure 1.
Environmental exposures and child outcome: passive gene–environment correlation, dynastic, and exposure effects. Shown is a schematic of passive gene–environment correlation for prenatal environmental exposures in the absence of assortative mating. “p” represents passive gene–environment correlation (i.e., where association between an exposure comes about because of shared inherited influences on environmental exposure in the parent generation and child outcome in the offspring generation) (pf denotes paternal effects and pm denotes maternal effects). “e” represents a direct environmental exposure effect on offspring outcome (ef denotes paternal effects and em denotes maternal effects). “d” represents the influence of nature on nurture (i.e., where parental inherited characteristics influence environmental exposures that may impact on offspring). The product of d and e gives an estimate of what have been termed “dynastic effects” where parental alleles may influence environmental exposure but the environment then has a direct impact on offspring independent of alleles shared between parent and offspring. (gf) father genes, (gm) mother genes, (gc) child genes.
In this work, we provide a brief review of the literature on maternal smoking during pregnancy and offspring ADHD and conduct problems for two reasons. First, there are systematic differences associated with exposure to maternal smoking during pregnancy, which suggests a potential contribution of unmeasured genetic and shared environmental confounding to associations observed between maternal smoking during pregnancy and offspring outcomes. Thus, mothers who smoke in pregnancy differ from those who quit during pregnancy or do not smoke at all (Orton et al. 2014), including on sociodemographic factors relevant to liability for ADHD and antisocial behavior. For example, mothers that smoke during pregnancy are younger, have higher rates of mental health difficulties including depression and antisocial behavior, report more stress during pregnancy, are more likely to live in deprived socioeconomic circumstances, and are more likely to be nicotine dependent (Maughan et al. 2004; Gilman et al. 2008a,b; D'Onofrio et al. 2014; Gustavson et al. 2017). Second, inconsistencies in the literature suggest that a narrative review of family-based genetically informed studies is likely to be useful.
Family-based, genetically informed designs take advantage of differing patterns of genetic similarity between relatives and aid causal inference because association between the exposure (e.g., cigarette smoking in pregnancy) and outcome (e.g., offspring health) are assessed after accounting for genetic and family-level environmental confounds. Table 1 summarizes a variety of family-based genetically informed designs and the sorts of environmental exposures that they can assess after disentangling these from inherited or shared familial factors.
Table 1.
Genetically informative designs and the sorts of environmental effects they can separate from inherited or familial factors
| Study design | Prenatal exposures | Postnatal and later-life exposures | Cross-generational transmission |
|---|---|---|---|
| Maternal vs. paternal exposure | + | ||
| Discordant sib pair design | + | + | |
| IVF design | + | + | + |
| Adoption design | + | + | |
| Classical twin designa | + | ||
| Children of twins designa (e.g., identical twin mothers each with at least one child) | + | + | + |
(IVF) in vitro fertilization.
aTwin design and its extensions are described in McAdams (2020).
Herein, we initially consider family-based designs that are informative for examining prenatal exposures and offspring outcome; these include the maternal versus paternal prenatal exposure, discordant sibling (Keyes et al. 2013), and in vitro fertilization (IVF) (Thapar et al. 2007) designs. These designs do not integrate genomic data. (For additional information, see Hwang et al. 2020, which looks at integrating family-based and genomic [Mendelian randomization] designs.) The crucial aspect of these designs is that they enable the contribution of the intrauterine environment to be separated from genetic or familial factors that the mother shares with her offspring (Rice et al. 2018). This is done either by separating the intrauterine environment from the maternal genome (as in the case of the IVF design) or by varying the intrauterine environment across separate pregnancies but holding the overall level of the mother–child genetic relationship constant (as in the case of the discordant sibling design). The separation of prenatal from genetic or shared familial factors cannot be achieved by classical twin or adoption study designs because any effects of the intrauterine environment cannot be isolated from maternal genetic factors that are shared with her offspring (twins or adopted-at-birth offspring). As discussed by us previously (Rice et al. 2018), the types of genetically informed designs that can separate the prenatal environment from genetic confounds are different to those that are able to pull apart postnatal or later-life environment and genetic confounds. We also discuss adoption-at-birth designs (Kendler et al. 2015) as these are invaluable for testing postnatal and later-life exposures but not prenatal ones. Each of these designs has different strengths and weaknesses and none is without its limitations (Table 2). Also, results from these studies cannot definitively infer causality because the same individual cannot be simultaneously exposed and unexposed (the counterfactual) to a risk or protective factor. Each of the genetically informative designs that we consider, as well as children-of-twins studies described in McAdams (2020), has a different pattern of strengths and limitations. When evidence from different designs is considered together, convergence of results from studies that use research designs with differing patterns of strength and weakness, often referred to as “triangulation” (Lawlor et al. 2016), strengthens confidence in causal inference. Where the pattern of results do not converge, we have argued that considering issues such as indicators of study quality including reliability and validity of measurement, adequate sample size, and tests as to whether the assumptions of the design are met are also important in aiding causal inference (Rice et al. 2018).
Table 2.
Strengths and limitations of family-based genetically informed designs
| Study design | Separation of prenatal exposures from shared familial factors including inherited confounds | Separation of prenatal exposures from inherited confounds | Representativeness of sample | Ability to account for shared environmental confounding | Ability to account for nonshared environmental confounding |
|---|---|---|---|---|---|
| Maternal vs. paternal exposure | + | +a | + | ||
| Discordant sib pair design | + | + | |||
| IVF design | + | + | |||
| Adoption design |
(+) Indicates where a particular study design has strengths. Random allocation to exposure (e.g., through a randomized controlled trial or a natural experiment involving an externally imposed external event) can account for nonshared environmental confounding. The selection of discordantly exposed siblings may mean that such siblings are selected to differ more greatly on nonshared confounding than the general population given similar exposure levels. (IVF) in vitro fertilization.
aAlthough theoretically representative, complete trio data (available father as well as mother and offspring) are likely to be affected by selection bias.
In this review, we focus on prenatal exposures. We summarize the genetically informed studies for maternal smoking during pregnancy and offspring ADHD and antisocial behavior/conduct problems. Exposure to maternal smoking in pregnancy has been viewed as a potentially causal exposure for many types of offspring mental health problems, in particular for ADHD and antisocial behavior. Associations between maternal smoking in pregnancy and ADHD and antisocial behavior have been observed repeatedly in prospective population cohort designs and clinical case–control studies, even when exposure to cigarette smoking is assessed by cotinine levels (e.g., Sourander et al. 2019). Meta-analyses of observational studies show association between smoking during pregnancy and an increased risk of offspring ADHD (OR [odds ratio] = 1.60; 95% CI = 1.45–1.76) (Huang et al. 2018) and conduct problems (OR = 2.06; 95% CI = 1.67, 2.54) (Ruisch et al. 2018). Nevertheless, the genetically informed studies provide a useful adjunct to observational studies and allow an investigation of causal inference controlling for genetic and shared familial effects on exposure and outcome. We include a summary of this literature because results have been interpreted as somewhat inconsistent, making a review useful. We have reviewed this literature in detail and systematically elsewhere (Rice et al. 2018). We also select other prenatal and postnatal exposures for illustrative purposes—focusing on examples where there is evidence supportive of a potentially causal relationship between exposure and outcome—to highlight areas where further research on the mechanisms underlying associations is warranted. We will next examine four family-based designs in turn: maternal versus paternal exposure during pregnancy, discordant sibling designs, IVF designs, and adoption designs.
MATERNAL VERSUS PATERNAL EXPOSURE DURING PREGNANCY
This design has been used for investigating prenatal exposures and compares maternal and paternal exposures during pregnancy and their associations with offspring outcome. An intrauterine contribution is possible for the mother–offspring association but not for associations observed between father and offspring. Mothers and fathers both share exactly 50% of their genetic material with their offspring, meaning that the genetic contribution shared between parent and offspring that contributes to the association between the prenatal exposure and offspring outcome is held constant. In this design, fathers serve as a negative control (Davey Smith 2012) for genetic factors and also as a control for family-level environmental confounding (Gage et al. 2016). Extensions of the design have involved including additional negative control variables such as varying the timing of exposure to maternal smoking (e.g., before and after pregnancy vs. during pregnancy) where an intrauterine effect on offspring is only plausible for prenatal exposure (Thapar et al. 2009; Gustavson et al. 2017). A strong association between maternal but not paternal exposure and the offspring outcome is consistent with a causal inference. For example, maternal smoking in pregnancy is associated with lower birth weight while paternal smoking during this time period does not show this association (Langley et al. 2012).
A recent systematic review identified four published studies that compared maternal and paternal smoking during pregnancy and examined effects on child ADHD (Langley et al. 2012; Keyes et al. 2014; Kovess et al. 2015; Gustavson et al. 2017). Two studies reported findings inconsistent with a causal effect of maternal smoking during pregnancy on offspring ADHD (Langley et al. 2012; Gustavson et al. 2017), while two studies reported results at least partially consistent with a causal effect (Keyes et al. 2014; Kovess et al. 2015); but those findings are challenging to interpret as we describe below. In a study of over 100,000 mothers and children, Gustavson and colleagues examined the association between maternal smoking during pregnancy and offspring diagnosis of ADHD and included three negative controls (paternal smoking during pregnancy, maternal grandmother smoking during pregnancy, and maternal smoking during previous pregnancies). The association with maternal smoking during pregnancy (where an intrauterine effect is plausible) was similar in size to the association for the three negative control variables. Similarly, in a sample of 8324 children, Langley and colleagues found that the association between maternal and paternal smoking in pregnancy with offspring symptoms of ADHD was very similar. Both of these studies therefore suggest that the association between maternal smoking during pregnancy and offspring ADHD is driven by shared inherited or familial factors. In a study of 1752 mothers and children by Keyes et al. (2014), results were initially inconsistent with a causal effect when no adjustments for confounders were made but adjusting for confounders attenuated the association with paternal smoking during pregnancy, indicating that maternal smoking during pregnancy might be more important for offspring ADHD. Nonetheless, within the same study, there was an association similar in magnitude with offspring ADHD in mothers who smoked before but not during pregnancy, which is inconsistent with a causal interpretation. The results of the study of 4517 mothers and children by Kovess and colleagues (2015) again suggested no causal relationship when no adjustments for confounders were made but adjusting for confounders attenuated the association with paternal smoking during pregnancy. Results also differed according to who rated the child's ADHD symptoms (mother or teacher), making it difficult to interpret results. Interestingly, the two studies that reported results at least partially consistent with a prenatal effect relied on retrospective maternal reports of paternal smoking during pregnancy and the reliability of this is not currently known (Keyes et al. 2014; Kovess et al. 2015).
Limitations
The maternal and paternal comparison is a useful design and it is possible to recruit large samples fairly easily. Limitations include that parents are more similar than expected for many phenotypes and exposures due to genetic and social factors, which is known as assortative mating. Also, it is limited to exposures that are relevant to both parents, and the assumptions of the design can be violated if the confounding structure of the maternal and paternal exposures differs (Keyes et al. 2014). Similarly, differential measurement error of the exposure in the two groups can bias results (Sanderson et al. 2018).
DISCORDANT SIBLING DESIGN
Sibling pairs who are reared together but discordant for exposure to a hypothesized risk factor provide the opportunity to examine whether the exposed individual shows higher rates of the outcome trait or condition than their unexposed sibling. This design is essentially a matched case–control comparison that takes advantage of siblings being “matched” for stable shared environmental variables. This design can be used to examine hypothesized exposures before and after birth and is useful for investigating prenatal exposures because the intrauterine environment varies but the overall level of the mother–child genetic relationship is held constant (full siblings share 50% of their genes with their mother). Given that the full complement of alleles inherited from the mother will differ between discordantly exposed siblings, large samples will be required to limit the influence of chance fluctuations in individual allele transmission. Although monozygotic twins, unlike sibling designs, entirely control for genetic confounding as emphasized earlier, twin designs cannot be used to assess prenatal exposures because twins are not differentially exposed (i.e., they develop in the same prenatal environment), at least in a way that is currently accessible to measurement.
Prenatal Exposures
The premise is that by investigating sibling pairs who are discordant for exposure to the hypothesized prenatal exposure (e.g., mother smokes in one pregnancy but not the other), the contribution of familial confounding to the association between the prenatal exposure and offspring outcomes is assessed. This design has been used to assess many different prenatal exposures, including maternal medications such as antidepressant use in the first trimester of pregnancy (Sujan et al. 2017) and complications of pregnancy such as inadequate maternal weight gain during pregnancy (Mackay et al. 2017). It has also been used to examine the effect of maternal smoking during pregnancy on offspring ADHD and conduct disorder problems. Typically, the association between exposure and outcome is examined in the full sample and compared to the association derived from discordantly exposed siblings.
A recent systematic review (Rice et al. 2018) yielded six informative published studies of ADHD in discordant sibling pairs who were differentially exposed in intrauterine life to cigarette smoke. Many of these studies utilized data from very large population-based samples. All observed an association between prenatal smoking and offspring ADHD at the population level as expected, but association was markedly attenuated in discordant sibling pairs. For example, the largest Danish registry-based study included ∼900,000 individuals (Obel et al. 2016). Analyses were performed using Cox regression and adjusted for a number of measured confounders (e.g., year of birth, child sex, maternal age at birth, and parity). The authors analyzed the full study population and compared these results with the discordantly exposed matched sibling analyses. In the text, we report the results obtained when adjusting for confounders. Conventional association analysis in the complete dataset yielded results in line with previous studies; the authors observed association between maternal smoking in pregnancy and ADHD (adjusted HR [hazard ratio] = 2.01; 95% CI = 1.94–2.07). In the sibling analysis, the association was substantially attenuated (adjusted HR = 1.07; 95% CI = 0.94–1.22). However, that was not the case for association with offspring birth weight where the association remained robust (adjusted OR = 1.68; 95% CI = 1.33–2.12). A number of methodological challenges to interpreting effects from sibling comparison designs have been highlighted (Frisell et al. 2012; Petersen and Lange 2020), which are discussed in the section on limitations below. A particular issue is the selection of discordantly exposed siblings, which has the effect of selecting pairs that differ on nonshared causes of the exposure (Frisell et al. 2012).
Since that systematic review, two additional sibling comparison papers have been published (Ekblad et al. 2017; Marceau et al. 2018). The study by Ekblad precludes any firm conclusions about the relationship between maternal smoking in pregnancy and offspring psychiatric outcomes because it examined the effect of change in maternal smoking during pregnancy status on the second sibling only. Thus, it did not compare rates of disorder in matched differentially exposed sibling pairs. Instead, it examined rates of disorder in the second sibling in mothers that changed smoking status across pregnancies (i.e., “quitters” or “starters”) and compared this to the rates of disorder in the offspring of mothers that smoked consistently across two pregnancies or nonsmokers. One comparison of 173 siblings found results inconsistent with a causal effect for symptom-based measures of ADHD, although one questionnaire measure reported a within-family effect for hyperactivity/impulsivity (Marceau et al. 2018). Collectively, the discordant sibling studies suggest that there is substantial genetic and/or family-level environmental confounding that contributes to the association between maternal smoking in pregnancy and offspring ADHD. Similar findings have been reported for offspring antisocial behavior, with all bar one (a smaller subsample of an initially negative study) of the seven discordant sibling studies finding results inconsistent with a causal explanation (see Rice et al. 2018 for further details).
The discordant sibling design has also been used to examine the association of maternal gestational weight gain and risk for psychosis in offspring (Mackay et al. 2017). That study found that inadequate maternal weight gain during pregnancy (but not maternal underweight, overweight, or obesity) was associated with an increased risk for nonaffective psychosis in offspring (HR = 1.32; 95% CI = 1.13, 1.54), an association that did not attenuate in the matched sibling analysis (HR = 1.61; 95% CI = 1.02, 2.56). These findings are consistent with those from studies where mothers were exposed to starvation during pregnancy following unfortunate historical events (Susser et al. 1996; St Clair et al. 2005) and suggest a role of maternal undernutrition in the development of psychosis, although the exact mechanisms remain unclear.
Postnatal Exposures
The sibling design and its extension, the co-relative study has also been used to examine exposures after birth. For example, in a recent large study, an extended sibling pair design that utilized Swedish registry data on siblings, half siblings, and cousins was employed to examine the relationship between cannabis use and psychiatric disorders (Giordano et al. 2015). The association between cannabis use and schizophrenia was observed as expected in the whole population (OR = 10.44; 95% confidence interval = 8.99–12.1). The association attenuated but did not disappear (OR in full siblings = 5.07; 95% CI = 4.17–6.16). The authors concluded that the findings suggest that observational designs overestimate the association between cannabis use and future schizophrenia because of familial confounding but do not remove the possibility that there is a causal link.
Limitations
Strengths of the discordant sibling design include the fact that siblings are “matched by nature” on many potential confounders and the existence of large population-based registries where family relationships can be linked have facilitated analyses in very large, representative samples. Nevertheless, as with all research designs, several limitations have been documented. First, there is the issue of selection for discordantly exposed siblings where, for prenatal exposures, it is necessary to select mothers that behave differently (or are affected differently) in different pregnancies. Therefore, discordant sibling designs are susceptible to confounding by nonshared factors that might lead to changes in the mother's behavior or health during pregnancy (Frisell et al. 2012). The selection of discordantly exposed sibling pairs thus implies that the sample includes pairs of individuals that are more likely to differ from each other on nonshared confounding than those similarly exposed but randomly selected from the general population (Frisell et al. 2012). Also, if the degree of measurement error differs for exposure and outcome, that can also lead to bias in results, and random measurement error will tend to increase misclassification in discordant sibling pairs (Frisell et al. 2012; Sanderson et al. 2018). These factors are likely to have the effect of biasing within family estimates toward the null (Frisell et al. 2012). Different siblings will be born at different time periods and thus exposed to different family- and population-level exposures including parental age. Sibling comparisons assume a stable family and social context. It is also assumed that one sibling's exposure does not influence the unexposed sibling, which seems unlikely for prenatal exposures (e.g., D'Onofrio et al. 2010; Skoglund et al. 2014) but plausible for later exposures. For instance, this phenomenon where the exposure and outcome of one child affects the exposure and outcome of his or her siblings (known as “carryover effects”) might plausibly exist for exposure to Caesarean section where a Caesarean section in one pregnancy might affect the likelihood in a subsequent pregnancy. Ultimately, this design on its own still cannot rule out that differences between siblings arise from some other exposure or experience and for exposures after birth, as genetic differences between siblings will also be a contributor. Finally, it is probably not possible to generalize results to the general population and estimates apply only to discordantly exposed siblings (Petersen and Lange 2020). Frisell and colleagues (2012) have suggested that sibling comparison designs may be most appropriate in situations where confounding factors are more likely to be shared between siblings than the exposure and avoided in situations where the opposite scenario is likely (i.e., greater sharing for the exposure than confounders).
IVF DESIGN
The IVF design is a prenatal cross-fostering design that separates the prenatal environment from genetic factors shared between mother and child (Table 3). It does this by comparing associations between a prenatal exposure and a child outcome in pairs of mothers and children who are related and unrelated. In the design, all children are conceived using IVF, with some mothers experiencing a pregnancy for a child to whom they are not genetically related (conceiving either by IVF with egg/embryo donation or gestational surrogacy). In the unrelated mother–child pairs where an unrelated mother or surrogate experiences the pregnancy, an association between a prenatal exposure and a child outcome must come about through intrauterine (rather than inherited) effects because while the mother/surrogate experiences the pregnancy, she does not pass on her genetic material to the baby. The IVF design therefore allows separation of genetic factors that mothers share with their offspring from the intrauterine/prenatal environment assuming that the oocyte/embryo donation or surrogacy is provided by an unrelated individual.
Table 3.
Maternal and paternal factors contributing to mother/father and offspring similarity in the IVF design
| Conception group | Mother contributions to offspring | Father contributions to offspring | Intrauterine environment provided by biological mother? | Postnatal rearing provided by biological mother? |
|---|---|---|---|---|
| Homologous IVF | Gm + I + E | Gf + E | Yes | Yes |
| IVF with sperm donation | Gm + I + E | E | Yes | Yes |
| IVF with oocyte donation | I + E | Gf + E | No | No |
| IVF with embryo donation | I + E | E | No | No |
| Gestational surrogacy | Gm + E | Gf + E | No | Yes |
With gestational surrogacy, the commissioning parents provide gametes and the rearing and an unrelated surrogate experiences the pregnancy.
(IVF) in vitro fertilization, (Gm) maternally provided genes, (Gf) paternally provided genes, (I) intrauterine environment, (E) postnatal and later-life-rearing environment.
Prenatal Exposures
The IVF design has been used to examine the association between maternal smoking during pregnancy with a range of offspring outcomes including birth weight, conduct problems, and symptoms of ADHD (Rice et al. 2009; Thapar et al. 2009). Those studies suggest that for birth weight, findings seem consistent with a causal relationship of maternal smoking during pregnancy; while for offspring conduct and ADHD, findings are inconsistent with a causal interpretation and appear as a result of genes shared been mother and child. Thus, infant birth weight is reduced to a similar extent when mothers smoke during pregnancy for related (β = −0.14, P < 0.01) and unrelated mother–child pairs (β = −0.11, P < 0.01), which is consistent with a prenatal environmental effect (Thapar et al. 2009). When looking at offspring ADHD, the association with maternal smoking during pregnancy was positive and greater in the related (β = 0.102, P < 0.02) than the unrelated (β = −0.052, P > 0.1) pairs (test for interaction between maternal smoking status and relatedness group β = −0.10, P < 0.05), suggesting that the association was due to shared genetic influences. Sensitivity checks assessing the role of related and unrelated paternal smoking during pregnancy also revealed a pattern of association consistent with a shared genetic influence on paternal smoking and offspring ADHD (similar to that observed for maternal smoking and offspring ADHD) (Thapar et al. 2009). For offspring conduct problems, a similar pattern of results was reported such that there was a positive association with maternal smoking during pregnancy and offspring antisocial behavior in related mother–child pairs (Cohen's d = 0.527) but not in the group of mothers who experienced the pregnancy but were genetically unrelated to their child (Cohen's d = −0.210). The magnitude of association was greater in the related mother–child pairs than the unrelated mother–child pairs (test for interaction between maternal smoking status and relatedness group F = 4.106, P = .04).
One study used an IVF design to assess the role of perceived maternal stress during late pregnancy on continuous measures of (maternally rated) childhood anxiety, conduct problems, and ADHD (Rice et al. 2010). Results differed for each childhood outcome examined. For birth weight and gestational age, results were consistent with a prenatal environmental effect with lower birth weight and gestational age for mothers with greater perceived stress during pregnancy irrespective of whether the mother experiencing the pregnancy shared genes with her baby. For offspring with ADHD, results consistent with a shared genetic link as an association was only observed in related mother–child pairs. For conduct problems (antisocial behavior), results were consistent with a prenatal environmental effect because similarly sized associations were observed in related and unrelated mother–child pairs. For child anxiety, while associations with maternal stress were observed in related and unrelated mother–child pairs, the pattern of findings suggested that postnatal maternal stress primarily explained the association with childhood anxiety (i.e., continuing maternal stress appeared to be important). A need to consider the continuation of maternal stress has been highlighted in a number of reviews and editorials on the fetal origins of mental health (Thapar and Rutter 2009; O'Donnell and Meaney 2017).
Postnatal Exposures
It is also possible to use the IVF design to study intergenerational transmission in a similar vein to a classic adoption study. The IVF design has been used to assess the intergenerational transmission of symptoms of anxiety, depression, and antisocial behavior in families (Harold et al. 2011; Lewis et al. 2011) and the role of gene–environment correlation with stressful life events and family processes (Harold et al. 2011; Rice et al. 2013). For example, Lewis and colleagues (2011) reported that the familial transmission of depression and anxiety symptoms was partly due to environmental processes independent of inherited effects. This finding is similar to many studies that have used the classic adoption-after-birth design to examine the transmission of depression in families (e.g., Tully et al. 2008).
The IVF design has also been used to test whether associations with prenatal risks such as maternal smoking are moderated by the postnatal later-life environment. For example, Gaysina and colleagues (2013) examined the relationship between maternally reported number of cigarettes smoked during pregnancy and offspring conduct problems. These authors also included an adoption-at-birth sample and observational cohort data. Consistent with what had been published previously in the same sample (Rice et al. 2009), in the analysis of the IVF unrelated mother–child pairs, no association between maternal smoking and offspring conduct problems was found and the correlation coefficient was zero for this group (r = 0.00, P = 0.98). Gaysina and colleagues additionally examined whether parenting practices (after birth) might influence the pattern of results observed—they did not. Thus, those results did not support the hypothesis that there is a causal effect of maternal smoking during pregnancy on offspring conduct problems. Nevertheless, it is worth noting that the findings reported by Gaysina et al. (2013) have been interpreted by others as being consistent with a causal effect (Slotkin 2013; Dolan et al. 2016) despite not reporting results consistent with such an interpretation, which has been highlighted elsewhere (Thapar and Rutter 2015; Rice et al. 2018). It seems likely this is due to confusion in assumptions that data from adoption-after-birth studies enable causal inferences for prenatal exposures—they do not (see below). This highlights the need for systematic review and clear reporting.
Limitations
The major strength of the IVF design is its ability to unambiguously separate the prenatal environment from maternal genes shared with the child by the inclusion of conception groups where a woman experiences the pregnancy for a child to whom she does not pass on her genetic material. This makes the IVF design a powerful approach to disentangling prenatal and maternally provided genetic effects. The main limitation concerns the extent to which findings are generalizable insofar as whether individuals conceiving via IVF are similar to those that conceive naturally. In terms of parental and child psychopathology and the family environment, the evidence shows strong similarities (Golombok and MacCallum 2003; Shelton et al. 2009; Golombok 2017). However, rates of perinatal complications are elevated in those conceiving via IVF and perhaps unsurprisingly the rates of exposure for some prenatal risks such as maternal smoking during pregnancy are low. A further limitation is that sample sizes are low in the informative groups (i.e., in unrelated mother–child pairs).
ADOPTION DESIGNS
Adoption designs involve comparing the similarity between children adopted after birth with their biological and rearing parents. Adoption-after-birth studies can be used to examine the role of the postnatal rearing environment on children's development (separately from genes shared between child and the parent who brings the child up). Where information on birth parents is available, this design is also valuable for examining the influence of evocative inherited child effects on the rearing environment (Ge et al. 1996; Rutter et al. 2001). As described above, adoption-after-birth studies are not informative for separating the effects of the prenatal environment from those shared between mother and child. This is because in the classic adoption design where the genetic mother experiences the pregnancy but the child is adopted after birth, there is no separation of the prenatal environment from the (biological) mother provided genetic effects as the biological mother provides genes and the prenatal environment to her offspring, but not the postnatal rearing (Table 1). Thus, the comparison between prenatal exposure and offspring outcome in the genetic mother whose child is then adopted is essentially the same as it would be in a standard observational design. As far as prenatal exposures are concerned, classic adoption designs can be used to examine whether the postnatal rearing environment has any moderating effect on the relationship between a prenatal exposure and an offspring outcome (Rice et al. 2007; Gaysina et al. 2013). It is important to note that mothers whose children are adopted away are different from mothers whose children are not, and this is likely to create differences in the prenatal environments of children who are adopted compared to children who are not. For instance, mothers whose children are adopted after birth typically show higher rates of smoking, alcohol, and drug use during pregnancy and higher rates of psychopathology than mothers whose children live with them after birth (Gaysina et al. 2013). As we have argued elsewhere, this may create a situation where the degree of familial confounding for prenatal risk exposures may be higher in adoption-after-birth studies than in population-based samples (Rice et al. 2018).
CONCLUSION
We have described several family-based genetically informed designs that have increasingly been used to strengthen causal inference and emphasized that the ones that are useful for assessing prenatal and after-birth exposures are not the same. Many studies that have used these informative designs have also tested associations using conventional approaches in the whole population. They have shown that including measured confounders in statistical analyses does not adequately capture genetic and shared environmental confounding, which further highlights the value of genetically informative approaches. Causality cannot be firmly concluded from findings from any of these designs. However, each of the genetically informed designs that we have considered (as well as those in the literature) has a different pattern of assumptions, strengths, and limitations. Thus, as mentioned earlier, consistent findings across different designs strengthens causal inference. For example, as we describe in a recent review (Rice et al. 2018), the vast majority of the published genetically informed studies of maternal smoking in pregnancy and offspring ADHD and conduct problems are consistent with associations being driven by shared familial and genetic confounding. The findings, when taken together, do not support the premise that smoking in pregnancy has a strong causal effect on offspring ADHD or conduct problems and contrast with findings for offspring birth weight. Instead, there is a need for independent replication and studies to elucidate potential mechanisms for different exposures where evidence suggests potentially causal effects on offspring health and behavior.
Until relatively recently, many of the designs discussed in this paper have been used to infer genetic contributions to medical disorders and traits. In the advent of large-scale molecular genetic studies, such designs may seem obsolete. However, these designs together with additional methods described in other papers, provide an invaluable approach for assessing causality and identifying environmental exposures that are appropriate targets for intervention.
Footnotes
Editors: George Davey Smith, Rebecca Richmond, and Jean-Baptiste Pingault
Additional Perspectives on Combining Human Genetics and Causal Inference to Understand Human Disease and Development available at www.perspectivesinmedicine.org
REFERENCES
*Reference is also in this collection.
- Academy of Medical Sciences. 2007. Identifying the environmental causes of disease: how should we decide what to believe and when to take action? https://acmedsci.ac.uk/file-download/34654-119615475058.pdf
- Davey Smith G. 2012. Negative control exposures in epidemiologic studies. Epidemiology 23: 350–351. 10.1097/EDE.0b013e318245912c [DOI] [PubMed] [Google Scholar]
- Davies NM, Howe LJ, Brumpton B, Havdahl A, Evans DM, Davey Smith G. 2019. Within family Mendelian randomization studies. Hum Mol Genet 28: R170–R179. 10.1093/hmg/ddz204 [DOI] [PubMed] [Google Scholar]
- Dolan CV, Geels L, Vink JM, van Beijsterveldt CE, Neale MC, Bartels M, Boomsma DI. 2016. Testing causal effects of maternal smoking during pregnancy on offspring's externalizing and internalizing behavior. Behav Genet 46: 378–388. 10.1007/s10519-015-9738-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Onofrio BM, Singh AL, Iliadou A, Lambe M, Hultman CM, Grann M, Neiderhiser JM, Långström N, Lichtenstein P. 2010. Familial confounding of the association between maternal smoking during pregnancy and offspring criminality: a population-based study in Sweden. Arch Gen Psychiatry 67: 529–538. 10.1001/archgenpsychiatry.2010.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Onofrio BM, Class QA, Lahey BB, Larsson H. 2014. Testing the developmental origins of health and disease hypothesis for psychopathology using family-based quasi-experimental designs. Child Dev Perspect 8: 151–157. 10.1111/cdep.12078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblad M, Lehtonen L, Korkeila J, Gissler M. 2017. Maternal smoking during pregnancy and the risk of psychiatric morbidity in singleton sibling pairs. Nicotine Tob Res 19: 597–604. 10.1093/ntr/ntx001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisell T, Öberg S, Kuja-Halkola R, Sjölander A. 2012. Sibling comparison designs bias from non-shared confounders and measurement error. Epidemiology 23: 713–720. 10.1097/EDE.0b013e31825fa230 [DOI] [PubMed] [Google Scholar]
- Gage SH, Munafò MR, Davey Smith G. 2016. Causal inference in developmental origins of health and disease (DOHaD) research. Annu Rev Psychol 67: 567–585. 10.1146/annurev-psych-122414-033352 [DOI] [PubMed] [Google Scholar]
- Gaysina D, Fergusson DM, Leve LD, Horwood J, Reiss D, Shaw DS, Elam KK, Natsuaki MN, Neiderhiser JM, Harold GT. 2013. Maternal smoking during pregnancy and offspring conduct problems: evidence from 3 independent genetically sensitive research designs. JAMA Psychiatry 70: 956–963. 10.1001/jamapsychiatry.2013.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Cadoret RJ, Conger RD, Neiderhiser JM, Yates W, Troughton E, Stewart MA. 1996. The developmental interface between nature and nurture: a mutual influence model of child antisocial behaviour and parent behaviors. Dev Psychol 32: 574–589. 10.1037/0012-1649.32.4.574 [DOI] [Google Scholar]
- Gilman SE, Breslau J, Subramanian SV, Hitsman B, Koenen KC. 2008a. Social factors, psychopathology, and maternal smoking during pregnancy. Am J Public Health 98: 448–453. 10.2105/AJPH.2006.102772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman SE, Gardener H, Buka SL. 2008b. Maternal smoking during pregnancy and children's cognitive and physical development: a causal risk factor? Am J Epidemiol 168: 522–531. 10.1093/aje/kwn175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano GN, Ohlsson H, Sundquist K, Sundquist J, Kendler KS. 2015. The association between cannabis abuse and subsequent schizophrenia: a Swedish national co-relative control study. Psychol Med 45: 407–414. 10.1017/S0033291714001524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golombok S. 2017. Parenting in new family forms. Curr Opin Psychol 15: 76–80. 10.1016/j.copsyc.2017.02.004 [DOI] [PubMed] [Google Scholar]
- Golombok S, MacCallum F. 2003. Practitioner review: outcomes for parents and children following non-traditional conception: what do clinicians need to know? J Child Psychol Psychiatry 44: 303–315. 10.1111/1469-7610.00123 [DOI] [PubMed] [Google Scholar]
- Gustavson K, Ystrom E, Stoltenberg C, Susser E, Surén P, Magnus P, Knudsen GP, Smith GD, Langley K, Rutter M, et al. 2017. Smoking in pregnancy and child ADHD. Pediatrics 139: e20162509. 10.1542/peds.2016-2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold GT, Rice F, Hay DF, Boivin J, van den Bree M, Thapar A. 2011. Familial transmission of depression and antisocial behavior symptoms: disentangling the contribution of inherited and environmental factors and testing the mediating role of parenting. Psychol Med 41: 1175–1185. 10.1017/S0033291710001753 [DOI] [PubMed] [Google Scholar]
- Huang L, Wang Y, Zhang L, Zheng Z, Zhu T, Qu Y, Mu D. 2018. Maternal smoking and attention-deficit/hyperactivity disorder in offspring: a meta-analysis. Pediatrics 141: e20172465. 10.1542/peds.2017-2465 [DOI] [PubMed] [Google Scholar]
- *.Hwang LD, Davies NM, Warrington NM, Evans DM. 2020. Integrating family-based and Mendelian randomization designs. Cold Spring Harb Perspect Med 10.1101/cshperspect.a039503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffee SR, Price T. 2008. Genotype–environment correlations: implications for determining the relationship between environmental exposures and psychiatric illness. Psychiatry 7: 496–499. 10.1016/j.mppsy.2008.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaaja RJ, Greer IA. 2005. Manifestations of chronic disease during pregnancy. JAMA 294: 2751–2757. 10.1001/jama.294.21.2751 [DOI] [PubMed] [Google Scholar]
- Kendler KS, Baker JH. 2007. Genetic influences on measures of the environment: a systematic review. Psych Med 37: 615–626. 10.1017/S0033291706009524 [DOI] [PubMed] [Google Scholar]
- Kendler KS, Turkheimer E, Ohlsson H, Sundquist J, Sundquist K. 2015. Family environment and the malleability of cognitive ability: a Swedish national home-reared and adopted-away cosibling control study. Proc Natl Acad Sci 112: 4612–4617. 10.1073/pnas.1417106112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Smith GD, Susser E. 2013. On sibling designs. Epidemiology 24: 473–474. 10.1097/EDE.0b013e31828c7381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes KM, Davey Smith G, Susser E. 2014. Associations of prenatal maternal smoking with offspring hyperactivity: causal or confounded? Psychol Med 44: 857–867. 10.1017/S0033291713000986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong A, Thorleifsson G, Frigge ML, Vilhjalmsson BJ, Young AI, Torgeirsson TE, Benonisdottir S, Oddsson A, Halldorsson BV, Masson G, et al. 2018. The nature of nurture: effects of parental genotypes. Science 359: 424–428. 10.1126/science.aan6877 [DOI] [PubMed] [Google Scholar]
- Kovess V, Keyes KM, Hamilton A, Pez O, Bitfoi A, Koç C, Goelitz D, Kuijpers R, Lesinskiene S, Mihova Z, et al. 2015. Maternal smoking and offspring inattention and hyperactivity: results from a cross-national European survey. Eur Child Adolesc Psychiatry 24: 919–929. 10.1007/s00787-014-0641-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley K, Heron J, Smith GD, Thapar A. 2012. Maternal and paternal smoking during pregnancy and risk of ADHD symptoms in offspring: testing for intrauterine effects. Am J Epidemiol 176: 261–268. 10.1093/aje/kwr510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DA, Tilling K, Davey Smith G. 2016. Triangulation in aetiological epidemiology. Int J Epidemiol 45: 1866–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis G, Rice F, Harold GT, Collishaw S, Thapar A. 2011. Investigating environmental links between parent depression and child depressive/anxiety symptoms using an assisted conception design. J Am Acad Child Adolesc Psychiatry 50: 451–459.e1. 10.1016/j.jaac.2011.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay E, Dalman C, Karlsson H, Gardner RM. 2017. Association of gestational weight gain and maternal body mass index in early pregnancy with risk for nonaffective psychosis in offspring. JAMA Psychiatry 74: 339–349. 10.1001/jamapsychiatry.2016.4257 [DOI] [PubMed] [Google Scholar]
- Marceau K, Bidwell LC, Karoly HC, Schettini Evans A, Todorov AA, Palmer RH, Health AC, Knopik VS. 2018. Within-family effects of smoking during pregnancy on ADHD: the importance of phenotype. J Abnorm Child Psychol 46: 685–699. 10.1007/s10802-017-0320-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan B, Taylor A, Caspi A, Moffitt TE. 2004. Prenatal smoking and early childhood conduct problems: testing genetic and environmental explanations of the association. Arch Gen Psychiatry 61: 836–843. [DOI] [PubMed] [Google Scholar]
- *.McAdams T. 2020. Twins and children of twins. Cold Spring Harb Perspect Med 10.1101/cshperspect.a039552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obel C, Zhu JL, Olsen J, Breining S, Li J, Grønborg TK, Gissler M, Rutter M. 2016. The risk of attention deficit hyperactivity disorder in children exposed to maternal smoking during pregnancy—a re-examination using a sibling design. J Child Psychol Psychiatry 57: 532–537. 10.1111/jcpp.12478 [DOI] [PubMed] [Google Scholar]
- O'Donnell KJ, Meaney MJ. 2017. Fetal origins of mental health: the developmental origins of health and disease hypothesis. Am J Psychiatry 174: 319–328. 10.1176/appi.ajp.2016.16020138 [DOI] [PubMed] [Google Scholar]
- Orton S, Bowker K, Cooper S, Naughton F, Ussher M, Pickett KE, Leonardi-Bee J, Sutton S, Dhalwani NN, Coleman T. 2014. Longitudinal cohort survey of women's smoking behaviour and attitudes in pregnancy: study methods and baseline data. BMJ Open 4: e004915. 10.1136/bmjopen-2014-004915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AH, Lange T. 2020. What is the causal interpretation of sibling comparison designs? Epidemiology 31: 75–81. 10.1097/EDE.0000000000001108 [DOI] [PubMed] [Google Scholar]
- Pingault JB, O'Reilly PF, Schoeler T, Ploubidis GB, Rijsdijk F, Dudbridge F. 2018. Using genetic data to strengthen causal inference in observational research. Nat Rev Genet 19: 566–580. 10.1038/s41576-018-0020-3 [DOI] [PubMed] [Google Scholar]
- Plomin R. 2018. Blueprint: how DNA makes us who we are. Allen Lane, London. [Google Scholar]
- Rice F, Jones I, Thapar A. 2007. The impact of gestational stress and prenatal growth on emotional problems in offspring: a review. Acta Psychiatr Scand 115: 171–183. 10.1111/j.1600-0447.2006.00895.x [DOI] [PubMed] [Google Scholar]
- Rice F, Harold GT, Boivin J, Hay DF, van den Bree M, Thapar A. 2009. Disentangling prenatal and inherited influences in humans with an experimental design. Proc Natl Acad Sci 106: 2464–2467. 10.1073/pnas.0808798106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice F, Harold GT, Boivin J, van den Bree M, Hay DF, Thapar A. 2010. The links between prenatal stress and offspring development and psychopathology: disentangling environmental and inherited influences. Psychol Med 40: 335–345. 10.1017/S0033291709005911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice F, Lewis G, Harold G, Thapar A. 2013. Examining the role of passive gene–environment correlation in childhood depression using a novel genetically sensitive design. Dev Psychopathol 25: 37–50. 10.1017/S0954579412000880 [DOI] [PubMed] [Google Scholar]
- Rice F, Langley K, Woodford C, Davey Smith G, Thapar A. 2018. Identifying the contribution of prenatal risk factors to offspring development and psychopathology: what designs to use and a critique of literature on maternal smoking and stress in pregnancy. Dev Psychopathol 30: 1107–1128. 10.1017/S0954579418000421 [DOI] [PubMed] [Google Scholar]
- Ruisch IH, Dietrich A, Glennon JC, Buitelaar JK, Hoekstra PJ. 2018. Maternal substance use during pregnancy and offspring conduct problems: a meta-analysis. Neurosci Biobehav Rev 84: 325–336. 10.1016/j.neubiorev.2017.08.014 [DOI] [PubMed] [Google Scholar]
- Rutter M, Pickles A, Murray R, Eaves L. 2001. Testing hypotheses on specific environmental causal effects on behavior. Psychol Bull 127: 291–324. 10.1037/0033-2909.127.3.291 [DOI] [PubMed] [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. 2006. Gene–environment interplay and psychopathology: multiple varieties but real effects. J Child Psychol Psychiatry 47: 226–261. 10.1111/j.1469-7610.2005.01557.x [DOI] [PubMed] [Google Scholar]
- Sanderson E, Macdonald-Wallis C, Davey Smith G. 2018. Negative control exposure studies in the presence of measurement error: implications for attempted effect estimate calibration. Int J Epidemiol 47: 587–596. 10.1093/ije/dyx213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton KH, Boivin J, Hay DF, van den Bree MMB, Rice F, Harold GT, Thapar A. 2009. Examining differences in psychological adjustment problems among children conceived by assisted reproductive technologies. Int J Behav Dev 33: 385–392. 10.1177/0165025409338444 [DOI] [Google Scholar]
- Skoglund C, Chen Q, D'Onofrio BM, Lichtenstein P, Larsson H. 2014. Familial confounding of the association between maternal smoking during pregnancy and ADHD in offspring. J Child Psychol Psychiatry 55: 61–68. 10.1111/jcpp.12124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA. 2013. Maternal smoking and conduct disorder in the offspring. JAMA Psych 70: 901–902. 10.1001/jamapsychiatry.2013.1951 [DOI] [PubMed] [Google Scholar]
- Sourander A, Sucksdorff M, Chudal R, Surcel HM, Hinkka-Yli-Salomäki S, Gyllenberg D, Cheslack-Postava K, Brown AS. 2019. Prenatal cotinine levels and ADHD among offspring. Pediatrics 143: e20183144. 10.1542/peds.2018-3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Clair D, Xu M, Wang P, Yu Y, Fang Y, Zhang F, Zheng X, Gu N, Feng G, Sham P, et al. 2005. Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959–1961. JAMA 294: 557–562. 10.1001/jama.294.5.557 [DOI] [PubMed] [Google Scholar]
- Sujan AC, Rickert ME, Öberg AS, Quinn PD, Hernández-Díaz S, Almqvist C, Lichtenstein P, Larsson H, D'Onofrio BM. 2017. Associations of maternal antidepressant use during the first trimester of pregnancy with preterm birth, small for gestational age, autism spectrum disorder, and attention-deficit/hyperactivity disorder in offspring. JAMA 317: 1553–1562. 10.1001/jama.2017.3413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susser E, Neugebauer R, Hoek HW, Brown AS, Lin S, Labovitz D, Gorman JM. 1996. Schizophrenia after prenatal famine. Further evidence. Arch Gen Psychiatry 53: 25–31. 10.1001/archpsyc.1996.01830010027005 [DOI] [PubMed] [Google Scholar]
- Thapar A, Rutter M. 2009. Do prenatal risk factors cause psychiatric disorder? Be wary of causal claims. Br J Psychiatry 195: 100–101. 10.1192/bjp.bp.109.062828 [DOI] [PubMed] [Google Scholar]
- Thapar A, Rutter M. 2015. Using natural experiments and animal models to study causal hypotheses in relation to child mental health problems. In Rutter's child and adolescent psychiatry, 6th ed. (ed. Pine DS, et al. ), pp. 145–162. Wiley, Somerset, NJ. [Google Scholar]
- Thapar A, Rutter M. 2019. Do natural experiments have an important future in the study of mental disorders? Psychol Med 49: 1079–1088. 10.1017/S0033291718003896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A, Harold G, Rice F, Ge X, Boivin J, Hay D, van den Bree M, Lewis A. 2007. Do intrauterine or genetic influences explain the foetal origins of chronic disease? A novel experimental method for disentangling effects. BMC Med Res Methodol 7: 25. 10.1186/1471-2288-7-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A, Rice F, Hay D, Boivin J, Langley K, van den Bree M, Rutter M, Harold G. 2009. Prenatal smoking might not cause attention-deficit/hyperactivity disorder: evidence from a novel design. Biol Psychiatry 66: 722–727. 10.1016/j.biopsych.2009.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully EC, Iacono WG, McGue M. 2008. An adoption study of parental depression as an environmental liability for adolescent depression and childhood disruptive disorders. Am J Psychiatry 165: 1148–1154. 10.1176/appi.ajp.2008.07091438 [DOI] [PMC free article] [PubMed] [Google Scholar]