Abstract
Non-Hodgkin lymphomas (NHLs) are a diverse group of entities, both clinically and molecularly. Here, we review the evolution of classification schemes in B-cell lymphoma, noting the now standard WHO classification system that is based on immune cell-of-origin and molecular phenotypes. We review how lymphomas arise throughout the B-cell development process as well as the molecular and clinical features of prominent B-cell lymphomas. We provide an overview of the major progress that has occurred over the past decade in terms of our molecular understanding of these diseases. We discuss treatment options available and focus on a number of the diverse research tools that have been employed to improve our understanding of these diseases. We discuss the problem of heterogeneity in lymphomas and anticipate that the near future will bring significant advances that provide a measurable impact on NHL outcomes.
Non-Hodgkin lymphomas (NHLs) are cancers that arise predominantly from mature B lymphocytes, white blood cells responsible for humoral immunity. These malignancies are common, affecting nearly 75,000 new patients each year in the United States alone. Lymphomas represent a striking degree of diversity in molecular origins and clinical behavior. Efforts to classify lymphomas were initially developed based on the recognition of the varying clinical aggressiveness of the disease. This early classification (“Working Formulation” [Robb-Smith 1982]) has since been supplanted by more biological approaches that have culminated in the now standard, but still evolving, World Health Organization (WHO) classification that is guided by immune cell lineage (Swerdlow et al. 2017).
B-cell development sets the stage for the acquisition of a number of different genetic alterations that result in a tumor with a stage-specific immunophenotype, including cell surface markers and a gene expression profile that reflects the normal cell of origin. This resemblance to the normal cell of origin in lymphoid development serves as the foundation for the WHO classification of lymphomas.
Here, we describe our current understanding of the pathogenesis, underlying biology, and clinical treatment of NHLs.
LYMPHOCYTE DEVELOPMENT AND LYMPHOMAGENESIS
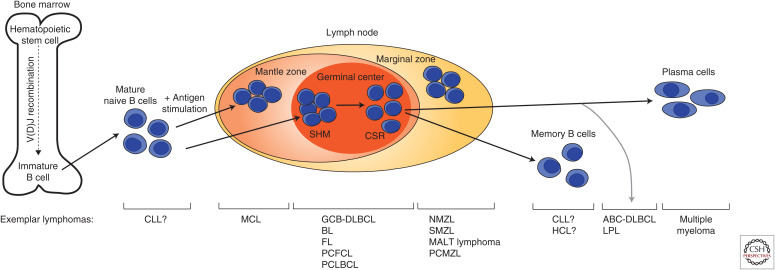
Although lymphomas can arise from either B or T cells, the vast majority of lymphomas arise from B cells. B cells undergo a complex and well-regulated program of development beginning in the bone marrow before terminally differentiating into mature effector cells following antigen stimulation (Fig. 1). In the bone marrow, the earliest progenitor B cells begin to undergo rearrangement of the immunoglobulin gene locus, giving rise to a unique antigen-binding B-cell receptor (BCR) comprised of heavy and light peptide chains that both contain variable and constant regions. The variable region of the heavy chain locus (IGH) is composed of variable (V), diversity (D), and joining (J) segments, whereas the variable regions of the light chain loci (IGK and IGL) contain only V and J segments. During B-cell differentiation, a random assortment of V, D, and J segments is chosen in each pre-B cell, which leads a BCR repertoire size on the order of 1010. Certain lymphomas show preferential selection of particular V(D)J segments. For instance, in particular, mucosa-associated lymphoid tissue (MALT) lymphomas frequently manifest identical V segments, whereas certain V(D)J recombinations are preferentially selected in B-cell chronic lymphocytic leukemias (B-CLLs) (Fais et al. 1998; Widhopf and Kipps 2001; Zucca and Bertoni 2004; Ghiotto et al. 2006). These data point to the role of particular antigens, including autoantibodies, in the development of these lymphomas.
Figure 1.
B-cell development. Progenitor B cells undergo V(D)J recombination in the bone marrow before exiting at the immature B-cell stage. Upon antigen stimulation, mature B cells undergo somatic hypermutation (SHM) and class-switch recombination (CSR) in the germinal center before undergoing terminal differentiation into plasma or memory B cells. Examples of lymphomas that can arise at each B-cell stage are noted. HSC, hematopoietic stem cell; CLL, chronic lymphocytic leukemia; MCL, mantle cell lymphoma; GCB-DLBCL, germinal center B-cell diffuse large B-cell lymphoma; BL, Burkitt lymphoma; FL, follicular lymphoma; PCFCL, primary cutaneous follicle center lymphoma; PCLBCL, primary cutaneous large B-cell lymphoma; NMZL, nodal marginal zone lymphoma; SMZL, splenic marginal zone lymphoma; MALT, mucosa-associated lymphoid tissue; PCMZL, primary cutaneous marginal zone lymphoma; HCL, hairy cell leukemia; ABC-DLBCL, activated B-cell like DLBCL; LPL, lymphoplasmacytic lymphoma.
Following initial recognition of a foreign antigen by the BCR, B-cell immunoglobulin genes undergo somatic hypermutation (SHM) in lymphoid tissue germinal centers (GCs) (Di Noia and Neuberger 2007). The process of SHM refines the affinity of the antibodies produced by B cells and further increases the antibody repertoire. The diversity of antibodies created during SHM is caused by direct mutagenesis of nucleotides in the variable region by the enzyme AID, encoded by AICDA (activation-induced cytidine deaminase) (Maul and Gearhart 2010). The SHM status of malignant cells in a tumor can be an additional indicator of the stage of B-cell maturation of the lymphoma cell of origin.
In parallel, at different stages of development, B-lineage cells express one of nine constant region gene segments corresponding to different immunoglobulin heavy chain isotypes with various effector functions. Only IgM is expressed in immature B cells. As B cells mature, they gain the ability to produce either IgM or IgD through alternative splicing mechanisms directed by AID. Upon activation, B cells in the GC undergo class switch recombination (CSR), which brings additional IG variable regions into proximity with one of the constant region gene segments to produce IgG, IgA, or IgE (Li et al. 2004).
The rearrangements associated with BCR formation in the GC can go awry and result in powerful oncogenic signals that transform B cells. Indeed, a majority of B-cell lymphomas arise from GC B cells. For instance, Burkitt lymphoma is associated with a translocation of the MYC gene to one of the IG loci, dysregulating the MYC gene more than 100-fold, a scale that makes Burkitt lymphoma one of the fastest growing tumors (Molyneux et al. 2012). Likewise, BCL2 is commonly deregulated in GC B-cell-derived lymphomas including follicular lymphoma and diffuse large B-cell lymphomas (Takata et al. 2014; Reddy et al. 2017; Chapuy et al. 2018; Schmitz et al. 2018).
CLASSIFICATION OF B-CELL LYMPHOMAS
Lymphomas can be broadly divided into four categories: B-cell lymphomas, T-cell lymphomas, cutaneous lymphomas, and lymphomas related to transplant and immunodeficiency. This section describes these categories and the most common lymphoma types in each category (summarized in Table 1).
Table 1.
The genetic features of B-cell lymphoma
| Type | Recurrent translocations (if known) | Recurrently mutated genes (if known) |
|---|---|---|
| Pre-germinal center origin | ||
| B-cell prolymphocytic leukemia (B-PLL) | t(8;14)(MYC/IGH), t(2;8)(IGK/MYC), t(8;22)(MYC/IGL) |
MYC, TP53 |
| Mantle cell lymphoma | t(11;14)(CCND1/IGH), t(2;11)(IGK/CCND1), t(11;22)(CCND1/IGL), t(8;14)(MYC/IGH), t(3;14)(BCL6/IGH) |
ATM, CCND1, KMT2D, NOTCH1/2, TP53 |
| Germinal center origin | ||
| Burkitt lymphoma | t(8;14)(MYC/IGH), t(2;8)(IGK/MYC), t(8;22)(MYC/IGL) |
MYC, ID3, DDX3X, TP53, TCF3, CCND3, RHOA, SMARCA4, ARID1A |
| Diffuse large B-cell lymphoma (DLBCL), NOS | BCL2, BCL6, and MYC rearrangements | EZH2, SGK1, GNA13, MYD88, ETV6, CD78B |
| Follicular lymphoma | t(14;18)(IGH/BCL2) | BCL2, KMT2D, EZH2, BCL6, CREBBP, EP300, FAS, MEF2B |
| Follicular lymphoma (pediatric type) | None | TNFRSF14, MAP2K1 |
| High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements | MYC, BCL2, and/or BCL6 rearrangements | TP53, MYD88, ID3 |
| Primary diffuse large B-cell lymphoma of the CNS | IG genes, BCL6 and MYC rearrangements | MYD88, CD79B, INPP5D, CBL, BLNK, CARD11, MALT1, BCL2 |
| Post-germinal center origin | ||
| ALK-positive large B-cell lymphoma | t(2;17)(ALK/CLTC), t(2;5)(ALK/NPM), ALK rearrangements with SQSTM1, SEC31A |
|
| Chronic lymphocytic leukemia (CLL)/Small lymphocytic lymphoma (SLL) | t(14;18)(IGH/BCL2), t(14;19)(IGH/BCL3) |
NOTCH1, SF3B1, TP53, ATM, BIRC3, POT1, MYD88 |
| Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) | t(11;18)(BIRC3/MALT1), t(14;18)(IGH/MALT1), t(1;14)(BCL10/IGH), t(3;14)(FOXP1/IGH) |
MYD88 |
| Hairy cell leukemia | None | BRAF, MAP2K1 |
| Hairy cell leukemia variant | TP53, MAP2K1 | |
| Lymphoplasmacytic lymphoma (LPL)/Waldenstrom's macroglobulinemia (WM) | None | MYD88, CXCR4, ARID1A, TP53, CD79B, KMT2D, MYBBP1A |
| Nodal marginal zone lymphoma | None | |
| Plasmablastic lymphoma | MYC rearrangements with IG genes | |
| Primary effusion lymphoma | None | BCL6 |
| Splenic marginal zone lymphoma | t(14;18)(IGH/BCL2), t(11;14)(CCND1/IGH), t(11;18)(BIRC3/MALT1), t(14;18)(IGH/MALT1), t(1;14)(BCL10/IGH), t(2;7)(IGK/CDK6) |
NOTCH2, KLF2 |
| Cutaneous lymphomas | ||
| Primary cutaneous diffuse large B-cell lymphoma, leg-type | t(3;14)(BCL6/IGH), t(8;14)(MYC/IGH) |
MYD88, CARD11, CD78B, TNFAIP3 |
| Primary cutaneous follicle center lymphoma | t(14;18)(IGH/BCL2) | CDKN2A, CDKN2B |
| Unknown origin | ||
| High-grade B-cell lymphoma, NOS | None | |
| Intravascular large B-cell lymphoma | ||
| Large B-cell lymphoma with IRF4 rearrangement | t(6;14)(IRF4/IGH), t(3;14)(BCL6/IGH) |
TP53 |
| Primary mediastinal (thymic) large B-cell lymphoma | MHC CIIA rearrangements with PDL1/2, PDL1/2 rearrangements | SOCS1, STAT6, PTPN1, BCL6, ITPKB, MFHAS1, XPO1 |
| Splenic diffuse red pulp small B-cell lymphoma | t(9;14)(PAX5/IGH) | TP53, CCND3, NOTCH1, MAP2K1, BRAF, SF3B1 |
| T-cell/histiocyte-rich large B-cell lymphoma | ||
Major B-cell lymphoma subtypes grouped by cell of origin with characteristic translocations and recurrently mutated genes.
NOS, Not otherwise specified; CNS, central nervous system; ALK, anaplastic lymphoma kinase.
As discussed above, the nature of the GC reaction leaves B cells vulnerable to genetic alterations that lead to lymphoma. The morphological, antigen-expression, and genetic features of each B-cell lymphoma subtype often mirror that of their normal cell of origin and are used as diagnostic markers.
Pre-GC B-Cell-Derived Lymphomas
Before entering germinal centers, (the pre-GC stage), B cells are primed for antigen exposure and migration. Such “naive” B cells often reside in the mantle zone of lymph nodes. These cells can transition from the mantle zone to germinal centers and sometimes back to the mantle zone. Mantle cell lymphoma (MCL) develops from such cells (Bertoni and Ponzoni 2007). This lymphoma is often associated with gastrointestinal and extranodal involvement. The genetic aspects that define MCL include t(11;14) translocation of the CCND1 gene to the IGH locus, high SOX11 expression, and mutations in ATM, KMT2C, NOTCH1, and TP53 (Rimokh et al. 1994; Ek et al. 2008; Kridel et al. 2012; Zhang et al. 2014). Interestingly, chronic lymphocytic leukemias (CLLs) can arise from pre- or post-GC cells, evidenced by patterns of somatic hypermutation, giving rise to distinct subgroups (“mutated” and “unmutated” CLL) that respond at different rates to standard chemotherapy. Genes across many pathways are recurrently mutated including MYD88, NOTCH1, and MAPK1 (Puente et al. 2011).
GC B-Cell-Derived Lymphomas
The GC represents the most vulnerable stage for the oncogenic transformation of B cells and is where a majority of B-cell lymphomas arise. These include the two most common forms of lymphoma: diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL), in addition to Burkitt lymphoma (BL) and others (Fig. 2). DLBCL comprises at least two subtypes arising from either GC B cells (GCB-DLBCL) or activated B cells (ABC-DLBCL), which likely arise from a pre- or post-GC stage associated with differentiation to plasma cells (Lenz et al. 2008). DLBCLs commonly manifest mutations in KMT2D, SPEN, PIM1, and CREBBP. Mutations in EZH2, SGK1, and GNA13 are associated with the GCB subtype, whereas mutations in MYD88, ETV6, and CD79B are associated with the ABC subtype (Morin et al. 2013; Zhang et al. 2013). A proportion of cases also present with chromosomal rearrangements involving BCL2, BCL6, or MYC, which are termed “double-hit” or “triple-hit” lymphomas and are associated with a poorer prognosis.
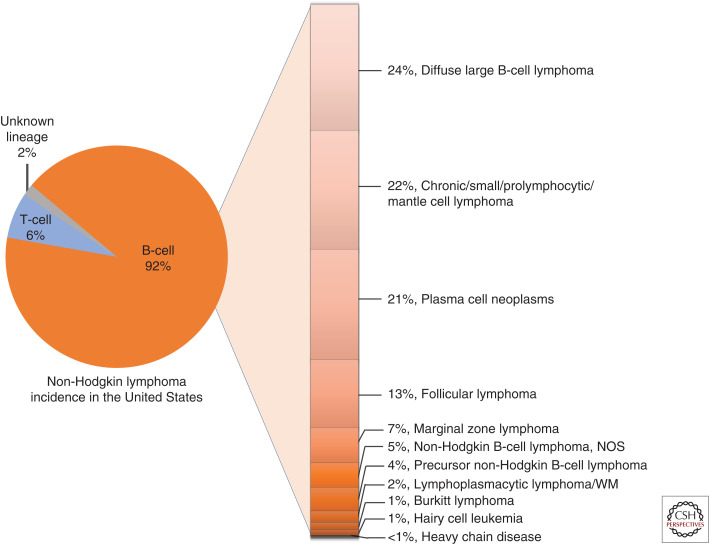
Figure 2.
Incidence of B-cell non-Hodgkin lymphomas. NOS, Not otherwise specified; WM, Waldenström's macroglobulinemia.
Follicular lymphoma can be an indolent disease that can transforms into DLBCL. The hallmark translocation observed in >90% of follicular lymphomas is t(14;18) translocation of the BCL2 gene to the IGH locus (Cleary and Sklar 1985). Commonly mutated genes in follicular lymphoma include BCL2, KMT2D, CREBBP, GNA13, and EZH2 (Okosun et al. 2014).
BL arises from GC cells and is comprised of three subtypes: sporadic, endemic (associated with EBV), and immunosuppression-associated (associated with HIV). BL is highly aggressive and can present in extranodal sites depending on the subtype. BLs present with hallmark t(8;14), t(2;8), or t(8;22) translocations of the MYC gene to the IGH, IGK, or IGL promoter sites, respectively (Taub et al. 1982). Commonly mutated genes include MYC, ID3, DDX3X, and TP53 (Love et al. 2012; Richter et al. 2012; Schmitz et al. 2012).
Post-GC and Marginal Zone B-Cell-Derived Lymphomas
Differentiated B cells that do not develop into plasma cells or memory B cells either undergo apoptosis or migrate to the mantle zone and marginal zones. These B cells may later differentiate into plasma cells or monocytoid B cells. Three types of lymphomas with distinct clinical behaviors arise from these B cells; they include nodal and splenic marginal zone lymphomas (NMZLs and SMZLs, respectively), and MALT lymphoma. NMZL manifests as peripheral lymphadenopathy in the head and neck lymph nodes. SMZL presents with splenomegaly with bone marrow involvement and can be associated with autoimmune thrombocytopenia or anemia. MALT lymphoma involvement is observed in the stomach, ocular adnexa, or thyroid, with involvement of multiple extranodal sites observed. Although there is no hallmark translocation, t(14;18) (IGH-MALT1) leads to deregulation of MALT1 and is found in ocular adnexa, orbit, and salivary gland sites (Clark et al. 1992). The t(3;14) translocation (FOXP1-IGH) is associated with deregulation of FOXP1 and is found in thyroid, ocular adnexa, orbit, and skin (Streubel et al. 2005).
Plasma cells represent terminally differentiated post-GC B cells. Multiple myeloma is the malignancy that arises from plasma cells. There are likely intermediate B-cell stages between the GC B cells and plasma cells as there are at least two distinct malignancies that are characterized by expression profiles intermediate between these cell types. First, WM or lymphoplasmacytic lymphoma (LPL) is derived from cells with plasmacytic differentiation. These cells may arise from memory B cells that can undergo differentiation to plasma cells outside GCs. Most WM cases have a mutation in genes in the B-cell receptor pathway including MYD88, CXCR4, and CD79B (Treon et al. 2012; Hunter et al. 2014). A subset of DLBCLs, ABC DLBCLs, arise from cells with an intermediate expression of genes that distinguish GC cells from plasma cells (Wright et al. 2003).
Primary Cutaneous Lymphoma
Primary cutaneous B-cell lymphoma (CBCL) is characterized by its sole localization in the skin at the time of diagnosis. CBCL consists of indolent types including primary cutaneous marginal zone B-cell lymphoma (PCMZL, 30% of cases) and primary cutaneous follicle center lymphoma (PCFCL, 48% of cases), and intermediate aggressive types such as primary cutaneous diffuse large B-cell lymphoma (PCLBCL, 20% of cases) (Willemze et al. 2005). These lymphomas are mainly derived from mature GC B cells. PCMZL arises as a tumor of marginal zone cells, lymphoplasmacytoid cells, and plasma cells on the trunk or extremities. PCFCL is characterized by centrocytes and centroblasts in a follicular or diffuse pattern on the head or truck. PCLBCL is characterized by skin lesions, mostly on the legs. These “leg-type” PCLBCLs resemble DLBCLs that arise from lymph nodes in terms of their cell of origin (Pham-Ledard et al. 2017). However, the origins of the immune cells that give rise to cutaneous lymphomas remain obscure.
Immunodeficiency-Related Lymphomas
Immunodeficiency diseases, both primary diseases caused by a genetic defect (PIDDs) and secondary, are associated with a 10-fold increased risk of lymphoma compared to the general population (Mayor et al. 2018). This increased lymphoma risk may be due to increased susceptibility to viral illness (including Epstein–Barr virus [EBV]), defective immune surveillance, DNA repair defects, tumor-suppressor gene defects, iatrogenic effects, or chronic inflammation (Gangemi et al. 2015; Mortaz et al. 2016). Ataxia telangiectasia, a disorder of DNA repair with mutations in ATM, has the highest rate of malignancy (mostly lymphoma) among PIDDs (Mortaz et al. 2016). Interestingly, ATM is also frequently mutated in a number of different lymphomas including DLBCL and CLL. Common variable immunodeficiency, Wiskott–Aldrich syndrome, and severe combined immunodeficiency (SCID) are other PIDDs associated with a higher risk for lymphomas. Lymphomas in PIDD patients are often associated with poorer outcomes than those without PIDDs.
HIV-AIDS, which results in immunodeficiency from the selective depletion of CD4+ helper T cells, is known to have an increased incidence of NHL and Hodgkin lymphoma compared to the general U.S. population (Seaberg et al. 2010). The most common types of NHL seen in this population include DLBCL and BL (Meister et al. 2018). Additional types of NHL include Burkitt-like lymphoma, extranodal MALT lymphoma, peripheral T-cell lymphoma (PTCL), primary effusion/body cavity lymphoma, plasmablastic lymphoma of the oral cavity, and polymorphic B-cell lymphoma (Rubinstein et al. 2014). An important mechanism in AIDS-related lymphoma development is chronic infection with EBV and/or HHV8 leading to chronic B-cell stimulation and production of a monoclonal B-cell population. EBV infection is associated with DLBCL (30%), plasmablastic DLBCL (90%), and primary effusion/body cavity lymphoma (100%). HHV8 infection is associated with 50% of plasmablastic lymphomas of the oral cavity. A patient's risk of lymphoma and response to therapy are correlated with the CD4 cell count, viral load, infections, and treatment with combination antiretroviral therapy (Meister et al. 2018).
Another state of secondary immunodeficiency is that which occurs as a result of iatrogenic immunosuppression to protect the graft after a solid organ or hematopoietic stem cell transplant (HSCT). Posttransplant lymphoproliferative disorders (PTLDs) comprise 20% of all cancers that occur after solid organ transplantation and are rare complications of HSCT (Nagle et al. 2017). Most cases of PTLD are due to EBV infection (either following primary EBV infection or reactivation of a previous infection), although PTLD can occur without the presence of EBV (Martinez and Krams 2017; Nagle et al. 2017). Iatrogenic immunosuppression allows EBV-induced B-cell proliferation and PTLD development (Martinez and Krams 2017). The main risk factors for PTLD include EBV status of the recipient and the degree of immunosuppression, specifically T-cell suppression. Therefore, treatment involves the reduction of immune suppression (Nagle et al. 2017). It may also involve the addition of the anti-CD20 antibody rituximab to control the proliferating B cells. There is no accepted system for determining prognosis, although tumor monoclonality, EBV negativity, and graft involvement are thought to predict a poorer outcome.
THERAPEUTIC APPROACHES IN B-CELL LYMPHOMA
Therapeutic Approaches and Promising Clinical Trials
For the clinician, the initial general therapeutic approach for the management of lymphoma is determined by the aggressiveness of disease and treatment intent. This guiding principle goes back to the original attempts to classify lymphoma (Working Formulation) that classified non-Hodgkin lymphomas as low-, intermediate-, or high-grade solely based on morphological and clinical characteristics of the disease (Robb-Smith 1982). Indolent, or slow-growing lymphomas (CLL, FL, and others), are generally considered incurable; the goal is to control the disease and prevent complications like organ dysfunction, cytopenias, malignant effusions, and symptoms attributable to the disease. In the absence of these problems, active observation is often pursued. In contrast, aggressive lymphomas (BL, DLBCL, PTCLs, etc.) are treated with curative intent. Here, multi-agent chemotherapy regimens are employed up front for several cycles with the goal of obtaining a complete response.
Lymphoma is a systemic disease. Although lymphocytes are exquisitely sensitive to radiation, radiation therapy is generally used in patients with localized disease in conjunction with chemotherapy and/or immunotherapy. Palliative radiation for relief of symptoms caused by bulky tumors remains an effective, if short-lived, therapy for patients.
Although aggressive B-cell lymphomas generally respond well to multi-agent chemotherapy, the complete response rate is far less in aggressive T-cell lymphomas, in which relapses are also more common. Therefore, some clinicians opt to give high-dose chemotherapy with autologous stem cell transplant upon attaining first complete remission in aggressive T-cell lymphomas, although there are no randomized data to support this practice.
The approach to subsequent line treatment of lymphoma is also largely guided by the intent to cure versus palliate. In large B-cell lymphomas and Hodgkin lymphomas that have relapsed or are refractory to first-line treatment, second-line multi-agent chemotherapy is given, and if response is attained, high-dose chemotherapy followed by autologous stem cell transplantation is considered standard of care. This approach offers the best chance for long-term remission in second-line treatment. With each subsequent relapse, the chance for long-term complete remission with chemotherapy decreases, and alternative small-molecule inhibitors, targeted agents, and cellular or immunotherapies may be used, even as the intent may not be to attempt a cure. In indolent and aggressive lymphomas alike, the optimal sequence of subsequent line therapies is not defined and is chosen based on urgency for disease control, toxicity profile, and patient or physician preference.
The current WHO classification of lymphoma integrates immunohistochemical and molecular characteristics of disease in addition to clinical and morphologic features. For example, molecular features like MYC, BCL2, and BCL6 translocations are associated with particularly aggressive disease, informing up-front treatment decisions while providing prognostic information as long-term remissions are difficult to obtain. Many clinicians tend to use more aggressive chemotherapy regimens, particularly in patients whose tumors manifest two or more translocations (“double hit”) simultaneously.
Determination of cell of origin in aggressive B-cell lymphomas by the Hans algorithm may provide the clinician some biological basis for selecting a subsequent line therapy for a given patient, although the optimal treatment for any given molecular subtype of lymphoma remains to be defined. Therefore, there is ample room for consideration of clinical trial options in the up-front treatment of high-risk disease or subsequent treatment of refractory disease. Outside of clinical trials, novel approaches using immune checkpoint blockade and chimeric antigen receptor modified T cells are appropriate in the relapsed or refractory setting after failing two lines of systemic therapy.
Chemotherapy
The most common treatment for NHL continues to be combination chemotherapy. Many of the compounds used in these therapies leverage the cytotoxic stress of DNA damage in the rapidly proliferating cancer cells in order to preferentially eliminate them. Alkylating agents (e.g., cyclophosphamide) and platins (e.g., cisplatin) form DNA cross-links that exploit the impaired ability of cancer cells to repair and replicate DNA with these genetic lesions. Purine analogs (e.g., fludarabine) act both as an antimetabolite of purines and as a DNA synthesis inhibitor once integrated into DNA. Pyrimidine antimetabolites (cytarabine) act in a similar manner to inhibit DNA synthesis at the incorporated base. Other antimetabolites (e.g., methotrexate) target folate metabolism that is required for the production of DNA/RNA bases and thymidylates (Rajagopalan et al. 2002). Anthracycline compounds (e.g., doxorubicin) intercalate into DNA, preventing topoisomerase II progression as well as evicting histones from transcriptionally active regions of the genome (Pommier et al. 2010; Pang et al. 2013). Vinca alkaloids (e.g., vincristine) are also effective in many lymphomas, inhibiting microtubule formation and thus disrupting mitosis. Many combination therapies for lymphoid neoplasms contain a corticosteroid agent (e.g., prednisone) that leverages the immunomodulating effects of the glucocorticoid pathway within the tumor cells.
Patients with NHL are typically treated with a combination of these chemotherapeutic agents. Common treatment regimens include CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) and EPOCH (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin). Escalated regimens for refractory or aggressive tumors include hyper-CVAD (cyclophosphamide, vincristine, doxorubicin, dexamethasone, methotrexate, and cytarabine) and DHAP (dexamethasone, cytarabine, and cisplatin). Many chemotherapy regimens are augmented with rituximab and/or targeted immunotherapy. Rituximab is an anti-CD20 monoclonal antibody that, when administered in conjunction with chemotherapy, is an effective component of curative standard care for B-cell lymphoma patients (>85% of all NHL patients) (Weiner 2010). Rituximab induces killing of CD20+ cells via multiple mechanisms including complement-mediated cytotoxicity and antibody-dependent cell-mediated cytotoxicity.
Brentuximab is an anti-CD30 antibody that is administered conjugated to a chemotherapeutic drug via protease-cleavable linker (Bhatt et al. 2013). This complex, brentuximab vedotin, can be used to treat some T-cell lymphomas in conjunction with CHOP or as an alternative treatment if lymphoma persists after utilizing other treatment options. Upon binding the CD30 antigen, brentuximab vedotin is internalized and transported to lysosomes, where the chemotherapeutic agent is released and binds to tubulin, causing cell cycle arrest and apoptosis.
Although these standard therapies are effective for a majority of patients, drug resistance and disease relapse in the remaining patients lead to poor clinical outcomes. Drug resistance can be intrinsic, treatment-acquired, or tumor microenvironment–mediated (for review, see Camicia et al. 2015). Major avenues of therapy resistance that arise via these mechanisms involve up-regulation of anti-apoptotic proteins (e.g., BCL2), down-regulation of pro-apoptotic factors (e.g., BAX), and down-regulation or mutation of drug targets (e.g., CD20). Disease relapse can arise from a clone that diverged either early or late from the dominant clone. Alterations that mediate immune evasion have been associated with relapse (Jiang et al. 2014). Resistant patients and the extreme side effects of treatment call for new, more effective treatment options to be added to the current treatment regimens.
Targeted Therapies
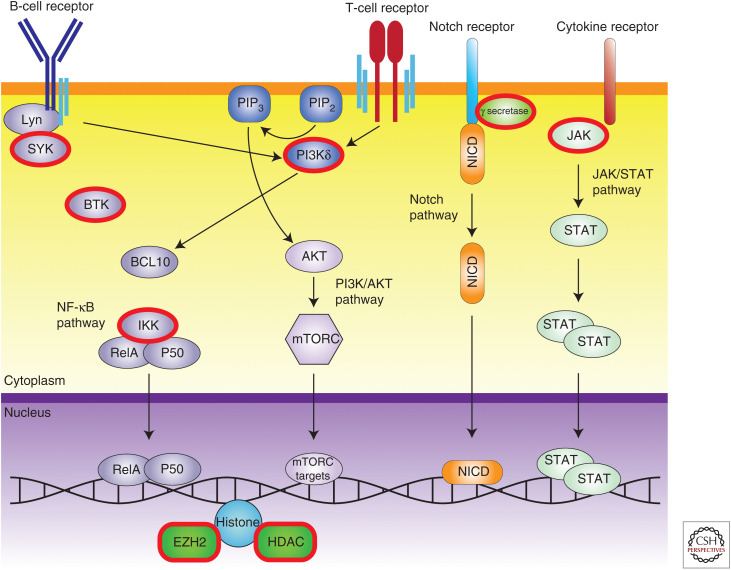
One of the promising avenues of targeted therapies in NHL is targeting endogenous and altered signaling pathways within the tumor. Recurrent genetic alterations indicate critical genes and pathways that drive the disease. Targeting these critical pathways with small molecules deprives the cancer cells of required signaling without the global off-target effects of other chemotherapy types such as genotoxic agents (Fig. 3).
Figure 3.
Targetable signaling pathways in non-Hodgkin lymphoma. Targetable nodes with clinically available small molecule inhibitors are ringed in red. BCR, B-cell receptor; SYK, spleen tyrosine kinase; BTK, Bruton's tyrosine kinase; PI3Kδ, phosphoinositide 3-kinases delta; NICD, Notch intracellular domain; JAK, Janus kinase; IKK, IκB kinase; HDAC, histone deacetylase.
There are several notable signaling pathways that are shared to differing degrees by NHLs. These include the JAK-STAT pathway, which is critical for lymphoid cell formation and differentiation and is a primary driver of activating signaling via cytokines (Liao et al. 2011). Constitutive STAT3/STAT5 activation is a feature of a number of different lymphoid malignancies (Migone et al. 1995; Buettner et al. 2002). JAK2 inhibition with ruxolitinib is currently in clinical trials in a number of lymphomas (Lee et al. 2018).
The major signaling networks in both B and T cells are modulated by surface immunoglobulin receptors that, once activated, generate powerful signaling cascades that induce growth, proliferation, and differentiation. The natural function of these immune cells requires an ability to rapidly divide upon antigen stimulation and then recede upon antigen clearance. Activation of the B-cell receptor (BCR) pathway is critical in B-cell neoplasms as it circumvents poised apoptosis of the unstimulated state (Lam et al. 1997). The BCR pathway contains a series of critical kinase nodes (SYK, PI3K, CD79A, and BTK) that are essential for proper BCR activation and have become important therapeutic targets (Seda and Mraz 2015). The Bruton's tyrosine kinase (BTK) inhibitor ibrutinib was shown to be effective in MCL and CLL, but other B-cell malignancies have not been as responsive, suggesting that more refined drugs or different signaling nodes should be targeted in these cases. Upstream in the signaling cascade, SYK has also been a target to disrupt tonic BCR signaling that drives lymphoma. Fostamatinib was developed as a potent SYK inhibitor and has shown response in a variety of NHL patients. The efficacy of these types of drugs demonstrate a path for disrupting the tonic endogenous growth signals that sustain lymphoma.
Further down the signaling cascade, both the B- and T-cell receptors signal through PI3K and NF-κB. Dysregulation of the PI3K pathway has been described in several lymphoid malignancies including Hodgkin lymphoma, MCL, and FL (Psyrri et al. 2009; Meadows et al. 2012; Yahiaoui et al. 2014). Idelalisib, a potent PI3Kδ inhibitor, was shown to be effective in preclinical models at not only reducing tonic BCR signaling, but also modulating the tumor microenvironment to be less tumor-supporting (Hoellenriegel et al. 2011). As a monotherapy, idelalisib was very effective in refractory patients; however, in combination, serious side effects halted further trials. DLBCL has been shown to be NF-κB-dependent, with mutations in genes such as CARD11 leading to constitutive NF-κB activation, making these related nodes attractive targets for targeted therapies (Davis et al. 2001; Milhollen et al. 2010; Staudt 2010).
Notch pathway mutations and dysregulation have been observed in many lymphoma subtypes, including DLBCL, SMZL, FL, MCL, and PTCL (Karube et al. 2014). Many of these changes involve loss of the PEST domain that regulates NOTCH degradation, leading to increased protein half-life and thus activity and related downstream signaling. The major targetable component of this pathway is γ-secretase; however, no clinical trials focused on lymphoma have been performed thus far.
The histone methyltransferase EZH2 is recurrently mutated in both DLBCL and FL (Morin et al. 2010; Bodor et al. 2011; Béguelin et al. 2016; Reddy et al. 2017). Interestingly, EZH2 mutations are largely gain-of-function alterations that impair the normal course of B-cell differentiation. In particular, the Y641 amino acid is a hotspot for missense mutations that result in increased levels of H3K27me3. A recent phase 1 trial of the inhibitor tazemetostat exhibited promising clinical response in patients with relapsed or refractory B-cell lymphomas (Italiano et al. 2018). EZH2 inhibitors might also be combined with HDAC inhibitors to relieve transcriptional repression of key target genes (Lue et al. 2019).
Whereas targeted therapies offer the ability to single out specific pathways that lymphomas rely on for growth and cell death escape, there is much to learn about translating the genetic and experimental findings into effective therapies. Unraveling the genetics provides a critical first step to this understanding.
Immunotherapy
Immunotherapy presents a highly promising new method for treating lymphomas. Immunotherapy consists of multiple approaches, chiefly (1) checkpoint inhibitor therapy, (2) chimeric antigen receptor (CAR) T-cell therapy, and (3) bispecific T-cell engager (BiTE) therapy.
Checkpoint inhibitor therapy targets proteins found on the surface of T cells or antigen-presenting cells that act to dampen T-cell activity in a normal immune response. Although this approach has proven to be effective in solid tumors and in Hodgkin lymphoma, the results in NHL have been disappointing. Ongoing work will help delineate the potential application of these therapies.
On the other hand, CARs are genetically engineered immune receptors that consist of a single peptide–containing V-heavy chain and V-light chain segments connected to an intracellular signaling chain. T cells are expanded from peripheral blood mononuclear cells (PBMCs) collected from patients, then transduced to express CARs targeting a tumor-associated antigen (TAA) of choice. One of the most widely used and most effective TAAs is CD19, which is widely expressed on NHLs. Early CAR T-cell clinical trials have demonstrated a striking degree of efficacy in patients, even as further advancements are needed to improve efficacy and reduce toxicity.
BiTE therapy also seeks to promote T-cell activity against tumor cells. As their name implies, BiTE antibodies bind two antigens simultaneously, generally the CD3 component of the T-cell receptor and a tumor antigen, resulting in T-cell activation and target cell lysis. To date, BiTE antibodies targeting CD19 (blinatumomab) and CD20 (e.g., mosunetuzumab) have been developed to treat B-cell malignancies. Blinatumomab is approved for relapsed/refractory acute lymphoblastic leukemia, but the severity of side effects, short half-life, and the advent of anti-CD19 CAR-T therapy have hindered its widespread adoption in NHL (Bacac et al. 2018; Yu et al. 2019). CD20-targeting BiTE antibodies with longer half-lives are still in early phases of investigation, although results have been promising (Chu et al. 2014; Sun et al. 2015; Schuster et al. 2019).
Much work is still needed in understanding the appropriate duration, intensity, and sequencing of different approaches in the appropriately risk-stratified patients with NHLs.
MODELING LYMPHOMAS
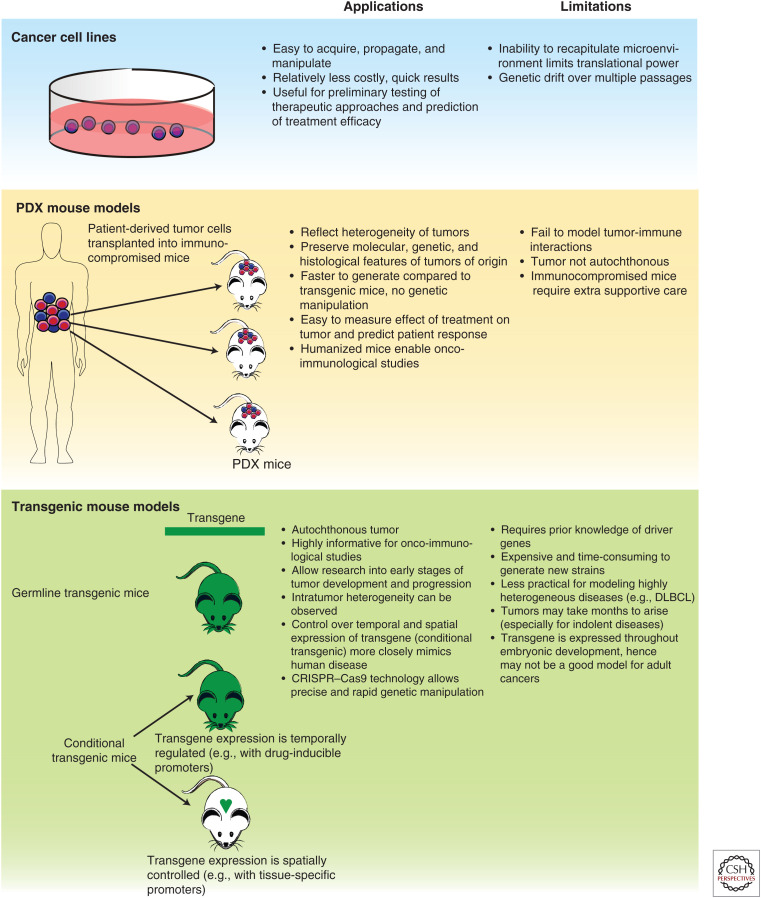
Preclinical models of cancer are essential tools that have furthered our understanding of the disease. These models allow for investigation of tumor biology and disease progression, evaluation of drug targets, and development of effective treatment strategies. There are currently three main approaches to model lymphomas, each representing a different set of trade-offs in recapitulating the complete tumor phenotypes: cell lines, patient-derived xenograft models (PDXs), and genetically engineered mouse models (GEMMs) (Fig. 4).
Figure 4.
Lymphoma models. Cell culture, patient-derived xenograft (PDX), and transgenic mouse models are used to model lymphomas in the research and clinical settings.
Cancer cell lines are derived by in vitro immortalization of lymphoma patient–derived tumor cells. As such, cancer cell lines represent the complete genotype of the tumor, in a way that is hard to achieve in engineered models. Many established lymphoma cell lines have well-characterized genetic alterations and cytogenetics, providing a platform to assess various genomic pathways. They allow for rapid and economical testing of therapeutic approaches and predictions of treatment efficacy. Cell lines, however, have a limited ability to translate findings to clinical practice because of their unregulated growth outside a normal tumor microenvironment, which can result in genetic divergence from the primary tumors of origin. Cell lines also lack the ability to model interactions with the surrounding stroma normally present within the microenvironment, which currently can only be replicated in vivo.
Some of these limitations are overcome in PDX mouse models, which are generated by implanting patient-derived tumor cells into immunocompromised mice. Propagation of PDXs does not require in vitro manipulation and has been shown to maintain the same biological, genetic, and histopathological features as the tumor of origin (Zhang et al. 2017). As such, PDXs have been used to predict drug response in patients and for confirming in vitro findings. Transplanted cells may take up to several months to engraft after implantation, and supportive care is needed for the maintenance of immunocompromised mice. Nevertheless, xenografts can be generated more rapidly than transgenic mice. A major drawback of xenografts is that due to the compromised immune system of host mice, treatment strategies such as immunotherapy cannot be evaluated. This limitation has spurred the development of humanized mice in which human hematopoietic stem progenitor cells (HSPCs) are co-transplanted with genetically manipulated cells or tumor cells into immunocompromised mice (Choi et al. 2018). These human HSPCs repopulate the host immune system, enabling investigations into tumor-immune system interactions. Although generation of humanized mice can be more time-consuming and expensive than traditional xenograft models, they may be more informative as they closely recapitulate the human disease and allow study of the tumor microenvironment.
Generation of GEMMs primarily involves introduction of “knock-out” mutations or “knock-in” of target genes into immunocompetent mice to study loss of function and gain of function, respectively. Germline transgenic mice express the transgene at all developmental stages and in all tissues. However, such systems may not accurately model adult cancers and may lead to embryonic lethality. Incorporation of specific gene-regulatory elements, such as drug-inducible and cell/tissue-specific promoters, allows for temporal or tissue-specific control of transgene expression. The advent of CRISPR–Cas9 technology has improved generation of in vivo models. CRISPR–Cas9 allows more rapid introduction of gene mutations in mouse zygotes, especially in situations with multiple target genes of interest (Wang et al. 2013; Aida et al. 2015). CRISPR–Cas9 editing also allows the mutated gene to remain under the control of its endogenous promoter. Newly developed Cas9 base editors allow investigators to make precise nucleotide changes and model hotspot or intergenic point mutations (Zafra et al. 2018). As the CRISPR–Cas9 toolbox expands, investigators will have more tools with which to model hematologic malignancies with increasing accuracy.
THE “HETEROGENEITY PROBLEM” IN LYMPHOMA
In spite of steady progress over the past decades in our understanding of the biology of lymphomas, the frontline therapies for most lymphomas have remained unchanged. A major reason for the lack of progress in these cancers is the biologic heterogeneity that underlies each lymphoma type. This problem is exemplified in the most common lymphoma, DLBCL.
DLBCLs have been extensively characterized to identify molecular subgroups and genomic correlates of prognosis following standard therapy. Gene expression profiling has revealed many competing approaches for classification (Kuze et al. 2000; Ando et al. 2002; Lossos et al. 2003; Rimsza et al. 2004; Hans et al. 2005; Natkunam et al. 2008)—two of the most widely cited include those based on cell of origin (Alizadeh et al. 2000) and those based on host response, B-cell receptor, and tumor metabolic states (Monti et al. 2005). The clinical application of these approaches has remained limited, even a decade after their initial description.
The advent of next-generation sequencing has provided a powerful approach to incorporate genetic alterations into prognostic models and subgroups. More than 100 genetic drivers have been described in DLBCL, with the average tumor comprising five to 10 distinct events simultaneously. Thus, no two patients appear to manifest identical sets of mutations, confounding our ability to subgroup patients and presenting daunting power calculations for samples needed to effectively discern the prognostic effects of diverse mutations.
The relatively high prevalence of DLBCL has enabled several landmark studies that used complementary approaches to understand the heterogeneity underlying survival differences in DLBCL (Table 2). There are two distinct approaches to genomic survival modeling in cancers including lymphoma. The first approach treats survival as a continuous variable that is modeled on individual mutations and critical expression features (e.g., MYC, BCL2, and cell of origin). The second approach treats survival as a reflection of underlying discrete subgroups with characteristic alterations that have distinct survival outcomes. The latter approach is particularly attractive because it enables us to consider such subgroups as the minimal entity to focus on for disease modeling and therapeutic targeting.
Table 2.
Recently published next-generation sequencing (NGS) studies of diffuse large B-cell lymphoma (DLBCL)
| References | Reddy et al. 2017 | Schmitz et al. 2018 | Chapuy et al. 2018 |
|---|---|---|---|
| Number of samples | 1001 | 574 | 304 |
| Paired tumor/normal samples | 401 | 0 | 167 |
| Percentage FFPE samples | 95% | 0% | 50% |
| Data types | Exome-seq, RNA-seq, CRISPR screen | Exome-seq, RNA-seq, aCGH | Exome-seq, targeted sequencing |
| Survival time | Overall survival | Overall survival, progression-free survival | Progression-free survival |
| Predictor type | Survival predictor | Supervised clustering | Supervised clustering |
| Number of subgroups | 3 | 4 | 5 |
FFPE, formalin-fixed paraffin-embedded; aCGH, array comparative genomic hybridization.
The first approach of treating survival as a continuous variable was utilized in a study of 1001 patients with DLBCL that showed that combinatorial blending of different genomic features generated very strong effects on overall patient survival. The second approach of treating survival as a function of distinct subgroups of genetic alterations was adopted by two different studies summarized in Table 2. Both studies were based on highly supervised approaches to analysis with somewhat different assumptions with relatively little overlap in the subgroups described between the two studies. The first study described five subgroups that collectively describe all 304 patients (i.e., no unclassified cases). The second study described four subgroups that collectively comprised fewer than one-half the patients with the remainder being unclassified. These subgroups offer a different window into DLBCL biology by highlighting mutually exclusive genetic events. Importantly, these described subgroups are themselves heterogeneous. Existing survival factors such as BCL2, MYC, cell of origin, EBV status, and many others remain prognostic within these subgroups, suggesting considerable molecular and clinical heterogeneity within these subgroups.
Interestingly, the issue of clinical and genetic heterogeneity has been studied in acute myeloid leukemia (AML) in large studies (Papaemmanuil et al. 2016; Gerstung et al. 2017). These studies have demonstrated that survival is better understood as a large set of combinatorial effects, rather than a few dominant subgroups. It appears that, in terms of overall survival, DLBCL shares this critical feature with AML. Ultimately, the utility of all survival models can only be established clinically.
CONCLUSION
Lymphomas collectively represent the fourth most common group of cancers and are a leading cause of cancer mortality. B-cell lymphomas represent a strikingly diverse group of malignancies that are best understood in the context of lineage. Recent work has given us greater insight into the molecular composition of these tumors. The coming years hold great promise for translating that knowledge into better clinical outcomes.
ACKNOWLEDGMENTS
The authors gratefully acknowledge support from the Lymphoma Research Foundation and the National Institutes of Health.
Footnotes
Editors: Michael G. Kharas, Ross L. Levine, and Ari M. Melnick
Additional Perspectives on Leukemia and Lymphoma: Molecular and Therapeutic Insights available at www.perspectivesinmedicine.org
REFERENCES
- Aida T, Chiyo K, Usami T, Ishikubo H, Imahashi R, Wada Y, Tanaka KF, Sakuma T, Yamamoto T, Tanaka K. 2015. Cloning-free CRISPR/Cas system facilitates functional cassette knock-in in mice. Genome Biol 16: 87. 10.1186/s13059-015-0653-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, et al. 2000. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403: 503–511. 10.1038/35000501 [DOI] [PubMed] [Google Scholar]
- Ando T, Suguro M, Hanai T, Kobayashi T, Honda H, Seto M. 2002. Fuzzy neural network applied to gene expression profiling for predicting the prognosis of diffuse large B-cell lymphoma. Jpn J Cancer Res 93: 1207–1212. 10.1111/j.1349-7006.2002.tb01225.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacac M, Colombetti S, Herter S, Sam J, Perro M, Chen S, Bianchi R, Richard M, Schoenle A, Nicolini V, et al. 2018. CD20-TCB with obinutuzumab pretreatment as next-generation treatment of hematologic malignancies. Clin Cancer Res 24: 4785–4797. [DOI] [PubMed] [Google Scholar]
- Béguelin W, Teater M, Gearhart MD, Calvo Fernández MT, Goldstein RL, Cárdenas MG, Hatzi K, Rosen M, Shen H, Corcoran CM, et al. 2016. EZH2 and BCL6 cooperate to assemble CBX8-BCOR complex to repress bivalent promoters, mediate germinal center formation and lymphomagenesis. Cancer Cell 30: 197–213. 10.1016/j.ccell.2016.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoni F, Ponzoni M. 2007. The cellular origin of mantle cell lymphoma. Int J Biochem Cell Biol 39: 1747–1753. 10.1016/j.biocel.2007.04.026 [DOI] [PubMed] [Google Scholar]
- Bhatt S, Ashlock BM, Natkunam Y, Sujoy V, Chapman JR, Ramos JC, Mesri EA, Lossos IS. 2013. CD30 targeting with brentuximab vedotin: a novel therapeutic approach to primary effusion lymphoma. Blood 122: 1233–1242. 10.1182/blood-2013-01-481713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodor C, O'Riain C, Wrench D, Matthews J, Iyengar S, Tayyib H, Calaminici M, Clear A, Iqbal S, Quentmeier H, et al. 2011. EZH2 Y641 mutations in follicular lymphoma. Leukemia 25: 726–729. [DOI] [PubMed] [Google Scholar]
- Buettner R, Mora LB, Jove R. 2002. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res 8: 945–954. [PubMed] [Google Scholar]
- Camicia R, Winkler HC, Hassa PO. 2015. Novel drug targets for personalized precision medicine in relapsed/refractory diffuse large B-cell lymphoma: a comprehensive review. Mol Cancer 14: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapuy B, Stewart C, Dunford AJ, Kim J, Kamburov A, Redd RA, Lawrence MS, Roemer MGM, Li AJ, Ziepert M, et al. 2018. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med 24: 679–690. 10.1038/s41591-018-0016-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Lee S, Kim K, Kim SH, Chung YJ, Lee C. 2018. Studying cancer immunotherapy using patient-derived xenografts (PDXs) in humanized mice. Exp Mol Med 50: 99. 10.1038/s12276-018-0115-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu TW, Yang J, Zhang R, Sima M, Kopecek J. 2014. Cell surface self-assembly of hybrid nanoconjugates via oligonucleotide hybridization induces apoptosis. ACS Nano 8: 719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark HM, Jones DB, Wright DH. 1992. Cytogenetic and molecular studies of t(14;18) and t(14;19) in nodal and extranodal B-cell lymphoma. J Pathol 166: 129–137. 10.1002/path.1711660208 [DOI] [PubMed] [Google Scholar]
- Cleary ML, Sklar J. 1985. Nucleotide sequence of a t(14;18) chromosomal breakpoint in follicular lymphoma and demonstration of a breakpoint-cluster region near a transcriptionally active locus on chromosome 18. Proc Natl Acad Sci 82: 7439–7443. 10.1073/pnas.82.21.7439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RE, Brown KD, Siebenlist U, Staudt LM. 2001. Constitutive nuclear factor κB activity is required for survival of activated B cell–like diffuse large B cell lymphoma cells. J Exp Med 194: 1861–1874. 10.1084/jem.194.12.1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Noia JM, Neuberger MS. 2007. Molecular mechanisms of antibody somatic hypermutation. Annu Rev Biochem 76: 1–22. 10.1146/annurev.biochem.76.061705.090740 [DOI] [PubMed] [Google Scholar]
- Ek S, Dictor M, Jerkeman M, Jirström K, Borrebaeck CA. 2008. Nuclear expression of the non-B-cell lineage Sox11 transcription factor identifies mantle cell lymphoma. Blood 111: 800–805. 10.1182/blood-2007-06-093401 [DOI] [PubMed] [Google Scholar]
- Fais F, Ghiotto F, Hashimoto S, Sellars B, Valetto A, Allen SL, Schulman P, Vinciguerra VP, Rai K, Rassenti LZ, et al. 1998. Chronic lymphocytic leukemia B cells express restricted sets of mutated and unmutated antigen receptors. J Clin Invest 102: 1515–1525. 10.1172/JCI3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangemi S, Allegra A, Musolino C. 2015. Lymphoproliferative disease and cancer among patients with common variable immunodeficiency. Leuk Res 39: 389–396. 10.1016/j.leukres.2015.02.002 [DOI] [PubMed] [Google Scholar]
- Gerstung M, Papaemmanuil E, Martincorena I, Bullinger L, Gaidzik VI, Paschka P, Heuser M, Thol F, Bolli N, Ganly P. 2017. Precision oncology for acute myeloid leukemia using a knowledge bank approach. Nat Genet 49: 332–340. 10.1038/ng.3756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiotto F, Fais F, Albesiano E, Sison C, Valetto A, Gaidano G, Reinhardt J, Kolitz JE, Rai K, Allen SL, et al. 2006. Similarities and differences between the light and heavy chain Ig variable region gene repertoires in chronic lymphocytic leukemia. Mol Med 12: 300–308. 10.2119/2006-00080.Ghiotto [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans CP, Weisenburger DD, Greiner TC, Chan WC, Aoun P, Cochran GT, Pan Z, Smith LM, Lynch JC, Bociek RG. 2005. Expression of PKC-β or cyclin D2 predicts for inferior survival in diffuse large B-cell lymphoma. Mod Pathol 18: 1377–1384. 10.1038/modpathol.3800434 [DOI] [PubMed] [Google Scholar]
- Hoellenriegel J, Meadows SA, Sivina M, Wierda WG, Kantarjian H, Keating MJ, Giese N, O'Brien S, Yu A, Miller LL, et al. 2011. The phosphoinositide 3′-kinase delta inhibitor, CAL-101, inhibits B-cell receptor signaling and chemokine networks in chronic lymphocytic leukemia. Blood 118: 3603–3612. 10.1182/blood-2011-05-352492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter ZR, Xu L, Yang G, Zhou Y, Liu X, Cao Y, Manning RJ, Tripsas C, Patterson CJ, Sheehy P, et al. 2014. The genomic landscape of Waldenström macroglobulinemia is characterized by highly recurring MYD88 and WHIM-like CXCR4 mutations, and small somatic deletions associated with B-cell lymphomagenesis. Blood 123: 1637–1646. 10.1182/blood-2013-09-525808 [DOI] [PubMed] [Google Scholar]
- Italiano A, Soria JC, Toulmonde M, Michot JM, Lucchesi C, Varga A, Coindre JM, Blakemore SJ, Clawson A, Suttle B, et al. 2018. Tazemetostat, an EZH2 inhibitor, in relapsed or refractory B-cell non-Hodgkin lymphoma advanced solid tumours: a first-in-human, open-label, phase 1 study. Lancet Oncol 19: 649–659. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Redmond D, Nie K, Eng KW, Clozel T, Martin P, Tan LH, Melnick AM, Tam W, Elemento O. 2014. Deep sequencing reveals clonal evolution patterns mutation events associated with relapse in B-cell lymphomas. Genome Biol 15: 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karube K, Martínez D, Royo C, Navarro A, Pinyol M, Cazorla M, Castillo P, Valera A, Carrió A, Costa D, et al. 2014. Recurrent mutations of NOTCH genes in follicular lymphoma identify a distinctive subset of tumours. J Pathol 234: 423–430. 10.1002/path.4428 [DOI] [PubMed] [Google Scholar]
- Kridel R, Meissner B, Rogic S, Boyle M, Telenius A, Woolcock B, Gunawardana J, Jenkins C, Cochrane C, Ben-Neriah S, et al. 2012. Whole transcriptome sequencing reveals recurrent NOTCH1 mutations in mantle cell lymphoma. Blood 119: 1963–1971. 10.1182/blood-2011-11-391474 [DOI] [PubMed] [Google Scholar]
- Kuze T, Nakamura N, Hashimoto Y, Sasaki Y, Abe M. 2000. The characteristics of Epstein–Barr virus (EBV)-positive diffuse large B-cell lymphoma: comparison between EBV+ and EBV− cases in Japanese population. Jpn J Cancer Res 91: 1233–1240. 10.1111/j.1349-7006.2000.tb00909.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam KP, Kühn R, Rajewsky K. 1997. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell 90: 1073–1083. 10.1016/S0092-8674(00)80373-6 [DOI] [PubMed] [Google Scholar]
- Lee S, Shah T, Yin C, Hochberg J, Ayello J, Morris E, van de Ven C, Cairo MS. 2018. Ruxolitinib significantly enhances in vitro apoptosis in Hodgkin lymphoma and primary mediastinal B-cell lymphoma and survival in a lymphoma xenograft murine model. Oncotarget 9: 9776–9788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz G, Wright GW, Emre NC, Kohlhammer H, Dave SS, Davis RE, Carty S, Lam LT, Shaffer AL, Xiao W, et al. 2008. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci 105: 13520–13525. 10.1073/pnas.0804295105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Woo CJ, Iglesias-Ussel MD, Ronai D, Scharff MD. 2004. The generation of antibody diversity through somatic hypermutation and class switch recombination. Genes Dev 18: 1–11. 10.1101/gad.1161904 [DOI] [PubMed] [Google Scholar]
- Liao W, Lin JX, Leonard WJ. 2011. IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr Opin Immunol 23: 598–604. 10.1016/j.coi.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lossos IS, Alizadeh AA, Rajapaksa R, Tibshirani R, Levy R. 2003. HGAL is a novel interleukin-4–inducible gene that strongly predicts survival in diffuse large B-cell lymphoma. Blood 101: 433–440. 10.1182/blood-2002-06-1931 [DOI] [PubMed] [Google Scholar]
- Love C, Sun Z, Jima D, Li G, Zhang J, Miles R, Richards KL, Dunphy CH, Choi WW, Srivastava G, et al. 2012. The genetic landscape of mutations in Burkitt lymphoma. Nat Genet 44: 1321–1325. 10.1038/ng.2468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue JK, Prabhu SA, Liu Y, Gonzalez Y, Verma A, Mundi PS, Abshiru N, Camarillo JM, Mehta S, Chen EI, et al. 2019. Precision targeting with EZH2 HDAC inhibitors in epigenetically dysregulated lymphomas. Clin Cancer Res 25: 5271–5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez OM, Krams SM. 2017. The immune response to Epstein–Barr virus and implications for posttransplant lymphoproliferative disorder. Transplantation 101: 2009–2016. 10.1097/TP.0000000000001767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul RW, Gearhart PJ. 2010. AID and somatic hypermutation. Adv Immunol 105: 159–191. 10.1016/S0065-2776(10)05006-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor PC, Eng KH, Singel KL, Abrams SI, Odunsi K, Moysich KB, Fuleihan R, Garabedian E, Lugar P, Ochs HD, et al. 2018. Cancer in primary immunodeficiency diseases: Cancer incidence in the United States Immune Deficiency Network Registry. J Allergy Clin Immunol 141: 1028–1035. 10.1016/j.jaci.2017.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows SA, Vega F, Kashishian A, Johnson D, Diehl V, Miller LL, Younes A, Lannutti BJ. 2012. PI3Kδ inhibitor, GS-1101 (CAL-101), attenuates pathway signaling, induces apoptosis, and overcomes signals from the microenvironment in cellular models of Hodgkin lymphoma. Blood 119: 1897–1900. 10.1182/blood-2011-10-386763 [DOI] [PubMed] [Google Scholar]
- Meister A, Hentrich M, Wyen C, Hübel K. 2018. Malignant lymphoma in the HIV-positive patient. Eur J Haematol 101: 119–126. 10.1111/ejh.13082 [DOI] [PubMed] [Google Scholar]
- Migone TS, Lin JX, Cereseto A, Mulloy JC, O'Shea JJ, Franchini G, Leonard WJ. 1995. Constitutively activated Jak-STAT pathway in T cells transformed with HTLV-I. Science 269: 79–81. 10.1126/science.7604283 [DOI] [PubMed] [Google Scholar]
- Milhollen MA, Traore T, Adams-Duffy J, Thomas MP, Berger AJ, Dang L, Dick LR, Garnsey JJ, Koenig E, Langston SP, et al. 2010. MLN4924, a NEDD8-activating enzyme inhibitor, is active in diffuse large B-cell lymphoma models: rationale for treatment of NF-κB-dependent lymphoma. Blood 116: 1515–1523. 10.1182/blood-2010-03-272567 [DOI] [PubMed] [Google Scholar]
- Molyneux EM, Rochford R, Griffin B, Newton R, Jackson G, Menon G, Harrison CJ, Israels T, Bailey S. 2012. Burkitt's lymphoma. Lancet 379: 1234–1244. 10.1016/S0140-6736(11)61177-X [DOI] [PubMed] [Google Scholar]
- Monti S, Savage KJ, Kutok JL, Feuerhake F, Kurtin P, Mihm M, Wu B, Pasqualucci L, Neuberg D, Aguiar RC, et al. 2005. Molecular profiling of diffuse large B-cell lymphoma identifies robust subtypes including one characterized by host inflammatory response. Blood 105: 1851–1861. 10.1182/blood-2004-07-2947 [DOI] [PubMed] [Google Scholar]
- Morin RD, Johnson NA, Severson TM, Mungall AJ, An J, Goya R, Paul JE, Boyle M, Woolcock BW, Kuchenbauer F, et al. 2010. Somatic mutations altering EZH2 (Tyr641) in follicular diffuse large B-cell lymphomas of germinal-center origin. Nat Genet 42: 181–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin RD, Mungall K, Pleasance E, Mungall AJ, Goya R, Huff RD, Scott DW, Ding J, Roth A, Chiu R, et al. 2013. Mutational and structural analysis of diffuse large B-cell lymphoma using whole-genome sequencing. Blood 122: 1256–1265. 10.1182/blood-2013-02-483727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortaz E, Tabarsi P, Mansouri D, Khosravi A, Garssen J, Velayati A, Adcock IM. 2016. Cancers related to immunodeficiencies: update and perspectives. Front Immunol 7: 365. 10.3389/fimmu.2016.00365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle SJ, Reshef R, Tsai DE. 2017. Posttransplant lymphoproliferative disorder in solid organ and hematopoietic stem cell transplantation. Clin Chest Med 38: 771–783. 10.1016/j.ccm.2017.08.001 [DOI] [PubMed] [Google Scholar]
- Natkunam Y, Farinha P, Hsi ED, Hans CP, Tibshirani R, Sehn LH, Connors JM, Gratzinger D, Rosado M, Zhao S. 2008. LMO2 protein expression predicts survival in patients with diffuse large B-cell lymphoma treated with anthracycline-based chemotherapy with and without rituximab. J Clin Oncol 26: 447–454. 10.1200/JCO.2007.13.0690 [DOI] [PubMed] [Google Scholar]
- Okosun J, Bödör C, Wang J, Araf S, Yang CY, Pan C, Boller S, Cittaro D, Bozek M, Iqbal S, et al. 2014. Integrated genomic analysis identifies recurrent mutations and evolution patterns driving the initiation and progression of follicular lymphoma. Nat Genet 46: 176–181. 10.1038/ng.2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang B, Qiao X, Janssen L, Velds A, Groothuis T, Kerkhoven R, Nieuwland M, Ovaa H, Rottenberg S, van Tellingen O, et al. 2013. Drug-induced histone eviction from open chromatin contributes to the chemotherapeutic effects of doxorubicin. Nat Commun 4: 1908. 10.1038/ncomms2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, Potter NE, Heuser M, Thol F, Bolli N. 2016. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med 374: 2209–2221. 10.1056/NEJMoa1516192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham-Ledard A, Prochazkova-Carlotti M, Deveza M, Laforet MP, Beylot-Barry M, Vergier B, Parrens M, Feuillard J, Merlio JP, Gachard N. 2017. Molecular analysis of immunoglobulin variable genes supports a germinal center experienced normal counterpart in primary cutaneous diffuse large B-cell lymphoma, leg-type. J Dermatol Sci 88: 238–246. 10.1016/j.jdermsci.2017.07.008 [DOI] [PubMed] [Google Scholar]
- Pommier Y, Leo E, Zhang H, Marchand C. 2010. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol 17: 421–433. 10.1016/j.chembiol.2010.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psyrri A, Papageorgiou S, Liakata E, Scorilas A, Rontogianni D, Kontos CK, Argyriou P, Pectasides D, Harhalakis N, Pappa V, et al. 2009. Phosphatidylinositol 3′-kinase catalytic subunit α gene amplification contributes to the pathogenesis of mantle cell lymphoma. Clin Cancer Res 15: 5724–5732. 10.1158/1078-0432.CCR-08-3215 [DOI] [PubMed] [Google Scholar]
- Puente XS, Pinyol M, Quesada V, Conde L, Ordóñez GR, Villamor N, Escaramis G, Jares P, Beà S, González-Díaz M, et al. 2011. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature 475: 101–105. 10.1038/nature10113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan PT, Zhang Z, McCourt L, Dwyer M, Benkovic SJ, Hammes GG. 2002. Interaction of dihydrofolate reductase with methotrexate: ensemble and single-molecule kinetics. Proc Natl Acad Sci 99: 13481–13486. 10.1073/pnas.172501499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy A, Zhang J, Davis NS, Moffitt AB, Love CL, Waldrop A, Leppa S, Pasanen A, Meriranta L, Karjalainen-Lindsberg ML, et al. 2017. Genetic and functional drivers of diffuse large B cell lymphoma. Cell 171: 481–494.e15. 10.1016/j.cell.2017.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter J, Schlesner M, Hoffmann S, Kreuz M, Leich E, Burkhardt B, Rosolowski M, Ammerpohl O, Wagener R, Bernhart SH, et al. 2012. Recurrent mutation of the ID3 gene in Burkitt lymphoma identified by integrated genome, exome and transcriptome sequencing. Nat Genet 44: 1316–1320. 10.1038/ng.2469 [DOI] [PubMed] [Google Scholar]
- Rimokh R, Berger F, Bastard C, Klein B, French M, Archimbaud E, Rouault JP, Santa Lucia B, Duret L, Vuillaume M, et al. 1994. Rearrangement of CCND1 (BCL1/PRAD1) 3′ untranslated region in mantle-cell lymphomas and t(11q13)-associated leukemias. Blood 83: 3689–3696. 10.1182/blood.V83.12.3689.3689 [DOI] [PubMed] [Google Scholar]
- Rimsza LM, Roberts RA, Miller TP, Unger JM, LeBlanc M, Braziel RM, Weisenberger DD, Chan WC, Muller-Hermelink HK, Jaffe ES. 2004. Loss of MHC class II gene and protein expression in diffuse large B-cell lymphoma is related to decreased tumor immunosurveillance and poor patient survival regardless of other prognostic factors: A follow-up study from the Leukemia and Lymphoma Molecular Profiling Project. Blood 103: 4251–4258. 10.1182/blood-2003-07-2365 [DOI] [PubMed] [Google Scholar]
- Robb-Smith AH. 1982. U.S. National Cancer Institute working formulation of non-Hodgkin's lymphomas for clinical use. Lancet 320: 432–434. 10.1016/S0140-6736(82)90454-8 [DOI] [PubMed] [Google Scholar]
- Rubinstein PG, Aboulafia DM, Zloza A. 2014. Malignancies in HIV/AIDS: from epidemiology to therapeutic challenges. AIDS 28: 453–465. 10.1097/QAD.0000000000000071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz R, Young RM, Ceribelli M, Jhavar S, Xiao W, Zhang M, Wright G, Shaffer AL, Hodson DJ, Buras E, et al. 2012. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature 490: 116–120. 10.1038/nature11378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz R, Wright GW, Huang DW, Johnson CA, Phelan JD, Wang JQ, Roulland S, Kasbekar M, Young RM, Shaffer AL, et al. 2018. Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med 378: 1396–1407. 10.1056/NEJMoa1801445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster SJ, Bartlett NL, Assouline S, Yoon SS, Bosch F, Sehn LH, Cheah CY, Shadman M, Gregory GP, Ku M, et al. 2019. Mosunetuzumab induces complete remissions in poor prognosis non-Hodgkin lymphoma patients, including those who are resistant to or relapsing after chimeric antigen receptor T-cell (CAR-T) therapies, is active in treatment through multiple lines. Blood 134: 6.31273004 [Google Scholar]
- Seaberg EC, Wiley D, Martínez-Maza O, Chmiel JS, Kingsley L, Tang Y, Margolick JB, Jacobson LP. 2010. Cancer incidence in the multicenter AIDS Cohort Study before and during the HAART era: 1984 to 2007. Cancer 116: 5507–5516. 10.1002/cncr.25530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seda V, Mraz M. 2015. B-cell receptor signalling and its crosstalk with other pathways in normal and malignant cells. Eur J Haematol 94: 193–205. 10.1111/ejh.12427 [DOI] [PubMed] [Google Scholar]
- Staudt LM. 2010. Oncogenic activation of NF-κB. Cold Spring Harb Perspect Biol 2: a000109. 10.1101/cshperspect.a000109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streubel B, Vinatzer U, Lamprecht A, Raderer M, Chott A. 2005. T(3;14)(p14.1;q32) involving IGH and FOXP1 is a novel recurrent chromosomal aberration in MALT lymphoma. Leukemia 19: 652–658. 10.1038/sj.leu.2403644 [DOI] [PubMed] [Google Scholar]
- Sun LL, Ellerman D, Mathieu M, Hristopoulos M, Chen X, Li Y, Yan X, Clark R, Reyes A, Stefanich E, et al. 2015. Anti-CD20/CD3 T cell–dependent bispecific antibody for the treatment of B cell malignancies. Sci Transl Med 7: 287ra270. [DOI] [PubMed] [Google Scholar]
- Swerdlow SHCE, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, ed. 2017. WHO classification of tumours of haematopoietic and lymphoid tissues, revised 4th ed. International Agency for Research on Cancer, Lyon. [Google Scholar]
- Takata K, Miyata-Takata T, Sato Y, Yoshino T. 2014. Pathology of follicular lymphoma. J Clin Exp Hematop 54: 3–9. 10.3960/jslrt.54.3 [DOI] [PubMed] [Google Scholar]
- Taub R, Kirsch I, Morton C, Lenoir G, Swan D, Tronick S, Aaronson S, Leder P. 1982. Translocation of the c-myc gene into the immunoglobulin heavy chain locus in human Burkitt lymphoma and murine plasmacytoma cells. Proc Natl Acad Sci 79: 7837–7841. 10.1073/pnas.79.24.7837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treon SP, Xu L, Yang G, Zhou Y, Liu X, Cao Y, Sheehy P, Manning RJ, Patterson CJ, Tripsas C, et al. 2012. MYD88 L265P somatic mutation in Waldenström's macroglobulinemia. N Engl J Med 367: 826–833. 10.1056/NEJMoa1200710 [DOI] [PubMed] [Google Scholar]
- Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R. 2013. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153: 910–918. 10.1016/j.cell.2013.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner GJ. 2010. Rituximab: mechanism of action. Semin Hematol 47: 115–123. 10.1053/j.seminhematol.2010.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widhopf GF II, Kipps TJ. 2001. Normal B cells express 51p1-encoded Ig heavy chains that are distinct from those expressed by chronic lymphocytic leukemia B cells. J Immunol 166: 95–102. 10.4049/jimmunol.166.1.95 [DOI] [PubMed] [Google Scholar]
- Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, Ralfkiaer E, Chimenti S, Diaz-Perez JL, Duncan LM, et al. 2005. WHO-EORTC classification for cutaneous lymphomas. Blood 105: 3768–3785. 10.1182/blood-2004-09-3502 [DOI] [PubMed] [Google Scholar]
- Wright G, Tan B, Rosenwald A, Hurt EH, Wiestner A, Staudt LM. 2003. A gene expression-based method to diagnose clinically distinct subgroups of diffuse large B cell lymphoma. Proc Natl Acad Sci 100: 9991–9996. 10.1073/pnas.1732008100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahiaoui OI, Nunès JA, Castanier C, Devillier R, Broussais F, Fabre AJ, Naimi D, Bouabdallah R, Olive D, Xerri L. 2014. Constitutive AKT activation in follicular lymphoma. BMC Cancer 14: 565. 10.1186/1471-2407-14-565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Wang W, Huang H. 2019. Efficacy safety of bispecific T-cell engager (BiTE) antibody blinatumomab for the treatment of relapsed/refractory acute lymphoblastic leukemia and non-Hodgkin's lymphoma: a systemic review and meta-analysis. Hematology 24: 199–207. [DOI] [PubMed] [Google Scholar]
- Zafra MP, Schatoff EM, Katti A, Foronda M, Breinig M, Schweitzer AY, Simon A, Han T, Goswami S, Montgomery E, et al. 2018. Optimized base editors enable efficient editing in cells, organoids and mice. Nat Biotechnol 36: 888–893. 10.1038/nbt.4194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Grubor V, Love CL, Banerjee A, Richards KL, Mieczkowski PA, Dunphy C, Choi W, Au WY, Srivastava G, et al. 2013. Genetic heterogeneity of diffuse large B-cell lymphoma. Proc Natl Acad Sci 110: 1398–1403. 10.1073/pnas.1205299110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Jima D, Moffitt AB, Liu Q, Czader M, Hsi ED, Fedoriw Y, Dunphy CH, Richards KL, Gill JI, et al. 2014. The genomic landscape of mantle cell lymphoma is related to the epigenetically determined chromatin state of normal B cells. Blood 123: 2988–2996. 10.1182/blood-2013-07-517177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Nomie K, Zhang H, Bell T, Pham L, Kadri S, Segal J, Li S, Zhou S, Santos D, et al. 2017. B-cell lymphoma patient-derived xenograft models enable drug discovery and are a platform for personalized therapy. Clin Cancer Res 23: 4212–4223. 10.1158/1078-0432.CCR-16-2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucca E, Bertoni F. 2004. MALT lymphomas. Landes Bioscience, Georgetown, TX. [Google Scholar]