Abstract
Observations of the incidence of tumors among chimney sweeps in the eighteenth century and later experiments with coal tars provided early evidence that carcinogens in the environment can promote cancer. Subsequent studies of individuals exposed to radiation, work on fly genetics, and the discovery that DNA was the genetic material led to the idea that these carcinogens act by inducing mutations in DNA that change the behavior of cells and ultimately cause cancer. In this excerpt from his forthcoming book, Joe Lipsick looks back at how the concepts of mutagenesis and carcinogenesis emerged, how these converged with development of the Ames test, and how biochemistry and crystallography ultimately revealed the underlying molecular basis.
AN UNCLEAN SWEEP
Despite all the chim chim-in-ey, chim chim-in-ey, chim chim cheeriness of Dick Van Dyke in the film Mary Poppins, it turns out that chimney sweeps were not as lucky as lucky can be. The poet William Blake was far closer to the mark than Disney's songwriters (Fig. 1). Indeed, it was only by a twist of plot that Oliver Twist, another boy who almost became a chimney sweep, escaped such a far more dismal fate than that eventually chronicled by Charles Dickens.
Figure 1.
On the plight of eighteenth-century English chimney sweeps. From William Blake's “The Chimney Sweeper,” in Songs of Experience, 1794.
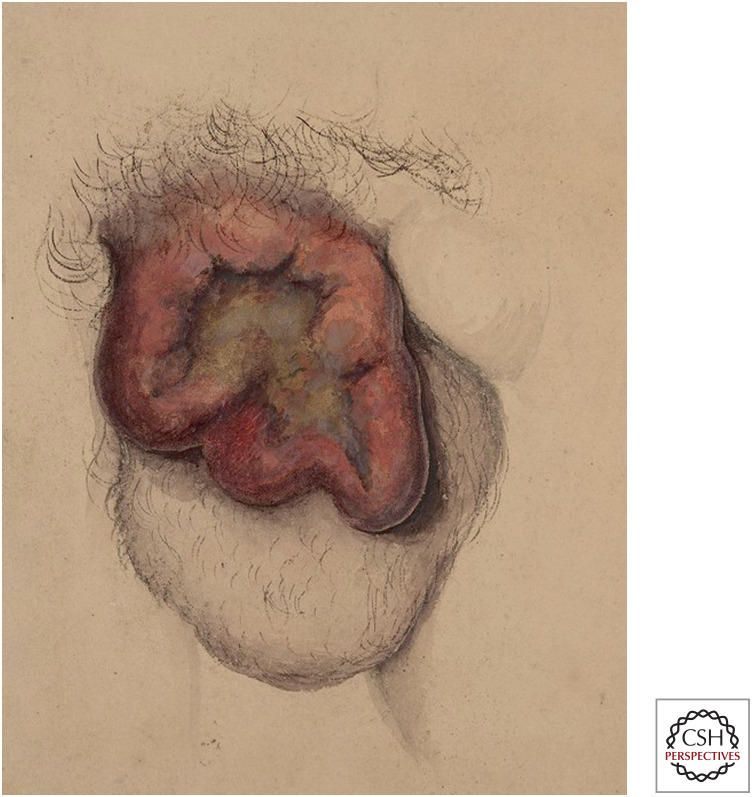
The hero of our own tale is Percival Pott, an English surgeon. In 1775, he reported that cancer of the scrotum occurred in chimney sweeps in London, but rarely if ever in anyone else (Fig. 2). This strong correlation between a rare disease and a relatively rare occupation suggested a causal relationship. Based on the anatomic location and course of the disease, Pott proposed that the causative agent was chimney soot that remained lodged in the furrowed folds of scrotal skin:
Figure 2.
Cancer of the scrotum, illustrated by Horace Benge Dobell in 1848. (Reproduced from https:// wellcomecollection.org/works/njqbesup.)
The fate of these people seems singularly hard; in their early infancy, they are most frequently treated with great brutality, and almost starved with cold and hunger; they are thrust up narrow, and sometime hot chimneys, where they are bruised, burned, and almost suffocated; and when they get to puberty, become peculiarly liable to a noisome, painful and fatal disease… the cancer of the scrotum and testicles. The disease, in these people, seems to derive its origin from a lodgment of soot in the rugae of the scrotum.—Percival Pott, 1775
Pott's observation was the first (but hardly the last) example of an occupational cancer, one caused by specific agents in the workplace. Because scrotal cancer was thought to develop in childhood, a law was enacted in 1788 prohibiting boys under the age of 8 from working as chimney sweeps. However, attempts to replace small boys at risk in chimneys with mechanical devices were defeated by the efforts of the Master Sweeps and insurance industry, the latter fearing a repeat of the Great Fire of London. Indeed, it was this catastrophic blaze that had resulted in the practice of constructing the long and tortuous chimneys, which in turn led to the use of climbing boys as chimney sweeps. In 1844, a law was finally passed prohibiting anyone under the age of 21 from sweeping chimneys. Nevertheless, this law was reportedly not enforced until 1875, exactly 100 years after Pott's initial report.
It was widely believed that the incidence of the “chimney sweeps’ cancer” declined rapidly after 1875, because the disease was thought to require extensive exposure to soot prior to puberty. However, a careful survey of actual hospital case records by Henry Butlin in 1892 strongly contradicted this prevalent view.
There has seldom been seen a more curious illustration of the steady growth and progress of a false impression than this in relation to the decline of chimney-sweeps’ cancer. One is irresistibly reminded by it of the growth of a slander or of that entertaining winter evening game in which the players sit in a long row and the first tells in a whisper to the second some short tale or anecdote. The second repeats it to the third, still in a whisper, and third to the fourth, and so on through the row, until the last player tells it aloud to the infinite amusement of the whole, who can scarcely recognise in the garbled version the story as it left them each in turn.—Butlin, 1892
Butlin's observations provide an early example of the importance of quantitative studies of cancer incidence and cancer deaths, as opposed to anecdotal evidence.
A puzzle arose regarding the chimney sweeps’ cancer—why England? Although chimney sweeps plied their trade all across Europe and in the United States, cancer of the scrotum was very rare outside of England. Various hypotheses were proposed to account for this difference: unusually narrow and tortuous English chimneys, local differences in the composition of coal, or the lack of protective clothing. However, the most likely explanation was the differing practice of personal hygiene in England. Richard Doll has noted that the medical records of a 25-year-old sweep treated for scrotal cancer in 1848 included the following statement: “He says he has worked in soot since boyhood and that when young he was never washed for 5 or 6 years at a time.” Doll further noted that although the average age of death from scrotal cancer had risen to 60 years by the 1920s, the annual mortality from this disease in chimney sweeps was still remarkably high—350 times higher in chimney sweeps than in agricultural workers. Pott's son-in-law James Earle later noted an unusually aggressive skin cancer on the hand of an English gardener. Because chimney soot appeared to cause scrotal cancer and because chimney soot was sometimes spread by gardeners to kill snails, Earle reasoned that the same agent might cause both types of these occupational cancers. However, at least one twentieth-century scholar has argued that exposure to ultraviolet light may have been responsible for this “soot cancer” of the hand.
COAL TAR—FROM OBSERVATION TO EXPERIMENT
Although chimney sweeps’ cancer was not often seen outside of England, cancers of the scrotum and other skin cancers did occur among workers in the coal distillation industry in Germany. In the late 1800s, Richard von Volkmann and others reported a correlation between exposure to coal tars and mineral oils and the development of these cancers. These reports stimulated attempts by experimental pathologists to induce cancer in animals with coal tars and mineral oils.
In the late nineteenth century there were several popular theories about the origin of cancer. Rudolph Virchow and his followers held that irritation or injury of a particular tissue was a direct cause of cancer. Julius Connheim proposed that cancers arose from remnants of embryonic tissue that are left behind during development and retain the capacity to proliferate. The tremendous success of Louis Pasteur, Robert Koch, and others in showing that infectious agents cause many diseases, including pneumonia, anthrax, and rabies, led to proposals that cancer might be an infectious disease.
The finding of parasitic worms in some human tumors led to the hypothesis that such worms might be the direct cause of some cancers. In 1913, Johannes Fibiger reported that he could cause cancers of the stomach and esophagus in rats by introducing a nematode worm, called Spiroptera carcinoma, found in cockroaches contaminating the animals’ food. Fibiger had studied with Robert Koch and was Professor and Director of the Institute of Anatomic Pathology at the University of Copenhagen. His work provided the first widely accepted evidence of an experimentally induced cancer. Fibiger was awarded the Nobel Prize for his discovery in 1926. However, others were unable to repeat his work. It eventually became clear that the worms were neither necessary nor sufficient to cause cancer. Rather, the changes Figiber had observed in the stomach and esophagus were not in fact cancers but metaplasia (the replacement of one type of epithelium with another, often in response to chronic inflammation).
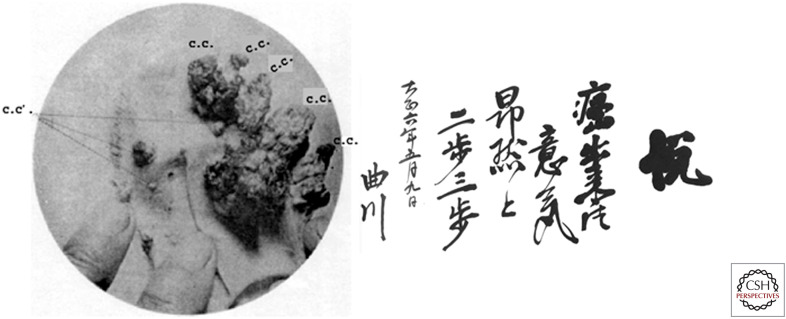
Meanwhile in Japan, Katsusabura Yamagiwa and Koichi Ichikawa were busy applying coal tar extracts to the ears of rabbits in an attempt to induce cancer. Yamagiwa had trained with Virchow in Germany and was himself a firm believer in Virchow's hypothesis that irritation was the major cause of cancer. Given that coal tar was the likely cause of cancer in chimney sweeps in England and in coal distillation workers in Germany, it seemed likely that coal tar might be just such a carcinogenic irritant. Many others had tried and failed to induce cancer in various animal species over the previous 50 years. However, Yamagiwa and Ichikawa were incredibly persistent in painting the inner ears of rabbits with coal tar every single day over the course of four long years. They eventually produced 16 carcinomas in 10 of these rabbits. Their findings were published in Japan in 1915, and then in the United States in 1918 (Fig. 3).
Figure 3.
(Left) A rabbit's ear with cancers induced by coal tar. (Right) Yamagiwa's haiku: “Cancer was produced! Proudly I walk a few steps.” (Left, Image from Fujiki H 2014. Cancer Science 105: 143–149; originally from Yamagiwa K, Ichikawa K. 1915. Mitteilungen of Medical Faculty of Imperial University of Tokyo 15: 295–344; right, from Shimkin MB 1977. “Contrary to Nature,” HHS Publication No. (NIH) 76-720, U.S. Department of Health, Education and Welfare, Washington, DC.)
Yamagiwa and Ichikawa had shown that coal tar could directly cause cancer at the site of repeated application. However, once a cancer formed, the application of coal tar was not required for further growth of the tumor. Importantly, Yamagiwa and Ichikawa were able to describe for the first time the progression of cancer—from benign proliferation, to carcinoma in situ, to invasive cancer, to metastasis. Being a poet as well as a scientist, Yamagiwa composed a haiku to celebrate his success.
Unfortunately, because Fibiger had erroneously beaten him to the punch in describing the first experimentally induced cancer, Yamagiwa was never awarded a Nobel Prize for his own discovery. Folke Hensche, a Swedish scientist who had argued in favor of awarding the Nobel Prize to Fibiger, would later cite a quotation from the Belgian pathologist Albert Dustin when lauding the groundbreaking work of Yamagiwa:
The man who solves the enigma of cancer does not need a Nobel Prize.
COOKING WITH GAS
Yamagiwa's experiments demonstrated unequivocally that coal tar could directly cause cancer. However, it remained unclear whether coal tar was just one of many irritants capable of causing cancer, or whether it contained a specific substance required for carcinogenesis. Butlin held that, “The very nature of soot renders it highly improbable that it contains within itself a cancerous element, even in suspension.” However, many investigators had tried and failed to induce cancer in animals with a variety of very irritating substances. Their experimental animals often displayed local inflammation, hyperplasia, and/or metaplasia, but not invasive cancer. These results indicated that there was indeed something special about coal tar, although the substance itself was chemically complex and variable.
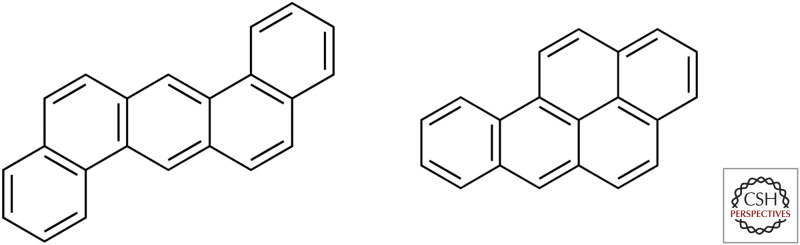
Bruno Block, a Swiss dermatologist, reported in 1921 that the carcinogenic component of coal tar had specific chemical properties. It had a high boiling point, could be extracted neither by acid nor by alkali, and contained neither nitrogen nor sulfur. Ernest Kennaway and his colleagues in London subsequently performed a series of careful experiments that allowed them to synthesize the first chemically pure carcinogen. They found that carcinogenic products could be obtained simply by heating simple hydrocarbons (e.g., isoprene or acetylene) to very high temperatures in a hydrogen atmosphere. This could also occur at much lower temperatures by starting with double-ringed naphthalene compounds and aluminum catalysts. The products of both types of reaction were highly fluorescent, and this property co-purified with their carcinogenic properties. The spectra of Kennaway's synthetic carcinogens were remarkably similar to those previously described for the four-ringed benzanthracene. Trials of different derivatives of benzanthracene demonstrated that several of these chemicals could indeed cause cancer in mice. In particular, a very highly purified sample of a related synthetic chemical, 1,2:5,6-dibenzanthracene, proved to be a particularly potent carcinogen (Fig. 4).
Figure 4.
Two polycyclic aromatic hydrocarbon carcinogens: 1,2:5,6-dibenzanthracene (left) and benzo[a]pyrene (right).
The isolation of the carcinogenic agent in coal tar required a cooperative effort between science and industry. Two tons of coal tar pitch were distilled at the Becton works of the Gas Light and Coke Company for this purpose in 1930. Using fluorescence as an assay, seven grams of a highly potent carcinogen were purified, a nearly one million-fold enrichment from the starting material. The active component was eventually identified by James Cook as benzo[a]pyrene (Fig. 4). Chemical synthesis of this compound confirmed both its identity and carcinogenicity. As a result of this work, chemically defined polycyclic aromatic hydrocarbons became the agents of choice for studies of carcinogenesis.
THE X FILES—RADIATION CAUSES CANCER
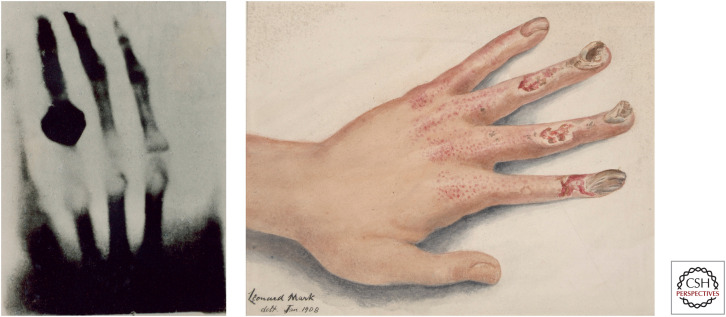
In 1895 Wilhem Roentgen, a German physicist, discovered X-rays while investigating the properties of vacuum tubes. He soon discovered that these rays could penetrate human soft tissues, but not bones (Fig. 5). Soon, physicians were using X-rays to examine broken bones and to search for metal bullets in soft tissues. X-rays became a widely used tool, from industrial analysis to dental diagnosis.
Figure 5.
(Left) Early X-ray of the hand of Wilhelm Roentgen's wife, Anna Berthe. (Right) Drawing of X-ray-induced damage to the hand. (Reproduced from Leonard Mark, 1908, from Saint Bartholomew's Hospital Museum and Archives; http://www.calmhosting01.com/BartsHealth/CalmView/Record.aspx?src=CalmView.Catalog&id=%2f35%2f22.)
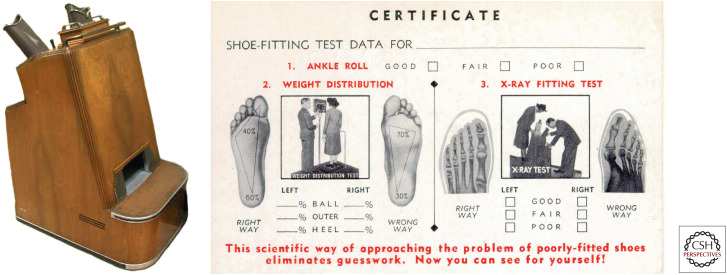
Like many technological advances, X-rays proved to be a double-edged sword. As early as 1902, physicians began to notice an increase in dermatitis and skin cancer among roentgenologists (radiologists). This occurred because they tested for X-ray production by seeing if the skin of their hand reddened when place in the path of the beam. These case reports prompted Pierre Edouard Jean Clunet to test whether X-rays alone could cause cancer in an experimental animal. In 1908 he irradiated four rats, repeating the treatment as soon as the radiation-induced ulcerations had healed. After four such cycles over a period of 14 months, an invasive cancer developed within the irradiated field in the two surviving animals. Although this experimental induction of cancer preceded those reported by Fibiger (erroneously) and by Yamagiwa, Clunet viewed his own accomplishment as a verification of a previous clinical observation rather than as a major scientific breakthrough. Despite Clunet's observations, the use of X-rays remained quite popular in a variety of settings of dubious merit, including the treatment of acne and the pedoscope, a fluoroscopic device designed to help in the fitting of shoes (Fig. 6). These devices were a standard attraction in many shoe stores in the United States from the 1920s until the 1960s. More recently questions have arisen about the risk versus benefit of various new high-energy computed tomography (CT) scans, particularly given the financial incentives involved.
Figure 6.
(Left) The pedoscope, a fixture in American shoe stores from the 1920s to the1960s. (Right) If the shoe fits, wear it. (Reprinted, with permission, from Oak Ridge Associated Universities [http://www.orau.org/ptp/collection/shoefittingfluor/shoe.htm], © ORAU.)
Because X-rays cause ulceration of the skin, one can imagine that proponents of the irritation theory of cancer would have viewed Clunet's observations as a confirmation of their hypothesis. However, a groundbreaking series of experiments in the 1920s suggested otherwise. The most successful experimental geneticists in the early twentieth century focused intensively on two model organisms—maize (Zea mays) and the fruit fly (Drosophila melanogaster). In both systems, the identification of new mutants resulted from random genetic variation coupled with keen observation. This was essentially the same method used by Gregor Mendel in his landmark studies of inheritance in the garden pea.
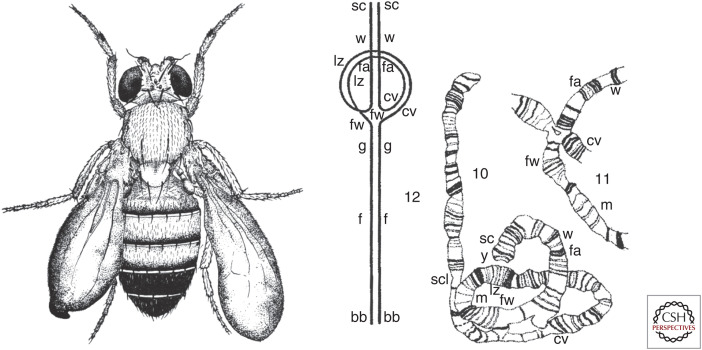
Hermann Muller had trained as a student in Thomas Morgan's fly group at Columbia University, but eventually became their fierce scientific competitor. At the University of Texas, Muller began to study the effects of radiation (using radium and X-rays) on mutation rates. High doses of X-rays caused sterility, but Muller found that lower doses permitted fertility while causing recessive lethal mutations. Eventually he was able to generate a remarkable array of X-ray-induced mutant phenotypes, many of them similar to those previously discovered via the laborious isolation of spontaneous mutants. Careful genetic analysis coupled with the cytologic staining of giant salivary gland polytene chromosomes, a method developed by Theophilus Painter, revealed that many of these X-ray-induced mutants contained gross chromosomal rearrangements (inversions, deletions, duplications, translocations, rings) (Fig. 7).
Figure 7.
(Left) Bloated wings caused by the X-ray-induced delta-49 inversion. (Right) Schematic and camera lucida drawings of paired X chromosomes from the larval salivary gland of a Drosophila that was heterozygous for the X chromosome with the delta-49 inversion. (Left, Reprinted from Muller HJ. 1930. J Genet 20: 299–334; right, reprinted from Painter TS. 1934. Genetics 19: 448–469.)
In the course of characterizing his new mutants, Muller discovered a number of important biological phenomena—gene dosage effects, dosage compensation for hemizygous genes on the male X chromosome, the presence of specialized telomeres at the ends of linear chromosomes, and epigenetic control of gene expression by position-effect variegation. Most importantly, X-rays provided a new tool for inducing mutations at will, rather than having to depend on rare spontaneous mutations.
Muller was a believer in eugenics and advocated artificial insemination, using sperm only 25 years after a donor's death to avoid passing on genetic defects that might become apparent late in life or in one's offspring. In the 1930s, he became disillusioned with racism and what he considered to be capitalist exploitation in the United States. He moved to Germany and then to Russia. However, the harsh reality of Stalinism and the destruction of Russian genetics by Lysenko caused him to flee in 1937. Because of his political views and perhaps because of his personality, Muller had great difficulty finding an academic position upon his return to the United States. After a stint as a lecturer at Amherst College, he was finally hired as a faculty member at Indiana University in 1945 at the age of 53. The following year he was awarded the Nobel Prize “for the discovery of the production of mutations by means of X-ray irradiation” that he had first published in 1927.
Oswald Avery and colleagues in 1944 showed that DNA was the genetic material, because it could transform the genotype of one bacterial strain into another. Additional support for this conclusion came from the “Waring blender” experiment performed by Alfred Hershey and Martha Chase in 1952, in which they showed that DNA rather than protein was the critical genetic component transferred during infection of bacteria by bacteriophage. The ensuing race to discover the structure of DNA was won by James Watson and Francis Crick, who postulated a double-helical structure. These results led to the conclusion that the X-ray-induced chromosomal rearrangements first seen by Muller were the results of double-stranded breaks in DNA.
Following the devastation caused by atomic bombs at Hiroshima and Nagasaki, Muller became a strong advocate for the control of nuclear weapons and for an end to open-air testing of atomic bombs. An increased incidence of cancer was seen in survivors of atomic bomb blasts in Japan, in American observers of atomic bomb tests, and eventually in American workers who had purified uranium for bomb production. Not surprisingly, the cancer cells of those irradiated individuals often showed chromosomal rearrangements.
In 1951, Muller proposed that cancer arose via multiple mutations in a single somatic cell, thus accounting for the long latency often seen between the initial exposure to a carcinogen and the appearance of cancer. In 1968, James Cleaver discovered that patients with an inherited predisposition to skin cancer (xeroderma pigmentosum) were unable to effectively repair DNA damaged by ultraviolet radiation, which was consistent with Muller's hypothesis.
PHYSICAL AND CHEMICAL: MOST CARCINOGENS ARE MUTAGENS
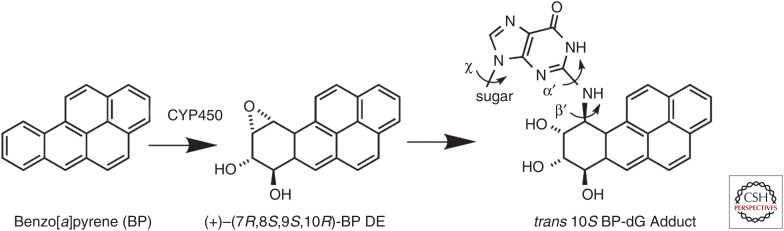
Studies of radiation in the early twentieth century led to the following line of reasoning: X-rays were carcinogens (Clunet); X-rays were mutagens (Muller); therefore, carcinogens were mutagens. It would, therefore, not have been a large leap of faith to think that chemical carcinogens might also act as mutagens. However, an influential review by Burdette in 1955 claimed the opposite. One of the major arguments was the failure of some very potent chemical carcinogens, such as benzo[a]pyrene, to act as mutagens in test systems, particularly in well-studied microorganisms. James and Elizabeth Miller in 1960 provided the key to this puzzle. They found that chemically active derivatives of benzopyrene were formed within the tissues of carcinogen-treated rats, and that inhibition of the metabolic activation of these carcinogens prevented formation of cancer (Fig. 8). Work by a number of laboratories then identified intracellular cytochrome P450 proteins of animals as the culprits. Normally these enzymes inactivated and detoxified various chemicals, but the same enzymes could cause the deleterious activation of other chemicals, including some carcinogens.
Figure 8.
Metabolically activated benzo[a]pyrene (BP) covalently bonds to DNA bases. (Modified, with permission, from Bauer J, et al. 2007. Proc Natl Acad Sci 104: 14905–14910, © National Academy of Sciences, USA.)
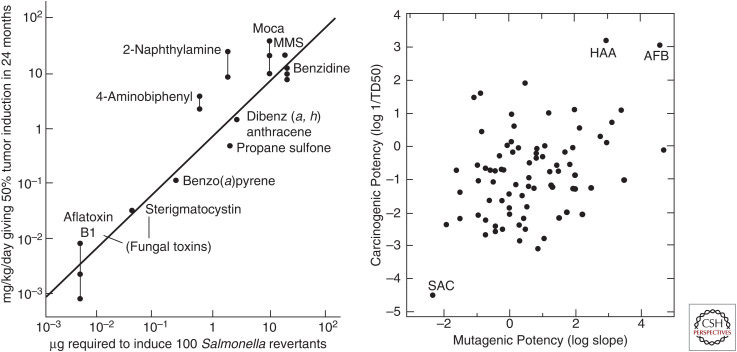
The circle was closed by Bruce Ames and coworkers, who showed in 1973 that many carcinogens were indeed mutagens. They used a simple bacterial assay in which Salmonella strains with different mutations in the histidine biosynthesis pathway were scored for genetic reversion via survival on histidine-free medium. One class of carcinogens (direct) scored positively in the assay with no further treatment. A second class of carcinogens (indirect) scored positively in this assay only after preincubation with a homogenate of rat liver containing enzymes capable of metabolic activation. This “Ames test” was rapidly adopted as a test for screening new and old chemical compounds for their mutagenic, and hence their likely carcinogenic, properties. However, some caveats emerged. First, not all known carcinogenic compounds appeared to act as mutagens (e.g., asbestos, estrogens, androgens). Second, despite initial reports of a strong correlation between mutagenesis in the Ames test and carcinogenesis in animal tests, additional work showed that this was not the case (Fig. 9). Finally, more than one-half of all compounds tested at high doses in rodents could cause cancer. Eventually both Ames and Lois Gold, who had spearheaded the development of a large database of potential carcinogens, questioned the wisdom of using either of these tests for governmental regulation of chemicals present at low concentrations in the environment.
Figure 9.
Mutagenicity versus carcinogenicity. (Left) An oft-cited early report showing a strong correlation (R = ∼0.9). (Right) A more thorough study showing a much weaker correlation (R = ∼0.3). (AFB) aflatoxin B1, (HAA) N-hydroxy-2-acetylaminofluorene, (SAC) saccharin. (Left, Reprinted from Meselson M, Russell K. 1977. In Origins of Human Cancer (ed. Hiatt H, et al.), pp. 1473–1481, © Cold Spring Harbor Laboratory Press; right, reprinted, with permission, from McCann, et al. 1988. Mutat Res 205: 183–195, © Elsevier.)
FLIPPING OUT—HOW BENZO[a]PYRENE CAUSES MUTATIONS
How did a polycyclic aromatic hydrocarbon like benzo[a]pyrene (BP) produce the mutations that ultimately cause cancer? Work in the 1950s and 1960s showed that BP could bind to proteins, RNA, and DNA. A comparison of the biochemical and biological activities of a series of related compounds indicated that binding to DNA correlated best with carcinogenesis. Careful chemical detective work in a number of laboratories in the 1970s resulted in a model in which BP was converted to a highly reactive epoxide that then became covalently bound to purine bases within DNA (Fig. 8).
Analysis of the mutations caused by metabolically activated BP revealed predominantly frameshifts (–1) and substitutions at guanine nucleotides. Peculiarly, the base 5′ to the BP-dG adduct determined the base incorporated opposite the lesion. For example, mutation of a 5′-TGC sequences results in a G=>T transversion to 5′-TTC >95% of the time.
The covalent adducts formed by BP and nucleic acid bases block the enzymatic synthesis of DNA. The high-fidelity DNA polymerases used to replicate genomic DNA generally cannot bypass bulky adducts like those formed by BP epoxides. However, the more error-prone Y-family DNA polymerases can bypass such lesions.
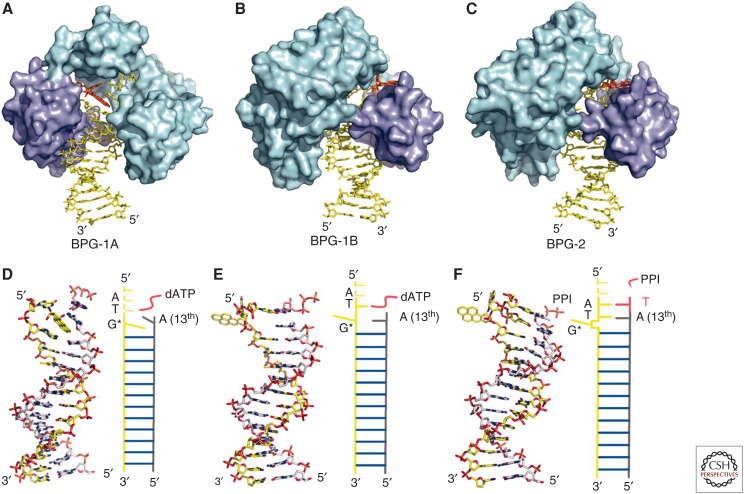
X-ray crystallography in the early twenty-first century revealed the structure of a BP-DNA adduct together with an active DNA polymerase and dATP. Three different structures were seen, presumably representing intermediates in trans-lesion DNA synthesis. In the first (Fig. 10A,D), the BP ring is stacked between two adjacent bases within the DNA double helix, thereby blocking DNA synthesis. In the second (Fig. 10B,E), the BP-dG adduct has flipped out of the DNA helix into a hydrophobic pocket within the polymerase. This permits the polymerase to add the next nucleotide to the primer strand using the base (T) adjacent to the BP-dG on the template strand to determine the next nucleotide added to the primer strand. The result is insertion of an A instead of a C. When the new strand itself is eventually replicated, this will result in the G=>T transversion typical of BP-induced mutagenesis. The ability of the Y-family polymerase to accommodate a flipped-out hydrophobic adduct appears to permit DNA synthesis to proceed beyond the BP-dG adduct. The third structure explains the –1 frameshift mutations also caused in vivo by BP (Fig. 10C,F). These crystallographic studies finally revealed in precise molecular detail how the chimney sweeps’ cancers described by Percival Pott in 1775 were caused by the soot in which they toiled.
Figure 10.
The hydrophobic BP-DNA adduct prefers to intercalate between the hydrophobic bases of the DNA double helix (A,D). However, a hydrophobic pocket peculiar to the Y-family DNA polymerases flips the BP-DNA adduct out of the double helix (B,C,E,F), thereby permitting bypass synthesis. (Reprinted, with permission, from Bauer J, et al. 2007. Proc Natl Acad Sci 104: 14905–14910, © National Academy of Sciences, USA.)
Footnotes
From the forthcoming volume Stalking the Enemy Within: A History of Cancer Research, by Joseph Lipsick
Additional Perspectives on A History of Cancer Research available at www.perspectivesinmedicine.org
SUGGESTED READING
Chimney Sweeps’ Cancer
- Brown JR, Thornton JL. 1957. Percival Pott (1714–1788) and chimney sweepers’ cancer of the scrotum. Br J Industrial Med 14: 68–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butlin HT. 1892. Three lectures on cancer of the scrotum in chimney-sweeps and others: delivered at the Royal College of Surgeons of England. J Brit Med Assoc 1: 1341–1346; July 2: 1–6; July 9: 66–71. 10.1136/bmj.1.1643.1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll R. 1975. Part III: 7th Walter Hubert lecture. Pott and the prospects for prevention. Br J Cancer 32: 263–272. 10.1038/bjc.1975.212 [DOI] [PMC free article] [PubMed] [Google Scholar]
Coal Tar–Induced Cancer
- Fujiki H. 2014. Gist of Dr. Katsusaburo Yamagiwa's papers entitled “Experimental study on the pathogenesis of epithelial tumors” (I to VI reports). Cancer Sci 105: 143–149. 10.1111/cas.12333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henschen F. 1968. Yamagiwa's tar cancer and its historical significance—from Percival Pott to Katsusaburo Yamagiwa. Gann 59: 447–451. [PubMed] [Google Scholar]
- Stolt CM, Klein G, Jansson AT. 2004. An analysis of a wrong Nobel Prize—Johannes Figiber, 1926: a study in the Nobel archives. Adv Cancer Res 92: 1–12. 10.1016/S0065-230X(04)92001-5 [DOI] [PubMed] [Google Scholar]
- Yamagiwa K, Ichikawa K. 1918. Experimental study of the pathogenesis of carcinoma. J. Cancer Res 3: 1–29. [Reprinted in CA Cancer J Clin27: 174–181 (1977).] [DOI] [PubMed] [Google Scholar]
Radiation-Induced Cancer
- Cleaver JE. 1968. Defective repair replication of DNA in xeroderma pigmentosum. Nature 218: 652–656. 10.1038/218652a0 [DOI] [PubMed] [Google Scholar]
- Muller HJ. 1927. Artificial transmutation of the gene. Science 66: 84–87. 10.1126/science.66.1699.84 [DOI] [PubMed] [Google Scholar]
- Mustacchi P, Shimkin MB. 1956. Radiation cancer and Jean Clunet. Cancer 9: 1073–1074. [DOI] [PubMed] [Google Scholar]
Chemical Carcinogens
- Ames BN, Gold LS. 2000. Paracelsus to parascience: the environmental cancer distraction. Mutation Res 447: 3–13. 10.1016/S0027-5107(99)00194-3 [DOI] [PubMed] [Google Scholar]
- Ames BN, Durston WE, Yamasaki E, Lee FD. 1973. Carcinogens are mutagens: a simple test system combining liver homogenates for activation and bacteria for detection. Proc Natl Acad Sci 70: 2281–2285. 10.1073/pnas.70.8.2281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer J, Xing G, Yagi H, Sayer JM, Jerina DM, Ling H. 2007. A structural gap in Dpo4 supports mutagenic bypass of a major benzo[a]pyrene dG adduct in DNA through template misalignment. Proc Natl Acad Sci 104: 14905–14910. 10.1073/pnas.0700717104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennaway E. 1955. The identification of a carcinogenic compound in coal-tar. Br Med J 2: 749–752. 10.1136/bmj.2.4942.749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malling HV. 2004. Incorporation of mammalian metabolism into mutagenicity testing. Mutation Res 566: 183–189. 10.1016/j.mrrev.2003.11.003 [DOI] [PubMed] [Google Scholar]
- Shear MJ. 1969. Yamagiwa's tar cancer and its historical significance—from Yamagiwa to Kennaway. Gann 60: 121–127. [PubMed] [Google Scholar]