Abstract
Although the sensitivity of reverse transcriptase-polymerase chain reaction (RT-PCR) is low in the diagnosis of coronavirus disease 2019 (COVID-19), it is the gold standard. Clinical improvement is prioritized in the follow-up of patients with COVID-19 who are followed as possible or definitive cases. Although the priority in the discharge decision is the resolution of complaints, it is also important to see radiological improvement and RT-PCR negativity. A total of 2 of our patients who were hospitalized and treated in our clinic with a diagnosis of COVID-19 were discharged after their complaints were resolved and their treatment was completed. The patients had 2 negative RT-PCR results at discharge. Both of them presented to the hospital with symptoms such as fever, cough, and shortness of breath after the discharge, and both showed positive RT-PCR results. Considering recurrent COVID-19 infection, we aimed to present treatment and the 2 cases we followed.
Keywords: Coronavirus disease 2019, false negativity, recurrent, re-infections, reverse transcriptase-polymerase chain reaction
INTRODUCTION
New-type coronavirus cases have rapidly spread all over the world. Coronavirus disease 2019 (COVID-19) affected approximately 4 million people and killed 276,000 people globally until May 9, 2020; 137,000 cases and 3,739 deaths were observed in Turkey [1].
Real-time reverse transcriptase-polymerase chain reaction (RT-PCR) is the gold standard for diagnosis of COVID-19. Virus detection rate in nasopharyngeal samples was determined as 63% and that for oropharyngeal samples was 32% [2]. Cases with repeat RT-PCR positivity have been reported in the literature [3–6].
In this study, we report 2 COVİD-19 cases with repeat RT-PCR positivity.
CASE PRESENTATIONS
Case 1
A 79-year-old man presented to the hospital on April 5, 2020, with complaints of cough, sputum, dyspnea, and a fever of 38°C. Saturation was 93% with nasal cannula with 2 L/min oxygen. There were no significant findings in his physical examination, presence of rhonchi during expiration, and sporadic rales in the bilateral lower zones. There was no history of travel or suspicious contact during the last 14 days. The patient had a history of chronic obstructive pulmonary disease and hypertension and was undergoing long-term oxygen treatment. He had a 36-pack/year smoking history and was an ex-smoker for 24 years. Nodular consolidation including ground-glass areas around the bilateral lower lobes was observed in the thoracic computed tomography (CT) (Figure 1. a, b). Table 1 presents the laboratory examination findings. The patient was hospitalized and treated with hydroxychloroquine and azithromycin after the detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the nasopharyngeal swab sample using the RT-PCR technique. After the completion of treatment and 2 RT-PCR control negativity tests, the patient was discharged from the hospital after 14 days of hospitalization with an instruction of 14-day home isolation.
Figure 1. a–d.
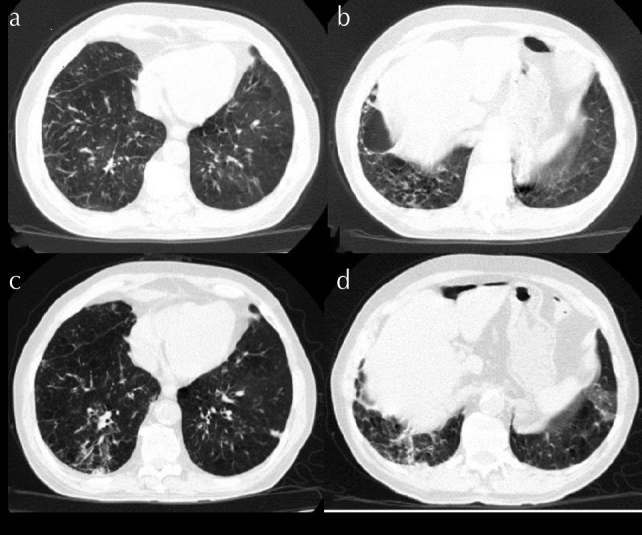
(a, b) Nodular consolidation areas including ground-glass areas around the bilateral lower lobes. (c, d) Progress observed in nodular consolidation areas on repeat computed tomography
Table 1.
Laboratory values
| Laboratory parameters | First hospitalization | Discharge | Second hospitalization | |
|---|---|---|---|---|
| Case 1 | White blood cell count K/uL | 16.41 | 9.50 | 10.71 |
| Lymphocyte count K/uL | 1.98 | 1.40 | 0.78 | |
| Neutrophil lymphocyte ratio (NLR) | 6.74 | 8.2 | 11.85 | |
| C-reactive protein mg/L | 204.8 | 60.7 | 42.04 | |
| Procalcitonin ng/mL | 0.2 | 0.122 | ||
| Ferritin ug/L | 112.98 | 340.5 | ||
| Lactate dehydrogenase U/L | 209 | 201 | ||
| D-dimer ng/mL | 420 | 196 | 353 | |
| Case 2 | White blood cell count K/uL | 4.99 | 7.77 | 14.64 |
| Lymphocyte count K/uL | 0.89 | 0.73 | 0.53 | |
| Neutrophil lymphocyte ratio (NLR) | 4.02 | 8.64 | 23.94 | |
| C-reactive protein mg/L | 19.06 | 42.95 | 96.96 | |
| Procalcitonin ng/mL | 0.051 | 0.86 | ||
| Ferritin ug/L | 290.9 | 321.9 | ||
| Lactate dehydrogenase U/L | 312 | 207 | 256 | |
| D-dimer ng/mL | 440 | 999 |
The patient revisited our hospital 1 week after discharge with complaints of cough with sputum for 3 days and fever of above 38°C for 1 day. No significant findings were observed during physical examination, except for rhonchi during expiration. The saturation of the patient was measured at 93% with nasal cannula on 2 L/min oxygen, whereas the respiratory rate was 28, and body temperature was 38°C. A repeat thoracic CT showed severe progression compared with the previous image (Figure 1. c, d). The patient reported no suspected contact and travel history after discharge. After repeated positivity in the nasopharyngeal RT-PCR and a PaO2/FiO2 ratio of 120, the patient was intubated and admitted to the intensive care unit. Oseltamivir, piperacillin-tazobactam, and favipiravir were started as treatment. Positivity was observed in the rectal RT-PCR sample on May 8, 2020. The patient has continuous acute respiratory distress syndrome as of May 20, and his follow-ups are ongoing with intubation in the prone position.
Case 2
A 77-year-old man presented to the hospital on March 26, 2020, with complaints of fever, dyspnea on exertion, and backache. It was determined upon assessment that he had a temperature of 37.3°C with an oxygen saturation of 94% via pulse oximeter on room air. The patient had no history of travel or suspicious contact during the last 14 days. It was determined that the patient had a history of diabetes mellitus, coronary artery disease, and coronary artery bypass graft along with 30-pack/year of smoking and that he was an ex-smoker for 15 years. Table 1 presents the laboratory examination. An area of ground-glass consolidation was observed in the subpleural right lower lobe on thoracic CT (Figure 2a, b). The patient was hospitalized after SARS-CoV-2 was determined using RT-PCR in the nasopharyngeal swab, and treatment was started with oseltamivir, hydroxychloroquine, and azithromycin. The patient was discharged from the hospital after his complaints were resolved with no fever and upon obtaining 2 consecutive RT-PCR negative results in 24-hour intervals after the completion of a 5-day treatment. Although there was no complete regression in radiological imaging at discharge, there was complete improvement of his complaints.
Figure 2. a–d.
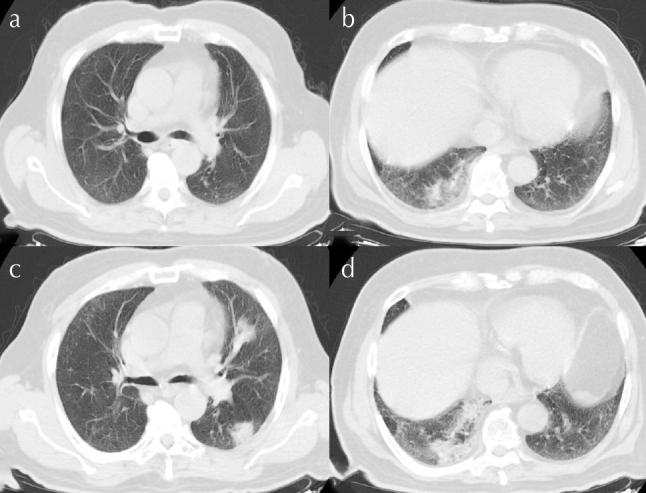
(a, b) Area of ground-glass consolidation observed in the subpleural right lower lobe. (c, d) Progress observed in the bilateral peripheral placement consolidation along with ground-glass areas
The patient was admitted to the emergency department with complaints of fever of 39°C, cough, and shortness of breath 4 days after discharge. The RT-PCR test was repeated, and the result was positive. Room air oxygen saturation was measured at 90%, and nasal oxygen support was given. When the repeat thoracic CT was compared with the previous one, there was progression in the bilateral peripheral placement consolidation along with ground-glass areas (Figure 2c, d). The patient reported no suspected contact or travel history after discharge. Favipiravir and tigecycline were started. Temperature returned to normal on the 2nd day of hospitalization after which the control RT-PCR test was observed to be negative on the 5th, 6th, and 8th days. At discharge, the room air oxygen saturation was 94%. The patient had no complaints after the completion of the 14-day home isolation and follow-up after discharge.
DISCUSSION
Repeat RT-PCR positivity was determined in 2 patients after discharge from among 107 patients followed up at our clinic between March 13 and May 9, 2020, in accordance with the diagnosis/treatment guideline [7] published by the Ministry of Health. A small number of similar cases were observed in the literature [3–6]. When these 2 patients were examined, there was evidence that some patients who have recovered from COVID-19 may have repeat infection. Reactivation of symptoms in both the patients and the absence of a suspicious contact history of the 2 patients suggest reactivation. In our cases, the time between negative and positive RT-PCR test was 4 and 7 days. This indicates that those with active disease and asymptomatic carriers, as well as healed patients, may still be carriers of the virus.
This indicates that even after the RT-PCR test becomes negative, a patient can be a carrier of the virus. This can be explained in 2 ways; the viral RNA cannot be completely eliminated with medical treatment and only viral replication is inhibited, i.e., viral load is reduced. This may be the reason that RT-PCR test was negative at discharge. The virus can continue replication after the treatment is stopped. Although this theory explains the repeat positivity, we still do not have clear information about viral cleansing after SARS-CoV-2 infection [5]. Another reason might be false negativity, and there are many studies in the literature, which indicate that the RT-PCR test has a low sensitivity despite its high specificity regarding the diagnosis of COVİD-19 infection [8]. The accuracy of RT-PCR test can be affected by many factors, such as respiratory tract viral load, sample source, sampling procedures and timing, quality control of the test, and the natural performance of the test kits [9]. The viral load in the throat samples peaks in 5–6 days, and the viral load in the sputum samples is significantly higher than that in the throat samples [10]. Hence, false negative results are frequently observed in the nasopharyngeal and oropharyngeal swab samples. Similarly, it was observed upon a literature survey that although the ratio of detecting RT-PCR samples in the sputum and bronchoscopic lung lavage is much greater than that in the nose/throat swabs because the main location of SARS-CoV-2 infection is the lower respiratory tract, it is necessary to compare the negative control RT-PCR nose/throat samples with that of bronchoscopic lavage, anorectal swabs, and feces [6].
The re-emergence of complaints and repeat RT-PCR positivity after the initial negative RT-PCR tests and complete resolution of complaints in our patients suggest reactivation. Our patients remained carriers of the virus after treatment, and the virus may have been reactivated because both the patients were older individuals with comorbidities. What renders our cases valuable is that these are the oldest patients in the literature to the best of our knowledge, and their re-infection was much more severe than other cases in the literature [3, 6]. In conclusion, it is necessary to maintain close follow-up for as long as possible owing to the possibility of recurrence of the disease, and all the patients should be quarantined at home for at least 14 days after discharge. We also recommend taking RT-PCR samples from different locations (sputum, bronchoalveolar lavage, and feces) to reduce false negativity rates.
MAIN POINTS
It would be appropriate to obtain clinical response especially before discharge from patients diagnosed with Covid 19 infection and to observe negativity in two pcr examined in different samples with 24 hours interval.
In recurrent covid 19 infection, recurrent clinical symptoms are more important than PCR positivity.
recurrent covid 19 infection may progress more severely in elderly patients.
Footnotes
Informed Consent: Written informed consent was obtained from the patients.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - E.U., N.Y, N.Ç.; Design - E.U., N.Y, N.Ç.; Supervision - E.U., N.D., S.B.Ö.; Resources - E.U., N.Y, N.Ç.; Materials - N.Y, N.Ç.; Data Collection and/or Processing - N.Y, N.Ç.; Analysis and/or Interpretation - E.U., N.Y, N.Ç.; Literature Review - E.U.; Writing Manuscript - E.U., N.Y, N.Ç.; Critical Review - N.D., S.B.Ö., İ.H.A.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.World Health Organization (WHO) Available from: http://www.who.int.
- 2.Loeffelholz MJ, Tang YW. Laboratory diagnosis of emerging human coronavirus infections - The state of the art. Emerg Microbes Infect. 2020;9:747–75. doi: 10.1080/22221751.2020.1745095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ye G, Pan Z, Pan Y, et al. Clinical characteristics of severe acute respiratory syndrome coronavirus 2 reactivation. J Infect. 2020;80:e14–e17. doi: 10.1016/j.jinf.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen D, Xu W, Lei Z, et al. Recurrence of positive SARS-CoV-2RNA in COVID-19: A case report. Int J Infect Dis. 2020;93:297–9. doi: 10.1016/j.ijid.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lan L, Xu D, Ye G, et al. Positive RT-PCR Test Results in Patients Recovered From COVID-19. JAMA. 2020;323:1502–3. doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao H, Ruan L, Liu J, et al. The clinical characteristic of eight patients of COVID-19 with positive RT-PCR test after discharge. J Med Virol. 2020 doi: 10.1002/jmv.26017. doi: 10.1002/jmv.26017. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turkey Ministry of Health. COVID-19 (SARS-CoV-2 Infection) Guide. [Access Date: 14 April 2020]. https://covid19bilgi.saglik.gov.tr.
- 8.Huang P, Liu T, Huang L, et al. Use of chest CT in combination with negative RT-PCR assay for the 2019 novel coronavirus but high clinical suspicion. Radiology. 2020;295:22–3. doi: 10.1148/radiol.2020200330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan JF, Yip CC, To KK, et al. Improved Molecular Diagnosis of COVID-19 by the Novel, Highly Sensitive and Specific COVID-19-RdRp/Hel Real-Time Reverse Transcription-PCR Assay Validated In Vitro and with Clinical Specimens. J Clin Microbiol. 2020;58:e00310–20. doi: 10.1128/JCM.00310-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan Y, Zhang D, Yang P, et al. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. 2020;20:411–2. doi: 10.1016/S1473-3099(20)30113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


