Abstract
OBJECTIVE
Bronchiectasis is characterized by chronic respiratory infection. The role of immunodeficiency in this disease is poorly studied in relation to clinical indices. The primary aim of this study was to determine the frequency of these neglected altered immune status by evaluating immunoglobulins, lymphocyte subsets, complement levels, and neutrophil function, and to assess its relationship with clinical parameters in adult patients with non-cystic fibrosis bronchiectasis (NCFB).
MATERIAL AND METHODS
A total of 74 (30 men and 44 women with a mean age of 47±17 years) adult patients with stable NCFB were enrolled in this study. The bronchiectasis severity index (BSI) and FACED (F:FEV1, A: Age, C: Chronic colonization, E: Extension, D: Dyspnea) scores were assessed. Peripheral blood samples were collected for the detection of total IgG, IgA, IgM, IgE, and IgG subclasses and C3 and C4 levels. The counts of CD3, CD4, CD8, CD19, CD16/56 expressing peripheral blood lymphocytes and neutrophil oxidative function were evaluated.
RESULTS
In the study population, BSI and FACED severity index scores increased with longer duration of the disease (p=0.01 and p=0.040, respectively). Of the 74 patients, 27 (37%) showed humoral aberrations. The number of male patients were higher in this group (p=0.03). High serum total IgE levels were associated with high scores in BSI (moderate-severe group versus mild group, p=0.030). Patients with bronchiectasis demonstrated lower CD3+ T cell count, lower CD4+ T helper cell percentage, and lower CD4+ T cell count (p=0.031, p=0.030, p=0.029, respectively) than healthy subjects. A significant negative correlation was found between the percentage and count of CD16/56+ natural killer (NK) cells and the number of exacerbations within the past year (r=−0.230, p=0.049 and r=−0.264, p=0.023, respectively).
CONCLUSION
Humoral aberrations in adult patients with NCFB were found to be frequent. IgE levels were related to high scores for disease severity indices. Furthermore, patients with low percentage and counts of NK cells had higher rates of exacerbations. These results emphasize the importance of immune function assessment in adult patients with NCFB.
Keywords: Non-cystic fibrosis bronchiectasis, primary immun disease, disease severity index
INTRODUCTION
Bronchiectasis has been defined as the abnormal dilatation of the air conducting bronchi of the lung, which is characterized by chronic respiratory tract infection and sputum production. Bronchiectasis in adults is one of the important causes of respiratory morbidity and mortality despite recent medical advances. Recently, the etiologic spectrum of these patients has changed remarkably with the new diagnostic tools, and new entities have been reported, such as adulthood cystic fibrosis, mucociliary system abnormalities, allergic bronchopulmonary aspergillosis, aspiration of irritants, common variable immune deficiency (CVID), collagen vascular diseases, inflammatory bowel disease, and various systemic diseases [1–4]. Although previous studies of immune defects in bronchiectasis have emphasized only the role of antibody deficiencies, major advances in immunology have improved our knowledge; various forms of immune deficiency have been described and categorized, such as primary immune deficiency disorders (PIDDs). Patients with PIDDs may present with recurrent infections affecting various organs, including lungs, along with organ-specific inflammation/autoimmunity and increased cancer risk [5]. Bronchiectasis itself is listed as the need for PIDD evaluation. Although reduced immunoglobulin secretion is the hallmark, there are remarkably few published data related to IgG subclass deficiencies, complement system, and neutrophil function deficiencies in patients with bronchiectasis [6–10]. In a study of Pasteur et al. [3], 27% of 150 patients with bronchiectasis were found to have immunodeficiency. King et al. [11] investigated immune system functions in 103 adult patients with bronchiectasis and found that a significant number of them had low levels of IgG3, B cell lymphocytes, and T helper lymphocytes in addition to the abnormality of the neutrophil oxidative burst. Almost half of the patients were reported to have immune deficiency disorders in that study.
These recent findings highlight the importance of immune system screening in adults with non-cystic fibrosis bronchiectasis (NCFB), whereas the diversity of PIDDs and the wide age range with different clinical occurrences may complicate the identification of these patients. The nature of the immune status in bronchiectasis and relation to the severity of the disease have not been well studied. This study aimed to determine the frequency of these neglected altered immune status by evaluating the levels of immunoglobulins, lymphocyte subsets, complement levels, and neutrophil function and to assess its relation with clinical parameters in adult patients with NCFB.
MATERIAL AND METHODS
Patients and Control Group
The research project was approved by Marmara University medical faculty clinical research ethics committee (NR 09.2015.120). Patients who met the criteria defined in this study protocol were enrolled into the study after obtaining written informed consents.
A total number of 74 adult patients who had been diagnosed for NCFB at pulmonology clinics of the xxxxx University hospital were enrolled in this cross-sectional study. The diagnosis of bronchiectasis was made on the basis of clinical history of mucopurulent sputum and radiological confirmation of high-resolution computed tomography (HRCT) scanning. A radiologist blinded to the study interpreted the HRCT scans and assessed the severity of radiologic presentation with the evaluation of each lobe. All patients had not had any exacerbation for at least 1 month. Sweat test was performed according to the international guidelines for exclusion of cystic fibrosis (CF) in all the participants. Patients with CF, malignancy, pregnancy, and already known secondary immunodeficiency were excluded from the study. Medical records were reviewed, and a follow-up form for each patient questioning their medical history, symptoms, smoking history, physical examination, medications and comorbidities, past history, duration of disease (time since diagnosis), the number of disease exacerbations and hospitalizations within the past year was filled out. Exacerbation was defined as a person with bronchiectasis with a deterioration in 3 or more of the following key symptoms for at least 48 hours; cough, sputum volume and/or consistency, sputum purulence, breathlessness and/or exercise tolerance, fatigue and/or malaise, hemoptysis, and a clinician determined that a change in bronchiectasis treatment was required [12]. All the patients underwent pulmonary function tests and carbon monoxide diffusion tests according to the American Thoracic Society criteria [13], their height and body weight were measured, and the modified medical research council (mMRC) dyspnea scales (grade 0: no dyspnea, grade 1: slight dyspnea, grade 2: moderate dyspnea: grade 3: severe dyspnea, and grade 4: very severe dyspnea) were assessed. Sputum smears and cultures were obtained from all the 74 patients during stable state. We investigated all respiratory bacteria, including mycobacteria. Bronchiectasis disease severity indices, such as bronchiectasis severity index (BSI) and FACED, were calculated. The BSI score was based on age, body mass index, FEV1 (Forced Expiratory Volume at first second) % of the predicted value, number of hospital admissions and exacerbations, mMRC scale, bacterial colonization, number of involved lobes (mild: 0–4 points, moderate: 5–8 points, severe: 9 and above points), and FACED score was based on F-FEV1% of predicted value, A-age, C-Chronic colonization of Pseudomonas aeruginosa, E-Extension-the number of involved lobes, and D-Dyspnea score as mMRC, (mild: 0–2 points, moderate: 3–4 points, severe: 5–7 points) [14, 15]. Charlson comorbidity indices weighting the clinical conditions present among secondary diagnoses (such as coronary artery disease 1 score and metastatic solid tumor 6 score, and so on) were calculated [16].
Immunological Analyses
Major clinical manifestations were screened for potential clinical features suggestive of an immunodeficiency in all patients using a questionnaire developed by the Jeffrey Modell Foundation. The questionnaire included frequencies of ear infection, bronchiectasis/pneumonia, deep skin infection, abscess, fungal infection, septicemia, need for intravenous antibiotics, and family history of primary immunodeficiency [5]. Immunologic evaluation was performed with absolute lymphocyte, neutrophil, and eosinophil counts; baseline levels of serum IgG, IgA, IgM, IgE, and IgG subclasses; C3, C4, lymphocyte subsets including T helper, T cytotoxic, B, and NK cell percentages; and absolute counts and oxidative burst capacity of neutrophils. The diagnosis of CVID, selective IgA deficiency, unclassified hypogammaglobulinemia, and IgG subclass deficiency were based on European Society of Immunodeficiencies-Pan-American Group for Immunodeficiency ESID-PAGID criteria [17]. A total of 17 healthy, age-matched donors were included as control into the study for the comparison of the lymphocyte subset analysis, including 11 women and 6 men aged between 27 and 52 (mean±standard deviation=38±8) years. None of these controls had a history of acute or chronic respiratory tract infection or primary immune deficiency warning signs.
Complete blood counts, including white cell differential counts, were determined with blood specimens using a hemocytometer (Abbot Diagnostics, USA). Total blood count analyses including hemoglobin level and number of thrombocytes were performed. Peripheral blood smears were stained with the Wright method, and differential blood counts (neutrophils, lymphocytes, and eosinophils) were checked microscopically.
Measurement of immunoglobulins, C3, C4 and α-1 antitrypsin levels
For the measurements of serum IgG, IgA, IgM, IgE, IgG subclasses; C3, C4; and α-1 antitrypsin levels, 10 cc of peripheral blood samples were collected. IgE levels were determined by solid phase enzyme-linked chemilumiscence immunometric assay (Immulite 2000, Siemens, USA). IgG, IgA, IgM, and IgG subclasses and α-1 antitrypsin levels were measured by immunonephelometry (BN Pro Spec Systems, Siemens Healthcare Diagnostics, Germany). The immunoglobulin results were compared with the normative data published by Kutukculer et al. [18]. An in vitro test was performed for the quantitative immunological determination of human complement C3 and C4 in serum obtained on a Cobas Integra 400 plus analyzer using the Cobas Integra Tina-quant Complement C3 and C4 (Roche, Mannheim, Germany). Reference values for complement and α-1 antitrypsin levels were based on our laboratory reference ranges.
Lymphocyte subsets
Lymphocyte subsets and complete blood count were performed to assess both percentages and absolute counts of T cells, B cells, and natural killer (NK) cells. Peripheral blood samples were collected from the patients into 2 mL ethylenediamine tetraacetic acid containing tubes, and 100 μL of whole blood was added to mixtures of directly conjugated fluorescent antibodies and incubated for 20 minutes in the dark at 22°C Red cells were then lysed using FACSlyse (Becton Dickinson, San Jose, CA, USA) and washed twice with CellWash (Becton Dickinson, San Jose, CA, USA) before acquisition. To determine lymphocyte subsets, the following monoclonal antibodies were used: fluorescein isothiocyanate-conjugated anti-CD3, CD4, CD45; phycoerythrin-conjugated anti-CD8, CD14, CD19, CD16/CD56; peridinin chlorophyll protein-conjugated anti-CD3; and allophycocyanin-conjugated anti-CD20. All these antibodies were obtained from BD Biosciences (San Diego, CA, USA). Analyses were performed and quadrant statistics were calculated on FACSCalibur cytometer using CellQuest software (Becton Dickinson, San Jose, CA, USA). CD3+ T cells, CD3+ CD4+ T helper, CD3+ CD8+ T cytotoxic, CD19+ B cell, CD16/CD56+ NK cell counts and percentages were determined. Absolute numbers were obtained by multiplying the percentages with Absolute Lymphocyte Count (ALC) concurrently, and percentage ratio and absolute numbers were compared with normative data previously created in our laboratory [19].
Neutrophil function-oxidative burst (dihydrorhodamine-1, 2, 3 test)
The dihydrorhodamine (DHR) assay was performed as described by Koker et al. [20]. Briefly, whole blood cells were lysed using FACSlyse and washed twice with Hank’s balanced salt solution. The cells were then incubated with DHR, stimulated with phorbol 12-myristate 13-acetate, and were analyzed using flow cytometry. The results were calculated as stimulation index (the ratio of mean fluorescence intensity of the stimulated cells and that of the unstimulated cells). Each patient sample was analyzed and assessed with a healthy sample as experimental control.
Statistical Analysis
All statistical analyses were performed using the Statistical Package for Social Sciences version 23.0 software program (IBM SPSS Corp.; Armonk, NY, USA). Descriptive statistics for normally distributed continuous data were shown as mean±standard deviation. Median (minimum-maximum) values were used to assess non-normally distributed data. Categorical variables were compared by the chi-square test, and the Fisher’s exact test was used if the number of expected values was lower than 5 in 4-cell tables. To compare the mean values of continuous variables between 2 groups, t-test was used for normally distributed and the Mann-Whitney U test was used for non-normally distributed data. Correlations between normally distributed data were tested by the Pearson correlation analysis, and Spearman correlation test was used for non-normally distributed data. The Tukey test was used for post-hoc analysis. A multivariant logistic regression analysis was performed to test the parameters that had significant correlations with the severity of bronchiectasis (BSI and FACED). For all analyses, p values <0.05 were considered statistically significant.
RESULTS
A total of 74 patients (44 women and 30 men) aged 18 years and above, who were referred to the pulmonary medicine outpatient clinic between September 01, 2015, and April 01, 2016, and were diagnosed with stable NCFB, were included in the study. Among the 92 patients with bronchiectasis who had been referred to the outpatient clinic, 3 patients with a known diagnosis of CVID in childhood and 10 patients with CF were excluded from the study, and 5 patients did not provide consent for participation.
Of the 74 patients with NCFB, 1 (1%) had a history of psoriasis, 5 (7%) had a history of COPD, 9 (12%) had a history of asthma, 5 (7%) had a history of rheumatoid arthritis, and 12 (15%) had a history of tuberculosis. Overall, 23 (31%) patients had normal, 20 (27%) patients had mixed, 13 (18%) had obstructive, and 18 (24%) had restrictive lung function tests. The sputum culture of 20 (25%) patients grew only Pseudomonas aeruginosa, and 41 (55%) patients had positivity for other microorganisms, including P. aeruginosa. Table 1 indicates the demographical characteristics, microorganisms in sputum culture, history of oxygen and non-Invasive Mechanical Ventilation (NIMV) use, Charlson comorbidity index, hospitalization and exacerbation rate within the past year, lung function test indices, and disease duration of the enrolled patients. According to the BSI severity index, 36 (49%) patients were classified as mild and 38 (51%) patients as moderate-severe and 48 (65%) patients as mild and 26 (35%) patients as moderate-severe on the basis of the FACED severity index. Frequency of patients with 10 years and longer duration of disease increased in the moderate-severe BSI and FACED index groups compared with mild BSI and FACED index groups (40% versus 11%, p=0.016 and 42% versus 17%, p=0.040, respectively).
Table 1.
Demographic and clinical characteristics of patients with NCFB
| Patients | n=74 |
|---|---|
| Age (years) | 47±17 |
| Sex F/M (%) | 44/30(59/41) |
| BMI (kg/m2) | 26±6 |
| Smoking history | |
| Non-smokers | 56 |
| Active smokers | 6 |
| Ex-smokers | 12 |
| History of tuberculosis | 12 |
| mMRC | 1 (0–4) |
| Colonization of microorganism | |
| Pseudomonas aeruginosa | 20 |
| Acromobacter | 1 |
| Haemophilus influenzae | 15 |
| Streptococcus pneumoniae | 1 |
| Klebsiella pneumoniae | 1 |
| Moraxella catarhalis | 1 |
| Methicillin-sensitive Staphylococcus aureus | 1 |
| H. influenza+streptococcus | 1 |
| Number of exacerbations within the past year | 3.11 (0–10) |
| Number of hospitalizations within the past year | 1.5 (0–4) |
| Disease duration (years) | 5 (1–40) |
| Charlson comorbidity index | 1 (1–6) |
| Lung function test results | |
| FEV1% | 70.2±29.5 |
| FEV1 (L) | 2.02±0.96 |
| FVC% | 78.4±24.9 |
| FVC (L) | 2.71±1.11 |
| FEV1/FVC % | 70.4±14.5 |
| MMEF % | 44±36 |
| MMEF (L) | 1.63±1.37 |
| DLCO % | 86±25 |
| DLCO L/min/mmHg | 7.47±2.65 |
NCFB: non-cystic fibrosis bronchiectasis; F/M: female/male; BMI: body mass index; mMRC: modified medical research council dyspnea score; FEV1: forced expiratory volume in the first second; FVC: forced vital capacity; MMEF: maximal mid-expiratory flow; DLCO: diffusing capacity of lung for carbon monoxide
Frequencies expressed as n (%), parametric data as mean±standard deviation, and non-parametric data as median (min–max) values.
A total of 12 (16%) patients had an eosinophil count of ≥300×103/mL. Eosinophil number and percentage was not found to be related to disease severity indices. None of the patients demonstrated neutropenia in the complete blood count. The summary of immunoglobulin levels is given in Table 2. A total of 27 (37%) patients were defined as having a humoral aberration, and only 1 patient had a low complement level (Table 3). Among the 74 patients with NCFB, we found 5 patients with probable CVID (2 patients with low IgA and low IgG levels and 3 patients with low IgG and IgM levels) and 22 patients with unclassified antibody deficiencies (low IgM, low IgA, and a combination of several low IgG subclass levels), and only 1 with IgG2 subclass deficiency (Table 3). Comparing the 2 groups as having humoral aberration/hypocomplementemia or not, the proportion of men was found to be more frequent than women in the first group (57% versus 43%, respectively, p=0.03). There was no difference between the ages of patients between these 2 groups. No significant differences were found in the setting of lung function patterns and severity indices between the 2 groups having humoral aberrant/hypocomplementemia or not. There was a range of microorganisms isolated from the sputum samples of patients, including P. aeruginosa, Moraxella catarrhalis, Streptococcus pneumonia, methicillin-sensitive Staphylococcus aureus, Haemophilus influenzae, Acromobacter, and Klebsiella pneumoniae. There was no difference between the frequency of Pseudomonas and other microorganisms between these 2 groups. Moreover, smoking status, mMRC, frequency of tuberculosis, duration of disease, exacerbation, and hospitalization rates did not result in any significant difference according to having humoral aberration/hypocomplementemia or not.
Table 2.
Immunoglobulins, complements, and α-1 antitrypsin levels in patients with NCFB
| Immunological data | Value |
|---|---|
| IgE (IU/L) | 46.9 (2.0–1643.0) |
| IgA (g/L) | 278.0 (6.0–834.0) |
| IgM (g/L) | 109.5 (27.0–370.0) |
| IgG (g/L) | 1360.0 (132.0–2230.0) |
| IgG1 (g/L) | 602.9±243.3 |
| IgG2 (g/L) | 506.4±153.4 |
| IgG3 (g/L) | 129.8±68.7 |
| IgG4 (g/L) | 0.45 (0.07–1.92) |
| C3 (g/L) | 1.16±0.22 |
| C4 (g/L) | 0.28 (0.10–0.61) |
| Alpha–1 antitrypsin (μg/mL) | 1.42 (0.21–2.09) |
NCFB: non-cystic fibrosis bronchiectasis
Parametric data presented as mean±standard deviation and non-parametric data as median (min–max) values.
Table 3.
Characteristics of immune-aberrant patients with NCFB
| Immunological data | Value |
|---|---|
| Probable CVID | 5 |
| Selective IgA deficiency and Low IgG | 1 |
| Low IgA and low IgG | 1 |
| Low IgM and low IgG | 3 |
| Unclassified antibody deficiencies | 22 |
| Low IgM | 12 |
| Low IgM and low IgG1 | 1 |
| Low IgA | 2 |
| Low IgA and low IgM | 1 |
| Low IgA and low IgG1 | 2 |
| Low IgM and low IgG3 | 1 |
| Low IgG and hypocomplementemia | 1 |
| Low IgM and hypocomplementemia | 1 |
| Only low IgG2 | 1 |
| Total humoral aberrations | 27(37) |
| Only hypocomplementemia | 1 (1) |
| 28 (38) |
NCFB: non-cystic fibrosis bronchiectasis, CVID: common variable immunodeficiency
Total IgE levels were higher than 100 IU/L in 27 (37%) patients. Median total IgE levels were higher in patients in the moderate-severe BSI index group than in those in the mild BSI severity index group (83.2 [2–1643] IU/L versus 37.3 [4–927] IU/L, respectively, p=0.030) (Figure 1), and the ratio of patients with high IgE was higher in the moderate-severe BSI index group than in the mild BSI index group (48% versus 25%, respectively, p=0.056).
Figure 1.
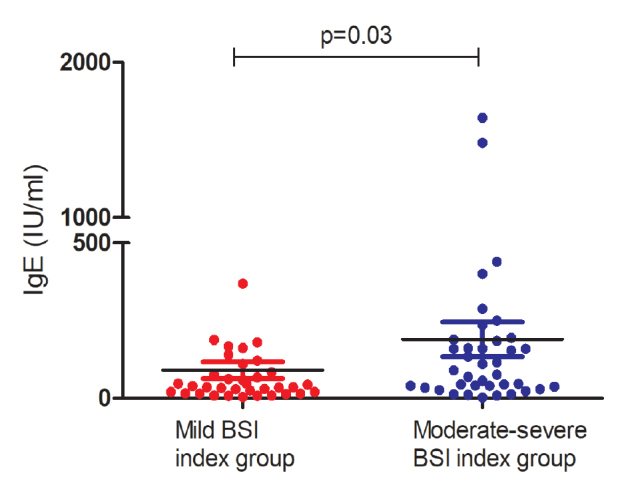
Mild and moderate-severe index groups according to bronchiectasis severity index in patients with non-cystic fibrosis bronchiectasis and serum IgE levels (p=0.03)
Peripheral blood CD3+ T cells, CD3+ CD4+ T helper, CD3+ CD8+ T cytotoxic, CD19+ B cells, CD16/CD56+ NK cell counts and percentages were determined in 74 patients. In the whole group, 22% of the patients had low CD3+ T cell percentage compared with lower limit of age-matched reference values, 18% of patients with low T helper cell percentage, 4% of patients with low T cytotoxic cell percentage, 16% of patients with low B cell percentage, and 9% of patients with low NK cell percentage. Compared with healthy individuals, patients with bronchiectasis demonstrated lower CD3+ T cell count and lower CD4+ T helper cell percentage and count (p<0.031, p<0.030, p<0.029, respectively) (Figure 2. a, b, and Supplement Table 1). Assessment of neutrophil oxidative activity with DHR test revealed a normal neutrophil oxidative burst capacity in all patients with NCFB.
Figure 2. a, b.
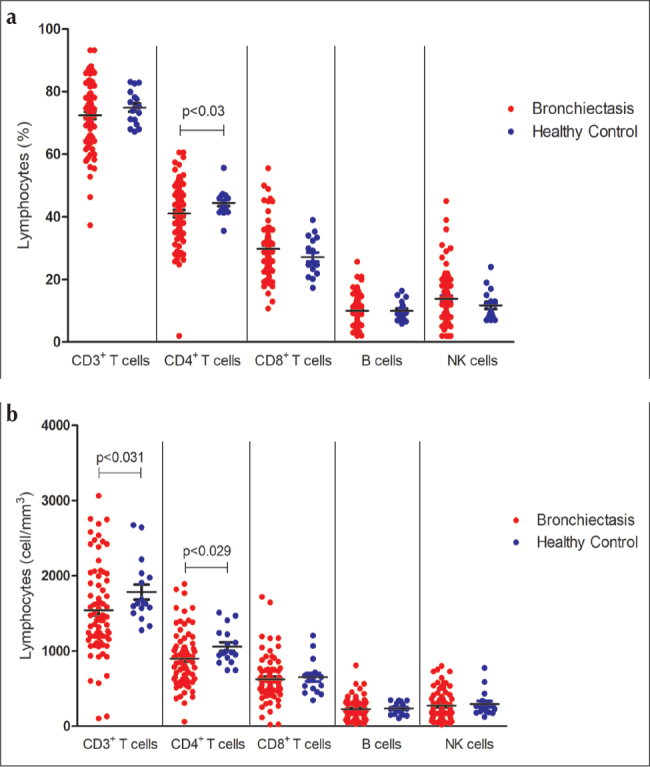
The (a) percentages and (b) counts of CD3+, CD4+, CD8+, CD 19+, and CD16/56+ cells in patients with non-cystic fibrosis bronchiectasis and healthy controls
No statistically significant results were obtained when the correlations were analyzed among immune parameters, BSI, and duration of disease in patients with NCFB. In this study, a statistically significant negative but weak correlation was found between CD16/56+ NK cell count and the number of exacerbations within the past year (r=−0.264, p=0.023). Moreover, the percentage of CD16/56+ NK cells and the number of exacerbations also had a negative weak correlation (r=−0.230, p=0.049).
In this study, we found 3 patients with low complement levels (Table 3). The levels of α−1 antitrypsin of all the patients were in normal reference ranges. The C3, C4, and α−1 antitrypsin values are presented in detail in Table 2.
DISCUSSION
Bronchiectasis still represents a major health concern worldwide, and post-infectious etiology still remains an important and frequent cause. Immunodeficiency may make these individuals more prone to recurrent respiratory infections. We aimed to determine the frequency of immune aberration in this patient group by assessing the lymphocyte subsets, immunoglobulins, neutrophil oxidative activity, α−1 antitrypsin, and complement levels as well as demonstrating its relationship with clinical outcomes. We did not find significant correlations between evaluated immunological parameters and BSI, except for IgE level, which was high in patients with moderate-severe BSI than in those with mild severity BSI. The immunoglobulin and complement levels were lower than normal in 28/74 of the patients, and male sex was more common than female sex in this immune-aberrant group of patients. In addition, flow cytometry analyses indicated that patients with NCFB had decreased CD3+ T cell count and lower CD4+ T helper cell percentage and count when compared with healthy individuals. Moreover, we found significant negative correlations between NK cell count/percentage and the number of disease exacerbations.
Primary antibody deficiency is the most common type of PIDD, and the spectrum is broad ranging from severe low serum immunoglobulins, such as agammaglobulinemia and CVID to patients with normal serum immunoglobulins reflecting selective antibody deficiency or patients with IgG subclasses deficiency. To date, limited information has been reported about the bronchiectasis observed in PIDDs. Touw et al. [4] have reviewed 26 studies that included 587 patients with CVID and found chronic bronchial changes, mostly bronchiectasis, in up to 73% of the patients. In the United Kingdom primary immunodeficiency registry, deficiency in immunoglobulins is found to be a common feature in patients with bronchiectasis [21]. King PT et al. [11] found only 2 patients with IgG and 3 patients with IgM deficiency in 103 patients with bronchiectasis. Similarly, 3/56 (5%) patients with bronchiectasis had low IgG levels without any IgA or IgM deficiency in another study [6]. In our study, there were 5 patients with probable CVID, 2 with low IgG and IgA levels, and 3 with low IgG and IgM levels. Selective IgA deficiency is common in individuals in western populations, and most of them are asymptomatic. In our study, only 6 patients had IgA deficiency, and 1 of them had selective IgA deficiency combined with low IgG level [22, 23]. However, a total of 22 patients with bronchiectasis showed various humoral aberrations with or without low complement levels in our study, which emphasizes the important role of antibodies in the development of bronchiectasis.
Previous studies highlighted a correlation between IgG subclass deficiency and the development of bronchiectasis and reported patients with IgG subclass deficiency associated with other immunoglobulin deficiencies and unresponsiveness to polysaccharide antigen. In one study, 13/56 patients of either a single IgG or combined subclasses deficiencies were reported to have bronchiectasis with predominantly IgG4 deficiency [6]. Another study involving 65 patients with idiopathic bronchiectasis found that 50% of the patients had IgG subtype deficiency (particularly IgG2), and this was shown to correlate with insufficient Haemophilus influenza vaccine antibody response [24]. Similarly, in the study by King et al. [11] including 103 patients with bronchiectasis, 17 had IgG subtype deficiency. However, other studies have reported IgG subtype deficiencies in adult patients with bronchiectasis with a wide range of frequencies [6–8, 24–27]. Similarly, in our study, we found 5 patients (7%) with IgG subclass deficiency, 2 with low IgM levels combined with low IgG1 and low IgG3 levels, 2 with low IgA levels combined with IgG1 level, and 1 patient with low IgG2 level. Our study confirmed that there was a higher risk of antibody deficiencies in the bronchiectasis group and emphasized the detection of immunoglobulins and IgG subclass in adult patients with NCFB.
The complement system that represents an important component of the innate system and plays a central role in the host defense was also investigated in patients with NCFB in this study. Deficiencies in complement functions are known to cause chronic bacterial infections. Primary complement system deficiencies are very rare in overall population and account for almost 2% of the PIDDs, and there are few reports on complement deficiencies in bronchiectasis [28]. In the study by King et al. [11], complement system deficiency was not detected in any of the 103 adult patients with NCFB. Similarly, this study did not show an increased prevalence of patients with low complement level, only 3 (2 with antibody deficiencies) of the patients had complement deficiency. Thus, we hypothesized that complement deficiency does not play a solo role but requires coexisting immune deficits to aid the pathogenesis of bronchiectasis in adult patients.
In this study, the disease severity indices, BSI and FACED, did not demonstrate any significant correlation with antibody deficiencies. We did not find any similar study in the literature evaluating a correlation between these parameters and the disease severity indices. However, when the total IgE levels were categorized as high if ≥100 IU/L, the BSI was found to be higher among patients with high total IgE levels in our study. In a study of the Danish national registry, it has been reported that IgE sensitization was associated with a significantly higher risk of chronic lower airway infective diseases [29]. However, no previous study in the literature has investigated the relationship between BSI and IgE levels. This is a novel finding and monitoring total IgE levels can be recommended during the follow-up of patients with bronchiectasis to predict the severity of the disease.
Bronchiectasis is characterized by recurrent airway infection; however, relatively little is known about the nature of systemic inflammation in bronchiectasis. It is a fact that lymphocytes are the main cells mediating the adaptive immune response, and there have been few studies analyzing lymphocyte subsets in these patients. In a study with 103 adult patients with bronchiectasis, assessment of the lymphocyte subsets revealed that 7 (7%) patients had CD4+ helper T cell count below normal range, 2 (2%) had CD8+ cytotoxic T cell count, and 6 (6%) had B cell count below the normal range [11]. However, in our study population, the count of CD3+ T cells and the count and percentage of CD4+ T cells significantly decreased compared with those of age- and sex-matched controls. Similar to the study by King et al. [11], there was no correlation between B cell count and percentages and immunoglobulin levels in this study. Similar to our findings in patients with CVID, T lymphocyte abnormalities were present in almost 20% of the patients, and the majority of patients had decreased numbers of CD3, CD4, and CD8 positive cells (7). In this study, these lymphocyte subset findings were not significantly related to bronchiectasis disease severity indices, duration of hospital stay, or colonization with P. aeruginosa or other microorganisms. However, a statistically significant negative relation was found between the count and percentage of cells expressing CD16/CD56 surface markers and the number of exacerbations within the past year, suggesting that the decrease in CD16/56+ NK cell percentage increased the number of hospitalizations within the past 1 year. This finding was not previously reported in the literature; to the best of our knowledge, our study is the first one to demonstrate this association.
Although bronchiectasis is characterized by airway neutrophil inflammation, the role of neutrophil functions in bronchiectasis has not been clearly assessed previously. It was suggested that a relation might exist between bronchiectasis and impaired neutrophil functions [30]. Assessment of the neutrophil phagocytosis functions of all the patients in our study indicated that all patients with NCFB had normal oxidative capacity levels by DHR. In contrast, in the study by King et al. [11] including 103 patients with bronchiectasis, DHR levels were reported to be decreased in 33 patients. Similar to our study, Pasteur et al. [3] did not find any major changes in neutrophil function compared with controls. These controversial results make it difficult to reach a conclusion.
The major limitation of our study was its small sample size and imbalance in the distribution of the sexes as the patients included in this study were randomly selected from among those referred to the outpatient clinics at the tertiary center. In addition, this was a cross-sectional study, and future studies should evaluate the immune status relative to the long-term follow-up results. Current literature confirmed that some patients with normal immunoglobulins have deficiency in specific antibody production; however, the immune response after vaccination was not evaluated in our patient group.
In this study, the lymphocyte subtypes indicated that patients with NCFB had decreased CD3+ T cell count and lower CD4+ T helper cell percentage and count than healthy individuals. One-third of the individuals had an abnormality of immunoglobulin classes (IgA, IgM, IgG, and IgG subclasses) and/or low complement levels. Patients with NCFB, who had decreased NK cell levels, experienced more frequent disease exacerbations; patients with elevated total IgE levels had higher BSI. Our study confirmed the need for a screening process and/or referring conditions with detailed immune tests including IgG subclasses and lymphocyte subset evaluation besides immunoglobulin levels to clinical immunology divisions for patients diagnosed with NCFB. On the basis of our findings, we believe that a correlation can be established by determining the immune status of patients with bronchiectasis as well as the number of exacerbations and their disease severity. Nevertheless, studies including larger patient populations are required to further support our conclusions.
MAIN POINTS
Humoral aberrations in adult patients with Non-CF Bronchiectasis were found to be frequent.
Lymphocyte subset analysis showed that there was fewer CD3+ T cell count and lower CD4+ T helper cell percentage and count in patients with non-CF Bronchiectasis when compared with healthy individuals.
Patients with low percentage and counts of Natural Killer cells had higher rates of exacerbations.
These results emphasize the importance of immune function assessment in adult patients with Non-CF Bronchiectasis.
Supplementary Data
Supplement Table 1.
Lymphocyte subsets in non-CF bronchiectasis patients and healthy controls. Statistical analyses represented with *: student-test, **: Mann Whitney U test)
| non-CF Bronchiectatic patients n=74 | Healthy Controls n=17 | p | |
|---|---|---|---|
| CD3+cell % | 72.39±10.61 | 74.88±5.32 | 0.169* |
| CD3+cell count/μL | 1543.57±602.51 | 1960.29±1015.58 | 0.031** |
|
| |||
| CD4+cell % | 41.03±9.91 | 44.42±4.15 | 0.030* |
| CD4+cell count/μL | 899.70±368.21 | 1152.63±545.30 | 0.029** |
| CD8+cell % | 29.79±8.50 | 27.15±6.05 | 0.225** |
| CD8+cell count/μL | 626.30±300.41 | 729.16±490.76 | 0.546** |
| CD19+cell % | 9.95±5.16 | 9.98±3.14 | 0.659** |
| CD19+cell count/μL | 228.42±143.84 | 257.52±131.47 | 0.447* |
| CD16/CD56+cell % | 13.88±8.74 | 11.62±4.67 | 0.560** |
| CD16/CD56+cellount/μL | 276.19±192.16 | 296.17±165.42 | 0.315** |
Acknowledgments
We would like to thank Dr. Şeyma Gorcin for the statistical analysis.
Footnotes
Ethics Committee Approval: Ethics Committee approval for the study was obtained from the Clinical Research Ethics Committee of Marmara University Medical Faculty (NR 09.2015.120).
Informed Consent: Written informed consent was obtained from the patients.
Peer-review: Externally peer-reviewed.
Conflict of Interest: The authors have no conflicts of interest to declare.
Author Contributions: Concept - E.K.A., M.B.; Design - İ.Ö.; Supervision - A.Ö., Ş.O.Y.; Fundings - M.B., D.K.; Materials - S.B.; Data Collection and/or Processing - M.B., İ.Ö.; Analysis and/or Interpretation - B.B.C., E.K.A., E.E.; Literature Review - İ.Ö.; Writing - B.B.C., M.B.; Critical Review - E.K.A., A.Ö.
Financial Disclosure: This work was supported by Marmara University Research Council (SAG-C-TUP-110915-0426).
REFERENCES
- 1.Brower KS, Del Vecchio MT, Aronoff SC. The etiologies of non-CF bronchiectasis in childhood: A systematic review of 989 subjects. BMC Pediatr. 2014;14:4. doi: 10.1186/s12887-014-0299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee E, Shim JY, Kim HY, et al. Clinical characteristics and etiologies of bronchiectasis in Korean children: A multicenter retrospective study. Respir Med. 2019;150:8–14. doi: 10.1016/j.rmed.2019.01.018. [DOI] [PubMed] [Google Scholar]
- 3.Pasteur MC, Helliwell SM, Houghton SJ, et al. An investigation into causative factors in patients with bronchiectasis. Am J Respir Crit Care Med. 2000;162:1277–84. doi: 10.1164/ajrccm.162.4.9906120. [DOI] [PubMed] [Google Scholar]
- 4.Touw CM, van de Ven AA, de Jong PA, et al. Detection of pulmonary complications in common variable immunodeficiency. Pediatr Allergy Immunol. 2010;21:793–805. doi: 10.1111/j.1399-3038.2009.00963.x. [DOI] [PubMed] [Google Scholar]
- 5.Costa-Carvalho BT, Grumach AS, Franco JL, et al. Attending to warning signs of primary immunodeficiency diseases across the range of clinical practice. J Clin Immunol. 2014;34:10–22. doi: 10.1007/s10875-013-9954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stead A, Douglas JG, Broadfoot CJ, et al. Humoral immunity and bronchiectasis. Clin Exp Immunol. 2002;130:325–30. doi: 10.1046/j.1365-2249.2002.01974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maarschalk-Ellerbroek LJ, Hoepelman AI, van Montfrans JM, et al. The spectrum of disease manifestations in patients with common variable immunodeficiency disorders and partial antibody deficiency in a university hospital. J Clin Immunol. 2012;32:907–21. doi: 10.1007/s10875-012-9671-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cereser L, De Carli M, d’Angelo P, et al. High-resolution computed tomography findings in humoral primary immunodeficiencies and correlation with pulmonary function tests. World J Radiol. 2018;10:172–83. doi: 10.4329/wjr.v10.i11.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King P, Bennett-Wood V, Hutchinson P, et al. Bactericidal activity of neutrophils with reduced oxidative burst from adults with bronchiectasis. APMIS. 2009;117:133–9. doi: 10.1111/j.1600-0463.2008.00028.x. [DOI] [PubMed] [Google Scholar]
- 10.Mooney D, Edgar D, Einarsson G, et al. Chronic lung disease in common variable immune deficiency (CVID): A pathophysiological role for microbial and non-B cell immune factors. Crit Rev Microbiol. 2017;43:508–19. doi: 10.1080/1040841X.2016.1268568. [DOI] [PubMed] [Google Scholar]
- 11.King PT, Hutchinson P, Holmes PW, et al. Assessing immune function in adult bronchiectasis. Clin Exp Immunol. 2006;144:440–6. doi: 10.1111/j.1365-2249.2006.03091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill AT, Haworth CS, Aliberti S, et al. EMBARC/BRR definitions working group. Pulmonary exacerbation in adults with bronchiectasis: A consensus definition for clinical research. Eur Respir J. 2017;49 doi: 10.1183/13993003.00051-2017. pii: 1700051. [DOI] [PubMed] [Google Scholar]
- 13.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 14.Costa JC, Machado JN, Ferreira C, et al. The Bronchiectasis Severity Index and FACED score for assessment of the severity of bronchiectasis. Pulmonology. 2018;24:149–54. doi: 10.1016/j.rppnen.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Minov J, Karadzinska-Bislimovska J, Vasilevska K, et al. Assessment of the Non-Cystic Fibrosis Bronchiectasis Severity: The FACED Score vs the Bronchiectasis Severity Index. Open Respir Med J. 2015;9:46–51. doi: 10.2174/1874306401509010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cordova-Rivera L, Gibson PG, Gardiner PA, et al. Extrapulmonary associations of health status in severe asthma and bronchiectasis: Comorbidities and functional outcomes. Respir Med. 2019;154:93–101. doi: 10.1016/j.rmed.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Picard C, Bobby Gaspar H, Al-Herz W, et al. International Union of Immunological Societies: 2017 Primary Immunodeficiency Diseases Committee Report on Inborn Errors of Immunity. J Clin Immunol. 2018;38:96–128. doi: 10.1007/s10875-017-0464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kutukcular N, Karaca NE, Demircioğlu O, et al. Increases in serum immunoglobulins to age related normal levels in children with IgA and/or IgG subclass deficiency. Pediatr Allergy Immunol. 2007;8:167–173. doi: 10.1111/j.1399-3038.2006.00491.x. [DOI] [PubMed] [Google Scholar]
- 19.Besci İÖ, Cicekkökü D, Kıykım A, et al. [Abstract] 3rd Clinical Immunology Congress; Izmir, Turkey. 2017. [Google Scholar]
- 20.Koker MY, Camcioglu Y, van Leeuwen K, et al. Clinical, functional, and genetic characterization of chronic granulomatous disease in 89 Turkish patients. J Allergy Clin Immunol. 2013;132:1156–63. doi: 10.1016/j.jaci.2013.05.039. [DOI] [PubMed] [Google Scholar]
- 21.Shillitoe B, Bangs C, Guzman D, et al. The United Kingdom Primary Immune Deficiency (UKPID) registry 2012 to 2017. Clin Exp Immunol. 2018;192:284–91. doi: 10.1111/cei.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lougaris V, Sorlini A, Monfredini C, et al. Clinical and laboratory features of 184 Italian pediatric patients affected with Selective IgA Deficiency (SIgAD): A Longitudinal Single-Center Study. J Clin Immunol. 2019;39:470–5. doi: 10.1007/s10875-019-00647-y. [DOI] [PubMed] [Google Scholar]
- 23.Dominguez O, Giner MT, Alsina L, et al. Clinical phenotypes associated with selective IgA deficiency: A review of 330 cases and a proposed follow-up protocol. An Pediatr (Barc) 2012;76:261–7. doi: 10.1016/j.anpedi.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Gracia J, Rodrigo MJ, Morell F, et al. IgG subclass deficiencies associated with bronchiectasis. Am J Respir Crit Care Med. 1996;153:650–5. doi: 10.1164/ajrccm.153.2.8564113. [DOI] [PubMed] [Google Scholar]
- 25.Tabatabaie P, Aghamohammadi A, Mamishi S, et al. Evaluation of humoral immune function in patients with bronchiectasis. Iran J Allergy Asthma Immunol. 2008;7:69–77. [PubMed] [Google Scholar]
- 26.Hodkinson JP, Bangs C, Wartenberg-Demand A, et al. Low IgA and IgM Is Associated with a Higher Prevalence of Bronchiectasis in Primary Antibody Deficiency. J Clin Immunol. 2017;37:329–31. doi: 10.1007/s10875-017-0381-y. [DOI] [PubMed] [Google Scholar]
- 27.Ozkan H, Atlihan F, Genel F, et al. IgA and/or IgG subclass deficiency in children with recurrent respiratory infections and its relationship with chronic pulmonary damage. J Investig Allergol Clin Immunol. 2005;15:69–74. [PubMed] [Google Scholar]
- 28.Walport MJ. Complement. Second of two parts. N Engl J Med. 2001;344:1140–4. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 29.Skaaby T, Husemoen LL, Thuesen BH, et al. IgE sensitization to inhalant allergens and the risk of airway infection and disease: A population-based study. PLoS One. 2017;12:e0171525. doi: 10.1371/journal.pone.0171525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Voglis S, Quinn K, Tullis E, et al. Human neutrophil peptides and phagocytic deficiency in bronchiectatic lungs. Am J Respir Crit Care Med. 2009;180:159–66. doi: 10.1164/rccm.200808-1250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Table 1.
Lymphocyte subsets in non-CF bronchiectasis patients and healthy controls. Statistical analyses represented with *: student-test, **: Mann Whitney U test)
| non-CF Bronchiectatic patients n=74 | Healthy Controls n=17 | p | |
|---|---|---|---|
| CD3+cell % | 72.39±10.61 | 74.88±5.32 | 0.169* |
| CD3+cell count/μL | 1543.57±602.51 | 1960.29±1015.58 | 0.031** |
|
| |||
| CD4+cell % | 41.03±9.91 | 44.42±4.15 | 0.030* |
| CD4+cell count/μL | 899.70±368.21 | 1152.63±545.30 | 0.029** |
| CD8+cell % | 29.79±8.50 | 27.15±6.05 | 0.225** |
| CD8+cell count/μL | 626.30±300.41 | 729.16±490.76 | 0.546** |
| CD19+cell % | 9.95±5.16 | 9.98±3.14 | 0.659** |
| CD19+cell count/μL | 228.42±143.84 | 257.52±131.47 | 0.447* |
| CD16/CD56+cell % | 13.88±8.74 | 11.62±4.67 | 0.560** |
| CD16/CD56+cellount/μL | 276.19±192.16 | 296.17±165.42 | 0.315** |


