Abstract
OBJECTIVE
Prone positioning (PP) has demonstrated to be a safe adjunctive therapy for severe acute respiratory distress syndrome (ARDS). There is limited evidence of PP effects on awake patients. This study aimed to investigate the effects and feasibility of PP on coronavirus disease 2019 (COVID-19)-associated awake patients with ARDS in a subintensive setting of care.
MATERIAL AND METHODS
This is a single-center case-control study involving patients with severe COVID-19 infection. A total of 29 patients underwent noninvasive ventilation, and PP was initiated 12 h from admission; 18 patients tolerated prone and side positioning for at least 10 h/d and cycled their position every 2 h, and 11 patients had no complaints with PP.
RESULTS
A total of 29 patients (25 men and 4 women) with a median age of 64 years showed the average baseline white blood cell count of 8.45×109 cells/L, C-reactive protein of 10.1 mg/L, lactate dehydrogenase of 366 mU/mL, and interleukin-6 of 172 pg/mL. Basal pO2/FiO2 ratio (P/F) was 95 (±56.5) and showed no linear correlation with any of the inflammatory markers tested. Computed tomography findings included ground-glass opacities in 100% (29/29) of patients. Consolidation/atelectasis was found in 58% (17/29) of patients. P/F was homogeneously distributed at baseline in patients with PP (96.5) and without PP (95). P/F during PP increased significantly compared with noncompliant controls (288 vs. 202; p=0.0002). Total duration of respiratory failure was significantly shorter in patients with PP (14 vs. 21 days; p=0.002). The number of days to recover from respiratory failure inversely correlated with PP P/F independently from baseline P/F.
CONCLUSION
COVID-19 can lead to a severe impairment of gas exchange regardless of inflammatory status. Therefore, respiratory support may play a major role in COVID-19 treatment. We documented substantial efficacy of PP when started early and for at least 10 h/d. On awake patients, PP feasibility strictly depends on patient’s compliance. The interface should be carefully chosen to best fit every patient.
Keywords: Acute respiratory distress syndrome, continuous positive airway pressure, severe coronavirus disease 2019, intensive care, respiratory failure
INTRODUCTION
Prone positioning (PP) has demonstrated to be a safe adjunctive therapy for severe acute respiratory distress syndrome (ARDS) because it increases gas exchange and decreases the risk of ventilator-associated pneumonia [1]. Several mechanisms can clarify this observation, including possible alveolar recruitment in the lung-dependent zones and homogeneity in alveolar inflation. In addition, PP may reduce the nonphysiological stress and strain associated with mechanical ventilation, thereby decreasing the risk of ventilator-induced lung injury and the overall mortality in severely hypoxemic patients with ARDS [2]. PROSEVA study, a prospective multicenter randomized controlled trial, also confirmed that early and prolonged application of PP can improve survival in severe ARDS [3].
Effects of PP have been widely described in invasively ventilated patients receiving intensive care. There is evidence of a more even tidal volume distribution and improvement of resting lung volume in the dorsocaudal regions by reducing the superimposed pressure of both the heart and the abdomen. In contrast, pulmonary perfusion remains preferentially distributed to the dorsal lung regions, thereby improving the overall alveolar ventilation/perfusion relationships [4]. There is limited evidence of PP effects on awake, nonintubated patients. Recently, the coronavirus disease 2019 (COVID-19) pandemic has brought the international community to adopt a more intensive care unit (ICU)–saving approach, and some cases of PP in nonintubated patients have been reported [5]. As COVID-19 shows a high rate of ARDS and mortality, this study aimed to investigate the effects and feasibility of PP on COVID-19–associated ARDS in a subintensive setting of care, where patients are awake and nonintubated.
MATERIAL AND METHODS
This is a single-center case-control study involving patients with severe COVID-19 infection. This study has been approved by the local ethics committee of the University of Campania “Luigi Vanvitelli” and A.O.R.N. Ospedali dei Colli in accordance with the 1976 Declaration of Helsinki and its later amendments. We retrospectively analyzed the medical records of patients admitted to the subintensive respiratory department from mid-March 2020 to April 2020. A total of 29 patients were affected by moderate-to-severe ARDS owing to COVID-19. Severe acute respiratory syndrome coronavirus 2 was detected by real-time reverse transcription polymerase chain reaction on nasopharyngeal swabs. The inflammatory status was investigated at baseline using the blood samples to determine white blood cell (WBC) count, C-reactive protein (CRP), lactate dehydrogenase (LDH), and interleukin 6 (IL-6). Patients also underwent a chest high-resolution computed tomography to identify ground-glass opacities (GGO), consolidation/atelectasis, crazy paving, and bronchiectasis.
All 29 patients received noninvasive ventilation (NIV) (10 Helmet continuous positive airway pressure [CPAP], 13 full-face mask CPAP, and 6 High Flow Nasal Cannula [HFNC]). PP was initiated 12 h from admission in the presence of hemodynamic stability. All patients were awake and cycled their position every 2 h between prone, right, and left lateral, and fowler’s semi-upright positions. Cycling was performed to prevent pressure wounds and joint pain. Two clinicians helped the patients at every cycling. Artery blood gases (ABG) were performed to measure PaO2/FiO2 ratio (P/F) at baseline, during NIV (after 2 h), and during PP (after 10 h), per protocol (Figure 1). ABG were subsequently performed daily to assess the need for invasive mechanical ventilation (IMV) and eventually determine the duration of respiratory failure in terms of days to reach a room air pO2≥60 mm Hg. All patients received a standard of care pharmacological treatment with hydroxychloroquine, azithromycin, antivirals, and low–molecular weight heparin. None of the patients received systemic steroids or tocilizumab during the first day of the protocol.
Figure 1.
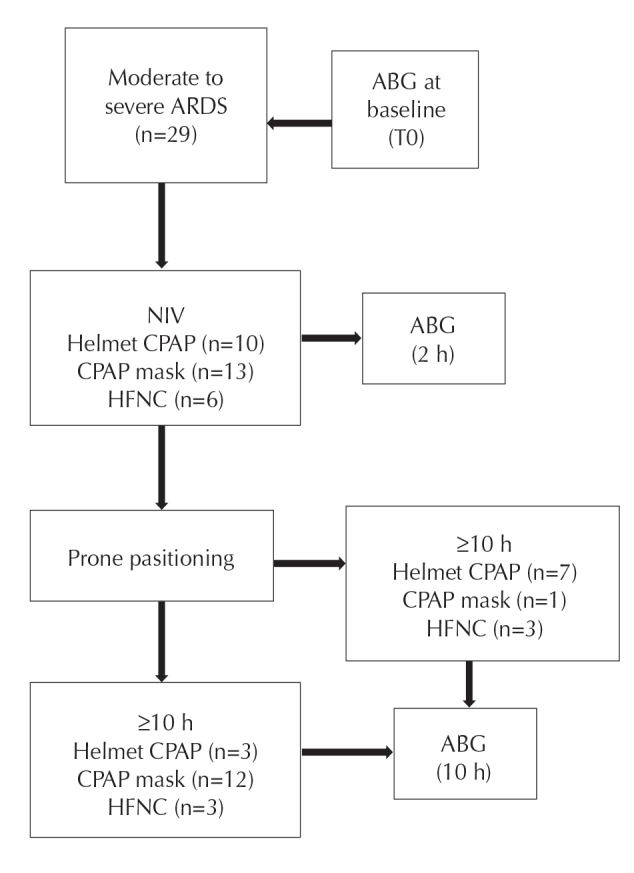
Study protocol
ARDS: acute respiratory distress syndrome, ABG: artery blood gases, NIV: non invasive ventilation, CPAP: continuous positive airway pressure, HFNC: high flow nasal cannula
Results are reported as numbers and percentages for categorical variables and medians and interquartile ranges for continuous variables. Differences between cases and controls were tested by the parametric unpaired Student t test. All correlations were expressed by a linear regression model and showed by dispersion graphs. A p-value <0.05 was considered statistically significant.
RESULTS
The study population included 29 patients with moderate-to-severe COVID-19 infection, mostly men (25 men and 4 women). The median age was 64 years (±22.5). Body mass index was 28 (±2.5). Laboratory testing at baseline showed median WBC count of 8.45×109 cells/L (±5.03), CRP of 10.1 mg/L (±11.1), LDH of 366 mU/mL (±139.5), and IL-6 of 172 pg/mL (±282.15). Basal P/F was 95 (±56.5). Baseline features did not differ between the 2 groups, as shown in Table 1. Furthermore, the severity of respiratory failure at baseline had no relation with inflammatory status. P/F did not correlate with any laboratory tests (Figure 2).
Table 1.
Baseline features. Results are expressed as median (interquartile range).
| Group P | Group nP | Total | |
|---|---|---|---|
| Age [years] | 61 (14) | 71 (10) | 64 (22.5) |
| BMI | 28 (2) | 28 (5) | 28 (2.5) |
| WBC [cells*109/L] | 7.725 (6.43) | 9.89 (3.75) | 8.45 (5.035) |
| LDH [mU/mL] | 362 (117) | 251 (123) | 366 (139.5) |
| CRP [mg/L] | 9.3 (9.9) | 13.4 (10.6) | 10.1 (11.1) |
| IL-6 [pg/mL] | 165.5 (156) | 204 (307) | 172 (282.15) |
| P/F | 96.5 (35) | 95 (92) | 95 (56.5) |
Figure 2. a–d.
(a) Linear regression shows no significant correlation between baseline P/F and white blood cells. (b) Linear regression shows no significant correlation between baseline P/F and C-reactive protein. (c) Linear regression shows no significant correlation between baseline P/F and lactate dehydrogenase. (d) Linear regression shows no significant correlation between baseline P/F and interleukin 6
Computed tomography findings included GGOs that were observed in 100% (29/29) of patients. GGO involved ≤2 lobes in 17% (5/29) of patients and >2 lobes in 83% (24/29) of patients. Consolidation/atelectasis was also found in 58% (17/29) of patients. When observed, consolidations were less extended than GGO. Crazy paving (10% [3/29]) and bronchiolectasis (7% [2/29]) were less common.
All patients underwent NIV, and PP was attempted in all patients. A total of 18 patients tolerated prone and side positioning for at least 10 h/d (group P); 11 patients did not compliant about PP and were considered as control group (group nP). The mean compliance in group nP was 3 h. Causes of intolerance to PP were interface displacement, oxygen desaturation, worsening of dyspnea, chest tightness, neck pain, and agitation. Baseline P/F was homogeneously distributed being 96.5 (±35) in group P and 95 (±92) in group nP. P/F during NIV considerably improved in both the groups, being 175.5 (±94) in group P and 175 (±136) in group nP. P/F during PP significantly increased in group P compared with group nP (288±80 vs. 202±122; mean difference, 115.0; p=0.0002) (Figure 3).
Figure 3.
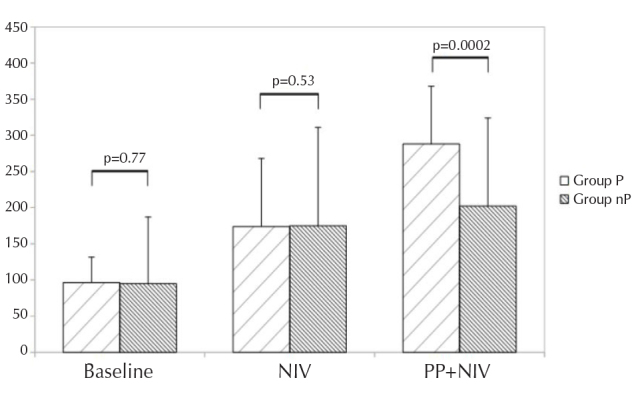
P/F at baseline, NIV and prone positioning+NIV
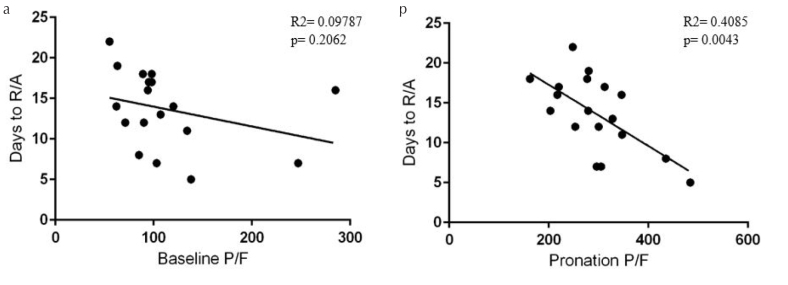
Total duration of respiratory failure was significantly different, with a median of 14 days (±7.5) in group P and 21 days (±6) in group nP (mean difference, −7.82; p=0.002). Moreover, in group P, duration of respiratory failure significantly correlated with PP P/F and not with baseline P/F (Figure 4), suggesting that recovery is related to good response to PP, independently from the baseline severity of the disease. Finally, in group P, only 1 (5.5%) patient deteriorated and needed IMV. In group nP, 2 (18%) patients needed IMV, and 3 (27%) patients died.
Figure 4. a, b.
Linear regression shows no significant correlation between number of days to room air and baseline P/F (a). Linear regression shows a significant negative correlation between number of days to room air and prone positioning P/F (b)
DISCUSSION
Our data show that COVID-19 can lead to a severe impairment of gas exchange at baseline, which is independent from immune and inflammatory status. This consideration may suggest that respiratory support plays a major role in COVID-19 treatment, regardless of antiinflammatory/antiviral therapies. We analyzed the blood gases at a very early stage and compared P/F within 24 h from hospital admission to minimize the impact of pharmacological therapies on the outcome. In our study, the association of PP and NIV improved oxygenation and total duration of respiratory failure. On the basis of the P/F values, we documented substantial efficacy of PP when started early and for at least 10 h/d.
Our data in patients with COVID-19 infection are consistent with a recent meta-analysis on all causes of ARDS in mechanically ventilated patients. The authors assessed that PP improves oxygenation when applied for 12 h daily [6]. Unfortunately, PP in ICU is labor-intensive and accounts for many potential complications [7].
According to our experience, when applied on awake patients, PP feasibility strictly depends on patient’s compliance more than the number of dedicated personnel. The interface used for NIV is also crucial. In our population, PP was better tolerated with full-face masks than helmet CPAP, as illustrated in Figure 1.
Furthermore, precocious optimization of ventilation with PP reduced the total duration of respiratory failure. We also highlighted that recovery from respiratory failure directly depends on P/F improvement during PP, thereby supporting the potential efficacy and prognostic value of this maneuver as adjunctive therapy in COVID-19-related ARDS. Furthermore, NIV failure is particularly relevant as outcome, especially during a pandemic when intensive care resources seem to be limited. Whether PP can prevent NIV failure is still controversial, but a recent case series from China suggested that this approach can reduce the need for intensive care [8]. We hypothesize that PP can reduce NIV failure and mortality, but further investigations are needed to confirm this result, considering the small sample size.
Finally, the selection of patients seems to be essential. Our patients presented mostly with severe oxygenation impairment; an extensive radiologic involvement was also detected, with diffuse GGO and consolidations. This observation seems to be consistent with a more physiological point of view, as previously reported [9].
In conclusion, PP may be an effective adjunctive therapy in patients with COVID-19-related ARDS. Oxygenation improves when PP is initiated early and performed for more than 10 h/d. A subintensive setting is optimal for awake patients and is labor saving. The patient’s compliance is crucial, and several attempts should be made to find the best interface to fit every patient. Finally, PP should be adopted in severely hypoxemic patients, especially when extensive GGO and consolidation/atelectasis are detected.
MAIN POINTS
Severity of gas exchange impairment in COVID-19 is not correlated to inflammatory status.
Respiratory support plays a major role in COVID-19 treatment.
Prone positioning and Non invasive ventilation improve oxygenation and reduce total duration of respiratory failure in COVID-19 patients.
Compliance, interface and dedicated personnel are crucial to perform prone positioning in awake patients.
Prone positioning should be performed when extensive ground glass opacity and consolidation are detected on computed tomography of the chest.
Footnotes
Ethics Committee Approval: Ethics Committee Approval for the study was obtained from the Local Ethics Committee of the University of Campania “Luigi Vanvitelli” and A.O.R.N. Ospedali dei Colli (Prot. 251/20) in accordance with the 1976 Declaration of Helsinki and its later amendments.
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - S.F., F.G.; Design - S.F., A.A., L.G.; Supervision - S.F, A.A., G.F.; Resources - L.G., M.M., M.S.; Materials - S.F., A.A., L.G., M.M., M.S.; Data Collection and/or Processing - S.F., A.A., M.M.; Analysis and/or Interpretation - S.F., A.A.; Literature Review - TS.F., A.A., L.G., M.M., M.S. F.G.; Writing - S.F., L.G.; Critical Review - A.A., M.M., F.G.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.Abroug F, Ouanes-Besbes L, Elatrous S, Brochard L. The effect of prone positioning in acute respiratory distress syndrome or acute lung injury: A meta-analysis. Areas of uncertainty and recommendations for research. Intensive Care Med. 2008;34:1002–11. doi: 10.1007/s00134-008-1062-3. [DOI] [PubMed] [Google Scholar]
- 2.Gattinoni L, Carlesso E, Taccone P, et al. Prone positioning improves survival in severe ARDS: A pathophysiologic review and individual patient meta-analysis. Minerva Anestesiol. 2010;76:448–54. [PubMed] [Google Scholar]
- 3.Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368:2159–68. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 4.Kallet RH. A Comprehensive Review of Prone Position in ARDS. Respir Care. 2015;60:1660–87. doi: 10.4187/respcare.04271. [DOI] [PubMed] [Google Scholar]
- 5.Sartini C, Tresoldi M, Scarpellini P, et al. Respiratory Parameters in Patients With COVID-19 After Using Noninvasive Ventilation in the Prone Position Outside the Intensive Care Unit. JAMA. 2020:e207861. doi: 10.1001/jama.2020.7861. doi: 10.1001/jama.2020.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munshi L, Del Sorbo L, Adhikari NKJ, et al. Prone Position for Acute Respiratory Distress Syndrome. A Systematic Review and Meta-Analysis. Ann Am Thorac Soc. 2017;14(Supplement_4):S280–S8. doi: 10.1513/AnnalsATS.201704-343OT. [DOI] [PubMed] [Google Scholar]
- 7.Kwee MM, Ho Y-H, Rozen WM. The Prone Position During Surgery and its Complications: A Systematic Review and Evidence-Based Guidelines. Int Surg. 2015;100:292–303. doi: 10.9738/INTSURG-D-13-00256.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng Z, Tay WC, Ho CHB. Awake Prone Positioning for Non-intubated Oxygen Dependent COVID-19 Pneumonia. Patients Eur Respir J. 2020 doi: 10.1183/13993003.01198-2020. doi: 10.1183/13993003.01198-2020. 2001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gattinoni L, Chiumello D, Rossi S. COVID-19 pneumonia: ARDS or not? Crit Care. 2020;24:154. doi: 10.1186/s13054-020-02880-z. [DOI] [PMC free article] [PubMed] [Google Scholar]