Abstract
We report on the development of an ultra-performance liquid chromatography–tandem mass spectrometry (UPLC-MS/MS) method for simultaneously measuring eight biomarkers of volatile organic compound (VOC) exposure, with potential application to e-cigarette aerosol biomonitoring. Phenylmercapturic acid (PMA) and trans, trans-muconic acid (tt-MA) are metabolites of benzene; 2-aminothiazoline-4-carboxylic acid (ATCA) is a metabolite of cyanide; N-2-furoylglycine (N2FG) is a metabolite of furfural and furfuryl alcohol; 5-hydroxymethylfuroic acid (HMFA), 5-hydroxymethyl-2-furoylglycine (HMFG), and 2,5-furandicarboxylic acid (FDCA) are metabolites of 5-hydroxymethylfurfural; and 5-hydroxy-N-methylpyrrolidone (5HMP) is a metabolite of N-methyl-2-pyrrolidone. A pentafluorophenyl-modified silica column was used for chromatographic separation. The overall run time for the method is about 6 min per sample injection. The method has low to sub-nanograms per milliliter sensitivity, linearity over 3 orders of magnitude, and precision and accuracy within 15%. The method was used to measure human urine samples. Results showed that people with known benzene exposure (daily cigarette smokers) had higher levels of tt-MA and PMA compared with non-smokers. The method is advantageous for high-throughput analysis of selected VOC metabolites in large-scale, population-based studies such as the National Health and Nutrition Examination Survey (NHANES). Quantifying these urinary biomarkers is important to public health efforts to understand human exposure to VOCs from various sources, including tobacco products and electronic nicotine delivery systems.
Keywords: Benzene, Furfural, Furfuryl alcohol, 5-Hydroxymethylfurfural, N-Methylpyrrolidone, Cyanide, VOC metabolites
Graphical Abstract
1. Introduction
Volatile organic compounds (VOCs) are ubiquitous in the environment. Chronic exposure to certain VOCs increases the risks for cancer, Parkinson’s disease, adverse reproductive outcomes, DNA mutations, respiratory irritation, cardiovascular toxicity, and developmental abnormalities [1–4]. Exposure to VOCs can come from occupational, residential, and environmental sources, including combusted tobacco products, e-cigarettes, and food contaminants [5–7]. Urinary VOC metabolites can be biomonitored to non-invasively assess parent VOC exposure. This work describes the development of a quantitative method for simultaneously measuring 8 metabolites of select parent VOCs: benzene, cyanide, furfural, furfuryl alcohol, 5-hydroxymethylfurfural (5HMF), and N-methyl-2-pyrrolidone (NMP).
Benzene is classified as a Group 1 carcinogen (known human carcinogen) by the International Agency for Research on Cancer [8]. Numerous studies have shown that benzene exposure can cause leukemia and other hematotoxic effects [7, 9, 10]. The most significant sources of benzene exposure to the general population are active and passive smoking and automobile use [9]. Occupational exposures to benzene from widespread industrial applications are prevalent, including use in the production of plastics, resins, synthetic fibers, dyes, detergents, drugs, and pesticides [11]. Additionally, benzene is present in crude oil and gasoline [7]. The metabolic pathway of benzene begins with catalytic oxidation aided by cytochrome P450 (CYP) 2E1. The benzene oxide intermediate then undergoes three different metabolic pathways leading to varying urinary excretion of the retained dose: 3.9% to trans,trans-muconic acid (tt-MA), 0.11% to S-phenyl mercapturic acid (PMA), and 40% to phenol and its conjugates [10–12]. PMA and tt-MA are the most commonly used urinary biomarkers of benzene exposure [10, 12].
Hydrogen cyanide and cyanide salts are well-known toxins. Exposure to cyanide can cause loss of consciousness, cardiac arrhythmias, and neurophysiological changes [13]. The acute neurotoxic effects of cyanide exposure include altered respiration, nausea, and vomiting. These result from cellular hypoxia, which can lead to convulsions, coma, and death [13, 14]. One of the major sources of exposure to cyanides is cigarette smoke [3]. Other sources include biomass burning, volcanic eruptions, industrial processing, and natural biogenic processes from plants, bacteria, and fungi [3, 15]. Rhodanese enzymatically converts 60–80% of the cyanide dose to thiocyanate. The other metabolic pathway involves cysteine, which converts approximately 15% of the dose to 2-aminiothiazoline-4-carboxylic acid (ATCA) and its tautomer, 2-iminothiazolidine-4-carboxylic acid (ITCA). ATCA is frequently used as a stable cyanide exposure biomarker [16, 17].
Furfural and furfuryl alcohol are reported to cause eye and mucous membrane irritation, abdominal pain, diarrhea, headache, and vomiting [18–22]. They are widely used for various industrial applications. They are neo-formed contaminants (i.e. intermediate in the Maillard reaction) produced during thermal decomposition of carbohydrate-rich food [19, 23]. Potential sources of their exposure include resins, adhesives, fungicides, inks, coffee, tobacco smoke, and e-cigarette aerosol [21, 24]. Furfural and furfuryl alcohol undergo reversible reactions in the gut of exposed humans. These include oxidation of furfuryl alcohol to furfural and vice-versa, followed by rapid oxidation to furoic acid. They are then primarily excreted as glycine conjugates (i.e. N-2-furoylglycine [N2FG]), with other minor metabolites, including furoic acid [19, 20, 25, 26].
5-hydroxymethylfurfural (5HMF) causes eye, upper respiratory tract, and mucous membrane irritation [4, 27, 28]. It is also a potential carcinogen [4, 29]. 5HMF is a neo-formed contaminant produced along with furfural and furfuryl alcohol during heating or cooking of carbohydrate-containing food. Potential sources of exposure to 5HMF include cooked food, food flavoring agents, dried fruits, coffee, caramel products, and e-cigarette aerosol [4, 23, 28]. 5HMF is believed to follow the same metabolic pathway as furfural metabolism. In animal models, 78–85% of the administered dose gets converted into 5-hydroxymethyl-2-furanoic acid (HMFA), 5–8% into 5-hydroxymethyl-2-furoylglycine (HMFG), and 2–6% into 2,5-furandicarboxylic acid (FDCA) [4, 28].
N-Methyl-2-pyrrolidone (NMP) can cause severe skin, eye, and respiratory tract irritation [30]. It is primarily used as an organic solvent for various industrial applications [31, 32]. It is a constituent of paint stripper and graffiti remover and is used as a polymer-resist stripping solvent in microelectronics fabrication. NMP is used as a formulating agent in pigments, dyes, and agricultural products such as insecticide, fungicides, and herbicides. It is also a pyrolysis product in tobacco smoke, and an additive in some tobacco products [33, 34]. Exposure to NMP can occur through inhalation, ingestion, and skin contact during its manufacturing, handling, and application. The major urinary metabolite of NMP is 5-hydroxy-N-methyl-2-pyrrolidone (5HMP), with the largest fraction (44%) excreted in urine [35]. Other metabolites include N-methylsuccinimide and 2-hydroxy-N-methylsuccinimide [31, 35].
Previously, several standalone analytical methods were developed to measure urinary biomarkers of exposure to the aforementioned VOCs [36–38]. Most of the established assays focused on measuring the metabolites of these VOCs and other ubiquitous VOCs [3, 6, 14, 36, 39, 40]. In our laboratory, we currently measure 28 VOC metabolites of exposure to 20 parent VOCs using a validated analytical assay [6]. The method comprises metabolites of exposure to combusted tobacco VOCs, including acrolein, acrylamide, acrylonitrile, 1,3-butadiene, and crotonaldehyde, along with non-tobacco-related VOCs. However, rapid increases in e-cigarette use among young adults and the presence of neo-formed contaminants in vaping aerosol [5, 23, 41] have shifted the focus of the biological monitoring beyond combustible VOCs. Quantitating exposures to furfural, furfuryl alcohol, 5HMP, and other harmful and potentially harmful constituents from electronic nicotine delivery systems is of significant interest to public health research. Therefore, we developed an ultra-performance liquid chromatography–tandem mass spectrometry (UPLC-MS/MS) method for the simultaneous measurement of 8 urinary biomarkers of exposure to benzene, cyanide, furfural, furfuryl alcohol, 5HMF, and NMP to characterize human exposure to these select VOCs. The method expands on the ability of our laboratory and others for the rapid and high-throughput analysis of VOC metabolites associated with environmental and dietary exposure to the corresponding parent VOCs.
2. Experimental
2.1. Materials.
HPLC-grade J.T. Baker water, LCMS Optima-grade ammonium formate, LCMS Optima-grade formic acid, and LCMS Optima-grade methanol were purchased from Fisher Scientific (Suwanee, GA). Analytical standard-grade FDCA was purchased from Sigma-Aldrich (St. Louis, MO). Analytical standard-grade HMFA was purchased from Santa Cruz Biotechnology (Dallas, TX). Analytical standard-grade ATCA, 5HMP, HMFG, N2FG, tt-MA, and PMA were purchased from Toronto Research Chemicals (Ontario, Canada). ATCA-[13C,15N2], HMFG-[13C2,15N], 5HMP-[2H3], FDCA-[13C6], N2FG-[2H3], and HMFA-[13C6] were purchased from Toronto Research Chemicals (Ontario, Canada). tt-MA-[13C6] was purchased from Sigma-Aldrich (St. Louis, MO). PMA-[13C6] was purchased from Cambridge Isotope Laboratories (Tewksbury, MA). Mass Spect Gold Urine was purchased from Golden West Diagnostics (Temecula, CA). ClinChek urine controls for occupational medicines, levels I/II (lyophilized), were purchased from IRIS Technologies International GmbH (Olathe, KS). A human urine pool was collected anonymously following a protocol approved by the CDC Institutional Review Board.
2.2. Calibration Solutions.
Individual master stocks of neat standards and internal standards were prepared in methanol. Master stocks were diluted in water to make working stocks. All calibration solutions were prepared by diluting the working stock solution in 5 mM ammonium formate buffer (pH 2.9). The buffer is prepared by mixing 50 mL of 200 mM ammonium formate solution, 3 mL of formic acid, and 1.947 L of water. A 200 mM ammonium formate solution is prepared by adding 3.15 g of neat ammonium formate to 250 mL HPLC grade water. All stock solutions were stored at −70 °C before use.
2.3. Sample Preparation.
A dilute-and-shoot method was selected to minimize sample preparation time. Urine samples were thawed, homogenized with a rugged rotator, and diluted 1:10 with 5 mM ammonium formate buffer before analysis.
2.4. UPLC-ESI-MS/MS Analysis.
The analytical run was performed using an Acquity I-Class UPLC system (Waters Corporation, Milford, MA) equipped with a Waters high-strength silica (HSS)-pentafluorophenyl (PFP) column (2.1 × 100 mm, 1.8 μm) and a Waters HSS-PFP VanGuard pre-column (Waters Corporation, Milford, MA). The UPLC system was coupled to a 5500 triple quadrupole mass spectrometer equipped with an electrospray ionization (ESI) source (Sciex, Framingham, MA). Chemical separation was performed using a solvent gradient of 5 mM ammonium formate buffer at pH 2.9 (mobile phase A) and methanol (mobile phase B) (Table 1). Column and sample manager temperatures were set to 30 °C and 25 °C, respectively. The injection volume was 2 μL using full loop injection mode. The mass spectrometer was operated in positive ion ESI and negative ion ESI scheduled multiple reaction monitoring modes using the polarity-switching feature. For each injection, data were collected in positive ion mode for the first 1.9 min before switching to negative ion mode for the remainder of the analysis time. The optimized ion source parameters were as follows: ESI voltage, 3.5 kV (positive mode) and −4.5 kV (negative mode); collisionally activated dissociation (CAD) gas, 7 psi; curtain gas flow, 20 psi; nebulizing gas (GS1) flow, 55 psi; heating gas (GS2) flow, 65 psi; and heater temperature, 650 °C. Fig. 1 shows the chemical structures of the urinary metabolites of interest.
Table 1.
Gradient elution table for chromatographic separation.
| Time (min) | Flow Rate (mL/min) | % Mobile Phase B (v/v) | Curve† |
|---|---|---|---|
| Initial | 0.4 | 2.5 | Initial |
| 0.6 | 0.4 | 2.5 | 6 (linear) |
| 2.8 | 0.4 | 35 | 6 (linear) |
| 4.5 | 0.4 | 80 | 5 (concave) |
| 5.5 | 0.4 | 2.5 | 1 (step) |
For the gradient curve algorithm refer to www.waters.com
Fig. 1.
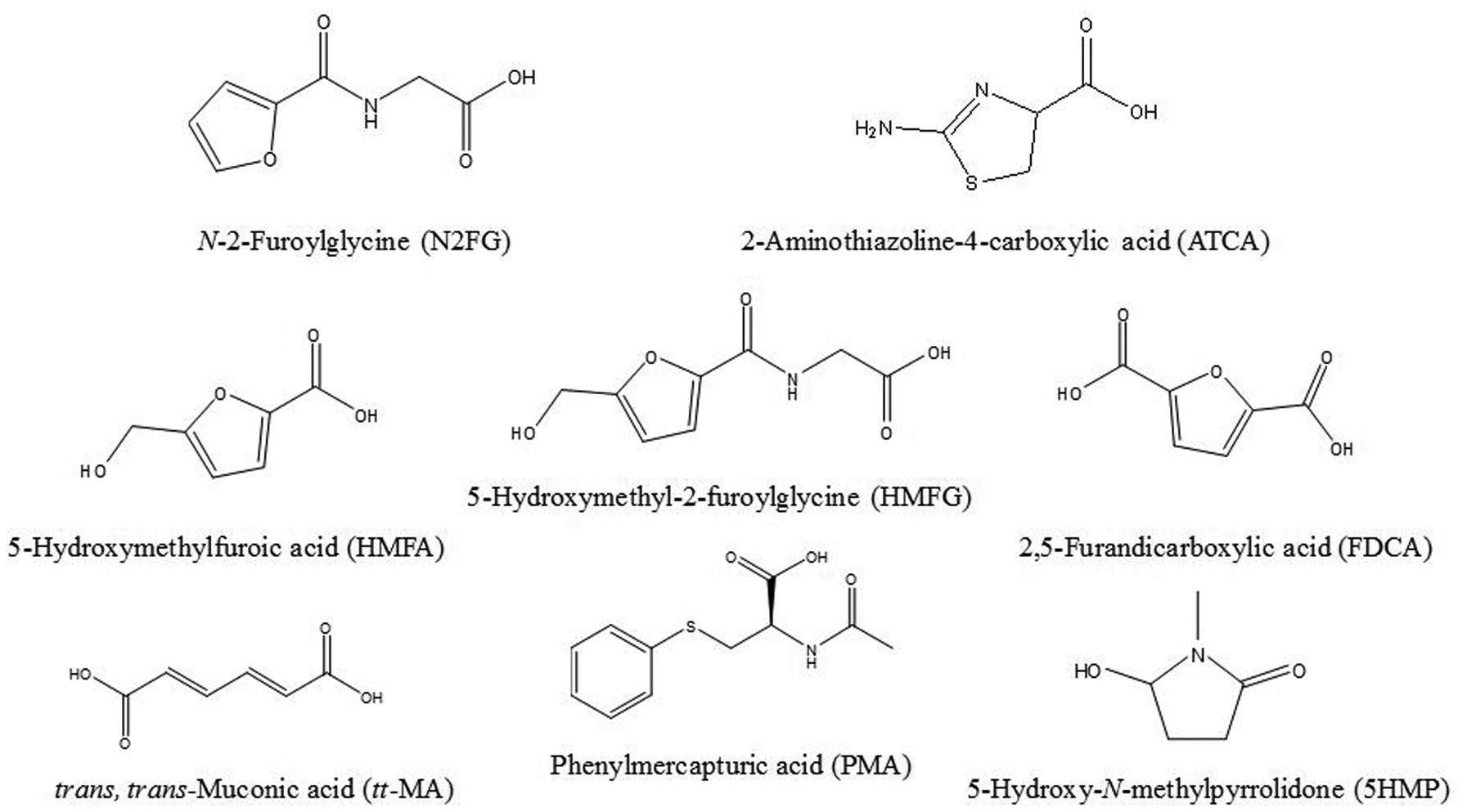
Chemical structures of urinary metabolites of interest.
2.5. Data Analysis.
All UPLC-MS/MS data were generated in Analyst 1.6.2 (Sciex, Framingham, MA) and processed in MultiQuant 3.0.3 (Sciex, Framingham, MA). Statistical analysis was performed with JMP 13 statistical software (SAS Institute, Cary, NC).
3. Results and Discussion
3.1. Method Development.
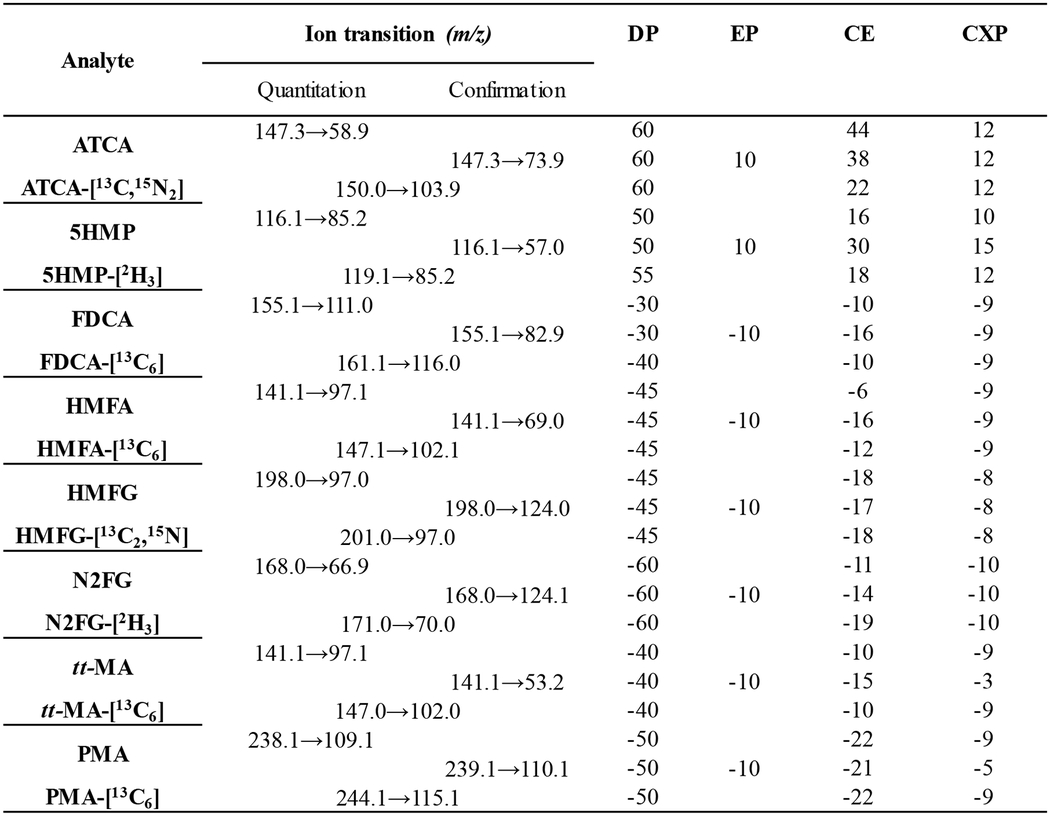
The compound-specific parameters (Table 2) for the triple quadrupole mass spectrometer were optimized by direct infusion of 0.1 μg/mL neat compound in methanol/water (50/50%, v/v) at a flow rate of 10 μL/min. The chromatographic separation was performed using a PFP-modified HSS column with reversed-phase gradient elution (Table 1). We chose the HSS-PFP column for the unique selectivity it provides for our compounds of interest. It offers various physiochemical interactions in addition to hydrophobic interactions, such as π-π, electrostatic, shape selectivity, and hydrogen bonding [42]. The buffer pH (mobile phase A) was optimized at pH 2.9 to maintain consistent reversed-phase type separation. This pH is especially important for analytes such as tt-MA, a dicarboxylic acid, which is doubly negatively charged at neutral pH. With a pKa1 and pKa2 of 3.87 and 4.65, respectively [43], and a drastic polarity difference, this dicarboxylic acid elutes closer to the void volume at pHs higher than 5 when performing a reversed-phase separation. This buffer pH was still optimal for all other analytes because their logP and logD values were comparable at pH 2.9 (Table 2). Converting these analytes to a neutral form did not affect their retention times, except diminished buffering capacity (e.g., below pH 2.7). For these reasons, pH 2.9 was optimal, and as such, analytes with the lower logD eluted first. As the percentage composition of methanol (mobile phase B) increased, the analytes with higher logD eluted sequentially, with a few exceptions (Fig. 2, Table 3). For example, N2FG and HMFG eluted later than expected, based on logD, but both compounds contain an aliphatic amide. This suggests that amide interactions might be preferentially involved in secondary retention mechanisms. The metabolites ATCA and 5HMP contain heterocycles or amine moieties, but followed the expected logD retention relationship.
Table 2.
Compound specific mass spectrometry parameters.
|
DP = declustering potential, EP = entrance potential, CE = collision energy, CXP= cell exit potential.
Fig. 2.
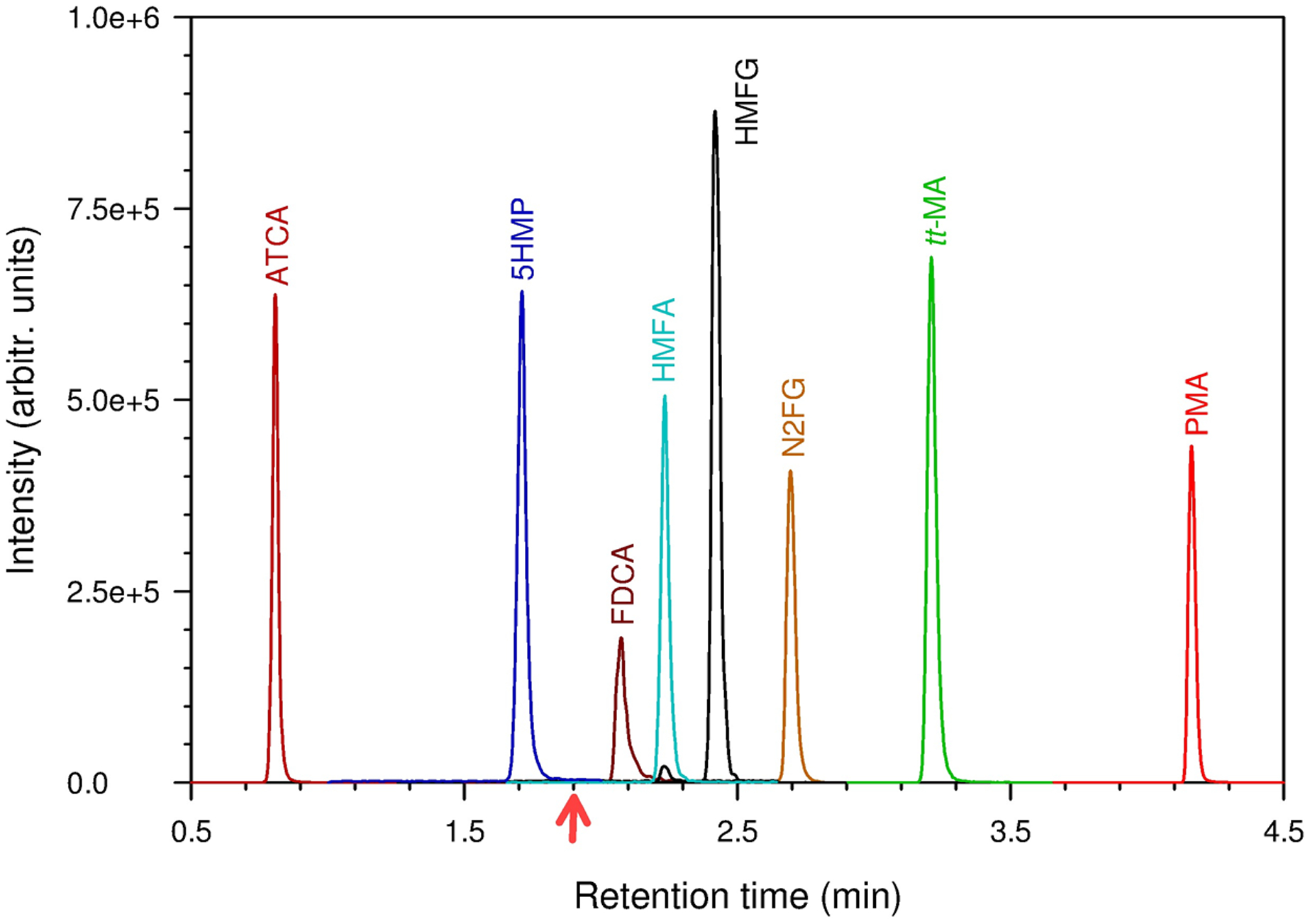
Extracted-ion chromatograms of the analytes of interest. Data were collected in positive ion electrospray ionization (ESI) mode until 1.9 min and in negative-ion ESI thereafter. Arrow on the bottom represents the time at which the ESI polarity was switched to negative ion mode from positive ion mode.
Table 3.
Physicochemical properties of analytes of interest [43]. The analytes are listed based on their chromatography elution order (Fig. 2). LogD is a logarithm of the ratio of chemical species in octanol to water at a specific pH. LogP is equivalent to logD for neutral species.
| Analytes | Strongest acidic pKa | Strongest basic pKa | logP | logD at pH=2.9 |
|---|---|---|---|---|
| ATCA | 2.28 | 8.15 | −1.75 | −1.825 |
| 5HMP | 13.49 | - | −0.95 | −0.949 |
| FDCA | 2.76 | - | 0.27 | −0.171 |
| HMFA | 2.96 | - | −0.16 | −0.364 |
| HMFG | 3.40 | - | −1.26 | −1.382 |
| N2FG | 3.34 | - | −0.41 | −0.577 |
| tt-MA | 3.87 | - | 0.49 | 0.412 |
| PMA | 3.80 | −2.00 | 1.05 | 1.002 |
Methanol (mobile phase B) is an important contributor to the column selectivity. Acetonitrile disrupts π-π interactions between solvated analytes and the PFP-modified surface by way of its carbon-nitrogen triple bond. Methanol resolved analytes more effectively because it does not inhibit such interactions [44].
3.2. Quantitative Analysis.
Quantitative studies were performed using calibration curves with stable isotope-labelled internal standards. The concentration of calibration curves spanned more than 3 orders of magnitude with the coefficient of determination (r2) exceeding 0.99 when fitted by linear regression on 1/x weighted data. Because the working stock calibrators were prepared in water, a matrix validation experiment was performed to investigate the use of non-urine–based calibrators. The slopes of two calibration curves, one made in water and one in pooled human urine, were compared for each analyte. The experiments were repeated 3 times. Table 4 shows the average percentage difference in the slopes for each analyte. The results were within 4% difference, which verified the use of non-urine–based calibrators for this assay. It is noteworthy that at least 10 times sample dilution was required to be able to use non-urine based calibrator.
Table 4.
Comparison of calibration curve slopes for different types of matrices.
| Analyte | Slope (N = 3) | Percent Difference | |
|---|---|---|---|
| Water | Pooled Urine | ||
| ATCA | 0.0026 | 0.0027 | 3.4 |
| 5HMP | 0.34 | 0.36 | 3.2 |
| FDCA | 0.017 | 0.017 | 0.10 |
| HMFA | 0.0040 | 0.0041 | 2.1 |
| HMFG | 0.0050 | 0.0049 | 2.1 |
| N2FG | 0.0036 | 0.0036 | 0.70 |
| tt-MA | 0.016 | 0.016 | 0.020 |
| PMA | 0.17 | 0.17 | 0.29 |
Table 5 shows the method limits of detection (LODs) and the range of linearity in non-urine matrix. The LODs were calculated based as 3S0, where S0 is the standard deviation at zero concentration from a standard deviation versus concentration plot [45]. The calculated LODs were in the low to sub-nanograms per milliliter range, with the lowest value (0.097 ng/mL) for PMA and the highest value (16.4 ng/mL) for FDCA. Because the injection volume was 2 μL, the on-column injection detection limit was between 0.19 pg/mL for PMA and 33 pg/mL for FDCA. The carryover effect was studied using the highest calibrator. Following a highest calibrator, no detectable peak was observed for any target analytes on the next injection of a blank solvent.
Table 5.
Method LODs and linearity range.
| Analytes | Method LOD (ng/mL) | Linearity Range (ng/mL) |
|---|---|---|
| ATCA | 0.835 | 0.184 – 582 |
| 5HMP | 0.274 | 0.0253 – 80.0 |
| FDCA | 16.4 | 9.99 – 3160 |
| HMFA† | 13.1 | 2.37 – 7500 |
| HMFG | 7.12 | 1.00 – 3160 |
| N2FG† | 6.71 | 3.95 – 12500 |
| tt-MA | 1.20 | 0.759 – 759 |
| PMA | 0.0970 | 0.00950 – 9.49 |
HMFA and N2FG instrumental parameters (collision energy) were detuned to avoid detector saturation.
Previous studies of humans who had no occupational benzene exposure have shown that the mean or median concentration of urinary tt-MA ranged from 30 to 300 μg/g creatinine and that tt-MA was successfully quantified using methods with LOD ≥ 5 ng/mL [10, 46]. Similarly, the central tendency of urinary PMA concentrations in non-occupationally exposed people ranged from 0.3 to 8.9 μg/g creatinine, and PMA was quantified using methods with LOD ≥ 0.2 ng/mL [10]. The LODs calculated in this method are lower than those reported values (Table 5). Our method has sufficient sensitivity for the proper assessment of biomarkers of environmental and occupational exposures to benzene. For the remaining metabolites, the method LODs or the average baseline concentration of the non-exposed urine samples were higher than the LODs calculated in our method [3, 4, 27, 39].
Accuracy was checked through spiking on two different urine specimens: 1) Mass Spect Gold Urine containing low endogenous levels of analytes and 2) a urine pool with higher endogenous levels of analytes. Each specimen was spiked with standard mix to make 3 samples of varied concentration. Samples were run in triplicate and repeated for 2 days. The results show that accuracy through spiking and the relative standard deviation (RSD) were within 15% (Table 6). Accuracy relative to the reference materials was determined when applicable. The ClinChek control reference materials containing benzene metabolites tt-MA and PMA were repeated for 5 days. The results were within 10.1% difference with respect to the nominal value provided by the vendors (Table 7). No other target analytes were available in the ClinChek controls. The interday precision of the method also was evaluated in spiked urine pools at two different concentrations for 10 days over 4 weeks. The percentage RSD varied between 2.2% for ATCA and 12.5% for PMA.
Table 6.
Accuracy through spiking.
| Analyte | Sample ID | Spiked Conc. (ng/mL) | Sample 1 (Mass Spect Gold Urine) | Spiked Conc. (ng/mL) | Sample 2 (Urine Pool) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Calculated Conc. (ng/mL) | Accuracy (% Error) | RSD (%) | Calculated Conc. (ng/mL) | Accuracy (% Error) | RSD (%) | ||||
| ATCA | Blank | 0 | 7.04 | NA | 4.7 | 0 | 101.25 | NA | 3.4 |
| Blank + Spike 1 | 29.1 | 34.7 | −3.96 | 2.2 | 29.1 | 126 | −2.99 | 2.2 | |
| Blank + Spike 2 | 92.0 | 96.8 | −2.26 | 2.7 | 92.0 | 195 | 0.66 | 2.4 | |
| Blank + Spike 3 | 920 | 99.3 | −0.738 | 2.6 | 920 | 997 | −2.37 | 2.8 | |
| 5HMP | Blank | 0 | 3.02 | NA | 4.0 | 0 | 13.3 | NA | 4.5 |
| Blank + Spike 1 | 12.6 | 16.6 | 5.61 | 2.6 | 12.7 | 25.9 | −0.184 | 2.9 | |
| Blank + Spike 2 | 40.0 | 43.4 | 0.771 | 1.3 | 40.0 | 55.6 | 4.19 | 1.8 | |
| Blank + Spike 3 | 400 | 403 | −0.010 | 2.6 | 400 | 403 | −2.48 | 2.0 | |
| FDCA | Blank | 0 | 0 | NA | 0.0 | 0 | 858 | NA | 4.0 |
| Blank + Spike 1 | 158 | 166 | 4.99 | 4.8 | 158 | 1020 | 0.110 | 5.8 | |
| Blank + Spike 2 | 500 | 472 | −5.59 | 5.6 | 500 | 1410 | 3.95 | 3.6 | |
| Blank + Spike 3 | 5000 | 5096 | 1.93 | 2.9 | 5000 | 5720 | −2.44 | 4.2 | |
| HMFA | Blank | 0 | 0 | NA | 0.0 | 0 | 1098 | NA | 8.8 |
| Blank + Spike 1 | 158 | 163 | 3.32 | 6.3 | 1580 | 2790 | 3.98 | 7.0 | |
| Blank + Spike 2 | 500 | 513 | 2.49 | 9.1 | 5000 | 5960 | −2.27 | 0.9 | |
| Blank + Spike 3 | 5000 | 5480 | 9.58 | 14.7 | 10000 | 11330 | 2.09 | 7.2 | |
| HMFG | Blank | 0 | 0.00 | NA | 0.0 | 0 | 148 | NA | 8.6 |
| Blank + Spike 1 | 158 | 166 | 5.24 | 3.9 | 1580 | 1690 | −2.27 | 7.7 | |
| Blank + Spike 2 | 500 | 547 | 9.44 | 5.2 | 5000 | 5170 | 0.41 | 6.1 | |
| Blank + Spike 3 | 5000 | 4990 | −0.166 | 6.2 | 10000 | 10040 | −1.05 | 6.5 | |
| N2FG | Blank | 0 | 0 | NA | 0.0 | 0 | 739 | NA | 6.4 |
| Blank + Spike 1 | 158 | 160 | 1.02 | 5.0 | 1580 | 2450 | 5.54 | 5.4 | |
| Blank + Spike 2 | 500 | 528 | 5.56 | 1.4 | 5000 | 5960 | 3.78 | 8.2 | |
| Blank + Spike 3 | 5000 | 5330 | 6.49 | 7.4 | 10000 | 10800 | 0.450 | 3.0 | |
| tt-MA | Blank | 0 | 0 | NA | 0.0 | 0 | 13.3 | NA | 7.5 |
| Blank + Spike 1 | 38.0 | 37.5 | −1.06 | 4.0 | 38.0 | 46.3 | −9.61 | 3.6 | |
| Blank + Spike 2 | 120 | 114 | −5.25 | 2.8 | 120 | 129 | −3.33 | 5.4 | |
| Blank + Spike 3 | 1200 | 1160 | −3.13 | 1.1 | 1200 | 1150 | −5.53 | 1.6 | |
| PMA | Blank | 0 | 0 | NA | 0.0 | 0 | 0 | NA | 0.0 |
| Blank + Spike 1 | 0.632 | 0.576 | −9.78 | 8.4 | 0.630 | 0.500 | −13.9 | 11 | |
| Blank + Spike 2 | 2.00 | 1.94 | −3.34 | 3.8 | 2.00 | 2.00 | −2.19 | 7.2 | |
| Blank + Spike 3 | 20.0 | 19.6 | −2.10 | 1.6 | 20.0 | 19.0 | −5.092 | 0.8 | |
Table 7.
Accuracy with respect to ClinChek control reference materials for PMA and tt-MA.
| Analyte | Nominal Concentration (ng/mL) | Level 1 (N = 10) | Nominal Concentration (ng/mL) | Level 2 (N = 10) | ||||
|---|---|---|---|---|---|---|---|---|
| Calculated Concentration (ng/mL) | Accuracy (% Error) | RSD (%) | Calculated Concentration (ng/mL) | Accuracy (% Error) | RSD (%) | |||
| PMA | 4.83 | 5.25 | 8.8 | 3.7 | 44 | 48.4 | 10.1 | 2.7 |
| tt-MA | 976 | 906 | −7.2 | 3.1 | 2990 | 2889 | −3.4 | 2.6 |
3.3. Stability.
The sample stability of spiked urine pools at two different concentrations and prepared in triplicate were examined to determine optimal handling and storage conditions. Bench-top stability was assessed for the length of time needed to handle study samples at room temperature (i.e. 4 h). The calculated percent error for the bench-top stability ranged from −0.8% (ATCA) to 11.4% (HMFG), with reference to the control (i.e. t = 0 h). Similarly, processed sample stability was evaluated on prepared samples stored in the autosampler at room temperature for 24 h. The percentage error for the processed sample stability compared with controls prepared the same day was within 10%.
Freeze-thaw stability was assessed for 3 freeze-thaw cycles. Samples were stored at −70 °C for at least 24 h between each cycle and then thawed for sample preparation. The percentage error ranged from −0.3% (5HMP) to 11.4% (HMFA).
3.4. Application to Human Specimens.
We measured urinary biomarkers of exposure to benzene in urine collected from 25 daily smokers and 25 non-smokers (Tennessee Blood Services). Cigarette smoke is known to contain benzene; smoking a pack of cigarettes leads to inhalation of low milligram quantities of benzene [47]. In this study, smokers and non-smokers were categorized based on the self-reported data provided by the vendor. The total detection frequencies for PMA and tt-MA were 70% and 100%, respectively. The calculated data below LOD were imputed as LOD/√(2) for the descriptive statistics [48]. The results were then logarithmically transformed for the Student’s t-test at 95% confidence interval (α = 0.05). Smokers had significantly higher urinary PMA and tt-MA compared with non-smokers. Table 8 summarizes the urinary geometric mean concentration and standard error, along with p-values for smokers and non-smokers samples. Our results are consistent with previously published reports of statistically higher urinary concentrations of PMA and tt-MA in smokers compared with non-smokers [10].
Table 8.
Urinary geometric mean (GM) concentration and standard error (SE) of benzene metabolites in 25 smoker and 25 non-smoker samples.
| Analyte | GM [SE] ng/mL | p-value | |
|---|---|---|---|
| Smoker | Non-smoker | ||
| PMA | 1.23 [0.737] | 0.298 [0.252] | 0.0002 |
| tt-MA | 144 [92.7] | 68.0 [16.0] | 0.0171 |
3.5. Sample Throughput.
This method is based on the dilute-and-shoot approach and requires minimal sample preparation. Including column equilibration and autosampler movement, the total sample analysis time per injection is 6 min. Therefore, the instrument time required to analyze 96 samples is 9.6 h. In addition, the sample preparation time per 96-well plate using an automated liquid handling system is about 1 h. In its current stage, the method can generate 768 analytical results in less than 12 h.
4. Conclusions
We developed a novel UPLC-ESI-MS/MS method to measure urinary metabolites of exposure to selected VOCs. This assay includes the assessment of urinary metabolites of known carcinogens, such as benzene, and other neo-formed toxicants of emerging interests. The method monitors parent compounds found in mainstream cigarette smoke (benzene, cyanide, furfuryl alcohol, NMP) and e-cigarette aerosol (furfural, 5HMP). Validation of the method’s accuracy, precision, and ruggedness showed it can be used as a rapid, high-throughput assay for nationally representative studies. The method showed that people with known benzene exposure (daily cigarette smokers) had higher levels of PMA and tt-MA compared with non-smokers. The method offers short run times via a simple dilute-and-shoot approach, but maintains superior selectivity and lower limits of detection.
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Use of trade names is for identification only and does not imply endorsement by the Centers for Disease Control and Prevention, the Public Health Service, or the U.S. Department of Health and Human Services.
References
- [1].Heinrich-Ramm R, Jakubowski M, Heinzow B, Christensen JM, Olsen E, Hertel O, Biological monitoring for exposure to volatile organic compounds (VOCs) (IUPAC Recommendations 2000), Pure Appl Chem, 2000, pp. 385. [Google Scholar]
- [2].Fowles J, Dybing E, Application of toxicological risk assessment principles to the chemical constituents of cigarette smoke, Tob Control, 12 (2003) 424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Vinnakota CV, Peetha NS, Perrizo MG, Ferris DG, Oda RP, Rockwood GA, Logue BA, Comparison of cyanide exposure markers in the biofluids of smokers and non-smokers, Biomarkers, 17 (2012) 625–633. [DOI] [PubMed] [Google Scholar]
- [4].Capuano E, Fogliano V, Acrylamide and 5-hydroxymethylfurfural (HMF): A review on metabolism, toxicity, occurrence in food and mitigation strategies, Food Sci Technol, 44 (2011) 793–810. [Google Scholar]
- [5].Margham J, McAdam K, Forster M, Liu C, Wright C, Mariner D, Proctor C, Chemical composition of aerosol from an E-Cigarette: A quantitative comparison with cigarette smoke, Chem Res Toxicol, 29 (2016) 1662–1678. [DOI] [PubMed] [Google Scholar]
- [6].Alwis KU, Blount BC, Britt AS, Patel D, Ashley DL, Simultaneous analysis of 28 urinary VOC metabolites using ultra high performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (UPLC-ESI/MSMS), Anal Chim Acta, 750 (2012) 152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Smith MT, Advances in Understanding Benzene Health Effects and Susceptibility, Annu Rev Public Health, 31 (2010) 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].IARC Monographs on the Identification of Carcinogenic Hazards to Humans, International Agency for Research on Cancer, https://monographs.iarc.fr/list-of-classifications-volumes/, 2018. [Google Scholar]
- [9].Wallace L, Environmental exposure to benzene: an update, Environ Health Perspect, 104 (1996) 1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Arnold SM, Angerer J, Boogaard PJ, Hughes MF, O’Lone RB, Robison SH, Robert Schnatter A, The use of biomonitoring data in exposure and human health risk assessment: benzene case study, Cri Rev Toxicol, 43 (2013) 119–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ghittori S, Maestri L, Fiorentino ML, Imbriani M, Evaluation of occupational exposure to benzene by urinalysis, Int Arch Occup Environ Health, 67 (1995) 195–200. [DOI] [PubMed] [Google Scholar]
- [12].Boogaard PJ, van Sittert NJ, Suitability of S-phenyl mercapturic acid and trans-trans-muconic acid as biomarkers for exposure to low concentrations of benzene, Environ Health Perspect, 104 (1996) 1151–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Newhouse K, Chiu N, Toxicological Review of Hydrogen Cyanide and Cyanide Salts, U.s. Environmental Protection Agency, Washington DC, 2010. [Google Scholar]
- [14].Giebułtowicz J, Rużycka M, Fudalej M, Krajewski P, Wroczyński P, LC-MS/MS method development and validation for quantitative analyses of 2-aminothiazoline-4-carboxylic acid – a new cyanide exposure marker in post mortem blood, Talanta, 150 (2016) 586–592. [DOI] [PubMed] [Google Scholar]
- [15].Taylor J, Roney N, Harper C, Fransen ME, Swarts S, Toxicological Profile for Cyanide, Agency for Toxic Substances and Disease Registry (ATSDR), 2006. [PubMed]
- [16].Logue BA, Hinkens DM, Baskin SI, Rockwood GA, The Analysis of Cyanide and its Breakdown Products in Biological Samples, Crit Rev Anal Chem, 40 (2010) 122–147. [Google Scholar]
- [17].Logue BA, Kirschten NP, Petrikovics I, Moser MA, Rockwood GA, Baskin SI, Determination of the cyanide metabolite 2-aminothiazoline-4-carboxylic acid in urine and plasma by gas chromatography–mass spectrometry, J Chromatogr B, 819 (2005) 237–244. [DOI] [PubMed] [Google Scholar]
- [18].Furfural, The National Institute of Occupational Safety and Health (NIOSH); Available at https://www.cdc.gov/niosh/ipcsneng/neng0276.html, 2015.
- [19].Cary R, Dobson S, Gregg N, 2-Furaldehyde, Concise international chemical assessment document, World Health Organization, Geneva, 2000. [Google Scholar]
- [20].Toxicology and Carcinogenesis Studies of Furfuryl Alcohol (CAS No. 98-00-0) in F344/N Rats and B6C3F1 Mice (Inhalation Studies), National Toxicology Program, 1999. [PubMed]
- [21].Nomeir AA, Silveira DM, McComish MF, Chadwick M, Comparative metabolism and disposition of furfural and furfuryl alcohol in rats, Drug Metab Dispos, 20 (1992) 198–204. [PubMed] [Google Scholar]
- [22].Furfural, IARC Monographs, https://www.cdc.gov/niosh/ipcsneng/neng0276.html.
- [23].Soussy S, El-Hellani A, Baalbaki R, Salman R, Shihadeh A, Saliba NA, Detection of 5-hydroxymethylfurfural and furfural in the aerosol of electronic cigarettes, Tob Control, 25 (2016) ii88–ii93. [DOI] [PubMed] [Google Scholar]
- [24].Sakuma H, Kusama M, Yamaguchi K, Sugawara S, The Distribution of Cigarette Smoke Components between Mainstream and Sidestream Smoke, Beitrage zur Tabakforschung International/Contributions to Tobacco Research, 12 (1984) 251–258. [Google Scholar]
- [25].Flek J, Sedivěc V, The Absorption, metabolism and excretion of furfural in man, Int Arch Occup Environ Health, 41 (1978) 159–168. [DOI] [PubMed] [Google Scholar]
- [26].Substance Evaluation Conclusion and Evaluation Report for Furfuryl Alcohol, http://echa.europa.eu/, 2018.
- [27].Hardt-Stremayr M, Mattioli S, Greilberger J, Stiegler P, Matzi V, Schmid MG, Wintersteiger R, Determination of metabolites of 5-hydroxymethylfurfural in human urine after oral application, J Sep Sci, 36 (2013) 670–676. [DOI] [PubMed] [Google Scholar]
- [28].Abraham K, Gürtler R, Berg K, Heinemeyer G, Lampen A, Appel KE, Toxicology and risk assessment of 5-Hydroxymethylfurfural in food, Mol Nutr Food Res, 55 (2011) 667–678. [DOI] [PubMed] [Google Scholar]
- [29].Sachse B, Meinl W, Sommer Y, Glatt H, Seidel A, Monien BH, Bioactivation of food genotoxicants 5-hydroxymethylfurfural and furfuryl alcohol by sulfotransferases from human, mouse and rat: a comparative study, Arch Toxicol, 90 (2016) 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Langworth S, Anundi H, Friis L, Johanson G, Lind ML, Söderman E, Åkesson BA, Acute health effects common during graffiti removal, Int Arch Occup Environ Health, 74 (2001) 213–218. [DOI] [PubMed] [Google Scholar]
- [31].Bader M, Wrbitzky R, Blaszkewicz M, Schäper M, van Thriel C, Human volunteer study on the inhalational and dermal absorption of N-methyl-2-pyrrolidone (NMP) from the vapour phase, Archiv Toxicol, 82 (2008) 13–20. [DOI] [PubMed] [Google Scholar]
- [32].Beaulieu HJ, Schmerber KR, M-Pyrol™ (NMP) Use in the Microelectronics Industry, Appl Occup Environ Hyg, 6 (1991) 874–880. [Google Scholar]
- [33].Schmeltz I, Hoffmann D, Nitrogen-Containing Compounds in Tobacco and Tobacco Smoke, Chem Rev, 77 (1977) 295–311. [Google Scholar]
- [34].Testing Status of N-Methyl-2-pyrrolidone M20172, National Toxicology Program; U.S. Department of Health and Human Services. [Google Scholar]
- [35].Åkesson B, Jönsson B.A.g., Major Metabolic Pathway for N-Methyl-2-Pyrrolidone in Humans, Drug Metab Dispos, 25 (1997) 267–269. [PubMed] [Google Scholar]
- [36].Paci E, Pigini D, Cialdella AM, Faranda P, Tranfo G, Determination of free and total S-phenylmercapturic acid by HPLC/MS/MS in the biological monitoring of benzene exposure, Biomarkers, 12 (2007) 111–122. [DOI] [PubMed] [Google Scholar]
- [37].Melikian AA, O’Connor R, Prahalad AK, Hu P, Li H, Kagan M, Thompson S, Determination of the urinary benzene metabolites S-phenylmercapturic acid and trans,trans-muconic acid by liquid chromatography-tandem mass spectrometry, Carcinogen, 20 (1999) 719–726. [DOI] [PubMed] [Google Scholar]
- [38].Darrall KG, Figgins JA, Brown RD, Determination of benzene and associated volatile compounds in mainstream cigarette smoke, Analyst, 123 (1998) 1095–1101. [DOI] [PubMed] [Google Scholar]
- [39].Åkesson B, Jönsson B.A.g., Biological monitoring of N-methyl-2-pyrrolidone using 5-hydroxy-N-methyl-2-pyrrolidone in plasma and urine as the biomarker, Scandinavian Journal of Work, Environment & Health, 26 (2000) 213–218. [DOI] [PubMed] [Google Scholar]
- [40].Ding YS, Blount BC, Valentin-Blasini L, Applewhite HS, Xia Y, Watson CH, Ashley DL, Simultaneous determination of six mercapturic acid metabolites of volatile organic compounds in human urine, Chem Res Toxicol, 22 (2009) 1018–1025. [DOI] [PubMed] [Google Scholar]
- [41].Kaur G, Muthumalage T, Rahman I, Mechanisms of toxicity and biomarkers of flavoring and flavor enhancing chemicals in emerging tobacco and non-tobacco products, Toxicol Lett, 288 (2018) 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Acquity UPLC Columns, http://www.waters.com/waters/en_US/ACQUITY-UPLC-Columns/nav.htm?locale=en_US&cid=513206.
- [43].www.chemicalize.com ChemAxon.
- [44].Nagy G, Peng T, Kabotso DEK, Novotny MV, Pohl NLB, Protocol for the purification of protected carbohydrates: Toward coupling automated synthesis to alternate-pump recycling high-performance liquid chromatography, Chem Commun, 52 (2016) 13253–13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Taylor JK, Quality Assurance of Chemical Measurements, CRC Press LLC; 1987. [Google Scholar]
- [46].Lee BL, Ong HY, Ong YB, Ong CN, A sensitive liquid chromatographic method for the spectrophotometric determination of urinary trans,trans-muconic acid, J Chromatog B, 818 (2005) 277–283. [DOI] [PubMed] [Google Scholar]
- [47].Pazo DY, Moliere F, Sampson MM, Reese CM, Agnew-Heard KA, Walters MJ, Holman MR, Blount BC, Watson CH, Chambers DM, Mainstream smoke levels of volatile organic compounds in 50 U.S. domestic cigarette brands smoked with the ISO and Canadian intense protocols, Nicotine Tob Res, 18 (2016) 1886–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Verbovsek T, a comparison of parameters below the limit of detection in geochemical analyses by substitution method, RMZ - Materials and Geoenvironment, 58 (2011) 393–404. [Google Scholar]


