Abstract
Considerable progress has been made over the last decades in the management of acute respiratory distress syndrome (ARDS). Mechanical ventilation(MV) remains the cornerstone of supportive therapy for ARDS. Lung-protective MV minimizes the risk of ventilator-induced lung injury (VILI) and improves survival. Several parameters contribute to the risk of VILI and require careful setting including tidal volume (VT), plateau pressure (Pplat), driving pressure (ΔP), positive end-expiratory pressure (PEEP), and respiratory rate. Measurement of energy and mechanical power allows quantification of the relative contributions of various parameters (VT, Pplat, ΔP, PEEP, respiratory rate, and airflow) for the individualization of MV settings. The use of neuromuscular blocking agents mainly in cases of severe ARDS can improve oxygenation and reduce asynchrony, although they are not known to confer a survival benefit. Rescue respiratory therapies such as prone positioning, inhaled nitric oxide, and extracorporeal support techniques may be adopted in specific situations. Furthermore, respiratory weaning protocols should also be considered. Based on a review of recent clinical trials, we present 10 golden rules for individualized MV in ARDS management.
Keywords: Acute respiratory distress syndrome (ARDS), Protective mechanical ventilation, Extracorporeal CO2 removal (ECCO2R), Extracorporeal membrane oxygenation (ECMO)
Introduction
Acute respiratory distress syndrome (ARDS) was first described more than 50 years ago [1]. Despite substantial research on effective causal/supportive therapies since then, ARDS remains hard to treat,with 33.2 deaths in every 100,000 ARDS-related cases in the United States and between 2.6 and 7.2 in every 100,000 people in Europe, with a declining annual rate [2].
It is estimated that more than 3 million people/year are affected by ARDS [3], accounting for up to 10% of intensive care unit (ICU) admissions each year globally and requiring mechanical ventilation (MV) that can itself damage the already injured ARDS lung [4]. Ventilator-induced lung injury (VILI) is the main consequence of injurious MV [5]. Great effort has been made to identify possible ventilatory strategies to mitigate VILI risk in critically ill patients with ARDS [6]. Several randomized controlled trials (RCTs) and observational studies have investigated the role of lung-protective MV on ARDS outcome, thus revolutionizing conventional ventilatory management [7], [8], [9], [10], [11]. Moreover, extracorporeal carbon dioxide removal (ECCO2R) [12], extracorporeal membrane oxygenation (ECMO) [13], inhaled vasodilators [14], neuromuscular blocking agents (NMBAs) [15], and prone positioning [16], [17] have been discussed by multidisciplinary groups in recent guidelines as potential rescue strategies for more severe cases [18], [19].
The aim of this review is to provide health practitioners with an up-to-date list of golden rules for diagnosing, classifying, and treating ARDS according to new findings in this research area.
Rule 1. Classification of severity
ARDS is a syndrome and not a disease [20], that is characterized by inflammatory lung injury resulting in parenchymal stiffening and consolidation, alveolar closure, altered vascular permeability, an increase in lung water content and, eventually, severe gas exchange failure with acute onset of hypoxemia. The most current definition of ARDS is the Berlin definition, proposed in 2012 by a consensus panel of experts [21], which ontlines the following 4 criteria that must be simultaneously met for a diagnosis of ARDS: (1) a certain degree of hypoxemia, evaluated by measuring the partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ratio; (2) acute onset of hypoxemia, with respiratory symptoms beginning within 1 week of clinical insult; (3) presence of bilateral opacities on chest imaging that are not fully explained by pleural effusion, alveolar/lobar collapse, or nodules; and (4) absence of cardiac failure and/or fluid overload. The Berlin definition also classifies ARDS severity based on the PaO2/FiO2 ratio with a positive end-expiratory pressure (PEEP) or continuous positive airway pressure >5 cmH2O: mild ARDS (PaO2/FiO2 ratio between 200 and 300), with a predicted mortality of 27%; moderate ARDS (PaO2/FiO2 ratio between 100 and 200), with a predicted mortality of 32%; and severe ARDS (PaO2/FiO2 ratio <100), with a predicted mortality of 45% [21]. In 2013, Villar et al. [22] modified the definition of ARDS severity with the aim of assessing ICU mortality risk according to the PaO2/FiO2 ratio; the authors tested 2 levels of PEEP and FiO2 (PEEP ≥5 and ≥10 cmH2O and FiO2 ≥0.5 and 1.0) at 24 h after ARDS diagnosis, and concluded that ARDS risk stratification is best achieved with PEEP ≥10 cmH2O and FiO2 ≥0.5, with a mortality rate of 17%, 41%, and 58% in mild, moderate, and severe ARDS groups, respectively. As with ARDS risk stratification, ARDS phenotypes have yet to be clearly defined. Calfee et al. [23] incorporated 2 phenotypes into their definition of ARDS. Phenotype 1 is characterized by less severe inflammation and shock. Phenotype 2 is characterized by higher plasma concentrations of inflammatory biomarkers, lower serum bicarbonate concentrations, more frequent use of vasopressors, and higher prevalence of sepsis; it is also associated with a higher mortality, fewer ventilator-free days, and different responses (e.g., mortality and ventilator-free days) to high PEEP vs. low PEEP, which is similar to the phenotype of coronavirus disease 2019(COVID-19) [24]. Thus, the Berlin definition and the classification of ARDS severity and prognostic accuracy remain controversial.
Rule 2. Tidal volume(VT), plateau pressure(Pplat), and driving pressure(ΔP)
The previous convention for MV in ARDS was a tidal volume (VT) of 10–15 ml/kg of predicted body weight (PBW) [8].Over the past decades, much has been learned concerning the detrimental sequelae of MV such as lung overdistention (e.g., in the case of a high VT) with subsequent volutrauma, which along with atelectrauma and biotrauma constitutethe basis for VILI [25], [26]. A multicenter RCT conducted in 2000 changed the clinical management of ARDS. The trial was interrupted after enrolling 861 patients because of a higher mortality rate and fewer ventilator-free days in patients treated with conventional VT (12 ml/kg of PBW and Pplat of 50 cmH2O) compared to those treated with a lower VT (6 ml/kg of PBW and Pplat of 30 cmH2O) [8]. The ARDSNet study attempted to maintain the partial pressure of carbon dioxide (PaCO2) as close to the normal range as possible, resulting in a higher respiratory rate (25–30 breaths/min), which is often required to maintain PaCO2 below 50 mmHg but can lead to dynamic hyperinflation and insufflation. Although hypercapnia can induce catecholamine release and increase pulmonary vascular resistance, it also suppresses inflammation and the production of free radicals [27]. Current guidelines suggest the use of a heated humidifier to control hypercapnia; however, VT can be increased over 6 ml/kg (PBW) in the case of marked and persistent hypercapnia with an already increased respiratory rate and reduced dead space [19]. A recent study comparing VT ≤ 6.5 ml/kg and ≥6.5 ml/kg found that an increase of 1 ml/kg PBW was associated with an increased risk of death (hazard ratio=1.23, 95% confidence interval [CI]: 1.06–1.44, P=0.008) [28]. In contrast, in the LUNG SAFE study, VT ≥ 7.1 ml/kg was not associated with increased mortality but Pplat, PEEP, and ΔP were shown to significantly influence outcome measures [29]. Thus, Pplat is an important parameter in the pathogenesis of VILI along with VT and PEEP, all of which are included in the calculation of static compliance [30]. A lower VT was associated with better survival but only if Pplat was <27 cmH2O [31]; on the other hand, a high VT was associated with increased oxygenation and improved compliance but also a higher rate of mortality [31], [32]. A recent study reported that Pplat was a more important determinant of mortality and outcome than ΔP[33]. ΔP is defined as VT/Crs (respiratory system compliance). In this formula, VT is normalized to Crs of the damaged respiratory system and may be a better predictor of survival than VT scaled to normal lung volume using PBW, which is determined by height and sex [34]. In other words, ΔP represents the distending pressure in the respiratory system when VT is delivered by the ventilator. VT, PEEP, and Pplat may contribute to VILI but can also interact in a complex manner; therefore, the relationship between any single parameter and mortality is unclear [35]. As Crs is directly associated with normal aerated lung volume, it was suggested the ΔP is the best parameter for predicting mortality in ARDS patients [36]. A posthoc analysis of published trials demonstrated that ΔP was highly correlated with mortality rate [34]. PEEP and VT may have protective effects only in association with a decreasd ΔP. Another study suggested targeting ΔP to below 13–15 cmH2O [37]. Whether PEEP should be set to minimize the value of ΔP is debated; this increased mortality rate in a recent trial [38]. Thus, setting parameters based on a reduction in ΔP is not recommended, and Pplat remains the most important parameter for protecting against lung damage [33]. Finally, the best ΔP should not be used to optimize MV in ARDS.
Rule 3. PEEP
PEEP is an essential aspect of ARDS management [21]. Benefits of using PEEP include alveolar recruitment, reduction of intrapulmonary shunt, and arterial oxygenation [39]; on the other hand, detrimental effects include an increased end-inspiratory lung volume and elevated risks of volutrauma and VILI [40]. Current guidelines recommend reserving high PEEP for patients with moderate or severe ARDS and avoiding it in mild cases [41]. In a secondary analysis of the Lung Open Ventilation Study, patients with ARDS who showed improved oxygenation with high PEEP had a lower risk of death (odds ratio=0.8; 95% CI: 0.72–0.89), while changes in compliance and dead space were unrelated to mortality [42]. The threshold for defining high vs. low PEEP is 12 cmH2O [19]. A recent meta-analysis comparing low VT combined with high or low PEEP found that a high PEEP improved survival (relative risk [RR]=0.58; 95% CI: 0.41–0.82; P=0.05) [43]. Three large RCTs comparing high and moderate PEEP levels in ARDS patients ventilated with low VT (6 ml/kg PBW) did not find any differences in mortality [44], [45], [46]. High PEEP was associated with lower mortality in patients with moderate and severe ARDS and higher mortality in those with mild ARDS [47]. A high PEEP level is associated with increased static stress, but is required to avoid repeated opening and closing of alveolar units [48]. The ART trial demonstrated that a PEEP value >15 cmH2O was associated with increased mortality, especially in patients with hemodynamic impairment and pneumonia [38]. Therefore, we do not recommend using an average PEEP level >15 cmH2O as this could compromise hemodynamic function and increase the need for fluids.
There are no definitive recommendations on how to set PEEP. In patients with moderate or severe ARDS, setting PEEP according to either transpulmonary pressure (PL) or PEEP/FiO2 did not influence mortality [38]. The best way to individualize PEEP is to use a low PEEP/PaO2/FiO2 table [49], as patients who require more PEEP have more recruitable lungs and vice versa. On the other hand, the use of the best ΔP or stress index, as well as PL at end expiration was associated with higher PEEP in less recruitable lungs and lower PEEP in more recruitable lungs. PEEP should be individualized, but without using ΔP and compliance as titration methods, giving that compliance decreases with lung volume and recruitment (and is influenced by VT); that is, the higher the compliance, the lower the ΔP. Higher PEEP increases intratidal recruitment, which in turn increases compliance (although this is undesirable). Changes in ΔP from airway pressure may be partly explained by changes in chest wall compliance in patients with high abdominal pressure. The following thresholds should be respected to minimize the risk of VILI: Pplat should be maintained as low as possible (<25–27 cmH2O); and ΔP should be low to reduce mechanical power (MP) in association with a reduction of VT, although a lower ΔP does not reduce MP in association with the optimal PEEP (set as ΔP). Finally, the outdated concept of high vs. low PEEP should be abandoned. In an experimental model, a higher PEEP increased static strain and VILI, while volutrauma caused more lung damage than atelectrauma [50], [51].
PEEP should be set at the lowest level that is needed to attain minimal acceptable oxygen saturation (SpO2) (88–92%) or PaO2 (55–70 mmHg) [52]. PaO2 and oxygen delivery can be optimized by increasing blood pHa and reducing PaCO2, which increases hemoglobin concentration, cardiac output, and arterial oxygen content. Clinicians should exercise caution when adopting lung-protective strategies, particularly with low oxygen targets and permissive hypercapnia [53]. PEEP should also be set to protect the right ventricle, because the recruitment of lung units leads to derecruitment of capillaries. At high PEEP, more fluids are needed to achieve capillary recruitment and improve right ventricle function and lymphatic flow drainage from the lungs is reduced [54].
Personalized ventilatory treatment optimized based on chest X-rays and computed tomography (CT) scans did not yield better outcomes and was even associated with a worse outcome [55], suggesting that chest imaging is not the best approach to optimize MV in ARDS patients.
Obese patients are at a particularly high risk of developing ARDS because of anatomic and physiologic alterations affecting the chest wall, lungs, pharynx, face, and neck [56]. These patients present with reduced functional residual capacity and lung compliance, hypoxia, and ventilation/perfusion mismatch. Applying PEEP in this population is important to mitigate atelectasis and distal airway closure. In this regard, airway occlusion at end-inspiration is a useful method for individualizing PEEP according to a patient's specific physiology [57], [58], [59].
We do not recommend using a PEEP level >15 cmH2O. Low VT combined with the minimum PEEP level needed to achieve saturation/PaO2 targets (88%–92%/55–70 mmHg) [52] is the best option to avoid repeated collapse and reopening of alveoli, essentially by closing down the lungs and keeping them at rest to minimize VILI [60]. The distinction between high and low PEEP should be abolished and PEEP should be individualized based on the functional characteristics of each ARDS patient.
Rule 4. Recruitment maneuvers(RMs)
The total weight of the lungs is increased in ARDS due to interstitial and alveolar edema. As a result, atelectasis in dependent areas of the lungs is common; the collapse of alveoli not only reduces the total lung surface available for gas exchange but also promotes lung injury by increasing shear stress in areas located at the interface between aerated and collapsed alveoli, which undergo cyclic recruitment and derecruitment [61]. RMs decrease the intrapulmonary shunt and improve oxygenation and compliance. Thus, RMs can be considered as a protective “open lung approach” to MV; although it can lead to hemodynamic impairment and overdistension, which is more harmful than atelectrauma [62], [63].
A recent meta-analysis of 6 RCTs involving 1423 ARDS patients showed a reduction in mortality with the use of RMs. Notably, 5 of the studies used a high PEEP in the intervention group, suggesting that RMs can be used in combination with an open lung-protection strategy. In the study that did not adopt the cointervention and used only periodic RMs without higher PEEP, mortality was reducted although the quality of evidence was low. All 6 studies showed improved oxygenation after 24 h (mean increase: 52 mmHg; 95% CI: 23–81 mmHg) [64]. In another meta-analysis of 10 trials using high PEEP only (n = 3), RMs only (n = 1), or their combination (n = 6), there was no differences in mortality rate (RR=0.96, 95% CI: 0.84–1.09, P = 0.5), or incidence of barotrauma (RR=1.22, 95% CI: 0.93–1.61, P = 0.16) [7]. Regarding the detrimental effects associated with RMs, there was no increase in the risk of barotrauma (4 trials; RR = 0.84; 95% CI: 0.46–1.55) or incidence of hemodynamic compromise (3 trials; RR=1.30; 95% CI: 0.92–1.78) [64]. Various lung RMs have been used including high airway pressure sustained for a limited amount of time, a stepwise increase in PEEP with fixed ΔP, etc [65], [66], [67], [68]; this heterogeneity may limit the accuracy of meta-analyses.
Further studies are needed to evaluate the beneficial effects of RMs; at present, they are not recommended in treatment guidelines for patients with severe ARDS [19].
Rule 5. Neuromuscular blocking agents(NMBA4)
NMBAs act by inhibiting patients’ active breathing. Patients with severe ARDS may benefit from NMBAs, especially those with higher APACHE-II score, alveolar-arterial oxygen gradients, and Pplat, who require rescue therapies such as prone position and ECMO [16], [17], [69]. NMBAs reduce patient-ventilator asynchronies and oxygen consumption and increase compliance, functional residual capacity, and regional distribution of VT, resulting in anti-inflammatory effects [16], [70]. NMBAs also play a critical role in limiting decruitment and maintaining PEEP, thereby reducing fluctuations in PL caused by strong inspiratory effort and expiratory alveolar collapse [71]. A major side effect of long-term NMBA administration is muscular weakness, which can be a detrimental during in weaning from MV.
A recent meta-analysis evaluating the effects of NMBAs on the outcome of ARDS patients found that NMBAs did not reduce mortality risk at 28 days (RR = 0.9; 95% CI: 0.78–1.03; P=0.12) and 90 days (RR = 0.81; 95% CI: 0.62–1.06; P=0.06), but significantly reduced ICU mortality risk (RR = 0.72; 95% CI: 0.57–0.91; P=0.007), ventilator-free-days, and duration of MV,and increased oxygenation (e.g., by decreasing the incidence of asynchronies) [72]. This meta-analysis did not include the ROSE trial [72], because the authors used a modified PEEP table and not the National Heart, Lung, and Blood Institute protocol used in the other RCTs; moreover, the patients were only lightly sedated, in contrast to the deep-sedation strategy used in the other trials. This could explain why in the ROSE trial, ARDS patients had a lower rate of vasopressor use, shorter intubation time, and lower mortality rate. In the ROSE trial, patients with moderate-to-severe ARDS were randomly divided into 2 groups(heavy sedation with NMBAs and light sedation with placebo); the same high PEEP ventilation and fluid conservation strategies were used in both groups to avoid the confounds of co-intervention. There were no differences in mortality rate at 28 and 90 days, and the incidence of muscle weakness was similar between groups [73]. In the ACURASYS trial, mortality at 90 days did not differ between the NMBA (31.6%; 95% CI: 25.2–38.8) and placebo (40.7%; 95% CI: 33.5–48.4) group (P=0.08), although mortality at 28 days differed slightly at 23.7% (95% CI: 18.1–30.5) and 33.3% (95% CI: 26.5–40.9) in the NMBA and placebo groups, respectively (P=0.05) [10].
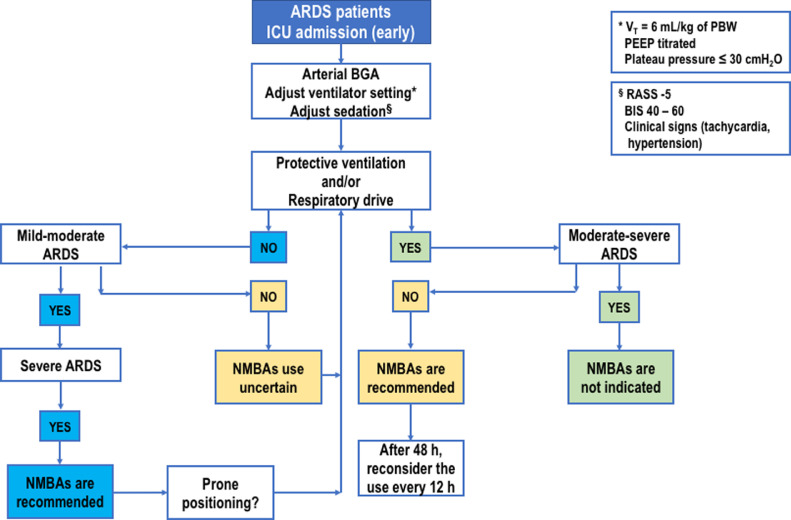
In summary, NMBAs do not reduce mortality risk at 28 and 90 days, ventilator-free days, or duration of MV, but improve oxygenation and reduce barotrauma without affecting ICU weakness. Fig. 1 outlines a management algorithm for the use of NMBAs in patients with moderate-to-severe ARDS.
Fig. 1.
Proposed algorithm for NMBAs use in ARDS patients. NMBA use is suggested when moderate to severe ARDS is present. NMBAs plays a pivotal role in limiting decruitment and maintaining PEEP, allowing a reduction in swings of transpulmonary pressure due to strong inspiratory effort and expiratory alveolar collapse. ARDS: Acute respiratory distress syndrome; BGA: Blood gas analysis; BIS: Bispectral index; ICU: Intensive care unit; NMBA: Neuromuscular blocking agent; PBW: Predicted body weight; PEEP: Positive end-expiratory pressure; RASS: Richmond Agitation Sedation Scale.
Rule 6. Assisted ventilation
In the acute phase of ARDS, it is reasonable to maintain the patients on continuous NMBA treatment in a protective, controlled ventilation mode. When there is clinical improvement, withdrawal from MV should be initiated. Spontaneous breathing has favorable effects such as reducing the wasting of respiratory muscles and improving oxygenation and compliance [74]. NMBAs and sedatives are first withdrawn until a spontaneous breathing effort is observed. Return to spontaneous ventilation is as inevitable as it is challenging. There are several problems associated with so-called pressure-support ventilation (PSV) modes. Spontaneous breathing can increase the inflammatory response and VILI [75] and an intense breathing effort due to exaggerated respiratory drive can worsen patient self-inflicted lung injury caused by the hyperinflation of aerated lung areas with increased strain. As a general rule, criteria for protection similar to those applied to controlled ventilation in ARDS [36] must be met for assisted spontaneous ventilation. With regard to the ventilator mode before weaning and extubation, a recent non-inferiority RCT comparing assisted ventilation without or with the sigh maneuver in acute hypoxemic patients showed that 23% of patients in the latter group failed to remain on pressure-control ventilation, compared to 30% in the assisted ventilation only group (absolute difference, −7%; 95% CI: −18% – 4%; P=0.015), highlighting the clinical benefit of using the sigh maneuver during assisted ventilation [76].
Rule 7. Prone positioning
The ventilation of dependent areas is severely impaired in the supine position in ARDS patients compared to non-ARDS patients [77]. Because of gravity, dependent areas are also more extensively perfused, resulting in hypoxemia due to ventilation/perfusion mismatch. Marked increases in oxygenation are frequently observed in ARDS patients in the prone position as a more homogeneous ventilation/perfusion ratio is achieved and intrapulmonary shunt is consequently diminished [78]. The prone position not only improves oxygenation but also reduces the risk of VILI [79]. Improved oxygenation with no change in PaCO2 leads to the redistribution of perfusion instead of recruitment because regional ventilation does not improve. On the other hand, improved oxygenation associated with reducted PaCO2 leads to recruitment and increases regional ventilation and survival [80].
Conflicting findings have been reported regarding the benefits of the prone position. The Prone-supine-II RCT [81], which enrolled 342 adult ARDS patients with moderate and severe hypoxemia, found no significant differences in overall survival at 28 days and 6 months between supine and prone patients (for 20 h/day); however, complications were significantly higher in the latter group. A recent meta-analysis of 8 RCTs also showed no difference in mortality between groups but in a subgroup analysis, mortality was lower in patients who were pronated for ≥ 12 h/day;moreover, PaO2/FiO2 ratio was higher and complications such as pressure sores and endotracheal tube obstruction were more frequent in the prone position group [82]. In the PROSEVA trial involving 466 patients with severe ARDS, the intervention group (237 patients) remained in the prone position for 16 h/day (an average of 4 sessions of prone positioning per patient); mortality was significantly lower in these patients at 28 days and at 90 days while the rate of complications was comparable to that in the supin groups, except for cardiac arrest , which occurred more frequently in the latter [83]. These data suggest that prone positioning may have clinical benefits in severe cases of ARDS, provided that it is maintained for at least 16 h.
Current guidelines recommend cycles of prone positioning lasting at least 16 h for patients with PaO2/FiO2 <150 in order to reduce mortality. Pronation is cost effective and relatively easy to implement, although the correct and safe positioning of patients requires technical skills and extreme caution [18]. Prone positioning for 1 day (12–18 h) repeated 3 times (2–5 days) is a reasonable schedule.
Prone positioning is the best technique for opening up the lungs and keeping them open, but at minimal acceptable oxygenation and airway pressure and lower PEEP. In this context, PEEP should be set to minimize injurious static strain.
Rule 8. Other rescue therapies
Rescue therapies for ARDS are indicated when other less invasive strategies are unsuccessful. ECCO2R with a blood flow up to 1.500 ml/min is an effective therapy for ARDS patients with either hypoxemic or hypercapnic respiratory failure. Artificial lungs are commercially available, that may be used within a conventional system of centrifugal pumps separate from or within a continuous renal replacement therapy circuit [84]. In our opinion, the circuits and pumps should be further improved in the near future. This system is attractive because it allows low-flow CO2 removal in severe cases of ARDS, while avoiding the invasiveness of high-flow ECMO. Low-flow CO2 removal maintains oxygenationwith less MP, and can be easily and safely applied at the bedside [84], [85]. ECCO2R protects against VILIg by reducing VT and Pplat while also controlling respiratory acidosis [86]; however, questions remain regarding its indications as most of the data come from observational studies of small case series or from retrospective analyses. A consensus statement published in 2020 on ECCO2R use in ARDS patients defined the target criteria for MV as follows: ΔP < 14 cmH2O, Pplat < 25 cmH2O, and a respiratory rate of 20–25 breaths/min. Indications for starting ECCO2R include ΔP > 15–20 cmH2O, Pplat > 30–35 cmH2O, PaCO2 ≥ 60 mmHg, pH < 7.25, respiratory rate > 20–30 breaths/min, PaO2/FiO2< 150, and PEEP > 8–15 cmH2O [86].
Inhaled nitric oxide (iNO) is another rescue strategy often used in ARDS patients who do not respond to conventional treatments. iNO was first reported in 1987 as an endogenous vasodilator to treat pulmonary hypertension and other pulmonary diseases; it was recently, shown to be advantageous for ventilation/perfusion mismatch. Current data indicate that iNO can be safely applied, although potential adverse effects include methemoglobinemia, reduced platelet aggregation, systemic vasodilation, and renal dysfunction. Thus, iNO should be used carefully in patients with renal diseases, and renal function should be strictly monitored during the treatment [87].
Rule 9. ECMO
While low-flow systems such as ECCO2R (0.5–1.5 L/min) provide adequate flow for both oxygenation and CO2 removal, high-flow systems such as ECMO (2–4 L/min) provide too much flow for minimal oxygenation and CO2 removal (for which a low blood flow is needed) [86], [87], [88]. In the EOLIA trial, ECMO was used in patients who were already pronated but did not show sufficient improvement. The trial failed to demonstrate a significant difference in 60-day mortality between ECMO and control groups [69]. A recent meta-analysis of 2 RCTs with a total of 429 patients reported a lower 60-day mortality in the venous-venous ECMO group (RR=0.73; 95% CI: 0.58–0.92; P=0.008), whereas 3 other studies reported a higher incidence of major hemorrhage in patients receiving ECMO [89]. Not all centers participating in these trials adopted the conventional rescue strategies for severe ARDS cases, and some lacked expertise in the use of ECMO. The latest Extracorporeal Life Support Organization guidelines for initiating ECMO include hypoxic respiratory failure with a mortality risk≥50% (PaO2/FiO2< 150 with FiO2> 90% and/or Murray score of 2–3, Age-Adjusted Oxygenation Index[AOI] score of 60, or APSS score [based on age, PaO2/FiO2, and the Pplat]), a risk of mortality ≥80% (PaO2/FiO2< 100 with FiO2> 90%, and/or Murray score 3–4, AOI score >80, or APSS score of 8); retention of PaCO2 despite maximal settings for MV; severe air leak syndrome; patients on the list for lung transplantation; or cardiac or respiratory collapse [90], [91], [92]. ECMO should also be used to reduce the risk of VILI by adopting an ultra-protective ventilator strategy [93]. While absolute contraindications are not available, relative contraindications should be considered such as >7 days of maximal MV settings; immunosuppression; central nervous system hemorrhage, damage or terminal malignancy; and increased age [88].
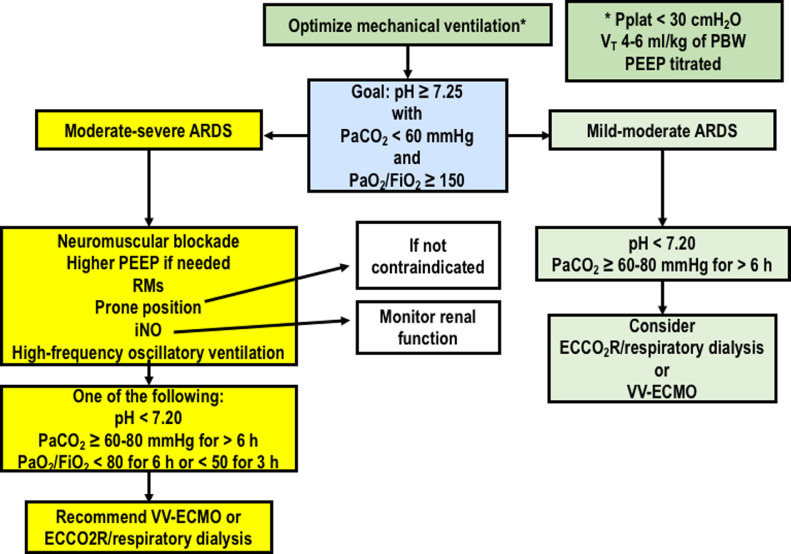
A strategy for selecting patients who may benefit from rescue strategies is presented in Fig. 2.
Fig. 2.
Proposed algorithm for rescue strategies. ECCO2R: Extracorporeal CO2 removal; FiO2: Fraction of inspired oxygen; iNO: Inhaled nitric oxide; PaCO2: Partial pressure of carbon dioxide; PaO2: Partial pressure of oxygen; VV-ECMO: Venous-venous extracorporeal membrane oxygenation.
Rule 10. Weaning from mechanical ventilation
Once lower desirable levels of pressure support under assisted ventilation have been achieved, sedatives and analgesics should be reduced and a spontaneous breathing trial (SBT) conducted. Post-extubation respiratory failure is associated with a high risk of mortality [94], [95], [96]. Daily interruption of sedation to assess the levels of agitation and pain has been adopted since 2000; this practice can reduce days on MV and length of ICU stay [97]. Weaning from MV can be categorized as simple, difficult, and prolonged [98]. Several methods have been proposed to predict successful weaning from MV, each with advantages and limitations; the most commonly used metric is the frequency/VT ratio [99].
Weaning strategies that are often used in general ICU patients include PSV or a T-tube trial. In an RCT comparing 30 min of low PSV (8 cmH2O and 0 PEEP) and 2 h with a T-tube, the former yielded greater success with extubation. However, although the decision to connect the patient to a high-flow nasal cannula or administer NIV after extubation, or to reconnect the patient 1 h before extubation was made during the randomization phase, the PSV arm received high-flow nasal cannulation or NIV for a longer period than the T-tube arm (25% vs. 19%; P=0.01), potentially confounding the final results [100]. Moreover, in some trials, patients were reconnected to the ventilator for a certain interval before extubation, whereas in others they were directly extubated after passing an SBT. Another RCT conducted in 2017 demonstrated that a 1-h rest period after passing an SBT reduced the rate of reintubation within 48 h after extubation [101]. A practical guideline for weaning is performing the SBT with inspiratory pressure augmentation and a PEEP level between 0 and 5 cmH2O followed by extubation and NIV in patients at high risk of extubation failure (e.g., patients with hypercapnia, chronic obstructive pulmonary disease, congestive heart failure, or other serious comorbidities) [102]. Personalized approaches for weaning general ICU patients need to be safer and faster. As specific studies on weaning in ARDS are not yet available, we recommend following local protocols based on current evidence obtained from the general ICU population. Additionally, the role of respiratory physiotherapy is critical in this setting. Chest physiotherapy should be initiated as soon as possible even during controlled MV to improve outcome and reduce complications. In particular, assisted mobilization, postural therapy, neuromuscular electrical stimulation, and respiratory muscle training can reduce muscle weakness in ICU patients, and while manual or ventilator hyperinflation [103], positioning [104], an active breathing cycle, and subglottic secretion drainage can reduce respiratory complications such as atelectasis, ventilator-associated pneumonia, and tracheobronchitis [105].
COVID-19 ARDS
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) was first identified in Wuhan, China in December 2019. It rapidly became a pandemic. Most cases of infection with the virus are limited to mild febrile illness, but some develop ARDS that requires ICU admission and critical care [106], [107]. The respiratory management of COVID-19 ARDS is based on distinct phenotypes according to chest CT findings [24], [108], [109], [110] and lung physiology; these include phenotype 1, with preserved lung compliance but few alveolar areas to recruit, along with high-perfusion areas; phenotype 2, with nonhomogeneously distributed atelectasis; and phenotype 3, featuring low compliance and inhomogeneous distribution of atelectasis(very similar to traditional ARDS) [108].
In addition to the protective MV strategy recommended for general ARDS patients, for phenotype 1 COVID-19 ARDS, we suggest using moderate PEEP to redistribute pulmonary blood flow from non-ventilated to more ventilated areas. For phenotype 2, we recommend using moderate-to-high PEEP to improve lung recruitment; rescue therapies can also be considered. For phenotype 3, we suggest adopting the current recommendations for typical (non-COVID) ARDS [[24], [105], [106], [107], [108], [109], [110], [111]].
Future perspectives
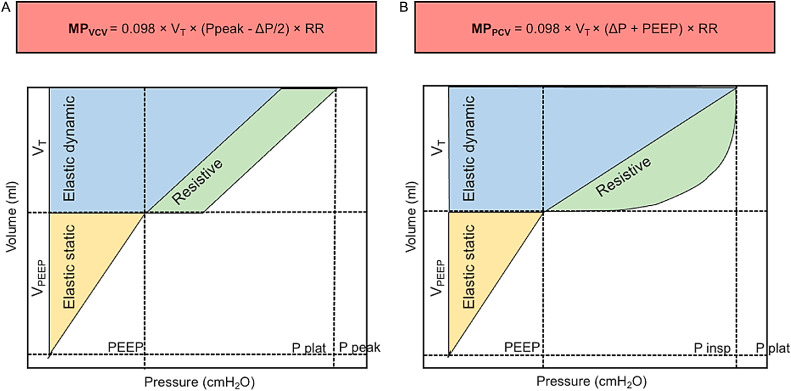
Recent studies have demonstrated that not only static parameters (PEEP, Pplat, and ΔP) but also dynamic parameters (airflow, inspiratory time, and respiratory rate) can cause lung damage [112]. MP, the product of mechanical energy and respiratory rate, is a measure of the amount of energy imparted to the patient by the mechanical ventilator. A related parameter, intensity, is MP normalized to the lung surface area [[5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106], [107], [108], [109], [110], [111], [112], [113], [114]]. For the same MP, intensity is higher for a smaller surface area [5]. Three or more equations have been proposed to calculate MP depending on the ventilatory setting. We propose that the simplest equations be adopted at the bedside in the case of pressure- and volume-control ventilation [115], [116], [117], [118] (Fig. 3). MP should be maintained below 12 J/min in ARDS, and below 17 J/min in non-ARDS. Moreover, MP levels >27 J/min should be considered during ECMO [119]. The concept of MP is new and still under investigation; although it is appealing, it may not be useful in clinical practice for setting MV parameters in ARDS patients.
Fig. 3.
Simplified formulas for mechanical power (MP) for volume-controlled and pressure-controlled ventilation. A: Mechanical power formula for volume-control ventilation. B: Mechanical power formula for pressure-controlled ventilation. Elastic static, dynamic, and resistive forces in yellow, blue, and green, respectively. MP: Mechanical power; PCV: Pressure-controlled ventilation; PEEP: Positive end-expiratory pressure; P peak: Peak pressure; Pplat: Plateau pressure; RR: Respiratory rate; VCV: Volume-controlled ventilation; VT: Tidal volume. Modified from Giosa et al. [120].
Conclusions
Over the last few decades there has been substantial progress in ARDS management. MV should follow the criteria for protective ventilation to minimize the risk of VILI. When it is impossible to optimize MV settings, rescue treatments should be initiated. These approaches must be undertaken by considering the treatment center's experience and benefits tor the patient. In general, VT, Pplat, ΔP, PEEP and PaO2 should be minimized, while increasing hemoglobin level, and permissive atelectasis and hypoxemia should be allowed. Thus, the strategy of closing down the lungs and keeping them resting can minimize VILI in ARDS.
Fundings
This work was funded by the Brazilian Council for Scientific and Technological Development (COVID-19-CNPq), Rio de Janeiro State Research Foundation (COVID-19- FAPERJ), Funding Authority for Studies and Projects (FINEP), and Brazilian Ministry of Science, Technology, and Information COVID-19 Network (RedeVírus MCTI).
Conflicts of Interest
All authors declare they have no conflicts of interest.
Acknowledgments
None.
Managing Editor: Jingling Bao
References
- 1.Ashbaugh DG, Bigelow DB, Petty TL, Levine BE. Acute respiratory distress in adults. Lancet. 1967;2(7511):319–323. doi: 10.1016/s0140-6736(67)90168-7. [DOI] [PubMed] [Google Scholar]
- 2.Cochi S.E., Kempker J.A., Annangi S., Kramer M.R., Martin G.S. Mortality trends of acute respiratory distress syndrome in the United States from 1999 to 2013. Ann Am Thorac Soc. 2016;13(10):1742–1751. doi: 10.1513/AnnalsATS.201512-841OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubenfeld G.D., Caldwell E., Peabody E., Weaver J., Martin D.P., Neff M., et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 4.Bellani G., Laffey J.G., Pham T., Fan E., Brochard L., Esteban A., et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 5.Silva P.L., Ball L., Rocco P.R.M., Pelosi P. Power to mechanical power to minimize ventilator-induced lung injury? Intensive Care Med Exp. 2019;7(Suppl 1):38. doi: 10.1186/s40635-019-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Battaglini D., Robba C., Rocco P., De Abreu M.G., Pelosi P., Ball L. Perioperative anaesthetic management of patients with or at risk of acute distress respiratory syndrome undergoing emergency surgery. BMC Anesthesiol. 2019;19(1):153. doi: 10.1186/s12871-019-0804-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ball L., Serpa Neto A., Trifiletti V., Mandelli M., Firpo I., Robba C., et al. Effects of higher PEEP and recruitment manoeuvres on mortality in patients with ARDS: a systematic review, meta-analysis, meta-regression and trial sequential analysis of randomized controlled trials. Intensive Care Med Exp. 2020;8(Suppl 1):39. doi: 10.1186/s40635-020-00322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Network Acute Respiratory Distress Syndrome, RG Brower, Matthay M.A., Morris A., Schoenfeld D., Thompson B.T., et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 9.Lemyze M., Mallat J., Thevenin D. Prone positioning in the acute respiratory distress syndrome. N Engl J Med. 2013;369(10):980. doi: 10.1056/NEJMc1308895. [DOI] [PubMed] [Google Scholar]
- 10.Papazian L., Forel J.M., Gacouin A., Penot-Ragon C., Perrin G., Loundou A., et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 11.Frat J.P., Thille A.W., Mercat A., Girault C., Ragot S., Perbet S., et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 12.Diehl J.L., Mercat A., Pesenti A. Understanding hypoxemia on ECCO(2)R: back to the alveolar gas equation. Intensive Care Med. 2019;45(2):255–256. doi: 10.1007/s00134-018-5409-0. [DOI] [PubMed] [Google Scholar]
- 13.Combes A., Schmidt M., Hodgson C.L., Fan E., Ferguson N.D., Fraser J.F., et al. Extracorporeal life support for adults with acute respiratory distress syndrome. Intensive Care Med. 2020;46(12):2464–2476. doi: 10.1007/s00134-020-06290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menk M., Estenssoro E., Sahetya S.K., Neto A.S., Sinha P., Slutsky A.S., et al. Current and evolving standards of care for patients with ARDS. Intensive Care Med. 2020;46(12):2157–2167. doi: 10.1007/s00134-020-06299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hraiech S., Yoshida T., Annane D., Duggal A., Fanelli V., Gacouin A., et al. Myorelaxants in ARDS patients. Intensive Care Med. 2020;46(12):2357–2372. doi: 10.1007/s00134-020-06297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guérin C., Albert R.K., Beitler J., Gattinoni L., Jaber S., Marini J.J., et al. Prone position in ARDS patients: why, when, how and for whom. Intensive Care Med. 2020;46(12):2385–2396. doi: 10.1007/s00134-020-06306-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guérin C., Mancebo J. Prone positioning and neuromuscular blocking agents are part of standard care in severe ARDS patients: yes. Intensive Care Med. 2015;41(12):2195–2197. doi: 10.1007/s00134-015-3918-7. [DOI] [PubMed] [Google Scholar]
- 18.Griffiths M., McAuley D.F., Perkins G.D., Barrett N., Blackwood B., Boyle A., et al. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Respir Res. 2019;6(1) doi: 10.1136/bmjresp-2019-000420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Papazian L., Aubron C., Brochard L., Chiche J.D., Combes A., Dreyfuss D., et al. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care. 2019;9(1):69. doi: 10.1186/s13613-019-0540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vincent J.L., Slutsky A.S. We've never seen a patient with ARDS! Intensive Care Med. 2020;46(12):2133–2135. doi: 10.1007/s00134-020-06255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 22.Villar J., Pérez-Méndez L., Blanco J., Añón J.M., Blanch L., Belda J., et al. A universal definition of ARDS: the PaO2/FiO2 ratio under a standard ventilatory setting–a prospective, multicenter validation study. Intensive Care Med. 2013;39(4):583–592. doi: 10.1007/s00134-012-2803-x. [DOI] [PubMed] [Google Scholar]
- 23.Calfee C.S., Delucchi K., Parsons P.E., Thompson B.T., Ware L.B., Matthay M.A. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2(8):611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robba C., Battaglini D., Ball L., Patroniti N., Loconte M., Brunetti I., et al. Distinct phenotypes require distinct respiratory management strategies in severe COVID-19. Respir Physiol Neurobiol. 2020;279 doi: 10.1016/j.resp.2020.103455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dreyfuss D., Soler P., Basset G., Saumon G. High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis. 1988;137(5):1159–1164. doi: 10.1164/ajrccm/137.5.1159. [DOI] [PubMed] [Google Scholar]
- 26.Slutsky A.S. Lung injury caused by mechanical ventilation. Chest. 1999;116(1 Suppl):9S–15S. doi: 10.1378/chest.116.suppl_1.9s-a. [DOI] [PubMed] [Google Scholar]
- 27.Nin N., Muriel A., Peñuelas O., Brochard L., Lorente J.A., Ferguson N.D., et al. Severe hypercapnia and outcome of mechanically ventilated patients with moderate or severe acute respiratory distress syndrome. Intensive Care Med. 2017;43(2):200–208. doi: 10.1007/s00134-016-4611-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Needham D.M., Yang T., Dinglas V.D., Mendez-Tellez P.A., Shanholtz C., Sevransky J.E., et al. Timing of low tidal volume ventilation and intensive care unit mortality in acute respiratory distress syndrome. a prospective cohort study. Am J Respir Crit Care Med. 2015;191(2):177–185. doi: 10.1164/rccm.201409-1598OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laffey J.G., Bellani G., Pham T., Fan E., Madotto F., Bajwa E.K., et al. Potentially modifiable factors contributing to outcome from acute respiratory distress syndrome: the LUNG SAFE study. Intensive Care Med. 2016;42(12):1865–1876. doi: 10.1007/s00134-016-4571-5. [DOI] [PubMed] [Google Scholar]
- 30.Webb H.H., Tierney D.F. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am Rev Respir Dis. 1974;110(5):556–565. doi: 10.1164/arrd.1974.110.5.556. [DOI] [PubMed] [Google Scholar]
- 31.Putensen C., Theuerkauf N., Zinserling J., Wrigge H., Pelosi P. Meta-analysis: ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injury. Ann Intern Med. 2009;151(8):566–576. doi: 10.7326/0003-4819-151-8-200910200-00011. [DOI] [PubMed] [Google Scholar]
- 32.Blanch L., Fernandez R., Vallés J., Solé J., Roussos C., Artigas A. Effect of two tidal volumes on oxygenation and respiratory system mechanics during the early stage of adult respiratory distress syndrome. J Crit Care. 1994;9(3):151–158. doi: 10.1016/0883-9441(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 33.Villar J., Martín-Rodríguez C., Domínguez-Berrot A.M., Fernández L., Ferrando C., Soler J.A., et al. A quantile analysis of plateau and driving pressures: effects on mortality in patients with acute respiratory distress syndrome receiving lung-protective ventilation. Crit Care Med. 2017;45(5):843–850. doi: 10.1097/CCM.0000000000002330. [DOI] [PubMed] [Google Scholar]
- 34.Pelosi P., Ball L. Should we titrate ventilation based on driving pressure? Maybe not in the way we would expect. Ann Transl Med. 2018;6(19):389. doi: 10.21037/atm.2018.09.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grasso S., Fanelli V., Cafarelli A., Anaclerio R., Amabile M., Ancona G., et al. Effects of high versus low positive end-expiratory pressures in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2005;171(9):1002–1008. doi: 10.1164/rccm.200407-940OC. [DOI] [PubMed] [Google Scholar]
- 36.Amato M.B., Meade M.O., Slutsky A.S., Brochard L., Costa E.L., Schoenfeld D.A., et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372(8):747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 37.Aoyama H., Pettenuzzo T., Aoyama K., Pinto R., Englesakis M., Fan E. Association of driving pressure with mortality among ventilated patients with acute respiratory distress syndrome: a systematic review and meta-analysis. Crit Care Med. 2018;46(2):300–306. doi: 10.1097/CCM.0000000000002838. [DOI] [PubMed] [Google Scholar]
- 38.Cavalcanti A.B., Suzumura É.A., Laranjeira L.N., Paisani D.M., Damiani L.P., Guimarães H.P., et al. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs Low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2017;318(14):1335–1345. doi: 10.1001/jama.2017.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sahetya S.K., Goligher E.C., Brower R.G. Fifty Years of Research in ARDS. setting positive end-expiratory pressure in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195(11):1429–1438. doi: 10.1164/rccm.201610-2035CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Slutsky A.S., Ranieri V.M. Ventilator-induced lung injury. N Engl J Med. 2013;369(22):2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 41.Fougères E., Teboul J.L., Richard C., Osman D., Chemla D., Monnet X. Hemodynamic impact of a positive end-expiratory pressure setting in acute respiratory distress syndrome: importance of the volume status. Crit Care Med. 2010;38(3):802–807. doi: 10.1097/CCM.0b013e3181c587fd. [DOI] [PubMed] [Google Scholar]
- 42.Goligher E.C., Kavanagh B.P., Rubenfeld G.D., Adhikari N.K., Pinto R., Fan E., et al. Oxygenation response to positive end-expiratory pressure predicts mortality in acute respiratory distress syndrome. a secondary analysis of the LOVS and ExPress trials. Am J Respir Crit Care Med. 2014;190(1):70–76. doi: 10.1164/rccm.201404-0688OC. [DOI] [PubMed] [Google Scholar]
- 43.Walkey A.J., Goligher E.C., Del Sorbo L., Hodgson C.L., Adhikari N., Wunsch H., et al. Low tidal volume versus non-volume-limited strategies for patients with acute respiratory distress syndrome. a systematic review and meta-analysis. Ann Am Thorac Soc. 2017;14(Supplement_4):S271–271S279. doi: 10.1513/AnnalsATS.201704-337OT. [DOI] [PubMed] [Google Scholar]
- 44.Mercat A., Richard J.C., Vielle B., Jaber S., Osman D., Diehl J.L., et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):646–655. doi: 10.1001/jama.299.6.646. [DOI] [PubMed] [Google Scholar]
- 45.Brower R.G., Lanken P.N., MacIntyre N., Matthay M.A., Morris A., Ancukiewicz M., et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 46.Meade M.O., Cook D.J., Guyatt G.H., Slutsky A.S., Arabi Y.M., Cooper D.J., et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):637–645. doi: 10.1001/jama.299.6.637. [DOI] [PubMed] [Google Scholar]
- 47.Briel M., Meade M., Mercat A., Brower R.G., Talmor D., Walter S.D., et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA. 2010;303(9):865–873. doi: 10.1001/jama.2010.218. [DOI] [PubMed] [Google Scholar]
- 48.Pelosi P., Andrea L., Vitale G., Pesenti A., Gattinoni L. Vertical gradient of regional lung inflation in adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;149(1):8–13. doi: 10.1164/ajrccm.149.1.8111603. [DOI] [PubMed] [Google Scholar]
- 49.Chiumello D., Cressoni M., Carlesso E., Caspani M.L., Marino A., Gallazzi E., et al. Bedside selection of positive end-expiratory pressure in mild, moderate, and severe acute respiratory distress syndrome. Crit Care Med. 2014;42(2):252–264. doi: 10.1097/CCM.0b013e3182a6384f. [DOI] [PubMed] [Google Scholar]
- 50.Samary C.S., Santos R.S., Santos C.L., Felix N.S., Bentes M., Barboza T., et al. Biological Impact of Transpulmonary Driving Pressure in Experimental Acute Respiratory Distress Syndrome. Anesthesiology. 2015;123(2):423–433. doi: 10.1097/ALN.0000000000000716. [DOI] [PubMed] [Google Scholar]
- 51.Güldner A., Braune A., Ball L., Silva P.L., Samary C., Insorsi A., et al. Comparative Effects of Volutrauma and Atelectrauma on Lung Inflammation in Experimental Acute Respiratory Distress Syndrome. Crit Care Med. 2016;44(9):e854–e865. doi: 10.1097/CCM.0000000000001721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barrot L., Asfar P., Mauny F., Winiszewski H., Montini F., Badie J., et al. Liberal or Conservative Oxygen Therapy for Acute Respiratory Distress Syndrome. N Engl J Med. 2020;382(11):999–1008. doi: 10.1056/NEJMoa1916431. [DOI] [PubMed] [Google Scholar]
- 53.Battaglini D., Brunetti I., Anania P., Fiaschi P., Zona G., Ball L., et al. Neurological Manifestations of Severe SARS-CoV-2 Infection: potential Mechanisms and Implications of Individualized Mechanical Ventilation Settings. Front Neurol. 2020;11:845. doi: 10.3389/fneur.2020.00845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bouferrache K., Vieillard-Baron A. Acute respiratory distress syndrome, mechanical ventilation, and right ventricular function. Curr Opin Crit Care. 2011;17(1):30–35. doi: 10.1097/MCC.0b013e328342722b. [DOI] [PubMed] [Google Scholar]
- 55.Constantin J.M., Jabaudon M., Lefrant J.Y., Jaber S., Quenot J.P., Langeron O., et al. Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): a multicentre, single-blind, randomised controlled trial. Lancet Respir Med. 2019;7(10):870–880. doi: 10.1016/S2213-2600(19)30138-9. [DOI] [PubMed] [Google Scholar]
- 56.De Jong A., Verzilli D., Jaber S. ARDS in Obese Patients: specificities and Management. Crit Care. 2019;23(1):74. doi: 10.1186/s13054-019-2374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Jong A., Wrigge H., Hedenstierna G., Gattinoni L., Chiumello D., Frat J.P., et al. How to ventilate obese patients in the ICU. Intensive Care Med. 2020;46(12):2423–2435. doi: 10.1007/s00134-020-06286-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ball L., Pelosi P. How I ventilate an obese patient. Crit Care. 2019;23(1):176. doi: 10.1186/s13054-019-2466-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schetz M., De Jong A., Deane A.M., Druml W., Hemelaar P., Pelosi P., et al. Obesity in the critically ill: a narrative review. Intensive Care Med. 2019;45(6):757–769. doi: 10.1007/s00134-019-05594-1. [DOI] [PubMed] [Google Scholar]
- 60.Pelosi P., Rocco P., Gama de Abreu M. Close down the lungs and keep them resting to minimize ventilator-induced lung injury. Crit Care. 2018;22(1):72. doi: 10.1186/s13054-018-1991-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muscedere J.G., Mullen J.B., Gan K., Slutsky A.S. Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med. 1994;149(5):1327–1334. doi: 10.1164/ajrccm.149.5.8173774. [DOI] [PubMed] [Google Scholar]
- 62.Fan E., Checkley W., Stewart T.E., Muscedere J., Lesur O., Granton J.T., et al. Complications from recruitment maneuvers in patients with acute lung injury: secondary analysis from the lung open ventilation study. Respir Care. 2012;57(11):1842–1849. doi: 10.4187/respcare.01684. [DOI] [PubMed] [Google Scholar]
- 63.Cipulli F., Vasques F., Duscio E., Romitti F., Quintel M., Gattinoni L. Atelectrauma or volutrauma: the dilemma. J Thorac Dis. 2018;10(3):1258–1264. doi: 10.21037/jtd.2018.02.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goligher E.C., Hodgson C.L., Adhikari N., Meade M.O., Wunsch H., Uleryk E., et al. Lung Recruitment Maneuvers for Adult Patients with Acute Respiratory Distress Syndrome. A Systematic Review and Meta-Analysis. Ann Am Thorac Soc. 2017;14(Supplement_4):S304–304S311. doi: 10.1513/AnnalsATS.201704-340OT. [DOI] [PubMed] [Google Scholar]
- 65.Silva P.L., Pelosi P., Rocco P.R. Recruitment maneuvers for acute respiratory distress syndrome: the panorama in 2016. Rev Bras Ter Intensiva. 2016;28(2):104–106. doi: 10.5935/0103-507X.20160023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Santos R.S., Silva P.L., Pelosi P., Rocco P.R. Recruitment maneuvers in acute respiratory distress syndrome: the safe way is the best way. World J Crit Care Med. 2015;4(4):278–286. doi: 10.5492/wjccm.v4.i4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chiumello D., Algieri I., Grasso S., Terragni P., Pelosi P. Recruitment maneuvers in acute respiratory distress syndrome and during general anesthesia. Minerva Anestesiol. 2016;82(2):210–220. [PubMed] [Google Scholar]
- 68.Rocco P.R., Pelosi P., de Abreu M.G. Pros and cons of recruitment maneuvers in acute lung injury and acute respiratory distress syndrome. Expert Rev Respir Med. 2010;4(4):479–489. doi: 10.1586/ers.10.43. [DOI] [PubMed] [Google Scholar]
- 69.Combes A., Hajage D., Capellier G., Demoule A., Lavoué S., Guervilly C., et al. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. N Engl J Med. 2018;378(21):1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 70.Yoshida T., Nakahashi S., Nakamura M., Koyama Y., Roldan R., Torsani V., et al. Volume-controlled Ventilation Does Not Prevent Injurious Inflation during Spontaneous Effort. Am J Respir Crit Care Med. 2017;196(5):590–601. doi: 10.1164/rccm.201610-1972OC. [DOI] [PubMed] [Google Scholar]
- 71.Guervilly C., Bisbal M., Forel J.M., Mechati M., Lehingue S., Bourenne J., et al. Effects of neuromuscular blockers on transpulmonary pressures in moderate to severe acute respiratory distress syndrome. Intensive Care Med. 2017;43(3):408–418. doi: 10.1007/s00134-016-4653-4. [DOI] [PubMed] [Google Scholar]
- 72.Ho A., Patolia S., Guervilly C. Neuromuscular blockade in acute respiratory distress syndrome: a systematic review and meta-analysis of randomized controlled trials. J Intensive Care. 2020;8:12. doi: 10.1186/s40560-020-0431-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moss M., Huang D.T., Brower R.G., Ferguson N.D., Ginde A.A., Gong M.N., et al. Early Neuromuscular Blockade in the Acute Respiratory Distress Syndrome. N Engl J Med. 2019;380(21):1997–2008. doi: 10.1056/NEJMoa1901686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carvalho N.C., Güldner A., Beda A., Rentzsch I., Uhlig C., Dittrich S., et al. Higher levels of spontaneous breathing reduce lung injury in experimental moderate acute respiratory distress syndrome. Crit Care Med. 2014;42(11):e702–e715. doi: 10.1097/CCM.0000000000000605. [DOI] [PubMed] [Google Scholar]
- 75.Yoshida T., Fujino Y., Amato M.B., Kavanagh B.P. Fifty Years of Research in ARDS. Spontaneous Breathing during Mechanical Ventilation. Risks, Mechanisms, and Management. Am J Respir Crit Care Med. 2017;195(8):985–992. doi: 10.1164/rccm.201604-0748CP. [DOI] [PubMed] [Google Scholar]
- 76.Mauri T., Foti G., Fornari C., Grasselli G., Pinciroli R., Lovisari F., et al. Sigh in Patients With Acute Hypoxemic Respiratory Failure and ARDS: the PROTECTION Pilot Randomized Clinical Trial. Chest. 2020 Nov 13 doi: 10.1016/j.chest.2020.10.079. S0012-3692(20)35138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lai-Fook S.J., Rodarte J.R. Pleural pressure distribution and its relationship to lung volume and interstitial pressure. J Appl Physiol. 1985;70(3):967–978. doi: 10.1152/jappl.1991.70.3.967. [DOI] [PubMed] [Google Scholar]
- 78.Pappert D., Rossaint R., Slama K., Grüning T., Falke K.J. Influence of positioning on ventilation-perfusion relationships in severe adult respiratory distress syndrome. Chest. 1994;106(5):1511–1516. doi: 10.1378/chest.106.5.1511. [DOI] [PubMed] [Google Scholar]
- 79.Santana M.C., Garcia C.S., Xisto D.G., Nagato L.K., Lassance R.M., Prota L.F., et al. Prone position prevents regional alveolar hyperinflation and mechanical stress and strain in mild experimental acute lung injury. Respir Physiol Neurobiol. 2009;167(2):181–188. doi: 10.1016/j.resp.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 80.Gattinoni L., Vagginelli F., Carlesso E., Taccone P., Conte V., Chiumello D., et al. Decrease in PaCO2 with prone position is predictive of improved outcome in acute respiratory distress syndrome. Crit Care Med. 2003;31(12):2727–2733. doi: 10.1097/01.CCM.0000098032.34052.F9. [DOI] [PubMed] [Google Scholar]
- 81.Taccone P., Pesenti A., Latini R., Polli F., Vagginelli F., Mietto C., et al. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2009;302(18):1977–1984. doi: 10.1001/jama.2009.1614. [DOI] [PubMed] [Google Scholar]
- 82.Munshi L., Del Sorbo L., Adhikari N., Hodgson C.L., Wunsch H., Meade M.O., et al. Prone Position for Acute Respiratory Distress Syndrome. A Systematic Review and Meta-Analysis. Ann Am Thorac Soc. 2017;14(Supplement_4):S280–280S288. doi: 10.1513/AnnalsATS.201704-343OT. [DOI] [PubMed] [Google Scholar]
- 83.Guérin C., Reignier J., Richard J.C., Beuret P., Gacouin A., Boulain T., et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 84.May A.G., Sen A., Cove M.E., Kellum J.A., Federspiel W.J. Extracorporeal CO2 removal by hemodialysis: in vitro model and feasibility. Intensive Care Med Exp. 2017;5(1):20. doi: 10.1186/s40635-017-0132-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Del Sorbo L., Cypel M., Fan E. Extracorporeal life support for adults with severe acute respiratory failure. Lancet Respir Med. 2014;2(2):154–164. doi: 10.1016/S2213-2600(13)70197-8. [DOI] [PubMed] [Google Scholar]
- 86.Combes A., Auzinger G., Capellier G., du Cheyron D., Clement I., Consales G., et al. ECCO(2)R therapy in the ICU: consensus of a European round table meeting. Crit Care. 2020;24(1):490. doi: 10.1186/s13054-020-03210-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ruan S.Y., Huang T.M., Wu H.Y., Wu H.D., Yu C.J., Lai M.S. Inhaled nitric oxide therapy and risk of renal dysfunction: a systematic review and meta-analysis of randomized trials. Crit Care. 2015;19(1):137. doi: 10.1186/s13054-015-0880-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shekar K., Badulak J., Peek G., Boeken U., Dalton H.J., Arora L., et al. Extracorporeal Life Support Organization Coronavirus Disease 2019 Interim Guidelines: a Consensus Document from an International Group of Interdisciplinary Extracorporeal Membrane Oxygenation Providers. ASAIO J. 2020;66(7):707–721. doi: 10.1097/MAT.0000000000001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Munshi L., Walkey A., Goligher E., Pham T., Uleryk E.M., Fan E. Venovenous extracorporeal membrane oxygenation for acute respiratory distress syndrome: a systematic review and meta-analysis. Lancet Respir Med. 2019;7(2):163–172. doi: 10.1016/S2213-2600(18)30452-1. [DOI] [PubMed] [Google Scholar]
- 90.Murray J.F., Matthay M.A., Luce J.M., Flick M.R. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138(3):720–723. doi: 10.1164/ajrccm/138.3.720. [DOI] [PubMed] [Google Scholar]
- 91.Dechert R.E., Park P.K., Bartlett R.H. Evaluation of the oxygenation index in adult respiratory failure. J Trauma Acute Care Surg. 2014;76(2):469–473. doi: 10.1097/TA.0b013e3182ab0d27. [DOI] [PubMed] [Google Scholar]
- 92.Villar J., Ambrós A., Soler J.A., Martínez D., Ferrando C., Solano R., et al. Age, PaO2/FIO2, and Plateau Pressure Score: a Proposal for a Simple Outcome Score in Patients With the Acute Respiratory Distress Syndrome. Crit Care Med. 2016;44(7):1361–1369. doi: 10.1097/CCM.0000000000001653. [DOI] [PubMed] [Google Scholar]
- 93.Abrams D., Schmidt M., Pham T., Beitler J.R., Fan E., Goligher E.C., et al. Mechanical Ventilation for Acute Respiratory Distress Syndrome during Extracorporeal Life Support. Research and Practice. Am J Respir Crit Care Med. 2020;201(5):514–525. doi: 10.1164/rccm.201907-1283CI. [DOI] [PubMed] [Google Scholar]
- 94.MacIntyre N. Discontinuing mechanical ventilatory support. Chest. 2007;132(3):1049–1056. doi: 10.1378/chest.06-2862. [DOI] [PubMed] [Google Scholar]
- 95.Frutos-Vivar F., Esteban A., Apezteguia C., González M., Arabi Y., Restrepo M.I., et al. Outcome of reintubated patients after scheduled extubation. J Crit Care. 2011;26(5):502–509. doi: 10.1016/j.jcrc.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 96.Esteban A., Alía I., Tobin M.J., Gil A., Gordo F., Vallverdú I., et al. Effect of spontaneous breathing trial duration on outcome of attempts to discontinue mechanical ventilation. Spanish Lung Failure Collaborative Group. Am J Respir Crit Care Med. 1999;159(2):512–518. doi: 10.1164/ajrccm.159.2.9803106. [DOI] [PubMed] [Google Scholar]
- 97.Kallet R.H., Zhuo H., Yip V., Gomez A., Lipnick M.S. Spontaneous Breathing Trials and Conservative Sedation Practices Reduce Mechanical Ventilation Duration in Subjects With ARDS. Respir Care. 2018;63(1):1–10. doi: 10.4187/respcare.05270. [DOI] [PubMed] [Google Scholar]
- 98.Boles J.M., Bion J., Connors A., Herridge M., Marsh B., Melot C., et al. Weaning from mechanical ventilation. Eur Respir J. 2007;29(5):1033–1056. doi: 10.1183/09031936.00010206. [DOI] [PubMed] [Google Scholar]
- 99.Telias I., Damiani F., Brochard L. The airway occlusion pressure (P(0.1)) to monitor respiratory drive during mechanical ventilation: increasing awareness of a not-so-new problem. Intensive Care Med. 2018;44(9):1532–1535. doi: 10.1007/s00134-018-5045-8. [DOI] [PubMed] [Google Scholar]
- 100.Subirà C., Hernández G., Vázquez A., Rodríguez-García R., González-Castro A., García C., et al. Effect of Pressure Support vs T-Piece Ventilation Strategies During Spontaneous Breathing Trials on Successful Extubation Among Patients Receiving Mechanical Ventilation: a Randomized Clinical Trial. JAMA. 2019;321(22):2175–2182. doi: 10.1001/jama.2019.7234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fernandez M.M., González-Castro A., Magret M., Bouza M.T., Ibañez M., García C., et al. Reconnection to mechanical ventilation for 1h after a successful spontaneous breathing trial reduces reintubation in critically ill patients: a multicenter randomized controlled trial. Intensive Care Med. 2017;43(11):1660–1667. doi: 10.1007/s00134-017-4911-0. [DOI] [PubMed] [Google Scholar]
- 102.Ouellette D.R., Patel S., Girard T.D., Morris P.E., Schmidt G.A., Truwit J.D., et al. Liberation from mechanical ventilation in critically ill adults: an official american college of chest physicians/American thoracic society clinical practice guideline: inspiratory pressure augmentation during spontaneous breathing trials, protocols minimizing sedation, and noninvasive ventilation immediately after extubation. Chest. 2017;151(1):166–180. doi: 10.1016/j.chest.2016.10.036. [DOI] [PubMed] [Google Scholar]
- 103.Li Bassi G., Martí J.D., Comaru T., Aguilera-Xiol E., Rigol M., Ntoumenopoulos G., et al. Short-term appraisal of the effects and safety of manual versus ventilator hyperinflation in an animal model of severe pneumonia. Respir Care. 2019;64(7):760–770. doi: 10.4187/respcare.06487. [DOI] [PubMed] [Google Scholar]
- 104.Meli A., Barbeta Viñas E., Battaglini D., Li Bassi G., Yang H., Yang M., et al. Lateral position during severe mono-lateral pneumonia: an experimental study. Sci Rep. 2020;10(1):19372. doi: 10.1038/s41598-020-76216-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Battaglini D., Robba C., Caiffa S., Ball L., Brunetti I., Loconte M., et al. Chest physiotherapy: an important adjuvant in critically ill mechanically ventilated patients with COVID-19. Respir Physiol Neurobiol. 2020;282 doi: 10.1016/j.resp.2020.103529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Robba C., Battaglini D., Pelosi P., Rocco P. Multiple organ dysfunction in SARS-CoV-2: mODS-CoV-2. Expert Rev Respir Med. 2020;14(9):865–868. doi: 10.1080/17476348.2020.1778470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Robba C., Battaglini D., Ball L., Valbusa A., Porto I., Della Bona R., et al. Coagulative Disorders in Critically Ill COVID-19 Patients with Acute Distress Respiratory Syndrome: a Critical Review. J Clin Med. 2021;10(1) doi: 10.3390/jcm10010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gattinoni L., Chiumello D., Caironi P., Busana M., Romitti F., Brazzi L., et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46(6):1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gattinoni L., Camporota L., Marini J.J. COVID-19 phenotypes: leading or misleading? Eur Respir J. 2020;56(2) doi: 10.1183/13993003.02195-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Azoulay E., Zafrani L., Mirouse A., Lengliné E., Darmon M., Chevret S. Clinical phenotypes of critically ill COVID-19 patients. Intensive Care Med. 2020;46(8):1651–1652. doi: 10.1007/s00134-020-06120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fan E., Beitler J.R., Brochard L., Calfee C.S., Ferguson N.D., Slutsky A.S., et al. COVID-19-associated acute respiratory distress syndrome: is a different approach to management warranted? Lancet Respir Med. 2020;8(8):816–821. doi: 10.1016/S2213-2600(20)30304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rocco P.R.M., Marini J.J. What have we learned from animal models of ventilator-induced lung injury? Intensive Care Med. 2020;46(12):2377–2380. doi: 10.1007/s00134-020-06143-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gattinoni L., Tonetti T., Cressoni M., Cadringher P., Herrmann P., Moerer O., et al. Ventilator-related causes of lung injury: the mechanical power. Intensive Care Med. 2016;42(10):1567–1575. doi: 10.1007/s00134-016-4505-2. [DOI] [PubMed] [Google Scholar]
- 114.Coppola S., Caccioppola A., Froio S., Formenti P., De Giorgis V., Galanti V., et al. Effect of mechanical power on intensive care mortality in ARDS patients. Crit Care. 2020;24(1):246. doi: 10.1186/s13054-020-02963-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Becher T., van der Staay M., Schädler D., Frerichs I., Weiler N. Calculation of mechanical power for pressure-controlled ventilation. Intensive Care Med. 2019;45(9):1321–1323. doi: 10.1007/s00134-019-05636-8. [DOI] [PubMed] [Google Scholar]
- 116.van der Meijden S., Molenaar M., Somhorst P., Schoe A. Calculating mechanical power for pressure-controlled ventilation. Intensive Care Med. 2019;45(10):1495–1497. doi: 10.1007/s00134-019-05698-8. [DOI] [PubMed] [Google Scholar]
- 117.Chiumello D., Gotti M., Guanziroli M., Formenti P., Umbrello M., Pasticci I., et al. Bedside calculation of mechanical power during volume- and pressure-controlled mechanical ventilation. Crit Care. 2020;24(1):417. doi: 10.1186/s13054-020-03116-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Giosa L., Busana M., Pasticci I., Bonifazi M., Macrì M.M., Romitti F., et al. Mechanical power at a glance: a simple surrogate for volume-controlled ventilation. Intensive Care Med Exp. 2019;7(1):61. doi: 10.1186/s40635-019-0276-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Serpa Neto A., Deliberato R.O., Johnson A., Bos L.D., Amorim P., Pereira S.M., et al. Mechanical power of ventilation is associated with mortality in critically ill patients: an analysis of patients in two observational cohorts. Intensive Care Med. 2018;44(11):1914–1922. doi: 10.1007/s00134-018-5375-6. [DOI] [PubMed] [Google Scholar]
- 120.Giosa L., Busana M., Pasticci I., Bonifazi M., Macrì M.M., Romitti F., et al. Mechanical power at a glance: a simple surrogate for volume-controlled ventilation. Intensive Care Med Exp. 2019;7(1):61. doi: 10.1186/s40635-019-0276-8. [DOI] [PMC free article] [PubMed] [Google Scholar]