Introduction
Coronavirus disease-2019 (COVID-19) originated in the Huanan South China Seafood Market in Wuhan and can present with a spectrum of clinical manifestations including fever, myalgia, cough, dyspnea and, less frequently, headache, diarrhea, nausea, and vomiting.1 Although respiratory symptoms predominate, multiple organ dysfunction may also occur with COVID-19. Coagulopathy has been found as a prominent feature of COVID-19. A high incidence of thromboembolic complications in patients hospitalized with COVID-19 (pulmonary embolism, deep vein thrombosis, ischemic stroke, acute limb ischemia, acute portal vein thrombosis, acute mesenteric ischemia and ischemic myocardial injury) has been reported.2, 3, 4, 5, 6
The patients who are on some form of anticoagulation therapy predisposes them to the development of bleeding complications. Bleeding is a spectrum and can range from minor to major, or even life-threatening. Major bleeding has a significant risk of immediate morbidity, regardless of the cause. We report on four cases of COVID-19-associated pneumonia in patients who were started on therapeutic anticoagulation for COVID-19-associated hypercoagulability and who developed bleeding at unusual sites.
Case presentation
We report on four cases of COVID-19-associated pneumonia complicated by bleeding at unusual locations. Table 1 summarizes the clinical characteristics, laboratory values, management and outcome of the four patients. The median age was 82 years (ranging from 67 to 88 years) and 50% were male. Three patients were Hispanic and 1 was white. All of them presented with respiratory symptoms, except one who presented them after a fall. All the patients were diagnosed with pneumonia. COVID-19 was diagnosed by RT-PCR in all four, except in one (suspected COVID-19). All of them were admitted to the non-ICU unit and none of them were intubated during the hospitalization. Inflammatory markers, the erythrocyte sedimentation rate (ESR) and c-reactive protein (CRP), were elevated in all patients All the four patients were started on therapeutic anticoagulation (LMWH) due to an elevated D-dimer and based on clinical judgment. Two patients had chest wall hematoma (Figure 1, Figure 2). One patient had hematoma involving the left adductor muscle, proximal hamstring muscles, piriformis and gluteus maximus (Figure 3) and another one had hematoma in the anterior compartment of the right thigh (Figure 4). Two patients underwent surgical evacuation of the hematoma, one underwent computed tomography-guided drainage by an intervention radiologist and one patient was managed conservatively. None of the patients had any trauma or thrombocytopenia or overt disseminated intravascular coagulation (DIC) at the time of the bleeding episode or history of prior bleeding. One patient had elevated creatinine at the time of the bleeding episode (Case 3). Only one patient was on antiplatelet drugs at the time of the bleeding episode (Case 4 — clopidogrel 75 mg po QD). All the patients were discharged in stable condition.
Table 1.
Summarizes the clinical characteristics, laboratory values, management and outcome of the four patients.
| Variable | Case 1 | Case 2 | Case 3 | Case 4 |
|---|---|---|---|---|
| Age (years) | 77 | 88 | 87 | 67 |
| Sex/Race | F/Hispanic | F/White | M/ Hispanic | M/Hispanic |
| Medical history | DM, HTN, DLD, gastritis, asthma, hypothyroidism, | HTN, dementia | HTN, DLD, schizophrenia, BPH, depression, | DM, HTN, DLD, CAD s/p CABG, HFrEF, hypothyroidism |
| Presenting complaints | Cough, SOB, dizziness | Fall | SOB | Cough, SOB |
| COVID-19 diagnostic test RT-PCR | Positive | Negative | Positive | Positive |
| Pneumonia | Yes | Yes | Yes | Yes |
| Intubated | No | No | No | No |
| Rx of COVID-19 | HCQ, tocilizumab, remdesivir methylprednisolone | Doxycycline, Ceftriaxone | HCQ | HCQ, azithromycin, methylprednisolone |
| Laboratory values On presentation | ||||
| WBC (K/mm3) | 8.1 | 9.6 | 10.2 | 10.8 |
| Hemoglobin (g/dL)/ hematocrit (%) | 13.88/41.3 | 10.7/33.4 | 13.6/42.1 | 13.1/38.4 |
| Platelets (K/mm3) | 211 | 180 | 224 | 332 |
| Sodium (mEq/ L) | 136 | 136 | 138 | 136 |
| Potassium (mEq/ L) | 3.7 | 3.8 | 4.9 | 4.7 |
| Chloride (mEq/ L) | 104 | 101 | 102 | 101 |
| Bicarbonate (mEq/ L) | 24 | 27 | 26 | 25 |
| BUN (mg/dL) | 15 | 13 | 33 | 28 |
| Creatinine (mg/dL) | 0.77 | 1.11 | 1.42 | 1.36 |
| AST/ALT (U/L) | 21/11 | 23/16 | 52/14 | 36/19 |
| Bilirubin (mg/dl) | 0.4 | 1.6 | 0.4 | 0.4 |
| PT/INR/PTT (sec) | 12.3/0.9/34.3 | ND | 13.6/1.0/32 | ND |
| Fibrinogen (mg/dl) | 542 | ND | 685 | ND |
| D-dimer (mcg/mL) | 0.94 | 20 | 4.29 | 1.69 |
| Ferritin (ng/mL) | 139 | 351 | 721 | 434 |
| CRP (mg/L) | 123.1 | 94.3 | 174.5 | 211 |
| ESR (mm/hr) | 40 | 81 | 63 | 112 |
| IL-6 (pg/mL) | 60.4 | ND | 28.6 | 27.7 |
| Troponin (ng/mL) | 0.01 | ND | 0.083 | 0.011 |
| Therapeutic anticoagulation- type/dose/day started/indication | Enoxaparin/85 mg Q12H SQ/Day 7/COVID-19- associated hypercoagulability | Enoxaparin/60 mg BID SQ/ Day 2/ COVID-19-associated hypercoagulability | Enoxaparin /80 mg Q12H SQ /Day 3/COVID-19-associated hypercoagulability | Enoxaparin/75 mg BID SQ/ Day 3/ COVID-19- associated hypercoagulability |
| Site of bleeding/size of hematoma | Left chest wall hematoma/Size – 15 × 13 cm | Left chest wall hematoma/Size –4.2 × 13.8 × 11.7 cm | Left adductor muscle, proximal hamstring muscles piriformis and gluteus maximus/size- NR | Anterior compartment of right thigh /size- 17 × 5 × 4 cm |
| Day of hospitalization corresponding to bleeding/ imaging used for diagnosis | Day 14/ CT chest angiography | Day 5/ CT chest angiography | Day 12/ CT abdomen pelvis without contrast | Day 14/ CT thigh with contrast |
| Treatment for bleeding | PRBC and FFP transfusion and CT-guided drainage of chest wall hematoma | PRBC and FFP transfusion and surgical drainage of chest wall hematoma | Supportive care – PRBC transfusion | PRBC transfusion and surgical evacuation of hematoma |
| Laboratory values at the time of bleeding episode | ||||
| WBC (K/mm3) | 14 | 9.8 | 18 | 7.6 |
| Hemoglobin (g/dL)/hematocrit (%) | 10.1/30.8 | 9.6/31.7 | 6.8/20.8 | 13.6/38.6 |
| Platelets (K/mm3) | 245 | 208 | 193 | 352 |
| BUN (mg/dL) | 20 | 30 | 51 | 23 |
| Creatinine (mg/dL) | 0.64 | 1.18 | 1.72 | 0.85 |
| PT/INR/PTT (sec) | 17.9/1.5/34.1 | 16.8/1.4/37.7 | 14/1.1/33.1 | 13.1/1.0/26.7 |
| Fibrinogen (mg/dl) | 101 | 193 | 500 | ND |
| FDP (mcg/mL) | ND | ND | <5 | ND |
| D-dimer (mcg/mL) | 0.26 | 5.04 | 1.59 | 1.46 |
| ISTH criteria | 1 | 2 | 2 | NA |
| Prior history of bleeding | No | No | No | No |
| Trauma | No | No (History of fall prior to admission) | No | No |
| Outcome | Discharged | Discharged | Discharged | Discharged |
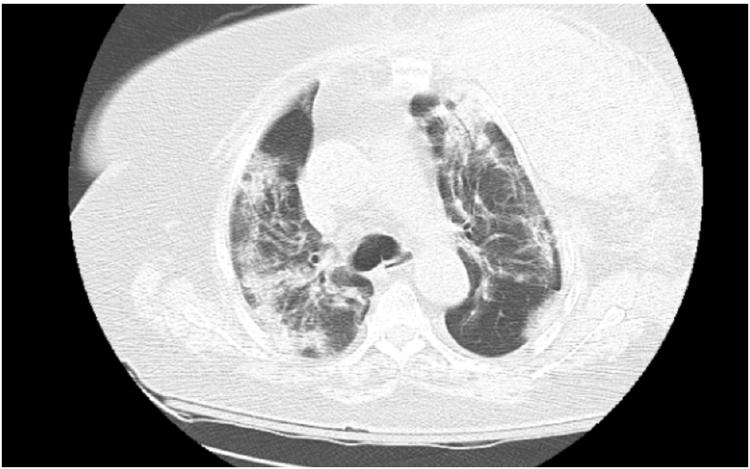
Figure 1.
CT chest angiography large left chest wall hematoma involving left breast tissue and left pectoral musculature.
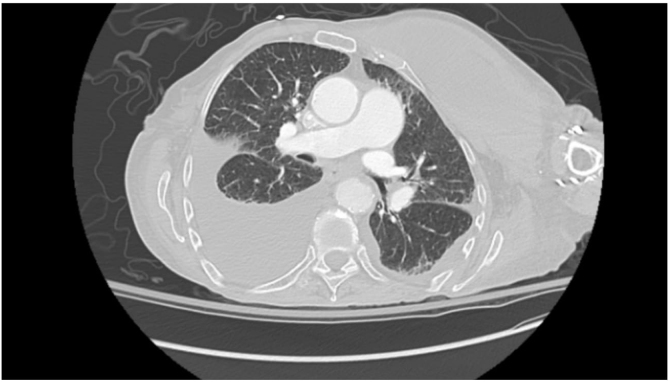
Figure 2.
CT chest angiography showing large multi-septated collection along left anterior to lateral chest wall with surrounding infiltration into latissimus dorsi and intercostal musculature.
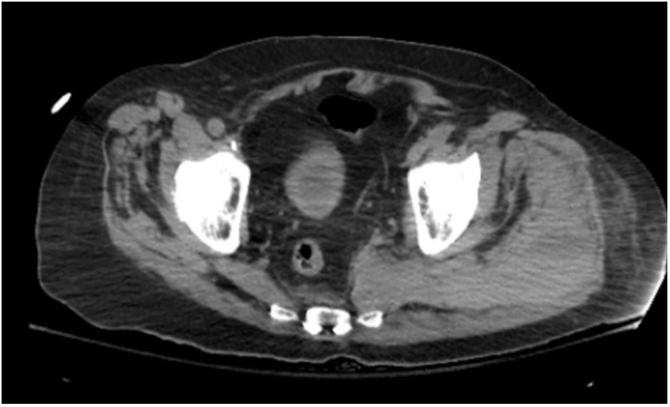
Figure 3.
CT abdomen pelvis without contrast showing marked edema with areas of increased density involving left adductor muscle, proximal hamstring muscles, piriformis and gluteus maximus.
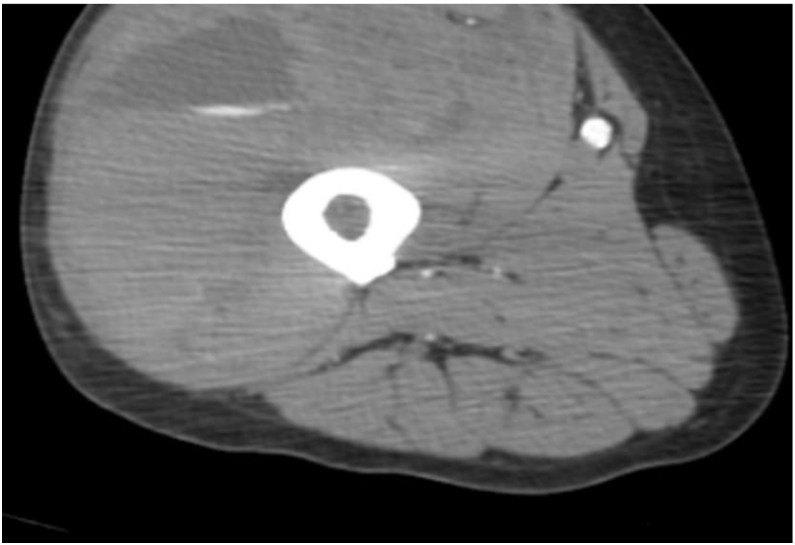
Figure 4.
CT thigh with contrast showing large hematoma in anterior compartment of right thigh.
Discussion
Bleeding is less common than thrombosis in patients with COVID-19, but it may occur, especially in the setting of anticoagulation. Bleeding diathesis is likely multifactorial, related to the COVID-19 illness severity or anticoagulation. In our case series, the patients received therapeutic anticoagulation and showed significant bleeding events at unusual locations (with no overt risk factors for bleeding, such as DIC or thrombocytopenia, at the time of the bleeding episode).
Shah et al. conducted a multicenter retrospective observational study evaluating 187 COVID-19 patients in intensive care units (ICUs) of four tertiary hospitals. A total of 178 patients (95.1%) were on either prophylactic or therapeutic anticoagulation. Fifteen patients (8.0%) developed hemorrhagic complications, of which 9 (4.8%) were classified as major bleeding. Nearly all patients were male (n = 14) and gastrointestinal bleeding was the most common site. Other sites in which bleeding was reported were intracranial, genitourinary, epistaxis and tracheostomy. Five patients who experienced major bleeding were on therapeutic anticoagulation and 4 patients were on standard thromboprophylaxis.7 No patients had overt DIC or a fibrinogen concentration of less than 1.5 g/L. In our case series, all the patients were admitted to the non-ICU unit, bleeding occurred at unusual sites and none of the patients had any trauma, thrombocytopenia or overt DIC at the time of the bleeding episode.
Tang et al. reported on a study in which 449 patients with severe COVID‐19 were enrolled, 99 of whom had received heparin (94 received LMWH 40–60 mg enoxaparin/d and five received unfractionated heparin 10,000–15,000 U/d for 7 days or longer), the 28‐day mortality of heparin users was lower than nonusers in patients with sepsis-induced coagulopathy SIC score ≥4 (40.0% vs. 64.2%, p = .029), or D‐dimer >6‐fold the normal upper limit (32.8% vs. 52.4%, p = .017), suggesting the anecdotal observation that thromboprophylaxis with heparin decreased mortality in patients with severe COVID-19 meeting sepsis-induced coagulopathy criteria or with markedly elevated D-dimer levels.8 In a study by Klok et al. on 184 ICU patients with COVID-19 pneumonia, a 31% incidence of thrombotic complications was found, however, none of the patients developed overt DIC.9
Erdinc et al. reported on a case of concurrent spontaneous retroperitoneal and massive acute deep vein thrombosis at the initial presentation of COVID-19.10 The patient was not on any anticoagulation medication before presentation. Mazzitelli et al. reported on three COVID-19 patients who developed spontaneous and severe muscle hematomas (psoas and adductor muscle). The patents were started on low molecular weight heparin for paroxysmal atrial fibrillation.11 Conti et al. reported on two cases of spontaneous ileo-psoas hematomas in hospitalized COVID-19 pneumonia patients. One patient was on prophylactic medication and one, on a therapeutic dose of anticoagulation medication for deep venous thrombosis.12
Daily monitoring of biomarkers, including D-dimer, as a means to guide intensity of anticoagulation management is not recommended.13 Despite the lack of prospective data, many institutions have adopted thromboprophylaxis protocols with intermediate-dose, or even therapeutic-dose, anticoagulation therapy. The American Society of Hematology (ASH) recommends that all hospitalized adults with COVID-19 receive pharmacologic thromboprophylaxis with LMWH over unfractionated heparin to reduce contact, unless the risk of bleeding outweighs the risk of thrombosis. Whether critically ill COVID-19 patients should receive therapeutic-intensity anticoagulation in the absence of confirmed or suspected venous thromboembolism (VTE) is currently unknown. Proper patient selection to identify patients at higher risk for bleeding, while at the same time weighing it against the risk of thrombosis, may help firmly establish the role of anticoagulation in patients with COVID-19. Randomized trials are ongoing to determine the optimal approach to thrombosis prevention in COVID-19 patients.
In conclusion, significant bleeding at unusual sites can occur in COVID-19 patients upon anticoagulation treatment (both prophylactic and therapeutic) and, therefore, a high degree of suspicion and careful clinical monitoring is required. Our case and review of the literature emphasize the importance of limiting anticoagulation to appropriate indications.
Funding
None.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh B., Kaur P., Maroules M. Splanchnic vein thrombosis in COVID-19: a review of literature. Dig Liver Dis. 2020;52(12):1407–1409. doi: 10.1016/j.dld.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh B., Mechineni A., Kaur P., Ajdir N., Maroules M., Shamoon F. Acute intestinal ischemia in a patient with COVID-19 infection. Korean J Gastroenterol. 2020;76(3):164–166. doi: 10.4166/kjg.2020.76.3.164. [DOI] [PubMed] [Google Scholar]
- 4.Singh B., Aly R., Kaur P., Gupta S., Vasudev R., Virk H.S. COVID-19 infection and arterial thrombosis: report of three cases. Ann Vasc Surg. 2021;70:314–317. doi: 10.1016/j.avsg.2020.08.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaur S., Bansal R., Kollimuttathuillam S., Gowda A.M., Singh B., Mehta D. The looming storm: blood and cytokines in COVID-19. Blood Rev. 2020 doi: 10.1016/j.blre.2020.100743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaur P., Posimreddy S., Singh B., Qaqa F., Habib H.A., Maroules M. COVID-19 presenting as acute limb ischaemia. Eur J Case Rep Intern Med. 2020;7(6) doi: 10.12890/2020_001724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah A., Donovan K., McHugh A., Pandey M., Aaron L., Bradbury C.A. Thrombotic and haemorrhagic complications in critically ill patients with COVID-19: a multicentre observational study. Critical Care. 2020;24(1):561. doi: 10.1186/s13054-020-03260-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klok F.A., Kruip M., van der Meer N.J., Arbous M.S., Gommers D.A., Kant K.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erdinc B., Raina J.S. Spontaneous retroperitoneal bleed coincided with massive acute deep vein thrombosis as initial presentation of COVID-19. Cureus. 2020;12(8) doi: 10.7759/cureus.9772. e9772-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazzitelli M., Serapide F., Tassone B., Laganà D., Trecarichi E.M., Torti C. Spontaneous and severe haematomas in patients with COVID-19 on low- molecular-weight heparin for paroxysmal atrial fibrillation. Mediterr J Hematol Infect Dis. 2020;12(1) doi: 10.4084/MJHID.2020.054. e2020054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conti C.B., Henchi S., Coppeta G.P., Testa S., Grassia R. Bleeding in COVID-19 severe pneumonia: the other side of abnormal coagulation pattern? Eur J Intern Med. 2020;77:147–149. doi: 10.1016/j.ejim.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flaczyk A., Rosovsky R.P., Reed C.T., Bankhead-Kendall B.K., Bittner E.A., Chang M.G. Comparison of published guidelines for management of coagulopathy and thrombosis in critically ill patients with COVID 19: implications for clinical practice and future investigations. Crit Care. 2020;24(1):559. doi: 10.1186/s13054-020-03273-y. [DOI] [PMC free article] [PubMed] [Google Scholar]