Abstract
Panstrongylus geniculatus (Latreille, 1811) is the triatomine with the largest geographic distribution in Latin America. It has been reported in 18 countries from southern Mexico to northern Argentina, including the Caribbean islands. Although most reports indicate that P. geniculatus has wild habitats, this species has intrusive habits regarding human dwellings mainly located in intermediate deforested areas. It is attracted by artificial light from urban and rural buildings, raising the risk of transmission of Trypanosoma cruzi. Despite the wide body of published information on P. geniculatus, many knowledge gaps exist about its biology and epidemiological potential. For this reason, we analysed the literature for P. geniculatus in Scopus, PubMed, Scielo, Google Scholar and the BibTriv3.0 databases to update existing knowledge and provide better information on its geographic distribution, life cycle, genetic diversity, evidence of intrusion and domiciliation, vector-related circulating discrete taxonomic units, possible role in oral T. cruzi transmission, and the effect of climate change on its biology and epidemiology.
Key words: Panstrongylus geniculatus, geographic distribution, genetic diversity, oral transmission of Trypanosoma cruzi, climate change
Chagas disease affects around 7 million people in Latin America and is considered one of the 10 most important neglected diseases in the region. 1 , 2 Currently, 154 species within the Triatominae subfamily (three extinct and 151 extant) have been described and are able to transmit Trypanosoma cruzi, the causative agent of Chagas disease under natural and experimental conditions. 2 , 3 , 4 , 5 , 6 , 7 , 8
Panstrongylus geniculatus (Latreille, 1811) is the triatomine with the greatest geographic distribution in the Americas, ranging from southern Mexico to northern Argentina and including the Caribbean islands. 9 , 10 This vector occupies extremely variable areas across the wild landscape, being found in different ecosystems within the same country. 11 Most records deal with the species occupying altitudes ranging from sea level to around 2,000 metres above sea level (masl); however, the species has been found at altitudes from 2,000 to 4,000 masl in some Andean countries (i.e., Colombia, Venezuela, Ecuador, Peru and Bolivia), thereby indicating the species’ great adaptive capability, probably enabling its shift in altitude as a consequence of lowland warming, regarding today’s climate changes. 12 , 13 , 14 , 15 , 16
The last few decades have seen much interest in the biology, ecology and epidemiology of P. geniculatus, thereby enabling effective control strategies for this species to be designed. Therefore, this review aims to update existing knowledge about P. geniculatus and address current information gaps. A search was conducted in Scopus, PubMed, Scielo, Google Scholar, BibTriv3.0 databases with no filters of language or time and until August 2020. All relevant studies on taxonomy, morphological variability, life cycle, geographical distribution, genetic diversity, intrusion and colonisation of human dwellings, oral transmission and discrete taxonomic units of T. cruzi and possible effects of climate change on P. geniculatus were chosen.
Taxonomy, morphology and life cycle
Taxonomy - Currently, P. geniculatus (Latreille, 1811) is the accepted scientific name for this species; however, the following synonyms have also been used reviewed by Patterson et al.: 17 Reduvius geniculatus (Latreille, 1811), Conorhinus lutulentus (Erichson, 1848), Lamus geniculatus (Stal, 1859), Conorhinus corticalis (Walker, 1873), Conorhinus geniculatus (Walker, 1873), Lamus corticalis (Lethierry & Severin, 1896), Triatoma geniculata (Chagas, 1912), Triatoma tenuis (Neiva, 1914), Triatoma fluminensis (Neiva and Pinto, 1922), Mestor geniculatus (Brindley, 1931) and Panstrongylus parageniculatus (Ortiz, 1971).
Fifteen species have been described in the Panstrongylus genus: P. chinai, P. diasi, P. geniculatus, P. guentheri, P. howardi, P. hispaniolae, P. humeralis, P. lenti, P. lignarius, P. lutzi, P. megistus, P. martinezorum, P. mitarakaensis, P. rufotuberculatus, P. tupynambai. 2 , 3 , 17 Studies on the taxonomy of this genus and the identification of P. geniculatus have been problematic because most are based on morphometry and karyotyping, but few have employed molecular markers. 17 , 18 , 19 , 20 ) The phylogenies based on morphological traits presents the Panstrongylus genus as a monophyletic group, whereas the ITS-2 (rDNA) phylogenetic trees suggest that it is polyphyletic group of Triatoma species from South, Central and North America. 9 , 21 In another study, using four mitochondrial markers (16S, COI, COII, Cytb) and two nuclear (18S and 28S) ones, Panstrongylus fell into two groups. One of the groups contained P. tupynambai, P. lutzi and P. geniculatus as sister taxa of Nesotriatoma, whereas the other was a highly supported group that includes Triatoma tibiamaculata and P. megistus. 22
The use of morphological characters to classify Panstrongylus species has led to the description of ‘new species’ in the genus. This is the case for two species described in French Guiana and Venezuela; namely, P. mitarakaensis and P. martinezorum, respectively. 23 , 24 They both look quite similar to P. geniculatus but no molecular methods have been used to confirm that they are the same species. 3 Another species in Venezuela, P. turpiali, was synonymised with P. chinai but both were then shown to phenotypically match P. geniculatus. 25 , 26 , 27 Therefore, it is necessary to use molecular approaches to clarify the taxonomic status of P. geniculatus and phenotypically similar species.
Morphological variability in P. geniculatus - Several authors have reported on the morphological characteristics of P. geniculatus. Its nymph and adult stages have been described using light and scanning electron microscopy. 9 , 17 , 19 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 Elsewhere, the geometric morphometric techniques applied to a sylvatic population and its laboratory descendants over five generations revealed size differences in P. geniculatus but not in its shape. Essentially, head size and wing size were both reduced from sylvatic and laboratory populations. 35 Another morphometric study on P. geniculatus showed a reduction in the size of its populations in Caracas city, when compared with sylvatic populations from other Venezuelan regions. 20 In this review we have included unpublished photographs representing the known morphological characteristics of the species, which we believe will help to a primary identification of individuals captured in sylvatic, peridomestic or domestic environments (Fig. 1A-G).
Fig. 1: (A) dorsal view of a female Panstrongylus geniculatus: (i) clipeus, (ii) antenna, (iii) compound eye, (iv) leg, (v) pronotum, (vi) scutellum, (vii) connexivum, (viii) wings (hemielytrons); (B) head; (C) pronotum and scutellum; (D) legs; (E) coxa and trochanter; (F) spicules; (G) connexivum. Source: authors.
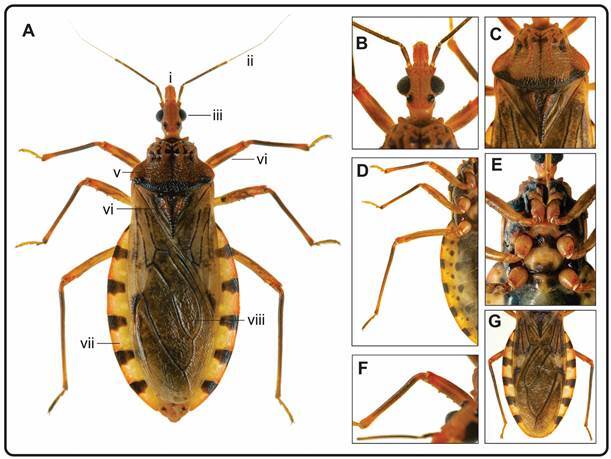
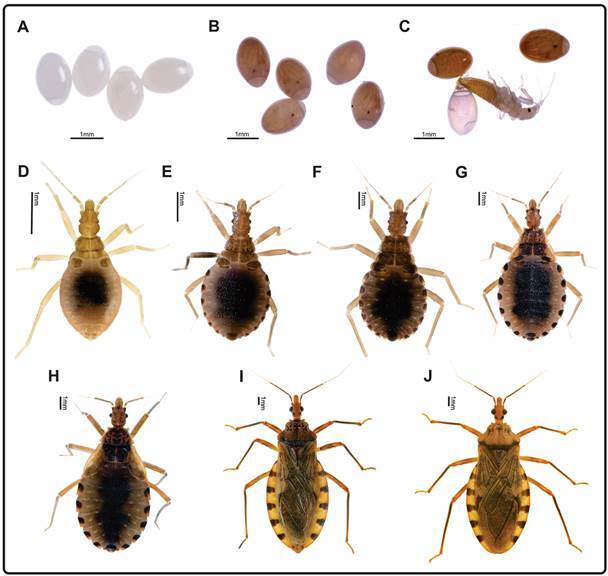
Adult P. geniculatus insects display sexual dimorphism. The total length of the male is 22-28 mm and the larger-bodied female is 22.5-29.5 mm. The posterior end of the male’s abdomen is rounded in shape, whereas it is tip-shaped in the female (Fig. 2I-J). Its colouration is light brown to orange brown and is interspersed with dark patches on various parts of the body, which do not follow a defined pattern in that the connexivum has two intercalated regions of colour: a flat black-coloured one and another coloured orange to ochre (yellow-orange) in the interior (Fig. 1A). 36
Fig. 2: stages of the life cycle of Panstrongylus geniculatus: (A) freshly oviposited eggs; (B) eggs near hatching; (C) hatching; (D) nymph stage N1; (E) nymph stage N2; (F) nymph stage N3; (G) nymph stage N4; (H) nymph stage N5; (I) adult (female); (J) adult (male). Source: authors.
The head is sub-conical-shaped, narrow at eye level, and shorter than the pronotum; the anteocular region is approximately twice as long as the postocular region. The dorsal surface is slightly rough. The general colouration is ochre, but in some individuals two dark stripes extending from the base of the jugae to the space between the ocelli are observed in the dorsal region. Anteniferous tubers are located near the anterior edges of the eyes, and the antennas vary from reddish brown to black. The first segment of the antenna subtly exceeds the apex level of the clypeus (Fig. 1A-B).
Behind the head is the orange-brown pronotum whose anterior lobe is slightly convex and rough. The pronotum features a central four-leaf clover-shaped mark and “1 + 1” side marks. The posterior lobe is irregularly rough, with a black band along the posterior margin, except in the humeral area (Fig. 1C). The femurs and tibiae are dark brown or black (Fig. 1A, D). The legs have light yellow or orange-yellow coxas and trochanters (Fig. 1E). The front and middle femurs have two to six (usually four) small subapical denticles arranged in two rows (Fig. 1F). The hemielytrons are light brown or light yellowish brown, with dark veins in the membranes (Fig. 1G).
Life cycle of P. geniculatus under laboratory conditions - P. geniculatus eggs do not adhere to the substrate; rather, they are oviposited free on a surface. They are oblong and symmetrically shaped, without lateral flattening or collar, and at one end is a prominent and convex operculum through which hatching is performed. The eggs are bright white for the first days after oviposition, and six-eight days later they turn pinkish yellow, pink, or pearl, becoming translucent after hatching (Fig. 2A-C). The nymphal and adult stages are shown in Fig. 2D-J.
The life cycle period under laboratory conditions has been shown to be dependent on the experimental conditions employed, such as temperature, relative humidity and food source in the colonies. Depending on the management of these variables, very heterogeneous records have been observed from 149.5 to 531 days, and even up to two years. When the experimental conditions are 21-26ºC and 90-100% for temperature and relative humidity, respectively, life cycle durations of 269, 297, 275.8, 273, 275.4 days have been reported (average, 278 days). 32 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 One of the first studies to investigate the life cycle of P. geniculatus reported that the total development time is approximately 2 years. 45 Another study reported on the difficulties of establishing the species as a colony and observed that the time taken for the life cycle is 531 days (75.8 weeks). 37
The 1990s brought advances in biological knowledge. The life cycle was studied under controlled laboratory conditions and reported that the average development time was 269 days (38.4 weeks) and 297 days (42.4 weeks) for nymphs fed on chickens (Gallus gallus) and opossums (Didelphis marsupialis), respectively. 38 In a life-cycle study using uncontrolled conditions, the insects were subjected to temperatures of 21-25ºC, relative humidities of 90-100%, and were fed ad libitum weekly on chickens (G. gallus). An N1 nymph stage to adulthood duration of 275.8 days (39.4 weeks) was reported. 39 The duration of the life cycle for specimens from the municipality of Amalfi in Antioquia, Colombia, which were subject to controlled temperature (29 ± 1ºC) and relative humidity (95 ± 1ºC) conditions, was 149.5 days (21.4 weeks). 32 Later on, evidence of the colonisation processes in Amalfi in Antioquia, Colombia, and estimated transmission risk indicator values were obtained. They also reported on the time required for blood intake by adults (mean, 67.8 minutes for males and 80.27 minutes for females). Blood consumption was on average 0.12 g for males and 0.26 g for females, with defecation times post-feeding of 55 minutes for females and 60 minutes for males. 40 Sylvatic intrusive insects captured in domestic environments have had high T. cruzi infection prevalence (see Table III in this review). In addition, a post-feeding time close to one hour for both males and females could be a determining factor for this species regarding the oral transmission of Chagas’ disease due to food contamination. As P. geniculatus has been repeatedly associated with orally transmitted outbreaks, this hypothesis could be ascertained by comparing this species’ post-feeding time characteristics and natural infection frequencies to those for other species invading human dwellings.
TABLE III. Panstrongylus geniculatus adult specimens reported inside human dwellings in Latin American countries, without evidence of colonization (eggs, nymphs and exuviae).
| Country | Department/State | Trypanosoma cruzi infection frequency | Reference |
| Argentina | Corrientes | ND | 95 |
| Bolivia | La Paz | 33% | 49 |
| Cochabamba | 62,5% | 51 | |
| Brazil | Paraná | Negative | 96 |
| Piauí | Positive | 54 | |
| Goiás | 1,40% | 97 | |
| Mato Grosso do Sul | 3,20% | 98 | |
| Rio de Janeiro | Negative | 99,100 | |
| Amazonas | Positive | 81,101 | |
| Amazonas | Negative | 102,103 | |
| Minas Gerais | ND-Negative | 104,105,106 | |
| Distrito Federal | Negative | 107 | |
| Goiás | 7,70% | 108 | |
| São Paulo | Negative | 109,110 | |
| Acre | Negative | 111 | |
| Colombia | Bolivar | ND | 112 |
| Meta | 70,6-100% | 93,113 | |
| Magdalena | 83% | 114 | |
| Casanare | ND | 115 | |
| Cordoba | 30-50% | 92,116 | |
| Santander | 56% | 117 | |
| Sucre | 58,82% | 118 | |
| Peru | Amazonas, Cajamarca | ND | 76 |
| Pasco | 18,20-50% | 119,120 | |
| Cusco | ND | 121 | |
| Loreto | ND | 122 | |
| San Martin | ND | 123 | |
| Surinam | Saramacca, Wanica | ND | 124 |
| Venezuela | Caracas | 38,86-53% | 125,126 |
| Merida | Positive | 127 | |
| Caracas, Merida, Vargas | 76,1% (average) | 128 | |
| Lara | ND | 129 | |
| Aragua | Negative | 130 | |
| Aragua, Caracas, Miranda, Vargas | 50,3% (average of the four states) | 84 | |
| Sucre | ND | 131 |
ND: no data.
The first statistical evaluation of the population dynamics of P. geniculatus under controlled laboratory conditions studied a cohort of 60 eggs placed at constant temperature (26 ± 3ºC) and relative humidity (90 ± 10%) that were fed every 15 days on chickens (G. gallus). The researchers observed that the hatching percentage was 88.9%, the average nymph development time was 273 days (39 weeks), and the longevity of the adults was 504 days (72 weeks). 41
In contrast, the need of optimising the ovipositors of female P. geniculatus to enable massive breeding of this species in the laboratory and for use in bioassays, compared the ovipositors of laboratory and field populations fed on chickens (G. gallus) and mice (Mus musculus, strain BALB/c). The authors found that the fertility of the first-generation laboratory population was twice that of the wild population when both were fed on chickens. 42 , 43
The most recent and detailed study on the vital statistics of P. geniculatus, estimated its development times by stages, vital statistics (mortality and fertility), and population growth parameters (intrinsic natural growth rate, finite population growth rate, net reproduction rate, and generational time). In a cohort of 100 eggs kept under constant temperature (26 ± 1ºC) and relative humidity (60 ± 10%) and fed every eight days with chicken blood, it was observed that the total development time by stage was 275.4 days (39.4 weeks), and mortality reached 58% in the initial stages (I, II, and III), which represents 60% of the total mortality. 44
Geographical distribution, ecotopes and genetic variability
Geographical distribution and ecotopes - P. geniculatus has the greatest geographical distribution of species belonging to this genus. It is found exclusively in Latin America between 21.1ºN, 30.3ºS, 38.8ºE, and 94.9ºW, covering an estimated 12 million km2. To date, its presence has been reported in 18 countries (Table I, Fig. 3), from southern Mexico to northern Argentina, including the Caribbean islands. 9 , 12 , 45 , 46 , 47
TABLE I. Geographic distribution of Panstrongylus geniculatus in Latin America.
| Country | Location | Reference |
| Argentina | Chaco, Corrientes, Entre Rios, Formosa, Misiones, Santa Fe and Santiago Del Estero | 9,10 |
| Bolivia | Beni, Cochabamba, La Paz, Santa Cruz and Tarija | 9,10,48,49,50,51 |
| Brazil | Acre, Amapá, Amazonas, Bahia, Brasília DF, Ceará, Espírito Santo, Goiás, Maranhão, Mato Grosso, Minas Gerais, Pará, Paraná, Piauí, Rio de Janeiro, Rondônia, Roraima, São Paulo and Tocantins | 9,10, 46, 52,53,54,55,56 |
| Colombia | Amazonas, Antioquia, Arauca, Atlantico, Bolivar, Boyaca, Caqueta, Casanare, Cauca, Cesar, Choco, Cordoba, Cundinamarca, Guainia, Guaviare, Huila, La Guajira, Magdalena, Meta, Norte de Santander, Putumayo, Risaralda, Santander, Tolima, Sucre, Valle del Cauca, Vaupes and Vichada | 9,10,15,57,58,59,60,61,62,63 |
| Costa Rica | Alajuela, Cartago, Guanacaste, Heredia, Limon, Puntarenas and San Jose | 9,11,64 |
| Ecuador | Esmeraldas, Imbabura, Manabi, Napo, Orellana, Pastaza, Pichincha and Sucumbios | 9,10,65,66,67,68,69 |
| Guatemala | ND | 9 |
| British Guiana | ND | 9 |
| French Guiana | Apotou, Awala-Yalimapo, Cayenne, Comopi, Grand-Santi, Kourou, Iracoubo, Macouria, Mana, Maripasoula, Matoury, Montsinery, Ouanry, Papaichton, Roura, Remire-Montjoly, Regina, Saint-Elie, Saint-Georges, Saint-Laurent, Sinnamary and Saul | 9,70,71 |
| Mexico | Chiapas, Veracruz and Yucatan | 10 |
| Nicaragua | Boaco and Zelaya | 9,72,73 |
| Panama | Panama | 9,74 |
| Paraguay | Alto Parana, Boqueron, Concepcion, Caaguazu, Nueva Asuncion and Paraguari | 9,10,46 |
| Peru | Amazonas, Ayacucho, Bagua, Cajamarca, Cerro de Pasco, Cusco, Huanuco, Jaen, Junin, Loreto, Madre de Dios, Martin, Puno, San Ignacio, San Pasca, Ucayali and Utcubama | 9,16,46,75,76,77 |
| Suriname | Commewijne, Para, Paramaribo, Saramacca, Sipaliwini and Wanica | 9,78,79 |
| Trinidad y Tobago | San Patricio and ST. George | 9,80 |
| Uruguay | ND | 9 |
| Venezuela | Amazonas, Anzoategui, Aragua, Bolivar, Carabobo, Distrito federal, Delta Amacuro, Falcon, Guarico, Lara, Merida, Miranda, Monagas, Tachira, Yaracuy and Zulia | 9,10,81 |
Fig. 3: continental distribution of Panstrongylus geniculatus. Maps were made with the database reported by Ceccarelli et al. 47 .
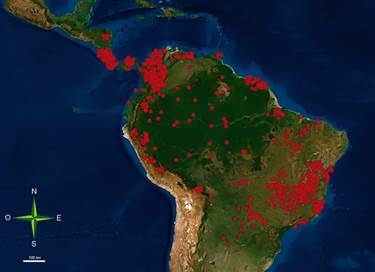
With its wide geographical distribution, P. geniculatus is found from sea level to 2,000-4,000 m above sea level in the Andean countries (Colombia, Venezuela, Ecuador, Peru and Bolivia) 14 , 15 , 16 and in places where the average annual temperature (minimum and maximum) ranges between -3.9 and 34.3ºC and precipitation reaches up to 4,000 mm/year. 9 Some authors have suggested that the morphological variations found among P. geniculatus populations result from the demands of the different environments. 18 Table II shows the presence of P. geniculatus above 2,000 masl in Colombia and Peru.
TABLE II. Records of Panstrongylus geniculatus presence above 2,000 masl in Colombia and Peru.
| Country | Locality | State | Coordinates | masl | Reference |
| Colombia | Boavita | Boyaca | 6.333333 N -72.666667 W | 2,350 | 14,15 |
| Susacon | Boyaca | 6.2298 N -72.6901 W | 2,480 | ||
| San Mateo | Boyaca | 6.3303 N -72.585 W | 2,200 | ||
| Casabianca | Tolima | 5.0796 N -75.1206 | 2,100 | ||
| Peru | Ninacaca | Pasco | -10.75819 S -76.17819 W | 4,100 | 14,16 |
| Tarma | Junin | -11.419335 S -75.68849 W | 3,050 | ||
| Cholon | Huanuco | -8.690057 S -76.733652 W | 2,350 | ||
| Cutervo | Cajamarca | -6.376045 S -78.821275 W | 2,600 | ||
| San Miguel | Ayacucho | -12.932081 S -73.747786 W | 2,660 | ||
| Huanta | Ayacucho | -12.938411 S -74.250212 W | 2,630 |
masl: metres above sea level.
Natural ecotopes vary from dry or very dry tropical forests to savanna and humid tropical forests, and P. geniculatus can be found in caverns, caves, and burrows in these environments where it is mainly associated with armadillos (Dasypodidae), anteaters (Myrmecophagidae), bats (Chiroptera), and a wide variety of other vertebrates. It is also found under bark, tree trunks and fallen trees and near bird nests in palm trees (Acrocomia aculeata, Syagrus romanzoffiana, Elaeis oleifera, and Leopoldinia piassaba) and in epiphytes. 9 , 17 , 82 , 83 , 84 , 85 Interestingly, it was reported that the species displays entomophagic behavior towards moths (Eacles spp.) in the forests of French Guyana for the first time, which confirms its versatility for survival in environments with low food availability. 86
Although the presence of P. geniculatus has been recorded in 18 countries, the recent availability of predictive maps can produce a robust summary of its distribution and show its spatial variation so that regions with a high probable risk of vector transmission and transmission through food contamination can be identified. 87
Genetic diversity in P. geniculatus - The Panstrongylus genus is classified as belonging to the Triatomini, a monophyletic tribe. However, the phylogenetic relationships inside this group are far to be cleared because current knowledge is limited and messy. 3 The Triatomini tribe is divided into three lineages: T. dispar, North American, and South American lineages. Panstrongylus is included in the Antillean Triatoma + Panstrongylus clade within the North American lineage. Three groups were tentatively distinguished in the Panstrongylus subclade, but only one of them includes P. geniculatus and its closest relatives P. mitarakaensis, P. martinezorum; 3 it also includes a set of south Amazonian species as P. lutzi (and its synonym P. sherlocki), P. lenti, P. diasi, P. guentheri and P. tupynambai. 9 , 17 , 23 , 24 , 88 , 89
Regarding karyotype variations in Panstrongylus species, it has been established that the species (P. chinai, P. geniculatus, P. lignarius, P. rufotuberculatus, P. tupynambai, P. herreri) have 20 autosomes (except P. megistus that has 18 autosomes), and X1X2Y chromosomes for males and X1X1X2X2 for females. In a study, three clusters were defined based on the C-band pattern and meiotic chromosomal behavior where P. geniculatus was not located in any of the groupings because it shares characteristics with each group. Consequently, the authors proposed that P. geniculatus should be considered as a complex of species comprising at least two different species because of its high chromosomal variability, wide distribution, and high phenotypic variation; 18 molecular evidence, however, is needed to confirm this hypothesis.
A Venezuelan study conducted a genetic variability analysis on P. geniculatus using Cytb as the molecular marker because it is extensively used to determine genetic polymorphisms in triatomines. The results showed that this marker is useful for describing genetic heterogeneity in P. geniculatus and revealed spatial and genetic patterns that agree with domiciliation processes for this species, but it could not resolve the evolutionary relationships. Even though this marker is informative about the population structure, other genes must be evaluated to study the genetic variability. 90
A very recent study used DNA markers and molecular data to study genetic diversity in P. geniculatus with the aim obtaining information about the processes that have shaped genetic diversity in this species. 91 Using mitochondrial DNA (mtDNA) fragments, 16S rRNA and Cytb, the authors reconstructed a phylogenetic tree that showed that P. geniculatus from Colombia and Venezuela form a monophyletic species with four clades concordant with its geographic distribution, which is partly explained by the Andes uplift (Fig. 4). However, other factors, including anthropogenic and eco-epidemiological effects must also be investigated to explain the existence of recent geographic P. geniculatus lineages. The authors also reconstructed the relationships within the Panstrongylus genus using various genetic markers. Using mtDNA markers (16S, ND4 and Cytb), they detected two clades: one includes P. lutzi and P. tupynambai, which is the sister clade of P. geniculatus (geniculatus group), and the other one includes P. lignarius and P. megistus, which represent the sister clade of P. rufotuberculatus, P. howardi and P. chinai (megistus group).
Fig. 4: genetic differentiation of Panstrongylus geniculatus in Colombia and Venezuela reported by Caicedo-Garzón et al. 91 .
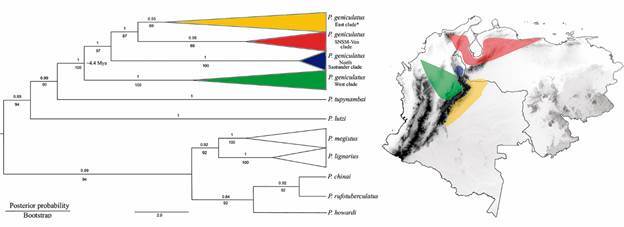
To conclude, further studies using more genetic markers and genomic approaches, along with widespread sampling from different geographic locations are needed to unravel the inter- and intra-specific relationships of the Panstrongylus genus, and specially for P. geniculatus considering its domiciliation reports, high infection rates with T. cruzi and incrimination in oral outbreaks of Chagas disease in Colombia and Venezuela. All this knowledge is relevant for the development of control and surveillance strategies for Chagas disease vectors.
Epidemiology, intrusion, colonisation of human habits and possible role in the oral transmission of T. cruzi
Evidence of dwelling intrusion by P. geniculatus in Latin America - Although P. geniculatus is considered a species of wild habits, several cases of intrusion into homes have been reported in different Latin American countries in recent decades. This new scenario implies an increased risk of transmission in endemic and non-endemic areas of Chagas disease, either by direct contact or via food contamination. 92 , 93 Table III shows 45 registered reports of intrusion by adult P. geniculatus in Bolivia, Brazil, Colombia, Peru, Surinam and Venezuela. Despite growing awareness of the relevance of the transmission dynamics of P. geniculatus, the drivers of house invasion remain poorly understood. However, it was observed that invasion by this species decreased with higher landscape disturbance levels and in hotter-day municipalities and increased somewhat in more places with intermediate disturbance, peaking in municipalities with average rainfall. 94
It is accepted that the increased interactions occurring between triatomines and humans are mainly related to losses in vegetation cover and increases in urban and rural populations invading natural ecotopes. Most of these intrusions occur at night when P. geniculatus fly in from wild environments to inhabited areas under the attraction of artificial lighting in search of food. 84 The author concluded that this occurred regardless of the type of housing, and that the invasion increased when high numbers of domestic animals were present and the distance to the forest was small. 84 Recently, 11 P. geniculatus specimens were captured in the city of Bucaramanga, a Colombian neighborhood, of which five out of nine tested were positive for T. cruzi (56%), which drew attention to the risk of infection by this vector settling in the periphery of homes and adjacent to the natural ecotopes where its life cycle in the wild occurs. 117
The ability of P. geniculatus to fly up to 2 km away has been verified. 125 In other study, was reported that P. geniculatus is attracted to artificial light sources. These researchers installed a light trap positioned 45 m above sea level in a wild area of Manaus in the Amazonas State of Brazil, and they operated it monthly for three consecutive nights over the course of a year. They found that the most commonly captured triatomine was P. geniculatus (38 individuals) in addition to six other species. 132 Likewise, Jacome et al., determined the risk associated with the dispersive nocturnal flights of sylvatic triatomines by installing artificial lights in a model house in the northeastern plains of Colombia and concluded that this nocturnal dynamic poses a risk for the domestic introduction of discrete typing units (DTUs) of T. cruzi associated with sylvatic transmission foci. 133
Evidence of intradomiciliary and peridomiciliary colonisation of P. geniculatus in Latin America
Although P. geniculatus is registered as an intrusive wild species in some regions of the Americas, in other regions it is registered as a species capable of colonising human dwellings. These differences in the behavior of P. geniculatus probably reflect the genetic differences in this species across Latin American populations. Generally, the reported number of P. geniculatus nymphs associated with human dwellings varies, and Table IV lists nine publications that have reported on the presence of P. geniculatus eggs and nymphs. Furthermore, two intradomiciled adults and three peridomiciled nymphs in Muñecas, La Paz (Bolivia) were recorded. 48
TABLE IV. Registration of Panstrongylus geniculatus adults, nymphs and eggs inside houses or peridomiciles in Latin American countries.
| Country | Department/State | ID | PD | Association with | Natural intection | Reference |
| Bolivia | La Paz | X | X | ND | Negative | 48 |
| Brazil | Pará | X | X | Pigs | 17% | 134 |
| Colombia | Antioquia | X | ND | ND | 21,2-50% | 40 |
| Atlantico | X | ND | ND | ND | 135 | |
| Casanare | X | ND | ND | Negative | 136 | |
| Venezuela | Miranda | X | ND | Rats | 20% | 137 |
| Lara | X | ND | ND | 9,0-5,07% | 138,139 | |
| Metropolitan District of Caracas | X | X | ND | 59,5% | 140 |
ND: no data; ID: intradomicily; PD: peridomicily.
Fifteen pigsties were examined in Muana, Pará State, Brazil, contained 207 females, 168 males, 2 N5, 7 NIII, 16 NII, 28 NI stages and 385 unhatched eggs, thereby evidencing a domiciliation process for P. geniculatus. In 10 of the 15 houses located up to a 10 m distant from the pigsties, 21 adults were collected in total. 134
The presence of P. geniculatus adults in 80 homes in Amalfi, Antioquia, Colombia, but only in one of the homes did they find nymphal stages of the insect was recorded. 40 Thirty-seven adults and a nymph of P. geniculatus in the intra-domicile of the studied dwellings in Atlántico, Colombia were reported. 135 The presence of adults and nymphs in development stages IV and V of P. geniculatus in the intra-domiciles in six of seven villages in the municipality of Tamara in Casanare Department (Colombia) was recorded. 136
Two males and one female P. geniculatus, 5NV, 8NIV, 3NIII, and 1NII stages, and seven eggs were captured in the tunnel of a rat (Rattus rattus) inside a house in Miranda, Venezuela. All the triatomines were abundantly blood-fed, with the exception of one recently molted male and one female, whose emergences were confirmed by the presence of NV-stage exuviae. Fecal examination of the three NV-stages and an adult male revealed the presence of T. cruzi. 137 In three houses examined in El Guamito, Lara State, Venezuela, were found one female, six N5-, two NIII- and two N2-stages and an overall T. cruzi infection rate of 9%. 138 In another study in the same state, P. geniculatus was found in 11 homes, registering 136 adults and two NIII stages, with a colonisation index of 18.18% and a 5.07% infection rate for T. cruzi. 139 3390 adult P. geniculatus specimens, 26 NV, 71 NIV and 27 NIII stages were captured in the Metropolitan District of Caracas between 2007 and 2013, of which 59,5% carried T. cruzi. The authors warned that dramatic modifications to the surrounding natural habitats had led to the establishment of a T. cruzi urban enzootic cycle, resulting in a high risk of transmission of Chagas disease in this capital city. 140
T. cruzi isolates from P. geniculatus, R. rattus and from humans infected during an orally-acquired Chagas disease outbreak in a non-endemic region of Venezuela belong to a same T. cruzi I genotype (TcId). The similarity in the parasite isolates from the three patients affected by the urban oral outbreak, the triatomine, and the rat captured at the guava juice preparation site allowed the authors to suggest that the source of infection was common among these persons and the vector, providing indirect evidence of P. geniculatus domiciliation in this region. 141
The colonisation capacity of P. geniculatus has been studied under laboratory conditions. One such study reported a ro/b index of 0.77, but concluded that as a “K” strategist with a low reproduction rate and a prolonged generational time, it was unlikely that P. geniculatus was an important disease vector at the household level, a finding that does not presently reflect the epidemiological importance of the species. 41 Nevertheless, another study compared the results they obtained from P. geniculatus (ro/b = 0.74) with two species known for their high colonising capacity, Rhodnius prolixus (0.74) and Triatoma infestans (0.65), and concluded that P. geniculatus has a remarkable colonising capacity, which should alert the relevant institutions to the risk presented by this species and the need to design new control strategies aimed at the wild species that in recent decades have been increasingly reported as having home intrusion and colonisation behavior. 44
The presence of sexual dimorphism was an indicator of home adaptation and the authors concluded that populations of P. geniculatus from Caracas, Venezuela, are morphologically adapted to home environments. 20 That the ability of the species to adapt to different environments in a few generations as was recently confirmed by Nakad-Bechara et al. 90 and poses a future risk to humans and should alert health institutions, especially taking into account the possible new scenarios of climate change and the specific anthropic processes of deforestation and landscape fragmentation.
P. geniculatus and oral transmission of T. cruzi
Over the last two decades, the oral form of disease transmission has changed from being a rare and unusual event to a public health concern after causing numerous deaths. 142 , 143
The difficulty in obtaining direct evidence incriminating the vector species responsible for the oral transmission of T. cruzi, is related to the fact that oral Chagas disease outbreaks occur several days after food contamination. Despite this difficulty, in some reported cases strong suspicions exist about the role of P. geniculatus in this transmission mechanism, because it has been observed as an abundant species in the area of the outbreak and it has been found invading the houses where contaminated food was prepared and, although in some cases the captured insects did not carry T. cruzi parasites, in other cases high levels of natural infection were observed. Table V lists 11 publications that report indirect evidence involving P. geniculatus in the oral transmission of T. cruzi.
TABLE V. Cases where Panstrongylus geniculatus has been reported as a highly probable transmitter of oral Trypanosoma cruzi infection in Latin America.
| Country | Location | Contaminated food | Cases/deaths | Reference |
| Restrepo (Meta) | Arepa and/or pineapple juice | 4/0 | 113 | |
| Antioquia (Turbo) | ND | 11/1 | 144 | |
| Santander (Bucaramanga) | tangerine juice and/or orange juice | 9/0 | 143 | |
| Colombia | Santander (Lebrija) | tangerine juice and/or orange juice | 10/2 | 145 |
| Santander (Piedecuesta) | ND | 5/0 | 146 | |
| San Vicente de Chucuri | ND | 3/0 | 143 | |
| Cesar (Aguachica) | ND | 11/0 | 147 | |
| Bolivia | Beni (Guayaramerin) | majo juice | 14/0 | 148 |
| Venezuela | Caracas | guava juice | 103/1 | 149 |
| Vargas | ND | 85/4 | 150 | |
| Merida (Antonio Pinto Salinas) | ND | 5/0 | 151 |
ND: no data.
Venezuela has experienced the most numerous and acute cases of parasite transmission by the oral route, with the source of infection generally being the intake of juices contaminated with triatomine feces. Infections have been reported in greater Caracas and the states of Falcon, Merida, Tachira, and Vargas where P. geniculatus has been the species linked with most of the outbreaks. Because it is the most abundant species in these localities, it has high T. cruzi infection rates and has been observed to defecate while returning to its shelter after feeding 128 , 140 , 152
In the municipality of Chacao in Caracas in 2007, 103 people from a school were infected with T. cruzi, and of those infected, 19% needed hospitalisation and one child died. The infection source was juice made in a house where T. cruzi-infected P. geniculatus insects were captured. 149 During 2009, another outbreak occurred in a school environment that affected 82 people, from which three children and one adult died. Again, the evidence from the case indicates that the infection source was juice contaminated with the feces of P. geniculatus. 150
In Colombia, several cases of infection by oral transmission have occurred in areas with low endemicity and presence of domiciled vectors. Thus, wild vectors such as P. geniculatus have become important in relation to this mechanism of infection and may be associated with outbreaks related to oral transmission in several Colombian municipalities. 153 In Lebrija, Santander, an acute case was presented, where ten infections and two deaths were reported. 145 A report on a new scenario for transmission in the department of Córdoba-Colombia in an area that was not considered to be a region at risk of T. cruzi transmission. This report was based on an acute case of the disease in which the patient did not present lesions on the skin or in the periocular region that indicated the bite of the insect. 92
The transmission sources for two oral outbreaks were identified, one of them in the Colombian town of Restrepo, Meta. By epidemiological analysis, genotyping and allele identification of seven T. cruzi microsatellites in the samples obtained from patients, as well as insects and reservoirs implicated in the outbreaks, they determined that this outbreak was caused by fecal contamination of arepa and pineapple juices by P. geniculatus. Their distance analysis allowed them to observe the grouping of patients with insects rather than with the reservoirs. Their findings were further explained by recent housing construction in the outbreak area, which altered the natural habitat of P. geniculatus and caused its intrusion into homes. 113
DTUs of T. cruzi detected in P. geniculatus
Natural infections of T. cruzi in P. geniculatus have been widely reported and quantified in different Latin American countries. Despite no clear trend, high rates of natural infection occur with this parasite, reaching 44% on average. Brazilian rates are 16.5%, 154 50%, 155 4.7% 156 and 27.3%. 157 The Trinidadian rate is 42.5%, 80 the Colombian rates are 50%, 40 70.6% 93 and 58.3%, 118 the Venezuelan rates are 20% 138 and 76.1%, 128 the Peruvian rate is 50%, 120 and the Bolivian rate is 62.5%. 51
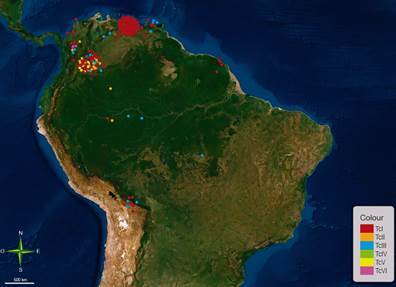
Currently, seven DTUs, TcI to TcVI and T.c.bat, are known to circulate in the vectors in different proportions. 158 , 159 The T. cruzi DTUs in P. geniculatus correspond to six of the seven described DTUs (TcI, TcII, TcIII, TcIV, TcV and TcVI), the geographic distributions of which in America for P. geniculatus are shown in Fig. 5. In Bolivia, insects infected with TcI and TcIII have been reported inside homes. 51 In Brazil, insects infected with TcII and TcIII have been identified. 157 In Colombia, the transmission dynamics of the primary and secondary vectors in six departments (Antioquia, Cesar, Guajira, Huila, Meta, and Norte de Santander) were studied and, with six circulating DTUs (TcI, TcII, TcIII, TcIV, TcV and TcVI). P. geniculatus was observed to be the species with the highest number of DTUs. 93 , 160 While TcI, TcIII and TcIV genotypes were isolated from the insects collected in the Colombian department of Casanare, 133 insects from Peru, Ecuador and French Guyana were only infected with TcI. 120 , 161 Last, TcI, TcIII and TcIV DTUs were identified in P. geniculatus collected from Venezuela. 140
Fig. 5: geographical distribution in America of Trypanosoma cruzi discrete typing units (DTUs) detected in Panstrongylus geniculatus.
Possible effects of climate change on P. geniculatus
Although controversy persists about the consequences of climate change on vector-transmitted infectious diseases, there is full consensus that the global temperatures are closely linked with the physiological processes of arthropod vectors. 162 , 163 Indeed, it has been shown that temperature and relative humidity can affect a vector insect by changing its original geographical distribution, altering the duration of its life cycle, or modifying its vector capacity, all which have consequences for the prevalence of infectious diseases. 164
To date, several studies have aimed to establish the effect of environmental variables on triatomines; however, attention has been mainly directed to T. infestans and R. prolixus. 165 , 166 , 167 No studies on P. geniculatus that assess the effect of climatic conditions on its life cycle or vector capacity have been reported under controlled laboratory conditions in the available databases. Only estimates of its potential distribution based on ecological niche modeling have been reported for some Latin American countries.
In Brazil, the geographic distribution of triatomine species in the Central West region was estimated and the climatic factors that influence their occurrence were analysed. The results indicate that among the variables analysed, temperature seasonality better explains the models of occurrence. The authors also reported that almost the entire region has the climatic conditions appropriate for at least one species and, according to the distribution maps, P. geniculatus was the most widespread species with the greatest potential for occurrence. 168 In Venezuela, the possible effects of climate change on the potential distribution of five species (Eratyrus mucronatus, P. geniculatus, T. maculata, R. robustus, and R. prolixus) were analysed. It was found that the variables best explaining the model were seasonal temperature (47.9%) and isometry (26.5%), and the highest relative vector competence values were those for R. prolixus (0.8) and P. geniculatus (0.1). However, they concluded that the possible future effects of climate change on the vulnerability of the Venezuelan P. geniculatus population show a slight downwards trend. 169
In Colombia, the geographical distribution of four species (P. geniculatus, R. pallescens, R. prolixus, and T. maculata) was estimated and identified a relationship between landscape structure and climate factors that influence their occurrence. According to the predictive maps on potential species distribution, the variables that best explain the model were altitude (26%) and precipitation seasonality (18%). 13 In light of the potential future geographical distributions, expressed as the suitability of a climate niche, several papers agree that P. geniculatus has a wide geographic distribution and has a greater distribution potential in possible climate change scenarios. 13 , 168 , 169
We recently observed that when P. geniculatus colonies are kept at 30ºC, they have higher mortality and lower fertility than those kept at 26 and 28ºC (Unpublished observations). Thus, it seems possible that under natural conditions, this species probably moves to areas where temperatures and altitudes favor its survival.
In Conclusion
All 15 of the species in the Panstrongylus genus have been described using morphological characters. Some of them are phenotypically similar to P. geniculatus, but molecular methods have not yet been used to determine whether or not they are the same species.
Geometric morphometry studies have revealed a reduction in the size of the populations studied in the urban areas of Caracas (Venezuela), when compared with sylvatic populations. The size reduction has also been verified in laboratory-raised populations, when compared with sylvatic populations; hence, this size reduction is likely associated with domiciliation processes.
Life-cycle timing under laboratory conditions has been shown to be dependent on the experimental conditions employed, such as temperature, relative humidity and the food source for the colonies. Depending on the management of these variables, very heterogeneous data have been recorded from 149.5-531 days to even up to two years. Other studies using temperature conditions of 21-26ºC and relative humidity of 90-100% have reported life cycle durations of 269, 297, 275.8, 273 and 275.4 days (average, 278 days).
Although P. geniculatus has been found in 18 countries, the recently generated predictive maps can produce a robust summary of its distributions. They can map its spatial variation to identify regions where a high risk of vector transmission of T. cruzi and transmission through food contamination are likely.
Based on the karyotype characteristics of P. geniculatus, which has high chromosomal variability, P. geniculatus is proposed to be a complex of species containing at least two different ones. The use of Cytb as a molecular marker has revealed spatial and genetic patterns related to domiciliation processes. The phylogenetic reconstruction of P. geniculatus from Colombia and Venezuela, using 16S rRNA and Cytb, showed that it comprises four phylogenetic clades concordant with its geographical distribution and the Andes uplift. However, it will be necessary to use other molecular markers and genomic approaches to identify intraspecific variations related to domiciliation of this species, high infection rates with T. cruzi and incrimination in oral outbreaks of Chagas disease in Colombia and Venezuela.
P. geniculatus is considered to have sylvatic habits; however, in recent decades at least 45 cases of intrusion into homes by adult insects have been reported in Bolivia, Brazil, Colombia, Peru, Surinam and Venezuela. This new scenario implies an increased transmission risk of the disease in endemic and non-endemic areas, either by direct contact or by food contamination. Nevertheless, in some Latin American regions the presence of nymphs, eggs and exuviae is evidence of colonisation of human dwellings, as reported in nine publications.(40,48,134,135,136, 137,138,139,140) These differences in species behavior probably reflect genetic differences between the populations.
The difficulty in obtaining direct evidence incriminating which vector species is responsible for oral transmission of T. cruzi, is related to the fact that orally-transmitted outbreaks of Chagas disease occur several days after food contamination. Despite this difficulty, in some reported cases there are strong suspicions about the role of P. geniculatus in this transmission mechanism, because it has been abundantly observed in outbreak areas. Indeed, it has been found invading houses where contaminated food is prepared and although in some cases the captured insects did not show infection with T. cruzi, in other cases high levels of natural infection were observed. At least 10 publications present indirect evidence involving P. geniculatus in the oral transmission of T. cruzi. 113 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151
Although it is not completely clear what the effect of climate change will be on the biology of P. geniculatus, it is known that the species has optimal development between 26 and 28ºC and between 90-100% relative humidity. That colonies kept at 30ºC have high mortality and low fertility means that increases in temperature may affect the distribution of the species such that it could move altitudinally to find places with more favorable temperatures.
ACKNOWLEDGEMENTS
To Sandra Cheesman, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/ac) and Jason Garry for editing a draft of this manuscript.
Footnotes
Financial support: Colombian Science, Technology and Innovation Department (Colciencias) project 120465843375 contract 063-2015 (http://www.colciencias.gov.co/node/1119) to FG, GAV and JDR; University of Tolima Research Fund (project 770115).
REFERENCES
- 1.WHO https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis)
- 2.Justi S, Galvão C. The evolutionary origin of diversity in Chagas disease vectors. Trends Parasitol. 2016;33(1):42–52. doi: 10.1016/j.pt.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monteiro F, Weirauch C, Felix M, Lazoski C, Abad-Franch F. Evolution, systematics, and biogeography of the Triatominae, vectors of Chagas disease. Adv Parasitol. 2018;99 doi: 10.1016/bs.apar.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Dorn P, Justi S, Dale C, Stevens L, Galvão C, Lima-Cordón R, et al. Description of Triatoma mopan sp. n. from a cave in Belize (Hemiptera, Reduviidae, Triatominae) ZooKeys. 2018 doi: 10.3897/zookeys.775.22553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poinar Jr A primitive triatomine bug, Paleotriatoma metaxytaxa gen. et sp. nov. (Hemiptera: Reduviidae: Triatominae), in mid-Cretaceous amber from northern Myanmar. Cretaceous Res. 2019;93 doi: 10.1016/j.cretres.2018.09.004. [DOI] [Google Scholar]
- 6.Nascimento J, Da Rosa J, Salgado-Roa F, Hernández C, Pardo-Diaz C, Alevi K, et al. Taxonomical over splitting in the Rhodnius prolixus (Insecta: Hemiptera: Reduviidae) clade: Are R. taquarussuensis (da Rosa et al., 2017) and R. neglectus (Lent, 1954) the same species? PLoS One. 2019;14(2) doi: 10.1371/journal.pone.0211285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jurberg J, Galvão C. Biology, ecology, and systematics of Triatominae (Heteroptera, Reduviidae), vectors of Chagas disease, and implications for human health. Neue. 2006;50:1096–1116. [Google Scholar]
- 8.Schofield C, Galvão C. Classification, evolution, and species groups within the Triatominae. Acta Trop. 2009;110(2-3) doi: 10.1016/j.actatropica.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Lent H, Wygodzinsky P. Revision of the Triatominae (Hemiptera: Reduviidae), and their significance as vectors of Chagas disease. Bull Am Museum Nat Hist. 1979;163(3):123–520. http://hdl.handle.net/2246/1282 [Google Scholar]
- 10.Galvão C, Carcavallo R, Rocha D, Jurberg J. A checklist of the current valid species of the subfamily Triatominae Jeannel, 1919 (Hemiptera, Reduviidae) and their geographical distribution, with nomenclatural and taxonomic notes. Zootaxa. 2003;202(1) doi: 10.11646/zootaxa.202.1.1.. [DOI] [Google Scholar]
- 11.Zeledón R, Ugalde J, Paniagua L. Entomological and ecological aspects of six sylvatic species of Triatomines (Hemiptera, Reduviidae) from the collection of the National Biodiversity Institute of Costa Rica, Central América. Mem Inst Oswaldo Cruz. 2001;96(6) doi: 10.1590/s0074-02762001000600002. [DOI] [PubMed] [Google Scholar]
- 12.Carcavallo RU, Curto de Casas S, Sherlock I, Galíndez I, Juberg J, Galvão C, et al. Fiocruz. Rio de Janeiro: 1998. Atlas of Chagas disease vectors in the Americas. [Google Scholar]
- 13.Parra-Henao G, Suárez-Escudero L, González-Caro S. Potential distribution of Chagas disease vectors (Hemiptera, Reduviidae, Triatominae) in Colombia, based on ecological niche modeling. J Trop Med. 2016 doi: 10.1155/2016/1439090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ceccarelli S, Balsalobre A, Cano ME, Vicente ME, Rabinovich JE, Medone P, et al. [DOI]
- 15.Guhl F, Aguilera G, Pinto N, Vergara D. Actualización de la distribución geográfica y ecoepidemiología de la fauna de triatominos (Reduviidae Triatominae) en Colombia. Biomédica. 2007;27(1):143–162. [PubMed] [Google Scholar]
- 16.Chávez J. Contribución al estudio de los triatominos del Perú distribución geográfica, nomenclatura y notas taxonómicas. An Fac Med. 2006;67(1):65–76. [Google Scholar]
- 17.Patterson J, Barbosa S, Feliciangeli M. On the genus Panstrongylus Berg 1879: evolution, ecology and epidemiological significance. Acta Trop. 2009;110(2-3) doi: 10.1016/j.actatropica.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Crossa R, Hernández M, Caraccio M, Rose V, Valente S, Valente V, et al. Chromosomal evolution trends of the genus Panstrongylus (Hemiptera, Reduviidae), vectors of Chagas disease. Infect Genet Evol. 2002;2(1) doi: 10.1016/S1567-1348(02)00063-1.. [DOI] [PubMed] [Google Scholar]
- 19.dos Santos C, Jurberg J, Galvão C, Rocha D. Morphometric study of the genus Panstrongylus Berg, 1879 (Hemiptera, Reduviidae. Triatominae) Mem Inst Oswaldo Cruz. 2003;98(7) doi: 10.1590/S0074-02762003000700014. [DOI] [PubMed] [Google Scholar]
- 20.Aldana E, Heredia E, Avendaño F, Lizano E, Concepción J, Bonfante R, et al. Análisis morfométrico de Panstrongylus geniculatus de Caracas, Venezuela. Biomédica. 2011;31(1) doi: 10.7705/biomedica.v31i1.341. [DOI] [PubMed] [Google Scholar]
- 21.Marcilla A, Bargues M, Abad-Franch F, Panzera F, Carcavallo R, Noireau F. Nuclear rDNA ITS-2 sequences reveal polyphyly of Panstrongylus species (Hemiptera Reduviidae: Triatominae), vectors of Trypanosoma cruzi. Infect Genet Evol. 2002;1(3):225–235. doi: 10.1016/s1567-1348(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 22.Justi S, Russo C, Mallet J, Obara M, Galvão C. Molecular phylogeny of Triatomini (Hemiptera Reduviidae: Triatominae) Parasit Vectors. 2014;7(149):1–12. doi: 10.1186/1756-3305-7-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bérenger J, Blanchet D. A new species of the genus Panstrongylus from French Guiana (Heteroptera; Reduviidae; Triatominae) Mem Inst Oswaldo Cruz. 2007;102(6) doi: 10.1590/S0074-02762007005000088. [DOI] [PubMed] [Google Scholar]
- 24.Ayala L. Una nueva especie de Panstrongylus Berg de Venezuela (Hemiptera Reduviidae, Triatominae) Entomotropica. 2009;24(3):105–109. [Google Scholar]
- 25.Valderrama A, Lizano E, Cabello D, Valera M. Panstrongylus turpiali, n sp. (Hemiptera: Reduviidae: Triatominae) from Venezuela. Caribb J Sci. 1996;32:142–144. [Google Scholar]
- 26.Lent H. Novos sinônimos de duas espécies de Triatominae da Venezuela (Hemiptera, Reduviidae) Entomol Vect. 1997;4:67–70. [Google Scholar]
- 27.Ayala J. Presencia de Panstrongylus Chinai (Del ponte, 1929) en Venezuela, con notas aclaratorias sobre su sinonimia con Panstrongylus Turpiali (Heteroptera Reduviidae: Triatominae) Boletín de la SEA. 2016;59:233–236. [Google Scholar]
- 28.Lent H, Jurberg J. Estudos morfológicos comparativos de Panstrongylus geniculatus (Latreille, 1811) e Panstrongylus megistus (Burmeister, 1835) e suas genitália externas (Hemiptera, Reduviidae, Triatominae) Rev Bras Biol. 1968;28(4):499–520. [Google Scholar]
- 29.Galíndez I, Carcavallo R, Valderrama A. Erwinilas o cerdas interomatidiales en la subfamilia Triatominae (Hemiptera Reduviidae) Entomol Vect. 1994;1(3):93–96. [Google Scholar]
- 30.Carcavallo RU, Galíndez Girón I, Catalá S, Jurberg J, Lent H, Galvão C, et al. Fiocruz. Rio de Janeiro: 1998. Atlas of Chagas disease vectors in the Americas. [Google Scholar]
- 31.Galíndez Girón I, Carcavallo RU, Jurberg J, Galvão C, Lent H, Barata J, et al. Fiocruz. Rio de Janeiro: 1998. Atlas of Chagas disease vectors in the Americas. [Google Scholar]
- 32.Wolff M, González C. Ciclo de vida de Panstrongylus geniculatus (Hemiptera: Reduviidae) en condiciones de laboratorio. Caldasia. 1998;20(1) https://revistas.unal.edu.co/index.php/cal/article/view/17471 [Google Scholar]
- 33.Soto-Vivas A. Clave pictórica de triatominos (Hemiptera Triatominae) de Venezuela. Bol Malariol Salud Amb. 2009;49(2):259–274. [Google Scholar]
- 34.Avendaño-Rangel F, Sandoval C, Aldana E. Descripción de setas cuticulares externas de cabeza, tórax, patas, abdomen y genitales en cuatro especies de Triatominae. Biomédica. 2016;36(3) doi: 10.7705/biomedica.v36i3.3122. [DOI] [PubMed] [Google Scholar]
- 35.Jaramillo N, Castillo D, Wolff M. Geometric morphometric differences between Panstrongylus geniculatus from field and laboratory. Mem Inst Oswaldo Cruz. 2002;97(5) doi: 10.1590/S0074-02762002000500015.. [DOI] [PubMed] [Google Scholar]
- 36.Jurberg J, Galvão C, Noireau F, Carcavallo R, Rocha D, Lent H. Uma iconográfica dos triatomíneos (Hemiptera Reduviidae) Entomol Vect. 2004;11(3):457–494. [Google Scholar]
- 37.Lent H, Jurberg J. Observacões sobre o ciclo evolutivo, em laboratorio, do Panstrongylus geniculatus (Latreille, 1811) (Hemiptera Reduviidae: Triatominae) An Acad Brasil Ciênc. 1969;41:125–131. [Google Scholar]
- 38.Galíndez I. Colonización y ciclo de vida de Panstrongylus geniculatus (Latreille, 1811) (Hemiptera: Reduviidae: Triatominae) Universidad de Los Andes. 1990 [Google Scholar]
- 39.Galíndez Girón I, Torres A, Márquez J, Carcavallo R. Colonización y ciclo de vida de Panstrongylus geniculatus (Latreille, 1811) (Hemiptera, Reduviidae, Triatominae) Entomol Vector. 1997;4(3):83–94. [Google Scholar]
- 40.Wolff M, Castillo D. Evidencias de domesticación y aspectos biológicos de Panstrongylus geniculatus (Latreille, 1811) (Hemiptera Reduviidae) Acta Entomol Chile. 2000;24:77–83. [Google Scholar]
- 41.Cabello D, Galíndez Girón I. Vital Statistics of Panstrongylus geniculatus (Latreille 1811) (Hemiptera: Reduviidae) under experimental conditions. Mem Inst Oswaldo Cruz. 1998;93(2) doi: 10.1590/S0074-02761998000200024. [DOI] [PubMed] [Google Scholar]
- 42.Esteban L, Angulo V. Estudio comparativo de la ovipostura de una cepa de laboratorio y una de campo de Panstrongylus geniculatus alimentadas con Gallus domesticus y Mus musculus BALB/c en condiciones de laboratorio. Biomédica. 2011;31(3) [Google Scholar]
- 43.Esteban L, Angulo V. Estandarización de las condiciones de ayuno y peso en ninfas de V instar de Panstrongylus geniculatus para su utilización en pruebas biológicas XX Congreso Latinoamericano de Parasitología. Biomédica. 2011;31(3):252–253. [Google Scholar]
- 44.Rabinovich J, Feliciangeli M. Vital statistics of Triatominae (Hemiptera: Reduviidae) under laboratory conditions: IV. Panstrongylus geniculatus. J Med Entomol. 2015;52(5) doi: 10.1093/jme/tjv112. [DOI] [PubMed] [Google Scholar]
- 45.Mayer M, Pifano C, Medina R. Aspectos epidemiológicos de la enfermedad de Chagas en Venezuela: bases para una campaña de saneamiento aplicable a zonas endémicas del medio rural venezolano. XII Conferencia Sanitaria Panamericana. 1946 http://iris.paho.org/xmlui/bitstream/handle/123456789/28807/CSP12_C30.pdf?sequence=1 [Google Scholar]
- 46.Leite G, dos Santos C, Falqueto A. Insecta, Hemiptera, Reduviidae, Panstrongylus geniculatus geographic distribution map. Checklist. 2007;3(2):147–152. [Google Scholar]
- 47.Ceccarelli S, Balsalobre A, Medone P, Cano M, Gonçalves R, Gurgel R, et al. DataTri, a database of American triatomine species occurrence. Scientific Data. 2018 doi: 10.1038/sdata.2018.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Depickère S, Durán P, López R, Chávez T. Presence of intradomicile colonies of the triatomine bug Panstrongylus rufotuberculatus in Muñecas, La Paz, Bolivia. Acta Trop. 2011;117(2) doi: 10.1016/j.actatropica.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 49.Depickère S, Durán P, López R, Martínez E, Chávez T. After five years of chemical control: colonies of the triatomine Eratyrus mucronatus are still present in Bolivia. Acta Trop. 2012;123(3) doi: 10.1016/j.actatropica.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 50.Waleckx E, Depickère S, Salas R, Aliaga C, Monje M, Calle H, et al. New discoveries of sylvatic Triatoma infestans (Hemiptera: Reduviidae) throughout the Bolivian Chaco. Am J Trop Med Hyg. 2012;86(3) doi: 10.4269/ajtmh.2012.11-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rojas M, Pinazo M, García L, Arteaga M, Uriona L, Gamboa S, et al. Trypanosoma cruzi infected Panstrongylus geniculatus and Rhodnius robustus adults invade households in the Tropics of Cochabamba region of Bolivia. Parasit Vectors. 2016;9(158) doi: 10.1186/s13071-016-1445-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Almeida F. Triatomíneos da Amazônia: encontro de três espécies naturalmente infectadas por Trypanosoma semelhante ao cruzi, no Estado do Amazonas (Hemiptera, reduviidae) Acta Amaz. 1971;1(1) doi: 10.1590/1809-43921971011089. [DOI] [Google Scholar]
- 53.Miles M, de Souza A, Póvoa M. Chagas disease in the Amazon basin. III. Ecotopes of ten triatomine bug species (Hemiptera: Reduviidae) from vicinity of Belém, Pará State, Brazil. J Med Entomol. 1981;18(4) doi: 10.1093/jmedent/18.4.266. [DOI] [PubMed] [Google Scholar]
- 54.Bento D, Farias L, Godoy M, Araújo J. Epidemiologia da doença de chagas na zona rural do município de Teresina - Piauí, Brasil. Rev Soc Bras Med Trop. 1992;25(1) doi: 10.1590/S0037-86821992000100008. [DOI] [PubMed] [Google Scholar]
- 55.de Souza K, Bavia M, Días A, Guimarães I, Soares Ê, Nascimento M, et al. Spatial distribution of triatomines (Reduviidae: Triatominae) in urban areas of the city of Salvador, Bahia, Brazil. Geospatial Health. 2011;5(2) doi: 10.4081/gh.2011.172. [DOI] [PubMed] [Google Scholar]
- 56.Santos F, Barreto W, de Macedo G, da Silva J, das Chagas S, Garcia C, et al. The reservoir system for Trypanosoma (Kinetoplastida, Trypanosomatidae) species in large neotropical wetland. Acta Trop. 2019;199 doi: 10.1016/j.actatropica.2019.105098. [DOI] [PubMed] [Google Scholar]
- 57.Ucrós H. Distribución de los Triatominae en Colombia. Rev Fac Med. 1960;28 https://revistas.unal.edu.co/index.php/revfacmed/article/viewFile/22596/23457 [PubMed] [Google Scholar]
- 58.D'Alessandro A, Barreto P, Saravia N, Barreto M. Epidemiology of Trypanosoma cruzi in the Oriental Plains of Colombia. Am J Trop Med Hyg. 1984;33(6) doi: 10.4269/ajtmh.1984.33.1084. [DOI] [PubMed] [Google Scholar]
- 59.Barreto M, Burbano M, Barreto P. Nuevos registros de flebotominos (Diptera: Psychodidae) y triatominos (Hemiptera: Reduviidae) para Risaralda, Cauca y Valle del Cauca, Colombia. Colombia Med. 1997;28(3) https://colombiamedica.univalle.edu.co/index.php/comedica/article/view/62 [Google Scholar]
- 60.Parra G, Flórez M, Angulo V. Vigilancia de Triatominae (Hemiptera: Reduviidae) Bogotá: Sic Editorial Ltda; 2015. [Google Scholar]
- 61.Escalante M, Gómez D, Silvera L, Sánchez G, Vanegas J. Detection of high percentage of Trypanosoma cruzi infection, the etiologic agent of Chagas disease, in wild populations of Colombian Caribbean Triatomines. Acta Parasitol. 2015;60(2) doi: 10.1515/ap-2015-0044. [DOI] [PubMed] [Google Scholar]
- 62.Esteban L, Montes J, Angulo V. Diversidad de triatominae (Hemiptera: reduviidae) en Santander, Colombia: implicaciones epidemiológicas. Biomédica. 2017;37(1) doi: 10.7705/biomedica.v37i1.3140. http://dx.doi.org/10.7705/bi omedica.v37i1.3140 [DOI] [PubMed] [Google Scholar]
- 63.León C, Ortiz M, Tovar C, Negrete J, Arroyo E, González C. Detection of Trypanosoma cruzi strains circulating in Córdoba department (Colombia) isolated from triatomines (Hemiptera: Reduviidae) collected by the community. Biomédica. 2019;39(2) doi: 10.7705/biomedica.v39i2.3973. [DOI] [PubMed] [Google Scholar]
- 64.Ayala J. Los triatominos de Costa Rica. Arquivos Entomoloxicos. 2017;18:189–215. [Google Scholar]
- 65.Espinoza L. Epidemiología de la enfermedad de Chagas en la República del Ecuador. Rev Ecuat Hig Med Trop. 1955;12:25–105. [PubMed] [Google Scholar]
- 66.Rodríguez J. Epidemiología de la enfermedad de Chagas en la República del Ecuador. Rev Goiana Medica. 1959;5:411–438. [Google Scholar]
- 67.Amunárriz M, Chico M, Guderian R. Chagas disease in Ecuador a sylvatic focus in the Amazon region. Am J Trop Med Hyg. 1991;94:145–149. [PubMed] [Google Scholar]
- 68.Chico H, Sandoval C, Guevara E, Calvopiña H, Cooper P, Reed S, et al. Chagas disease in Ecuador: evidence for disease transmission in an indigenous population in the Amazon region. Mem Inst Oswaldo Cruz. 1997;92(3) doi: 10.1590/S0074-02761997000300002. [DOI] [PubMed] [Google Scholar]
- 69.Aguilar V, Abad-Franch F, Paucar C, Racines V. Epidemiology of Chagas disease in Ecuador. A brief review. Mem Inst Oswaldo Cruz. 1999;94(1) doi: 10.1590/S0074-02761999000700076. [DOI] [PubMed] [Google Scholar]
- 70.Chippaux J, Dedet J, Geoffroy G, Tavakilian G, Pajot F. La maladie de Chagas en Guyane Française. La nature et l'homme en Guyane. Institut Pasteur. 1983 http://horizon.documentation.ird.fr/exl-doc/pleins_textes/divers11-11/15460.pdf [Google Scholar]
- 71.Bérenger J, Pluot-Sigwalt D, Blanchet D, Aznar C. The Triatominae species of French Guiana (Heteroptera: Reduviidae) Mem Inst Oswaldo Cruz. 2009;104(8) doi: 10.1590/S0074-02762009000800007. [DOI] [PubMed] [Google Scholar]
- 72.Maes J. Los triatominos (Heteroptera Reduviidae) en Nicaragua. Rev Nica Ent. 1992;21:1–8. [Google Scholar]
- 73.Maes J, Ratcliffe B, Jameson M. Fauna entomológica de la reserva natural Bosawas, Nicaragua XIII. Panstrongylus rufotuberculatus, un triatomino (Heteroptera-Reduviidae) nuevo para la fauna de Nicaragua. Rev Nica Ent. 1997;41:19–22. [Google Scholar]
- 74.Rodríguez I, Loaiza J. American trypanosomiasis, or Chagas disease, in Panama: a chronological synopsis of ecological and epidemiological research. Parasit Vectors. 2017;10 doi: 10.1186/s13071-017-2380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guillén Z, Cáceres I, Elliot A, Ramírez J. Triatominos del norte peruano y su importancia como vectores de Trypanosoma spp. Rev Per Ent. 1988;31 http://sisbib.unmsm.edu.pe/BVRevistas/entomologia/v31/pdf/a06v31.pdf [Google Scholar]
- 76.Cáceres A, Troyes L, Gonzáles-Pérez A, Llontop E, Bonilla C, Murias E. Enfermedad de Chagas en la región nororiental del Perú I. Triatominos (Hemiptera Reduviidae) presentes en Cajamarca y Amazonas. Rev Peru Med Exp Salud Publica. 2002;19(1):17–23. [Google Scholar]
- 77.Náquira C, Cabrera R. Breve reseña histórica de la enfermedad de Chagas, a cien años de su descubrimiento y situación actual en el Perú. Rev Peru Med Exp Salud Publica. 2009;26(4):494–504. [Google Scholar]
- 78.Lent H. Triatomideos da Guiana holandesa Redescrição de Panstrongylus lignarius (Walker, 1873) Mem Inst Oswaldo Cruz. 1943;38(3):485–496. [Google Scholar]
- 79.Hiwat H. Triatominae species of Suriname (Heteroptera: Reduviidae) and their role as vectors of Chagas disease. Mem Inst Oswaldo Cruz. 2014;109(4) doi: 10.1590/0074-0276130408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Omah-Maharaj I. Studies on vectors of Trypanosoma cruzi in Trinidad, West Indies. Med Vet Entomol. 1992;6(2) doi: 10.1111/j.1365-2915.1992.tb00586.x. [DOI] [PubMed] [Google Scholar]
- 81.Noya-Alarcón O, Botto C, Cortez J, Ferrer E, Viettri M, Herrera L. Primer registro de Panstrongylus geniculatus (Latreille, 1811) en los municipios Alto Orinoco y Atures, estado Amazonas, Venezuela. Bol Malariol Salud Amb. 2011;51(1):81–85. [Google Scholar]
- 82.Carcavallo RU, Rodríguez M, Salvatella R, Curto de Casas S, Rocha D, Sherlock I, et al. Fiocruz. Rio de Janeiro: 1998. Atlas of Chagas disease vectors in the Americas. [Google Scholar]
- 83.Carcavallo RU, Rocha D, Galíndez Girón I, Sherlock I, Galvão C, Lent H, et al. Fiocruz. Rio de Janeiro: 1998. Atlas of Chagas disease vectors in the Americas. [Google Scholar]
- 84.Reyes M. Panstrongylus geniculatus Latreille 1811 (Hemiptera Reduviidae: Triatominae), vector de la enfermedad de Chagas en el ambiente domiciliario del centro-norte de Venezuela. Rev Biomed. 2009;20:180–205. [Google Scholar]
- 85.Abad-Franch F, Lima M, Sarquis O, Gurgel R, Sánchez M, Calzada J, et al. On palms, bugs, and Chagas disease in the Americas. Acta Trop. 2015;151 doi: 10.1016/j.actatropica.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 86.Garrouste R. La première observation in natura de l'entomophagie de Panstrongylus geniculatus (Latreille 1811) hématophage vecteur de la maladie de Chagas (Hemiptera: Reduviidae) Ann Soc Entomol Fr (NS) 2009;45(3) doi: 10.1080/00379271.2009.10697614. [DOI] [Google Scholar]
- 87.Bender A, Python A, Lindsay S, Golding N, Moyes C. Modelling geospatial distributions of the triatomine vectors of Trypanosoma cruzi in Latin America. PLoS Negl Trop Dis. 2020;14(8) doi: 10.1371/journal.pntd.0008411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Barrett T. Advances in triatomine bug ecology in relation to Chagas disease. In K Harris, org. Advances in disease vector research. Springer-Verlag. 1991 [Google Scholar]
- 89.Jurberg J, Carcavallo R, Lent H. Panstrongylus sherlocki sp n. do estado da Bahia, Brasil (Hemiptera, Reduviidae, Triatominae) Entomol Vect. 2001;8(2):261–274. [Google Scholar]
- 90.Nakad-Bechara C, Londoño J, Segovia M, León M, Martínez P, Rodríguez R, et al. Genetic variability of Panstrongylus geniculatus (Reduviidae: Triatominae) in the Metropolitan District of Caracas, Venezuela. Infect Genet Evol. 2018;66 doi: 10.1016/j.meegid.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 91.Caicedo-Garzón V, Salgado-Roa F, Sánchez-Herrera M, Hernández C, Arias-Giraldo L, García L, et al. Genetic diversification of Panstrongylus geniculatus (Reduviidae: Triatominae) in northern South America. PLoS One. 2019;14(10) doi: 10.1371/journal.pone.0223963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.C Negrete J, González C León C, Ortiz M Chacón J, et al. New scenarios of Chagas disease transmission in northern Colombia. J Parasitol Res. 2017 doi: 10.1155/2017/3943215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hernández C, Salazar C, Brochero H, Teherán A, Buitrago L, Vera M, et al. Untangling the transmission dynamics of primary and secondary vectors of Trypanosoma cruzi in Colombia: parasite infection, feeding sources and discrete typing units. Parasit Vectors. 2016;9(1) doi: 10.1186/s13071-016-1907-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brito R, Gorla D, Diotaiuti L, Gomes A, Souza R, Abad-Franch F. Drivers of house invasion by sylvatic Chagas disease vectors in the Amazon-Cerrado transition: A multi-year, state-wide assessment of municipality-aggregated surveillance data. PLoS Negl Trop Dis. 2017;11(11) doi: 10.1371/journal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Damborsky M, Bar M, Oscherov E. Detección de triatominos (Hemiptera: Reduviidae) en ambientes domésticos y extradomésticos, Corrientes, Argentina. Cad Saude Publica. 2001;17(4) doi: 10.1590/S0102-311X2001000400018. [DOI] [PubMed] [Google Scholar]
- 96.Barretto M. Estudos sobre reservatorios e vetores silvestres do Trypanosoma cruzi XXI. Observações sobre a ecología do Panstrongylus geniculatus (Latreille, 1811). (Hemiptera, Reduviidae) Rev Bras Biol. 1967;27(4):337–348. [PubMed] [Google Scholar]
- 97.de Oliveira A, da Silva I. Distribuição geográfica e indicadores entomológicos de triatomíneos sinantrópicos capturados no Estado de Goiás. Rev Soc Bras Med Trop. 2007;40(2) doi: 10.1590/S0037-86b2007000200011. [DOI] [PubMed] [Google Scholar]
- 98.Almeida P, Santos H, Barata J, Obara M, Cerretti W. Ocorrência de Panstrongylus guentheri Berg (Hemiptera: Reduviidae) no Mato Grosso do Sul. Neotrop Entomol. 2008;37(1) doi: 10.1590/S1519-566X2008000100019. [DOI] [PubMed] [Google Scholar]
- 99.Lorosa E, Santos C, Jurberg J. Foco de doença de Chagas em São Fidélis, no estado do Rio de Janeiro. Rev Soc Bras Med Trop. 2008;41(4) doi: 10.1590/s0037-86822008000400020. https://www.arca.fiocruz.br/handle/icict/27550 [DOI] [PubMed] [Google Scholar]
- 100.Peixoto R, Rocha D, Dale C, Galvão C. Panstrongylus geniculatus (Latreille, 1811) (Hemiptera, Reduviidae, Triatominae): first record on Ilha Grande, Rio de Janeiro, Brazil. CheckList. 2020;16(2) doi: 10.15560/16.2.391. [DOI] [Google Scholar]
- 101.Naiff M, Naiff R, Barret T. Vetores selváticos de doença de Chagas na área urbana de Manaus (AM): atividade de vôo nas estações secas e chuvosas. Rev Soc Bras Med Trop. 1998;31(1) doi: 10.1590/S0037-86821998000100014. [DOI] [PubMed] [Google Scholar]
- 102.Fé N, Magalhães L, Fé F, Arakian S, Monteiro W, Barbosa M. Ocorrência de triatomíneos em ambientes silvestres e domiciliares do município de Manaus, Estado do Amazonas. Rev Soc Bras Med Trop. 2009;42(6) doi: 10.1590/S0037-86822009000600006.. [DOI] [PubMed] [Google Scholar]
- 103.Batista D, Britto C, Monte G, Baccaro F. Occurrence of triatomines (Hemiptera: Reduviidae) in domestic and natural environments in Novo Remanso, Itacoatiara, Amazonas, Brazil. J Braz Soc Trop Med. 2019;52 doi: 10.1590/0037-8682-0063-2019. [DOI] [PubMed] [Google Scholar]
- 104.de Assis G, Azeredo B, Gorla D, Diotaiuti L, Lana M. Entomological surveillance of Chagas disease in Berilo municipality, Jequitinhonha Valley, State of Minas Gerais, Brazil. Rev Soc Bras Med Trop. 2009;42(6) doi: 10.1590/S0037-86822009000600001. [DOI] [PubMed] [Google Scholar]
- 105.de Paula M, da Costa I, Freitas P, Limongi J, Neto A, Pinto R, et al. Occurrence of positivity for Trypanosoma cruzi in triatomine from municipalities in Southeastern Brazil, from 2002 to 2004. Rev Soc Bras Med Trop. 2010;43(1) doi: 10.1590/S0037-86822010000100003. [DOI] [PubMed] [Google Scholar]
- 106.Dias J, Queiroz D, Martins H, Gorla D, Pires H, Diotaiuti L. Spatial distribution of triatomines in domiciles of an urban area of the Brazilian Southeast Region. Mem Inst Oswaldo Cruz. 2016;111(1) doi: 10.1590/0074-02760150352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maeda M, Knox M, Gurgel-Gonçalves R. Occurrence of synanthropic triatomines (Hemiptera: Reduviidae) in the Federal District of Brazil. Rev Soc Bras Med Trop. 2012;45(1) doi: 10.1590/S0037-86822012000100014. [DOI] [PubMed] [Google Scholar]
- 108.Minuzzi-Souza T, Nitz N, Cuba-Cuba C, Santalucia M, Knox M, Hagström L, et al. Synanthropic triatomines as potential vectors of Trypanosoma cruzi in central Brazil. Rev Soc Bras Med Trop. 2017;50(6) doi: 10.1590/0037-8682-0199-2017. [DOI] [PubMed] [Google Scholar]
- 109.Barretto M. Estudos sobre reservatorios e vetores silvestres do Trypanosoma cruzi XXI. Observações sobre a ecología do Panstrongylus geniculatus (Latreille, 1811). (Hemiptera, Reduviidae) Rev Bras Biol. 1967;27(4):337–338. [PubMed] [Google Scholar]
- 110.Ceretti-Junior W, Vendrami D, de M, Matos-Junior, Rimoldi-Ribeiro A, Alvarez J, Marques S, et al. Occurrences of triatomines (Hemiptera: Reduviidae) and first reports of Panstrongylus geniculatus in urban environments in the city of São Paulo, Brazil. Rev Inst Med Trop São Paulo. 2018;60 doi: 10.1590/S1678-9946201860033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ribeiro M, Castro G, de Souza J, da Rosa J, Camargo L, Meneguetti D. Occurrence of triatomines in an urban residential complex in the municipality of Rio Branco, Acre, South-Western Amazon. Rev Soc Bras Med Trop. 2019;52 doi: 10.1590/0037-8682-0177-2018. [DOI] [PubMed] [Google Scholar]
- 112.Cortés L, Suárez H. Triatominos (Reduviidae: Triatominae) en un foco de enfermedad de Chagas en Talaigua Nuevo (Bolívar, Colombia) Biomédica. 2005;25(4) doi: 10.7705/biomedica.v25i4.1383. [DOI] [PubMed] [Google Scholar]
- 113.Hernández C, Vera M, Cucunubá Z, Flórez C, Cantillo O, Buitrago L. High-Resolution molecular typing of Trypanosoma cruzi in 2 large outbreaks of acute Chagas disease in Colombia. J Infect Dis. 2016;214(8):1252–1255. doi: 10.1093/infdis/jiw360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dib J, Barnabe C, Tibayrenc M, Triana O. Incrimination of Eratyrus cuspidatus (Stal) in the transmission of Chagas disease by molecular epidemiology analysis of Trypanosoma cruzi isolates from a geographically restricted area in the north of Colombia. Acta Trop. 2009;111(3) doi: 10.1016/j.actatropica.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 115.Angulo V, Esteban L, Luna K. Attalea butyracea próximas a las viviendas como posible fuente de infestación domiciliaria por Rhodnius prolixus (Hemiptera: Reduviidae) en los Llanos Orientales de Colombia. Biomédica. 2012;32(2) doi: 10.7705/biomedica.v32i2.430. [DOI] [PubMed] [Google Scholar]
- 116.León C, Ortiz M, Tovar C, Negrete J, Arroyo E, González C. Detection of Trypanosoma cruzi strains circulating in Cordoba department (Colombia) isolated from triatomines (Hemiptera: Reduviidae) collected by the community. Biomédica. 2019;39(2) doi: 10.7705/biomedica.v39i2.3973. [DOI] [PubMed] [Google Scholar]
- 117.Reyes M, Torres A, Esteban L, Flórez M, Angulo V. Riesgo de transmisión de la enfermedad de Chagas por intrusión de triatominos y mamíferos silvestres en Bucaramanga, Santander, Colombia. Biomédica. 2017;37(1) doi: 10.7705/biomedica.v37i1.3051. [DOI] [PubMed] [Google Scholar]
- 118.Ayala C, Hernández C, Eyes M, Romero L, Álvarez R, Blanco P. Detección de infección natural por Trypanosoma cruzi (Trypanosomatidae) en triatominos del municipio de Colosó, Colombia. Acta Biol Col. 2019;24(1) doi: 10.15446/abc.v24n1.72306. [DOI] [Google Scholar]
- 119.Vega S, Mendoza A, Cabrera R, Cáceres A, Campos E, Ancca J. Primer caso de enfermedad de Chagas aguda en la Selva Central del Perú investigación de colaterales, vectores y reservorios. Rev Peru Med Exp Salud Publica. 2006;23(4):288–292. [Google Scholar]
- 120.Padilla C, Alvarado U, Ventura G, Luna-Caipo D, Suárez M, Tuñoque J, et al. Detección de unidades discretas de tipificación de Trypanosoma cruzi en triatominos recolectados en diferentes regiones naturales de Perú. Biomédica. 2017;37(2) doi: 10.7705/biomedica.v37i0.3559. [DOI] [Google Scholar]
- 121.Torres D, Cabrera R. Geographical distribution and intra-domiciliary capture of sylvatic triatomines in La Convencion province, Cusco, Peru. Rev Inst Med Trop São Paulo. 2010;52(3) doi: 10.1590/S0036-46652010000300008. [DOI] [PubMed] [Google Scholar]
- 122.Cabrera R, Vega S, Cáceres A, Ramal C, Álvarez C, Ladera P, et al. Epidemiological investigation of an acute case of Chagas disease in an area of active transmission in Peruvian Amazon region. Rev Inst Med Trop São Paulo. 2010;52(5) doi: 10.1590/S0036-46652010000500009. [DOI] [PubMed] [Google Scholar]
- 123.Cáceres A, Vega S, Ancca J, Pinto J, Vela G, Cárdenas V. Aspectos entomológicos de la enfermedad de Chagas en Huallaga y Picota, San Martín, Perú. An Fac Med. 2010;71(1):28–36. [Google Scholar]
- 124.Hiwat H. Triatominae species of Suriname (Heteroptera: Reduviidae) and their role as vectors of Chagas disease. Mem Inst Oswaldo Cruz. 2014;109(4) doi: 10.1590/0074-0276130408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pifano F. El potencial enzoótico silvestre del complejo ecológico Schizotrypanum cruzi - Didelphis marsupialis - Panstrongylus geniculatus y sus incursiones a la vivienda humana del valle de Caracas - Venezuela. Bol Acad Cienc Fis Mat Nat. 1986;46:143–144. [Google Scholar]
- 126.Londoño J, Briceño L, Carrasco H, Rodríguez M. Riesgo de transmisión urbana de Trypanosoma cruzi por Panstrongylus geniculatus XV Congreso Colombiano de Parasitología y Medicina Tropical, Colombia. Biomédica. 2011;31(3):51–52. [Google Scholar]
- 127.Añez N, Saavedra C, Crisante G, Rojas A, Lizano E. Infección natural por Trypanosoma cruzi en Panstrongylus geniculatus (Latreille, 1811) de la región montana de Mérida, Venezuela. Bol Malariol Salud Amb. 2005;45(2) http://www.saber.ula.ve/handle/123456789/29808 [Google Scholar]
- 128.Carrasco H, Torrellas A, García C, Segovia M, Feliciangeli M. Risk of Trypanosoma cruzi I (Kinetoplastida: Trypanosomatidae) transmission by Panstrongylus geniculatus (Hemiptera: Reduviidae) in Caracas (Metropolitan District) and neighboring States, Venezuela. Int J Parasitol. 2005;35(13) doi: 10.1016/j.ijpara.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 129.Rojas M, Várquez P, Villarreal M, Velandia C, Vergara L, Moran-Borges Y, et al. Estudio seroepidemiológico y entomológico sobre la enfermedad de Chagas en un área infestada por Triatoma maculata (Erichson 1848) en el centro-occidente de Venezuela. Cad Saude Publica. 2008;24(10) doi: 10.1590/S0102-311X2008001000013. [DOI] [PubMed] [Google Scholar]
- 130.Serrano O, Mendoza F, Suárez B, Soto A. Seroepidemiología de la enfermedad de Chagas en dos localidades del municipio Costa de Oro, estado Aragua, Venezuela. Biomédica. 2008;28(1) doi: 10.7705/biomedica.v28i1.113. [DOI] [PubMed] [Google Scholar]
- 131.García-Jordán N, Berrizbeitia M, Concepción J, Aldana E, Cáceres A, Quiñones W. Estudio entomológico de vectores transmisores de la infección por Trypanosoma cruzi en la población rural del estado Sucre, Venezuela. Biomédica. 2015;35(2) doi: 10.7705/biomedica.v35i2.2390. [DOI] [PubMed] [Google Scholar]
- 132.Castro M, Barrett T, Santos W, Abad-Franch F, Rafael J. Attraction of Chagas disease vectors (Triatominae) to artificial light sources in the canopy of primary Amazon rainforest. Mem Inst Oswaldo Cruz. 2010;105(8) doi: 10.1590/s0074-02762010000800019. [DOI] [PubMed] [Google Scholar]
- 133.Jácome-Pinilla D, Hincapié-Peñaloza E, Ortiz M, Ramírez J, Guhl F, Molina J. Risks associated with dispersive nocturnal flights of sylvatic Triatominae to artificial lights in a model house in the northeastern plains of Colombia. Parasit Vectors. 2015;8 doi: 10.1186/s13071-015-1209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Valente V, Valente S, Noireau F, Carrasco H, Miles M. Chagas disease in the Amazon Basin: Association of Panstrongylus geniculatus (Hemiptera: Reduviidae) with domestic pigs. J Med Entomol. 1998;35(2) doi: 10.1093/jmedent/35.2.99. [DOI] [PubMed] [Google Scholar]
- 135.Maestre-Serrano R, Eyes-Escalante M. Actualización de la presencia y distribución de triatominos en el departamento del Atlántico-Colombia 2003-2010. Bol Malariol Salud Amb. 2012;52(1):125–128. [Google Scholar]
- 136.Montenegro D, Vera M, Zuleta L, Llanos I, Junqueira A. Estrategia para determinar la línea base en áreas de interrupción vectorial de la enfermedad de Chagas. Rev Panam Salud Publica. 2016;39(6):341–351. [PubMed] [Google Scholar]
- 137.Reyes M, Rodríguez A. Domiciliation of selvatic Chagas disease vector Panstrongylus geniculatus (Latreille, 1811) (Triatominae: Reduviidae) in Venezuela. Trans R Soc Trop Med Hyg. 2000;94(5) doi: 10.1016/s0035-9203(00)90068-3. [DOI] [PubMed] [Google Scholar]
- 138.Feliciangeli M, Carrasco H, Patterson J, Suárez B, Martínez C, Medina M. Mixed domestic infestation by Rhodnius prolixus Stal, 1859 and Panstrongylus geniculatus (Latreille, 1811), vector incrimination, and seroprevalence for Trypanosoma cruzi among inhabitants in El Guamito, Lara state, Venezuela. Am J Trop Med Hyg. 2004;71(4) doi: 10.4269/ajtmh.2004.71.501. [DOI] [PubMed] [Google Scholar]
- 139.Rodríguez-Bonfante C, Amaro A, García M, Wohlert L, Guillén P, García R, et al. Epidemiología de la enfermedad de Chagas en el municipio Andrés Eloy Blanco, Lara, Venezuela: infestación triatomínica y seroprevalencia en humanos. Cad Saude Publica. 2007;23(5) doi: 10.1590/S0102-311X2007000500015. [DOI] [PubMed] [Google Scholar]
- 140.Carrasco H, Segovia M, Londoño J, Ortegoza J, Rodríguez M, Martínez C. Panstrongylus geniculatus and four other species of triatomine bug involved in the Trypanosoma cruzi enzootic cycle: high risk factors for Chagas disease transmission in the Metropolitan District of Caracas, Venezuela. Parasit Vectors. 2014;7(602) doi: 10.1186/s13071-014-0602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Díaz-Bello Z, Thomas M, López M, Zavala-Jasper R, Noya O, Alarcón de Noya B, et al. Trypanosoma cruzi genotyping supports a common source of infection in a school-related oral outbreak of acute Chagas disease in Venezuela. Epidemiol Infect. 2014;142(1) doi: 10.1017/S0950268813000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nicholls R, Cucunubá Z, Knudson A, Flórez A, Montilla M, Puerta C, et al. Enfermedad de Chagas aguda en Colombia, una entidad poco sospechada. Informe de 10 casos presentados en el periodo 2002 a 2005. Biomédica. 2007;27(1) doi: 10.7705/biomedica.v27i1.244. [DOI] [PubMed] [Google Scholar]
- 143.Rueda K, Trujillo J, Carranza J, Vallejo G. Transmisión oral de Trypanosoma cruzi: una nueva situación epidemiológica de la enfermedad de Chagas en Colombia y otros países suramericanos. Biomédica. 2014;34(4) doi: 10.7705/biomedica.v34i4.2204. [DOI] [PubMed] [Google Scholar]
- 144.Ríos J, Arboleda M, Montoya A, Alarcón E, Parra-Henao G. Probable brote de transmisión oral de enfermedad de Chagas en Turbo, Antioquia. Biomédica. 2011;31(2) doi: 10.7705/biomedica.v31i2.302. [DOI] [PubMed] [Google Scholar]
- 145.Hernández L, Ramírez A, Cucunubá Z, Zambrano P. Brote de Chagas agudo en Lebrija, Santander, 2008. Revista del Observatorio de Salud Pública de Santander. 2009;4(1):28–36. [Google Scholar]
- 146.Zambrano P, Cucunubá Z, Montilla M, Flórez C, Parra E, Cortés L. Brotes de síndrome febril asociado a miocarditis aguda chagásica de posible transmisión oral en el departamento de Santander, diciembre de 2008 a mayo de 2009. Inf Quinc Epidemiol Nac. 2008;15:17–32. [Google Scholar]
- 147.Soto H, Tibaduiza T, Montilla M, Triana O, Suárez D, Torres M, et al. Investigación de vectores y reservorios en brote de Chagas agudo por posible transmisión oral en Aguachica, Cesar, Colombia. Cad Saude Publica. 2014;30(4) doi: 10.1590/0102-311X00024013. [DOI] [PubMed] [Google Scholar]
- 148.Santalla J, Oporto P, Espinoza E, Ríos T, Brutus L. Primer brote reportado de la enfermedad de Chagas en la Amazonía Boliviana reporte de 14 casos agudos por transmisión oral de Trypanosoma cruzi en Guayaramerín, Beni-Bolivia. Biofarbo. 2011;19(1):52–58. [Google Scholar]
- 149.Alarcón de Noya B, Díaz-Bello Z, Colmenares C, Ruiz-Guevara R, Mauriello L, Zavala-Jaspe R, et al. Large urban outbreak of orally acquired acute Chagas disease at a school in Caracas, Venezuela. J Infect Dis. 2010;201(9) doi: 10.1086/651608. [DOI] [PubMed] [Google Scholar]
- 150.Suárez J, de Suárez C. Alarcón de Noya B.Espinosa R.Chiurillo M.Villaroel A Enfermedad de Chagas sistémico en fase aguda por transmisión oral diagnóstico integral de un caso autopsiado. Gac Med Caracas. 2010;118(3):212–222. [Google Scholar]
- 151.Añez N, Crisante G, Rojas A, Dávila D. Brote de enfermedad de Chagas agudo de posible transmisión oral en Mérida, Venezuela. Bol Malariol Salud Amb. 2013;53(1):1–10. [Google Scholar]
- 152.Alarcón de Noya B, Díaz-Bello Z, Colmenares C, Ruiz-Guevara R, Mauriello L, Muñoz A, et al. Update on oral chagas disease outbreaks in Venezuela: Epidemiological, clinical and diagnostic approaches. Mem Inst Oswaldo Cruz. 2015;110(3) doi: 10.1590/0074-02760140285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ramírez J, Montilla M, Cucunubá Z, Floréz A, Zambrano P, Guhl F. Molecular epidemiology of human oral Chagas disease outbreaks in Colombia. PLoS Negl Trop Dis. 2013;7(2) doi: 10.1371/journal.pntd.0002041. http://dx.doi/10.1371/journal.pntd.0002041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Valente V. Potential for domestication of Panstrongylus geniculatus (Latreille, 1811) (Liemiptera, Reduviidae, Triatominae) in the municipality of Muaná, Marajó Island, State of Pará, Brazil. Mem Inst Oswaldo Cruz. 1999;94(1) doi: 10.1590/S0074-02761999000700078. [DOI] [PubMed] [Google Scholar]
- 155.Dario M, Lisboa C, Costa L, Moratelli R, Nascimento M, Costa L, et al. High Trypanosoma spp. diversity is maintained by bats and triatomines in Espírito Santo state, Brazil. PLoS One. 2017;12(11) doi: 10.1371/journal.pone.0188412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ferro A, Sobral-Souza T, Vancine M, Muylaert R, de Abreu A, Pelloso S, et al. Spatial prediction of risk areas for vector transmission of Trypanosoma cruzi in the State of Paraná, southern Brazil. PLoS Negl Trop Dis. 2018;12(10) doi: 10.1371/journal.pntd.0006907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Dario M, Andrade T, dos Santos C, Fux B, Brandão A, Falqueto A. Molecular characterization of Trypanosoma cruzi samples derived from Triatoma vitticeps and Panstrongylus geniculatus of the Atlantic rainforest, southeast Brazil. Parasite. 2018;25 doi: 10.1051/parasite/2018060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zingales B, Andrade S, Briones M, Campbell D, Chiari E, Fernandes O, et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz. 2009;104(7) doi: 10.1590/s0074-02762009000700021. [DOI] [PubMed] [Google Scholar]
- 159.Marcili A, Lima L.Cavazzana JrJunqueira A, Veludo H, Da Silva F, et al. A new genotype of Trypanosoma cruzi associated with bats evidenced by phylogenetic analyses using SSU rDNA, cytochrome b and Histone H2B genes and genotyping based on ITS1 rDNA. Parasitology. 2009;136(6) doi: 10.1017/S0031182009005861. [DOI] [PubMed] [Google Scholar]
- 160.Messenger LA, Ramírez JD, Llewellyn MS, Guhl F, Miles MA. Importation of hybrid human-associated Trypanosoma cruzi strains of southern South American origin, Colombia. Emerg Infect Dis. 2016;22(8):1452–1455. doi: 10.3201/eid2208.150786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Barnabé C, Brisse S, Tibayrenc M. Population structure and genetic typing of Trypanosoma cruzi, the agent of Chagas disease: a multilocus enzyme electrophoresis approach. Parasitology. 2000;120(5) doi: 10.1017/s0031182099005661. [DOI] [PubMed] [Google Scholar]
- 162.Liang L, Gong P. Climate change and human infectious diseases: a synthesis of research findings from global and spatio-temporal perspectives. Environ Int. 2017;103 doi: 10.1016/j.envint.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 163.Laferty K. The ecology of climate change and infectious diseases. Ecology. 2009;90(4) doi: 10.1890/08-0079.1. [DOI] [PubMed] [Google Scholar]
- 164.Gage K, Burkot T, Eisen R, Hayes E. Climate and vector-borne diseases. Am J Prev Med. 2008;35(5) doi: 10.1016/j.amepre.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 165.Roca M, Lazzari C. Effects of relative humidity on the haematophagous bug Triatoma infestans: hygropreference and eclosion success. J Insect Phys. 1994;40(10) doi: 10.1016/0022-1910(94)90024-8. [DOI] [Google Scholar]
- 166.Luz C, Fargues J, Grunewald J. Development of Rhodnius prolixus (Hemiptera: Reduviidae) under constant and cyclic conditions of temperature and humidity. Mem Inst Oswaldo Cruz. 1999;94(3) doi: 10.1590/S0074-02761999000300022. [DOI] [PubMed] [Google Scholar]
- 167.Tamayo L, Guhl F, Vallejo G, Ramírez J. The effect of temperature increase on the development of Rhodnius prolixus and the course of Trypanosoma cruzi metacyclogenesis. PLoS Negl Trop Dis. 2018;12(8) doi: 10.1371/journal.pntd.0006735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pereira J, Almeida P, Sousa A, Paula A, Machado R, Gurgel-Gonçalves R. Climatic factors influencing triatomine occurrence in Central-West Brazil. Mem Inst Oswaldo Cruz. 2013;108(3) doi: 10.1590/S0074-02762013000300012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ceccarelli S, Rabinovich J. Global climate change effects on Venezuela's vulnerability to Chagas disease is linked to the geographic distribution of five Triatomine species. J Med Entomol. 2015;52(6) doi: 10.1093/jme/tjv119. [DOI] [PubMed] [Google Scholar]


