Abstract
Coronavirus disease 19 (COVID-19) is placing a major burden on healthcare, economy and social systems worldwide owing to its fast spread and unacceptably high death toll. The unprecedented research effort has established the role of a deregulated immune response to the severe acute respiratory syndrome coronavirus 2, resulting in systemic inflammation. After that, the immunomodulatory approach has been placed in the top list of the research agenda for COVID-19. Corticosteroids have been used for more than 70 years to modulate the immune response in a broad variety of diseases. These drugs have been shown to prevent and attenuate inflammation both in tissues and in circulation via non-genomic and genomic effects. At the bedside, numerous observational cohorts have been published in the past months and have been inconclusive. Randomized controlled trials with subsequent high quality meta-analyses have provided moderate to strong certainty for an increased chance of survival and relief from life supportive therapy with corticosteroids given at a dose of 6 mg per day dexamethasone or equivalent doses of hydrocortisone or methylprednisolone. The corticotherapy was not associated with an increased risk of bacterial infection or of delayed viral clearance. In daily practice, physicians may be encouraged to use corticosteroids when managing patients with COVID-19 requiring oxygen supplementation.
Keywords: Coronavirus, Corticosteroids, Inflammatory mediators, Trials, Survival, Superinfections
Introduction
Coronavirus disease 19 (COVID-19), a disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was first described in Wuhan China in December 2019 [1]. Then, it rapidly became a pandemic, as of November 9th 2020, affects 50,446,517 people worldwide and has killed 1,256,869 people [2]. SARS-CoV-2 is a single stranded RNA virus, and classified as a beta coronavirus. Its subunit S1 of the spike, a structural protein of the virus, is responsible for virus binding to host cell receptors such as angiotensin-converting enzyme 2 receptor, and the subunit S2 contributes to the fusion of the virus membrane with the cell's membrane [3]. After the binding process, the spike proteins are activated by proteases, particularly at furin cleavage site, ubiquitously expressed, making SARS-CoV-2 very pathogenic. The penetration of the virus into lung epithelial cells will release danger associated molecular patterns that will activate resident macrophages and dendritic cells with subsequent release of the inflammasome [4]. As long as the virus's direct and indirect cytoxicity remains located in the epithelium, the disease remains characterized by mild to moderate lung inflammation. When the endothelium is damaged by the direct viral invasion of endothelial cells or by the inflammasome [5], then systemic hyper-inflammation and multi-organs dysfunction become the hallmark of COVID-19 [6]. Therefore, modulating the immune host response to SARS-CoV-2 has been rapidly considered a top priority for the international research agenda in the fight against the COVID-19 [7]. Among the drugs that target the immune system, corticosteroids are the most commonly used in the routine management of a broad variety of acute and chronic inflammatory disorders and autoimmune diseases. This article summarizes the current knowledge about the rationale and practical modalities for using corticosteroids in patients with COVID-19.
Rationale for the corticotherapy of COVID-19
Clinical evidence for excessive systemic inflammation in COVID-19
Physicians rapidly recognized that in oxygen-dependent COVID-19 patients, circulating levels of the acute phase reaction proteins, C-reactive protein and serum amyloid A, were increased and prealbumin levels were decreased [8]. Likewise, these patients have increased circulating levels of proinflammatory cytokines such as interleukin (IL)−6, tumor necrosis (TNF)-α, IL-1−β, IL-2, IL-7, and IL-17 [9,10]. The deregulated interferon(IFN)-I response also likely contributes to pathological damages to the lungs and other organs in severe COVID-19 [11]. More specifically, abnormal up-regulation of neutralizing auto-antibodies against type I IFNs may contribute to critical illness in COVID-19 patients by impairing the binding of type I IFNs to their receptor downstream signaling [12]. The increase in the proinflammatory cytokines parallels clinical severity and the higher IL-6 and TNF-α the higher the risk of death from COVID-19 [13]. While plasma levels of proinflammatory cytokines have been described as being higher than in other community acquired pneumonia [14], they may mimic those reported in sepsis or acute respiratry distress syndrme(ARDS) [15]. In practice, the degree of circulating pro-inflammatory mediators varied fairly between studies [16]. On the one hand, comprehensive immune analysis of peripheral blood cells in severe COVID-19 found alterations similar to those observed during sepsis, e.g. decreased expression of CD16 by neutrophils, monocytes, granulocytes and decreased expression of human leukocyte antigen(HLA)-DR on monocyte surface [17]. On the other hand, the same analyses found alterations specific to SARS-CoV-2 such as the expansion of plasmablasts and activated T cells [18]. The immune response to SARS-CoV-2 appears complex and heterogeneous. Some patients showed robust T and B cells activation and proliferation while in about one out of five patients there was minimal immune response compared to controls [18]. Moreover, among patients who mounted a lymphocytes response there was substantial heterogeneity in the immune profiles with potentially three distinct immune responses, with robust activation of CD4 T cells (immunotype 1), mild activation of CD4 T cells (immunotype 2) or lack of detectable lymphocytes response (immunotype 3) [18]. Immunotype 1 was associated with more severe COVID-19.
Mechanisms of action of corticosteroids relevant to COVID-19
Corticosteroids have pleiotropic effects resulting from complex molecular mechanisms including non-genomic and genomic effects [19]. We have recently summarized the molecular basis underlying the benefits of corticosteroids in severe infections [20]. Briefly, glucocorticoids exert anti-inflammatory by stimulating the synthesis and release of anti-inflammatory proteins and by inhibiting that of pro-inflammatory proteins. Glucocorticoids bind to the glucocorticoid receptor (GR) located in the cytoplasm of almost all cells. Upon binding with glucocorticoids, the GR dissociates from chaperone proteins heat-shock proteins 70 (Hsp70), Hsp90, and immunophilin [21]. Then, it enters into the nucleus to interact with specific DNA sequences (glucocorticoid responsive elements) of the regulatory region of target genes with subsequent chromatin remodeling [22,23]. Activated GR represses the expression of pro-inflammatory genes by inhibiting histone acetyltransferases and activating histone deacetylases. For instance, the expression of the interferon regulatory factor 3(IRF3) transcription factor, implicated in interferon production and viral protection, is down-regulated by glucocorticoids [24,25]. The GR-glucocorticoid complex also inhibits the production of pro-inflammatory proteins by sequestration of nuclear factor-κB(NF-κB) within the cytosol through increased expression of the inhibitory protein IκBα [26]. Glucocorticoids induce the expression of glucocorticoid-induced leucine zipper (GILZ), an inhibitor of NF-κB [27], and of the anti-inflammatory protein mitogen-activated protein(MAP) kinase phosphatase 1, which inhibits nuclear translocation of transcription factor GATA-3 implicated in T helper (Th)2 type cytokine expression [28]. They promote the production of annexin 1, thereby inhibiting the expression of phospholipase A2, and enhancing the resolution of inflammation and the phagocytosis by macrophages of apoptotic neutrophils [29].
Glucocorticoids suppress the production of acute phase reactants and chemokines [30,31], thereby preventing leucocyte recruitment. They suppress the expression of endothelial-leukocyte adhesion molecule 1 (ELAM-1), intracellular adhesion molecule 1 (ICAM-1) and vascular adhesion molecule 1 (VCAM-1), opposing to leucocytes diapedesis [32,33]. Target cells for glucocorticoids include (1) myeloid cells: macrophages, monocytes, tissue resident, migratory and plasmacytoid dendritic cells (DC), and granulocytes; (2) lymphocytes: CD8, Th1, Th2 and Th17 as well as Treg and B cells [19,20]. Glucocorticoids repress the maturation, differentiation and proliferation of all subtypes of leucocytes. They reduce the number of monocytes/macrophages, of DC and of eosinophil and basophil granulocytes [34]. Glucocorticoids increase neutrophils released by the bone marrow and demargination, and increase DC release of the anti-inflammatory cytokines IL-10 and transforming growth factor β (TGF-β) [35]. They reduce the membrane expression of major-histocompatibility-complex (MHC) class II and Fc receptors [36,37] and suppress antigen presenting to T cells [38].
Glucocorticoids prevent B cell lymphocytes activation, proliferation and release of immunoglobulins [39]. They deplete thymic stroma cells and T cells by apoptosis [40,41]. They change the polarization of naive T-cell toward anti-inflammatory Th 2 and T-reg phenotypes preventing the polarization to pro-inflammatory Th1 and Th17 phenotypes [42], [43], [44]. Subsequently, glucocorticoids suppress lymphocytes, production of the pro-inflammatory cytokines IL-2, IL-4, IL-5, IL-13 and IFN [19,20,45].
Summary of the clinical evidence of corticosteroids effects in patients with COVID-19
Methods
Although this was a narrative review, the author searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2020 Issue 12) using the search terms 'COVID', 'COVID-19, ‘coronavirus’, ‘SARS’, ‘SARS-CoV’, 'steroids' and 'corticosteroids'. We also searched (up to December 2020) MEDLINE, EMBASE and Latin American Caribbean Health Sciences Literature (LILACS). The author included observational cohort and randomized trials where the participants were patients with suspected or proven COVID-19, and the interventions were oral or intravenous corticosteroids. The author focused on all-cause mortality in the short-term as the primary outcome. The risk for secondary infections, more specifically ventilator associated pneumonia and bacteremia, and the risk for delayed viral clearance were also analyzed. We calculated a weighted treatment effect across trials. We expressed results as risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous outcomes. We considered methods based on the random-effects model for all analyses, as serious heterogeneity across studies was expected. We analyzed separately observational cohorts and randomized trials. In the analysis of randomized trials, sensitivity analysis was performed according to the use of placebo or usual care as the comparator. All analyses were done using Review Manager 5.3 software.
Observational cohorts
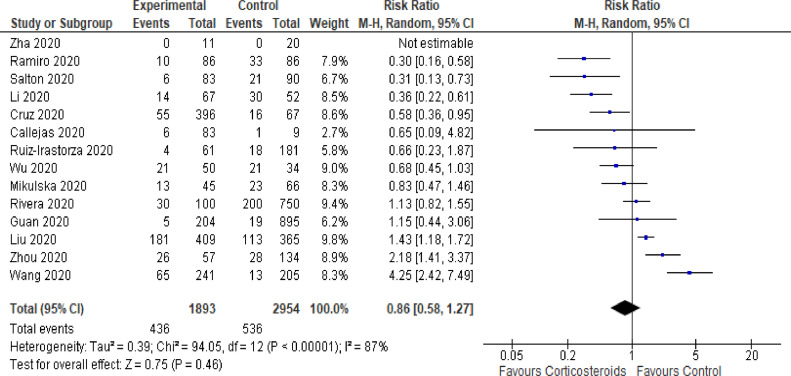
There were several reports about observational cohorts on the use of corticosteroids in patients with COVID-19 [1,[46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58]]. Table 1 summarizes the main characteristics of these observational studies. Most studies were performed in China. Most studies were single center, and the sample size varied from 31 to 2135 patients. These cohort studies provided data on mortality in the short term, e.g. in-hospital mortality, or mortality at two weeks or one month. When authors provided details about the interventions, methylprednisolone was the most commonly used, at an average dose of 0.5 to 2 mg/kg/day for an average duration of 5 to 10 days. Studies have enrolled adults with a median age from 39 to 68 years, and a majority of males. Studies have occasionally enrolled severe COVID-19 requiring invasive mechanical ventilation. Data from 14 observational studies accounting for 4847 patients with COVID-19 found a RR for short term mortality of 0.86 (95% CI: 0.58–1.27; random effect models) (Fig. 1). There were 436/1893 vs. 536/2954 deaths in the corticosteroid-treated patients compared to corticosteroid-free patients. There was strong heterogeneity across the studies (I 2=87%) mainly related to the heterogeneity of disease severity and of treatment modalities.
Table 1.
Effects of corticosteroids on mortality in patients with COVID-19 - Observational studies.
| Studies | Date (month year) | Design | Population | Main Outcomes | Comments |
|---|---|---|---|---|---|
| Guan et al. [1] | February 2020 | Country : China Retrospective 552 centers Primary outcome: composite of ICU admission, need for invasive MV, mortality Descriptive statistics |
Screened, 7736 Included, 1099 Median age: 46 years Male: 58.1% Invasive MV: 2.3% Admission to ICU, 5% |
In-hospital mortality Corticosteroids: 5/204 (2.5%) No corticosteroids: 10/895 (1.1%) |
No data on type of corticosteroids, dose, duration |
| Zhou et al. [46] | March 2020 | Country : China Retrospective Two centers Primary outcome : in-hospital mortality Logistic regression |
Screened, 813 Included, 191 Median Age : 56 years Male: 62% Median Time to hospitalization: 11 days Median SOFA: 2 Invasive MV: 17% |
In-hospital mortality: Corticosteroids: 26/57 (45.6%) No corticosteroids: 28/134 (20.9%) |
No data on type of corticosteroids, dose, duration |
| Wu et al. [47] | March 2020 | Country : China Retrospective Single center Primary outcome : development of ARDS and mortality Survival analyses, Cox models |
Included, 201 Median age: 51 years Male: 63.7% Invasive MV: 3% |
In-hospital mortality Corticosteroids: 21/50 (46%) No corticosteroids: 21/34 (61.8%) |
Methylprednisolone |
| Zha et al. [48] | March 2020 | Country : China Retrospective Two centers Primary outcome : time to virus clearance Secondary outcomes: time to clinical recovery; hospital length of stay Survival analyses |
Included, 31 Median age: 39 years Male: 68% Time to hospitalization: 4 days |
In-hospital mortality Corticosteroids: 0/11 No corticosteroids: 0/20 |
Methylprednisolone intravenously, 40 mg once or twice per day), administered within 24 h of admission for a median 5 days (interquartile range: 4.5–5.0 days) |
| Wang et al. [49] | April 2020 | Country : China Retrospective Single center Primary outcome: 15-day mortality Cox models |
Included, 548 Median age: 63 years Male: 51% |
15-day mortality Corticosteroids: 65/241 (19.1%) No corticosteroids: 13/205 (6.3%) |
Low-dose glucocorticoid treatment (< 1 mg/ kg) or no glucocorticoid use was associated with a lower hazard ratio of 15 day mortality compared with high-dose treatment (≥ 1 mg/kg) There is no information on the type and duration of corticotherapy |
| Callejas Rubio et al. [50] | April 2020 | Country : Spain Retrospective Single center ferritin > 300 _g/l, IL-6 > 40 pg/ml D-dimers > 1 mg/l Tryglycerides > 300 mg/dl Primary outcome In-hospital mortality Secondary outcome Need for invasive MV Logistic regression |
Included, 92 Mean age: 64 years Male: 63% |
In-hospital mortality Corticosteroids: 6/83 (7.2%) No corticosteroids: 1/9 (11.1%) |
Methylprednisolone pulse therapy for 3 days at dose of 2 mg/kg/d (36,5%), or 250 mg/d(32,9%) or 500 mg/d(31,7%) |
| Ramiro et al. [51] | July 2020 | Country : Netherlands Prospective cohort with historical controls Single center The primary outcome, ≥2 stages of improvement on a 7-item WHO-endorsed scale, or discharge from the hospital Secondary outcomes, hospital mortality and mechanical ventilation. Time to event analyses, Cox models |
Included, 172 (86 in each group) Median age: 67 years Male: 79% MV: 8% |
14 day mortality Corticosteroids: 10/86 (11.6%) No corticosteroids: 33/86 (38.4%) |
Methylprednisolone 250 mg intravenously on day 1, followed by 80 mg on days 2–5, and an option for a 2-day extension if considered necessary and safe Tocilizumab between day 2 and day 5 (single-dose 8 mg/kg intravenous, max 800 mg), if lack of improvement |
| Li et al. [52] | August 2020 | Country : China Retrospective Two cohorts 4 centers Primary outcome : need for invasive MV Secondary outcomes: safety Logistic regression analyses |
Shanghai cohort, screened 311, included 68 Mean age: 58 years Male: 62% Validation cohort, screened 187, included 51 |
Invasive MV Shanghai cohort: Corticosteroids: 5/47 (10.6%) No corticosteroids: 7/21 (33.3%) Validation cohort Corticosteroids: 9/20 No corticosteroids 23/31 (74.2%) |
Methylprednisolone 40–80 mg/d (0.75–1.5 mg/kg/day) for 3 days, then was tapered to 20 mg/day, with a total treatment period of less than 7 days |
| Fernández-Cruz-Cruz et al. [53] | August 2020 | Country : Spain Retrospective, Single center Primary outcome : need for invasive MV Secondary outcomes: safety |
screened 848, included 463 Mean age: 58 years Male: 62% |
In-hospital mortality Corticosteroids: 55/396 (13.9%) No corticosteroids 16/67 (23.9%) |
Methylprednisolone (or the equivalent) 1 mg/kg/day (22.5% of them received steroid pulses later on) and 86 (21.7%) received pulses from the beginning |
| Mikulska et al. [54] | August 2020 | Country : Italy Retrospective, Single center Primary outcome : time to failure, defined as intubation and mechanical ventilation or death, whichever occurred first, within 30 days from the hospital admission |
Screened 295, Included 195, Median age: 67.9 years Male: 67.4% |
Short term mortality Corticosteroids: 13/45 (28.9%) Corticosteroids/ Tocilizumab : 5/56 (8.9%) No corticosteroids : 23/66 (34.8%) |
Methylprednisolone (1 mg/kg for 5 days intravenously, then 0.5 mg/kg for 5 days) Tocilizumab was administered intravenously at the dose of 8 mg/kg (maximum 800 mg), with the possibility of repeating the dose after 24 h if no response was obtained:30 |
| Ruiz-Irastorza et al. [55] | September 2020 | Country : Spain Retrospective Single center Primary outcome, time to mortality, time to mortality or invasive MV Survival analyses, Cox models |
Screened, 343 Included, 242 Mean age: 64 years Male: 62% Mean time to hospitalization: 6.6 days |
In-hospital mortality Corticosteroid: 4/61 (6.6%) No corticosteroids: 18/181 (9.9%). |
Methylprednisolone doses around 1 mg/Kg/d during several days and later as, 125 to 250 mg/d for 3 consecutive days, |
| Salton et al. [56] | September 2020 | Country : Italy multicenter, observational, longitudinal study Primary outcome composite endpoint of admission to ICU, need for invasive MV, or all-cause mortality by day 28 ARDS Berlin definition Survival analysis, Cox models |
Screened, 322 Included, 173 Median age: 65 years Male: 69% Median SOFA: 3 |
28-day mortality Methylprednisolone: 6/83 (7.2%) Control: 21/90 (23.3%) adjusted HR=0.29, 95% CI: 0.12–0.73 |
Methylprednisolone, loading dose of 80 mg intravenously at study entry (baseline), followed by an infusion of 80 mg/d in 240 ml of normal saline at 10 ml/h for at least 8 days, until achieving either a PaO2:FiO2 >350 mmHg or a CRP <20 mg/L; after which, oral administration at 16 mg or 20 mg iv twice daily until CRP reached <20% of the normal range or a PaO2:FiO2 >400 (alternative SatHbO2 ≥95% on room air). |
| Rivera et al. [57] | October 2020 | Country : USA Retrospective Multicenter Primary outcome 30-day mortality Cancer patients with COVID-19 Logistic regression |
Screened, 2956 Included, 2186 Median age: 67 years Male: 49% |
30-day mortality 329 (92%) died within 30 days OR=2.8, 95% CI: 0.77–10.15 Corticosteroids: 30/100 (30%) No corticosteroids: 200/750 (26.7%) |
NA |
| Liu et al. [58] | November 2020 | Country : China Retrospective Five centers Primary outcome 28-day all cause mortality Time to event analyses, Cox models, logistic regression |
Screened, 2537 Included, 774 Median age: 64 years Male: 58% Median SOFA: 11 Invasive MV: 1.4% |
28-day all cause mortality Corticosteroids: 181/409 (44.3%) No corticosteroids: 113/365 (31.0%) |
Methylprednisolone 396/409 Prednisolone 32/409 Dexamethasone 12/409 Hydrocortisone 2/409 Median duration 6 days Median dose 200 mg equivalent Hydrocortisone |
ICU: Intensive care unit; MV: Mechanical ventilation; SOFA: Sequential Organ Failure Assessment; ARDS: Acute respiratory distress syndrome; PaO2: Arterial partial pressure of oxygen; FiO2: Fraction of inspired oxygen; SatHbO2: Oxygen saturation level of hemoglo-bin; CRP: C-reactive protein; NA: not available.
Fig. 1.
Corticosteroids associated mortality in observational cohorts.
Forest plot showing risk ratio for mortality in the short term for corticotherapy versus usual care in observational cohorts.
Randomized controlled trials
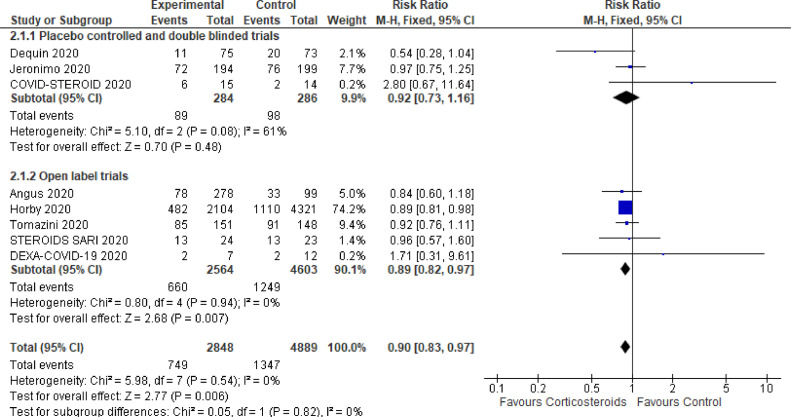
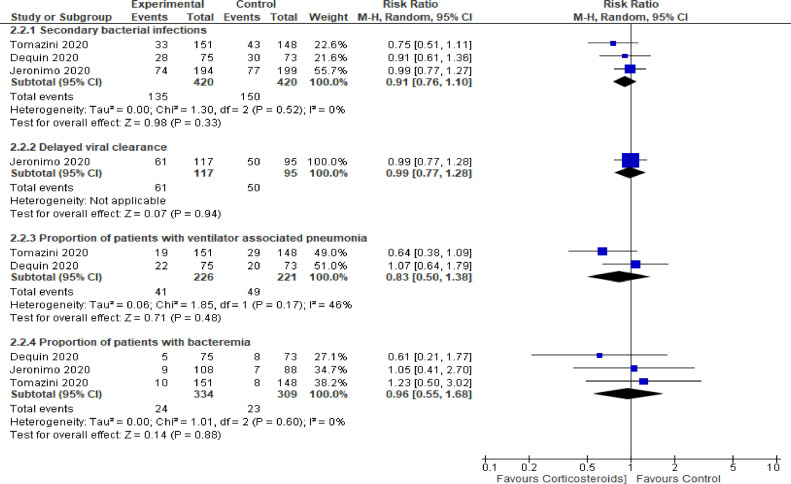
Eight randomized controlled trials have investigated the benefit and risk of corticotherapy in patients with COVID-19 [59], [60], [61], [62], [63], [64], [65], [66]. The main characteristics of these trials are summarized in Table 2. There were three placebo-controlled, double blind trials [63], [64], [65] and the remaining trials have compared corticotherapy to usual care. There were 2 platform trials with several domains covering the investigations of numerous potential interventions for COVID-19 [59,60]. One trial was conducted at an international level with countries from four continents [60], the remaining trials were performed in Brazil [62,63], China [64], Denmark [65], France [61], United Kingdom [59] and Spain [66]. These trials accounted for 7737 patients with COVID-19. There were 749/2848 (26.3%) and 1347/4889 (27.6%) deaths in the short-term in the corticosteroids-treated and corticosteroids-free patients, respectively. The corresponding pooled relative risk for short term mortality was 0.90 (95% CI: 0.83–0.97, P = 0.006, I2=0%, random effect models) in favor or corticosteroids (Fig. 2). Corticosteroids were generally associated with improved respiratory support free days [60], [61], [62] and improved cardiovascular support free days [60]. There was no evidence from data available in randomized controlled trials for any increase in the risk of superinfection with corticotherapy (Fig. 3). The pooled relative risk for any secondary bacterial infection or sepsis was 0.91 (three trials; 95% CI: 0.76–1.10, P = 0.33, I2=0%, random effect models). The RR for ventilator associated pneumonia and for bacteremia were 0.83 (95% CI : 0.50–1.38) and 0.96 (95% CI: 0.55 –1.68), respectively. Likewise, data from one study did not suggest any delay in viral clearance from the airway by corticotherapy [63]. One trial suggested that patients who do not require oxygen supplementation draw no benefit from or might be harmed by dexamethasone as well as those whom duration of disease was lower than 7 days at time of randomization [59]. Another trial found that patients older than 60 years are more likely to draw survival benefits than younger patients [63].
Table 2.
Eight randomized controlled trials of the benefit and risk of corticotherapy in patients with COVID-19.
| Studies acronyms | Date (month year) | Design | Population | Interventions | Main Outcomes | Comments |
|---|---|---|---|---|---|---|
| RECOVERY [59] | June 2020 | Country: United Kingdom Platform trial Multicenter, randomized Non blinded |
Planned n = NA Actual n = 6425 Suspected or confirmed COVID-19 Hospitalized |
Exp: Dexamethasone 6 mg/d orally or Intravenously For 5 to 10 days Control: usual care |
28-day mortality Corticosteroids: 482/2104 (22.9%) No corticosteroids: 1110/4321 (25.7%) (age-adjusted rate ratio = 0.83; 95% CI: 0.75 –0.93 |
Trial stopped prematurely for efficacy Treatment response was significantly greater in patients who required respiratory support |
| DEXA-COVID 19* | Not published | Country: Spain Multicenter, randomized Non blinded |
Planned n = 200 Actual n = 19 Invasive MV Moderate to severe ARDS per Berlin criteria Confirmed COVID-19 |
Exp: Dexamethasone 20 mg/d intravenously × 5 d and then 10 mg/d intravenously × 5 d Control: usual care |
28-day mortality Corticosteroids: 2/7 (28.6%) No corticosteroids: 2/12 (16.7%) OR=2.00, 95% CI: 0.21–18.69 |
Trial was stopped prematurely following external information from the RECOVERY trial |
| CoDEX [62] | September 2020 | Country: Brazil Multicenter, randomized Non blinded |
Planned n = 350 Actual n = 256 Invasive MV Moderate to severe ARDS per Berlin criteria Onset of ARDS <48 h before randomization Probable or confirmed COVID-19 |
Exp: Dexamethasone 20 mg/d intravenously × 5 d and then 10 mg/d intravenously × 5 d Control: usual care |
28-day mortality Corticosteroids: 85/151 (56.3%) No corticosteroids: 91/148 (61.5%) RR=0.86, 95% CI: 0.64–1.15 |
Trial was stopped prematurely following external information from the RECOVERY trial |
| CAPE COVID [61] | September 2020 | Country: France Multicenter, Embedded, randomized Double-blinded placebo |
Planned n = 290 Actual n = 256 Admitted to ICU or intermediate care unit Oxygen (≥6 L/min) Probable or confirmed COVID-19 |
Exp : Hydrocortisone, Continuous intravenous infusion × 8 d or 14 d (200 mg/d × 4 d or 7 d; 100 mg/d × 2 d or 4 d; 50 mg/d × 2 d or 3 d) Control: Placebo |
21-day mortality Corticosteroids : 11/75 Placebo: 20/73 OR=0.46, 95% CI: 0.20–1.04 |
Trial was stopped prematurely following external information from the RECOVERY trial |
| COVID STEROID [65] | Unpublished | Country: Denmark Multicenter, randomized Double-blinded placebo |
Planned n = 1000 Actual n = 29 Oxygen (≥10 L/min) Confirmed COVID-19 |
Exp: Hydrocortisone 200 mg/d intravenously × 7 d (continuous or bolus dosing every 6 h) Control: Placebo |
28-day mortality Corticosteroids: 6/15 Placebo: 2/14 OR=4.00, 95% CI: 0.65–24.66 |
Trial was stopped prematurely following external information from the RECOVERY trial |
| REMAP-CAP [60] | September 2020 | Country: Europe, USA, Canada, Australia, New Zealand, Saudi Arabia Platform trial Multicenter, randomized Non blinded Three arms Bayesian analyses |
Planned n = NA Actual n = 403 Admitted to ICU receiving high-flow nasal oxygen with FIO2 ≥0.4 at ≥30 L/min, noninvasive or invasive ventilatory support, or receiving vasopressors Probable or confirmed COVID-19 |
Exp: Hydrocortisone intravenously fixed 7-day course of 50 mg or 100 mg every 6 h) (n = 143), OR a shock-dependent course of 50 mg every 6 h when shock was clinically evident) (n = 152) Control: Usual care (n = 108). |
28-day mortality Corticosteroids: 78/278 No corticosteroids: 33/99 RR=0.84, 95% CI: 0.60– 1.18 |
Trial was stopped prematurely following external information from the RECOVERY trial |
| Steroids-SARI* | Unpublished | Country : China Randomized Non blinded |
Planned n = 80 Actual n = 47 Admitted to ICU with PaO2:FIO2 <200 mmHg on positive pressure ventilation (invasive or noninvasive) or high-flow nasal canulae >45 L/min Confirmed COVID-19 |
Exp : Methylprednisolone 40 mg intravenously every 12 h × 5 d Control: Usual care |
30-day mortality Corticosteroids: 13/24 (54.2%) No corticosteroids: 13/23 (56.5%) OR= 0.91, 95%CI: 0.29–2.87 |
Trial was stopped prematurely following external information from the RECOVERY trial |
| MetCOVID [63] | August 2020 | Country: Brazil Multicenter, randomized Double-blinded Placebo |
Planned n=378 Actual n=393 suspicion of COVID-19, SpO2 ≤ 94% with room air, required supplementary oxygen, or required invasive mechanical ventilation |
Exp: intravenous sodium succinate methylprednisolone 0.5 mg/kg twice daily for 5 days Control: saline solution twice daily for 5 days |
28-day mortality methylprednisolone: 72/194 (37.1%) Placebo: 76/199 (38.2%) |
Post hoc analysis suggested survival benefit from methylprednisolone in patients of >60 years old whereas younger patients may have increased risk of death with methylprednisolone |
ARDS: Acute respiratory distress syndrome; CAPE COVID: Community-Acquired Pneumonia: Evaluation of Corticosteroids in Coronavirus Disease; CoDEX: COVID-19 Dexamethasone; COVID-19: Coronavirus disease 2019; COVID STEROID: Hydrocortisone for COVID-19 and Severe Hypoxia; DEXA-COVID 19: Efficacy of Dexamethasone Treatment for Patients With ARDS Caused by COVID-19; FIO2: Fraction of inspired oxygen; ICU, intensive care unit; NA, not applicable; RECOVERY: Randomized Evaluation of COVID-19 Therapy; REMAP-CAP: Randomized, Embedded, Multifactorial Adaptive Platform Trial for Community-Acquired Pneumonia; Sepsis-3: Third International Consensus Definitions for Sepsis and Septic Shock; Steroids-SARI: Glucocorticoid Therapy for COVID-19 Critically Ill Patients With Severe Acute Respiratory Failure; MV: Mechanical ventilation; Exp: Experimental group; OR: Odd ratio: RR: Relative risk; CI: Confidence interval.
data were extracted from Sterne et al. [76]. JAMA 2020.
Fig. 2.
Corticosteroids effects on mortality in randomized controlled trials.
Forest plot showing risk ratio for mortality in the short term for corticotherapy versus usual care or placebo in randomized controlled trials.
Fig. 3.
Serious infections associated with corticosteroids in randomized controlled trials.
Forest plot showing risk ratio for serious infectious complications with corticotherapy versus usual care or placebo in randomized controlled trials.
Systematic reviews and meta-analyses
Table 3 summarizes the characteristics and main findings from 13 systematic reviews and meta-analyses published since March 2020 [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79]. Two of these systematic reviews included only randomized controlled trials [76, 77]. The number of accumulated patients ranged from 542 to 21,350. These systematic reviews variably found that corticosteroids increased, reduced or had no effect on survival. This heterogeneity in the results of the systematic reviews was related to the date of publication, target population and type of studies. The two systematic reviews that included only randomized controlled trials found an odd ratio for short term deaths of 0.66 (95% CI: 0.53–0.82) [76] and of 0.87 (95% CI: 0.77–0.98) [77] in favor of corticosteroids. These meta-analyses suggested that corticosteroids were not associated with the increased rate of serious adverse events. The WHO prospective meta-analysis found no evidence that response to corticosteroids may vary with age, gender, duration of the disease before initiating treatment, the dose and type of corticosteroids [76].
Table 3.
Systematic reviews and meta-analyses.
| Reference | Date of publication(month year) | Type of studies, number | Populations Category, n | Main Outcomes | Certainty of evidence | Comments |
|---|---|---|---|---|---|---|
| Veronese et al. [67] | April 2020 | Observational, 4 | Severe COVID-19, 542 Mean age: 52 years Male: 55.7% |
1 study reported increased risk of ICU admission, 1 study reported delayed viral clearance from the airways 1 study found no difference between patients with and without corticosteroids, 1 study found increased chance of survival in methylprednisolone treated patients |
Very low | There was no pooled analysis |
| Yang et al. [68] | April 2020 | Observational cohorts, 15 |
COVID-19, 5270 |
No quantitative data for COVID-19 | Very low | This review also included patients with SARS, MERS, |
| Ye et al. [69] | May 2020 | Observational cohorts, 6 Randomized controlled trials, 0 |
COVID-19 without ARDS, 679 COVID-19 with ARDS, 84 |
In-hospital mortality MP : 21/50 (46%) No MP : 21/34 (61.8%) HR=0.38, 95% CI: 0.20–0.72 HR=2.30, 95% CI: 1.00–5.29 |
Very low Very low |
This review also included patients with SARS, MERS, ARDS from any cause, and community acquired pneumonia from any cause |
| Li et al. [70] | May 2020 | Observational cohorts, 10 Randomized controlled trials, 1 |
COVID-19, 5249 | In-hospital mortality No separate data for the COVID-19 population Mixed population RR=1.07,95% CI: 0.81– 1.42; I2 = 80% |
Very low | This review also included patients with SARS, MERS, |
| Singh et al. [71] | June 2020 | Observational cohorts, 5 |
COVID-19, Hospitalized, 1832 |
3 trials found survival benefits 1 trial found no difference between corticosteroid-treated patients and controls 1 trial found delayed viral clearance from the airways |
Very low | This review also included patients with ARDS not related to COVID-19 There was no quantitative pooled estimation |
| Hasan et al. [72] | July 2020 | Observational, 23 Randomized trials, Reports, 2 |
COVID-19 with or without ARDS | NA | NA | This review provided no pooled estimate for the association between corticosteroids and mortality |
| Cheng et al. [73] | August 2020 | Observational cohorts, 13 Case-series, 7 |
COVID-19 broad range of severity, 2840 | In-hospital mortality RR=1.59; 95% CI: 0.69–3.66, I2=93.5% Clinical recovery RR=1.30, 95% CI: 0.98–1.72 |
Very low Very low |
All except two studies were from China, This review also suggested corticosteroids reduced length of hospital stay and did not alter viral clearance or duration of mechanical ventilation |
| Pei et al. [74] | August 2020 | Observational cohorts, 5 | COVID-19, 943 | In-hospital mortality OR=2.43; 95% CI: 1.44–4.1, I2=61.9% |
Very low | This review also examined other interventions for COVID-19, e.g. antivirals |
| Tlayjeh et al. [75] | September 2020 | Observational, 9 Randomized trials, 1 |
COVID-19 broad range of severity, 10,278 | Short term mortality – 8 trials adjusted RR=0.92, 95% CI: 0.69–1.22, I2=81.94% |
Very low | This review used published and unpublished data |
| Composite outcome of mortality, ICU admission and mechanical ventilation – 4 trials Adjusted 0.41 ; 95% CI: 0.23−0.73, I2=78.69% |
Very low | |||||
| Delayed viral clearance – 6 trials adjusted RR= 1.47, 95% CI: 1.11–1.93, I2=43.38% |
Very low | |||||
| Sterne et al. [76] | September 2020 | Randomized controlled trials, 7 | Critically ill COVID-19, 1703 Median age: 60 years Male: 61% |
30-day mortality Corticosteroids: 222/678 (32.7%) No corticosteroids: 425/1025 (41.5%) OR= 0.66, 95% CI: 0.53–0.82 |
Moderate | No variation in treatment response with respect to age, gender, duration of disease, type of corticosteroids (dexamethasone or hydrocortisone), low versus high dose Corticosteroids might be more effective is vasopressor-free or invasive ventilation free patients |
| Siemieniuk et al. [77] | September 2020 | Randomised clinical trials, Bayesian network meta-analysis |
Suspected, probable, or confirmed COVID-19 | Survival benefit with corticosteroids OR=0.87, 95% CI: 0.77–0.98; |
Moderate | Living meta-analysis of intervention trials for COVID-19 |
| Budhathoki et al. [78] | September 2020 | Observational cohorts, 32 Case-series, 9 |
COVID-19 Hospitalized, ICU and non-ICU patients, patients with and without ARDS |
Short term mortality RR=2.01, 95% CI: 1.12–3.63; participants=4451; studies=14; I2= 92% |
Very low | This review was based only on observational cohorts It showed also prolonged hospital stay and delayed conversion to negative PCR with corticosteroids |
| Cano et al. [79] | October 2020 | Observational, 72 Randomized controlled trials, 1 |
COVID-19 hospitalized patients, 21,350 | 32/73 studies contributed to mortality analysis OR=2.30, 95% CI: 1.45–3.63; with squared I2 = 90% |
Very low | 55 Chinese studies contributed to 43% of the sample size |
| 8/73 studies in ARDS related to COVID-19 OR=0.65, 95% CI 0.51–0.83, P = 0.0006 with I2 = 29% |
low |
ICU: Intensive care unit; SARS: Severe Acute Respiratory Syndrome;MERS: Middle East Respiratory Syndrome;ARDS: Acute respiratory distress syndrome; MP: Methylprednisolone; HR: Hazard ratio; CI: Confidence interval;RR: Relative risk; OR: Odd ratio; NA: not available.
Suggestions for practice
In COVID-19, like in bacterial sepsis, the deregulation of the immune host response results in the most severe cases in overwhelming systemic inflammation. Corticosteroids are potent immunomodulatory drugs that may via genomic and non-genomic effects help preventing or attenuating the hyper-inflammation state that characterized severe SARS-CoV-2 infections. While data from observational cohorts were inconclusive, data from randomized controlled trials and high quality systematic reviews and meta-analyses strongly support the use of corticosteroids in patients with COVID-19 that require oxygen support. Corticosteroids should not be given to patients who do not require oxygen supplementation. Corticosteroids should be given intravenously or orally, pending clinical severity of illness, as dexamethasone, hydrocortisone, or methylprednisolone, at a dose equivalent to 6 mg of dexamethasone and for 5 to 10 days.
Conflicts of Interest
The author declares that he has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Managing Editor: Jingling Bao
Footnotes
Given his role as Associate Editor, Prof. Djillali Annane had no involvement in the peer-review of this article and has no access to information regarding its peer-review. Prof. Jiao Liu took the responsibility for peer-review progress. Prof. Jean-Louis Teboul who is the co-editor-in-chief made the final decision.
References
- 1.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johns Hopkins University . 2020. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University.https://coronavirus.jhu.edu/map.html Available at. Accessed November 12. [Google Scholar]
- 3.Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: A review. Clin Immunol. 2020;215 doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohn MK, Hall A, Sepiashvili L, Jung B, Steele S, Adeli K. Pathophysiology of COVID-19: mechanisms underlying disease severity and progression. Physiology (Bethesda) 2020;35(5):288–301. doi: 10.1152/physiol.00019.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teuwen LA, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20(7):389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polidoro RB, Hagan RS, de Santis, Santiago R, Schmidt NW. Overview: systemic inflammatory response derived from lung injury caused by SARS-CoV-2 infection explains severe outcomes in COVID-19. Front Immunol. 2020;11:1626. doi: 10.3389/fimmu.2020.01626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO R&D Blueprint novel Coronavirus . Vol. 12. 2020. https://www.who.int/publications/m/item/who-working-group-therapeutics-prioritization (WHO Working Group – Therapeutics Prioritization for COVID-19. Available at). Accessed November. [Google Scholar]
- 8.Li L, Chen C. Contribution of acute-phase reaction proteins to the diagnosis and treatment of 2019 novel coronavirus disease (COVID-19) Epidemiol Infect. 2020;148:e164. doi: 10.1017/S095026882000165X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in wuhan, china. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Annane D, Heming N, Grimaldi-Bensouda L, Frémeaux-Bacchi V, Vigan M, Roux AL, et al. Eculizumab as an emergency treatment for adult patients with severe COVID-19 in the intensive care unit: A proof-of-concept study. EClinicalMedicine. 2020;28 doi: 10.1016/j.eclinm.2020.100590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JS, Park S, Jeong HW, Ahn JY, Choi SJ, Lee H, et al. Immunophenotyping of COVID-19 and influenza highlights the role of type I interferons in development of severe COVID-19. Sci Immunol. 2020;5(49) doi: 10.1126/sciimmunol.abd1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515):eabd4585. doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McElvaney OJ, McEvoy NL, McElvaney OF, Carroll TP, Murphy MP, Dunlea DM, et al. Characterization of the inflammatory response to severe COVID-19 illness. Am J Respir Crit Care Med. 2020;202(6):812–821. doi: 10.1164/rccm.202005-1583OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson JG, Simpson LJ, Ferreira AM, Rustagi A, Roque J, Asuni A, et al. Cytokine profile in plasma of severe COVID-19 does not differ from ARDS and sepsis. JCI Insight. 2020;5(17) doi: 10.1172/jci.insight.140289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leisman DE, Ronner L, Pinotti R, Taylor MD, Sinha P, Calfee CS, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020;8(12):1233–1244. doi: 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuri-Cervantes L, Pampena MB, Meng W, Rosenfeld AM, Ittner C, Weisman AR, et al. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci Immunol. 2020;5(49) doi: 10.1126/sciimmunol.abd7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathew D, Giles JR, Baxter AE, Oldridge DA, Greenplate AR, Wu JE, et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. 2020;369(6508):eabc8511. doi: 10.1126/science.abc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nat Rev Immunol. 2017;17(4):233–247. doi: 10.1038/nri.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heming N, Sivanandamoorthy S, Meng P, Bounab R, Annane D. Immune effects of corticosteroids in sepsis. Front Immunol. 2018;9:1736. doi: 10.3389/fimmu.2018.01736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Picard D, Khursheed B, Garabedian MJ, Fortin MG, Lindquist S, Yamamoto KR. Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature. 1990;348(6297):166–168. doi: 10.1038/348166a0. [DOI] [PubMed] [Google Scholar]
- 22.Nissen RM, Yamamoto KR. The glucocorticoid receptor inhibits NFkappaB by interfering with serine-2 phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev. 2000;14(18):2314–2329. doi: 10.1101/gad.827900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luecke HF, Yamamoto KR. The glucocorticoid receptor blocks P-TEFb recruitment by NFkappaB to effect promoter-specific transcriptional repression. Genes Dev. 2005;19(9):1116–1127. doi: 10.1101/gad.1297105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reily MM, Pantoja C, Hu X, Chinenov Y, Rogatsky I. The GRIP1:IRF3 interaction as a target for glucocorticoid receptor-mediated immunosuppression. EMBO J. 2006;25(1):108–117. doi: 10.1038/sj.emboj.7600919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogawa S, Lozach J, Benner C, Pascual G, Tangirala RK, Westin S, et al. Molecular determinants of crosstalk between nuclear receptors and Toll-like receptors. Cell. 2005;122(5):707–721. doi: 10.1016/j.cell.2005.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKay LI, Cidlowski JA. Molecular control of immune/inflammatory responses: interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr Rev. 1999;20(4):435–459. doi: 10.1210/edrv.20.4.0375. [DOI] [PubMed] [Google Scholar]
- 27.Berrebi D, Bruscoli S, Cohen N, Foussat A, Migliorati G, Bouchet-Delbos L, et al. Synthesis of glucocorticoid-induced leucine zipper (GILZ) by macrophages: an anti-inflammatory and immunosuppressive mechanism shared by glucocorticoids and IL-10. Blood. 2003;101(2):729–738. doi: 10.1182/blood-2002-02-0538. [DOI] [PubMed] [Google Scholar]
- 28.Lasa M, Abraham SM, Boucheron C, Saklatvala J, Clark AR. Dexamethasone causes sustained expression of mitogen-activated protein kinase (MAPK) phosphatase 1 and phosphatase-mediated inhibition of MAPK p38. Mol Cell Biol. 2002;22(22):7802–7811. doi: 10.1128/mcb.22.22.7802-7811.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vago JP, Nogueira CR, Tavares LP, Soriani FM, Lopes F, Russo RC, et al. Annexin a1 modulates natural and glucocorticoid-induced resolution of inflammation by enhancing neutrophil apoptosis. J Leukoc Biol. 2012;92(2):249–258. doi: 10.1189/jlb.0112008. [DOI] [PubMed] [Google Scholar]
- 30.Miyamasu M, Misaki Y, Izumi S, Takaishi T, Morita Y, Nakamura H, et al. Glucocorticoids inhibit chemokine generation by human eosinophils. J Allergy Clin Immunol. 1998;101(1):75–83. doi: 10.1016/S0091-6749(98)70196-4. 1 Pt. [DOI] [PubMed] [Google Scholar]
- 31.Pype JL, Dupont LJ, Menten P, Van Coillie E, Opdenakker G, Van Damme J, et al. Expression of monocyte chemotactic protein (MCP)-1, MCP-2, and MCP-3 by human airway smooth-muscle cells. modulation by corticosteroids and t-helper 2 cytokines. Am J Respir Cell Mol Biol. 1999;21(4):528–536. doi: 10.1165/ajrcmb.21.4.3660. [DOI] [PubMed] [Google Scholar]
- 32.Cronstein BN, Kimmel SC, Levin RI, Martiniuk F, Weissmann G. A mechanism for the antiinflammatory effects of corticosteroids: the glucocorticoid receptor regulates leukocyte adhesion to endothelial cells and expression of endothelial-leukocyte adhesion molecule 1 and intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1992;89(21):9991–9995. doi: 10.1073/pnas.89.21.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pitzalis C, Pipitone N, Bajocchi G, Hall M, Goulding N, Lee A, et al. Corticosteroids inhibit lymphocyte binding to endothelium and intercellular adhesion: an additional mechanism for their anti-inflammatory and immunosuppressive effect. J Immunol. 1997;158(10):5007–5016. [PubMed] [Google Scholar]
- 34.Boumpas DT, Chrousos GP, Wilder RL, Cupps TR, Balow JE. Glucocorticoid therapy for immune-mediated diseases: basic and clinical correlates. Ann Intern Med. 1993;119(12):1198–1208. doi: 10.7326/0003-4819-119-12-199312150-00007. [DOI] [PubMed] [Google Scholar]
- 35.Stary G, Klein I, Bauer W, Koszik F, Reininger B, Kohlhofer S, et al. Glucocorticosteroids modify langerhans cells to produce TGF-β and expand regulatory T cells. J Immunol. 2011;186(1):103–112. doi: 10.4049/jimmunol.1002485. [DOI] [PubMed] [Google Scholar]
- 36.Bianchi M, Meng C, Ivashkiv LB. Inhibition of IL-2-induced JAK-STAT signaling by glucocorticoids. Proc Natl Acad Sci U S A. 2000;97(17):9573–9578. doi: 10.1073/pnas.160099797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Piemonti L, Monti P, Allavena P, Sironi M, Soldini L, Leone BE, et al. Glucocorticoids affect human dendritic cell differentiation and maturation. J Immunol. 1999;162(11):6473–6481. [PubMed] [Google Scholar]
- 38.DeKruyff RH, Fang Y, Umetsu DT. Corticosteroids enhance the capacity of macrophages to induce Th2 cytokine synthesis in CD4+ lymphocytes by inhibiting IL-12 production. J Immunol. 1998;160(5):2231–2237. [PubMed] [Google Scholar]
- 39.Cupps TR, Gerrard TL, Falkoff RJ, Whalen G, Fauci AS. Effects of in vitro corticosteroids on B cell activation, proliferation, and differentiation. J Clin Invest. 1985;75(2):754–761. doi: 10.1172/JCI111757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Vliet E, Melis M, van Ewijk W. The influence of dexamethasone treatment on the lymphoid and stromal composition of the mouse thymus: a flowcytometric and immunohistological analysis. Cell Immunol. 1986;103(2):229–240. doi: 10.1016/0008-8749(86)90086-9. [DOI] [PubMed] [Google Scholar]
- 41.Wang D, Müller N, McPherson KG, Reichardt HM. Glucocorticoids engage different signal transduction pathways to induce apoptosis in thymocytes and mature T cells. J Immunol. 2006;176(3):1695–1702. doi: 10.4049/jimmunol.176.3.1695. [DOI] [PubMed] [Google Scholar]
- 42.Ramírez F, Fowell DJ, Puklavec M, Simmonds S, Mason D. Glucocorticoids promote a Th2 cytokine response by CD4+ T cells in vitro. J Immunol. 1996;156(7):2406–2412. [PubMed] [Google Scholar]
- 43.Franchimont D, Galon J, Gadina M, Visconti R, Zhou Y, Aringer M, et al. Inhibition of Th1 immune response by glucocorticoids: dexamethasone selectively inhibits IL-12-induced STAT4 phosphorylation in T lymphocytes. J Immunol. 2000;164(4):1768–1774. doi: 10.4049/jimmunol.164.4.1768. [DOI] [PubMed] [Google Scholar]
- 44.Liberman AC, Refojo D, Druker J, Toscano M, Rein T, Holsboer F, et al. The activated glucocorticoid receptor inhibits the transcription factor T-bet by direct protein-protein interaction. FASEB J. 2007;21(4):1177–1188. doi: 10.1096/fj.06-7452com. [DOI] [PubMed] [Google Scholar]
- 45.Maneechotesuwan K, Yao X, Ito K, Jazrawi E, Usmani OS, Adcock IM, et al. Suppression of GATA-3 nuclear import and phosphorylation: a novel mechanism of corticosteroid action in allergic disease. PLoS Med. 2009;6(5) doi: 10.1371/journal.pmed.1000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. Mar 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in wuhan, china. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zha L, Li S, Pan L, Tefsen B, Li Y, French N, et al. Corticosteroid treatment of patients with coronavirus disease 2019 (COVID-19) Med J Aust. 2020;212(9):416–420. doi: 10.5694/mja2.50577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang K, Zhang Z, Yu M, Tao Y, Xie M. 15-day mortality and associated risk factors for hospitalized patients with COVID-19 in wuhan, china: an ambispective observational cohort study. Intensive Care Med. 2020;46(7):1472–1474. doi: 10.1007/s00134-020-06047-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Callejas Rubio JL, Luna Del Castillo JD, de la Hera Fernández J, Guirao Arrabal E, Colmenero Ruiz M, Ortego Centeno N. Effectiveness of corticoid pulses in patients with cytokine storm syndrome induced by SARS-CoV-2 infection. Med Clin (Barc) 2020;155(4):159–161. doi: 10.1016/j.medcli.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramiro S, Mostard RLM, Magro-Checa C, van Dongen CMP, Dormans T, Buijs J, et al. Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: results of the CHIC study. Ann Rheum Dis. 2020;79(9):1143–1151. doi: 10.1136/annrheumdis-2020-218479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Y, Zhou X, Li T, Chan S, Yu Y, Ai JW, et al. Corticosteroid prevents COVID-19 progression within its therapeutic window: a multicentre, proof-of-concept, observational study. Emerg Microbes Infect. 2020;9(1):1869–1877. doi: 10.1080/22221751.2020.1807885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fernández-Cruz A, Ruiz-Antorán B, Muñoz-Gómez A, Sancho-López A, Mills-Sánchez P, Centeno-Soto GA, et al. A retrospective controlled cohort study of the impact of glucocorticoid treatment in SARS-CoV-2 infection mortality. Antimicrob Agents Chemother. 2020;64(9) doi: 10.1128/AAC.01168-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mikulska M, Nicolini LA, Signori A, Di Biagio A, Sepulcri C, Russo C, et al. Tocilizumab and steroid treatment in patients with COVID-19 pneumonia. PLoS One. 2020;15(8) doi: 10.1371/journal.pone.0237831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruiz-Irastorza G, Pijoan JI, Bereciartua E, Dunder S, Dominguez J, Garcia-Escudero P, et al. Second week methyl-prednisolone pulses improve prognosis in patients with severe coronavirus disease 2019 pneumonia: an observational comparative study using routine care data. PLoS One. 2020;15(9) doi: 10.1371/journal.pone.0239401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salton F, Confalonieri P, Meduri GU, Santus P, Harari S, Scala R, et al. Prolonged low-dose methylprednisolone in patients with severe COVID-19 pneumonia. Open Forum Infect Dis. 2020;7(10) doi: 10.1093/ofid/ofaa421. ofaa421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rivera DR, Peters S, Panagiotou OA, Shah DP, Kuderer NM, Hsu CY, et al. Utilization of COVID-19 treatments and clinical outcomes among patients with cancer: A COVID-19 and cancer consortium (ccc19) cohort study. Cancer Discov. 2020;10(10):1514–1527. doi: 10.1158/2159-8290.CD-20-0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu J, Zhang S, Dong X, Li Z, Xu Q, Feng H, et al. Corticosteroid treatment in severe COVID-19 patients with acute respiratory distress syndrome. J Clin Invest. 2020;130(12):6417–6428. doi: 10.1172/JCI140617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with COVID-19 - preliminary report. N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Angus DC, Derde L, Al-Beidh F, Annane D, Arabi Y, Beane A, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: the remap-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020;324(13):1317–1329. doi: 10.1001/jama.2020.17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dequin PF, Heming N, Meziani F, Plantefève G, Voiriot G, Badié J, et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: A randomized clinical trial. JAMA. 2020;324(13):1298–1306. doi: 10.1001/jama.2020.16761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the coDEX randomized clinical trial. JAMA. 2020;324(13):1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jeronimo C, Farias M, Val F, Sampaio VS, Alexandre M, Melo GC, et al. Methylprednisolone as adjunctive therapy for patients hospitalized with COVID-19 (metcovid): A randomised, double-blind, phase iib, placebo-controlled trial. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.International Severe Acute Respiratory and Emerging Infections Consortium. COVID-19 report: 13 July 2020. Accessed November 24, 2020. https://media.tghn.org/medialibrary/2020/07/ISARIC_Data_Platform_COVID-19_Report_13JUL20.pdf.
- 65.Scandinavian Critical Care Trials Group Hydrocortisone for COVID-19 and Severe Hypoxia (COVID STEROID) https://clinicaltrials.gov/ct2/show/NCT04348305. Accessed November 24 11, 2020.
- 66.DEXA-ARDS Network. Efficacy of Dexamethasone Treatment for Patients With ARDS Caused by COVID-19 (DEXA-COVID19). https://clinicaltrials.gov/ct2/show/NCT04325061. Accessed November 24 11, 2020.
- 67.Veronese N, Demurtas J, Yang L, Tonelli R, Barbagallo M, Lopalco P, et al. Use of corticosteroids in coronavirus disease 2019 pneumonia: A systematic review of the literature. Front Med (Lausanne) 2020;7:170. doi: 10.3389/fmed.2020.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang Z, Liu J, Zhou Y, Zhao X, Zhao Q, Liu J. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Infect. 2020;81(1):e13–e20. doi: 10.1016/j.jinf.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ye Z, Wang Y, Colunga-Lozano LE, Prasad M, Tangamornsuksan W, Rochwerg B, et al. Efficacy and safety of corticosteroids in COVID-19 based on evidence for COVID-19, other coronavirus infections, influenza, community-acquired pneumonia and acute respiratory distress syndrome: a systematic review and meta-analysis. CMAJ. 2020;192(27):E756–E767. doi: 10.1503/cmaj.200645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li H, Chen C, Hu F, Wang J, Zhao Q, Gale RP, et al. Impact of corticosteroid therapy on outcomes of persons with SARS-CoV-2, SARS-CoV, or MERS-CoV infection: a systematic review and meta-analysis. Leukemia. 2020;34(6):1503–1511. doi: 10.1038/s41375-020-0848-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singh AK, Majumdar S, Singh R, Misra A. Role of corticosteroid in the management of COVID-19: A systemic review and a clinician's perspective. Diabetes Metab Syndr. 2020;14(5):971–978. doi: 10.1016/j.dsx.2020.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hasan SS, Capstick T, Ahmed R, Kow CS, Mazhar F, Merchant HA, et al. Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use: a systematic review and meta-analysis. Expert Rev Respir Med. 2020;14(11):1149–1163. doi: 10.1080/17476348.2020.1804365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cheng W, Li Y, Cui L, Chen Y, Shan S, Xiao D, et al. Efficacy and safety of corticosteroid treatment in patients with COVID-19: A systematic review and meta-analysis. Front Pharmacol. 2020;11 doi: 10.3389/fphar.2020.571156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pei L, Zhang S, Huang L, Geng X, Ma L, Jiang W, et al. Antiviral agents, glucocorticoids, antibiotics, and intravenous immunoglobulin in 1142 patients with coronavirus disease 2019: a systematic review and meta-analysis. Pol Arch Intern Med. 2020;130(9):726–733. doi: 10.20452/pamw.15543. [DOI] [PubMed] [Google Scholar]
- 75.Tlayjeh H, Mhish OH, Enani MA, Alruwaili A, Tleyjeh R, Thalib L, et al. Association of corticosteroids use and outcomes in COVID-19 patients: A systematic review and meta-analysis. J Infect Public Health. 2020;13(11):1652–1663. doi: 10.1016/j.jiph.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA. 2020;324(13):1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Siemieniuk RA, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Kum E, et al. Drug treatments for COVID-19: living systematic review and network meta-analysis. BMJ. 2020;370:m2980. doi: 10.1136/bmj.m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Budhathoki P, Shrestha DB, Rawal E, Khadka S. Corticosteroids in COVID-19: is it rational? A systematic review and meta-analysis. SN Compr Clin Med. 2020:1–21. doi: 10.1007/s42399-020-00515-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cano EJ, Fonseca Fuentes X, Corsini Campioli C, O'Horo JC, Abu Saleh O, Odeyemi Y, et al. Impact of Corticosteroids in Coronavirus Disease 2019 Outcomes: Systematic Review and Meta-analysis. Chest. S0012-3692(20)35107-2. doi: 10.1016/j.chest.2020.10.054. [DOI] [PMC free article] [PubMed]