Abstract
Invasive liver abscess syndrome (ILAS) is caused by Klebsiella pneumoniae and is typically seen in people from East Asia, often with diabetes and gallstones. ILAS includes metastatic sequelae of the infection, commonly to the eyes. The case described below occurred in a London hospital. The patient’s abscess was diagnosed on CT and MRI and he developed endophthalmitis secondary to metastatic spread of the infection. He was treated with intravenous and intravitreal antibiotics and discharged with a plan for vitrectomy and cholecystectomy as an outpatient. We discuss the epidemiology, risk factors, pathogenesis, prognosis and management of this rare condition. There have been a number of recent reports of cases of this nature outside of Asia and we believe greater awareness is required. A high index of suspicion should be held for the potential development of metastases in patients of this demographic presenting with abscesses of this nature.
Keywords: general surgery, infection (gastroenterology), infectious diseases, ophthalmology
Background
Invasive liver abscess syndrome (ILAS) was described in the 1980s,1 but it was not until 2012 that it was defined as a distinct syndrome.2 ILAS is typically seen in patients from East Asia with 40%–75% having diabetes mellitus3–7 and a similar proportion with gallstones.8 9
Almost all severe cases of ILAS: bacteraemia, liver abscesses and extrahepatic infections, are caused by Klebsiella pneumoniae serotypes K1 or K2, but not all infections with K1 or K2 serotypes result in this syndrome.2
There is a significant morbidity and mortality associated with the condition. Overall 10.6% develop extrahepatic metastatic infection, and common complications include central nervous system involvement, necrotising fasciitis and endophthalmitis.4 5 10 Ophthalmic complications are often severe, particularly in those with diabetes, with more than 85% of patients developing a severe visual deficit.2 11
ILAS is endemic in Taiwan,10 and increasingly recognised across North America and Europe,2 6 12–18 although we are aware of only a handful of cases from the UK.13 14 19 20
Case presentation
A 57-year-old Vietnamese man presented to the emergency department of a South London hospital in March 2020 with a 3-day history of rigours, profuse sweating and right-sided abdominal pain. He had a background of a left hemisphere meningioma resection, Grave’s disease and type 2 diabetes controlled with metformin and gliclazide. His most recent travel to Asia was in December 2014.
On examination, he was alert and orientated. Auscultation of the chest revealed good bilateral air entry with slight left basal crackles and normal heart sounds. His abdomen was soft but tender in the right upper quadrant and he was passing concentrated urine. His initial observations were; temperature 37.5°C, heart rate 126 bpm, blood pressure 125/79 mm Hg, saturations 97% on room air and respiratory rate 27 breaths per minute.
His initial blood results revealed a C reactive protein (CRP) of 242 mg/L and an estimated glomerular filtration rate of 47, assumed to be an acute kidney injury in the context of an acute inflammatory process. Full blood count showed a normal white cell count (WCC) of 4.4 ×109/L and haemoglobin of 149 g/L. His liver function tests were; bilirubin 18 mg/dL, alkaline phosphatase 123 u/L and alanine aminotransferase 85 u/L while international normalised ratio was 1.3. His blood glucose was 20.9 mmol/L and glycated haemoglobin (HbA1c) was 51. At this point, the differential diagnoses included common general surgical pathology such as appendicitis, cholecystitis or another hepatobiliary infectious process, as well as malignancy.
Blood cultures taken on admission grew a gram-negative organism after 9 hours that was resistant to amoxicillin and, based on sensitivity results, he was commenced on intravenous piperacillin/tazobactam 4.5 g three times a day with the addition of gentamicin 5 mg/kg/day to cover for organisms with inducible beta-lactamase activity. K. pneumoniae was subsequently isolated from this blood culture while his urine cultured no organisms. The following day, a CT scan of his thorax, abdomen and pelvis with contrast revealed a hypoattenuating, hypoenhanced, inhomogeneous lesion in the parahilar region of the central part of the liver, measuring 6.3×5.2 cm (figure 1). A specialist gastrointestinal radiologist deemed it most likely that the lesion was an abscess although not amenable to drainage at this stage. By then, his WCC had risen to 14.9×109/L (with a neutrophilia of 13.7×109/L). With continued intravenous fluids and antibiotics, his inflammatory markers trended downwards and renal function normalised.
Figure 1.
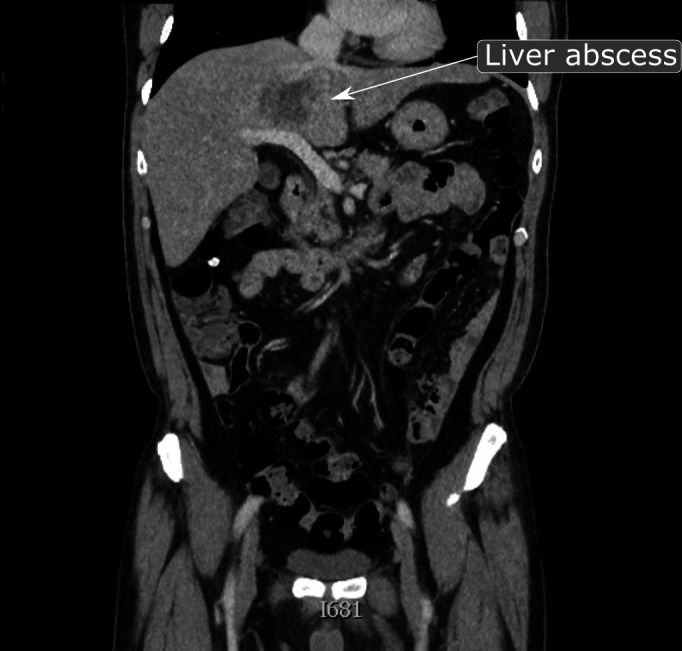
A CT abdomen and pelvis in the coronal plane, 2 days after presentation: There is the hypoattenuating-hypoenhanced lesion in the central part of the liver (parahilar region), measuring at least 6.3×5.2 cm, which shows inhomogeneity. There are a few, slightly prominent retroperitoneal lymph nodes but with no significant enlargement.
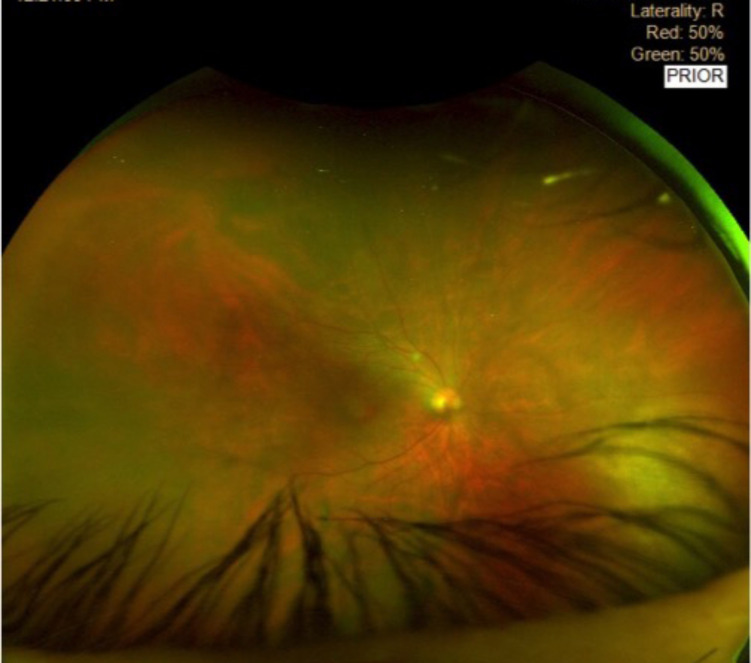
On the third day of admission, he had blurred vision in his right eye, with conjunctival injection and pain in response to light and movement. Ophthalmology findings were of visual acuity 6/24 in the right eye and 6/9 in the left eye; localised vitritis and active nasal retinitis were detected and endogenous endophthalmitis was diagnosed. Of note, diabetic retinopathy grading was not sufficient to produce visual impairment in either eye. The patient was commenced on flucytosine 200 mg/kg/daily four times a day and ketorolac 10 mg three times a day for pain.
One week later, his vitritis slightly increased with further deterioration of vision (3/60). Vitreous biopsy and intravitreal cefuroxime 2 mg/0.1 mL and vancomycin 1 mg/0.1 mL was given. At this point, the ketorolac was held and a week’s course of dexamethasone drops 0.1% four times a day and ofloxacin drops 0.3% four times a day were commenced alongside cyclopentolate 1% two times a day for 10 days. His right eye improved and evidence of infection and inflammation in the vitreous decreased within a few days (figure 2).
Figure 2.
Funduscopy of the right eye on day 22 postpresentation showing endophthalmitis after initial intravitreal antibiotic injections.
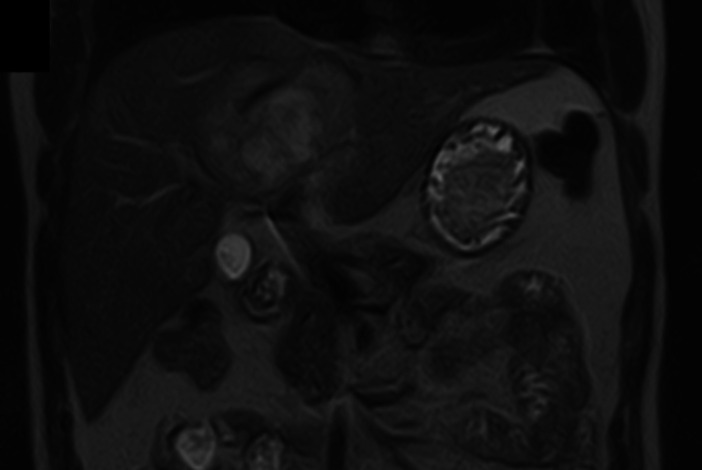
Despite initial improvement, his WCC rose on day nine to 12.8 ×109/L. MR cholangiography showed a complex multiloculated lesion within liver segments IV and VIII, measuring 7.8×5.9 cm in diameter (figure 3). It also revealed calculi within the gallbladder. A liver biopsy was not performed however a radiologically guided drain was inserted and the same organism was isolated from a pus sample.
Figure 3.
An MRI (T2 weighted, T2W) abdomen and pelvis in the coronal plane, 17 days after presentation: there is a complex multiloculate lesion with restricted diffusion within segments IV and VIII, measuring 7.8x 5.9 cm in diameter. There are calculi within the gallbladder with no biliary dilatation.
Outcome and follow-up
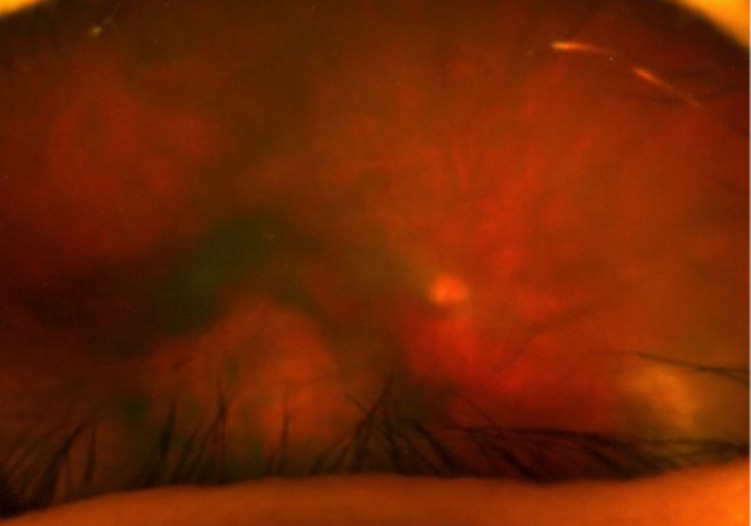
On day 18, an ultrasound of his liver showed the abscess had resolved. At this point his WCC count was 6.2 ×109/L and CRP was 6 mg/L. The patient was discharged 2 days later with a 5-day course of oral ciprofloxacin 500 mg two times a day, based on culture sensitivities and an abdominal drain in situ. A week later, he was reviewed as a surgical outpatient, the drain was removed and he was found to be making good progress. He has been booked for an elective laparoscopic cholecystectomy. The eye has continued to improve (figure 4), though at time of writing his visual acuity has not improved beyond 6/24 in the right eye, and the plan is to offer vitrectomy to clear the vitreous and hopefully improve the vision once the anterior chamber is completely free of inflammation.
Figure 4.
Funduscopy of the right eye on day 37 post-presentation showing ongoing endophthalmitis after further intravitreal antibiotic injections.
Discussion
Epidemiology and differential diagnoses
When first described over 30 years ago,1 ILAS was exclusively seen in East Asia, and while it remains a rare condition in other continents, its prevalence is rising. Between 1992 and 2002, >900 cases of K. pneumoniae liver abscess were reported in Asia compared with 23 elsewhere, but in the following 10 years there were 38 cases described across two series in the USA alone.2 12 Authors from Switzerland18 and Ireland6 have since published case series and our report adds to the growing number of those from the UK.13 14 19 20
Both imaging and culture techniques—to isolate the specific causative organisms—are used to delineate between malignant and infectious causes.3 Ultrasound alone is used in nearly half of cases of pyogenic liver abscess21 but in cases where the diagnosis is more difficult, MRI is regarded as the most sensitive imaging technique.22 However, even MRI cannot differentiate between pyogenic and amoebic abscesses, highlighting the need for tissue sampling.22 In addition, some infectious lesions, such as Bacteroides fragilis abscesses, can mimic malignant metastases.23 Of the infectious causes of liver abscesses, two broad categories can be used24; those caused by bacteria (including amoeba) and parasites including hydatiform cysts and cystic echinococcosis and alveolar echinococcosis, both caused by helminths.25
Risk factors and virulence
Asian ethnicity appears to be a risk factor.2 6 It has been theorised liver abscesses might occur when bacteria from the intestinal flora translocate across the intestinal epithelium into the portal system. Those of Asian descent have an increased prevalence of the virulent K. pneumoniae in faecal samples.2 14 26 Specifically, capsular polysaccharide serotypes K1 and K2 and an increased resistance to phagocytosis and intracellular killing were seen in samples from Taiwanese subjects.27 Host genetic factors may also play a role as two genes that convey resistance to Klebsiella (PHG1 and KIL1) have been identified.28
Patients with Klebsiella liver abscess are often diabetic3 27–29 and it is a comorbidity in approximately 40%–75% of reported cases of ILAS.4 6 7 10 12 13 15 30 The immunocompromise seen in diabetes is due to impairment of various components of the immune system. Specifically, patients with poorly controlled type 2 diabetes show impairment in the phagocytosis of both capsular serotypes and the chemotaxis of polymorphs.31 Also, their leucocytes have reduced bactericidal effect due to impaired antioxidant systems.30 Furthermore, increased blood glucose levels can be preferential for the replication of bacteria as well as weakening human immunity.3 4 30 A study in mice models suggested that a diabetic phenotype provides a specialised environment that facilitates dissemination of Klebsiella from the gut into the circulation as described above.32 As seen in this case, gallstone disease is also associated with biliary infections and subsequent pyogenic liver abscesses. Studies have shown this to be the case in over half on incidences and that it is associated with a more severe condition.8 9 24 33
Metastases
Metastases of infections are commonly seen with Klebsiella liver abscesses (8%–24%).2 3 Specifically, meningitis, endophthalmitis, septic pulmonary emboli and empyema have been well-described plus rarer examples such as osteomyelitis, soft-tissue abscesses and necrotising fasciitis.2 Cases of ILAS with septic metastasis have a 20-fold increased association with diabetes compared with non-invasive disease15 30 and this comorbidity worsens the prognosis for this already life-threatening syndrome, leading to a higher rate of intensive care admission and mortality.2 4
Roughly half of cases of K. pneumoniae endophthalmitis originate from a liver abscess13 and it is strongly associated with K1 and K2 serotypes.34 Diabetes is the leading risk factor and it can be devastating for patients’ vision with only 89% of patients left able to simply perceive light11 and diabetes is also associated with a poorer outcome.31
Regarding potential mechanisms of metastasis, haematogenous seeding is a possibility35 while direct spread into the chest via abscess rupture or through a hepatobronchial fistula has also been postulated.36–38 Crucially, the harmful metastatic sequalae of ILAS can be difficult to pick up early as neither biochemical markers nor physical examination findings necessarily correlate. In fact, those with metastatic infections may be less likely to develop a fever or right upper quadrant pain and tenderness; perhaps a sign of a lesser immune response due to the likely presence of diabetes as a comorbidity.38
Prognosis and management
While rare in the western world, 1.0–2.3/100 000 population,39 we believe ILAS is increasingly common, in part due to greater migration but also recognition of approximately equal incidences in both Asians and non-Asians outside Asia.2 Furthermore, this syndrome carries significant risk of morbidity and mortality; the average hospital stay is 18.7±13.1 days with 24.9% requiring intensive care and approximately 5%–11% of cases result in death, compared with 2.5% in non-invasive cases of Klebsiella liver abscess.2 4 15 Alarmingly, patients with metastatic infection often go longer before initiation of antibiotics.38 It is, therefore, urgent that the cluster of infectious foci is recognised as a syndrome that must be treated promptly and aggressively with antibiotics, with good tissue penetration.4 Cephalosporins and aminoglycosides make up the mainstay of treatment in Asia whereas in the USA, fluoroquinolones and metronidazole are often included,2 however, it is crucial to base selection on in-vitro sensitivities.
Abscess drainage is also effective and resolution of the infection can be assessed with ultrasound, as described in this case.2 Fortunately, extended spectrum beta lactamase producing K. pneumoniae are rarely seen in cases of this nature but the threat is increasing as antimicrobial resistance broadens.2 15 Regarding endophthalmitis, early identification is key, and intravitreal sampling will guide appropriate intravitreal antibiotic regimen.13 Finally, identification of ILAS may present an opportunity to pick up previously undiagnosed diabetes and good control of blood sugar levels in known patients with diabetes can prevent metastatic spread of infection.2 30
Conclusion
In conclusion, K. pneumoniae is an important cause of ILAS, a condition strongly associated with gallstones and diabetes, which has devastating effects elsewhere in the body, notably in the eye, via metastatic spread. There is growing evidence that this syndrome, once almost exclusive to East Asia is becoming more prevalent globally. Epidemiological studies are required to quantify this, meanwhile greater awareness of the condition, its relation to diabetes and poor blood sugar control, and its effective management are warranted.
Learning points.
Klebsiella pneumonia liver abscesses can metastasise and cause invasive liver abscess syndrome.
Invasive liver abscess syndrome (ILAS) is most prevalent in East Asians due to the make up of their gut commensals and genetics.
ILAS is typically seen in diabetics due to the associated immunocompromise.
Endophthalmitis is a common and important sequelae.
Prompt treatment with highly penetrant antibiotics is required.
Footnotes
Contributors: RK: planning, conduct/clinical care, interpretation of case and writing/editing. SH: planning, conduct/clinical care, acquisition of images, writing/editing. ALP: planning, conduct/clinical care, interpretation of case, reporting, conception and design, and writing/editing. MD: planning, conduct/clinical care, data and image collection, interpretation of case, reporting, conception and design, and writing/editing.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Liu YC, Cheng DL, Lin CL. Klebsiella pneumoniae liver abscess associated with septic endophthalmitis. Arch Intern Med 1986;146:1913–6. 10.1001/archinte.1986.00360220057011 [DOI] [PubMed] [Google Scholar]
- 2.Siu LK, Yeh K-M, Lin J-C. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis 2012;12:887. 10.1016/S1473-3099(12)70205-0 [DOI] [PubMed] [Google Scholar]
- 3.Qian Y, Wong C, Lai S. Klebsiella pneumoniae invasive liver abscess syndrome with purulent meningitis and septic shock : A case from mainland 2016;22:2861–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qian Y, Wong CC, Lai S, et al. A retrospective study of pyogenic liver abscess focusing on Klebsiella pneumoniae as a primary pathogen in China from 1994 to 2015. Sci Rep 2016;6:12. 10.1038/srep38587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin J-C, Yeh K-M, Chang F-Y. The distant metastasis of pyogenic liver abscess caused by Klebsiella pneumoniae serotype K2 and the underlying disease of diabetes mellitus should be carefully interpreted. Clin Infect Dis 2007;45:1531–2. 10.1086/523008 [DOI] [PubMed] [Google Scholar]
- 6.Moore R, O'Shea D, Geoghegan T, et al. Community-Acquired Klebsiella pneumoniae liver abscess: an emerging infection in Ireland and Europe. Infection 2013;41:681–6. 10.1007/s15010-013-0408-0 [DOI] [PubMed] [Google Scholar]
- 7.Xu SB, Yang SY, Weng SS, et al. [Clinical characteristics of patients with Klebsiella pneumoniae pyogenic liver abscess]. Zhonghua Nei Ke Za Zhi 2020;59:439–44. 10.3760/cma.j.cn112138-20190610-00403 [DOI] [PubMed] [Google Scholar]
- 8.Peng Y-C, Lin C-L, Sung F-C. Risk of pyogenic liver abscess and endoscopic sphincterotomy: a population-based cohort study. BMJ Open 2018;8:e018818–13. 10.1136/bmjopen-2017-018818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng S, Florescu S, Mendoza M. Klebsiella pneumoniae invasive syndrome in a diabetic patient with gallbladder abscess. Clin Case Rep 2020;8:1940–2. 10.1002/ccr3.3038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lau YJ, Hu BS, Wu WL, et al. Identification of a major cluster of Klebsiella pneumoniae isolates from patients with liver abscess in Taiwan. J Clin Microbiol 2000;38:412–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang C-S, Tsai H-Y, Sung C-S, et al. Endogenous Klebsiella endophthalmitis associated with pyogenic liver abscess. Ophthalmology 2007;114:876–80. 10.1016/j.ophtha.2006.12.035 [DOI] [PubMed] [Google Scholar]
- 12.Ko W-C, Paterson DL, Sagnimeni AJ, et al. Community-Acquired Klebsiella pneumoniae bacteremia: global differences in clinical patterns. Emerg Infect Dis 2002;8:160–6. 10.3201/eid0802.010025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soon WC, Pouncey A, Ashley E, et al. Klebsiella pneumoniae infection: a virulent cause of visual loss. Case Rep Ophthalmol 2014;5:468–73. 10.1159/000370145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdul-Hamid A, Bailey SJ. Klebsiella pneumoniae liver abscess and endophthalmitis. BMJ Case Rep 2013:2–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braiteh F, Golden MP. Cryptogenic invasive Klebsiella pneumoniae liver abscess syndrome. Int J Infect Dis 2007;11:16–22. 10.1016/j.ijid.2005.10.006 [DOI] [PubMed] [Google Scholar]
- 16.Lapinsky S, Leen J, Mak J, et al. A challenging case of non-resolving pneumonia: keeping antisynthetase syndrome in the differential diagnosis. Can Journ Gen Int Med 2019;14:25–8. 10.22374/cjgim.v14i1.278 [DOI] [Google Scholar]
- 17.Van Keer J, Van Keer K, Van Calster J, et al. More than meets the eye: Klebsiella pneumoniae invasive liver abscess syndrome presenting with endophthalmitis. J Emerg Med 2017;52:e221–3. 10.1016/j.jemermed.2017.01.043 [DOI] [PubMed] [Google Scholar]
- 18.Babouee Flury B, Donà V, Buetti N, et al. First two cases of severe multifocal infections caused by Klebsiella pneumoniae in Switzerland: characterization of an atypical non-K1/K2-serotype strain causing liver abscess and endocarditis. J Glob Antimicrob Resist 2017;10:165–70. 10.1016/j.jgar.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 19.Maybury B, Powell-Chandler A, Kumar N. Two cases of Klebsiella pneumoniae liver abscess necessitating liver resection for effective treatment. Ann R Coll Surg Engl 2015;97:e37–8. 10.1308/003588414X14055925060037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez-Villar S, Fife A, Baldwin C, et al. Antibiotic-resistant hypervirulent Klebsiella pneumoniae causing community- acquired liver abscess : an emerging disease 2019:206–11. [DOI] [PMC free article] [PubMed]
- 21.Serraino C, Elia C, Bracco C, et al. Characteristics and management of pyogenic liver abscess. Medicine 2018;97:e0628–6. 10.1097/MD.0000000000010628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anesi JA, Gluckman S. Amebic liver abscess. Clinical Liver Disease 2015;6:41–3. 10.1002/cld.488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lonardo A, Grisendi A, Pulvirenti M, et al. Right colon adenocarcinoma presenting as Bacteroides fragilis liver abscesses. J Clin Gastroenterol 1992;14:335–8. 10.1097/00004836-199206000-00013 [DOI] [PubMed] [Google Scholar]
- 24.Akhondi H, Sabih DE. Liver abscess. Treasure Island (FL, 2020. [Google Scholar]
- 25.Can H, İnceboz T, Caner A, et al. [Detection of Echinococcus granulosus and Echinococcus multilocularis in cyst samples using a novel single tube multiplex real-time polymerase chain reaction]. Mikrobiyol Bul 2016;50:266–77. 10.5578/mb.21005 [DOI] [PubMed] [Google Scholar]
- 26.Siu LK, Fung C-P, Chang F-Y, et al. Molecular typing and virulence analysis of serotype K1 Klebsiella pneumoniae strains isolated from liver abscess patients and stool samples from noninfectious subjects in Hong Kong, Singapore, and Taiwan. J Clin Microbiol 2011;49:3761–5. 10.1128/JCM.00977-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin J-C, Chang F-Y, Fung C-P, et al. High prevalence of phagocytic-resistant capsular serotypes of Klebsiella pneumoniae in liver abscess. Microbes Infect 2004;6:1191–8. 10.1016/j.micinf.2004.06.003 [DOI] [PubMed] [Google Scholar]
- 28.Benghezal M, Fauvarque M-O, Tournebize R, et al. Specific host genes required for the killing of Klebsiella bacteria by phagocytes. Cell Microbiol 2006;8:139–48. 10.1111/j.1462-5822.2005.00607.x [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Yan Y, Xue X, et al. Comparison of pyogenic liver abscesses caused by hypermucoviscous Klebsiella pneumoniae and Non-Klebsiella pneumoniae pathogens in Beijing: a retrospective analysis. J Int Med Res 2013;41:1088–97. 10.1177/0300060513487645 [DOI] [PubMed] [Google Scholar]
- 30.Lin Y-T, Wang F-D, Wu P-F, et al. Klebsiella pneumoniae liver abscess in diabetic patients: association of glycemic control with the clinical characteristics. BMC Infect Dis 2013;13:1. 10.1186/1471-2334-13-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheu S-J, Kung Y-H, Wu T-T, et al. Risk factors for endogenous endophthalmitis secondary to Klebsiella pneumoniae liver abscess: 20-year experience in southern Taiwan. Retina 2011;31:2026–31. 10.1097/IAE.0b013e31820d3f9e [DOI] [PubMed] [Google Scholar]
- 32.Lin Y-C, Lu M-C, Tang H-L, et al. Assessment of hypermucoviscosity as a virulence factor for experimental Klebsiella pneumoniae infections: comparative virulence analysis with hypermucoviscosity-negative strain. BMC Microbiol 2011;11:50 http://www.biomedcentral.com/1471-2180/11/50 10.1186/1471-2180-11-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jun JB. Klebsiella pneumoniae Liver Abscess. Infect Chemother 2018;50:210–8. 10.3947/ic.2018.50.3.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin J-C, Siu LK, Fung C-P, et al. Impaired phagocytosis of capsular serotypes K1 or K2 Klebsiella pneumoniae in type 2 diabetes mellitus patients with poor glycemic control. J Clin Endocrinol Metab 2006;91:3084–7. 10.1210/jc.2005-2749 [DOI] [PubMed] [Google Scholar]
- 35.Cheng DL, Liu YC, Yen MY, et al. Septic metastatic lesions of pyogenic liver abscess. their association with Klebsiella pneumoniae bacteremia in diabetic patients. Arch Intern Med 1991;151:1557–9. [PubMed] [Google Scholar]
- 36.Nesper M, McGahan JP. Hepatobronchial fistula with percutaneous pyogenic abscess drainage of the liver. Gastrointest Radiol 1985;10:131. 10.1007/BF01893086 [DOI] [PubMed] [Google Scholar]
- 37.Yang DM, Kim HN, Kang JH, et al. Complications of pyogenic hepatic abscess: computed tomography and clinical features. J Comput Assist Tomogr 2004;28:311–7. 10.1097/00004728-200405000-00002 [DOI] [PubMed] [Google Scholar]
- 38.Chen S-C, Lee Y-T, Lai K-C, et al. Risk factors for developing metastatic infection from pyogenic liver abscesses. Swiss Med Wkly 2006;136:119–26. doi:2006/07/smw-11341 [DOI] [PubMed] [Google Scholar]
- 39.Chong VH, Hassan H, Chong C. Rare complications of pyogenic liver abscess. Singapore Med J 2010;51. [PubMed] [Google Scholar]