Abstract
Background
Immune checkpoint inhibitors (ICIs) are being used after allogeneic hematopoietic stem cell transplantation (alloHCT) to reverse immune dysfunction. However, a major concern for the use of ICIs after alloHCT is the increased risk of graft-versus-host disease (GVHD). We analyzed the association between GVHD prophylaxis and frequency of GVHD in patients who had received ICI therapy after alloHCT.
Methods
A retrospective study was performed in 21 patients with acute myeloid leukemia (n=16) or myelodysplastic syndromes (n=5) who were treated with antiprogrammed cell death protein 1 (16 patients) or anticytotoxic T lymphocyte-associated antigen 4 (5 patients) therapy for disease relapse after alloHCT. Associations between the type of GVHD prophylaxis and incidence of GVHD were analyzed.
Results
Four patients (19%) developed acute GVHD. The incidence of acute GVHD was associated only with the type of post-transplantation GVHD prophylaxis; none of the other variables included (stem cell source, donor type, age at alloHCT, conditioning regimen and prior history of GVHD) were associated with the frequency of acute GVHD. Twelve patients received post-transplantation cyclophosphamide (PTCy) for GVHD prophylaxis. Patients who received PTCy had a significantly shorter median time to initiation of ICI therapy after alloHCT compared with patients who did not receive PTCy (median 5.1 months compared with 26.6 months). Despite early ICI therapy initiation, patients who received PTCy had a lower observed cumulative incidence of grades 2–4 acute GVHD compared with patients who did not receive PTCy (16% compared with 22%; p=0.7). After controlling for comorbidities and time from alloHCT to ICI therapy initiation, the analysis showed that PTCy was associated with a 90% reduced risk of acute GVHD (HR 0.1, 95% CI 0.02 to 0.6, p=0.01).
Conclusions
ICI therapy for relapsed acute myeloid leukemia/myelodysplastic syndromes after alloHCT may be a safe and feasible option. PTCy appears to decrease the incidence of acute GVHD in this cohort of patients.
Keywords: transplantation Immunology, immunotherapy, hematologic neoplasms
Introduction
Allogeneic hematopoietic stem cell transplantation (alloHCT) remains a curative approach for acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) owing to the antileukemic properties of T cells, which leads to a graft-versus-leukemia effect. However, up to 50% of AML/MDS patients relapse after alloHCT,1 and therapy options for these patients remain limited. Among patients with relapsed AML, the 1-year postrelapse overall survival (OS) rate is less than 20% despite subsequent therapies.2 Thus, novel therapeutic strategies for these patients are urgently needed.
AML relapse after alloHCT is mediated through several unique mechanisms. Toffalori et al demonstrated that disease relapse may occur after alloHCT owing to the ability of the AML cells to escape immune control by expressing inhibitory immune checkpoint proteins B7-1/B7-2 and programmed death ligand 1/2 (PD-L1/PD-L2), which impair antitumor immunity by inducing T cell exhaustion and downregulating the activity of cytotoxic T cells that are mediating the graft-versus-leukemia effect.3–5 Given these findings, immune checkpoint inhibitors (ICIs) are now being tested in patients who have undergone alloHCT to reverse the immune dysfunction that is driving the AML to relapse, and to augment the graft-versus-leukemia effect. However, a major concern with using ICIs after alloHCT is the increased risk of graft-versus-host disease (GVHD).
A phase I/Ib study highlighted the efficacy of cytotoxic T lymphocyte-associated antigen 4 receptor (CTLA-4) blockade following alloHCT for patients with relapsed AML.6 In that study, 28 patients with relapsed hematological malignancies after alloHCT (12 patients with AML, including 3patients with leukemia cutis) were treated with single-agent ipilimumab. Of the 22 patients who received the higher ipilimumab dose (10 mg/kg), complete responses were reported in five patients (23%), including three patients with leukemia cutis and two patients with AML. In the remaining six patients, who received the lower dose of ipilimumab (3 mg/kg), no clinical responses were reported. Immune-related adverse events (irAEs) of grades 2–4 were reported in six patients (21%), including one death, and acute GVHD (aGVHD) and chronic GVHD (cGVHD) following ipilimumab administration was reported in four patients (14%).
Programmed cell death protein 1 (PD-1) receptor blockade after alloHCT has also been tested as a therapeutic strategy. In a phase I clinical trial of nivolumab in 28 patients with relapsed hematological malignancies after alloHCT (11 patients with AML), two of the six patients who were treated with nivolumab at a dose of 1 mg/kg developed irAEs. Moreover, among patients who received nivolumab at a dose of 0.5 mg/kg, four patients had dose-limiting toxicity, including two cases of grade 3 aGVHD resulting in death. Of the 19 evaluable patients who received nivolumab at a dose of 0.5 mg/kg, the response rate was 16% (one patient with AML achieved partial response).7
Irrespective of the use of ICIs, several pretransplantation and post-transplantation factors are known to play a crucial role in inciting GVHD, including the type of GVHD prophylaxis that is used. Several reports have established the efficacy of post-transplantation cyclophosphamide (PTCy) in GVHD prophylaxis to selectively deplete alloreactive T cells after T cell-replete alloHCT,8–10 including after haploidentical alloHCT.11 12 PTCy use after alloHCT is associated with a lower incidence of grades 3–4 aGVHD and cGVHD, and some studies have even reported lower rates of GVHD in patients who underwent haploidentical alloHCT with PTCy compared with those who underwent alloHCT from matched related or matched unrelated donors using GVHD prophylaxis that omitted PTCy.9 13–16 We, therefore, hypothesize that using PTCy for GVHD prophylaxis will reduce the incidence of GVHD in patients who receive ICI therapy for AML/MDS relapse after alloHCT.
In this retrospective analysis, we describe our experience with ICI therapy after alloHCT in AML/MDS patients. We analyzed the association between GVHD prophylaxis and frequency of GVHD in this cohort of patients, and we observed reduced incidence of GVHD in patients who received PTCy for GVHD prophylaxis.
Methods
Patient selection
After institutional review board approval, MD Anderson databases were searched to identify cancer patients who had received ICI at any time between January 1 2004 and March 31 2019. All patients with transplantation claims were included in the initial search. Standard International Classification of Diseases 9 and 10 diagnostic codes were used to identify patients who may have undergone alloHCT. Medical records with at least one relevant code were reviewed in depth. We included in our final analysis AML/MDS patients who had a confirmed alloHCT prior to the initiation of at least one dose of ICI. We Identified 21 patients with either AML (n=16) or MDS (n=5) treated with ICIs after alloHCT relapse. Clinical response and disease status were defined using International Working Group recommendations. All patients provided written informed consent for their treatment and were treated in accordance with the Declaration of Helsinki. Patient demographics, transplantation characteristics, relapse and treatment details were collected, along with last follow-up and cause of death.
GVHD prophylaxis consisted of either tacrolimus and mini-methotrexate or PTCy at a dose of 50 mg/kg on days 3 and 4 after stem cell infusion, as well as tacrolimus with or without mycophenolate mofetil. GVHD was diagnosed and graded histologically, confirmed by board-certified pathologists at MD Anderson. The grade/stage of aGVHD was scored according to consensus and National Institutes of Health criteria.17 Any irAEs were graded using Common Terminology Criteria for Adverse Events (V.4.0).
Statistical analyses
Frequencies and percentages were used to summarize patient characteristics. Differences in demographic/clinical/treatment features were compared between two cohorts using the Fisher’s exact test for categorical variables and Wilcoxon rank-sum test for continuous variables. Cumulative incidence of GVHD measured from ICI therapy initiation was estimated for each cohort, and comparisons were made using the Gray test, where death and relapse were considered competing risks. The Kaplan-Meier method and corresponding log-rank test were used to compare OS and progression-free survival (PFS) between cohorts, where OS was measured from ICI initiation to death and PFS was measured from ICI therapy initiation to disease progression/relapse or death. Patients not experiencing an outcome were censored at time of last follow-up for both OS and PFS. Univariate and multivariable competing risk Cox regression modeling was used to calculate cause-specific hazard ratios of plausible risk factors for GVHD, and conventional univariate and multivariable Cox regression were used to analyze PFS and OS. For each outcome, variables with p<0.10 in the univariate analysis were considered for inclusion in multivariable modeling.
Results
Patient characteristics
Twenty-one patients with AML (n=16) or MDS (n=5) were treated with ICIs for relapse of myeloid malignancies after alloHCT. Demographics and baseline characteristics of the patients are shown in table 1. The median age at the time of transplantation was 54 years (range 29–75 years). Fourteen patients (67%) were male and seven patients (33%) were female. ICI therapy was administered in the form of PD-1 blockade or CTLA-4 blockade. Sixteen patients (76%) received PD-1 blockade (nivolumab or pembrolizumab) and five patients received CTLA-4 blockade (ipilimumab) (online supplemental table 1). The median time from transplantation to initial treatment with ICIs in the entire cohort was 9.7 months (range 1.3–134.4 months). Before initiation of ICI therapy, five patients (24%) had previously had aGVHD: one patient had grade 1 aGVHD, two patients had grade 2 and two patients had grade 3. None of the patients included in our study had previously had cGVHD. Only one patient received ICI prior to alloHCT in the form of combination ipilimumab and nivolumab.
Table 1.
Baseline characteristics of patients in our study who did and did not receive PTCy as GVHD prophylaxis
| Characteristic | No (%) | P value* | ||
| All patients, n=21 | PTCy, n=12 | No PTCy, n=9 | ||
| Median age at alloHCT (range) | 54 years (29–75 years) | 53 years (29–75 years) | 57 years (42–68 years) | 0.7 |
| Sex | 0.9 | |||
| Male | 14 (67) | 7 (58) | 7 (78) | |
| Female | 7 (33) | 5 (42) | 2 (22) | |
| Disease type | 0.9 | |||
| AML | 16 (76) | 9 (75) | 7 (78) | |
| MDS | 5 (24) | 3 (25) | 2 (22) | |
| ICI | 0.3 | |||
| Nivolumab | 16 (76) | 8 (67) | 8 (89) | |
| Ipilimumab | 5 (24) | 4 (33) | 1 (11) | |
| Median interval between alloHCT and ICI therapy initiation (range) | 9.7 months (1.3–134.4 months) | 5.1 months (1.8–57 months) | 26.6 months (5.5–134 months) | 0.04 |
| Median interval between post-alloHCT relapse and ICI therapy initiation | 2.4 months (0.1–16.4 months) | 2 months (0.2–16.4 months) | 4.7 months (0.1–14.6 months) | 0.3 |
| Hematopoietic stem cell source | ||||
| PBSCs | 14 (67) | 7 (58) | 7 (78) | 0.3 |
| BM | 7 (33) | 5 (42) | 2 (22) | |
| Donor type | ||||
| Matched related | 9 (43) | 5 (42) | 4 (44) | |
| Matched unrelated | 9 (43) | 5 (42) | 4 (44) | 1.0 |
| Haploidentical | 3 (14) | 2 (17) | 1 (12) | |
| Donor/recipient CMV status† | ||||
| R/R | 8 (40) | 3 (25) | 5 (63) | |
| R/NR or NR/R | 10 (50) | 7 (58) | 3 (38) | 0.3 |
| NR/NR | 2 (10) | 2 (17) | 0 (0) | |
| Conditioning regimen | 0.18 | |||
| Myeloablative | 15 (72) | 7 (58) | 8 (89) | |
| Non-myeloablative | 6 (28) | 5 (42) | 1 (11) | |
| Hematopoietic cell transplantation–specific comorbidity index‡ | ||||
| 0 | 3 (16) | 3 (27) | 0 (0) | |
| 1–2 | 10 (53) | 7 (64) | 3 (38) | 0.04 |
| >2 | 6 (32) | 1 (9) | 5 (63) | |
| Prior history of GVHD before ICI initiation | ||||
| None | 16 (76) | 10 (83) | 6 (67) | 0.6 |
| Acute | 5 (24) | 2 (17) | 3 (33) | |
| Chronic | 0 (0) | 0 (0) | 0 (0) | |
*P values shown in bold represent p<0.05.
†In one patient, the donor/recipient CMV status was not reported.
‡In two patients, the hematopoietic cell transplantation-specific comorbidity index was not reported.
alloHCT, allogeneic hematopoietic stem cell transplantation; AML, acute myeloid leukemia; BM, bone marrow; CMV, cytomegalovirus; GVHD, graft-versus-host disease; ICI, immune checkpoint inhibitor; MDS, myelodysplastic syndromes; NR, non-reactive; PBSCs, peripheral blood stem cells; PTCy, post-transplantation cyclophosphamide; R, reactive.
jitc-2020-001818supp001.pdf (55.8KB, pdf)
Of the 21 patients analyzed, 14 patients (67%) had received peripheral blood stem cell grafts and seven patients (33%) had received bone marrow grafts. Fifteen patients (72%) had received myeloablative conditioning regimen and six patients (28%) had received non-myeloablative regimen. Donor types included matched related in nine patients (43%), matched unrelated in nine patients (43%) and haploidentical in three patients (14%). Patients were analyzed on the basis of whether they received PTCy as GVHD prophylaxis. Post-transplantation GVHD prophylaxis consisted of tacrolimus and mini methotrexate in nine patients (43%) and PTCy and tacrolimus with (n=2) or without (n=10) mycophenolate mofetil in 12 patients (57%). Two of the three recipients of grafts from haploidentical donors (67%) received PTCy, along with five of the nine patients (56%) with grafts from matched unrelated donors and five of the nine patients (56%) with grafts from matched related donors.
Patients received a median of two doses of ICI therapy (range 1–12 doses). Four patients (19%) developed aGVHD (three of whom had previously had aGVHD). Two patients with preexisting aGVHD not requiring immunosuppressive therapy at baseline showed no exacerbation of symptoms after ICI infusion (both patients received ipilimumab). All four cases of aGVHD were seen following nivolumab therapy. Three patients had gastrointestinal GVHD, one patient with grade 2 gastrointestinal GVHD and two patients with grade 3 gastrointestinal GVHD, and one patient developed grade 2 skin GVHD. The frequency of grades 1–2 aGVHD was 9.5%, and grades 3–4 aGVHD, 9.5%, with a median time from initiation of nivolumab to onset of aGVHD of 24 days (range 12–31 days). The median number of nivolumab doses received prior to onset of aGVHD was one dose (range 1–2 doses), and the median time from alloHCT to onset of nivolumab-induced aGVHD was 188 days (range 109–419 days). None of the patients in our cohort of AML/MDS patients developed cGVHD.
All four patients who developed aGVHD were treated with corticosteroids. Two patients were able to stop steroids following resolution of their aGVHD, while the other two patients died of sepsis and disease progression. Of the four patients who received corticosteroids for treatment of aGVHD, only one patient continued receiving ICI while on steroids, and after eight doses, nivolumab was stopped due to disease progression. One patient stopped ICI on diagnosis of skin aGVHD, and the other two patients stopped nivolumab because of disease progression. Although we cannot confidently determine if corticosteroid use affected the efficacy of ICI in our cohort of AML/MDS patients, several studies have shown that the use of high doses of corticosteroids while initiating ICI therapy dampen the efficacy of ICI in patients with melanoma and non-small cell lung cancers.18 19
Two patients developed irAEs distinct from GVHD that were potentially attributable to nivolumab. After a single infusion of nivolumab, one patient developed grade 1 skin rash, and another patient developed grade 2 pneumonitis after two infusions of nivolumab. Both irAEs responded to corticosteroid therapy. No grade 3–4 irAEs were observed. Neither GVHD nor irAEs were seen in any of the patients who received ipilimumab. ICIs were discontinued owing to progressive disease (n=12), toxicity due to irAE or aGVHD (n=3), infections (n=2), physician decision (n=1), patient decision (n=1), and second alloHCT (n=1), and one patient completed five cycles.
According to univariate and multivariable analysis, the incidence of grades 2–4 aGVHD was correlated only with post-transplantation GVHD prophylaxis; none of the other variables included in the analysis (stem cell source, donor type, age at alloHCT, conditioning regimen, cytomegalovirus serostatus and prior history of GVHD) were associated with the frequency of grades 2–4 aGVHD (online supplemental table 2).
Incidence of aGVHD after PTCy
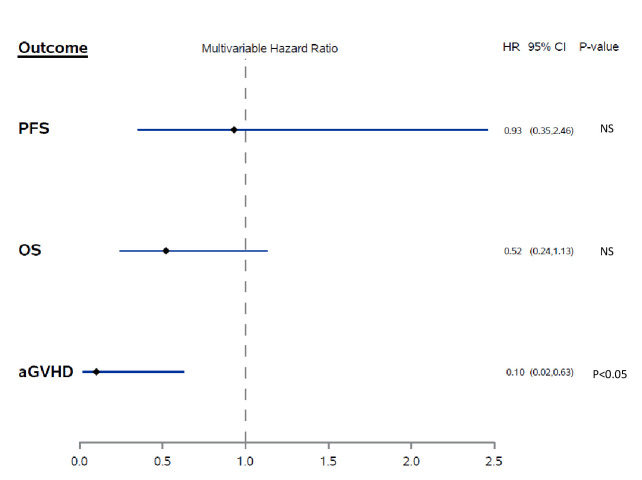
Baseline disease and alloHCT characteristics were similar between patients who received PTCy and those who did not receive PTCy, with the exception of a lower median HCT-specific Comorbidity Index (HCT-CI) (1, range 0–3) in patients who received PTCy compared with patients who did not receive PTCy (3, range 1–5; p=0.04). As expected, due to the concern that PTCy may add to the toxicity of the conditioning regimen, patients with a higher HCT-CI score were found in the non-PTCy group. Moreover, patients who received PTCy had a significantly shorter median time to initiation of ICI therapy after alloHCT compared with patients who did not receive PTCy (median 5.1 months compared with 26.6 months, p=0.04; table 1). Patients who received PTCy also had a lower observed cumulative incidence of grades 2–4 aGVHD (16%) compared with patients who did not receive PTCy (22%), although this difference was not statistically significant (p=0.7). However, after controlling for the comorbidity index and time from alloHCT to ICI therapy initiation, we found that PTCy was associated with a 90% reduced risk of aGVHD (HR 0.1, 95% CI 0.02 to 0.6, p=0.01; figure 1).
Figure 1.
Multivariable analysis of the association between post-transplantation cyclophosphamide for GVHD prophylaxis and aGVHD, progression-free survival (PFS) and overall survival (OS). aGVHD, acute graft-versus-host disease; NS, not significant.
Efficacy of ICIs
Eighteen patients were evaluable for response: the tumor response was not reported in one patient owing to early death and in two patients owing to toxicity-related discontinuation of the ICI after a single infusion. Of the 13 evaluable patients who received nivolumab, four (31%) demonstrated an objective response; three patients achieved a complete response and one patient achieved a partial response that lasted for 2 months. Of the three patients with a complete response, one patient relapsed and underwent a second alloHCT, and in the other two, the complete response lasted less than 1 month. The other nine patients had progressive disease. Of the five patients who received ipilimumab, one patient (20%) achieved a complete response that lasted for 5 months; the remaining four patients had progressive disease. The clinical responses did not differ between patients who received a PD-1 inhibitor and those who received a CTLA4 inhibitor (p=0.65). Moreover, the objective response rate did not differ between patients who developed aGVHD and those who did not (0% compared with 33%, p=0.39), and the objective response rate did not differ between patients who received PTCy and those who did not (20% compared with 37%, p=0.41). Additionally, the two patients who developed irAEs did not achieve an objective response to ICI.
During the study, 19 patients (90%) died. Thirteen patients (62%) died from relapse/progressive disease, and six patients (28%) died from other causes; three patients died due to sepsis, two patients from infection (one from COVID-19 and one from disseminated acanthamoeba infection), and one patient died due to a secondary malignancy. No death was attributed to aGVHD.
PFS and OS
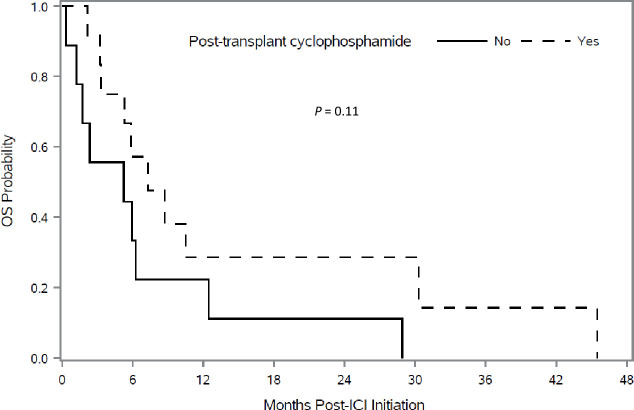
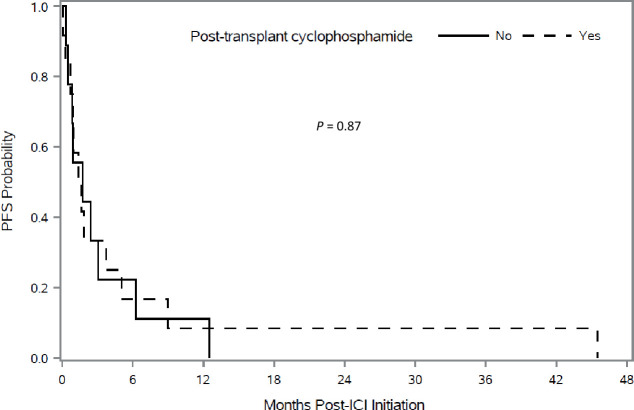
Kaplan-Meier plots showed better OS in patients who received PTCy. Figure 2 shows OS in patients according to the use of PTCy. Patients who received PTCy had a longer median OS, but this was not statistically significant (7.3 months compared with 5.2 months, p=0.11). Multivariable Cox regression analysis adjusting for hematopoietic stem-cell-specific comorbidity index, hematopoietic stem cell source and sex match/mismatch found that PTCy was associated with a nearly 50% lower risk of death with borderline statistical significance (HR 0.52, 95% CI 0.24 to 1.13, p=0.098) (figure 1). PFS according to the use of PTCy is shown in figure 3. Based on univariate analysis, the median PFS was not significantly different for patients who received PTCy versus those who did not receive PTCy (1.5 months compared with 1.7 months, p=0.87). Multivariable Cox regression analysis showed no association between PTCy and PFS (HR 0.93; 95% CI 0.35 to 2.46, p=0.88) (figure 1).
Figure 2.
Kaplan-Meir analysis of overall survival (OS) after immune checkpoint inhibitor (ICI) therapy initiation according to use of post-transplantation cyclophosphamide as graft-versus-host disease prophylaxis.
Figure 3.
Kaplan-Meir analysis of progression-free survival (PFS) after initiation of immune checkpoint inhibitor (ICI) therapy according to use of post-transplantation cyclophosphamide as graft-versus-host disease prophylaxis.
Discussion
The use of ICIs in patients with hematological malignancies is increasing. However, ICI use after alloHCT has been hampered owing to the fear of eliciting GVHD, which is unique to alloHCT recipients.20–24 A variety of pretransplantation and post-transplantation factors can play a crucial role in exacerbating GVHD, including the dose of ICIs administered, allograft donor source, conditioning intensity, prior history of GVHD and GVHD prophylaxis. We performed a retrospective analysis to report our experience with ICI use after alloHCT in AML/MDS patients treated at our institution, with a focus on the impact of PTCy GVHD prophylaxis. Our analysis shows that the use of ICIs for AML/MDS relapse after alloHCT is a safe and feasible option, and that PTCy appears to decrease the frequency of aGVHD in this patient population.
Activation of graft immunity after alloHCT through ICIs carries a risk of triggering GVHD. A few phases I clinical trials have investigated the efficacy and safety of ICIs in patients with relapsed disease after alloHCT.6 7 25 26 In a phase I/Ib trial of 28 patients with relapsed hematological malignancies, including AML/MDS (n=14), Hodgkin lymphoma (n=7), non-Hodgkin’s lymphoma (n=4), multiple myeloma (n=1), myeloproliferative neoplasm (n=1) and acute lymphoblastic leukemia (n=1), who were treated with ipilimumab (3 mg/kg or 10 mg/kg) after alloHCT, four patients (14%) developed GVHD, including three with cGVHD and one with aGVHD.6 More recently, Davids et al reported the first prospective clinical trial of nivolumab (0.5 mg/kg or 1 mg/kg) for relapsed hematological malignancies in 28 patients, including those with AML/MDS (n=17), Hodgkin lymphoma (n=5), non-Hodgkin's lymphoma (n=3), chronic lymphocytic leukemia (n=1), myeloproliferative disorder (n=1) and leukemia of unspecified type (n=1), after alloHCT. Eleven patients (39%) developed GVHD; two patients developed aGVHD (7%), eight patients (29%) developed cGVHD and one patient (4%) developed both.7 Patients enrolled in that study had undergone alloHCT ≥6 months prior to study enrolment to minimize GVHD risk, which is believed to be increased with early initiation of ICI therapy after alloHCT.27
However, because many patients with AML/MDS relapse within a few months of alloHCT and require prompt initiation of salvage therapy, it is often not feasible to wait for an extended period to initiate ICI therapy after alloHCT. Our results showed that early initiation of ICI therapy after alloHCT was safe, particularly in patients who had received PTCy GVHD prophylaxis. In our cohort, patients who received PTCy had a significantly shorter median time to initiation of ICI therapy after alloHCT compared with patients who did not receive PTCy (5.1 months compared with 26.6 months). Despite early initiation of ICI therapy in our cohort, patients who received PTCy had a lower incidence of GVHD than did patients who did not receive PTCy. Because of the small number of patients and the retrospective nature of our study, prospective studies are needed to definitively confirm this observation.
In our entire cohort, 19% of patients developed aGVHD following ICI therapy initiation, and no deaths were attributed to GVHD. Not only was the rate of aGVHD in our cohort of patients lower than what has been reported in previous studies, but also none of the patients in our cohort developed cGVHD. Although the pathology reports confirmed GVHD, we recognize that it may be difficult to distinguish GVHD from some forms irAEs. However, based on clinical data and overall clinical assessment of the patients, in addition to pathology, we strongly believe that the reported events were more likely GVHD than irAEs. Two retrospective studies reported the outcomes of PD-1 inhibition in patients with Hodgkin lymphoma after alloHCT. In the first study, which included 20 patients, aGVHD occurred in six patients (30%), including two deaths due to aGVHD.28 In the second study, which included 31 patients, aGVHD occurred in 10 patients (32%), including four deaths attributed to aGVHD.29 Consistent with our data, in the latter study, Haverkos et al made the observation that of the five patients who received PTCy GVHD prophylaxis, none developed aGVHD following initiation of nivolumab.29 More recently, Ijaz et al analyzed the use of ICIs in patients with relapsed hematological malignancies after alloHCT in a comprehensive literature review of 24 articles (13 case reports and 11 research articles).30 Of the 176 patients who received ICIs after alloHCT, 44 patients (25%) developed GVHD; 25 patients (14%) developed aGVHD and 19 patients (11%) developed cGVHD. Furthermore, the investigators reported 10 GVHD-related deaths. The authors did not report any association between GVHD prophylaxis regimen and GVHD.
Use of PTCy after HLA‐matched alloHCT has been shown to be safe and efficacious,16 and is now a standard for GVHD prophylaxis at our institution in patients deemed to be medically fit to receive PTCy. At the time of this study, patients received PTCy based on the clinical judgment of their primary stem cell transplant physician, considering their HCT-CI score, performance status and the risk of GVHD. PTCy was first used for GVHD prophylaxis in recipients of haploidentical alloHCT11 12 31 and has since been adopted in patients receiving grafts from matched related and matched unrelated donors.32–34 Recently, a randomized phase II clinical trial compared the efficacy of three GVHD prophylaxis regimens, including PTCy, in patients who had undergone alloHCT with matched related and matched unrelated donors.31 Of the three regimens compared, only the PTCy-containing regimen attenuated the risk of severe aGVHD and cGVHD. There are multiple studies that showed that PTCy does not increase relapse rate in the matched or mismatched setting, For instance, our group had previously compared PTCy to traditional GVHD prophylaxis in 7/8 mismatched unrelated donor donors and reported encouraging results in patients receiving PTCy, showing no difference in non-relapse mortality compared with patients who did not receive PTCy.35
Efficacy of PTCy in reducing GVHD was also shown in patients who received ICI therapy prior to alloHCT. In a retrospective analysis of 43 patients with AML/MDS who were treated at our institution with ICIs prior to alloHCT, Oran et al reported that patients who received PTCy had a lower incidence of grades 3–4 aGVHD than did patients who did not receive PTCy (5% compared with 22%).36 Similar findings also were reported by Schoch et al, who reported no grades 3–4 aGVHD with the use of PTCy in a series of 14 patients who received ICI therapy prior to alloHCT.37 Our data corroborate these reports and support the use of PTCy to reduce GVHD after ICI therapy. Although we recognize that the conditioning regimens used in lymphoid malignancies prior to alloHCT differ from the conditioning regimens used in myeloid malignancies, our study may be applicable primarily to patients with AML/MDS who receive myeloablative or non-myeloablative conditionings that incorporates PTCy as GVHD prophylaxis.
Several mechanisms may be mediating the decrease in aGVHD incidence following PTCy. In patients who undergo haploidentical alloHCT, a preferential increase in the frequency of regulatory T cells was reported in those who received PTCy.38 This was also validated in murine models, in which PTCy successfully restored T cell homeostasis and reduced GVHD induced by PD1−/− donor T cells, rescuing PD-1−/− regulatory T cells from apoptosis.39 The use of PTCy for GVHD prophylaxis therefore may be abrogating ICI-induced T cell-mediated GVHD by selectively abolishing alloreactive T cells, stimulating regulatory T cells and promoting long-term tolerance.39–42 Therefore, PTCy GVHD prophylaxis may increase the safety of using ICI therapy in patients who experience relapse after alloHCT.
Even with the limitations of the retrospective nature of our analysis, ours is the first study to investigate the effects of PTCy on outcomes of AML/MDS patients who receive ICI therapy for disease relapse after alloHCT. Our data suggest that irAEs associated with the use of ICI therapy after alloHCT may be lessened in patients who receive PTCy as GVHD prophylaxis. Given the small number of patients in our study, further prospective investigation with a larger number of patients is needed to determine the magnitude of the effect of PTCy on reducing aGVHD following ICI therapy.
Acknowledgments
We are grateful to Erica Goodoff from Editing Services, at The University of Texas MD Anderson Cancer Center for her valuable contributions.
Footnotes
Twitter: @chantal.saberian, @may_daher, @hsafaMD, @garciamanero, @daver_leukemia
CS and NA-W contributed equally.
AD and GA-A contributed equally.
Presented at: Presented at the 2020 Annual Meeting of the Society of Hematology Oncology in Houston, Texas, USA.
Contributors: CS collected, analyzed and interpreted the data and wrote the manuscript; SG performed statistical analysis; HR, JJ and GR collected data and edited the manuscript; NA-W, FF, MD, LW, HS, MS, MS-A, CK, AMG, MM, RM, MK, MO, FR, GR, GG-M, BO, URP, AMA, ND and RC edited the manuscript; GA-A and AD designed the research, analyzed and interpreted the data, and edited the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: AD has received research funds from Bristol Myers Squibb, Pfizer, Apexigen, Nektar Therapeutics and Idera Therapeutics. FR has received research funding and honoraria from Bristol-Myers Squibb.
Patient consent for publication: Not required.
Ethics approval: The study protocol was approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center (IRB number PA15–007).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: No data are available.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.de Lima M, Porter DL, Battiwalla M, et al. Proceedings from the National cancer Institute's second International workshop on the biology, prevention, and treatment of relapse after hematopoietic stem cell transplantation: Part III. prevention and treatment of relapse after allogeneic transplantation. Biol Blood Marrow Transplant 2014;20:4–13. 10.1016/j.bbmt.2013.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bejanyan N, Weisdorf DJ, Logan BR, et al. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: a center for international blood and marrow transplant research study. Biol Blood Marrow Transplant 2015;21:454–9. 10.1016/j.bbmt.2014.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toffalori C, Zito L, Gambacorta V, et al. Immune signature drives leukemia escape and relapse after hematopoietic cell transplantation. Nat Med 2019;25:603–11. 10.1038/s41591-019-0400-z [DOI] [PubMed] [Google Scholar]
- 4.Dermime S, Mavroudis D, Jiang YZ, et al. Immune escape from a graft-versus-leukemia effect may play a role in the relapse of myeloid leukemias following allogeneic bone marrow transplantation. Bone Marrow Transplant 1997;19:989–99. 10.1038/sj.bmt.1700778 [DOI] [PubMed] [Google Scholar]
- 5.Simonetta F, Pradier A, Bosshard C, et al. Dynamics of expression of programmed cell death protein-1 (PD-1) on T cells after allogeneic hematopoietic stem cell transplantation. Front Immunol 2019;10:1034. 10.3389/fimmu.2019.01034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davids MS, Kim HT, Bachireddy P, et al. Ipilimumab for patients with relapse after allogeneic transplantation. N Engl J Med 2016;375:143–53. 10.1056/NEJMoa1601202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davids MS, Kim HT, Costello C. A multicenter, phase I study of nivolumab for relapsed hematologic malignancies after allogeneic transplantation. Blood. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson TM, O’Donnell PV, Fuchs EJ, et al., . Haploidentical bone marrow and stem cell transplantation: experience with post-transplantation cyclophosphamide. Seminars in hematology. Elsevier, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciurea SO, Zhang M-J, Bacigalupo AA, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood 2015;126:1033–40. 10.1182/blood-2015-04-639831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasamon YL, Bolaños-Meade J, Prince GT, et al. Outcomes of nonmyeloablative HLA-haploidentical blood or marrow transplantation with high-dose post-transplantation cyclophosphamide in older adults. J Clin Oncol 2015;33:3152–61. 10.1200/JCO.2014.60.4777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Donnell PV, Luznik L, Jones RJ, et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 2002;8:377–86. 10.1053/bbmt.2002.v8.pm12171484 [DOI] [PubMed] [Google Scholar]
- 12.Luznik L, O'Donnell PV, Symons HJ, et al. HLA-Haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 2008;14:641–50. 10.1016/j.bbmt.2008.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moiseev IS, Pirogova OV, Alyanski AL, et al. Graft-Versus-Host disease prophylaxis in unrelated peripheral blood stem cell transplantation with post-transplantation cyclophosphamide, tacrolimus, and mycophenolate mofetil. Biol Blood Marrow Transplant 2016;22:1037–42. 10.1016/j.bbmt.2016.03.004 [DOI] [PubMed] [Google Scholar]
- 14.Carnevale-Schianca F, Caravelli D, Gallo S, et al. Post-Transplant cyclophosphamide and Tacrolimus-Mycophenolate mofetil combination prevents graft-versus-host disease in allogeneic peripheral blood hematopoietic cell transplantation from HLA-matched donors. Biol Blood Marrow Transplant 2017;23:459–66. 10.1016/j.bbmt.2016.12.636 [DOI] [PubMed] [Google Scholar]
- 15.Kanate AS, Mussetti A, Kharfan-Dabaja MA, et al. Reduced-intensity transplantation for lymphomas using haploidentical related donors vs HLA-matched unrelated donors. Blood 2016;127:938–47. 10.1182/blood-2015-09-671834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greco R, Lorentino F, Morelli M, et al. Posttransplantation cyclophosphamide and sirolimus for prevention of GVHD after HLA-matched PBSC transplantation. Blood 2016;128:1528–31. 10.1182/blood-2016-06-723205 [DOI] [PubMed] [Google Scholar]
- 17.Przepiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant 1995;15:825–8. [PubMed] [Google Scholar]
- 18.Faje AT, Lawrence D, Flaherty K, et al. High-Dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 2018;124:3706–14. 10.1002/cncr.31629 [DOI] [PubMed] [Google Scholar]
- 19.Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed Death-Ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol 2018;36:2872–8. 10.1200/JCO.2018.79.0006 [DOI] [PubMed] [Google Scholar]
- 20.Armand P. Immune checkpoint blockade in hematologic malignancies. Blood 2015;125:3393–400. 10.1182/blood-2015-02-567453 [DOI] [PubMed] [Google Scholar]
- 21.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med Overseas Ed 2015;373:23–34. 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med Overseas Ed 2015;373:1627–39. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med Overseas Ed 2019;381:2020–31. 10.1056/NEJMoa1910231 [DOI] [PubMed] [Google Scholar]
- 24.Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol 2019;20:1370–85. 10.1016/S1470-2045(19)30413-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bashey A, Medina B, Corringham S, et al. Ctla4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation. Blood 2009;113:1581–8. 10.1182/blood-2008-07-168468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khouri IF, Fernandez Curbelo I, Turturro F, et al. Ipilimumab plus lenalidomide after allogeneic and autologous stem cell transplantation for patients with lymphoid malignancies. Clin Cancer Res 2018;24:1011–8. 10.1158/1078-0432.CCR-17-2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herbaux C, Merryman R, Devine S, et al. Recommendations for managing PD-1 blockade in the context of allogeneic hCT in Hodgkin lymphoma: taming a necessary evil. Blood 2018;132:9–16. 10.1182/blood-2018-02-811174 [DOI] [PubMed] [Google Scholar]
- 28.Herbaux C, Gauthier J, Brice P, et al. Efficacy and tolerability of nivolumab after allogeneic transplantation for relapsed Hodgkin lymphoma. Blood 2017;129:2471–8. 10.1182/blood-2016-11-749556 [DOI] [PubMed] [Google Scholar]
- 29.Haverkos BM, Abbott D, Hamadani M, et al. Pd-1 blockade for relapsed lymphoma post-allogeneic hematopoietic cell transplant: high response rate but frequent GVHD. Blood 2017;130:221–8. 10.1182/blood-2017-01-761346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ijaz A, Khan AY, Malik SU, et al. Significant risk of graft-versus-host disease with exposure to checkpoint inhibitors before and after allogeneic transplantation. Biol Blood Marrow Transplant 2019;25:94–9. 10.1016/j.bbmt.2018.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bolaños-Meade J, Reshef R, Fraser R, et al. Three prophylaxis regimens (tacrolimus, mycophenolate mofetil, and cyclophosphamide; tacrolimus, methotrexate, and bortezomib; or tacrolimus, methotrexate, and maraviroc) versus tacrolimus and methotrexate for prevention of graft-versus-host disease with haemopoietic cell transplantation with reduced-intensity conditioning: a randomised phase 2 trial with a non-randomised contemporaneous control group (BMT ctn 1203). Lancet Haematol 2019;6:e132–43. 10.1016/S2352-3026(18)30221-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luznik L, Bolaños-Meade J, Zahurak M, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood 2010;115:3224–30. 10.1182/blood-2009-11-251595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanakry CG, O'Donnell PV, Furlong T, et al. Multi-Institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol 2014;32:3497–505. 10.1200/JCO.2013.54.0625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanakry CG, Tsai H-L, Bolaños-Meade J, et al. Single-Agent GVHD prophylaxis with posttransplantation cyclophosphamide after myeloablative, HLA-matched BMT for AML, all, and MDS. Blood 2014;124:3817–27. 10.1182/blood-2014-07-587477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehta RS, Saliba RM, Chen J, et al. Post-Transplantation cyclophosphamide versus conventional graft-versus-host disease prophylaxis in mismatched unrelated donor haematopoietic cell transplantation. Br J Haematol 2016;173:444–55. 10.1111/bjh.13977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oran B, Garcia-Manero G, Saliba RM, et al. Posttransplantation cyclophosphamide improves transplantation outcomes in patients with AML/MDS who are treated with checkpoint inhibitors. Cancer 2020;126:2193–205. 10.1002/cncr.32796 [DOI] [PubMed] [Google Scholar]
- 37.Schoch LK, Cooke KR, Wagner-Johnston ND, et al. Immune checkpoint inhibitors as a bridge to allogeneic transplantation with posttransplant cyclophosphamide. Blood Adv 2018;2:2226–9. 10.1182/bloodadvances.2018019208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberto A, Castagna L, Zanon V, et al. Role of naive-derived T memory stem cells in T-cell reconstitution following allogeneic transplantation. Blood 2015;125:2855–64. 10.1182/blood-2014-11-608406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ganguly S, Ross DB, Panoskaltsis-Mortari A, et al. Donor CD4+ Foxp3+ regulatory T cells are necessary for posttransplantation cyclophosphamide-mediated protection against GVHD in mice. Blood 2014;124:2131–41. 10.1182/blood-2013-10-525873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayumi H, Umesue M, Nomoto K. Cyclophosphamide-induced immunological tolerance: an overview. Immunobiology 1996;195:129–39. 10.1016/S0171-2985(96)80033-7 [DOI] [PubMed] [Google Scholar]
- 41.Luznik L, O'Donnell PV, Fuchs EJ, . Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Seminars in oncology. Elsevier, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cieri N, Peccatori J, Oliveira G, et al. Tracking T cell dynamics in the first month after Haplo-HSCT with post-transplant cyclophosphamide reveals a predominant contribution of memory stem T cells to the early phase of immune reconstitution. Blood 2013;122:4615. 10.1182/blood.V122.21.4615.4615 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2020-001818supp001.pdf (55.8KB, pdf)