SUMMARY
TCF1 plays a critical role in T lineage commitment and the development of αβ lineage T cells, but its role in γδ T cell development remains poorly understood. Here, we reveal a regulatory axis where T cell receptor (TCR) signaling controls TCF1 expression through an E-protein-bound regulatory element in the Tcf7 locus, and this axis regulates both γδ T lineage commitment and effector fate. Indeed, the level of TCF1 expression plays an important role in setting the threshold for γδ T lineage commitment and modulates the ability of TCR signaling to influence effector fate adoption by γδ T lineage progenitors. This finding provides mechanistic insight into how TCR-mediated repression of E proteins promotes the development of γδ T cells and their adoption of the interleukin (IL)-17-producing effector fate. IL-17-producing γδ T cells have been implicated in cancer progression and in the pathogenesis of psoriasis and multiple sclerosis.
Graphical Abstract
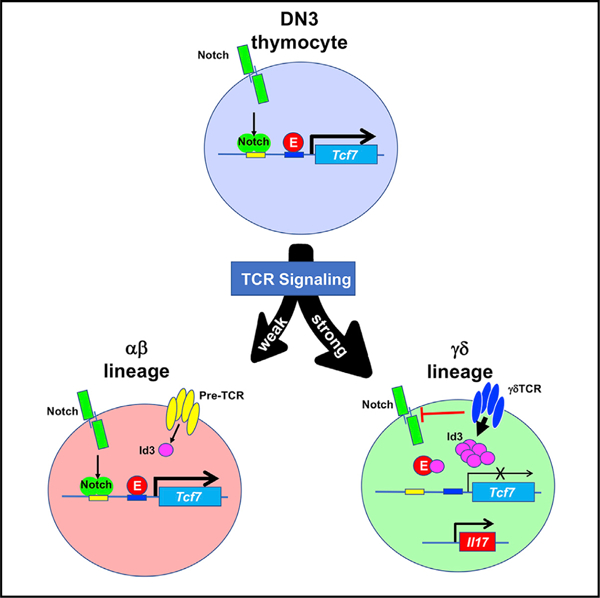
In Brief
Fahl et al. reveal a regulatory axis where T cell receptor (TCR) signaling controls TCF1 expression through an E-protein-bound regulatory element, and this regulatory axis modulates γδ lineage commitment and effector fate. This finding provides mechanistic insight into how TCR signals promote adoption of the γδ17 effector fate.
INTRODUCTION
γδ lineage T cells play a critical role in host defense that is at least partially distinct from that of αβ T cells (Chien et al., 2014; Nielsen et al., 2017; Vantourout and Hayday, 2013). Both of these T cell lineages arise from a common CD4−CD8− (double-negative; DN) progenitor pool in the thymus (Ciofani et al., 2006; Petrie et al., 1992). A critical factor instructing separation of these lineages is the nature of T cell receptor (TCR) signals experienced by immature progenitors, with weak and strong TCR signals promoting the adoption of the αβ and γδ T cell fates, respectively (Haks et al., 2005; Hayes et al., 2005). The signaling cascade that is differentially induced to specify these alternative lineage fates comprises the activation of tyrosine kinases, mitogen-activated protein kinases (MAPKs), early growth response (Egr) transcription factors (TFs), and inhibitor of DNA binding (Id) family members (Id2 and Id3) (Lauritsen et al., 2009; Ueda-Hayakawa et al., 2009; Zhang et al., 2014). The TCR-mediated induction of Id proteins then represses the activity of E box DNA-binding proteins (E proteins) in a graded manner in proportion to TCR signal strength (Bain et al., 2001; Lauritsen et al., 2009). However, the critical E protein targets differentially affected by these distinct fate-determining signals remain to be identified.
In addition to commitment to the γδ T cell lineage, the effector fate of most γδ T cells—either interferon-γ (IFN-γ) or interleukin (IL)-17 production—is also determined in the thymus (Jensen and Chien, 2009; Muñoz-Ruiz et al., 2017; Prinz et al., 2013; Vantourout and Hayday, 2013; Wiest, 2016). The molecular events controlling the specification of γδ T cell effector fate remain controversial, specifically regarding the relative importance of developmental context versus TCR signaling. While there are reports indicating that TCR-independent developmental context impacts the potential of a precursor to adopt the IL-17-producing effector fate (Haas et al., 2012; Spidale et al., 2018), there is substantial, widely accepted evidence that TCR signaling plays a key role in the intrathymic generation of functional γδ T cell effectors. Indeed, strong γδTCR signals are thought to be required for the development of IFN-γ-producing γδ T cells, while IL-17-producing fate is thought to be incompatible with strong γδTCR signals, instead requiring weak TCR signals (Fahl et al., 2018; Jensen et al., 2008; Muñoz-Ruiz et al., 2016; Sumaria et al., 2017). The signals of differing intensity that lead to the adoption of these effector fates have been linked to the TF, whose function is important for the particular effector fate. Specifically, IFN-γ producers depend on the function of Egr3, which is induced by strong TCR signals, while IL-17 producers depend on the actions of RORγt, SOX13, and c-MAF (Gray et al., 2013; Malhotra et al., 2013; Turchinovich and Hayday, 2011; Zuberbuehler et al., 2019). Nevertheless, accumulating evidence suggests that the paradigm of strong and weak TCR signals promoting the IFN-γ- and IL-17-producing effector fates, respectively, may be too simplistic. Indeed, unlike the IL-17 producers that develop in response to weak signals in the absence of ligand, the Hayday laboratory reported that some IL-17-producing γδ T cells are dependent on strong TCR signals, as evidenced by the impairment of their development by attenuation of TCR signaling (Wencker et al., 2014). RORγt and SOX13, along with Sox4, have been implicated in the development of this population of innate-like IL-17-producing γδ T cells (Gray et al., 2013; Wencker et al., 2014), which show limited capacity to produce IL-17 in response to TCR engagement but exhibit robust production of IL-17 in response to cytokine stimulation (IL-1 and IL-23) (Wencker et al., 2014). Although this report is seemingly at odds with several other studies indicating that the IL-17 fate is incompatible with strong TCR signals (Fahl et al., 2018; Jensen et al., 2008; Muñoz-Ruiz et al., 2016; Sumaria et al., 2017), two recent reports provide a potential explanation for this apparent discrepancy (In et al., 2017; Zuberbuehler et al., 2019). The Ciofani laboratory determined that the TF c-Maf is required for the development of IL-17-producing γδ T cells and determined that c-Maf induction was inversely associated with γδTCR signal strength, as defined by CD5 induction (Zuberbuehler et al., 2019); however, c-Maf is also robustly induced by ectopic expression of activated mutants of the signaling molecules protein kinase C (PKC) and Ras (Zuberbuehler et al., 2019). Ectopic expression of these activated signaling molecules could not reasonably be described as generating weak signals but might rather be regarded as producing distinct signals, compatible with c-Maf induction. Another study from the Anderson laboratory suggests that there are two distinct developmental pathways for IL-17-producing γδ T cells, and these pathways are distinguished by CD73 expression (In et al., 2017). CD73 expression, which is induced by γδTCR-ligand engagement and strong TCR signals, marks the commitment of most γδ precursors to the γδ lineage (Coffey et al., 2014; In et al., 2017); however, some IL-17-producing γδ T cells do not pass through a CD73+ stage, consistent with their adoption of the IL-17-producing effector fate in response to weaker or distinct TCR signals (In et al., 2017). Taken together, these data suggest that there are two pathways of IL-17 γδ T cell development, one that is compatible with stronger, ligand-induced γδTCR signals and another driven by a weaker, or perhaps distinct, constellation of signals.
The molecular link between the γδTCR signals that promote development of IL-17-producing γδ T cells and the TFs responsible for orchestrating the IL-17-producing differentiation program, HEB, c-Maf, Sox4, and Sox13, remains unclear (Gray et al., 2013; In et al., 2017; Malhotra et al., 2013; Turchinovich and Hayday, 2011; Zuberbuehler et al., 2019). Analysis of the molecular mechanism through which these TFs orchestrate the development of IL-17-producing γδ cells has centered primarily on their capacity to regulate expression of the critical TF, RORγt (Malhotra et al., 2013; Zuberbuehler et al., 2019). The capacity of Sox4, Sox13, and c-Maf to promote RORγt expression is antagonized by the action of TCF1 (Malhotra et al., 2013; Zuberbuehler et al., 2019). TCF1 is a high-mobility group (HMG) box TF that has been demonstrated to play an important role in the Notch-mediated commitment of thymic progenitors to the T cell fate (Germar et al., 2011; Weber et al., 2011). It does so by remodeling the epigenetic landscape and transactivating the expression of gene targets critical for the commitment and development of αβ T cell progenitors (Emmanuel et al., 2018; Goux et al., 2005; Johnson et al., 2018); however, TCF1 loss was not reported to adversely affect fetal γδ T cell development (Okamura et al., 1998). TCF1 function has been reported to antagonize the development of IL-17-producing γδ T cells (Malhotra et al., 2013). Accordingly, the development of IL-17-producing γδ T cells appears to be controlled by the antagonistic balance of the positive regulatory action of Sox14, Sox13, and c-Maf versus the negative regulation of TCF1. The mechanism by which this balance is set and influenced by TCR signaling remains a critical unanswered question.
Here, we report that TCF1 plays a critical role not only in regulating γδ T cell effector function but also in γδ T lineage commitment. Importantly, the negative regulatory effect of TCF1 is mitigated by γδTCR signals, which repress TCF1 expression during γδ T lineage commitment and maturation. TCR signals do so through a regulatory E-protein-bound element (EPE) located in the first intron of the Tcf7 locus. Collectively, these data suggest that TCR signals promote adoption of the γδ T cell fate and tip the regulatory balance in favor of the positive regulators of IL-17 effector function, do so by attenuating the expression of the antagonist TF, TCF1.
RESULTS
TCF1 expression levels are reduced during γδ T cell development
The contribution of TCF1 to separation of the αβ and γδ T cell fates has not been explored. Quantitative PCR (qPCR) analysis revealed that levels of mRNA encoding TCF1 (Tcf7) decreased as adult bipotential, γδ TCR-expressing cells (CD24+CD73−; uncommitted [Uncom.]) commit to the γδ lineage (CD24+CD73+; committed [Com.]) and mature (CD24−CD73+mature [Mat.]) (Figure 1A; Coffey et al., 2014). Similar changes in Tcf7 expression were also observed using a Tcf7 reporter (Figure 1B) (Xu et al., 2017) and by intracellular staining (Figure S1A). Tcf7 expression also declined during differentiation of fetal progenitors into committed γδ lineage cells, and this was accompanied by reduced expression of its target genes, including Bcl11b (Figure 1C). Interestingly, Tcf7 expression was not reduced in αβ lineage cells, as they traversed the β-selection checkpoint and differentiated to the CD4−CD8−CD44−CD25− DN4 stage (Figures 1D and S1A), indicating that the reduction in expression is restricted to γδ T cell development. Taken together, these data indicate that Tcf7 levels are selectively downmodulated during γδ T cell development.
Figure 1. Tcf7 expression decreases during γδ T cell development.

(A) Quantitative real-time PCR analysis of Tcf7 mRNA on electronically sorted adult Thy1+ CD4− CD8− TCRδ+ CD24+ CD73− (uncommitted [Uncom.]), CD24+ CD73+ (γδ lineage committed [Com.]), and CD24− CD73+ (mature [Mat.]) γδ T cell subsets. mRNA levels were normalized to the expression of Gapdh. Data are representative of three independent experiments. p values were calculated from triplicate measurements within each experiment. Error bars represent ± SD. *p < 0.05; **p < 0.005.
(B) γδ developmental intermediates from adult Tcf7GFP/+ mice were analyzed for GFP expression: Thy1+ CD4− CD8− TCRδ+ CD24+ CD73− (Uncom.), CD24+ CD73+ (Com.), and CD24− CD73+ (Mat.) cells.
(C) Heatmap depicting TCF1 target genes in fetal DN3, γδTCR+ CD24+ CD73− (Uncom.), and γδTCR+ CD24+ CD73+ (Com). RNA-seq was performed on fetal liver progenitor-derived Rag2−/− DN3 cells and KN6 γδTCR-transduced Rag2−/− CD24+ CD73− and CD24+ CD73+ cells (two independent replicates each). Data are reads per kilobase million (RPKM) values for each gene and are normalized to the RPKM values in DN3 cells.
(D) Thymocytes from Tcf7GFP/+ mice were analyzed for GFP expression in Thy1+ CD4− CD8− TCRδ− CD44− CD25+ CD27− (CD27− DN3), CD25+ CD27+ (CD27+ DN3), and CD25− (DN4) cells. Gray shaded histogram represents GFP expression in Thy1+ CD4− CD8− TCRδ+ thymocytes from Tcf7+/+ mice. n = 4 mice.
TCF1 deficiency markedly alters γδ T cell development
To determine whether the reduction in Tcf7 expression that occurs during γδ T cell development plays an important role in regulating γδ lineage commitment and effector fate, we analyzed development in TCF1-deficient (Tcf7−/−) mice. Indeed, TCF1 deficiency markedly altered γδ T cell development in the thymus. The total number of γδ T cells was significantly reduced in TCF1-deficient thymi, owing largely to the loss of CD24+CD73− uncommitted and CD24+CD73+ committed γδ T cell progenitors (Figures 2A and 2B). Importantly, the number of mature γδ T cells was markedly increased, relative to that in controls, in both proportion and number (Figures 2A and 2B). Similar changes in these subsets were already evident in neonates, where the total number of γδ T cells was unchanged, but there was a marked skewing toward committed and mature γδ T cells (Figures S1B–S1E). Mature γδ T cell proportion and number were also increased in adult Tcf7+/− mice, although the effect was not as pronounced as in Tcf7−/− mice (Figures 2A and 2B). The representation of particular Vγ subsets was altered by TCF1 deficiency. The Vγ1 and Vγ2 subsets were increased and decreased, respectively, while representation of the Vγ4 and Vγ5 subsets was unchanged (Figures 2C and S2A). Interestingly, the Vγ3 subset, which is generated in the first fetal wave of development, migrates to the skin and is never observed in adult thymus; however, the Vγ3+ cells were markedly increased in the thymi of TCF1-deficient adult mice, where their abundance was on par with that of the Vγ1+ and Vγ2+ subsets (Figure 2C). Vγ3 cells were depleted from the skin, raising the possibility that their presence in the thymus resulted from a failure to exit the thymus (Figures S2B and S2C). Consistent with this possibility, expression of the chemokine receptor required for skin migration, Ccr10 (Xia et al., 2010), was reduced in γδ T cells from TCF1-deficient mice (Figure S2D). The effects of TCF1 deficiency are entirely cell-autonomous, as they were reproduced upon conditional ablation of Tcf7 during fetal development of hematopoietic progenitors using Vav-Cre (Figures 2D, 2E, and S3A) (de Boer et al., 2003). Notably, when Tcf7 ablation was mediated by CD2-Cre, after progenitors have entered the thymus, the skewing toward mature (CD73+CD24−) γδ T cell populations is also evident but is not associated with loss of immature γδ T cell progenitors, indicating that their absence upon Vav-Cre or germline ablation of Tcf7 likely results from loss of TCF1 support for the early phases of T lineage commitment (Figures S3B and S3C) (Germar et al., 2011; Weber et al., 2011). Co-deletion of both members of the TCF/LEF family of transcriptional regulators, Tcf7 and Lef1, using CD2-Cre, exacerbated the skewing toward mature γδ T cell populations, suggesting that LEF1 partially compensates for TCF1 deficiency (Figures S3B and S3C). The effect of TCF1/LEF1 double deficiency was most prominent among Vγ2+ γδ T cells and those lacking both Vγ1 and Vγ2 (Figure S3C). Taken together, these data demonstrate that TCF1 deficiency alters γδ T cell development, suggesting that the expression level of TCF1 plays a critical role in this process.
Figure 2. TCF1 deficiency alters γδ T cell development.
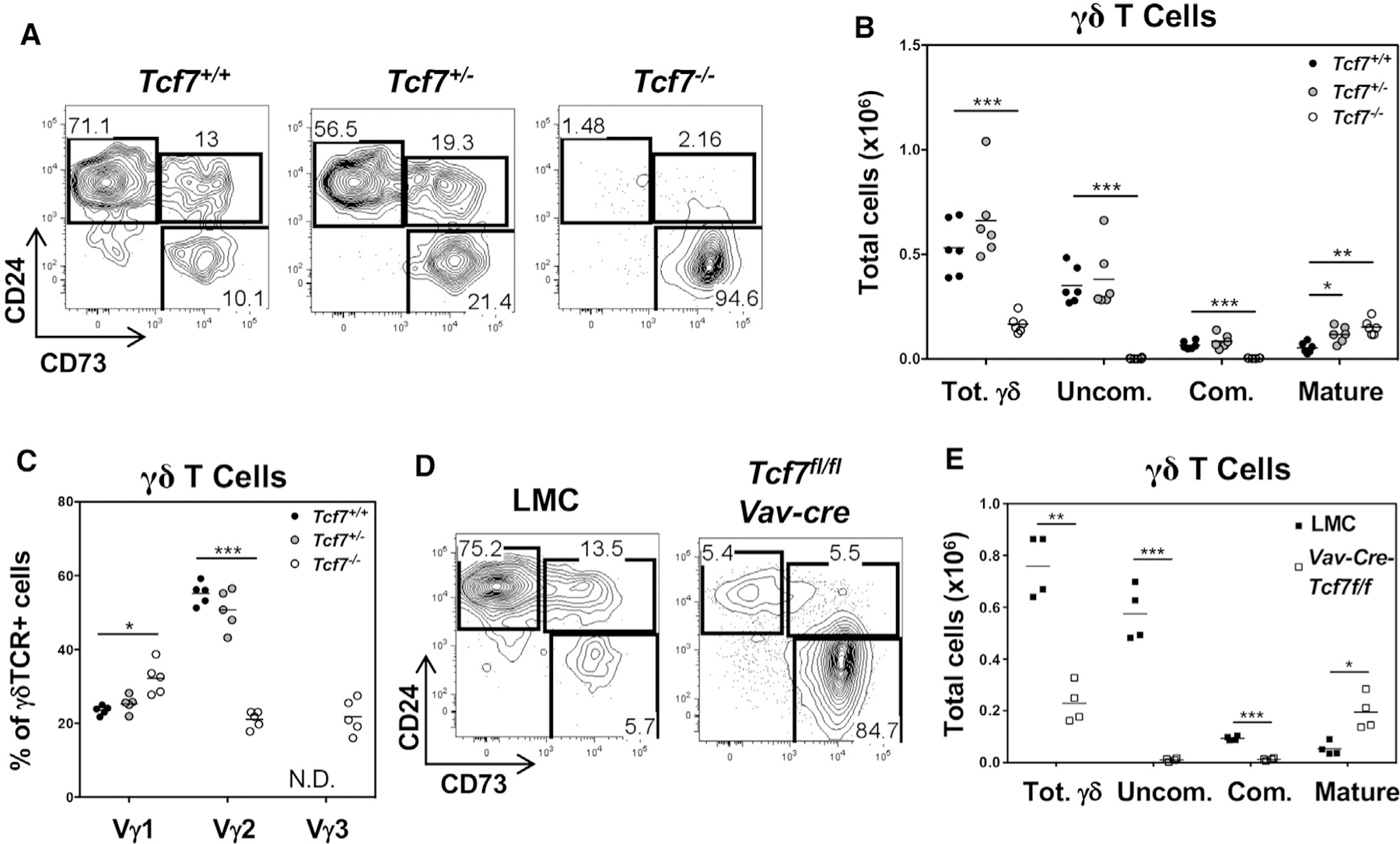
(A) Thymocytes from Tcf7+/+, Tcf7+/−, and Tcf7−/− mice were analyzed for surface expression of Thy1, CD4, CD8, TCRδ, CD24, and CD73. Flow plots depict subsets defined by CD24 and CD73 that were pre-gated on Thy1+ CD4− CD8− TCRδ+ cells.
(B) Absolute numbers of total γδ (Thy1+ CD4− CD8− TCRδ+), uncommitted γδ (Uncom.; Thy1+ CD4− CD8− TCRδ+ CD24+ CD73−), committed γδ (Com.; Thy1+ CD4− CD8− TCRδ+ CD24+ CD73+), and mature γδ (Thy1+ CD4− CD8− TCRδ+ CD24− CD73+) T cells in the thymus of Tcf7+/+, Tcf7+/−, and Tcf7−/− mice were calculated from gate frequencies.
(C) Percentage of Thy1+ CD4− CD8− TCRδ+ Vγ1+, Vγ2+, and Vγ3+ γδ T cells in the thymus of Tcf7+/+, Tcf7+/−, and Tcf7−/− mice. N.D., not detected.
(D) Thymocytes from Tcf7fl/fl Vav-cre mice and littermate controls (LMCs) were analyzed for surface expression of Thy1, CD4, CD8, TCRδ, CD24, and CD73. Flow plots displaying populations defined by CD24 and CD73 expression were pre-gated on Thy1+ CD4− CD8− TCRδ+ cells.
(E) Absolute numbers of total γδ, uncommitted γδ (Uncom.), committed γδ (Com.), and Mat. γδ T cells in the thymus of Tcf7fl/fl Vav-cre mice, and LMCs were quantified from gate frequencies and are depicted as scatterplots.
n = 6 mice (A and B), 5 mice (C), and 4 mice (D and E) per genotype. Each point represents an individual mouse. *p < 0.01; **p < 0.001; ***p < 0.0001.
TCF1 expression levels influence T cell fate
The capacity of TCF1 deficiency to cause cell-autonomous increases in committed and mature γδ lineage T cells raised the possibility that TCF1 might regulate γδ lineage commitment. To test this possibility, we used the KN6 γδTCR transgenic (Tg) mouse model, where fate specification can be controlled by manipulating access to ligand (Pereira et al., 1992). When KN6 γδ T lineage progenitors encounter the H2-T22d ligand, they adopt the γδ fate and mature into IFN-γ-producing γδ T cells; however, upon removal of ligand, this same γδTCR redirects progenitors to adopt the αβ T cell fate and differentiate to the CD4+CD8+ double-positive (DP) stage (Fahl et al., 2018). Consequently, we sought to determine whether short hairpin RNA (shRNA)-mediated knockdown of TCF1 expression enhanced the capacity of γδ T lineage progenitors to adopt the γδ fate, even under suboptimal TCR signaling conditions, produced by limiting access to ligand. Indeed, KN6 γδTCR-expressing progenitors that had been transduced with Tcf7 shRNA displayed a profound increase in the proportion of progenitors that committed to the γδ lineage (CD24+CD73+ and CD24−CD73+) relative to control (Figures 3A, S4A, and S4B). However, because TCF1 knockdown impairs the development of early progenitors, the absolute number of committed γδ T lineage progenitors was not increased (data not shown). To eliminate the effect of TCF1 loss on early T lineage progenitors, we used pTα-iCre-mediated ablation of Tcf7, since this occurs after the initial steps of T lineage commitment (Luche et al., 2013). Interestingly, pTα-iCre-mediated marking of progenitors using a lox-stop-lox ZsGreen (LSL-ZsG) allele revealed few CD73+, committed γδ T lineage T cells; however, pTα-iCre-mediated ablation of Tcf7 markedly increased both the proportion and number of committed and mature γδ T lineage T cells, while simultaneously reducing the number of αβ-lineage CD4+CD8+ DP thymocytes (Figures 3B and 3C). The increase in committed and mature γδ T lineage T cells was not associated with increased proliferation (Figure S4C). This suggests that, in addition to its role in facilitating T lineage commitment, TCF1 also influences the subsequent step of αβ/γδ lineage commitment. To directly test this in vivo, we used pTα-iCre ablation of Tcf7 in the KN6 model to determine whether TCF1 loss promoted γδ lineage commitment even when TCR signaling was severely attenuated by the absence of ligand. Indeed, TCF1 deficiency increased the proportion and number of KN6 Tg T lineage progenitors that induced CD73 expression and adopted the γδ T lineage, despite being deprived of TCR ligand stimulation (Lig−; Figure 3D). Taken together, these data suggest that, in addition to its ability to promote T lineage commitment, TCF1 also plays a subsequent role in the separation of the αβ and γδ T lineages, with reduced TCF1 expression facilitating adoption of the γδ T cell fate.
Figure 3. TCF1 insufficiency alters γδ T cell selection.

(A) KN6 γδTCR+ Rag2−/− fetal liver progenitors were transduced with MLS and MLS-shTcf7 and cultured for 5 days on OP9-DL1 monolayers lacking the KN6 TCR ligand (Lig-), in the presence of IL-7 and Flt3L. Electronically gated Thy1+ GFP+ CD4− CD8− cells, stained as indicated, are depicted (left panels). Graphs represent the proportion of CD4− CD8− CD24+ CD73+ (γδCom.) and CD4− CD8− CD24− CD73+ (γδ Mat.) cells within the Thy1+ population. Data are representative of three independent experiments. p values were calculated for duplicate measurements within each experiment. Error bars represent ± SD. *p < 0.01; **p < 0.005.
(B and C) Thymocytes from pTa-iCre Tcf7+/+ LSL-ZsG+ and pTa-iCre Tcf7f/f LSL-ZsG+ mice were stained as described above, and electronically gated ZsG+ CD4−CD8−TCRδ+ (B) or ZsG+ (C) cells are displayed. Scatterplots representing the absolute number of αβ lineage CD4+CD8+ (DP), CD4− CD8− TCRδ+ (Total γδ), CD4− CD8− TCRδ+ CD24+ CD73− (γδUncom.), CD4− CD8− TCRδ+ CD24+ CD73+ (γδ Com.), and CD4− CD8− CD4− CD8− TCRδ+ CD24− CD73+ (γδ Mat.) cells within the Thy1+ population were calculated based on gate frequencies. Each symbol represents an individual mouse (filled squares, Cre+Tcf7+/+; open squares, Cre+Tcf7f/f). *p = 0.1; **p = 0.004; ***p = 0.003; and ****p = 0.0001.
(D) Thymocytes from KN6 Tg+ H2t−/− Tcf7fl/fl and KN6 Tg+ H2t−/− pTa-iCre Tcf7f/f mice were analyzed, and data were presented as in (C). *p < 0.05; **p < 0.005.
TCF1 deficiency disrupts the specification of effector fate
TCF1 deficiency has previously been implicated in the regulation of γδ T cell effector fate in conjunction with signals involving the Wnt/β-catenin pathway (Malhotra et al., 2013). To investigate this possibility, we evaluated the impact of TCF1 deficiency on the generation of the CD27/NK1.1 phenotypes of γδ T cells linked to distinct effector fates (Ribot et al., 2009). We found that TCF1 deficiency markedly diminished the CD27+ IFN-γ-producing effectors while markedly increasing the CD27− subset linked to IL-17 production (Figures 4A and 4B). Indeed, TCF1 deficiency caused a striking increase in IL-17-producing γδ T cells (Figures 4C and 4D), which was recapitulated by conditional ablation of Tcf7 in hematopoietic cells, indicating that the effect is cell autonomous (Figures S5A and S5B). Importantly, the increase in IL-17 producers occurred regardless of Vγ usage, since, in addition to being observed among the Vγ2 subset—which exhibits a modest IL-17 bias in normal mice—it was also observed in the Vγ1 and Vγ3 subsets, which typically exhibit an IFN-γ bias (Figures S5C and S5D). Moreover, TCF1 deficiency was capable of markedly enhancing the generation of IL-17-producing γδ T cells in the KN6 γδTCR Tg model, where ligand-engagement normally favors adoption of the IFN-γ-producing effector fate (Figure 4E) (Fahl et al., 2018). It should be noted that pTa-iCre-mediated ablation of Tcf7 in this model did not diminish the frequency or number of IFN-γ-producing γδ cells, suggesting that Tcf7 ablation after the DN1d stage, which has enhanced IL-17-producing fate potential, limits the capacity of Tcf7 ablation to alter effector fate (Spidale et al., 2018). Together, these data indicate that TCF1 loss markedly enhances the development of IL-17-producing γδ T cells. In doing so, it is able to supersede TCR signals that promote development of other effector fates, but its capacity to do so is limited by developmental context.
Figure 4. TCF1 deficiency alters γδ T cell effector function.

(A) Thymocytes from Tcf7+/+, Tcf7+/−, and Tcf7−/− mice were analyzed for surface expression of Thy1, CD4, CD8, TCRδ, CD27, and NK1.1. Flow plots are pre-gated on Thy1+ CD4− CD8− TCRδ+ cells.
(B) Absolute numbers of Thy1+ CD4− CD8− TCRδ+ CD27+NK1.1−, Thy1+ CD4− CD8− TCRδ+ CD27−NK1.1−, and Thy1+ CD4− CD8− TCRδ+ CD27+NK1.1+ γδ T cells in the thymus of Tcf7+/+, Tcf7+/−, and Tcf7−/− mice were derived from gate frequencies and are represented as a scatterplot.
(C and D) Thymocytes from Tcf7+/+, Tcf7+/−, and Tcf7−/− mice were depleted of CD4+ and CD8+ cells and stimulated with PMA and ionomycin at 37°C for 4 h. Cells were analyzed by intracellular staining for expression of IL-17 and IFNγ. Flow plots were pre-gated on CD4− CD8− TCRδ+ cells. The frequency of cytokine-producing cells was derived from gate frequencies and is represented as a scatterplot.
(E) Thymocytes from KN6 Tg ligand-expressing (Lig+) mice were crossed to pTa-iCre Tcf7f/f mice, stimulated by PMA and ionomycin, and analyzed for cytokine production as in (C) and (D).
n = 6 mice (A and B), 5 mice (C and D), or 6 mice (E) per genotype. Each point represents an individual mouse. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
The function of TCF1 in regulating the generation of IL-17-producing γδ T cells was previously suggested to be mediated by Wnt signaling through the activation of the β/γ-catenin cofactors (Malhotra et al., 2013). To assess the role of β/γ-catenin function in regulating γδ T cell development and effector fate, we examined γδ T cell development in Tcf7 mutant mice (p45−/−) lacking the β/γ-catenin interacting domain (Xu et al., 2017). Interestingly, the p45−/− mutant mice did not display the profound alternations in γδ T cell development observed in TCF1-deficient mice (Figure 5), suggesting that β/γ-catenin interaction by TCF1—and, by extension, Wnt signaling—is not absolutely essential. However, the p45−/− mutant mice did exhibit a 2-fold reduction in γδ T cell numbers and an increase in the representation and number of mature γδ T cells (Figures 5A and 5B). There was also an increase in IL-17-producing γδ T cells (Figures 5C–5F). Vγ usage was altered in that the Vγ1 subset was increased while the Vγ2 subset was decreased, as was observed in the TCF1-deficient mice (Figure S6A); however, Vγ3+ cells were not observed in the thymus (Figure S6A). Finally, analysis of competitive reconstitution of γδ T cell development by β/γ-catenin double-deficient mice revealed no gross alterations in γδ T cells (Figure S6B). While our analysis of the p45 mutant mice suggests that the β/γ-catenin interacting domain of TCF1 may play some role in γδ T cell development, the expression level of the p45 TCF1 isoform is reduced (Xu et al., 2017), raising the possibility that the observed effects are due to reduced protein levels and not the loss of the β/γ-catenin binding capacity of TCF1 (Xu et al., 2017).
Figure 5. The catenin-interacting domain of TCF1 is not essential to support γδ T cell development and function.
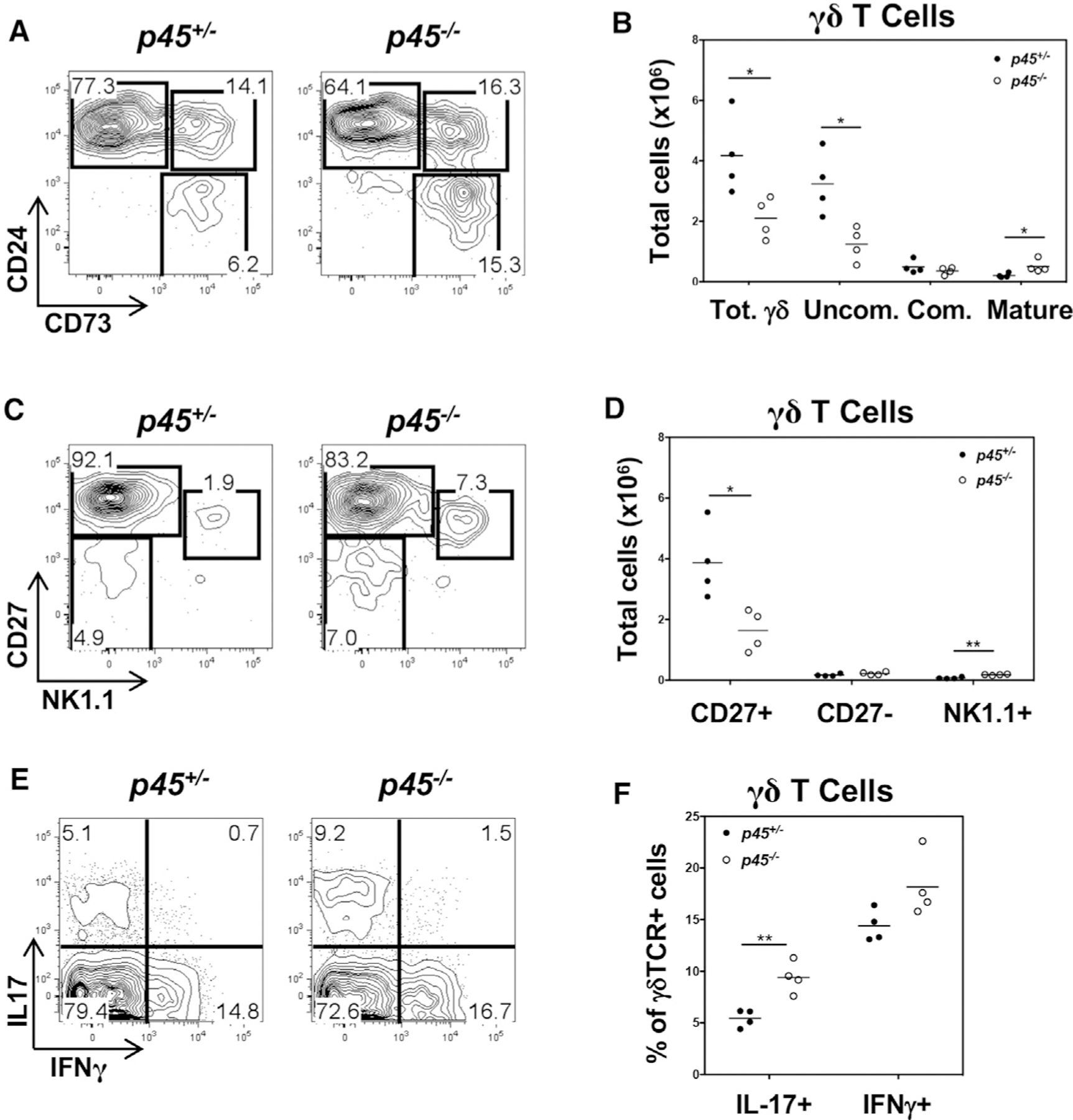
(A) Thymocytes from p45+/− and p45−/− mice were analyzed for surface expression of Thy1, CD4, CD8, TCRδ, CD24, and CD73. Flow plots are pre-gated on Thy1+ CD4− CD8− TCRδ+ cells.
(B) Absolute numbers of total γδ (Thy1+ CD4− CD8− TCRδ+), γδ-uncommitted (γδ-Uncom.; Thy1+ CD4− CD8− TCRδ+ CD24+ CD73−), γδ committed (γδ Com.; Thy1+ CD4− CD8− TCRδ+ CD24+ CD73+), and γδ Mat. (Thy1+ CD4− CD8− TCRδ+ CD24− CD73+) T cells in the thymus of p45+/− and p45−/− mice were calculated using gate frequencies and are depicted in a scatterplot.
(C) Thymocytes from p45+/− and p45−/− mice were analyzed for surface expression of Thy1, CD4, CD8, TCRδ, CD27, and NK1.1. Flow plots are pre-gated on Thy1+ CD4− CD8− TCRδ+ cells.
(D) Absolute numbers of Thy1+ CD4− CD8− TCRδ+ CD27+ NK1.1−, Thy1+ CD4− CD8− TCRδ+ CD27− NK1.1−, and Thy1+ CD4− CD8− TCRδ+ CD27− NK1.1+ γδ T cells in the thymus of p45+/− and p45−/− mice were determined and are depicted as above.
(E and F) Thymocytes from p45+/− and p45−/− mice were depleted of CD4+ and CD8+ cells and stimulated with PMA and ionomycin at 37°C for 4 h. Cells were analyzed for IL-17 and IFN-γ production by intracellular staining. Flow plots are pre-gated on CD4− CD8− TCRδ+ cells, and cytokine-producing cells were quantified and are depicted as above.
n = 4 mice per genotype. Each point represents an individual mouse. *p < 0.05; **p < 0.01.
E protein binding regulates Tcf7 expression
Collectively, our data indicate that the decline in Tcf7 expression during γδ development plays an important role in γδ T lineage commitment and specification of effector fate. Because the suppression of E-box DNA binding protein activity by TCR signaling has been demonstrated to play an important role in γδ T cell lineage commitment and maturation (Lauritsen et al., 2009; Ueda-Hayakawa et al., 2009; Verykokakis et al., 2010), we wished to determine whether the repression of E protein activity was responsible for the decline in Tcf7 expression. To investigate this possibility, we performed chromatin immunoprecipitation sequencing (ChIP-seq) analysis to identify the genomic sites of E protein binding. This analysis revealed an element in intron 1 (EPE) of the Tcf7 locus in that is bound by both E2A and HEB in DN3 thymocytes (Figure 6A). Importantly, that binding was lost, as progenitors express the γδTCR (CD73−) and commit to the γδ fate (CD73+) (Figure 6A). Moreover, the repression of E protein function in TCR-deficient Rag2−/− fetal precursors by the E protein antagonist Id3 was sufficient to both repress E protein binding and Tcf7 expression (Figures 6B and 6C).
Figure 6. TCF1 expression is regulated by E proteins in response to γδTCR signaling.
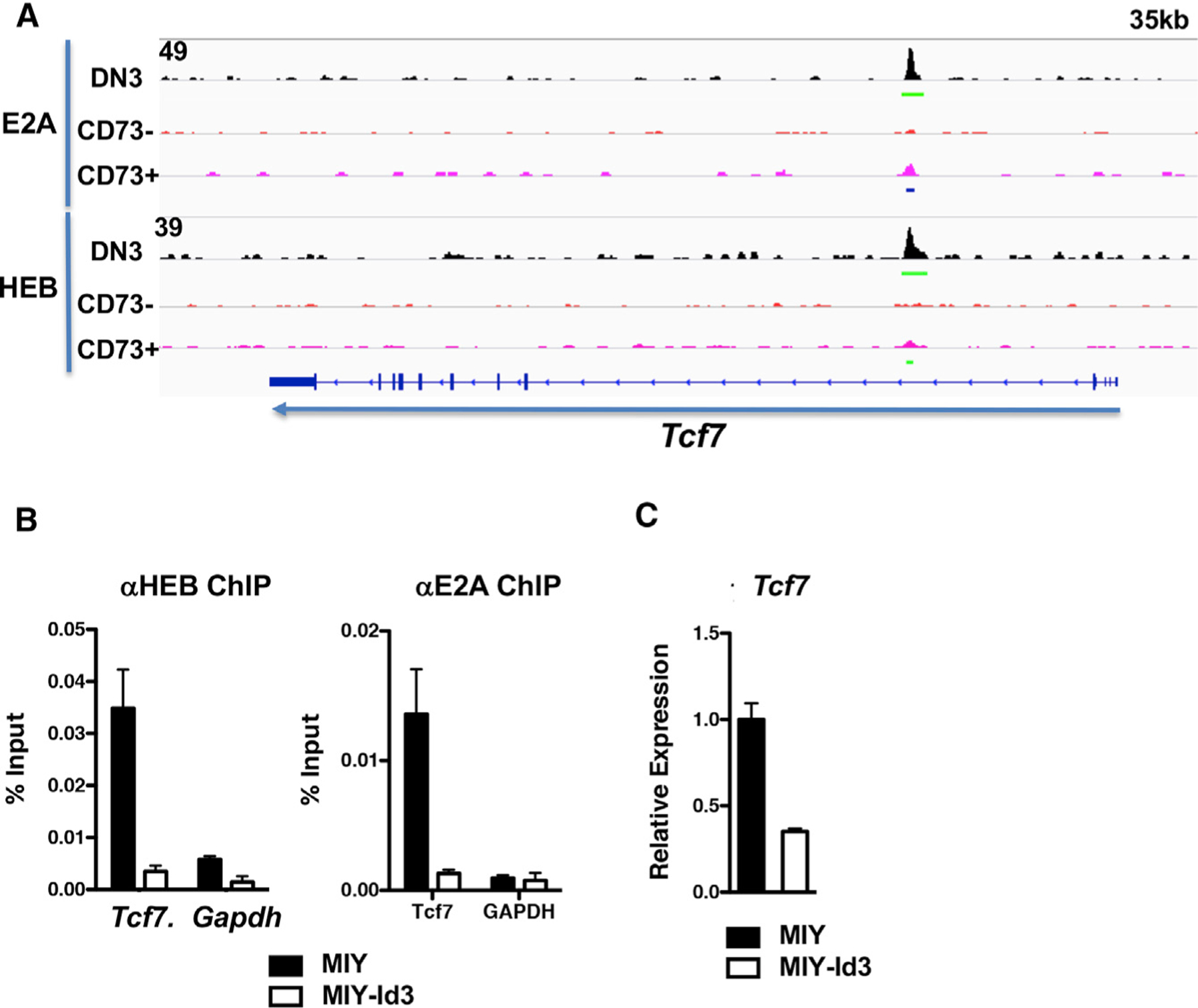
(A) ChIP-seq analysis of E2A and HEB binding within the Tcf7 locus in fetal liver progenitor-derived Rag2−/− DN3 cells and KN6 γδTCR-transduced Rag2−/− CD24+ CD73− and CD24+ CD73+ cells. Data are representative of two independent experiments.
(B) ChIP-qPCR of E2A and HEB binding within the Tcf7 and Gapdh loci was performed on fetal liver progenitor-derived Rag2−/− DN3 cells transduced with MIY or MIY-Id3 and cultured on OP9-DL1 monolayers in the presence of IL-7 and Flt3L at 37°C for 3 days.
(C) Quantitative real-time PCR analysis for Tcf7 mRNA was performed on the samples in (B). mRNA levels were normalized to the expression of Gapdh. Data are representative of three independent experiments. Error bars represent ± SD of triplicate measurements within each experiment.
To determine whether the EPE plays an important role in the regulation of Tcf7 expression and γδ T cell development, we used CRISPR-based mutagenesis to delete a 500-bp fragment encompassing the E protein sites in intron 1 (EPE; Figure 7A). Importantly, ablation of the EPE fragment reduced TCF1 expression in developing γδ, but not αβ, lineage progenitors (Figures 7B and S7A). EPE ablation did not affect mouse viability, thymic cellularity, or αβ T cell development (Figure S7B); however, the ablation of EPE did alter γδ T cell development. Indeed, ablation of the EPE increased the proportion and number of mature γδ T cells and skewed their effector function, as it increased the proportion and number of IL-17-producing γδ T cells (Figure 7C). The skewing toward IL-17 production was associated with an increase in the proportion and number of CD27− γδ T cells (Figure S7C). The number of IFN-γ-producing γδ T cells was not altered in ΔEPE mice (Figure S7C). The effect on γδ T cell development and specification of effector fate was cell autonomous, as it was recapitulated in fetal liver chimeras (Figure S7D). Together, these observations link the decline in Tcf7 expression that accompanies γδ T commitment and maturation to a critical regulatory EPE in the Tcf7 locus (Figure 7D).
Figure 7. Ablation of an E-protein-binding regulatory element in the Tcf7 locus diminishes Tcf7 expression and alters γδ T cell development.

(A) Schematic of E protein binding sites within the regulatory E-protein-bound element (EPE) in the Tcf7 locus. E box sites are underlined.
(B) Quantitative real-time PCR analysis for Tcf7 mRNA in electronically sorted Thy1+ CD4− CD8− TCRδ+ CD24+ CD73− (Uncom.), CD24+ CD73+ (Com.), and CD24− CD73+ (Mat.) γδ T cell subsets from C57BL/6 and ΔEPE mice. mRNA levels were normalized to Gapdh. Data are representative of 3 independent experiments. Error bars represent ± SD of triplicate measurements within each experiment.
(C) Top panel: thymocytes from C57BL/6 and ΔEPE mice were analyzed for surface expression of Thy1, CD4, CD8, TCRδ, CD24, and CD73. Flow plots are pre-gated on Thy1+ CD4− CD8− TCRδ+ cells. Bottom panel: thymocytes from C57BL/6 and ΔEPE mice were depleted of CD4+ and CD8+ cells and stimulated with PMA and ionomycin at 37°C for 4 h. Cells were analyzed for cytokine production by intracellular staining for IL-17. Flow plots are pre-gated on CD4− CD8− TCRδ+ cells, and the indicated populations were quantified as in Figure 5. n = 10 mice (C57BL/6) and 11 mice (ΔEPE). Each point represents an individual mouse. *p < 0.05; **p < 0.01; ***p < 0.005.
(D) Schematic depicting a model of the influence of strong TCR signaling on E protein function, TCF expression, and the function of TFs that orchestrate the CD73+ IL-17-producing effector fate. CoF, a potential cofactor other than β/γ-catenin that may associate with TCF1 through its catenin-binding domain.
DISCUSSION
TCF1 had previously been shown to play a critical role in Notch-mediated specification of the T cell fate in the thymus (Germar et al., 2011; Weber et al., 2011). Moreover, TCF1 plays a critical role in supporting αβ T cell development, which is severely attenuated by TCF1 deficiency (Goux et al., 2005; Okamura et al., 1998); however, the role of TCF1 in γδ T cell development is much less well understood. We report here that although TCF1 expression is maintained in developing αβ T cells, TCF1 significantly declines as T cell progenitors commit to the γδ fate and mature. The decline in TCF1 expression influences both γδ lineage commitment and effector fate. Indeed, genetic attenuation of TCF1 expression promotes adoption of the γδ fate and maturation of γδ T cells. We note that this occurs even in response to suboptimal γδTCR signals. TCF1 deficiency also radically alters γδ effector fate, converting essentially all γδ cells into IL-17-producing effectors, regardless of Vγ gene usage; however, this is somewhat mitigated after the receipt of strong TCR signals that usually promote IFN-γ production and when TCF1 loss is induced later in development (Sumaria et al., 2017). These findings indicate that the capacity of TCF1 loss to promote the IL-17-producing effector fate, even in the context of strong TCR signals, is more constrained in cells that have developed beyond the DN1d stage, a subpopulation of cells with increased IL-17 precursor potential (Spidale et al., 2018). Finally, we have identified a novel E-protein-bound regulatory element that selectively controls TCF1 expression in γδ, but not αβ, T cell progenitors. Specifically, we determined that TCF1 expression declines during γδ development, because of the suppression of E protein activity, presumably in response to TCR-induced expression of the Id family of E protein antagonists, and this is regulated by the EPE in the first intron of the Tcf7 locus.
It is well established that TCF1 expression is induced by Notch signaling in T cell precursors and plays a critical role as a molecular effector of Notch-mediated orchestration of T lineage commitment (Mercer et al., 2011; Weber et al., 2011). We find that the elevated levels of TCF1 are maintained during the development of αβ T cells, consistent with the marked impairment of αβ T cell development caused by TCF1 deficiency (Okamura et al., 1998; Verbeek et al., 1995). By contrast, we found that expression of TCF1 declines during γδ commitment. γδ T cell development is less dependent upon Notch signaling (Ciofani et al., 2006), and Notch expression is reduced during γδ T cell development (Lauritsen et al., 2009). Consequently, it was possible that the attenuation of Notch expression was contributing to the decrease in TCF1 expression observed in developing γδ cells. Importantly, we have previously demonstrated that the Notch independence of γδ T cell development is dependent upon the induction of the E protein antagonist Id3 by strong/prolonged signals transduced by the γδTCR (Lauritsen et al., 2009). This suggested that Id3-dependent antagonism of E protein function might be important in regulating TCF1 expression during γδ T cell development. Indeed, we detected E protein binding to a cluster of E protein binding sites in the first intron of the Tcf7 locus and determined that binding by E2A and HEB declined during γδ lineage commitment. E2A binding to this site has also been observed in naive CD8 T cells (Masson et al., 2013). Importantly, genetic ablation of the regulatory EPE containing this cluster of E protein binding sites was sufficient to phenocopy some of the developmental alterations caused by TCF1 deficiency. Specifically, ablation of this element caused a cell-autonomous increase in the generation of mature γδ cells and skewed them to the IL-17-producing effector fate, albeit to a lesser extent than was observed in TCF1-deficient mice. It is worth noting that we also observed additional E2A/HEB binding peaks 5′ to the Tcf7 locus that are also lost during γδ T cell development (data not shown). This suggests either that these E-protein-bound elements do not play a critical role in maintaining TCF1 expression in developing γδ T cells or that loss of the EPE disables the capacity of E proteins to regulate TCF1 expression through those other elements. A recent report revealed that an element nearby one of those E protein peaks plays an important role in supporting TCF1 expression in developing αβ lineage progenitors and innate lymphoid cells (Harly et al., 2020), raising the possibility that different elements are used to support TCF1 expression in different cell types.
Although the decline in TCF1 expression that accompanies γδ T cell development plays an important role in separation of the αβ and γδ T cell lineages, the basis by which TCF1 does so remains unclear. TCF1 has recently been shown to be capable of initiating the epigenetic identity of T cells (Johnson et al., 2018). Moreover, TCF1 function is known to be regulated by Wnt signaling through association with β-catenin (Staal and Sen, 2008), and its loss in the thymus has previously been linked to skewing of γδ function to the IL-17-producing effector fate (Malhotra et al., 2013), implicating Wnt signaling as an important environmental cue that influences γδ fate and function. Consistent with this notion, mutant mice in which TCF1 lacks the β-catenin-interacting domain did manifest some of the developmental anomalies associated with TCF1 deficiency, including enhanced γδ maturation and IL-17 production. However, because the p45 mutant TCF1 isoform that cannot bind β/γ-catenin is expressed at reduced levels, the alterations in γδ T cell development observed in these mice may result from reduced expression rather than the inability to bind catenins (Xu et al., 2017). This would be consistent with the absence of obvious alterations in γδ T cell development in mice doubly deficient for β- and γ-catenin. Taken together, these data suggest that Wnt signaling is unlikely to play a significant role in modulating TCF1 function in developing T cells. Nevertheless, it remains possible that, if the catenin binding domain of TCF1 does play a role, it probably does so through interaction with a distinct partner that influences TCF1 function in developing T cells, as a number of other potential partners have been identified (Figure 7D) (Steinke et al., 2014).
It is well established that commitment of bipotent DN T cell progenitors to the αβ or γδ fate is predominantly determined by the nature of the TCR signals transduced, with weak and strong signals favoring the αβ and γδ fates, respectively (Haks et al., 2005; Hayes et al., 2005). We found that TCF1 loss facilitated the adoption of the γδ fate, even in response to severely attenuated TCR signals. Consequently, it is likely that declining TCF1 expression during γδ development influences γδ fate specification by modulating either the nature of the TCR signal or its effect on gene expression. Although TCF1 has not been implicated in the regulation of TCR signaling itself, it has been implicated in regulating the expression of genes that are selectively required for the development of αβ lineage cells (Mercer et al., 2011; Weber et al., 2011). In particular, Bcl11b is a TCF1 target whose expression is markedly diminished during γδ development and whose function is predominantly required for αβ T cell development, since Bcl11b deficiency markedly attenuates αβ T cell development but is dispensable for most γδ T cells (Ikawa et al., 2010; Li et al., 2010a, 2010b). The loss of BCL11B and other TCF1 targets during γδ development presumably contributes to the loss of αβ fate potential by CD73+ γδ lineage cells (Coffey et al., 2014). Nevertheless, the loss of αβ fate potential alone does not explain the capacity of progenitors to adopt the γδ fate in response to suboptimal TCR signals and suggests that the remodeling of expression of other TCF1 targets also decreases the threshold to adoption of the γδ fate. The TCF1 targets responsible for this change in threshold remain to be identified.
Finally, we also contend that our findings provide a potential explanation for the observation that the development of CD73+ IL-17-producing γδ T cells does not depend upon the E protein HEB, which supports expression of critical IL-17-promoting targets Sox4, Sox13, and RORγt (Malhotra et al., 2013; Wencker et al., 2014; In et al., 2017) (Figure 7D). Indeed, TCF1 has been demonstrated to be an antagonist of the HMG box family members Sox4 and Sox13, which promote the IL-17-producing γδ effector fate (Malhotra et al., 2013). Consequently, we advance a model in which the strong TCR signals that promote adoption of CD73+ IL-17-producing γδ T cells do so by limiting the capacity of TCF1 to antagonize Sox4 and Sox13 function. This is mediated by blocking E protein binding to the Tcf7 locus and reducing TCF1 expression. The reduced level of TCF1 is sub-stoichiometric to that required to antagonize the capacity of even the lower levels of Sox4 and Sox13 to promote adoption of CD73+ IL-17-producing γδ T cells.
In summary, our study reveals a critical role for the expression level of TCF1 in influencing the αβ versus γδ lineage choice by T lineage progenitors in the thymus. Moreover, we reveal new insights into how TCF1 expression and function are controlled. Although the function of TCF1 is most often regulated by Wnt activation of β-catenin (Staal and Sen, 2008), this axis is dispensable in developing γδ T cells. Furthermore, we have determined that TCF1 levels at this lineage branch point do not appear to be controlled by Notch, as is the case at earlier developmental stages, but instead are controlled by E protein binding and depend on a regulatory element within the first intron of the Tcf7 locus. This suggests that TCF1 expression in γδ T cell progenitors is modulated by TCR-mediated induction of E protein antagonists, including Id3. This mode of regulation may be restricted to developing γδ T cell progenitors, since a distinct element appears to be utilized to support TCF1 expression in developing αβ and innate lymphoid cells (Harly et al., 2020). It is also possible that this mode of regulation is important in other contexts, including the generation of memory CD8 T cells, where several E-protein-bound elements have been reported near the Tcf7 locus (Masson et al., 2013; Miyazaki et al., 2017). Together, our findings link TCR signaling, the E-Id axis, and TCF1 in a common framework that underpins the specification and developmental progression of the γδ T cell lineage.
STAR★METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, David Wiest (David.Wiest@FCCC.edu)
Materials availability
All reagents and novel mouse strains used in this study are available upon request from the Lead Contact, David Wiest.
Data and code availability
The accession number for the ChIP-Seq and RNA-Seq data reported in this study is GEO: GSE162292.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animals
All mice were maintained in AALAC-accredited laboratory animal facilities at Fox Chase Cancer Center, National Institute of Aging, National Cancer Institute, or the University of Iowa. All experiments were conducted under approved institutional animal care and use committee (IACUC) approved protocols on both male and female age and sex matched mice 6–8 weeks of age. KN6 Tg mice, Ptcra-iCre Tg, Tcf7−/−, Tcf7GFP, Tcf7fl/fl and p45−/− mice were previously described(Abbas et al., 1991; Haks et al., 2005; Luche et al., 2013; Steinke et al., 2014; Verbeek et al., 1995; Xu et al., 2017; Yang et al., 2015).
Generation of mutant mice with CRISPR-Cas9 system
EPE Mutant mice were generated as previously described(Wang et al., 2013). Oligos were ordered from Integrated DNA Technologies and the gRNA sequences used were: TCF-EPE gRNA1-ATAACTGCCGAGGTTAGATT and TCF-EPE gRNA2-AAGCAGCG-TATCTACGGCAG. gRNA were synthesized in vitro with HiScribe T7 High Yield RNA Synthesis Kit (New England Biolabs, #E2040S), and purified with RNA Clean & Concentrator (Zymo Research, #R1015) according to manufacturer’s protocol. Cas9 protein (PNA Bio, #CP03) 30 ng/µl, two gRNAs each 0.3 µM were mixed according to PNA Bio protocol. 10 µl of the mixture was micro-injected into each embryonic zygote. Genomic DNA was isolated from transgenic mice tail with DNeasy Blood & Tissue Kits (QIAGEN, #69506) and used for genotyping using the following oligos: TCF-EPE F - AGTCACAGGAGGGCGTACGG and TCF-EPE R – GCAGCCTGTCCTAGTCCCAGG. Purified PCR products were further cloned into pGEM®-T Vector Systems (Promega, #A3600), transformed into DH5a competent cells (Thermo Fisher Scientific, #18258012), and sequenced by GENEWIZ.
METHOD DETAILS
Flow cytometry and proliferation analysis
Flow cytometry was performed on single cell suspensions from thymus and epidermis. Cells were isolated and stained with the following antibodies: anti-CD4 (GK1.5), anti-CD8 (53–6.7), anti-CD24 (M1/69), anti-CD25 (PC61), anti-CD27 (LG.3A10), anti-CD44 (IM7), anti-CD73 (TY/11.8), anti-CD127 (A7R34) anti-NK1.1 (PK136), anti-Thy1.2 (53–2.1), anti-TCRδ (GL3), anti-Vγ1 (2.11), anti-Vγ2 (UC3–10A6), anti-Vγ3 (536), anti-IL17A (TC11–18H10.1) and anti-IFNγ (XMG1.2). The antibodies were purchased from eBioscience or BioLegend. Intracellular flow cytometry for cytokine production was performed as previously described(Coffey et al., 2014). Intracellular staining TCF1 was performed on thymocytes first stained with antibodies to the indicated cell surface proteins, fixed with BD Fixation/Permeabilization Solution for 20min at 4°C, washed with BD Perm/Wash Buffer, and then stained internally with anti-TCF1 (AF647-TCF1, R&D Systems) for 40 min at 4°C. Analysis of proliferation was performed using the Click-iT Plus EdU assay (Invitrogen) according to manufacturer’s specifications. Briefly, thymocytes were incubated with 10 µM EdU for 2 hours, washed with 1% BSA/PBS, and stained with the indicated fluorochrome-coupled antibodies. Following fixation with 4% paraformaldehyde for 15 min, the cells were incubated with Pacific Blue picolyl azide for 30 min at room temperature to visualize incorporated EdU. Multi-parametric flow cytometric analysis was performed on all samples using an LSRII flow cytometer (BD Biosciences) with FACSDiva software. Dead cells were excluded from analyses of unfixed populations using propidium iodide (PI). Data were analyzed using FlowJo software. Cell populations to be purified by flow cytometry were isolated using a FACSAria Cell Sorter (BD Biosciences).
Retroviral transduction and OP9-DL1 cultures
Retroviral particles were produced by transient calcium phosphate transfection of Phoenix cells. pMiG and pMiG-Id3 vectors have been previously described(Lauritsen et al., 2009; Xing et al., 2016). shRNAs targeting Tcf7 were expressed in the MSCV-based vector MLS(Dickins et al., 2005). OP9-DL1 cocultures were performed as previously described(Coffey et al., 2014). Fetal livers were harvested from KN6 γδTCR+ Rag2−/− mice at day 14.5 of gestation and cell suspensions were centrifuged over a cushion of Lympholyte-M (Cederlane Labs). Isolated cells were expanded on OP9-DL1 cells for 4 days in the presence of 5 ng/ml IL-7 and 5 ng/ml Flt3L (R&D Systems). At day 4 of culture, cells were transduced by spin infection using retroviral supernatant treated with 8 µg/ml polybrene (Sigma-Aldrich). Infected cells were cultured overnight, and GFP+ DN3 (Thy1.2+ CD4- CD8- CD25+ CD44-) cells were electronically sorted and cultured for 5 days on OP9-DL1 Ligand-monolayers(Coffey et al., 2014). At day 5 of culture, cells were harvested and analyzed by flow cytometry. In vitro cytokine stimulation with IL1β and IL23 was performed as previously described(Wencker et al., 2014).
ChIP-qPCR, ChIP-seq, and RNA-seq analysis
Rag2−/− DN3 cells were derived from fetal liver progenitors cultured on OP9-DL1 monolayers as previously described(Coffey et al., 2014). To generate γδTCR+ CD24+ CD73− and γδTCR+ CD24+ CD73+ cells, Rag2−/− fetal liver progenitors were cultured on OP9-DL1 monolayers for 7 days in the presence of 5 ng/ml IL7 and 5 ng/ml Flt3L (R&D Systems). At day 7 of culture, Rag2−/− DN3 cells were harvested and cultured overnight on MIY-KN6 γδTCR-producing GP+E cells in the presence of 5 ng/ml IL7, 5 ng/ml Flt3L, and 8 µg/ml polybrene (Sigma Aldrich). YFP+ DN3 (Thy1.2+ CD4− CD8− CD25+ CD44−) cells were electronically sorted and cultured for 3 days on OP9-DL1 monolayers. At day 3 of culture, cells were harvested and fixed for 10 minutes at 20°C with 1% (wt/vol) formaldehyde, following which the Thy1+ YFP+ CD4− CD8− CD24+ CD73− and Thy1+ YFP+ CD4− CD8− CD24+ CD73+ subsets were purified by flow cytometry. Chromatin immunoprecipitation was performed as previously described(In et al., 2017; Miyazaki et al., 2011). Cells were lysed and sonicated, and sonicated chromatin was immunoprecipitated with 10 µg anti-E2A (affinity-purified rabbit polyclonal sera raised against the last 12 amino acids of the C terminus of E2A) or 10 µg anti-HEB (affinity-purified rabbit polyclonal sera raised against the last 12 amino acids of the C terminus of HEB). Samples were washed and bound chromatin was eluted. Crosslinking was reversed overnight at 65°C. Samples were treated with RNase A and proteinase K, and DNA was purified using the ChIP DNA Clean and Concentrator Kit (Zymo). For ChIP-qPCR, quantitative real time PCR was performed with QuantiTect SYBR Green PCR Kit (QIAGEN) on an ABI Prism 7700 Real-Time PCR machine (Applied Biosystems). Primer sequences were as follows: Tcf7 (TGACGCTCCTGTGACCTGAT, AGTCACACCCCCTCACACCT), Gapdh (TGGCGTAGCAATCTCCTTTT, CTCCTGGCTTCTGTCTTTGG). For ChIP-Seq, purified DNA was ligated to an adaptor, amplified by PCR (Illumina) and sequenced. ‘Reads’ were aligned to the mm10 assembly with Bowtie software for the alignment of short DNA sequences. Custom tracks generated by the UCSC Genome Browser were used for visualization. For RNA-Seq, DN3, Thy1+ YFP+ CD4− CD8− CD24+ CD73−, and Thy1+ YFP+ CD4− CD8− CD24+ CD73+ subsets were produced as above, isolated by flow cytometry and lysed in TRIzol (Invitrogen). Reads were aligned using STAR and analyzed using HOMER. E protein ChIP-Seq and RNA-Seq datasets have been deposited in GEO (GEO: GSE162292).
Q-PCR and immunoblotting
RNA was purified using the RNeasy MiniPrep Kit (QIAGEN) per manufacturer’s instructions and converted to cDNA using the Super-Script II kit (Invitrogen) with oligo dT(12–18) primers (Invitrogen). Expression of indicated genes was measured by real time PCR using stock TaqMan primer/probe sets on an ABI Prism 7700 Real Time PCR machine (Applied Biosystems). Analysis was performed in triplicate, normalized by Gapdh, and converted into fold difference. Primer and probes were from Applied Biosystems: Gapdh, Mm99999915_g1; Tcf7, Mm00493445_m1; Ccr10, Mm01292449_m1. SCID.adh cells were transduced with MLS, MLS-shTcf.7 #1, or MLS-sh Tcf.7 #3 and lysed in RIPA Buffer (20 mM HEPES [pH 7], 150 mM NaCl, 1% deoxycholate, 1% Nonidet P-40, 0.1% SDS, 1 mM Na2VO4, 2 mM EDTA, and a complete protease inhibitor mixture; Roche). Samples were resolved on NuPage Novex Bis-Tris gels (Invitrogen) and blotted with the following antibodies: anti-TCF1 (C63D9, Cell Signaling) and anti-GAPDH (6C5, Millipore).
Fetal liver chimeras
100,000 Fetal liver progenitors were transferred i.v. into each irradiated, allotype-marked (CD45.2) recipient. 6 weeks after engraftment, thymic explants were analyzed by flow cytometry to assess developmental progression and by intracellular staining to measure cytokine production, as described(Fahl et al., 2018).
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical significance of alterations in cell populations or changes in gene expression was assessed using the two-tailed Student t test. Details of all statistical analysis can be found in the legends of both the main and supplemental figures, including the statistical tests used, the exact value of n (number of mice, unless defined otherwise), and the definition of confidence intervals.
Supplementary Material
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Polyclonal rabbit anti-mouse E2A | This lab; this report | Custom |
| Polyclonal rabbit anti-mouse HEB | This lab; this report | Custom |
| Monoclonal anti-mouse TCF1 AF647 | R&D Systems | RRID:AB_2888931 |
| Biological samples | ||
| Fetal liver derived murine CD44-CD25+DN3 cells | Coffey et al., 2014; this lab | PMID: 24493796 |
| Fetal liver derived murine γδTCR+CD73- cells | Coffey et al., 2014; this lab | PMID: 24493796 |
| Fetal liver derived murine γδTCR+CD73+ cells | Coffey et al., 2014; this lab | PMID: 24493796 |
| Chemicals, peptides, and recombinant proteins | ||
| C-terminal mouse HEB 12aa peptide immunogen | Alpha Diagnostics | Custom |
| C-terminal mouse E2A 12aa peptide immunogen | Alpha Diagnostics | Custom |
| Interleukin-7 | R&D Systems | Cat# 407-ML |
| Flt3 Ligand | R&D Systems | CatE 427-FL |
| Cas9 protein | PNA bio | Cat# CP03 |
| Critical commercial assays | ||
| Click-iT Plus EdU assay | ThermoFisher | Cat# C10418 |
| Deposited data | ||
| DN3, CD73−, CD73+ RNA Sep, E2A ChIP-Seq, HEB ChIP-Seq | GEO | GSE162292 |
| Experimental models: organisms/strains | ||
| KN6 Tg mice | Haks et al., 2005; this lab | PMID: 15894277 |
| Ptcra-iCre mice | Luche et al., 2013 | PMID: 23509324 |
| Tcf7−/− mice | Verbeek et al., 1995 | PMID: 7870176 |
| Tcf7GFP mice | Yang et al., 2015 | PMID: 26280998 |
| Tcf7f/f mice | Steinke et al., 2014 | PMID: 24836425 |
| Tcf7p45−/− mice | Xu et al., 2017 | PMID: 28348272 |
| EPE−/− mice | This lab; this study | N/A |
| Oligonucleotides | ||
| SYBR Green QPCR primer: Tcf7 (TGACGCTCCTGTGACCTGAT, AGTCACACCCCCTCACACCT) | IDT | Custom |
| SYBR QPCR primer: Gapdh (TGGCGTAGCAATCTCCTTTT, CTCCTGGCTTCTGTCTTTGG) | IDT | Custom |
| TAQMAN Probe Gapdh | Applied Biosystems | Cat# Mm99999915_g1 |
| TAQMAN Probe Tcf7 | Applied Biosystems | Cat# Mm00493445_m1 |
| TAQMAN Probe Ccr10 | Applied Biosystems | Cat# Mm01292449_m1 |
| EPE gRNA-1 - ATAACTGCCGAGGTTAGATT | IDT | Custom |
| EPE gRNA-2 - AAGCAGCGTATCTACGGCAG | IDT | Custom |
| EPE genotyping primer F-AGTCACAGGAGGGCGTACGG | IDT | Custom |
| EPE genotyping primer R - GCAGCCTGTCCTAGTCCCAGG | IDT | Custom |
| Recombinant DNA | ||
| MIY-KN6 γδCR | Coffey et al., 2014; this lab | PMID: 24493796 |
Highlights.
TCF1 expression levels regulate γδ lineage commitment and effector function
TCF1 expression in developing γδ T cells is controlled by TCR induction of Id3
Id3 induction represses TCF1 by blocking E protein binding to an intronic element
This regulatory element controls TCF1 expression in γδ but not αβ precursors
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health (NIH), United States, grants P01AI102853 and P30CA006927, an appropriation from the Common-wealth of Pennsylvania, and the Bishop Fund to D.L.W. J.M.S. was supported by the Intramural Research Program of the National Institute on Aging, United States. H.-H.X. was supported by NIH grants AI112579, AI121080, and AI139874. We are grateful to the Wiest laboratory for helpful suggestions and Dr. Ranjan Sen for critical evaluation of the manuscript. These studies were supported by the following core facilities at Fox Chase: Cell Culture, Flow Cytometry, Biostatistics and Bioinformatics, Genomics, and Laboratory Animal.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information can be found online at https://doi.org/10.1016/j.celrep.2021.108716.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Abbas AK, Williams ME, Burstein HJ, Chang TL, Bossu P, and Lichtman AH (1991). Activation and functions of CD4+ T-cell subsets. Immunol. Rev 123, 5–22. [DOI] [PubMed] [Google Scholar]
- Bain G, Cravatt CB, Loomans C, Alberola-Ila J, Hedrick SM, and Murre C (2001). Regulation of the helix-loop-helix proteins, E2A and Id3, by the Ras-ERK MAPK cascade. Nat. Immunol 2, 165–171. [DOI] [PubMed] [Google Scholar]
- Chien YH, Meyer C, and Bonneville M (2014). γδ T cells: first line of defense and beyond. Annu. Rev. Immunol 32, 121–155. [DOI] [PubMed] [Google Scholar]
- Ciofani M, Knowles GC, Wiest DL, von Boehmer H, and Zúñiga-Pflücker JC (2006). Stage-specific and differential notch dependency at the alphabeta and gammadelta T lineage bifurcation. Immunity 25, 105–116. [DOI] [PubMed] [Google Scholar]
- Coffey F, Lee SY, Buus TB, Lauritsen JP, Wong GW, Joachims ML, Thompson LF, Zúñiga-Pflücker JC, Kappes DJ, and Wiest DL (2014). The TCR ligand-inducible expression of CD73 marks γδ lineage commitment and a metastable intermediate in effector specification. J. Exp. Med 211, 329–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer J, Williams A, Skavdis G, Harker N, Coles M, Tolaini M, Norton T, Williams K, Roderick K, Potocnik AJ, and Kioussis D (2003). Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur. J. Immunol 33, 314–325. [DOI] [PubMed] [Google Scholar]
- Dickins RA, Hemann MT, Zilfou JT, Simpson DR, Ibarra I, Hannon GJ, and Lowe SW (2005). Probing tumor phenotypes using stable and regulated synthetic microRNA precursors. Nat. Genet 37, 1289–1295. [DOI] [PubMed] [Google Scholar]
- Emmanuel AO, Arnovitz S, Haghi L, Mathur PS, Mondal S, Quandt J, Okoreeh MK, Maienschein-Cline M, Khazaie K, Dose M, and Gounari F (2018). TCF-1 and HEB cooperate to establish the epigenetic and transcription profiles of CD4+CD8+ thymocytes. Nat. Immunol 19, 1366–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahl SP, Coffey F, Kain L, Zarin P, Dunbrack RL Jr., Teyton L, Zúñiga-Pflücker JC, Kappes DJ, and Wiest DL (2018). Role of a selecting ligand in shaping the murine γδ-TCR repertoire. Proc. Natl. Acad. Sci. USA 115, 1889–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germar K, Dose M, Konstantinou T, Zhang J, Wang H, Lobry C, Arnett KL, Blacklow SC, Aifantis I, Aster JC, and Gounari F (2011). T-cell factor 1 is a gatekeeper for T-cell specification in response to Notch signaling. Proc. Natl. Acad. Sci. USA 108, 20060–20065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goux D, Coudert JD, Maurice D, Scarpellino L, Jeannet G, Piccolo S, Weston K, Huelsken J, and Held W (2005). Cooperating pre-T-cell receptor and TCF-1-dependent signals ensure thymocyte survival. Blood 106, 1726–1733. [DOI] [PubMed] [Google Scholar]
- Gray EE, Ramírez-Valle F, Xu Y, Wu S, Wu Z, Karjalainen KE, and Cyster JG (2013). Deficiency in IL-17-committed Vγ4(+) γδ T cells in a spontaneous Sox13-mutant CD45.1(+) congenic mouse substrain provides protection from dermatitis. Nat. Immunol 14, 584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas JD, Ravens S, Düber S, Sandrock I, Oberdörfer L, Kashani E, Chennupati V, Föhse L, Naumann R, Weiss S, et al. (2012). Development of interleukin-17-producing γδ T cells is restricted to a functional embryonic wave. Immunity 37, 48–59. [DOI] [PubMed] [Google Scholar]
- Haks MC, Lefebvre JM, Lauritsen JP, Carleton M, Rhodes M, Miyazaki T, Kappes DJ, and Wiest DL (2005). Attenuation of gammadeltaTCR signaling efficiently diverts thymocytes to the alphabeta lineage. Immunity 22, 595–606. [DOI] [PubMed] [Google Scholar]
- Harly C, Kenney D, Wang Y, Ding Y, Zhao Y, Awasthi P, and Bhandoola A (2020). A Shared Regulatory Element Controls the Initiation of Tcf7 Expression During Early T Cell and Innate Lymphoid Cell Developments. Front Immunol 11, 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SM, Li L, and Love PE (2005). TCR signal strength influences alphabeta/gammadelta lineage fate. Immunity 22, 583–593. [DOI] [PubMed] [Google Scholar]
- Ikawa T, Hirose S, Masuda K, Kakugawa K, Satoh R, Shibano-Satoh A, Kominami R, Katsura Y, and Kawamoto H (2010). An essential developmental checkpoint for production of the T cell lineage. Science 329, 93–96. [DOI] [PubMed] [Google Scholar]
- In TSH, Trotman-Grant A, Fahl S, Chen ELY, Zarin P, Moore AJ, Wiest DL, Zúñiga-Pflücker JC, and Anderson MK (2017). HEB is required for the specification of fetal IL-17-producing γδ T cells. Nat. Commun 8, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KD, and Chien YH (2009). Thymic maturation determines gammadelta T cell function, but not their antigen specificities. Curr. Opin. Immunol 21, 140–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KD, Su X, Shin S, Li L, Youssef S, Yamasaki S, Steinman L, Saito T, Locksley RM, Davis MM, et al. (2008). Thymic selection determines gammadelta T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon gamma. Immunity 29, 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JL, Georgakilas G, Petrovic J, Kurachi M, Cai S, Harly C, Pear WS, Bhandoola A, Wherry EJ, and Vahedi G (2018). Lineage-Determining Transcription Factor TCF-1 Initiates the Epigenetic Identity of T Cells. Immunity 48, 243–257.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritsen JP, Wong GW, Lee SY, Lefebvre JM, Ciofani M, Rhodes M, Kappes DJ, Zúñiga-Pflücker JC, and Wiest DL (2009). Marked induction of the helix-loop-helix protein Id3 promotes the gammadelta T cell fate and renders their functional maturation Notch independent. Immunity 31, 565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Leid M, and Rothenberg EV (2010a). An early T cell lineage commitment checkpoint dependent on the transcription factor Bcl11b. Science 329, 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Burke S, Wang J, Chen X, Ortiz M, Lee SC, Lu D, Campos L, Goulding D, Ng BL, et al. (2010b). Reprogramming of T cells to natural killer-like cells upon Bcl11b deletion. Science 329, 85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luche H, Nageswara Rao T, Kumar S, Tasdogan A, Beckel F, Blum C, Martins VC, Rodewald HR, and Fehling HJ (2013). In vivo fate mapping identifies pre-TCRα expression as an intra- and extrathymic, but not prethymic, marker of T lymphopoiesis. J. Exp. Med 210, 699–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra N, Narayan K, Cho OH, Sylvia KE, Yin C, Melichar H, Rashighi M, Lefebvre V, Harris JE, Berg LJ, and Kang J; Immunological Genome Project Consortium (2013). A network of high-mobility group box transcription factors programs innate interleukin-17 production. Immunity 38, 681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson F, Minnich M, Olshansky M, Bilic I, Mount AM, Kallies A, Speed TP, Busslinger M, Nutt SL, and Belz GT (2013). Id2-mediated inhibition of E2A represses memory CD8+ T cell differentiation. J. Immunol 190, 4585–4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer EM, Lin YC, Benner C, Jhunjhunwala S, Dutkowski J, Flores M, Sigvardsson M, Ideker T, Glass CK, and Murre C (2011). Multilineage priming of enhancer repertoires precedes commitment to the B and myeloid cell lineages in hematopoietic progenitors. Immunity 35, 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki M, Rivera RR, Miyazaki K, Lin YC, Agata Y, and Murre C (2011). The opposing roles of the transcription factor E2A and its antagonist Id3 that orchestrate and enforce the naive fate of T cells. Nat. Immunol 12, 992–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki M, Miyazaki K, Chen K, Jin Y, Turner J, Moore AJ, Saito R, Yoshida K, Ogawa S, Rodewald HR, et al. (2017). The E-Id Protein Axis Specifies Adaptive Lymphoid Cell Identity and Suppresses Thymic Innate Lymphoid Cell Development. Immunity 46, 818–834.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Ruiz M, Ribot JC, Grosso AR, Gonçalves-Sousa N, Pamplona A, Pennington DJ, Regueiro JR, Fernández-Malavé E, and Silva-Santos B (2016). TCR signal strength controls thymic differentiation of discrete proinflammatory γδ T cell subsets. Nat. Immunol 17, 721–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Ruiz M, Sumaria N, Pennington DJ, and Silva-Santos B (2017). Thymic Determinants of γδ T Cell Differentiation. Trends Immunol 38, 336–344. [DOI] [PubMed] [Google Scholar]
- Nielsen MM, Witherden DA, and Havran WL (2017). γδ T cells in homeostasis and host defence of epithelial barrier tissues. Nat. Rev. Immunol 17, 733–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura RM, Sigvardsson M, Galceran J, Verbeek S, Clevers H, and Grosschedl R (1998). Redundant regulation of T cell differentiation and TCRalpha gene expression by the transcription factors LEF-1 and TCF-1. Immunity 8, 11–20. [DOI] [PubMed] [Google Scholar]
- Pereira P, Zijlstra M, McMaster J, Loring JM, Jaenisch R, and Tonegawa S (1992). Blockade of transgenic gamma delta T cell development in beta 2-microglobulin deficient mice. EMBO J 11, 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie HT, Scollay R, and Shortman K (1992). Commitment to the T cell receptor-alpha beta or -gamma delta lineages can occur just prior to the onset of CD4 and CD8 expression among immature thymocytes. Eur. J. Immunol 22, 2185–2188. [DOI] [PubMed] [Google Scholar]
- Prinz I, Silva-Santos B, and Pennington DJ (2013). Functional development of γδ T cells. Eur. J. Immunol 43, 1988–1994. [DOI] [PubMed] [Google Scholar]
- Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, Girardi M, Borst J, Hayday AC, Pennington DJ, and Silva-Santos B (2009). CD27 is a thymic determinant of the balance between interferongamma- and interleukin 17-producing gammadelta T cell subsets. Nat. Immunol 10, 427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spidale NA, Sylvia K, Narayan K, Miu B, Frascoli M, Melichar HJ, Zhihao W, Kisielow J, Palin A, Serwold T, et al. (2018). Interleukin-17-Producing γδ T Cells Originate from SOX13+ Progenitors that Are Independent of γδTCR Signaling. Immunity 49, 857–872.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal FJ, and Sen JM (2008). The canonical Wnt signaling pathway plays an important role in lymphopoiesis and hematopoiesis. Eur. J. Immunol 38, 1788–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinke FC, Yu S, Zhou X, He B, Yang W, Zhou B, Kawamoto H, Zhu J, Tan K, and Xue HH (2014). TCF-1 and LEF-1 act upstream of Th-POK to promote the CD4(+) T cell fate and interact with Runx3 to silence Cd4 in CD8(+) T cells. Nat. Immunol 15, 646–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumaria N, Grandjean CL, Silva-Santos B, and Pennington DJ (2017). Strong TCRγδ Signaling Prohibits Thymic Development of IL-17A-Secreting γδ T Cells. Cell Rep 19, 2469–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchinovich G, and Hayday AC (2011). Skint-1 identifies a common molecular mechanism for the development of interferon-γ-secreting versus interleukin-17-secreting γδ T cells. Immunity 35, 59–68. [DOI] [PubMed] [Google Scholar]
- Ueda-Hayakawa I, Mahlios J, and Zhuang Y (2009). Id3 restricts the developmental potential of gamma delta lineage during thymopoiesis. J. Immunol 182, 5306–5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vantourout P, and Hayday A (2013). Six-of-the-best: unique contributions of γδ T cells to immunology. Nat. Rev. Immunol 13, 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeek S, Izon D, Hofhuis F, Robanus-Maandag E, te Riele H, van de Wetering M, Oosterwegel M, Wilson A, MacDonald HR, and Clevers H (1995). An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature 374, 70–74. [DOI] [PubMed] [Google Scholar]
- Verykokakis M, Boos MD, Bendelac A, Adams EJ, Pereira P, and Kee BL (2010). Inhibitor of DNA binding 3 limits development of murine slam-associated adaptor protein-dependent ‘‘innate’’ gammadelta T cells. PLoS ONE 5, e9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, and Jaenisch R (2013). One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153, 910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber BN, Chi AW, Chavez A, Yashiro-Ohtani Y, Yang Q, Shestova O, and Bhandoola A (2011). A critical role for TCF-1 in T-lineage specification and differentiation. Nature 476, 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wencker M, Turchinovich G, Di Marco Barros R, Deban L, Jandke A, Cope A, and Hayday AC (2014). Innate-like T cells straddle innate and adaptive immunity by altering antigen-receptor responsiveness. Nat. Immunol 15, 80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiest DL (2016). Development of γδ T Cells, the Special-Force Soldiers of the Immune System. Methods Mol. Biol 1323, 23–32. [DOI] [PubMed] [Google Scholar]
- Xia M, Qi Q, Jin Y, Wiest DL, August A, and Xiong N (2010). Differential roles of IL-2-inducible T cell kinase-mediated TCR signals in tissue-specific localization and maintenance of skin intraepithelial T cells. J. Immunol 184, 6807–6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing S, Li F, Zeng Z, Zhao Y, Yu S, Shan Q, Li Y, Phillips FC, Maina PK, Qi HH, et al. (2016). Tcf1 and Lef1 transcription factors establish CD8(+) T cell identity through intrinsic HDAC activity. Nat. Immunol 17, 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Xing S, Shan Q, Gullicksrud JA, Bair TB, Du Y, Liu C, and Xue HH (2017). Cutting Edge: β-Catenin-Interacting Tcf1 Isoforms Are Essential for Thymocyte Survival but Dispensable for Thymic Maturation Transitions. J. Immunol 198, 3404–3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Li F, Harly C, Xing S, Ye L, Xia X, Wang H, Wang X, Yu S, Zhou X, et al. (2015). TCF-1 upregulation identifies early innate lymphoid progenitors in the bone marrow. Nat. Immunol 16, 1044–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Lin YY, Dai M, and Zhuang Y (2014). Id3 and Id2 act as a dual safety mechanism in regulating the development and population size of innate-like γδ T cells. J. Immunol 192, 1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuberbuehler MK, Parker ME, Wheaton JD, Espinosa JR, Salzler HR, Park E, and Ciofani M (2019). The transcription factor c-Maf is essential for the commitment of IL-17-producing γδ T cells. Nat. Immunol 20, 73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The accession number for the ChIP-Seq and RNA-Seq data reported in this study is GEO: GSE162292.


