Abstract
Environmental arsenic exposure in adults and children has been associated with a reduction in the expression of club cell secretory protein (CC16) and an increase in the expression of matrix metalloproteinase-9 (MMP-9), both biomarkers of lung inflammation and negative respiratory outcomes. The objectives of this study were to determine if the levels of serum CC16 and MMP-9 and subsequent respiratory infections in children are associated with the ingestion of arsenic by drinking water. This cross-sectional study included 216 children from three Yaqui villages, Potam, Vicam, and Cocorit, with levels of arsenic in their ground water of 70.01 ± 21.85, 23.3 ± 9.99, and 11.8 ± 4.42 μg/L respectively. Total arsenic in water and urine samples was determined by inductively coupled plasma/optical emission spectrometry. Serum was analyzed for CC16 and MMP-9 using ELISA. The children had an average urinary arsenic of 79.39 μg/L and 46.8 % had levels above of the national concern value of 50 μg/L. Increased arsenic concentrations in drinking water and average daily arsenic intake by water were associated with decreased serum CC16 levels (β = − 0.12, 95% CI − 0.20, − 0.04 and β = − 0.10, 95% CI − 0.18, − 0.03), and increased serum MMP-9 levels (β = 0.35, 95% CI 0.22, 0.48 and β = 0.29, 95% CI 0.18, 0.40) at significant levels (P < 0.05). However, no association was found between levels of these serum biomarkers and urinary arsenic concentrations. In these children, reduced serum CC16 levels were significantly associated with increased risk of respiratory infections (OR = 0.34, 95% CI 0.13, 0.90). In conclusion, altered levels of serum CC16 and MMP-9 in the children may be due to the toxic effects of arsenic exposure through drinking water.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11356-021-13070-x.
Keywords: Arsenic, MMP-9, CC16, Drinking water, Yaqui children, Respiratory infections
Introduction
Arsenic is a common element in the environment with many industrial uses. It is, however, a known carcinogen and number one on the priority list of hazardous substances issued by the US Agency for Toxic Substances and Disease Registry (ATSDR 2019). Arsenic exposure is linked to a variety of cancers in humans, as well as skin lesions, diabetes, cardiovascular diseases, kidney and liver problems, and other pathologies (Chiou et al. 1995; Ng et al. 2003). Arsenic has been linked to both malignant and non-malignant respiratory illnesses following ingestion. Chronic arsenic exposure by drinking water increases the risk of developing lung cancer (Ferreccio et al. 2000; Smith et al. 2006).
In humans, chronic arsenic exposure has been associated with a high prevalence and a wide variety of respiratory symptoms such as chronic cough and shortness of breath, chest sounds, and blood in sputum as well as the development of non-malignant respiratory diseases such as pneumonia, chronic obstructive pulmonary disease (Mazumder 2007; Ramsey 2015; George et al. 2015). Early arsenic exposure can be implicated with abnormal DNA methylation and posterior genetic expression related with lung function impairment (Gonzalez-Cortes et al. 2017). Children from La Comarca Lagunera in Mexico who were exposed to high levels of arsenic from drinking water excreted high concentrations of arsenic in urine and showed restrictive spirometric patterns compared with children with low arsenic levels in urine (Recio-Vega et al. 2015). Ahmed et al. (2017) found a relation between prenatal arsenic exposure and impaired lung function in children from Bangladesh. They also observed a greater number of cases of airway inflammation in boys when compared with girls. Other studies carried out on early life arsenic exposure showed greater numbers of lung diseases and mortality in young adults, who generally had reduction in their lung capacity (Dauphiné et al. 2011; Smith et al. 2006).
Drinking water is considered the most common and significant non-occupational arsenic exposure. Millions of people are chronically exposed worldwide via this exposure media (Farzan et al. 2013; Garcia-Rico et al. 2019; Meza et al. 2004). The increased risk of developing lung cancer is similar whether the arsenic is ingested or inhaled (Smith et al. 2009). Some studies have shown that the presence of this metalloid in inhaled dust and ingested food plays an important role in increased health risk (Garcia-Rico et al. 2019; Kurzius-Spencer et al. 2016). Recently, Witten et al. (2019) reported significant changes in the lung functions and lung structure of a mouse model exposed to low levels of arsenic by inhalation.
In several regions of Mexico, including La Comarca Lagunera located in Durango and Coahuila but also in the states of Sonora, Michoacan and Hidalgo, levels of arsenic in drinking water are higher than limits set by national and international legislation (Mejía-González et al. 2014). Excessive arsenic levels are found in the groundwater in some regions in Mexico. Respiratory diseases are an important public health problem in this country with 79,383 deaths attributed to respiratory illness in 2015 alone.
In southern Sonora, there are reports of low and moderate arsenic levels in the ground water of 57 villages located in the Yaqui and Mayo valleys. The Yaqui tribe is an ethnic community that resides in southern Sonora and is organized into eight villages. Studies done in some of these indigenous villages showed levels of arsenic in drinking water between 2.7 and 98.7 μg As/L. Children from the communities with higher arsenic levels in groundwater showed DNA damage. An increase of both non-cancer and cancer risks from chronic arsenic exposure was detected (Burgess et al. 2013, 2007; Garcia-Rico et al. 2019; Maldonado Escalante et al. 2018; Meza-Montenegro et al. 2012).
Biomarkers are thought to be measurable indicators that can help determine the relationship between exposure to contaminants and the toxicity as well as the potential for the development of diseases. Biomarkers can be used to determine the risk in a population exposed to pollutants and the probability of members of that community developing chronic diseases (Burgess et al. 2013; Farzan et al. 2017; States et al. 2011; Strimbu and Tavel 2010; Wang et al. 2018). It has been shown that exposure to drinking water with arsenic concentrations in the range of 10–50 μg/L modifies the gene expression of some proteins reducing the ability to repair wounds of the respiratory tracts. Thus, these proteins could be candidate biomarkers for arsenic exposure (Olsen et al. 2008).
Club cell secretory protein (CC16) is an anti-inflammatory biomarker secreted by club (formerly Clara) cells and is expressed at high levels in the human respiratory epithelium unique to the lung and small bronchi and bronchioles. CC16 plays an important protective role in the respiratory tract against oxidative stress and inflammatory response (Ahmed et al. 2017; Lakind et al. 2007). CC16 is also of major interest as a peripheral lung marker for assessing the cellular integrity or the permeability of the lung epithelium (Broeckaert et al. 2000). Changes in serum CC16 concentrations can be used as biomarkers for pulmonary disease states and pulmonary stress/inflammatory response, respectively (Lakind et al. 2007). There are some reports which show that CC16 levels are significantly lower in patients with acute pulmonary injury (Kropski et al. 2009).
Studies on arsenic exposure and CC16 in humans are scarce. Parvez et al. (2008) studied adults in Bangladesh and found a wide range of arsenic in their drinking water (0.1 to 761 μg/L) and reduction in the levels of serum CC16. Ahmed et al. (2017) reported that among Bangladesh children, arsenic exposure by drinking water altered their lung function and caused airway inflammation evidenced by the increase of spirometric indices when CC16 levels increased. Decreasing serum CC16 levels were observed in these children when their arsenic urinary levels increased. Though rarely studied, other important arsenic exposure media in humans are soil and dust. Beamer et al. (2016) reported that children from Arizona, USA, showed a negative association between urine CC16 levels and increases of arsenic exposure via soil.
Metalloproteinases (MMP) are collagenases present in the lungs at low concentrations which are produced by bronchial epithelial and alveolar cells. These have been used as sensitive biomarkers of lung inflammation in humans and have been associated with lung remodeling causing alteration in the extracellular matrix because of their capacity to cleave structural proteins such as collagens and elastin (Atkinson and Senior 2003). In an in vitro model (human bronchial epithelial cells), acute exposure to arsenic in a wide range of concentrations (15–290 μg/L), an increase in the activity and expression of MMP-9 was observed, which altered the airway epithelial barrier impeding the proper wound repair of bronchial cells (Olsen et al. 2008). MMP-9 also has been implicated in cancer progression, including breast cancer, invasion, and metastasis (Provatopoulou et al. 2009).
There are few investigations in human populations which relate the arsenic exposure with increased levels of MMP-9 (Josyula et al. 2006; Lantz et al. 2007). Two studies have demonstrated increased levels of MMP-9 in sputum associated with increased arsenic exposure. Kurzius-Spencer et al. (2016) carried out a study in adults from Arizona, USA, exposed at low levels of arsenic via drinking water (< 10 μg/L). Burgess et al. (2013) reported a positive association between serum MMP-9 levels and arsenic concentrations in drinking water among, adult residents of Sonora, Mexico. Only two studies have focused on these factors among children in Mexico: Children from Coahuila showed a positive association between sputum MMP-9 levels and arsenic exposure in drinking water and negative association with lung indices (Olivas-Calderon et al. 2015). Children from Hermosillo, Sonora, showed a positive association between serum MMP-9 levels and arsenic concentrations in playground dust (Garcia-Rico et al. 2019).
In Mexico, there are no reports which relate arsenic exposure via drinking water to both serum CC16 and MMP-9 levels. This is the first study carried out in indigenous Yaqui children exposed to low and moderate levels of arsenic which report the association with these two lung inflammation biomarkers. The objectives of this study were to determine if the levels of serum CC16 and MMP-9 and subsequent respiratory infections in children are associated with the ingestion of arsenic by drinking water.
Method
Study zone
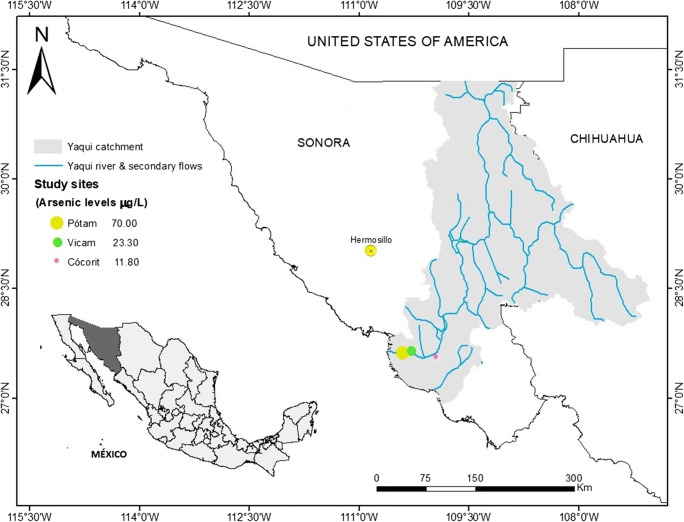
The study was done in three Yaqui communities located in southern Sonora, Mexico: Vicam, Potam, and Cocorit (Fig. 1). Cocorit had the lowest arsenic levels and more advanced water purification technology and thus better drinking water quality than the other two communities.
Fig. 1.
Location and spatial distribution of arsenic levels from the three study villages: Pótam, Vícam, and Cócorit in southern Sonora, Mexico
Drinking water sampling
The drinking water sampling was done using the method proposed by Meza et al. (2004). The water was allowed to flow for approximately five minutes, then was collected and preserved with 1 mL of pure nitric acid in polypropylene jars (500 mL) previously washed in extran (2%) and nitric acid (10%) for 24 h and transported to the Instituto Tecnológico de Sonora (ITSON) in coolers at 4 °C, remaining in frozen storage at − 20 °C until the analysis was done.
Ethical considerations
The protocol, questionnaires, and consent forms used were approved by the Bioethical Committee of Instituto Tecnológico de Sonora identifying the nature of the project and the subjects’ benefits and rights according to Helsinki Declaration. A total of 216 children were recruited. The participation of children in the study required the permission of their parents or guardians. These consent forms were signed by both the parents/guardians as well as the participants in the study. The inclusion criteria were age between 6 and 12 years, resident of the study zone for at least 5 years, consumption of drinking water from the communities well, and willingness to donate blood. Questionnaires were used to obtain anthropometric characteristics, general health information, nutritional habits, and a socioeconomic status. The anthropometrics measures were recorded. We obtained specific respiratory health data (frequency to year and duration of cough, cold, throat and ear infection, respiratory insufficiency and previous health conditions like asthma, allergy, and pneumonia); this information is included as supplementary material.
Urine sampling
Urine samples were collected in sterile vessels delivered to the participants the previous day with instructions on how to obtain the first urine of the morning. Aliquots of 4 mL were obtained from the original samples and kept frozen in Falcon tubes at − 20 °C until the analysis was done.
Blood sampling
Blood samples were collected by venipuncture using a syringe and a serum separator tube. Samples were allowed to clot at room temperature for 30 min before centrifugation for 15 min at 3200×g. Serum was removed and separated into 0.5 mL aliquots and stored at − 80 °C until analysis.
Determination of total arsenic
Urinary arsenic was measured as reported by Garcia-Rico et al. (2019), using inductively coupled plasma/optical emission spectrometry (Thermo Scientific ICP/ OES) after nitric acid sample digestion. Briefly, urine samples (4 mL) were placed in a microwave digestion lineal vessel, and 4 mL of concentrated nitric acid (HNO3) and 2 mL of hydrogen peroxide (H2O2) were added and digested (CEM Corp., Matthews, NC) for 35 min at 200 °C. After the microwave digestion, the samples were adjusted to a final volume of 25 mL with HPLC-grade water. For quality control purposes, blanks, duplicate samples, and a Standard Reference Material SRM 3669, Arsenic Species in Frozen Human Material Urine (NIST, Gaithersburg, MD) were also analyzed. In the quality control analysis, arsenic recovery was 82–106% and the coefficient of variation was 5.1%. The limit of quantification (LOQ) for arsenic averaged 1.0 μg/L.
Determination of serum CC16 and MMP-9 levels
Analysis of serum CC16 and MMP-9 levels was done using ELISA (R & D Systems, Minneapolis, MN, catalog numbers DUGB00 and DMP900, respectively). The detection limits were 0.313 ng/mL and 0.78 ng/mL, respectively, and were read to 450 nm with a background correction of 540 to 570 nm (Broeckaert et al. 2000; Matrisian 1992). Due to technical issues, only serum CC16 levels were analyzed in 186 serum samples of the total (N = 216 samples).
Determination of ADD or ADI
Daily water ingestion of arsenic by consumption was calculated by the equation (USEPA 1989):
| 1 |
ADD or ADI = Average daily dose or average daily intake (mg Kg-1 day-1).
AsW= Arsenic concentration in groundwater (mg/L).
IR= Ingestion rate (L).
EF= Exposure frequency (days/year).
ED= Exposure duration (years).
BW= Body weight (Kg).
AT= Average time/life expectancy.
Determination of HQ
The non-cancer hazard quotient (HQ) for arsenic by drinking water was determined using the equation (USEPA 1989):
| 2 |
Where:
ADD or ADI= Average daily dose or average daily intake (mg Kg-1 day-1).
RfD= Oral reference dose of arsenic (3.0 x 10-4 mg Kg−1day−1)(USEPA, 2015).
Statistical analysis
To evaluate the associations between arsenic (urinary, water, and estimated arsenic intake from water) and biomarkers (CC16 and MMP-9) predicting negative health outcomes (cold and throat infections), we used a variety of statistical tools, including Pearson correlation coefficients, linear & logistic mixed effects regression (LMER), general linear models (GLM), and logistic regression. We report the crude associations of CC16 and MMP9 with arsenic exposure using Pearson correlation coefficients. Since community wells serve as the primary source of drinking water for all members of the community, and water arsenic levels were measured from these wells (one per community), we estimated associations of arsenic in water with health outcomes using general linear models. Since estimated arsenic intake from water was derived from this variable, we also used GLM to model associations for this variable. However, for urinary arsenic and for CC16 and MMP-9, we used linear mixed effects regression. Cold and throat infections were dichotomized, such that the variable indicates 2 or more infections in the past year, and logistic regressions were used for these outcomes. Continuous variables were logged. All linear mixed effects models were adjusted for age, sex, tobacco/smoking, and weight, with a random effect for community. All GLM models were adjusted for the same variables, but without the random effect for community. We used a P value cut off of 0.05 for all interpretations of significance.
Results and discussion
The spatial distribution of the arsenic levels in the well from each village is shown in Fig. 1. The levels of arsenic were significantly different among the three study villages (P < 0.0001). Potam showed the highest concentration of arsenic with a mean value of 70.01 ± 21.85 μg/L, exceeding both Mexican and international standards of 25 μg/L and 10 μg/L respectively. Vicam and Cocorit were within the national standard with levels of 23.3 ± 9.99 and 11.8 ± 4.42 μg/L respectively, but both exceeded the international limit (Table 1). In our study area, a decrease pattern of arsenic levels from the north to the south was observed (Fig. 1): i.e. Potam > Vicam > Cocorit. Previous studies done using a higher number of wells, including these villages, have reported the same spatial distribution pattern which is primarily due to geogenic contamination (Navarro-Espinoza et al. 2021; García-Rico et al. 2019). In addition, similar concentrations have been reported in these villages in previous studies in the last 15 years (Meza et al. 2004; Meza-Montenegro et al. 2008; Maldonado Escalante et al. 2018; Garcia-Rico et al. 2019).
Table 1.
Anthropometric measures, intake rate of water, average daily intake, arsenic in well water, urinary arsenic, and hazard quotient (HQ)
| Variable | Cocorit (n = 73) |
Vicam (n = 53) |
Potam (n = 90) |
Overall (n = 216) |
|
|---|---|---|---|---|---|
| Age (years) | Mean | 9.97 | 10.26 | 9.93 | 10.03 |
| S.D | 2.18 | 2.84 | 2.55 | 2.50 | |
| Median | 10 | 11 | 9.5 | 10 | |
| Weight (Kg) | Mean | 38.33 | 41.31 | 39.07 | 39.37 |
| S.D | 15.91 | 4.44 | 13.42 | 15.10 | |
| Median | 32 | 18.31 | 37 | 35.50 | |
| BMI | Mean | 18.61 | 19.26 | 18.47 | 18.71 |
| S.D | 4.30 | 4.44 | 4.16 | 4.27 | |
| Median | 16.84 | 18.31 | 18.07 | 18.07 | |
| Intake rate of water (L/day) | Mean | 1.63 | 1.71 | 1.76 | 1.70 |
| S.D | 0.51 | 0.45 | 0.55 | 0.52 | |
| Median | 1.75 | 1.50 | 2.00 | 1.75 | |
| ADD or ADI ( mg Kg-1 d-1) | Mean | 1.35 × 10-3 | 1.11 × 10-3 | 3.31 × 10-3* | 2.11 × 10-3 |
| S.D | 1.16 × 10-3 | 4.33 × 10-4 | 1.30 × 10-3 | 1.50 × 10-3 | |
| Median | 8.04 × 10-4 | 9.76 × 10-4 | 3.22 × 10-3 | 1.85 × 10-3 | |
| As in well water (μg L-1) | Mean | 11.8 | 23.3 | 70.01* | |
| S.D | 4.42 | 9.99 | 21.85 | ||
| Median | 13.21 | 22.32 | 76.04 | ||
| Urinary As (μg L-1) | Mean | 37.30 | 47.04 | 132.57* | 79.39 |
| S.D | 30.58 | 65.16 | 99.25 | 86.38 | |
| Median | 33.43 | 27.05 | 102.53 | 44.92 | |
| HQ | Mean | 2.16* | 3.54* | 9.74* | 5.66 |
| S.D | 1.04 | 1.37 | 3.84 | 4.38 | |
| Median | 1.95 | 3.13 | 9.48 | 3.74 |
BMI body mass index, ADI average daily intake, ADD average daily dose
*Statistically significant difference by ANOVA test at P value < 0.05
Table 1 shows the general characteristics and results of urinary arsenic excretion, water consumption, arsenic intake, rate of water intake (water consumption/day), average daily intake (ADI), and hazard quotient (HQ) obtained for these children. There were not statistically significant differences by village in the measures of age, weight, and BMI in the children, similar to results reported by Maldonado Escalante et al. (2018). Regarding ADI and HQ, significant differences among the three villages (P < 0.05) were found. Potam had the highest arsenic exposure via drinking water and also showed the highest ADI value = 3.31 × 10-3 mg Kg-1 day-1. Differences between ADI and sex were not found, probably due to similarities in age and water consumption (Table 1). Children from these three villages had higher ADI values (2.11 × 10-3 mg Kg-1 day-1) compared with a population from Zacatecas, Mexico, also exposed to arsenic via drinking water with an average of ADI = 5.4 × 10-4 mg Kg-1day-1 (Martinez-Acuna et al. 2016). Increases in arsenic concentration and exposure frequency raise the ADI values which increase probabilities for diseases, including cancer (Phan et al. 2010; Sharma et al. 2016). Regarding risk assessment, significantly high variation in the values of health risk obtained for each village was found. The HQs were 9.74, 3.54, and 2.16 for Potam, Vicam, and Cocorit respectively (Table 1). This variation in risk assessment was noticed also in a population from southern Taiwan exposed to arsenic by ground water and seafood consumption (Liang et al. 2016; Liang et al. 2013). This variability could be explained basically by the differences in arsenic levels in drinking water for each village, the volume of water consumption, and weight for each participant which are key variables in Eqs. 1 and 2, to calculate ADI and HQ respectively (Table 1). In our study, the values of intake rate of water and weight were obtained directly from the questionnaires applied to each child. Significant differences in these variables by town were not observed. In this context, the most important predictor of the differences in HQ is the level of arsenic in each well. Potam had the highest arsenic levels in water and the highest values of HQ while Cocorit, with the lowest value of HQ had the lowest level of arsenic in drinking water. These results suggest a potential health risk for children who live in Vicam and Cocorit, but children from Potam have the highest risk of developing a chronic illness associated with arsenic exposure.
The highest urinary arsenic excretion was for children from Potam, the village with higher arsenic in their drinking water with an average of urinary arsenic of 132.57 μg/L. The same behavior was observed for Vicam and Cocorit with values of 47.04 and 37.30 μg/L respectively (Table 1); 46.8% of children had arsenic levels in urine above the national concern value of 50 μg/L. Some studies have reported similar results observing an increase of urinary arsenic excretion when the concentration of arsenic in drinking water increased as well (Ahsan et al. 2000; Gardner et al. 2011; Meza-Montenegro et al. 2008). Kippler et al. (2016), however, found that urinary arsenic excretion does not necessarily diminish when the arsenic levels in drinking water are reduced, perhaps due to other sources of arsenic exposure. For arsenic excreted in urine, the children in this study showed a statistically significant difference by gender (P < 0.05), males presented higher levels than females with a mean concentration of 107.11 μg/L and 61.33 μg/L respectively. These results are opposite to previously reported studies where adult women had higher urinary arsenic excretion capacity when compared with men, probably due to the possible hormonal influence on the methylation ability of the metalloid in these women (Lindberg et al. 2008). Meza-Montenegro et al. (2008) did not observe significant differences in urinary arsenic excretion by sex, in children from the Yaqui valley exposed to arsenic via drinking water. In some studies, arsenic methylation efficiency has been correlated with BMI in adult women; however, there was no correlation between these two variables among the children from this study (Gomez-Rubio et al. 2011).
Alteration in serum CC16 and MMP-9 levels in our children shows the toxic effect of this metalloid when the arsenic exposure increased via drinking water. Figure 2 shows the results of serum CC16 and MMP-9 levels from the three villages. Children from Cocorit with the lowest arsenic exposure presented the highest expression of CC16 (40.74 ng/mL), followed by Potam and Vicam, with values of 32.26 ng/mL and 27.36 ng/mL respectively (Fig. 2). These results are consistent with previous reports where reduced serum CC16 levels have been interpreted as a biomarker of club cell toxicity in environments of chronic exposures (Beamer et al. 2016; Burgess et al. 2013; Parvez et al. 2008). Serum CC16 levels in children from our study were higher than values reported by Zhai et al. (2018) with levels of 8.42 ng/mL in children of 11 years old and for adults of 22, 26, and 33 years old with concentrations of 11.28, 9.54, and 10.61 ng/mL, respectively. Beamer et al. (2019) also found lower serum CC16 levels (range 7.9 to 11.5 ng/mL) for children and adults in the age range of 6 to 32 years. However, in a recent study carried out in healthy adults, and stable chronic obstructive pulmonary disease adult patients, the serum CC16 levels were 111.3 ± 17.9 and 94.2 ± 20.9 ng/mL respectively, higher levels than those obtained from our children (Rong et al. 2020).
Fig. 2.
Serum MMP-9 and CC16 levels in the Yaqui children by village (LSD show a significant difference, P-value < 0.05
The serum MMP-9 levels increased with increased arsenic exposure. Potam, the village with the highest arsenic in drinking water, showed a significant increase in expression of serum MMP-9 (593.59 ng/mL), followed by Vicam and Cocorit with values of 452.45 and 411.52 ng/mL respectively (Fig. 2). This result is consistent with previous studies carried out in populations exposed to arsenic by drinking water where a similar pattern was observed. Higher MMP-9 levels were observed when arsenic exposure by drinking water increased (Islam et al. 2015; Olivas-Calderon et al. 2015; Kurzius-Spencer et al. 2016; Burgess et al. 2013).
Serum CC16 levels showed significant negative correlation with the average daily arsenic intake (ρ = − 0.2190, P = 0.0031) and arsenic in drinking water (ρ = − 0.2256, P = 0.0023) but not in urine (Table 2), the opposite of results reported by Parvez et al. (2008) and Ahmed et al. (2017) who found that serum CC16 levels in children had a negative correlation with urinary arsenic, but not with arsenic in drinking water. Table 2 shows that serum MMP-9 levels in the children were positively correlated with the average daily arsenic intake (ρ = 0.2787, P = 0.0000), arsenic in drinking water (ρ = 0.3214, P = 0.0000) and arsenic excreted in the urine (ρ = 0.1355, P = 0.0467). Our results were similar to those reported for adults by Burgess et al. (2013) who had a significant positive correlation between serum MMP-9 levels and arsenic intake and among serum MMP-9 levels and arsenic concentrations in drinking water. Josyula et al. (2006) found that sputum MMP-9 levels were related positively with urinary arsenic in adults similar to our results with children. On the other hand, in the children, serum MMP-9 levels had a significant correlation with HQ (ρ = 0.2430, P = 0.0003), similar to results reported by Martinez-Acuna et al. (2016) who reported HQ increases when the arsenic exposure was higher.
Table 2.
Pearson correlation between CC16 and MMP-9 and As exposure
| Parameter | Correlation | P-value | |
|---|---|---|---|
| CC16 | ADI by drinking water | − 0.2190 | 0.0031 |
| As in drinking water | − 0.2256 | 0.0023 | |
|
Total As in urine HQ |
− 0.0587 − 0.1176 |
0.4330 0.1158 |
|
| MMP-9 | ADI by drinking water | 0.2787 | 0.0000 |
| As in drinking water | 0.3214 | 0.0000 | |
| Total As in urine | 0.1355 | 0.0467 | |
| HQ | 0.2430 | 0.0003 |
Table 3 shows a linear association of arsenic exposure with CC16 and MMP-9. This model showed a negative association between CC16, urinary arsenic, and average daily arsenic intake. Our results are consistent with previous reports which found that the concentration of CC16 decreases when arsenic exposure increases via drinking water (Parvez et al. 2008). Unexpectedly, we found that serum MMP-9 levels and urinary arsenic excretion had a negative association. Farzan et al. (2017) also found a negative association between arsenic concentrations in toenails and plasma MMP-9 levels, but they had a positive association between urinary arsenic and MMP-9 levels similar to two previous studies (Burgess et al. 2013; Kurzius-Spencer et al. 2016).
Table 3.
Linear associations of arsenic exposure with CC16 and MMP-9
| CC16 (N = 180) | MMP-9 (N = 216) | |
|---|---|---|
| Urinary arsenic | − 0.03 (− 0.09, 0.03) | − 0.03 (− 0.14, 0.08) |
| Arsenic in water | − 0.12 (− 0.20, − 0.04) | 0.35 (0.22, 0.48) |
| ADI by drinking water | − 0.05 (− 0.20, 0.10) | 0.29 (0.18, 0.40) |
The effects for arsenic in water, and arsenic intake from water models, were estimated in general linear models and adjusted for age, sex, tobacco/smoking, and weight. The effects for urinary arsenic models were adjusted for these covariates and additionally included a random effect for community. P value cut off of 0.05 for all interpretations of significance
Table 4 shows the models explaining the association between arsenic exposure and respiratory infections as well as the OR values with non-significant associations between urinary arsenic and cold infection (OR = 1.25 95% CI 0.87, 1.80), arsenic in drinking water and cold infection (OR = 0.95 95% CI 0.87, 1.05), and average daily arsenic intake by drinking water and cold infection (OR = 0.97 95% CI 0.69, 1.35). The same non-significant associations were shown for urinary arsenic and throat infection (OR = 1.33 95% CI 0.92, 1.92), arsenic in drinking water and throat infection (OR = 0.97 95% CI 0.89, 1.07), and average daily arsenic intake by drinking water and throat infection (OR = 0.90 95% CI 0.62, 1.32). Table 5 shows a statistically significant association between serum CC16 levels and cold infections (OR = 0.34 95% CI 0.13, 0.90), but not serum MMP-9 levels and cold infections (Table 5). The association between CC16 and throat infections was not significant (OR = 0.91 95% CI 0.40, 2.07).
Table 4.
Logistic models for associations of arsenic exposure with respiratory infections
| N = 216 | Cold infections OR (95%CI) |
Throat infections OR (95%CI) |
|---|---|---|
| Urinary arsenic | 1.25 (0.87, 1.80) | 1.33 (0.92, 1.92) |
| Arsenic in water | 0.96 (0.87, 1.05) | 0.97 (0.89, 1.07) |
| ADI by drinking water | 0.97 (0.69, 1.35) | 0.90 (0.62, 1.32) |
The effects for arsenic in water, and arsenic intake from water models, were estimated in general linear models and adjusted for age, sex, tobacco/smoking, and weight. The effects for urinary arsenic models were adjusted for these covariates and additionally included a random effect for community. P value cut off of 0.05 for all interpretations of significance
Table 5.
Logistic model for associations of CC16 and MMP-9 with respiratory infections
| Cold infections OR (95%CI) |
Throat infections OR (95%CI) |
|
|---|---|---|
| CC16 (N = 180) | 0.34 (0.13, 0.90) | 0.91 (0.40, 2.07) |
| MMP9 (N = 216) | 0.80 (0.55, 1.18) | – |
N sample size. The MMP9/Throat Infection model failed to converge and thus is not presented here. Models were adjusted for age, sex, tobacco/smoking, and weight and included a random effect for community. P value cut off of 0.05 for all interpretations of significance
There is compelling evidence that chronic exposure to high levels of arsenic via drinking water increases the risk of developing respiratory disease and that the lungs are the major target for arsenic toxicity (Lantz 2020; Ramsey 2015). Rahman et al. (2011) found that arsenic exposure during pregnancy was linked with increased morbidity in infectious diseases during infancy. Some studies have reported a higher prevalence of chronic bronchitis and cough in residents from Bangladesh exposed to high arsenic levels compared with unexposed residents, and this rate increased when the concentrations of arsenic in water increased as well (Milton et al. 2001; Milton and Rahman 2002). Positive association between high arsenic exposure via drinking water and respiratory symptoms such as dyspnea, asthma, eye irritation, headache, sore throat, dry cough, and cough with phlegm as well as others was found in adults from India (Das et al. 2014). A positive and significant association between respiratory symptoms and urinary arsenic in children was reported by Farzan et al. (2017). In adults from Chile exposed to arsenic by drinking water was observed that 38% of them experienced shortness of breath while they were walking, but found no associations between arsenic exposure and chronic cough, phlegm, or chronic bronchitis (Dauphiné et al. 2011).
The mechanisms determining how arsenic causes these respiratory diseases are not clear, but some studies have shown that oral arsenic exposure alters gene expression and DNA methylation patterns, causes inhibition of DNA repair, compromises the respiratory epithelial barrier integrity by increasing systemic translocation of inhaled pathogens and small molecules, exacerbates tissue inflammation, and induces respiratory function impairment by oxidative stress, causing changes in the immune response by altering developmental signaling pathways (Henderson et al. 2017; Farzan et al. 2016; Olivas-Calderón et al. 2015; Burgess et al. 2013; Andrew et al. 2008; Reynolds et al. 2007). Arsenic exposure even at low levels has been found to be associated with alterations in a variety of proteins implicated in lung remodeling resulting in an increase in the potential for adverse effects in the respiratory system (Lantz et al. 2007, 2009). Sherwood et al. (2013) reported a model mouse with relevant alterations in epithelial airway cells, re-ordering of proteins and loss of barrier functions, when animals were exposed to arsenic. Arsenic exposure has also been shown to increase mortality in mice following influenza infection (Kozul et al. 2009). Exposure to arsenic by drinking water at low levels (10–50 μg/L) modifies the gene expression of some proteins reducing the ability to repair wounds of the respiratory tracts. Clara cells have the ability to act as a mechanical barrier to prevent infection by certain common pathogens, but when the concentration of CC16 is decreasing, lung tissue and its components are exposed and their functions are compromised, being more susceptible to respiratory infections. This is the first study which reports a negative and significant association between CC16 levels and colds in a population exposed to arsenic. Our children had reduction of CC16 levels by moderate arsenic exposure, as a consequence, may were more vulnerable to developing colds (OR = 0.34, P < 0.05). MMP-9 is related to the wound healing process, and as part of the lung inflammatory response, it is continually secreted in the airways. The exact mechanism for the association between arsenic exposure and increased MMP-9 is unknown. A likely scenario is that MMP-9 modulates other enzymes and cytokines to fine-tune both destruction and repair; other possibilities are activation of activating protein-1 receptor sites in the promoter region of MMP-9 or altered gene methylation (Burgess et al. 2013). Atkinson and Senior (2003) and Ohbayashi and Shimokata (2005) reported that MMP-9 increased its levels in some lung diseases such as asthma, idiopathic pulmonary fibrosis, and chronic obstructive pulmonary disease. Ueland et al. (2020) found early increase in circulating MMP-9 in COVID-19 patients with respiratory failure. In our study, we found a positive association between increase of serum MMP-9 levels with cold infections in children, but it was not significant (Table 5).
Conclusion
Arsenic concentrations in drinking water and average daily arsenic intake by water were negatively associated with serum CC16 levels, but were positively associated with serum MMP-9 levels, and no association was found between urinary arsenic concentrations with serum MMP-9 and CC16 levels. Serum CC16 levels, but not MMP-9 levels, showed an inverse association with colds. In children exposed to arsenic via drinking water, alteration of levels of these biomarkers may cause toxic effects.
Supplementary Information
(DOCX 26 kb)
Acknowledgements
The authors would like to thank each of the Governors of the Yaqui Tribe and participants of this study and also to Ana Lilia López Duarte and Jazmir Ariadne Yocupicio for their laboratory assistance. Special thanks to Dr. Paul W. Kilpatrick for his support with the English edition.
Author contribution
Meza-Montenegro conceptualized and supervised the study. Meza-Montenegro, Beamer, P., Burgess, J., O´Rourke, MK., Vega-Millán, Dévora-Figueroa, and Lantz, C. designed the study and acquired the data. Vega-Millán, Furlong, M., Dévora-Figueroa, Meza-Figueroa, Meza-Montenegro, and Balderas-Cortés conducted the data analysis and interpreted the data. Vega-Millán, Meza-Montenegro, and Burgess, J. prepared the first draft. Melissa, F., García-Rico, O´Rourke, MK., and Meza-Escalante participated in critical revision of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by funds provided by the National Council of Science and Technology (CONACYT) through the following grant: FONSALUD-S0008-2014-1-233976. This research was also supported by funds from University of Arizona Grant No. SWEHSC-2018-P30-ES06694 and Instituto Tecnológico de Sonora Grant No. ITSON/PROFAPI_2020_0039. There was no participation of these funding institutions in the design of the study and collection, analysis, and interpretation of data nor in writing the manuscript.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval
The protocol, questionnaires, and consent forms used were approved by the Bioethical Committee of Instituto Tecnológico de Sonora identifying the nature of the project and the subjects’ benefits and rights according to the Helsinki Declaration. The authors confirm that the manuscript has been read and approved by all authors. The authors declare that this manuscript has not been published and not under consideration for publication elsewhere.
Consent to participate
The participation of children in the study required the permission of their parents or guardians. These consent forms were signed by both the parents/guardians as well as the participants in the study. On the other hand, the authors have been personally and actively involved in substantive work leading to the manuscript and will hold themselves jointly and individually responsible for its content.
Consent for publication
Consent is not applicable for our study subjects. The information for each child was kept confidential, as was specified in the consent form. We only include averages of the results of the total population who participated in our study. All authors, however, consent to the publication of this research.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmed S, Akhtar E, Roy A, von Ehrenstein OS, Vahter M, Wagatsuma Y, Raqib R. Arsenic exposure alters lung function and airway inflammation in children: a cohort study in rural Bangladesh. Environ Int. 2017;101:108–116. doi: 10.1016/j.envint.2017.01.014. [DOI] [PubMed] [Google Scholar]
- Ahsan H, Perrin M, Rahman A, Parvez F, Stute M, Zheng Y, Hasnat Milton A, Brandt-Rauf P, van Geen A. Associations between drinking water and urinary arsenic levels and skin lesions in Bangladesh. J Occup Environ Med. 2000;42(12):1195–1201. doi: 10.1097/00043764-200012000-00016. [DOI] [PubMed] [Google Scholar]
- Andrew AS, Jewell DA, Mason RA, Whitfield ML, Moore JH, Karagas MR. Drinking-water arsenic exposure modulates gene expression in human lymphocytes from a U.S. population. Environ Health Perspect. 2008;116(4):524–531. doi: 10.1289/ehp.10861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson JJ, Senior RM. Matrix metalloproteinase-9 in lung remodeling. Am J Respir Cell Mol Biol. 2003;28(1):12–24. doi: 10.1165/rcmb.2002-0166TR. [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR). 2019 priority list of hazardous substances. Available online: https://www.atsdr.cdc.gov/spl/#2019spl.
- Beamer P, Klimecki WT, Loh M, Ornelas Van Horne Y, Sugeng A, Lothrop N, Billheimer D, Guerra S, Lantz RC, et al. Association of Children’s Urinary CC16 Levels with Arsenic Concentrations in Multiple Environmental Media. Int J Environ Res Public Health. 2016;13:521. doi: 10.3390/ijerph13050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beamer PI, Furlong M, Lothrop N, Guerra S, Billheimer D, Stern DA, Zhai J, Halonen M, Wright AL, Martinez FD. CC16 levels into adult life are associated with nitrogen dioxide exposure at birth. Am J Respir Crit Care Med. 2019;200(5):600–607. doi: 10.1164/rccm.201808-1488OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeckaert F, Clippe A, Knoops B, Hermans C, Bernad A. Clara cell secretory protein (CC16): features as a peripheral lung biomarker. Ann N Y Acad Sci. 2000;923(1):68–77. doi: 10.1111/j.1749-6632.2000.tb05520.x. [DOI] [PubMed] [Google Scholar]
- Burgess JL, Meza MM, Josyula AB, Poplin GS, Kopplin MJ, McClellen HE, Sturup S, Lantz RC. Environmental arsenic exposure and urinary 8-OHdG in Arizona and Sonora. ClinToxicol (Phila) 2007;45(5):490–498. doi: 10.1080/15563650701354119. [DOI] [PubMed] [Google Scholar]
- Burgess JL, Kurzius-Spencer M, O'Rourke MK, Littau SR, Roberge J, Meza-Montenegro MM, Gutiérrez-Millán LE, Harris RB (2013) Environmental arsenic exposure and serum matrix metalloproteinase-9. Journal of Exposure Science and Environmental Epidemiology 23:163–169. 10.1038/jes.2012.1072012.107 [DOI] [PMC free article] [PubMed]
- Chiou HY, Hsueh YM, Liaw KF, Horng SF, Chiang MH, Pu YS, et al. Incidence of internal cancers and ingested inorganic arsenic: a seven-year follow-up study in Taiwan. Cancer Res. 1995;55:1296–1300. [PubMed] [Google Scholar]
- Das D, Bindhani B, Mukherjee B, Saha H, Biswas P, Dutta K, Prasad P, Sinha D, Ray MR. Chronic low-level arsenic exposure reduces lung function in male population without skin lesions. International Journal of Public Health. 2014;59(4):655–663. doi: 10.1007/s00038-014-0567-5. [DOI] [PubMed] [Google Scholar]
- Dauphiné DC, Ferreccio C, Guntur S, Yuan Y, Hammond SK, Balmes J, Smith AH, Steinmaus C. Lung function in adults following in utero and childhood exposure to arsenic in drinking water: preliminary findings. Int Arch Occup Environ Health. 2011;84(6):591–600. doi: 10.1007/s00420-010-0591-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzan SF, Korrick S, Li Z, Enelow R, Gandolfi AJ, Madan J, Nadeau K, Karagas MR. In utero arsenic exposure and infant infection in a United States cohort: a prospective study. Environ Res. 2013;126:24–30. doi: 10.1016/j.envres.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzan SF, Li Z, Korrick SA, Spiegelman D, Enelow R, Nadeau K, Baker E, Karagas MR (2016) Infant infections and respiratory symptoms in relation to arsenic exposure in a U.S. cohort. Environ Health Perspect 124(6):840–847. 10.1289/ehp.1409282 [DOI] [PMC free article] [PubMed]
- Farzan SF, Howe CG, Zens MS, Palys T, Channon JY, Li Z, Chen Y, Karagas MR. Urine arsenic and arsenic metabolites in U.S. adults and biomarkers of inflammation, oxidative stress, and endothelial dysfunction: a cross-sectional study. Environ Health Perspect. 2017;125(12):127002. doi: 10.1289/ehp2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreccio C, Gonzalez C, Milosavjlevic V, Marshall G, Sancha AM, Smith AH. Lung cancer and arsenic concentrations in drinking water in Chile. Epidemiology. 2000;11(6):673–679. doi: 10.1097/00001648-200011000-00010. [DOI] [PubMed] [Google Scholar]
- Garcia-Rico L, Meza-Figueroa D, Gandolfi AJ, Del Rivero CI, Martinez-Cinco MA, Meza-Montenegro MM. Health risk assessment and urinary excretion of children exposed to arsenic through drinking water and soils in Sonora, Mexico. Biol Trace Elem Res. 2019;187(1):9–21. doi: 10.1007/s12011-018-1347-5. [DOI] [PubMed] [Google Scholar]
- Gardner R, Hamadani J, Grander M, Tofail F, Nermell B, Palm B, Kippler M, Vahter M. Persistent exposure to arsenic via drinking water in rural Bangladesh despite major mitigation efforts. Am J Public Health. 2011;101(Suppl 1):S333–S338. doi: 10.2105/ajph.2010.300025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George CM, Brooks WA, Graziano JH, Nonyane BAS, Hossain L, Goswami D, Zaman K, Yunus M, Khan AF, Jahan Y, Ahmed D, Slavkovich V, Higdon M, Deloria-Knoll M, O’ Brien KL. Arsenic exposure is associated with pediatric pneumonia in rural Bangladesh: a case control study. Environ Health. 2015;14(1):83. doi: 10.1186/s12940-015-0069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Rubio P, Roberge J, Arendell L, Harris RB, O'Rourke MK, Chen Z, Cantu-Soto E, Meza-Montenegro MM, Billheimer D, Lu Z, Klimecki WT. Association between body mass index and arsenic methylation efficiency in adult women from southwest U.S. and northwest Mexico. Toxicol Appl Pharmacol. 2011;252(2):176–182. doi: 10.1016/j.taap.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Cortes T, Recio-Vega R, Lantz RC, Chau BT. DNA methylation of extracellular matrix remodeling genes in children exposed to arsenic. ToxicolApplPharmacol. 2017;329:140–147. doi: 10.1016/j.taap.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson MW, Madenspacher JH, Whitehead GS, Thomas SY, Aloor JJ, Gowdy KM, Fessler MB. Effects of orally ingested arsenic on respiratory epithelial permeability to bacteria and small molecules in mice. Environ Health Perspect. 2017;125(9):097024. doi: 10.1289/ehp1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MS, Mohanto NC, Karim MR, Aktar S, Hoque MM, Rahman A, Jahan M, Khatun R, Aziz A, Salam KA, Saud ZA, Hossain M, Rahman A, Mandal A, Haque A, Miyataka H, Himeno S, Hossain K. Elevated concentrations of serum matrix metalloproteinase-2 and -9 and their associations with circulating markers of cardiovascular diseases in chronic arsenic-exposed individuals. Environ Health. 2015;14(1):92. doi: 10.1186/s12940-015-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josyula AB, Poplin GS, Kurzius-Spencer M, McClellen HE, Kopplin MJ, Stürup S, Lantz RC, Burgess JL. Environmental arsenic exposure and sputum metalloproteinase concentrations. Environ Res. 2006;102(3):283–290. doi: 10.1016/j.envres.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Kippler M, Skroder H, Rahman SM, Tofail F, Vahter M. Elevated childhood exposure to arsenic despite reduced drinking water concentrations—a longitudinal cohort study in rural Bangladesh. Environ Int. 2016;86:119–125. doi: 10.1016/j.envint.2015.10.017. [DOI] [PubMed] [Google Scholar]
- Kozul CD, Ely KH, Enelow RI, Hamilton JW. Low-dose arsenic compromises the immune response to influenza A infection in vivo. Environ Health Perspect. 2009;117(9):1441–1447. doi: 10.1289/ehp.0900911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropski JA, Fremont RD, Calfee CS, Ware LB. Clara cell protein (CC16), a marker of lung epithelial injury, is decreased in plasma and pulmonary edema fluid from patients with acute lung injury. Chest. 2009;135(6):1440–1447. doi: 10.1378/chest.08-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzius-Spencer M, Harris RB, Hartz V, Roberge J, Hsu C-H, O'Rourke MK, Burgess JL. Relation of dietary inorganic arsenic to serum matrix metalloproteinase-9 (MMP-9) at different threshold concentrations of tap water arsenic. Journal of exposure science & environmental epidemiology. 2016;26(5):445–451. doi: 10.1038/jes.2014.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakind JS, Holgate ST, Ownby DR, Mansur AH, Helms PJ, Pyatt D, Hays SM. A critical review of the use of Clara cell secretory protein (CC16) as a biomarker of acute or chronic pulmonary effects. Biomarkers. 2007;12(5):445–467. doi: 10.1080/13547500701359327. [DOI] [PubMed] [Google Scholar]
- Lantz RC (2020) Arsenic exposure, CC16 and its effect on pulmonary function. NIH project. Arizona, USA. https://tools.niehs.nih.gov/portfolio/index.cfm/portfolio/grantDetail/grant_number/. Accessed 23 Jan 2021
- Lantz RC, Lynch BJ, Boitano S, Poplin GS, Littau S, Tsaprailis G, Burgess JL. Pulmonary biomarkers based on alterations in protein expression after exposure to arsenic. Environ Health Perspect. 2007;115(4):586–591. doi: 10.1289/ehp.9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz RC, Chau B, Sarihan P, Witten ML, Pivniouk VI, Chen GJ. In utero and postnatal exposure to arsenic alters pulmonary structure and function. Toxicol Appl Pharmacol. 2009;235(1):105–113. doi: 10.1016/j.taap.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang CP, Jang CS, Chen JS, Wang SW, Lee JJ, Liu CW. Probabilistic health risk assessment for ingestion of seafood farmed in arsenic contaminated groundwater in Taiwan. Environ Geochem Health. 2013;35(4):455–464. doi: 10.1007/s10653-012-9507-6. [DOI] [PubMed] [Google Scholar]
- Liang CP, Wang SW, Kao YH, Chen JS. Health risk assessment of groundwater arsenic pollution in southern Taiwan. Environ Geochem Health. 2016;38(6):1271–1281. doi: 10.1007/s10653-016-9794-4. [DOI] [PubMed] [Google Scholar]
- Lindberg AL, Ekstrom EC, Nermell B, Rahman M, Lonnerdal B, Persson LA, Vahter M. Gender and age differences in the metabolism of inorganic arsenic in a highly exposed population in Bangladesh. Environ Res. 2008;106(1):110–120. doi: 10.1016/j.envres.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Maldonado Escalante JF, Meza Figueroa D, Dévora Figueroa AG, García Rico L, Burgess JL, Lantz RC, Yañez Estrada L, Martínez Cinco MA, Balderas Cortés JJ (2018) An integrated health risk assessment of indigenous children exposed to arsenic in Sonora, Mexico. Human and Ecological Risk Assessment: An International Journal:1–16. 10.1080/10807039.2018.1449098
- Martinez-Acuna MI, Mercado-Reyes M, Alegria-Torres JA, Mejia-Saavedra JJ. Preliminary human health risk assessment of arsenic and fluoride in tap water from Zacatecas, Mexico. Environ Monit Assess. 2016;188(8):476. doi: 10.1007/s10661-016-5453-6. [DOI] [PubMed] [Google Scholar]
- Matrisian LM. The matrix-degrading metalloproteinases. Bio Essays. 1992;14(7):455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- Mazumder DNG. Arsenic and non-malignant lung disease. J Environ Sci Health A. 2007;42(12):1859–1867. doi: 10.1080/10934520701566926. [DOI] [PubMed] [Google Scholar]
- Mejía-González, M. Á., González-Hita, L., Briones-Gallardo, R., Cardona-Benavides, A., & Soto-Navarro, P. (2014). Mecanismos que liberan arsénico al agua subterránea de la Comarca Lagunera, estados de Coahuila y Durango, México. Tecnología y ciencias del agua, 5, 71-82. Retrieved from http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S2007-24222014000100005&nrm=iso
- Meza MM, Kopplin MJ, Burgess JL, Gandolfi AJ. Arsenic drinking water exposure and urinary excretion among adults in the Yaqui Valley, Sonora, Mexico. Environ Res. 2004;96(2):119–126. doi: 10.1016/j.envres.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Meza-Montenegro MM, Kopplin MJ, Burgess JL, Gandolfi AJ. Urinary arsenic methylation profile in children exposed to low arsenic levels through drinking water. Toxicol Environ Chem. 2008;90(5):957–970. doi: 10.1080/02772240701782140. [DOI] [Google Scholar]
- Meza-Montenegro MM, Gandolfi AJ, Santana-Alcantar ME, Klimecki WT, Aguilar-Apodaca MG, Del Rio-Salas R, De la O. VM., Gomez-Alvarez, A., Mendivil-Quijada, H. Metals in residential soils and cumulative risk assessment in Yaqui and Mayo agricultural valleys, northern Mexico. Sci Total Environ. 2012;433:472–481. doi: 10.1016/j.scitotenv.2012.06.083. [DOI] [PubMed] [Google Scholar]
- Milton AH, Rahman M. Respiratory effects and arsenic contaminated well water in Bangladesh. Int J Environ Health Res. 2002;12(2):175–179. doi: 10.1080/09603120220129346. [DOI] [PubMed] [Google Scholar]
- Milton AH, Hasan Z, Rahman A, Rahman M. Chronic Arsenic poisoning and respiratory effects in Bangladesh. J Occup Health. 2001;43(3):136–140. doi: 10.1539/joh.43.136. [DOI] [Google Scholar]
- Navarro-Espinoza S, Angulo-Molina A, Meza-Figueroa D, López-Cervantes G, Meza-Montenegro M, Armienta A, Soto-Puebla D, Silva-Campa E, Burgara-Estrella A, Álvarez-Bajo O, Pedroza-Montero M. Effects of untreated drinking water at three indigenous Yaqui Towns in Mexico: insights from a murine model. Int J Environ Res Public Health. 2021;18(2):805. doi: 10.3390/ijerph18020805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng JC, Wang J, Shraim A. A global health problem caused by arsenic from natural sources. Chemosphere. 2003;52(9):1353–1359. doi: 10.1016/s0045-6535(03)00470-3. [DOI] [PubMed] [Google Scholar]
- Ohbayashi H, Shimokata K. Matrix metalloproteinase-9 and airway remodeling in asthma. Curr Drug Targets Inflamm Allergy. 2005;4(2):177–181. doi: 10.2174/1568010053586246. [DOI] [PubMed] [Google Scholar]
- Olivas-Calderon E, Recio-Vega R, Gandolfi AJ, Lantz RC, Gonzalez-Cortes T, Gonzalez-De Alba C, Froines JR, Espinosa-Fematt JA. Lung inflammation biomarkers and lung function in children chronically exposed to arsenic. Toxicol Appl Pharmacol. 2015;287(2):161–167. doi: 10.1016/j.taap.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen CE, Liguori AE, Zong Y, Lantz RC, Burgess JL, Boitano S. Arsenic upregulates MMP-9 and inhibits wound repair in human airway epithelial cells. Am J Physiol Lung Cell Mo lPhysiol. 2008;295(2):L293–L302. doi: 10.1152/ajplung.00134.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvez F, Chen Y, Brandt-Rauf PW, Bernard A, Dumont X, Slavkovich V, Argos M, D'Armiento J, Foronjy R. Nonmalignant respiratory effects of chronic arsenic exposure from drinking water among never-smokers in Bangladesh. Environ Health Perspect. 2008;116(2):190–195. doi: 10.1289/ehp.9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan K, Sthiannopkao S, Kim KW, Wong MH, Sao V, Hashim JH, Mohamed Yasin MS, Aljunid SM. Health risk assessment of inorganic arsenic intake of Cambodia residents through groundwater drinking pathway. Water Res. 2010;44(19):5777–5788. doi: 10.1016/j.watres.2010.06.021. [DOI] [PubMed] [Google Scholar]
- Provatopoulou X, GA, Kalogera E, Zagouri F, Flessas I, Goussetis E, Nonni A, Papassotiriou I, Zografos G (2009) Circulating levels of matrix metalloproteinase-9 (MMP-9), neutrophil gelatinase-associated lipocalin (NGAL) and their complex MMP-9/NGAL in breast cancer disease. Biomedical Central 9(390). 10.1186/1471-2407-9-390 [DOI] [PMC free article] [PubMed]
- Rahman A, Vahter M, Ekström EC, Persson LÅ. Arsenic exposure in pregnancy increases the risk of lower respiratory tract infection and diarrhea during infancy in Bangladesh. Environ Health Perspect. 2011;119(5):719–724. doi: 10.1289/ehp.1002265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey K. 13 - Arsenic and respiratory disease. In: Flora SJS, editor. Handbook of Arsenic Toxicology. Oxford: Academic Press; 2015. pp. 335–347. [Google Scholar]
- Recio-Vega R, Gonzalez-Cortes T, Olivas-Calderon E, Lantz RC, Gandolfi AJ, Gonzalez-De Alba C. In utero and early childhood exposure to arsenic decreases lung function in children. J ApplToxicol. 2015;35(4):358–366. doi: 10.1002/jat.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds SD, Reynolds PR, Snyder JC, Whyte F, Paavola KJ, Stripp BR. CCSP regulates cross talk between secretory cells and both ciliated cells and macrophages of the conducting airway. Am J Physiol Lung Cell Mol Physiol. 2007;293(1):L114–L123. doi: 10.1152/ajplung.00014.2007. [DOI] [PubMed] [Google Scholar]
- Rong B, Fu T, Gao W, Li M, Rong C, Liu W, Liu H. Reduced serum concentration of CC16 is associated with severity of chronic obstructive pulmonary disease and contributes to the diagnosis and assessment of the disease. International journal of chronic obstructive pulmonary disease. 2020;15:461–470. doi: 10.2147/COPD.S230323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Kaur J, Nagpal AK, Kaur I. Quantitative assessment of possible human health risk associated with consumption of arsenic contaminated groundwater and wheat grains from Ropar Wetand and its environs. Environ Monit Assess. 2016;188(9):506. doi: 10.1007/s10661-016-5507-9. [DOI] [PubMed] [Google Scholar]
- Sherwood CL, Liguori AE, Olsen CE, Lantz RC, Burgess JL, Boitano S. Arsenic compromises conducting airway epithelial barrier properties in primary mouse and immortalized human cell cultures. PLoS One. 2013;8(12):e82970. doi: 10.1371/journal.pone.0082970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AH, Marshall G, Yuan Y, Ferreccio C, Liaw J, von Ehrenstein O, Steinmaus C, Bates MN, Selvin S. Increased mortality from lung cancer and bronchiectasis in young adults after exposure to arsenic in utero and in early childhood. Environ Health Perspect. 2006;114(8):1293–1296. doi: 10.1289/ehp.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AH, Ercumen A, Yuan Y, Steinmaus CM. Increased lung cancer risks are similar whether arsenic is ingested or inhaled. J Expo Sci Environ Epidemiol. 2009;19(4):343–348. doi: 10.1038/jes.2008.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- States JC, Barchowsky A, Cartwright IL, Reichard JF, Futscher BW, Lantz RC. Arsenic toxicology: translating between experimental models and human pathology. Environ Health Perspect. 2011;119(10):1356–1363. doi: 10.1289/ehp.1103441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strimbu K, Tavel JA. What are biomarkers? CurrOpin HIV AIDS. 2010;5(6):463–466. doi: 10.1097/COH.0b013e32833ed177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueland T, Holter JC, Holten AR, Müller KE, Lind A, Bekken GK, Dudman S, Aukrust P, Dyrhol- Riise, AM., & Heggelund, L. Distinct and early increase in circulating MMP-9 in COVID-19 patients with respiratory failure. The Journal of infection. 2020;81(3):163–169. doi: 10.1016/j.jinf.2020.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Environmental Protection Agency (USEPA) (1989). Risk assessment guidance for superfund volume 1 human health evaluation manual (part a) interim final EPA/540/l −89/002 (Vol. I). Washington, DC: United States Environmental Protection Agency.
- United States Environmental Protection Agency (USEPA). (2015). Risk based screening table-generic, summary table. United States Environmental Protection Agency. https://www.epa.gov/risk/regional-screening-levels-rsls-generic-tables
- Wang Y, Duan H, Meng T, Shen M, Ji Q, Xing J, Wang Q, Wang T, Niu Y, Yu T, Liu Z, Jia H, Zhan Y, Chen W, Zhang Z, Su W, Dai Y, Zhang X, Zheng Y. Reduced serum club cell protein as a pulmonary damage marker for chronic fine particulate matter exposure in Chinese population. Environ Int. 2018;112:207–217. doi: 10.1016/j.envint.2017.12.024. [DOI] [PubMed] [Google Scholar]
- Witten ML, Chau B, Sáez E, Boitano S, Lantz RC. Early life inhalation exposure to mine tailings dust affects lung development. Toxicol Appl Pharmacol. 2019;365:124–132. doi: 10.1016/j.taap.2019.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai J, Stern DA, Sherrill DL, Spangenberg AL, Wright AL, Morgan WJ, Halonen M, Martinez FD, Guerra S. Trajectories and early determinants of circulating CC16 from birth to age 32 years. Am J Respir Crit Care Med. 2018;198(2):267–270. doi: 10.1164/rccm.201712-2398LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 26 kb)
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.