Abstract
The aim of this study was to investigate the antifungal activity of a cyclic tetrapeptide from Bacillus velezensis CE 100 against anthracnose-causing fungal pathogen Colletotrichum gloeosporioides. Antifungal compound produced by B. velezensis CE 100 was isolated and purified from ethyl acetate extract of B. velezensis CE 100 culture broth using octadecylsilane column chromatography. The purified compound was identified as cyclo-(prolyl-valyl-alanyl-isoleucyl) based on mass spectrometer and nuclear magnetic resonance analyses. This is the first report of the isolation of a cyclic tetrapeptide from B. velezensis CE 100 culture filtrate. Cyclic tetrapeptide displayed strong antifungal activity at concentration of 1000 µg/mL against C. gloeosporioides mycelial growth and spore germination. Our results demonstrate that the antifungal cyclic tetrapeptide from B. velezensis CE 100 has potential in bioprotection against anthracnose disease of plants caused by C. gloeosporioides.
Keywords: antagonistic bacteria, antifungal cyclic tetrapeptide, anthracnose disease, mycelial growth, spore germination, biocontrol agent
1. Introduction
Phytopathogenic fungi are serious threats to crops. They can reduce the quality and yield of agricultural products [1,2]. Generally, the ideal way to prevent fungal invasion is by the application of fungicides as they require less time to reduce serious crop losses [3,4]. However, fungicides have detrimental impacts, including the emergence of fungicide-resistant pathogens, decline of soil physio–chemical properties, accumulation of toxic compounds, and long residual periods [5,6,7,8,9]. Due to increasing demand of consumers for fungicide-free products, the need for alternative disease control strategies, such as biological control has been emphasized [10,11]. Biological control agents are environment friendly and sustainable for protecting plants against pathogens [8,12,13].
The role of active metabolites derived from biocontrol agents as viable and reliable alternatives to chemical fungicides cannot be underestimated [9,14,15,16,17]. Among various biocontrol agents, Bacillus species show strong abilities to restrict the growth of plant pathogens by synthesizing hydrolytic enzymes and antifungal compounds [14,15,16,18,19]. Moreover, Bacillus species are known to produce peptide antibiotics [20,21,22] used for biocontrol of agricultural crops [23]. Peptide antibiotic compounds have received particular attention as candidates for plant protection products [24,25]. These compounds can permeate and disrupt fungal cell membranes [26,27], thus reducing the likelihood of developing resistance compared to traditional antibiotics [28]. Bacillus velezensis also produce peptide antibiotics that can inhibit the growth of various fungi [16,22]. Its potential utility as a biocontrol agent against several fungal plant pathogens has recently been investigated [16,29,30].
Colletotrichum fungi causing anthracnose disease in many economical crops worldwide, is categorized as one among the top 10 fungal pathogens [31]. These pathogens can invade host plants via melanized appressoria and spread infection by forming primary and secondary hyphae to colonize host plant cells, leading to the development of dark or water-soaked lesions with sunken necrotic tissues at infected areas. Colletotrichum infections are visible as lesions on leaves, fruits, and other parts of plants, resulting in yield losses and reduced crop marketability [32]. Colletotrichum consists of approximately 189 known species with a broad host range and high genetic diversities [33]. Although many Colletotrichum species are seed-borne pathogens, they can exist in soil and dead plant parts as saprophytes. Their spores can be dispersed through water splashing and by wind [33,34]. Anthracnose caused by C. gloeosporioides has been reported from valuable crop plants such as strawberry, dragon fruit, cassava, mango, guava, apple, coffee, avocado, almond, jujube, etc., and causes a serious economic constraint till harvest [35]. Considering the challenges posed by the disease, reliable and cost-efficient biocontrol agents are advocated. Further, the mechanisms of action of biocontrol agent-derived antibiotics against anthracnose caused by C. gloeosporioides remains poorly understood [36].
Numerous microorganisms have been used for controlling fungal diseases including the diseases caused by C. gloeosporioides [14,15,16]. However, many researchers are focusing on antagonistic microorganisms that can more effectively control C. gloeosporioides and improve crop production and quality. Bacillus strains possess the advantage of sporulation which confers heat resistance, desiccation tolerance, and the ability to successfully colonize the plant micro-environment [37,38], thereby restricting pathogen infection [39]. The objectives of this study was to isolate and identify an antifungal cyclic tetrapeptide from B. velezensis CE 100 and, subsequently, investigate its inhibitory effects on mycelial growth and spore germination of plant pathogen C. gloeosporioides.
2. Results
2.1. Antifungal Activities of B. velezensis CE 100 Culture Filtrate against Phytopathogenic Fungi
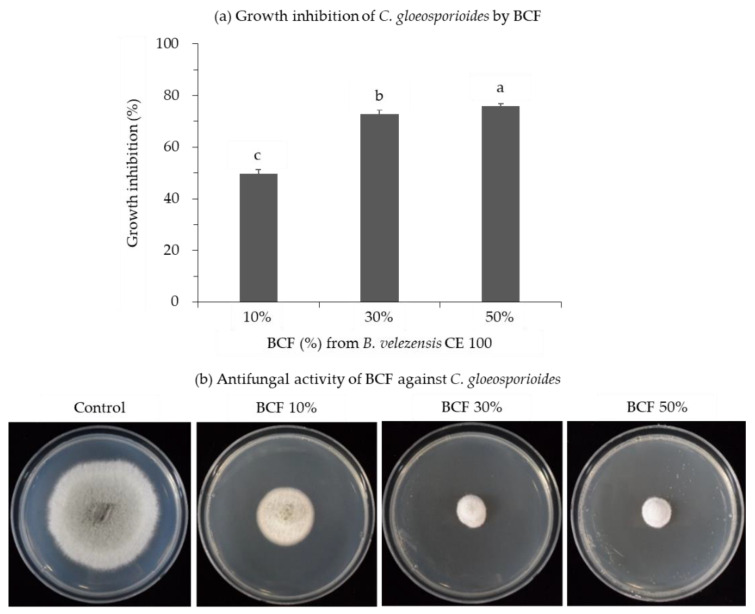
Various concentrations of B. velezensis CE 100 culture filtrate (BCF) were tested for antifungal properties against the plant pathogen C. gloeosporioides (Figure 1). BCF at all concentrations was able to inhibit the growth of the fungal pathogens. This is substantiated by the fact that the mycelial growth inhibition significantly increased with increasing concentration of BCF. BCF at a concentration of 50% showed the highest inhibition (75.9%) of mycelial growth while the inhibition was 49.7% at 10% of BCF concentration (Figure 1).
Figure 1.
Mycelial growth inhibition of C. gloeosporioides by bacterial culture filtrate (BCF) from B. velezensis CE 100. (a) Growth inhibition of C. gloeosporioides by BCF. (b) Antifungal activity of BCF against C. gloeosporioides. Error bars represent standard deviation of the mean. Calculated mean values are from three replicates. Mean with the different letter are significantly different at p < 0.05 when compared using least significant difference test.
2.2. Isolation and Identification of Antifungal Cyclic Tetrapeptide
The antifungal compound (EE3-3) was purified from the ethyl acetate fraction of B. velezensis CE 100 culture broth by medium pressure liquid chromatography (MPLC) coupled with an octadecylsilane (ODS) column. The molecular weight of EE3-3 (380.2) was established by observing a sodiated molecular ion peak at m/z 403.2 (M+Na)+ in the electrospray ionization-mass spectrometry (ESI-MS) spectral analysis. 1H and 13C nuclear magnetic resonance (NMR) spectra of EE3-3 were similar to those of a cyclic tetrapeptide cyclo-(prolyl-leucyl-alanyl-isoleucyl) reported previously [40,41].
Further, the NMR spectral analysis identified EE3-3 to contain a valine [cyclo (prolyl valyl alanyl isoleucyl)] instead of leucine cyclo-(prolyl-leucyl-alanyl-isoleucyl). The 13C spectrum of EE3-3 showed the presence of 19 carbons (Table 1), including four amide carbonyl carbons at δ 172.7 (C-1), 171.4 (C-1″), 169.4 (C-1‴), and 167.7 (C-1′). The 1H NMR spectrum showed four methine protons at δ 4.21 (1H, t, J = 7.5 Hz, H-2), 4.02–4.04 (1H, m, H-2′), 4.01–4.06 (1H, m, H-2″), 3.91 (1H, d, J = 2.5 Hz, H-2‴), and five methyl protons at δ 0.94–1.45. Individual amino acids were assigned based on 1H-1H correlation spectroscopy (COSY; Figure 2, bold lines). Connectivities of the antifungal cyclic tetrapeptide were further confirmed by heteronuclear single quantum correlation (HSQC) and heteronuclear multiple-bond correlation (HMBC, Figure 2, arrows) experiments. The antifungal cyclic tetrapeptide was thus confirmed to be [cyclo-(prolyl-valyl-alanyl-isoleucyl)].
Table 1.
1H (500 MHz) and 13C (125 MHz) NMR data of the antifungal compound in deuterated methanol (CD3OD).
| Residue | Position | δH (Int., Multi., J in Hz) | δC |
|---|---|---|---|
| Proline | 1 | a | 172.7 |
| 2 | 4.21 (1H, t, 7.5) | 60.2 | |
| 3 | 2.31−2.35 (1H, m), 1.91−1.94 (1H, m) b | 29.7 | |
| 4 | 2.03−2.06 (1H, m), 1.91−1.94 (1H, m) b | 23.4 | |
| 5 | 3.52−3.54 (2H, m) | 46.3 | |
| Valine | 1′ | a | 167.7 |
| 2′ | 4.02−4.04 (1H, m) c | 61.7 | |
| 3′ | 2.48−2.51 (1H, m) | 30.0 | |
| 4′ | 1.10 (3H, d, 7.0) | 19.0 | |
| 5′ | 0.94 (3H, d, 6.5) | 16.8 | |
| Alanine | 1″ | a | 171.4 |
| 2″ | 4.01−4.06 (1H, m) c | 51.7 | |
| 3″ | 1.45 (3H, d, 7.0) | 21.1 | |
| Isoleucine | 1‴ | a | 169.4 |
| 2‴ | 3.91 (1H, d, 2.5) | 61.1 | |
| 3‴ | 1.93−1.96 (1H, m) | 40.4 | |
| 4‴ | 1.50−1.55 (1H, m), 1.23−1.29 (1H, m) | 25.8 | |
| 5‴ | 1.03 (3H, d, 6.0) | 15.7 | |
| 6‴ | 0.96 (3H, t, 7.5) | 12.3 |
a No proton signal. b Signals of H-3 and H-4 overlapped. c Signals of H-2′ and H-2″ overlapped. Carbon position is represented as ′ for valine, ″ for alanine, and ‴ for isoleucine.
Figure 2.
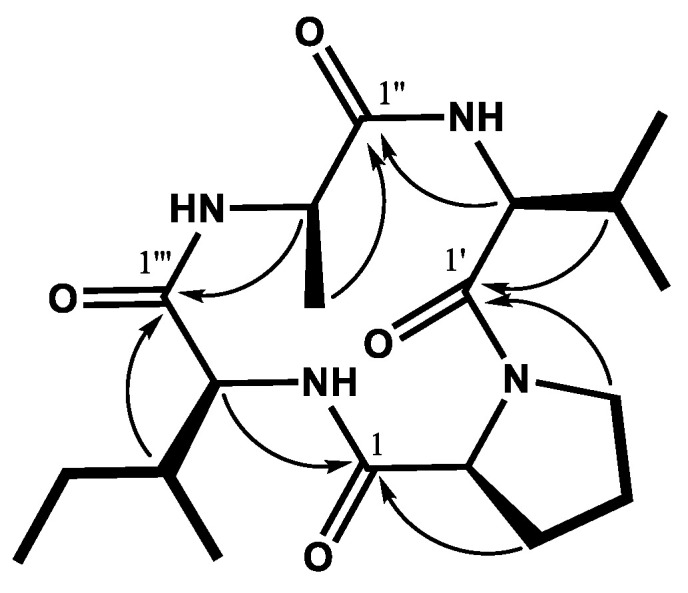
Chemical structure of the antifungal cyclic tetrapeptide [cyclo-(prolyl-valyl-alanyl-isoleucyl)] isolated from B. velezensis CE 100 culture broth underlying 1H-1H COSY (bold lines) and HMBC (arrows) correlations. Carbon position is represented as ′ for valine, ″ for alanine, and ‴ for isoleucine.
2.3. Antifungal Properties of the Cyclic Tetrapeptide against C. gloeosporioides
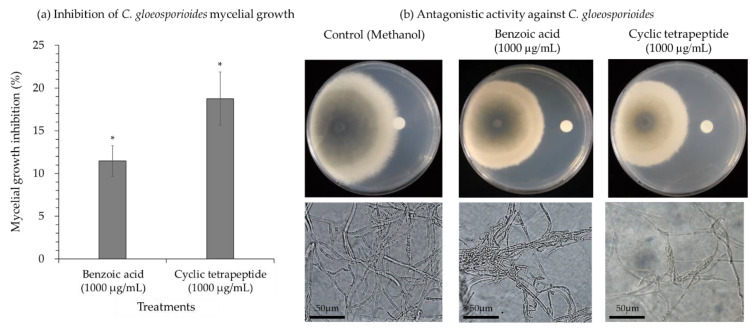
The C. gloeosporioides hyphae were incubated with 1000 µg/mL of the cyclic tetrapeptide and benzoic acid (from B. licheniformis MH48) as a positive control. The degradation and deformation of mycelia were observed (Figure 3). The inhibition rate of the cyclic tetrapeptide on the mycelial growth of C. gloeosporioides was 18.8% in 1000 µg/mL, with significant differences compared to benzoic acid (11.5%) (Figure 3a). Mycelium morphology was observed under light microscopy and showed buckle formation and swelling growth in C. gloeosporioides hyphae in both the treatment conditions (Figure 3b).
Figure 3.
Antifungal efficacy of the cyclic tetrapeptide against C. gloeosporioides. (a) Inhibition of C. gloeosporioides mycelial growth. (b) Antagonistic activity against C. gloeosporioides. The experiment included methanol (as a negative control), benzoic acid derived from B. licheniformis MH148 (as a positive control), and the cyclic tetrapeptide from B. velezensis CE 100 against the fungal pathogen C. gloeosporioides. Error bars represent standard deviation of the mean. Calculated mean values are from three replicates. Asterisk indicates a significant difference between treatments as observed by t-test at p < 0.05.
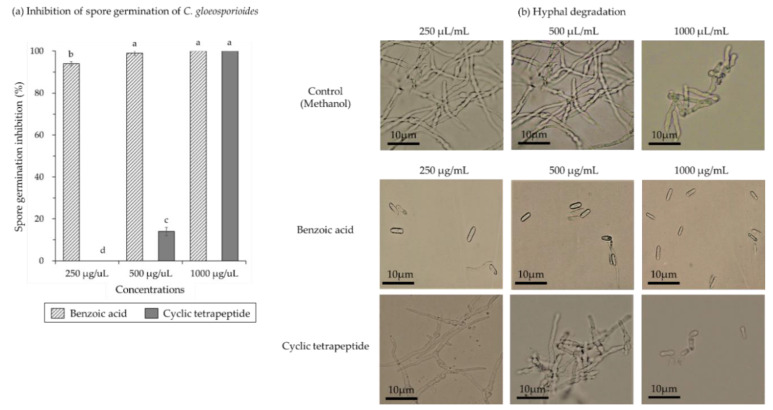
C. gloeosporioides spores germination was significantly inhibited with increasing concentrations of benzoic acid and the cyclic tetrapeptide (Figure 4). The spore germination inhibition was 94.0% at 250 µg/mL of benzoic acid and complete inhibition at 1000 µg/mL (Figure 4a). The spores germinated and developed into hyphae at 250 µg/mL of cyclic tetrapeptide concentration that means no inhibition of C. gloeosporioides spore germination (Figure 4b). At 500 µg/mL, cyclic tetrapeptide concentration 14.0% inhibition of spore germination was observed. The C. gloeosporioides spores germination was totally inhibited at 1000 µg/mL of the cyclic tetrapeptide concentration (Figure 4a).
Figure 4.
Antifungal efficacy of a cyclic tetrapeptide against spore germination of C. gloeosporioides. (a) Spore germination inhibition and (b) hyphal degradation of C. gloeosporioides. The experiment included methanol (as a negative control), benzoic acid derived from B. licheniformis MH148 (as a positive control), and the cyclic tetrapeptide from B. velezensis CE 100 against the fungal pathogen C. gloeosporioides. Error bars represent standard deviation of the mean. Calculated mean values are from three replicates. Mean with the different letters are significantly different at p < 0.05 when compared using least significant difference test.
3. Discussion
Bacillus species demonstrating wide array of bioactive metabolites have been recognized as effective candidates to control several phytopathogens [14,15,16,42,43,44,45]. In this study, the BCF of B. velezensis CE 100 displayed significant antifungal activities against the pathogen, C. gloeosporioides, which causes anthracnose disease in plants. Results of in vitro tests suggested the involvement of secondary metabolites, consistent with findings from several previous studies stating the promiscuous action of the secondary metabolites against fungal pathogens [14,15,16,42,43,44,45]. In this study, we identified a cyclic tetrapeptide compound from B. velezensis CE 100 culture filtrate based on one- and two-dimensional NMR spectral analysis. This is the first report showing the isolation of the cyclic tetrapeptide from B. velezensis CE 100 culture filtrate. 1H and 13C nuclear magnetic resonance (NMR) spectra were similar to those of cyclo-(prolyl-leucyl-alanyl-isoleucyl) as reported previously [40,41].
Cyclic tetrapeptide caused significant antifungal activities against the fungal plant pathogen C. gloeosporioides revealed by the mycelial growth and spore germination results. Especially, the cyclic tetrapeptide inhibited about 1.6-fold of mycelial growth compared to that with the positive control benzoic acid. Moreover, the spore germination was totally inhibited by the action of the cyclic tetrapeptide at 1000 µg/mL. The germination of the hyphae also was inhibited by the action of the cyclic tetrapeptide. However, benzoic acid at 250 µg/mL showed strong inhibition of C. gloeosporioides spore germination. As reported earlier, BCF from B. licheniformis MH48 involves benzoic acid, which inhibits the growth of C. gloeosporioides by 63.1% at 50% of the BCF [14]. Further, at 50% of the BCF from B. velezensis CE 100 a strong antifungal activity (75.9%) was noticed against C. gloeosporioides. The present findings indicate that the mycelial growth and spore germination inhibition could be effective to control the plant pathogen C. gloeosporioides.
4. Materials and Methods
4.1. Bacterial Culture and Fungal Pathogen
The antagonistic bacterial strain B. velezensis CE 100 was isolated from pot soil of tomato plant [44]. The strain was streaked onto tryptone soy agar medium to obtain single colonies. Subsequently, a single colony was inoculated in tryptone soy broth (TSB) and incubated at 30 °C and 130 rpm for 2 days. The resulting cultural broth (107 colony-forming unit (CFU)/mL) was mixed with 50% sterile glycerin and maintained at −80 °C for further experiments. The fungal pathogen C. gloeosporioides KACC 40896 used in this study was provided by Korean Agricultural Culture Collection and sub-cultured on potato dextrose agar (PDA) medium for 7 days at 25 °C.
4.2. Antifungal Activity of B. velezensis CE 100 Culture Filtrate
One single colony of B. velezensis CE 100 was inoculated in TSB medium at 30 °C and 130 rpm for 3 days. Then 500 µL of inoculated culture broth (107 CFU/mL) was inoculated again into fresh TSB medium (500 mL) at 30 °C and 130 rpm with shaking incubator for 7 days. Three replications of the inoculation were maintained. The B. velezensis CE 100 culture broth was centrifuged at 12,000 rpm for 15 min at 4 °C. The supernatant was collected and filtered through four layers of filter paper (Whatman No. 6). The endospore remnants in the culture filtrate were removed using a syringe filter (0.2 µm). The obtained filtrate was used for antifungal studies against C. gloeosporioides.
The PDA medium with different water levels (90%, 70%, and 50%) was prepared in different conical flasks, autoclaved at 121 °C for 15 min, and keep until 60 °C. Then, B. velezensis CE 100 culture filtrate concentrations (10%, 30%, and 50%) was added into each flask, mixed thoroughly, and poured into sterile petri dishes. PDA plates without the culture filtrate were used as controls. A mycelial plug from culture of C. gloeosporioides was placed at the center of the PDA plate and incubated at 25 °C for 7 days. Three replications were maintained for each assay. Mycelial growth inhibition percentage was calculated as (R − r)/R × 100, where R is the radius of the fungal colony in the control plate and r was the radius of the fungal colony in the treatment plate.
4.3. Purification and Characterization of Antifungal Compound
B. velezensis CE 100 was cultured in 12 L of TSB medium for 14 days at 30 °C. The culture broth was centrifuged at 6000 rpm for 30 min and the supernatant was filtered through a filter paper (Whatman No. 6). The culture filtrate was then acidified with concentrated HCl solution to pH 3 and then partitioned successively with n-hexane, chloroform, ethyl acetate, and water-saturated n-butanol (each 12 L). These fractions were concentrated with a vacuum rotary evaporator. The ethyl acetate fraction was found suitable for the inhibition of the growth of C. gloeosporioides by conducting paper disc method.
The antifungal compound was purified from the ethyl acetate fraction using MPLC (Isolera one, Biotage, Sweden) coupled with SNAP Ultra C18 120 g column (Isolera one, Biotage, Sweden) with gradient elution of H2O (A) and MeCN (B). The compounds separated were monitored at 254 and 220 nm with a flow rate of 25 and 50 mL/min. Nine fractions (EA−EI) were separated from the ethyl acetate fraction (5.0 g) by a linear gradient elution of initial 0% B for 5 min→100% B for 30 min. Fraction EE (retention time tR of 15–18 min, 632 mg) was re-eluted by a linear gradient elution of 15% B→35% B for 18 min→35% B for 28 min to obtain seven subfractions (EE1−EE7). The antifungal compound (EE3-3, tR 7.6–8.1 min, 17.2 mg) was finally purified from subfraction EE3 (tR 7.6–8.1 min, 632 mg) by linear gradient elution of 10% B for 12 min→25% B for 36 min (flow rate 25 mL/min).
The antifungal compound (EE3-3, white amorphous powder) was analyzed by MS and NMR experiments. Mass spectra were obtained on a hybrid ion-trap time-of-flight mass spectrometer (SYNAPT G2, Waters, Cambridge, UK) equipped with an electrospray ionization source at Korea Basic Science Institute (KBSI, Ochang, Cheongju, Korea). The antifungal compound was dissolved in deuterated methanol (CD3OD). 1H (500 MHz) and 13C (125 MHz) NMR spectra were acquired using an unityINOVA 500 spectrometer (Varian, Walnut Creek, CA, USA) in Korean Basic Science Institute, Gwangju Center, Korea. The structure of compound was determined by the 1H−1H correlation spectroscopy (COSY), heteronuclear multiple-quantum coherence (HMQC) and heteronuclear multiple-bond correlation (HMBC) experiments.
4.4. Antifungal Properties of the Purified Compound
The purified cyclic tetrapeptide was assayed for the inhibition of the mycelial growth and spore germination against pathogen C. gloeosporioides. The B. licheniformis MH48 derived benzoic acid acted as positive control and was purchased from Daejung Chemicals, Siheung, Korea. The C. gloeosporioides mycelial growth inhibition by the purified cyclic tetrapeptide was assayed using the paper disc method. The purified cyclic tetrapeptide and benzoic acid were dissolved in methanol at 1000 µg/mL. A paper disc was placed on one side of the PDA plate and 50 µL from methanol (negative control), benzoic acid (positive control), and the purified cyclic tetrapeptide was loaded on the paper disc. Then, a mycelial plug (5 mm diameter) of C. gloeosporioides was inoculated at 4 cm distance from the paper disc on the same PDA plate. The experiment was conducted in three replications, wherein the plates were incubated at 25 °C for 7 days. The mycelial growth inhibition of C. gloeosporioides was measured using the following formula: mycelial growth inhibition (%) = (M − m)/M × 100, where M is the radial growth of C. gloeosporioides in the control plate (methanol) and m the radial growth of C. gloeosporioides in the treatment plate (benzoic acid and cyclic tetrapeptide). A small piece of mycelium from the border of C. gloeosporioides colony inhibited by concentrations of the purified cyclic tetrapeptide and benzoic acid was used to observe the deformation of hyphal structures under a light microscope (Olympus BX41TF, Tokyo, Japan).
The spore suspension was prepared using the C. gloeosporioides culture spread on PDA plates for 7 days at 25 °C. The surface of fully sporulated fungal colony was flooded with 10 mL of sterile distilled water and gently scrubbed with sterile spatula. The fungal suspension was filtered through sterile gauze to remove mycelia. The resulting spore suspension was adjusted to 1 × 106 spore/mL using a hemocytometer cell-counting chamber. To measure the effects of the cyclic tetrapeptide on C. gloeosporioides spore germination, 2 mg of the purified cyclic tetrapeptide was first dissolved in 200 µL of methanol. Next, 2 mg of benzoic acid was dissolved in 200 µL of methanol as the positive control. Subsequently, the purified cyclic tetrapeptide and benzoic acid was serially diluted with sterile PDB medium to obtain concentrations of 250, 500, and 1000 µg/mL. Spore suspension of C. gloeosporioides (100 µL) was added to each vial containing different concentrations of purified cyclic tetrapeptide and benzoic acid. The vial with different concentration of methanol (without cyclic tetrapeptide and benzoic acid) was used as a negative control. Each treatment was set as three replicates. The vials were incubated at 25 °C for 10 h. A total of 100 spores from each replication of each treatment were examined using an Olympus BX41 light microscope. Numbers of germinated spores in each treatment were counted. Spore germination inhibition (%) was calculated as (S − s)/S × 100, where S and s represents the number of germinated spore in the control vials (methanol) and treatment vials (cyclic tetrapeptide and benzoic acid), respectively.
4.5. Statistical Analysis
To determine the differences in the mycelial growth inhibitions between benzoic acid and cyclic tetrapeptide against C. gloeosporioides, we used t-test at p < 0.05. The mycelial growth inhibition of the BCF concentrations and the spore germination inhibition of compounds against C. gloeosporioides were determined with the analysis of variance. Mean values were compared using least significant difference test at p < 0.05. All data were performed using SAS 9.0 software (SAS Institute, Cary, NC, USA).
Author Contributions
Conceptualization, funding acquisition, and project administration, Y.S.A.; investigation and experiments, V.C., C.E.H.M., S.-J.W., and J.-H.M.; data analysis, V.C., C.E.H.M., S.-J.W., J.-H.M., and J.-Y.C.; original draft preparation, V.C., K.Y.K., Y.S.H., J.-Y.C., and Y.S.A.; review and editing, V.C., K.Y.K., Y.S.H., J.-Y.C., and Y.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grant no. 2018122B10-1820-AB01 and no. 2020183C10-2022-AA02 of the R&D program for Forest Science Technology funded by the Korea Forest Service (Korea Forestry Promotion Institute). It was also supported by grant no. 2018R1D1A1B07050052 of the National Research Foundation (NRF) of the Basic Science Research Program funded by the Korean government.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All the raw data is available and provided upon request.
Conflicts of Interest
The authors have no conflicts of interest to disclose.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Savary S., Ficke A., Aubertot J.N., Hollier C. Crop losses due to diseases and their implications for global food production losses and food security. J. Food Secur. 2012;4:519–537. doi: 10.1007/s12571-012-0200-5. [DOI] [Google Scholar]
- 2.Almeida F., Rodrigues M.L., Coelho C. The still underestimated problem of fungal diseases worldwide. Front. Microbiol. 2019;10:214. doi: 10.3389/fmicb.2019.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen Y., Coffey M.D. Systemic fungicides and the control of oomycetes. Annu. Rev. Phytopathol. 1986;24:311–338. doi: 10.1146/annurev.py.24.090186.001523. [DOI] [Google Scholar]
- 4.Brent K.J., Hollomon D.W. Fungicide Resistance: The Assessment of Risk. 2nd ed. Fungicide Resistance Action Committee; Brussels, Belgium: 2007. pp. 1–48. [Google Scholar]
- 5.Agrios G.N. Plant Pathology. 3rd ed. Academic Press Inc.; New York, NY, USA: 1988. p. 803. [DOI] [Google Scholar]
- 6.Cook R.J. Making greater use of introduced microorganisms for biological control of plant pathogens. Annu. Rev. Phytopathol. 1993;31:53–80. doi: 10.1146/annurev.py.31.090193.000413. [DOI] [PubMed] [Google Scholar]
- 7.Chae D.H., Jin R.D., Bo H.J., Kim Y.W., Kim Y.C., Park R.D., Krishnan H.B., Kim K.Y. Control of late blight (Phytophthora capsici) in pepper plant with a compost containing multitude of chitinase–producing bacteria. BioControl. 2005;51:339–351. doi: 10.1007/s10526-005-2934-x. [DOI] [Google Scholar]
- 8.Heydari A., Misaghi I.J., Balestra G.M. Pre-emergence herbicides influence the efficacy of fungicides in controlling cotton seedling damping-off in the field. Int. J. Agric. Res. 2007;2:1049–1053. doi: 10.3923/ijar.2007.1049.1053. [DOI] [Google Scholar]
- 9.Shafi J., Hui T., Mingshan J. Bacillus species as versatile weapons for plant pathogens: A review. Biotechnol. Biotechnol. Equip. 2017;31:446–459. doi: 10.1080/13102818.2017.1286950. [DOI] [Google Scholar]
- 10.Baker K.F. Evolving concepts of biological control of plant pathogens. Annu. Rev. Phytopathol. 1987;25:67–85. doi: 10.1146/annurev.py.25.090187.000435. [DOI] [Google Scholar]
- 11.Cook R.J., Bruckart W.L., Coulson J.R., Goettel M.S., Humber R.A., Lumsden R.D., Maddox J.V., McManus M.L., Moore L., Meyer S.F., et al. Safety of microorganisms intended for pest and plant disease control: A framework for scientific evaluation. Biol. Control. 1996;7:333–351. doi: 10.1006/bcon.1996.0102. [DOI] [Google Scholar]
- 12.Haggag M., Wafaa M.H. Sustainable agriculture management of plant diseases. Online J. Biol. Sci. 2002;2:280–284. doi: 10.3923/jbs.2002.280.284. [DOI] [Google Scholar]
- 13.Romanazzi G., Lichter A., Gabler F.M., Smilanick J.L. Recent advances on the use of natural and safe alternatives to conventional methods to control postharvest gray mold of table grapes. Postharvest Biol. Technol. 2012;63:141–147. doi: 10.1016/j.postharvbio.2011.06.013. [DOI] [Google Scholar]
- 14.Jeong M.H., Lee Y.S., Cho J.Y., Ahn Y.S., Moon J.H., Hyun H.N., Cha G.S., Kim K.Y. Isolation and characterization of metabolites from Bacillus licheniformis MH48 with antifungal activity against plant pathogens. Microb. Pathog. 2017;110:645–653. doi: 10.1016/j.micpath.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 15.Xu B.H., Lu Y.Q., Ye Z.W., Zheng Q.W., Wei T., Lin J.F., Guo L.Q. Genomics-guided discovery and structure identification of cyclic lipopeptides from the Bacillus siamensis JFL15. PLoS ONE. 2018;13:e0202893. doi: 10.1371/journal.pone.0202893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin P., Wang H., Tan Z., Xuan Z., Dahar G.Y., Li Q.X., Miao W., Liu W. Antifungal mechanism of bacillomycin D from Bacillus velezensis HN-2 against Colletotrichum gloeosporioides Penz. Pestic. Biochem. Physiol. 2019;163:102–107. doi: 10.1016/j.pestbp.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 17.Maracahipes Á.C., Taveira G.B., Sousa–Machado L.Y., Machado O., Rodrigues R., Carvalho A.O., Gomes V.M. Characterization and antifungal activity of a plant peptide expressed in the interaction between Capsicum annuum fruits and the anthracnose fungus. Biosci. Rep. 2019;39 doi: 10.1042/BSR20192803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Won S.J., Choub V., Kwon J.H., Kim D.H., Ahn Y.S. The Control of Fusarium Root Rot and Development of Coastal Pine (Pinus thunbergii Parl.) Seedlings in a Container Nursery by Use of Bacillus licheniformis MH48. Forests. 2019;10:6. doi: 10.3390/f10010006. [DOI] [Google Scholar]
- 19.Won S.J., Kwon J.H., Kim D.H., Ahn Y.S. The effect of Bacillus licheniformis MH48 on control of foliar fungal diseases and growth promotion of Camellia oleifera seedlings in the coastal reclaimed land of Korea. Pathogens. 2019;8:6. doi: 10.3390/pathogens8010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang X., Li B., Wang Y., Guo Q., Lu X., Li S., Ma P. Lipopeptides, a novel protein, and volatile compounds contribute to the antifungal activity of the biocontrol agent Bacillus atrophaeus CAB-1. Appl. Microbiol. Biotechnol. 2013;97:9525–9534. doi: 10.1007/s00253-013-5198-x. [DOI] [PubMed] [Google Scholar]
- 21.Cao Y., Pi H., Chandrangsu P., Li Y., Wang Y., Zhou H., Xiong H., Helmann J.D., Cai Y. Antagonism of two plant–growth promoting Bacillus velezensis isolates against Ralstonia solanacearum and Fusarium oxysporum. Sci. Rep. 2018;8:4360. doi: 10.1038/s41598-018-22782-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anthony T., Rajesh T., Kayalvizhi N., Gunasekaran P. Influence of medium components and fermentation conditions on the production of bacteriocin(s) by Bacillus licheniformis AnBa9. Bioresour. Technol. 2009;100:872–877. doi: 10.1016/j.biortech.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 23.Chen X.H., Koumoutsi A., Scholz R., Schneider K., Vater J., Süssmuth R., Piel J., Borriss R. Genome analysis of Bacillus amyloliquefaciens FZB42 reveals its potential for biocontrol of plant pathogens. J. Biotechnol. 2009;140:27–37. doi: 10.1016/j.jbiotec.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Marcos J.F., Muñoz A., Perez-Paya E., Misra S., López-García B. Identification and rational design of novel antimicrobial peptides for plant protection. Annu. Rev. Phytopathol. 2008;46:273–301. doi: 10.1146/annurev.phyto.121307.094843. [DOI] [PubMed] [Google Scholar]
- 25.Yuan J., Li B., Zhang N., Waseem R., Shen Q., Huang Q. Production of bacillomycin-and macrolactin-type antibiotics by Bacillus amyloliquefaciens NJN-6 for suppressing soilborne plant pathogens. J. Agric. Food Chem. 2012;60:2976–2981. doi: 10.1021/jf204868z. [DOI] [PubMed] [Google Scholar]
- 26.Regente M.C., Giudici A.M., Villalaín J., De la Canal L. The cytotoxic properties of a plant lipid transfer protein involve membrane permeabilization of target cells. Lett. Appl. Microbiol. 2005;40:183–189. doi: 10.1111/j.1472-765X.2004.01647.x. [DOI] [PubMed] [Google Scholar]
- 27.Lee J., Lee D.G. Antifungal properties of the peptide derived from the signal peptide of the HIV-1 regulatory protein. Rev. FEBS Lett. 2009;583:1544–1547. doi: 10.1016/j.febslet.2009.03.063. [DOI] [PubMed] [Google Scholar]
- 28.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 29.Grady E.N., MacDonald J., Ho M.T., Weselowski B., McDowell T., Solomon O., Renaud J., Yuan Z.C. Characterization and complete genome analysis of the surfactin-producing, plant-protecting bacterium Bacillus velezensis 9D-6. BMC Microbiol. 2019;19:5. doi: 10.1186/s12866-018-1380-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park G., Nam J., Kim J., Song J., Kim P.I., Min H.J., Lee C.W. Structure and mechanism of surfactin peptide from Bacillus velezensis antagonistic to fungi plant pathogens. Bull. Korean Chem. Soc. 2019;40:704–709. doi: 10.1002/bkcs.11757. [DOI] [Google Scholar]
- 31.Dean R., Van Kan J.A., Pretorius Z.A., Hammond-Kosack K.E., Di Pietro A., Spanu P.D., Rudd J.J., Dickman M., Kahmann R., Ellis J., et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012;13:414–430. doi: 10.1111/j.1364-3703.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma M., Kulshrestha S. Colletotrichum gloeosporioides: An anthracnose causing pathogen of fruits and vegetables. Biosci. Biotech. Res. Asia. 2015;12:1233–1246. doi: 10.13005/bbra/1776. [DOI] [Google Scholar]
- 33.Baroncelli R., Talhinhas P., Pensec F., Sukno S.A., Le Floch G., Thon M.R. The Colletotrichum acutatum species complex as a model system to study evolution and host specialization in plant pathogens. Front. Microbiol. 2017;8:2001. doi: 10.3389/fmicb.2017.02001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cannon P.F., Damm U., Johnston P.R., Weir B.S. Colletotrichum–current status and future directions. Stud. Mycol. 2012;73:181–213. doi: 10.3114/sim0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Erpelding J.E. Field Assessment of Anthracnose disease response for the Sorghum germplasm collection from the Mopti region. Am. J. Agri. Biol. Sci. 2010;5:363–369. doi: 10.3844/ajabssp.2010.363.369. [DOI] [Google Scholar]
- 36.Vinale F., Sivasithamparam K., Ghisalberti E.L., Marra R., Woo S.L., Lorito M. Trichoderma plant pathogen interactions. Soil Biol. Biochem. 2008;40:1–10. doi: 10.1016/j.soilbio.2007.07.002. [DOI] [Google Scholar]
- 37.Molina–Romero D., Baez A., Quintero–Hernández V., Castañeda–Lucio M., Fuentes–Ramírez L.E., Del Rocío Bustillos–Cristales M., Rodríguez–Andrade O., Morales–García Y.E., Munive A., Muñoz–Rojas J. Compatible bacterial mixture, tolerant to desiccation, improves maize plant growth. PLoS ONE. 2017;12:e0187913. doi: 10.1371/journal.pone.0187913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bressuire–Isoard C., Broussolle V., Carlin F. Sporulation environment influences spore properties in Bacillus: Evidence and insights on underlying molecular and physiological mechanisms. FEMS Microbiol. Rev. 2018;42:614–626. doi: 10.1093/femsre/fuy021. [DOI] [PubMed] [Google Scholar]
- 39.Wu L., Wu H.J., Qiao J., Gao X., Borriss R. Novel routes for improving biocontrol activity of Bacillus based bioinoculants. Front. Microbiol. 2015;6:1395. doi: 10.3389/fmicb.2015.01395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rungprom W., Siwu E.R.O., Lambert L.K., Dechsakulwatana C., Barden M.C., Kokpol U., Blanchfield J.T., Kita M., Garson M.J. Cyclic tetrapetides from marine bacteria associated with the seaweed Diginea sp. and the sponge Halisarca ectofibrosa. Tetrahedron. 2008;64:3147–3152. doi: 10.1016/j.tet.2008.01.089. [DOI] [Google Scholar]
- 41.Napitupulu O.I., Sumiarsa D., Subroto T., Nurleasari, Harneti D., Supratman U., Maharani R. Synthesis of cyclo-PLAI using a combination of solid- and solution-phase methods. Synth. Commun. 2019;49:308–315. doi: 10.1080/00397911.2018.1554148. [DOI] [Google Scholar]
- 42.Thaniyavarn J., Roongsawang N., Kameyama T., Haruki M., Imanaka T., Morikawa M., Kanaya S. Production and characterization of biosurfactants from Bacillus licheniformis F2.2. Biosci. Biotechnol. Biochem. 2003;67:1239–1244. doi: 10.1271/bbb.67.1239. [DOI] [PubMed] [Google Scholar]
- 43.Souto G.I., Correa O.S., Montecchia M.S., Kerber N.L., Pucheu N.L., Bachur M., García A.F. Genetic and functional characterization of a Bacillus sp. strain excreting surfactin and antifungal metabolites partially identified as iturinlike compounds. J. Appl. Microbiol. 2004;97:1247–1256. doi: 10.1111/j.1365-2672.2004.02408.x. [DOI] [PubMed] [Google Scholar]
- 44.Korenblum E., von der Weid I., Santos A.L.S., Rosado A.S., Sebastián G.V., Coutinho C.M., Magalhães F.C., Paiva M.M., Seldin L. Production of antimicrobial substances by Bacillus subtilis LFE-1, B. firmus H2O-1 and B. licheniformis T6-5 isolated from an oil reservoir in Brazil. J. Appl. Microbiol. 2005;98:667–675. doi: 10.1111/j.1365-2672.2004.02518.x. [DOI] [PubMed] [Google Scholar]
- 45.Choi T.G., Maung C.E.H., Lee D.R., Henry A.B., Lee Y.S., Kik K.Y. Role of bacterial antagonists of fungal pathogens, Bacillus thuringiensis KYC and Bacillus velezensis CE 100 in control of root-knot nematode, Meloidogyne incognita and subsequent growth promotion of tomato. Biocontrol. Sci. Technol. 2020;30:685–700. doi: 10.1080/09583157.2020.1765980. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the raw data is available and provided upon request.