Abstract
Protein lipoxidation is a non-enzymatic post-translational modification that consists of the covalent addition of reactive lipid species to proteins. This occurs under basal conditions but increases in situations associated with oxidative stress. Protein targets for lipoxidation include metabolic and signalling enzymes, cytoskeletal proteins, and transcription factors, among others. There is strong evidence for the involvement of protein lipoxidation in disease, including atherosclerosis, neurodegeneration, and cancer. Nevertheless, the involvement of lipoxidation in cellular regulatory mechanisms is less understood. Here we review basic aspects of protein lipoxidation and discuss several features that could support its role in cell signalling, including its selectivity, reversibility, and possibilities for regulation at the levels of the generation and/or detoxification of reactive lipids. Moreover, given the great structural variety of electrophilic lipid species, protein lipoxidation can contribute to the generation of multiple structurally and functionally diverse protein species. Finally, the nature of the lipoxidised proteins and residues provides a frameshift for a complex interplay with other post-translational modifications, including redox and redox-regulated modifications, such as oxidative modifications and phosphorylation, thus strengthening the importance of detailed knowledge of this process.
Keywords: lipoxidation, electrophilic lipids, oxidative stress, cell signalling, regulation, selectivity, post-translational modifications
1. Introduction
Lipids constitute a structurally and functionally heterogeneous group of hydrophobic biomolecules that, among other species, include fatty acids, triacylglycerols, phospholipids, and sterols. Lipids are essential components of cellular membranes, serve as key molecules for the storage of energy, and play important metabolic and signalling functions [1,2]. Lipids can undergo various metabolic transformations, which contribute to their great structural and functional variety. Among these reactions, lipid oxidation is a common transformation that occurs in physiological conditions as a consequence of cellular metabolism but is usually increased under conditions of oxidative stress, i.e., in situations where there is a redox imbalance potentially leading to cellular damage. Both enzymatic and non-enzymatic mechanisms can be involved in lipid oxidation, and may occur by radical or non-radical attack [3]. Oxidized lipids play important roles in inflammation, atherosclerosis, cancer and ageing [4,5,6]. Importantly, some oxidized lipids are or lead to the formation of reactive or electrophilic molecules that can form covalent adducts with other macromolecules, including proteins. Electrophilic lipids can also arise from dehydration or nitration [7,8]. Thus, the term protein lipoxidation refers to the non-enzymatic post-translational modification (PTM) of proteins by reactive or electrophilic lipid species, which frequently arise from lipid oxidation. There continues to be some confusion in the literature between the terms lipid oxidation and lipoxidation, with some researchers erroneously assuming they are synonymous. In addition, it is important to distinguish both terms from protein lipidation. This process refers to the PTM of proteins by lipid moieties, which usually occurs through enzymatic mechanisms, can involve structurally varied lipids, such as glycosylphosphatidylinositol, fatty acids, isoprenoids, and cholesterol, and generally affects protein hydrophobicity and localization and/or protein-membrane or protein-protein interactions [9]. Lipidation can take place at the N- or C-terminus as well as at cysteine, serine, and lysine residues [9,10]. Moreover, lipids can be non-covalently associated with proteins forming complex particles known as lipoproteins, which are constituted by a cholesterol-triglyceride core surrounded by phospholipids, other lipids, and embedded proteins [11]. Lipoproteins are essential elements in lipid transport and metabolism, as well as in cardiovascular pathophysiology, and both their lipid and protein components can undergo various oxidations [12]. In this article, we will clarify the terminology (Box 1) and explain the process of protein lipoxidation, before addressing advanced aspects of the effects of this PTM and pointing out as yet unanswered questions in the field.
Box 1. Terminology and Definitions.
Lipid Oxidation: an overall term encompassing both radical and non-radical (electrophilic) reactions and leading to an increase in the number of oxygens and other heteroatoms (such as nitrogen or chlorine) or a decrease in the hydrogen content of the lipid.
Lipid Peroxidation: a specific form of radical attack, usually at bis-allylic sites in an unsaturated hydrocarbon chain, that leads first to a carbon-centred radical and then the addition of molecular oxygen to form a peroxyl radical (-O-O•) on that carbon. The peroxyl radical remains reactive and can abstract hydrogens from adjacent molecules, resulting in a chain reaction and propagation of damage.
Lipoxidation: covalent reaction of reactive and electrophilic lipid products, mostly arising from lipid oxidation, for example, aldehydes or α,β-unsaturated breakdown products such as acrolein and 4-hydrononenal, or cyclopentenone-containing lipids (e.g., 15-deoxy-Δ12,14-prostaglandin J2) with macromolecules. The targets of lipoxidation include proteins, DNA or head groups of phospholipids.
Advanced Lipoxidation End-products (ALEs): the covalent adducts formed by the process of lipoxidation.
Protein lipoxidation: the modification of proteins by electrophilic lipids. Although is not an oxidative modification per se, it frequently contributes to the damage to proteins under oxidative stress conditions.
Protein lipidation: enzymatically-catalysed covalent modification of proteins by lipids, which usually enable the proteins to associate with membranes. Typical examples include N-myristoylation, S-palmitoylation, or S-prenylation, as well as the addition of a glycosylphosphatidylinositol anchor.
Lipoproteins: particles formed by amphipathic proteins embedded in a phospholipid monolayer and surrounding an inner core of cholesterol, cholesterol esters and triacylglycerols. They function as lipid transporters and are commonly found in plasma.
2. Lipid Oxidation and Protein Lipoxidation
Lipid oxidation can occur enzymatically, catalysed by cyclooxygenases (COX-1/2/3), lipoxygenases (LOX), and cytochrome P450-dependent enzymes (CYP450), or non-enzymatically, when it is mediated by carbon and oxygen-centred radicals [13,14]. Enzymatic pathways give rise to bioactive mediators, such as prostaglandins (PG), thromboxanes and leukotrienes, among others, which have been broadly studied and can act as physiological signalling molecules, with an important role as immunomodulators [15]. Non-enzymatic mechanisms are adventitious oxidations that commonly occur on phospholipids present in cellular membranes and on lipoproteins [12]. Lipids containing unsaturated fatty acyl chains, and particularly those that are polyunsaturated fatty acids (PUFAs), such as linoleic and arachidonic acids, are more vulnerable to attack by different reactive species of oxygen, nitrogen and halogens [16]. In lipid peroxidation, peroxyl radicals are formed as intermediary products [3]. Hence, in a phospholipid bilayer environment, a single radical attack can entail a chain reaction based on a cascade of radical hydrogen abstractions and oxidations. Peroxyl radicals, and to a lesser extent hydroperoxides, are unstable and undergo subsequent reactions including further oxidation, cleavage and cyclization reactions to form a variety of secondary oxidation products (reviewed by [17,18]).
Many of these secondary products, including full-chain length oxidized phospholipids, phospholipids with a truncated fatty acyl chain, and non-esterified breakdown products, are reactive electrophilic products that undergo further rearrangements. Both full-length and shortened fatty acyl chains can contain epoxides, hydroxides, and carboxylic acids as well as reactive carbonyl moieties and α,β-unsaturated alkenal moieties, which are highly reactive [3,19,20,21]. In general, alkenals, and hydroxy- or oxo-alkenals are the most reactive and versatile in terms of their reactivity [17,22,23,24]. Certain reactive lipid products can be formed by enzymatic and/or non-enzymatic reactions. Dehydration of PG synthetised via COX enzymes or non-enzymatically through the isoprostane pathway leads to the generation of cyclopentenone prostaglandins (cyPG) or of keto-PG, which contain unsaturated carbonyl moieties in the cyclopentenone ring and/or in the lateral chains [25,26,27,28]. Lipids can also be attacked by reactive nitrogen species (RNS) to give rise to nitro-alkenals that also can covalently modify proteins [29].
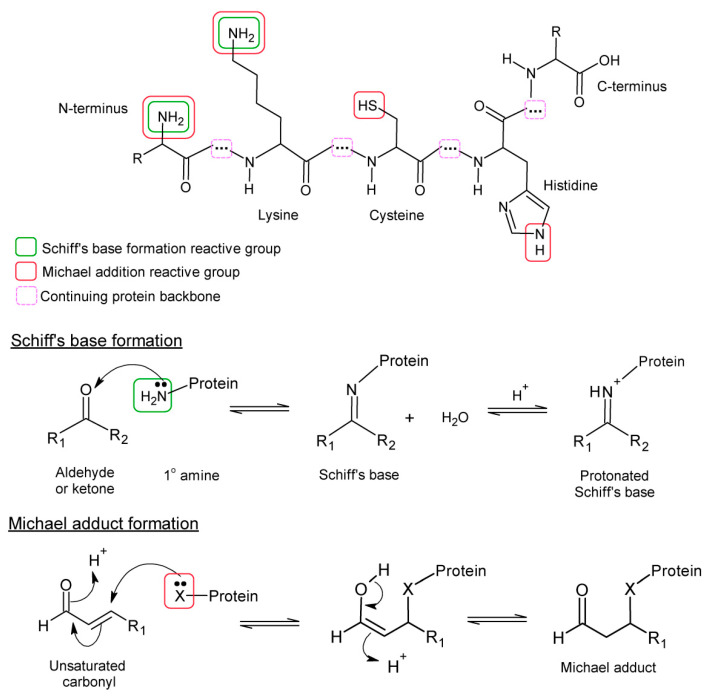
Protein lipoxidation involves the formation of Schiff’s bases or Michael adducts. Schiff’s bases are formed by the reaction between carbonyls (aldehydes or ketones) and primary amines, and consequently can only form on lysine or amino-terminal residues in proteins. In contrast, Michael adducts are formed by reaction of a nucleophile with the β-carbon of an α,β-alkenal, and reactivity is enhanced by the presence of an electron-withdrawing group on the γ carbon, such as in 4-hydroxynonenal (HNE) or 4-oxononenal (ONE). In proteins, the nucleophilic group is most commonly cysteine (in the thiolate form), lysine (primary amine in deprotonated form) or histidine (secondary amine in deprotonated form). Adducts with asparagine and glutamine side chains have been reported for certain lipid species despite the lower nucleophilicity of the amino group in an amide [30]. Arginine can form Michael adducts only when deprotonated, which is rare in physiological conditions as the guanidino group is highly basic. This different reactivity of protein residues and lipid species influences the selectivity of protein lipoxidation as will be discussed below. A summary of the mechanisms is given in Figure 1, while detailed reaction mechanisms for the formation of these adducts and their subsequent rearrangements can be found in other reviews [19,31]. Some of the most studied and interesting electrophilic lipids involved in protein lipoxidation are considered briefly below and in Table 1.
Figure 1.
Formation of Schiff’s base and Michael adducts with protein residues. The structures of the lysine, cysteine and histidine residues are shown at the top, with the moieties involved in nucleophilic attack indicated. The histidine imidazole ring exists in 2 resonance forms where the hydrogen can reside on either nitrogen, so either nitrogen can undertake nucleophilic attack. Schiff’s base formation with an amino group is shown in the centre. The Schiff’s base reactions are reversible, involving a hydrolysis reaction. The bottom panel shows Michael adduct formation by nucleophilic group X, where X represents a primary or secondary amine or a thiol. Michael adducts can also decompose by reversal of these reactions, although they are more stable than Schiff’s bases.
Table 1.
Examples of electrophilic lipid products that can cause lipoxidation.
| Reactive Lipid Product | Type | Source | Reactions Reported With | Cross-Linking |
|---|---|---|---|---|
| Malondialdehyde | bis-aldehyde, isomerizes to β-hydroxy-acrolein | Polyunsaturated chains with ≥3 double bonds | Lys (Michael and Schiff’s) His, Arg, Cys (Michael) | √ |
| Acrolein | Alkenal (3 carbons) (α-β-unsaturated aldehyde) | Polyunsaturated lipids but also other environmental sources | Lys (Michael and Schiff’s) His, Cys (Michael) | √ |
| Crotonaldehyde | Alkenal (4 carbons) (α-β-unsaturated aldehyde) | ω-3 unsaturated lipids (α-linolenic, eicosapentaenoic or docosahexaenoic acid) | Lys (Michael and Schiff’s) His, Cys (Michael) | √ |
| 4-hydroxy-2- hexenal (HHE) | 4-hydroxy-alkenal (α-β-unsaturated aldehyde) | ω-3 polyunsaturated lipids (α-linolenic, eicosapentaenoic or docosahexaenoic acid) |
Lys (Michael and Schiff’s) His, Cys (Michael) |
√ |
| 4-hydroxy-2-nonenal (HNE) | 4-hydroxy-alkenal (α-β-unsaturated aldehyde) |
ω-6 polyunsaturated lipids (γ-linolenic or arachidonic acid) |
Lys (Michael and Schiff’s) His, Cys (Michael) |
√ |
| 4-oxo-2-nonenal (ONE) | 4-oxo-alkenal (α-β-unsaturated aldehyde) |
ω-6 polyunsaturated lipids (γ-linolenic or arachidonic acid) |
Lys (Michael and Schiff’s) His, Cys (Michael) |
√ |
| 15-deoxy-Δ12,14-prosta-glandin J2 (15d-PGJ2) | Cyclopentenone prostaglandin (cyPG) | Arachidonic acid | His, Cys (Michael) | √ |
| 15-keto-prostaglandin E2 | Prostaglandin | Arachidonic acid | Cys (Michael) | X |
| Palmitoyl-oxovaleroyl phosphatidylcholine (POVPC) | Esterified alkenal | ω-6 polyunsaturated lipids (γ-linolenic or arachidonic acid) | Lys (Michael and Schiff’s) His, Cys (Michael) | X |
| Palmitoyl-oxononanoyl phosphatidylcholine (PONPC) | Esterified alkenal | ω-6 polyunsaturated lipids (γ-linolenic or arachidonic acid) | Lys (Schiff’s base only) | X |
| Isolevuglandins (isoLGs) and Isoketals | γ-keto-aldehydes | Arachidonic acid and docosahexenoic acid | Lys (Schiff’s base only) | √ |
| Nitro-oleate and nitro-linoleate | Nitro-fatty acids (NO2-FAs) (can be esterified in PLs) | Unsaturated fatty acyl chains (e.g., oleoyl or linoleoyl) | Lys, His, Cys (Michael) (nitro-alkylation) | X |
| Chloro-hexadecanal or chloro-octadecanal | Chloro-fatty aldehydes | Plasmenyl phospholipids (palmitate or stearate attached by vinyl ether bond) | Lys (Schiff’s base only) | X |
Diverse electrophilic lipid species from different origins can covalently bind to proteins (protein lipoxidation) through the formation of Michael and/or Schiff’s adducts with nucleophilic residues. Some of the reported species can induce protein crosslinking. For detailed information, please see [23,24,49,50,51].
Reactive lipid products can be grouped into chemical families according to their reactive chemical groups, which determine their reactivity in lipoxidation reactions. Owing in part to their availability, as well as their biological actions, some reactive lipid products have been much more extensively studied than others. The small, non-esterified aldehydes malondialdehyde (MDA), acrolein, and HNE fall into this category [23,32]. Of these, HNE is the most toxic, acrolein is the most reactive, and MDA is the most mutagenic [33,34,35], reviewed in [10,22,36]; these effects ultimately relate to their potential to cause lipoxidation. In contrast, there are many fewer publications on other aldehydes such as crotonaldehyde, pentanal, hexenal, 4-hydroxy-hexenal (HHE) and 4-hydroxy-dodecadienal, although some of them may be formed physiologically in sufficient amounts to have biological effects and evidence is emerging that they also modify proteins and affect their functions. Substantial research has also been devoted to long-chain species, especially isoprostanes, isolevuglandins, PG species such as cyPG, and nitrated fatty acids (NO2-FAs), in part due to their signalling properties [37,38,39,40]. Whereas isoprostanes are important as biomarkers of oxidative stress [41], the behaviour of certain eicosanoids including cyPG, and of NO2-FAs as transcription factor agonists and mediators of inflammatory resolution has raised high interest in their potential therapeutic applications. Moreover, cyPG have been used as model compounds for the identification of lipoxidation targets in proteomic studies [27]. Interest in oxidized and nitrated phospholipids as potential agents of lipoxidation is more recent but nevertheless of emerging physiological importance. In summary, the propensity of a lipoxidation adduct to be formed depends on the reactivity of the lipid oxidation product, the nucleophilicity of the target amino acid in the protein, and the stability of the product generated [42]. Furthermore, the initial adducts can undergo additional rearrangements, including reactions with other nucleophilic groups to cause inter- or intra-molecular cross-links, resulting in linear or cyclic stable products [19,43]. Thus, protein lipoxidation contributes to the generation of protein diversity through PTMs, with a variety of structural and functional consequences.
Oxidation of lipid components of membranes occurs in all living organisms. Therefore, the process of protein lipoxidation would be expected to be universal. Indeed, although much work has focused on mammalian and clinical samples, protein lipoxidation has also been studied in plants and microorganisms. For example, immunoblot analysis using monoclonal antibodies against reactive aldehyde-derived protein modifications showed that in spinach leaves grown in normal conditions the oxygen-evolving complex protein 33 was modified by MDA, acrolein and crotonaldehyde [44], while salt stress in Arabidopsis thaliana resulted in modification of soluble proteins by HNE, HHE, crotonaldehyde, acrolein and MDA [45]. Excellent reviews that highlight the importance of this PTM in plants are available [46,47]. In contrast, little information on natural lipoxidation and its effects are available for fungi and bacteria, although it has been reported that bactericidal antibiotic treatments lead to the formation of MDA adducts [48]. The chemistry and generic consequences of lipoxidation on protein function are expected to be similar in all of these organisms and will depend on the context and the protein target. However, most of the examples provided in this review will be related to animal models in general and human health in particular, in relation to the pathophysiological consequences of this modification.
3. Functional Consequences of Lipoxidation
Proteins serve a wide array of functions in the cellular and extracellular contexts, which are subjected to complex control, often involving enzymatically generated PTMs, such as phosphorylation, glycosylation, ubiquitination, methylation and lipidation, to mention just a few. Non-enzymatic modifications of proteins also involve a plethora of chemical transformations of protein residues that, especially at high levels, can have deleterious effects on protein structure and function. Nevertheless, oxidative and electrophilic PTMs, including lipoxidation, may also contribute to the regulation of protein function and play an important role in redox signalling [19,52,53]. Below, the different effects of lipoxidation of proteins are considered, together with their potential as physiological regulatory processes. Table 2 provides examples of protein targets of lipoxidation and of its functional consequences, which are schematically illustrated in Figure 2.
Table 2.
Examples of lipoxidation targets.
| Category | Protein | Lipid | Residue | Implication | Reference |
|---|---|---|---|---|---|
| Cytoskeletal protein | Vimentin | 15d-PGJ2, PGA1 HNE | Cys328 | Filament reorganisation |
[73,74] [75,76] |
| GFAP | 15d-PGJ2, PGA1 | Cys294 | Filament reorganisation |
[77] | |
| Actin | HNE PGA1 15d-PGJ2 Acrolein |
Cys374 Cys374 Cys374, His87, His173 |
Electrophilic scavenger, filament disruption |
[78] [79] [80] [81] |
|
| Tubulin | HNE | Cys295 | Filament reorganisation |
[75] | |
| Metabolic enzymes |
AKR1B1 AKR1B AKR1B10 |
Acrolein HNE PGA1 |
Cys298 Cys298 Cys299 |
Activation Inhibition Inhibition |
[56] [57] [82] |
| α-Enolase | HNE 15d-PGJ2 |
? * | Inhibition | [83] [80] |
|
| Soluble epoxide hydrolase | 15d-PGJ2 | Cys521 | Inhibition | [84] | |
| Pyruvate kinase | 15d-PGJ2 Acrolein, HHE, MDA HNE, ONE |
? Cys152, Cys358, Cys423, Cys474 Cys424, His439 |
? Inhibition Inhibition |
[74] [33] [85] |
|
| Pin1 | HNE | Cys113 | Inhibition | [86] | |
| Chaperones | Hsp 90 | 15d-PGJ2 PGA1 HNE, ONE |
? Cys572 |
Inhibition | [74] [73,87] [88] |
| Hsp 70 | cyPG HNE |
? Cys267 |
Inhibition | [84,87] [89] |
|
| Transcription factor | PPARγ | NO2-FAs 15d-PGJ2 | Cys285 Cys285 |
Activation | [90] [91,92] |
| P53 | 15d-PGJ2 | Cys277 | Inhibition | [93] | |
| NF-κB | 15d-PGJ2, PGA1 | Cys38 (p65) and Cys62 (p50) | Inhibition | [94,95] | |
| STAT3 | 15-keto-PGE2 | Cys259 | Inhibition | [96] | |
| AP-1 | 15d-PGJ2 | Cys269 (c-Jun) | Inhibition | [97] | |
| Membrane receptor | Estrogen receptor α | 15d-PGJ2 | Cys227, Cys240 | Inhibition | [98] |
| EGFR | HNE | ? | Activation (low levels); inhibition (high levels) | [99] | |
| TRPA | 15d-PGJ2 | Cys421, Cys621 | Activation | [100] | |
| Regulatory proteins | Keap1 | NO2-FAs HNE 15d-PGJ2 |
Cys151, 273, 288 Cys 273, Cys288 |
Inhibition | [101,102] [103] [101,102,103,104] |
| IKK | HNE cyPG |
? Cys179 |
Inhibition | [105] [106] |
|
| H-Ras | 15d-PGJ2, PGA1 | Cys118, Cys181, Cys184 |
Activation | [107,108] | |
| Signalling protein | PTEN | Acrolein, HNE, PGA2, 15d-PGJ2 | Cys71, Lys327 | Inhibition | [58,109] |
| Akt | HNE | His196, His267, Cys311 | Inhibition | [110] | |
| PP2A | HNE | ? | Inhibition | [60] | |
| Epigenetic regulation | Sirt2 | Acrolein, HNE | Cys482 | Inhibition | [111] |
| HDACs | Acrolein HNE, 15d-PGJ2 |
Cys274 Cys 274 |
Inhibition Inhibition |
[112] [61] |
|
| Mitochondrial proteins | DRP1 | 15d-PGJ2 | At least Cys644 | Fission inhibition | [113] |
| Cytochrome c | HNE | His196, His267, Lys87 | In vitro modification | [114] | |
| Aconitase | HNE | Cys99, Cys358, Cys421, Cys424, Cys565 | Inhibition | [115] | |
| Others | Albumin | Δ12-PGJ2 HNE, acrolein |
His146 Cys34 |
? | [116] [117] |
* ? indicates that either the site of adduction or the biological effect were not determined in this study.
Figure 2.
Overview of the biochemical effects of protein lipoxidation, which are highly interrelated.
Lipoxidation of residues located at or near the active site of enzymes can bring about changes in enzymatic activity, for example through alterations of their active conformation or by blocking the binding of substrates [54]. Lipoxidation-induced enzyme inactivation has been reported for aldehyde dehydrogenase (ALDH2) [55] and pyruvate kinase [33], and may simply represent damage. In contrast, both activation or inactivation have been documented for aldo-ketoreductase B1 (AKR1B1), depending on the size of the electrophilic moiety causing the adduct [56,57]. As well as reacting with metabolic enzymes, electrophilic lipids can target proteins and enzymes involved in signal transduction, such as the phosphatases phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase (also known as phosphatase and tensin homolog PTEN) and protein phosphatase 2 (PP2A). PTEN can be modified by acrolein, HNE, prostaglandin A2 (PGA2) or 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) [58,59], whereas PP2A has been recently reported to be modified by HNE [60], resulting in both cases in inhibition, which indirectly affects the phosphorylation status of their targets and therefore, their downstream signalling pathways. Certain histone deacetylases (HDACs) can also be inhibited by HNE and 15d-PGJ2, which affects gene expression [61]. In contrast, activation of metalloprotease-9 by acrolein has been reported [62], with potential implications for tissue damage in a variety of inflammatory conditions.
Electrophilic lipids can also induce protein conformational changes, which may affect activity indirectly, lead to partial unfolding or alter protein-protein interactions. Conformational changes often cause changes in secondary structure, such as an unfolding or increase in β-sheet content, which tend to favour the formation of amyloid-like structures and aggregation of proteins [63]. Examples of specific proteins undergoing these changes upon lipoxidation are the ubiquitin hydrolase ubiquitin carboxy-terminal hydrolase L1 (UCH-L1), which is present in neurofibrillary tangles or Lewy bodies in Parkinson disease [64], and glutathione-S-transferase (GST), which is cross-linked in the presence of 15d-PGJ2 [65]. Moreover, an increased immunoreactivity with anti-ALE antibodies has been observed in a number of protein aggregates associated with pathophysiological conditions, including β2-microglobulin amyloid deposits associated with uremic complications [66]. This suggests a role for lipoxidation in the pathophysiology of these conditions. Although lipoxidation is more likely to affect nucleophilic residues located at the protein surface, small aldehydes can gain access into protein folds or binding pockets, leading to protein instability and unfolding. This can increase the exposure of hidden residues, rendering the protein more vulnerable to further modification [31]. As a result, the unfolded protein response (UPR) may be activated [67,68,69,70]. In addition, cross-linking or aggregation of proteins prevents their degradation via the 20S proteasome; inhibition of proteasome function may then occur, which directly affects cell viability and commonly results in cell death [71,72].
In some cases, conformational changes induced by binding of electrophilic lipids cause protein activation, either through direct or indirect mechanisms. The transcription factor peroxisome proliferator-activated receptor γ (PPARγ), which is redox-sensitive [90], illustrates this point nicely. Conformational changes in PPARγ during its activation trigger heterodimerization with retinoid X receptor, thus promoting the induction of anti-inflammatory genes and repression of pro-inflammatory genes such as nitric oxide synthase (iNOS) [118,119]. Several electrophilic lipids, including NO2-FAs, oxo-octadecadienoic acid (oxo-ODE) and cyPG cause covalent modification of PPARγ by Michael addition at the Cys285 residue and promote conformational changes necessary for its full activation [90,91,92,120]. Another example is the nuclear factor erythroid 2–related factor 2 (Nrf2), a transcription factor that is central in the antioxidant response and is indirectly activated by lipoxidation. Normally, Nrf2 is bound to its regulator Keap-1, an adaptor for ubiquitination that enables Nrf2 proteasomal degradation under non-stressed conditions. Modification of critical cysteines in Keap-1 causes a conformational change disrupting the degradation machinery and allowing nuclear translocation of newly synthetized Nrf2 and activation of its target genes [121,122]. Therefore, lipoxidation-induced conformational changes can also affect protein–protein interactions. The assembly of cytoskeletal proteins provides clear examples. Lipoxidation of a single cysteine residue (Cys328) in the intermediate filament protein vimentin can alter filament morphology or cause a complete disruption of assembly with the formation of aggregates [123]. Similarly, lipoxidation of actin at Cys374 can disrupt microfilaments if it occurs in a substantial proportion of molecules [80]. Lipoxidation of tubulin alters the morphology of microtubules, inducing a thinner appearance [74]. Some of these alterations could be due to steric hindrance caused by the addition of bulky moieties. In addition, changes in protein charge due to lipoxidation can also affect protein-protein interactions as reported for the binding of lipoxidised albumin to the receptor of advanced glycation end products (RAGE) [124]. Finally, lipoxidation can alter protein–DNA interactions, as is the case for transcription factor NF-κB, which is responsible for the signalling cascade that controls the expression of many proinflammatory genes. Direct lipoxidation of subunit p65 (Cys38) or p50 (Cys62) by 15d-PGJ2 or PGA1 has been reported to inhibit NF-κB binding to the DNA [94,95], thus reducing expression of proinflammatory genes.
As mentioned above, lipoxidation can influence protein subcellular localization indirectly through changes in protein interactions or degradation. However, the addition of electrophilic lipid moieties can also alter membrane targeting, either directly by the action of the bound lipid or indirectly if lipoxidation occurs on residues or domains involved in subcellular targeting or alters the transport mechanisms. Lipoxidation could increase the hydrophobicity of the molecule by altering its charge or introducing acyl groups, which could mimic the effects of lipidation and thus influence membrane interaction. The protein H-Ras poses an interesting example because it can be modified by cyPG at Cys181 and Cys184 residues [107,108], which are sites of palmitoylation and therefore important for subcellular targeting. Indeed, modification of these residues in H-Ras by different moieties has been shown to correlate with its localization to the plasma membrane or endomembranes [125]. In turn, lipoxidation of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), although it inactivates the enzyme, induces its translocation to the nucleus where it is involved in the induction of apoptosis [62]. Interestingly, lipoxidation of Chromosomal Maintenance 1 (CRM1) inhibits nuclear protein export [126], therefore inducing nuclear accumulation of its substrates.
Although this review is more focused on lipoxidation in the cellular context, protein lipoxidation in the extracellular milieu and the bloodstream has important consequences, including increased immunogenicity, transfer of proinflammatory and damage signals and contribution to a variety of pathophysiological processes [12,127]. In summary, lipoxidation can impact essential processes including cell signalling and metabolism, cytoskeletal function, protein degradation and gene expression. Moreover, regulation of these processes by lipoxidation is often double-sided, with either protective or deleterious effects depending on the protein target, the nature and the levels of the electrophilic lipid species and cellular context factors, which will be discussed below.
4. Selectivity and Protein Targets of Lipoxidation
Investigations of reactive oxidized lipid-protein adducts on entire proteomes have shown that not all proteins of a proteome are subject to lipoxidation [75,87,128], thus suggesting that this process is both site-specific and protein selective. Protein lipoxidation appears to occur on specific sets of proteins within the cellular proteome, which act as “hot spots”. In the circulation, albumin seems to be very susceptible to lipoxidation because of its abundance and of the high reactivity and accessibility of some nucleophilic residues (Cys34 and Lys199) [129]. In the cellular environment, the chaperones Hsp70 and Hsp90, Keap1, and the cytoskeletal proteins tubulin, actin and vimentin are frequent targets of lipoxidation [74,130]. Also, adducts seem to be more common in the cytosol and nucleoplasm than in the membrane, although this may depend on the type of lipid and on the difficulties to analyse membrane proteins [73,131,132,133]. In addition, certain cellular pathways, such as defence responses, or subcellular localizations appear particularly susceptible. Studies on the mitochondrial proteome showed that respiratory chain and tricarboxylic acid cycle (TCA) proteins, as well as transporters, are the most represented proteins undergoing lipoxidation [134,135]. Codreanu et al. identified HNE and ONE protein adducts in THP-1 and RKO cell lines and performed a Gene Ontology (GO) analysis, which showed that their function was predominantly involved in folding, RNA metabolic and glucose catabolic processes, cytoskeletal regulation and protein synthesis and turnover [136]. This is in agreement with previous studies that identified proteins related to the cytoskeleton, stress and immune responses, metabolic processes and glycolysis, regulation of translation and RNA binding as targets for HNE or cyPG in various cellular models [74,75,87]. Table 2 gives also examples of the site-specificity of lipoxidation on some target proteins, as determined in studies performed mostly in vivo or in cellulo, using physiological or pathophysiological treatment levels of electrophilic lipids and employing mutagenesis approaches to investigate the biological effect. Interestingly, information on sites of modification has also been obtained from in vitro studies, which have provided fundamental information on relative residue susceptibility and functional consequences, although in some cases yielded a higher number of modified residues. Some examples are shown in Table 3.
Table 3.
Multiple modification mapping studies in vitro.
| Protein | Targeted Residue (Position) | Electrophile | Type of Adduction | Reference |
|---|---|---|---|---|
| Pyruvate kinase | Cys 49, 152, 326, 358, 423, 474 | Acrolein, HHE and MDA | Michael, Schiff’s or FDP adduction | [33] |
| Lys 66, 115, 135, 166, 188, 207, 224, 247, 270, 305, 367, 393, 475 | ||||
| His 379, 391, 464 | ||||
| Cyclin-dependent Kinase 2 | Cys 177 | HNE | Michael | [85] |
| Lys 129 | ||||
| His 60, 71, 161, 268, 283, 295 | ||||
| Serum Albumin | Cys 53, 62, 75, 101, 124, 245, 246, 253, 269, 270, 277, 514 | HNE and MDA | Michael and Schiff’s (N-propenal-lysine adduct with MDA) | [137,138] |
| Lys 73, 106, 136, 174, 233, 240, 281, 378, 525, 541, 545 | ||||
| His 67, 105, 128, 242, 247, 510 | ||||
| Apolipoprotein E | Lys 64, 67, 68, 135, 138, 149, 155, 254 | Acrolein | Michael and Schiff’s | [139] |
| Creatine kinase | Cys 141, 145, 254, 283 | HNE | Michael and Schiff’s | [140] |
| Lys 86, 101 | ||||
| His 7, 26, 29, 66, 97, 191, 219, 234, 276, 296, 305 |
Why are some proteins more susceptible to lipoxidation than others? Some of the proteins mentioned above (albumin, chaperones, cytoskeletal and glycolytic proteins) are highly abundant in cells; as chemical reactions are concentration-dependent, there is a higher probability that abundant proteins will be both modified and detected during the analysis. However, this is not always the explanation, as illustrated by the lipoxidation of transcription factors and signalling proteins, which are minor cellular components. Instead, the biochemical characteristics of the protein or enzyme come into play. An important factor is the reactivity of amino acid sidechains by Schiff’s base formation or Michael addition, which is determined by their nucleophilicity [24,141]. Generally, the high nucleophilicity of the cysteine thiol makes it more reactive than the imidazole in histidine or amino group in lysine [134]. This agrees with the observation that out of 398 residue sites targeted by HNE in HEK293T cells, most (85.9%) were cysteines (342 residues), and only 27 were histidines (6.8%) and 29 lysines (7.3%) [142]. Moreover, in proteins with multiple cysteine residues frequently only one or two of them are targets for lipoxidation. For instance, Cys34 of albumin and Cys374 of actin are the most reactive and commonly modified residues of these two proteins [129], while the cysteine residues located in the C-terminal segments of several proteins of the Ras superfamily, including H- and N-Ras and Rac1, are lipoxidised [107,143]. This selectivity can arise because of a low pKa of the cysteine, which is influenced by its chemical microenvironment; the proximity of basic amino acids, such as positively-charged lysines, a metal centre, a catalytic triad or aromatic amino acids, lower the pKa and favour the formation of the more nucleophilic thiolate form, which is more prone to oxidation and lipoxidation [144,145,146,147,148]. Consequently, those thiols can act as redox sensors because they are highly responsive to various oxidative modifications. Examples of proteins with unusually low cysteine pKas include protein tyrosine phosphatases, thioredoxin (Trx) and peroxiredoxins (Prx). Lysine and histidine sidechains are commonly positively charged at physiological pH, but their pKas can also be modulated by their local environment through hydrogen bonding and charge stabilization, though as yet this has been less studied.
Another factor important in determining target residues in a protein is their solvent accessibility. A meta-analysis of human proteins identified as targets of HNE and acrolein modification showed that adducted residues were, on average, more accessible than the unreactive ones [141]. Similar findings were reported for the modification of pyruvate kinase by 3 small aldehydes [33]. The influence of nucleophilic residue accessibility was studied in the context of the modification of mitochondrial proteins by endogenous 2-alkenals [134], and it was found that local flexibility (B-factor values) and solvent accessibility areas were generally higher on 4 out of 5 cysteine residues that were found adducted on mitochondrial malate dehydrogenase. Interestingly, it has been reported that adducted residues are surrounded by a greater number of aromatic residues and fewer aliphatic residues than unreactive nucleophile residues [141].
Clearly, the nature and concentration of the electrophilic lipid species also determine the nucleophilic side chains targets in proteins, as explained above [33,42,115] and illustrated by the information in Table 2. There is good evidence that size and structure play an important role in the selectivity of protein modification. A study on cultured fibroblasts found that the closely related cyPG PGA1 and 15d-PGJ2 modified distinct and not totally overlapping subsets of proteins, with some targets clearly being preferentially modified by one of the cyPG [82,149]. Molecular simulations and docking studies have provided insight into the structural basis of the interaction between electrophilic lipids and proteins, documenting the basis for selectivity. PGA1 undergoes interactions with residues at the active site of AKR1B1 or B10, which favour the formation of a Michael adduct [82]. Similarly, favourable interactions have been proposed for the addition of 15-keto-PGE2 to signal transducer and activator of transcription 3 (STAT3) [96].
Overall, it can be seen that lipoxidation “hot spots” occurring at the level of the proteome and the protein are highly interdependent, and influenced both by the nature of the protein, the electrophilic lipid and their concentrations. The situation is further complicated by the fact that other aspects of the cellular environment can influence properties of the electrophilic lipids or the adducts, including their availability and stability, thus impacting on the selectivity of the modification. This is considered further in the next sections and illustrated in Figure 3.
Figure 3.
Scheme showing the main factors affecting lipoxidation, from the generation of reactive species and PTM crosstalk on the left to mechanisms of detoxification and reversibility on the right. Please, see text for details and abbreviations.
5. The Emerging Role of Lipoxidation in Cellular Regulation
PTMs involved in signalling are generally considered to be fast, reversible and specific, for these properties provide a high degree of control over the downstream processes. As a non-enzymatic modification, the role of protein lipoxidation as a signalling mechanism has been controversial, due in part to an incomplete understanding of the possibilities of regulation of this process. Nevertheless, protein lipoxidation can be modulated at several levels, including at the level of generation of the reactive species or their precursors, through their metabolism/detoxification, which influences their availability, or at the level of the stability of adducts, which can be regulated by adduct reversal. Some electrophilic lipids, including cyPG, derive from the dehydration of PG, which in turn are synthesized by regulated enzymatic processes that can be induced under situations of inflammation. Knockout of key enzymes involved in cyPG generation, such as PGD synthase, results in a decreased production and action of these electrophilic lipids [150]. Consequently, inhibitors of the phospholipases, COX and/or PG synthases involved in the enzymatic steps of PG synthesis may result in a reduction of the generation of the electrophilic lipids derived from them [151,152].
The metabolism or detoxification of reactive lipids or their precursors can be catalysed by diverse enzymes, thus influencing their availability and therefore the extent of lipoxidation. GSTs constitute a well-characterized family of enzymes that catalyse the conjugation of reduced glutathione (GSH) to electrophilic lipids to generate more soluble species that can be exported by multidrug resistance transporters, thus reducing their cellular availability [153,154,155,156]. Several electrophilic lipids, including cyPG and HNE are substrates of GST [153,154,156,157], for which enzymatic and non-enzymatic conjugation GSH has been shown to decrease their levels and activity [153,156].
Other enzymes that have been proposed as mediators of lipid detoxification include soluble epoxide hydrolase (sEH), which can metabolise epoxy fatty acids (PUFAs) [158], phospholipid hydroperoxide glutathione peroxidase and the Prxs [29]. A wide and diverse group of enzymes can detoxify aldehyde-containing electrophilic lipids. For instance, several isoforms of the aldo-keto reductase (AKR) family use NAD(P)H to reduce aldehyde groups of some electrophilic lipids such as acrolein, HNE or cyPG precursors [159,160], thus decreasing their availability and biological effects. Other enzymes that can reduce the aldehyde group of HNE, including aldose/aldehyde reductase (ALR), alcohol dehydrogenase (ADH), aldehyde dehydrogenase (ALDH), alkenal reductase (AER), alkenal hydrogenase (ALH), and alkenal/one reductase (ACR) have been reported to reduce its bioavailability and reactivity in both plants and humans [32,46]. Thus HNE detoxification can occur both by conjugation with GSH or direct detoxification by ADH or ALDH [32,161]. Importantly, several enzymes involved in detoxification of electrophilic lipids, including GST, AKR and soluble epoxide hydrolase are targets for reactive lipids themselves, which increases the complexity of these interactions [65,82,84].
A key feature of mechanisms considered to participate in cell signalling is that they need to be reversible, either directly or indirectly; lipoxidation shows potential reversibility through several mechanisms. Although both Schiff’s and Michael adducts are chemically reversible, Schiff’s adducts are more labile and reversal can occur spontaneously in aqueous solution [31], whereas Michael adducts are in general more stable. However, retro-Michael reactions are also possible under some circumstances. An adduct formed between AKR1B1 enzyme and a biotinylated analogue of PGA1 is partially reversed by incubation in the presence of an excess GSH in vitro [162]. Furthermore, Michael adducts generated by HNE and ONE can be reverted in vitro and in cells as demonstrated by quantitative chemoproteomic analysis [163] and kinetic studies [164]. In cells, the involvement of enzymatic mechanisms in the reversal of lipoxidation has been proposed. Acrolein protein adducts are reversed in bronchiolar epithelial cells by mechanisms dependent on GSH and Trx 1 [165]. In addition, the deacetylase Sirt2 has been reported to catalyse the enzymatic reversion of acrolein lipid adducts [166,167], as revealed by quantitative analysis [163]. NO2-FAs are a special case, since their adducts with cysteine residues are reversible, and there is consensus that they can behave as signalling mediators [168,169], although there is still much to be learned about the potential regulation of their generation and site of action.
The functional reversal of lipoxidation can also be achieved indirectly by degradation of the lipoxidised proteins and substitution by newly synthesized proteins with the consequence of the recovery of the biological effect [50,170]. As stated above, lipoxidation is frequently associated with inhibition of proteasomal degradation. Therefore, removal of protein-lipid adducts has been proposed to occur through lysosomal degradation and autophagy [171], especially of those containing α,β-unsaturated carbonyl groups or aldehydes.
Specificity is an important aspect of regulatory processes. Besides the selective aspects of lipoxidation discussed above, several lines of evidence indicate that in some cases protein lipoxidation can display distinct structure-function relationships. Lipoxidation of members of the AKR family by different species can lead to distinct functional consequences. Whereas modification by small moieties such as acrolein at Cys298 of AKR1B1 increases its catalytic activity [56], the addition of bigger reactive lipids such as HNE or certain cyPG promotes the inactivation of these enzymes [57,162]. The assembly of cytoskeletal protein vimentin is also sensitive to modulation by lipoxidation of its single cysteine residue and displays differential features depending on the structure of the adducted lipid [123]. However, whether this is a physiological mechanism of signalling is not known at present.
6. The Dependence of Lipoxidation on the Cellular Environment
Reactive lipids can exert both beneficial and detrimental effects on the cell [38]. Which of these predominates depends on many factors that influence the generation and final fate of the lipids and their adducts, including the cell type, the status of the antioxidant defences, and the concurrence of other reactive species or stimuli. The complexity of this balance is illustrated by the fact that electrophilic lipids can induce the expression of antioxidant defence enzymes, but at the same time influence their activity either directly, through lipoxidation, or indirectly, by triggering the production of reactive oxygen species (ROS) (see [172] for review).
The antioxidant system of the cell includes both enzymatic and non-enzymatic elements. Enzymes contributing to the antioxidant defence include the superoxide dismutase (SOD), heme oxygenase-1 (HO-1), catalases, glutathione peroxidase, Prxs, glutathione reductases and Trx. Small-molecule antioxidants include GSH, vitamins, lipoic acid and several cations such as Mn, Fe, Cu or Zn [63,173]. An example of the interplay between these factors and lipoxidation is provided by the cell- and species-dependent subcellular compartmentalization of cyPG accumulation and effects. For instance, differences in the main site of inhibition of the NF-κB activation pathway (through either cytoplasmic or nuclear events) have been attributed to the distinct subcellular distribution and abundance of antioxidant defences, i.e., GST activity and GSH content, in different cell types (see [172] for review). Therefore, increased levels of GSH, or enzymes involved in cyPG metabolism such as GST, are associated with lower levels of cyPG modification, and vice versa. Moreover, the stability of the adducts between electrophilic lipids and GSH depends on the lipid species [174], for which GSH levels will not affect the availability of electrophilic lipids uniformly. In addition, the observation that non-hydrolysable GSH analogues protect certain proteins, e.g., GSTp, from lipoxidation suggests the involvement of steric effects or induction of conformational changes in the protective effects of GSH [65]. Finally, these elements are dynamic, which increases the complexity of these interactions. For instance, cytosolic GSTs can translocate to the nucleus, altering the location of protection [175,176].
The complexity of these interactions is even higher since electrophilic lipids also influence the activity of the detoxifying enzymes. Certain electrophilic lipids can bind and inactivate GST and/or induce its crosslinking [65,177]. In addition, the reduced form of Prx is a direct target of HNE [178] whereas Trx can be modified by acrolein and HNE at the non-catalytic Cys73 [179] and by cyPG at Cys35 and Cys69 [180]. Moreover, TrxR is also a target for lipoxidation [181]. In most cases, lipoxidation is associated with inhibition of these targets, thus inducing the accumulation of cellular ROS. Nevertheless, as stated above, interaction with GSH can protect these enzymes from lipoxidation.
Vitamins may act as both pro- and anti-oxidants and their interactions with electrophilic lipids and lipoxidation appear to be complex and dependent on the experimental system. Examples of these interactions include reports on vitamin E decreasing lipid peroxidation in clinical trials or studies [182] and the ability of vitamin B6 to sequester intermediates of lipid peroxidation and reduce the formation of lipoxidation adducts [183,184]. Nevertheless, some actions of vitamins are controversial and the reader is referred to specialized reviews on this topic [170,173,185].
Divalent cations such as iron, copper, zinc or manganese also influence the redox state of the cell through various mechanisms including radical generation through the Fenton reaction (iron and copper), radical scavenging (manganese) or acting as cofactors for antioxidant enzymes (reviewed in [173]). In the context of lipoxidation, zinc presents special interest. Zinc competes with iron and copper in their coordination environments and suppresses their redox activity in Fenton chemistry. Interestingly, Zn2+ can interact with the thiolate group of cysteine, with important implications in Redox Biol, and the imidazole group of histidine [186], both of which are strong nucleophiles and frequent targets of lipoxidation. Zinc binding can affect the reactivity of cysteine residues and/or protect them from chemical modification, including lipoxidation [187,188]. The cytoskeletal protein vimentin provides an example of this protection both in vitro and in cells, since zinc availability in the physiological range protects the single cysteine residue of vimentin from alkylation, oxidation or lipoxidation in vitro, and preserves the integrity of the network in cells [188]. In turn, oxidation or lipoxidation of cysteine residues involved in the interaction with zinc releases this metal and contributes to zinc toxicity in cells [189]. On the other hand, metal-ion chelators inhibit lipoxidation reactions through the elimination of metal ions [170]. Some examples of compounds which can act as metal-ion chelators include citric acid (relatively non-specific chelator) [170], polyphenols and flavonoids [173].
Among other factors related to the cellular or extracellular context that can modulate lipoxidation is the presence of scavengers or quenchers. While the two terms are often used interchangeably, scavengers could be considered non-covalent binders of electrophilic lipids, whereas quenchers would be strong nucleophilic compounds reacting with the electrophilic derivatives leading to unreactive products. Thus, scavenging or quenching of electrophilic lipids could prevent protein lipoxidation. Therefore, in addition to endogenous compounds entailing this activity, exogenous natural and synthetic quenchers are being studied as potential therapeutic tools [170,190]. One of the best-studied examples is the dipeptide carnosine composed of β-alanine and histidine, which has served as the basis for the synthesis of more stable analogues, one which, known as carnosinol, has been found to decrease lipoxidation and showed beneficial effects in animal models of disease [191].
Finally, the presence of other reactive species, either endogenous or exogenous, such as drugs and their metabolites can influence lipoxidation by causing alterations in the cellular antioxidant systems or the protein targets, and even compete for target residues contributing to PTMs crosstalk. Therefore, factors from the cellular context may influence the extent and the site of protein lipoxidation, contributing to its selectivity and accounting for potential differences in the results from in vitro and in in vivo studies.
7. Interplay among Post-Translational Modifications
Lipoxidation can induce oxidative stress, thus eliciting the formation of further reactive species, responsible for additional PTMs leading to chain reactions with implications in different cellular processes [192]. Moreover, lipoxidation of enzymes involved in PTMs, such as phosphatases, kinases or deacetylases (see above), can affect PTMs. Therefore, a complex interplay between PTMs can take place involving lipoxidation, modifications by other reactive species, and activation or inhibition of proteins catalysing other PTMs. Moreover, direct cooperation or competition among PTMs can occur on the same proteins or residues, which could result in an increase of protection from lipoxidation, thus contributing to the generation of highly diverse proteoforms and the complexity of events determining the overall outcome.
Among reactive species potentially competing with electrophilic lipids for modification of proteins are species derived from the oxidation of sugars, ROS and RNS and other small molecules, like metabolites of certain amino acids, or even drugs. The modification of cysteine residues can provide numerous examples of this potential competition, given their capacity to accommodate multiple modifications [193,194]. In general, it could be considered that cysteine oxidation in its various forms, including formation of disulphide bonds, sulfenic and sulfonic acids, nitrosation, etc., would make the residue less available for lipoxidation. Nevertheless, sulfenic acids have been reported to be more reactive towards certain electrophilic compounds [195], while some disulfides are highly reactive with oxidants [196]. Therefore, in certain cases, cysteine reversible modifications, including disulphide formation, glutathionylation, nitrosation, or addition of NO2-FAs, could confer protection against more deleterious ones involving the formation of stable adducts, protein crosslinks, unfolding or aggregation [197,198].
Several previously discussed lipoxidation targets provide examples of these protective mechanisms. The enzyme AKR1B1 possesses seven cysteine residues, two of which, Cys298 and 303, are close to the active site. Formation of a disulphide bond between these cysteine residues reversibly inactivates the enzyme. Nevertheless, this modification could prevent a more stable modification causing either activation or inactivation of the enzyme. For instance, the cyPG PGA1 forms an adduct with Cys298 resulting in inhibition. The single cysteine residue of vimentin, Cys328 can also be the target for a wide variety of modifications. Reversible modifications of this residue include disulphide formation, nitrosation or glutathionylation, which can have different functional consequences [199]. Nitrosation, in particular, appears to elicit only minor alterations of vimentin assembly in vitro [200]. Therefore, it would be interesting to explore whether this reversible modification can play a protective role against more disruptive modifications such as CyPG addition. Interestingly, in vitro incubation of vimentin or a PPARγ construct with the nitrated phospholipid 1-palmitoyl-2-oleyl-phosphatidylcholine (NO2-POPC) shields their cysteine residues from alkylation [201]. Whether this is due to the occurrence of competing modifications requires further study.
Lipoxidation maintains an important interplay with phosphorylation through various mechanisms. As briefly discussed above, several kinases and phosphatases contain reactive thiols that are subjected to redox control and can be targets for several electrophilic species. Examples of kinases with reactive thiols include protein kinase A (PKA), PKG, PKC and Ca2+/calmodulin-dependent protein kinase II (CaMKII) [202,203]. Moreover, both 5′ AMP-activated kinase and AKT have been shown to be direct targets for lipoxidation by HNE [110,204]. Moreover, kinase cascades can be indirectly activated by lipoxidation. Monomeric GST binds and sequesters several stress kinases such as c-Jun N-terminal kinase (JNK) or Traf-2 or binds to their substrates [205,206] in such a way that oxidation or lipoxidation-induced GST crosslinking results in the activation of the corresponding stress signalling pathways [65,205,207]. In turn, lipoxidation of Ras proteins elicits their activation and that of downstream kinase cascades, including MAPKs, phosphoinositide 3-kinase (PI3K) and AKT [107,208].
In addition, several serine and tyrosine phosphatases can be regulated by redox mechanisms and are targets for lipoxidation, which can result in activation or inactivation of phosphatase activity, generally leading to reciprocal changes in the phosphorylation level of its substrates and modulation of the corresponding pathways [209]. Examples of phosphatases subjected to this control are PP2B, PP1, PP2A and PTEN. Lipoxidation of PP2A by PGA1 through the formation with a Michael adduct at Cys377 reduces the phosphorylation state of Tau [210]. In contrast, several electrophilic lipids, including acrolein, HNE and cyPG covalent modify and inactivate PTEN, resulting in activation of the AKT pathway and increased proliferation in several cancer cell lines [58,59]. Recently, the formation of an adduct of 15d-PGJ2 with Cys136 of PTEN has been reported [211]. Importantly, the possibility that electrophilic lipids can alter the expression levels of kinases or phosphatases provides an additional layer for interplay [212]. Therefore, alterations in protein phosphorylation status could be commonly associated with lipoxidation, and the occurrence of both modifications on the same target would influence the final outcome. For instance, in the case of vimentin, lipoxidation and phosphorylation appear to cooperate to induce filament disassembly [123]. However, in the case AKT, HNE indirectly promotes its phosphorylation, which would normally lead to activation but at the same time, directly modifies the enzyme, resulting in inhibition [110].
Importantly, direct competition between lipoxidation and phosphorylation could occur at histidine residues, which can be targets for both kinds of modification [213], although this potential interplay, to the best of our knowledge, has not been explored in detail. Other unusually phosphorylated amino acids include lysine and arginine.
Lipoxidation can also affect protein acetylation. Several HDACs are targets for lipoxidation. Indeed, a feedback mechanism controlling the expression of stress genes has been proposed that depends on the modification of certain HDACs by cyPG and HNE [61]. Moreover, lipoxidation of Sirt3 by HNE associates with mitochondrial protein hyperacetylation [214]. Notably, as lysine residues are targets for both lipoxidation and acetylation, the interplay between both modifications could occur also at this level. Similar interactions that could affect other modifications such as lysine ubiquitination or formylation deserve investigation.
8. Conclusions
In summary, modification of proteins by lipoxidation can elicit varied functional consequences and affect a myriad of intracellular processes. Being a non-enzymatic modification, envisaging potential regulatory roles of lipoxidation is controversial. The chain reaction provoked by lipid oxidation could expand in a flood-like manner affecting multiple proteins and pathways. Nevertheless, accumulating evidence indicates that protein lipoxidation is not a random process, which could be subjected to regulation at several levels. Indeed, low or moderate level protein lipoxidation appears to be involved in cellular defence responses and adaptation to stress. Currently, it is not clear how cells could harness this process in physiological situations. However, the interplay with generation of antioxidant defences, such as GSH, with detoxifying and repairing enzymes, and with other PTMs are unveiling further possibilities for modulation of the effects of lipoxidation. Detailed knowledge of these processes will be necessary to understand its involvement in pathophysiology as well as the possibilities for therapeutic intervention.
Acknowledgments
We thank A.R. Pitt for helpful comments and discussion. Feedback from EU COST Actions CA19105 EpiLipidNet and CA15214 EuroCellNet is gratefully acknowledged.
Abbreviations
| ACR | Alkenal/one reductase |
| ADH | Alcohol dehydrogenase |
| AER | Alkenal reductase |
| AKR | Aldo-keto reductase |
| AKR1B1 | Aldo-ketoreductase B1 |
| ALDH | Aldehyde dehydrogenase |
| ALDH2 | Aldehyde dehydrogenase 2 |
| ALEs | Advanced lipoxidation end products |
| ALH | Alkenal hydrogenase |
| ALR | Aldose/aldehyde reductase |
| CaMKII | Ca2+/calmodulin-dependent protein kinase II |
| COX | Cyclooxygenases |
| CRM1 | Chromosomal maintenance 1 |
| cyPG | Cyclopentenone prostaglandin(s) |
| CYP450 | Cytochrome P450 |
| FDP | Nε-(3-formyl-3,4-dehydropiperidino) |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GFAP | Glial fibrillary acidic protein |
| GO | Gene ontology |
| GSH | Reduced glutathione |
| GST | Glutathione S-transferase |
| HDACs | Histone deacetylases |
| HNE | 4-hydroxynonenal |
| Hsp70 | Heat shock protein 70 |
| Hsp90 | Heat shock protein 90 |
| iNOS | Nitric oxide synthase |
| LOX | Lipoxygenases |
| MDA | Malondialdehyde |
| NF-κB | Nuclear factor-κB |
| NO2-FA | Nitrate fatty acid |
| NO2-POPC | Nitrated phospholipid 1-palmitoyl-2-oleyl-phosphatidylcholine |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| ONE | 4-oxononenal |
| oxo-ODE | Oxooctadecadienoic acid |
| PG | Prostaglandin(s) |
| PGA1 | Prostaglandin A1 |
| PGA2 | Prostaglandin A2 |
| PGD | Prostaglandin D |
| PGE2 | Prostaglandin E2 |
| PI3K | Phosphoinositide 3-kinase |
| PKA | Protein kinase A |
| PKC | Protein kinase C |
| PKG | Protein kinase G |
| PP1 | Protein phosphatase 1 |
| PP2A | Protein phosphatase 2 |
| PP2B | Protein phosphatase 2B |
| PPARγ | Peroxisome proliferator-activated receptor γ |
| Prx | Peroxiredoxin |
| PTEN | Phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase |
| PTM | Post-translational modification |
| PUFAs | Polyunsaturated fatty acids |
| RAGE | Receptor of advanced glycation end products |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| sEH | soluble epoxide hydrolase |
| SOD | Superoxide dismutase |
| STAT3 | Signal transducer and activator of transcription 3 |
| TCA | Tricarboxylic acid cycle |
| Trx | Thioredoxins |
| UPR | Unfolded protein response |
| 15d-PGJ2 | 15-deoxy-Δ12,14-prostaglandin J2 |
Author Contributions
Design and coordination, D.P.-S.; conceptualization, C.M.S. and D.P.-S., writing, Á.V.-P., P.G.-J., O.L., I.C.-M., C.M.S. and D.P.-S.; writing-review and editing, C.M.S. and D.P.-S.; funding acquisition, C.M.S. and D.P.-S. All authors have read and agreed to the published version of the manuscript.
Funding
Work at DPS laboratory is supported by RTI2018-097624-B-I00 from Agencia Estatal de Investigación, MICINN/ERDF, and RETIC ARADYAL RD16/0006/0021 from ISCIII/ERDF, Spain Work at CMS laboratory (including I.C.M. and O.L.) is supported by funding from the European Union’s Horizon 2020 research and innovation programme under Marie Sklodowska-Curie grant agreement No 847419 (MemTrain). Á.V.P. and P.G.J. are the recipients of predoctoral contracts BES-2016-076965 and PRE2019-088194, respectively, from MICINN, Spain.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Burdge G.C., Calder P.C. Introduction to fatty acids and lipids. World Rev. Nutr. Diet. 2015;112:1–16. doi: 10.1159/000365423. [DOI] [PubMed] [Google Scholar]
- 2.Zhang C., Wang K., Yang L., Liu R., Chu Y., Qin X., Yang P., Yu H. Lipid metabolism in inflammation-related diseases. Analyst. 2018;143:4526–4536. doi: 10.1039/C8AN01046C. [DOI] [PubMed] [Google Scholar]
- 3.Spickett C.M. Formation of Oxidatively Modified Lipids as the Basis for a Cellular Epilipidome. Front. Endocrinol. 2020;11:602771. doi: 10.3389/fendo.2020.602771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Que X., Hung M.Y., Yeang C., Gonen A., Prohaska T.A., Sun X., Diehl C., Maatta A., Gaddis D.E., Bowden K., et al. Oxidized phospholipids are proinflammatory and proatherogenic in hypercholesterolaemic mice. Nature. 2018;558:301–306. doi: 10.1038/s41586-018-0198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomita K., Takashi Y., Ouchi Y., Kuwahara Y., Igarashi K., Nagasawa T., Nabika H., Kurimasa A., Fukumoto M., Nishitani Y., et al. Lipid peroxidation increases hydrogen peroxide permeability leading to cell death in cancer cell lines that lack mtDNA. Cancer Sci. 2019;110:2856–2866. doi: 10.1111/cas.14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hochstein P., Jain S.K. Association of lipid peroxidation and polymerization of membrane proteins with erythrocyte aging. Fed. Proc. 1981;40:183–188. [PubMed] [Google Scholar]
- 7.Fam S.S., Murphey L.J., Terry E.S., Zackert W.E., Chen Y., Gao L., Pandalai S., Milne G.L., Roberts L.J., Porter N.A., et al. Formation of highly reactive A-ring and J-ring isoprostane-like compounds (A4/J4-neuroprostanes) in vivo from docosahexaenoic acid. J. Biol. Chem. 2002;277:36076–36084. doi: 10.1074/jbc.M205638200. [DOI] [PubMed] [Google Scholar]
- 8.O’Donnell V.B., Eiserich J.P., Chumley P.H., Jablonsky M.J., Krishna N.R., Kirk M., Barnes S., Darley-Usmar V.M., Freeman B.A. Nitration of unsaturated fatty acids by nitric oxide-derived reactive nitrogen species peroxynitrite, nitrous acid, nitrogen dioxide, and nitronium ion. Chem. Res. Toxicol. 1999;12:83–92. doi: 10.1021/tx980207u. [DOI] [PubMed] [Google Scholar]
- 9.Chen B., Sun Y., Niu J., Jarugumilli G.K., Wu X. Protein Lipidation in Cell Signaling and Diseases: Function, Regulation, and Therapeutic Opportunities. Cell Chem. Biol. 2018;25:817–831. doi: 10.1016/j.chembiol.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hentschel A., Zahedi R.P., Ahrends R. Protein lipid modifications--More than just a greasy ballast. Proteomics. 2016;16:759–782. doi: 10.1002/pmic.201500353. [DOI] [PubMed] [Google Scholar]
- 11.Feingold K.R. Introduction to Lipids and Lipoproteins. In: Feingold K.R., Anawalt B., Boyce A., Chrousos G., de Herder W.W., Dungan K., Grossman A., Hershman J.M., Hofland J., Kaltsas G., et al., editors. Endotext. MDText.com. Inc.; South Dartmouth, MA, USA: 2000. [Google Scholar]
- 12.Afonso C.B., Spickett C.M. Lipoproteins as targets and markers of lipoxidation. Redox Biol. 2018:101066. doi: 10.1016/j.redox.2018.101066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Bus I., Witkamp R., Zuilhof H., Albada B., Balvers M. The role of n-3 PUFA-derived fatty acid derivatives and their oxygenated metabolites in the modulation of inflammation. Prostaglandins Lipid Mediat. 2019;144:106351. doi: 10.1016/j.prostaglandins.2019.106351. [DOI] [PubMed] [Google Scholar]
- 14.Vigor C., Bertrand-Michel J., Pinot E., Oger C., Vercauteren J., Le Faouder P., Galano J.M., Lee J.C., Durand T. Non-enzymatic lipid oxidation products in biological systems: Assessment of the metabolites from polyunsaturated fatty acids. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014;964:65–78. doi: 10.1016/j.jchromb.2014.04.042. [DOI] [PubMed] [Google Scholar]
- 15.Malmsten C.L. Prostaglandins, thromboxanes, and leukotrienes in inflammation. Am. J. Med. 1986;80:11–17. doi: 10.1016/0002-9343(86)90073-2. [DOI] [PubMed] [Google Scholar]
- 16.Zhong S., Li L., Shen X., Li Q., Xu W., Wang X., Tao Y., Yin H. An update on lipid oxidation and inflammation in cardiovascular diseases. Free Radic. Biol. Med. 2019;144:266–278. doi: 10.1016/j.freeradbiomed.2019.03.036. [DOI] [PubMed] [Google Scholar]
- 17.Reis A., Spickett C.M. Chemistry of phospholipid oxidation. Biochim. Biophys. Acta. 2012;1818:2374–2387. doi: 10.1016/j.bbamem.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Galano J.M., Lee J.C., Gladine C., Comte B., Le Guennec J.Y., Oger C., Durand T. Non-enzymatic cyclic oxygenated metabolites of adrenic, docosahexaenoic, eicosapentaenoic and alpha-linolenic acids; bioactivities and potential use as biomarkers. Biochim. Biophys. Acta. 2015;1851:446–455. doi: 10.1016/j.bbalip.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Domingues M.R., Domingues P., Melo T., Pérez-Sala D., Reis A., Spickett C. Lipoxidation adducts with peptides and proteins: Deleterious modifications or signalling mechanisms? J. Proteom. 2013;92:110–131. doi: 10.1016/j.jprot.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Schneider C., Boeglin W.E., Yin H., Porter N.A., Brash A.R. Intermolecular peroxyl radical reactions during autoxidation of hydroxy and hydroperoxy arachidonic acids generate a novel series of epoxidized products. Chem. Res. Toxicol. 2008;21:895–903. doi: 10.1021/tx700357u. [DOI] [PubMed] [Google Scholar]
- 21.Salomon R.G., Batyreva E., Kaur K., Sprecher D.L., Schreiber M.J., Crabb J.W., Penn M.S., DiCorletoe A.M., Hazen S.L., Podrez E.A. Isolevuglandin-protein adducts in humans: Products of free radical-induced lipid oxidation through the isoprostane pathway. Biochim. Biophys. Acta. 2000;1485:225–235. doi: 10.1016/S1388-1981(00)00038-X. [DOI] [PubMed] [Google Scholar]
- 22.Esterbauer H., Schaur R.J., Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 23.Sousa B.C., Pitt A.R., Spickett C.M. Chemistry and analysis of HNE and other prominent carbonyl-containing lipid oxidation compounds. Free Radic. Biol. Med. 2017;111:294–308. doi: 10.1016/j.freeradbiomed.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Gueraud F., Atalay M., Bresgen N., Cipak A., Eckl P.M., Huc L., Jouanin I., Siems W., Uchida K. Chemistry and biochemistry of lipid peroxidation products. Free Radic. Res. 2010;44:1098–1124. doi: 10.3109/10715762.2010.498477. [DOI] [PubMed] [Google Scholar]
- 25.Shibata T., Kondo M., Osawa T., Shibata N., Kobayashi M., Uchida K. 15-Deoxy-Delta 12,14-prostaglandin J2. A PROSTAGLANDIN D2 METABOLITE GENERATED DURING INFLAMMATORY PROCESSES. J. Biol. Chem. 2002;277:10459–10466. doi: 10.1074/jbc.M110314200. [DOI] [PubMed] [Google Scholar]
- 26.Uchida K., Shibata T. 15-Deoxy-Delta(12,14)-prostaglandin J2: An electrophilic trigger of cellular responses. Chem. Res. Toxicol. 2008;21:138–144. doi: 10.1021/tx700177j. [DOI] [PubMed] [Google Scholar]
- 27.Stamatakis K., Pérez-Sala D. Prostanoids with cyclopentenone structure as tools for the characterization of electrophilic eicosanoid-protein interactomes. Ann. N. Y. Acad. Sci. 2006;1091:548–570. doi: 10.1196/annals.1378.096. [DOI] [PubMed] [Google Scholar]
- 28.Hammond V.J., Morgan A.H., Lauder S., Thomas C.P., Brown S., Freeman B.A., Lloyd C.M., Davies J., Bush A., Levonen A.L., et al. Novel keto-phospholipids are generated by monocytes and macrophages, detected in cystic fibrosis, and activate peroxisome proliferator-activated receptor-gamma. J. Biol. Chem. 2012;287:41651–41666. doi: 10.1074/jbc.M112.405407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schopfer F.J., Cipollina C., Freeman B.A. Formation and signaling actions of electrophilic lipids. Chem. Rev. 2011;111:5997–6021. doi: 10.1021/cr200131e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao J., Chen J., Zhu H., Xiong Y.L. Mass spectrometric evidence of malonaldehyde and 4-hydroxynonenal adductions to radical-scavenging soy peptides. J. Agric. Food Chem. 2012;60:9727–9736. doi: 10.1021/jf3026277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spickett C.M., Pitt A.R. Modification of proteins by reactive lipid oxidation products and biochemical effects of lipoxidation. Essays Biochem. 2020;64:19–31. doi: 10.1042/EBC20190058. [DOI] [PubMed] [Google Scholar]
- 32.Ayala A., Munoz M.F., Arguelles S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014;2014:360438. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sousa B.C., Ahmed T., Dann W.L., Ashman J., Guy A., Durand T., Pitt A.R., Spickett C.M. Short-chain lipid peroxidation products form covalent adducts with pyruvate kinase and inhibit its activity in vitro and in breast cancer cells. Free Radic. Biol. Med. 2019;144:223–233. doi: 10.1016/j.freeradbiomed.2019.05.028. [DOI] [PubMed] [Google Scholar]
- 34.Liu-Snyder P., Borgens R.B., Shi R. Hydralazine rescues PC12 cells from acrolein-mediated death. J. Neurosci. Res. 2006;84:219–227. doi: 10.1002/jnr.20862. [DOI] [PubMed] [Google Scholar]
- 35.Niedernhofer L.J., Daniels J.S., Rouzer C.A., Greene R.E., Marnett L.J. Malondialdehyde, a product of lipid peroxidation, is mutagenic in human cells. J. Biol. Chem. 2003;278:31426–31433. doi: 10.1074/jbc.M212549200. [DOI] [PubMed] [Google Scholar]
- 36.Esterbauer H., Eckl P., Ortner A. Possible mutagens derived from lipids and lipid precursors. Mutat Res. 1990;238:223–233. doi: 10.1016/0165-1110(90)90014-3. [DOI] [PubMed] [Google Scholar]
- 37.Roberts L.J., 2nd, Morrow J.D. Products of the isoprostane pathway: Unique bioactive compounds and markers of lipid peroxidation. Cell. Mol. Life Sci. 2002;59:808–820. doi: 10.1007/s00018-002-8469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pérez-Sala D. Electrophilic eicosanoids: Signaling and targets. Chem. Biol. Interact. 2011;192:96–100. doi: 10.1016/j.cbi.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Schopfer F.J., Vitturi D.A., Jorkasky D.K., Freeman B.A. Nitro-fatty acids: New drug candidates for chronic inflammatory and fibrotic diseases. Nitric Oxide. 2018;79:31–37. doi: 10.1016/j.niox.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salomon R.G. Levuglandins and isolevuglandins: Stealthy toxins of oxidative injury. Antioxid. Redox Signal. 2005;7:185–201. doi: 10.1089/ars.2005.7.185. [DOI] [PubMed] [Google Scholar]
- 41.Montuschi P., Barnes P.J., Roberts L.J., 2nd Isoprostanes: Markers and mediators of oxidative stress. FASEB J. 2004;18:1791–1800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- 42.Martyniuk C.J., Fang B., Koomen J.M., Gavin T., Zhang L., Barber D.S., Lopachin R.M. Molecular mechanism of glyceraldehyde-3-phosphate dehydrogenase inactivation by alpha,beta-unsaturated carbonyl derivatives. Chem. Res. Toxicol. 2011;24:2302–2311. doi: 10.1021/tx200437y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sayre L.M., Lin D., Yuan Q., Zhu X., Tang X. Protein adducts generated from products of lipid oxidation: Focus on HNE and one. Drug Metab. Rev. 2006;38:651–675. doi: 10.1080/03602530600959508. [DOI] [PubMed] [Google Scholar]
- 44.Yamauchi Y., Sugimoto Y. Effect of protein modification by malondialdehyde on the interaction between the oxygen-evolving complex 33 kDa protein and photosystem II core proteins. Planta. 2010;231:1077–1088. doi: 10.1007/s00425-010-1112-2. [DOI] [PubMed] [Google Scholar]
- 45.Mano J., Nagata M., Okamura S., Shiraya T., Mitsui T. Identification of oxidatively modified proteins in salt-stressed Arabidopsis: A carbonyl-targeted proteomics approach. Plant Cell Physiol. 2014;55:1233–1244. doi: 10.1093/pcp/pcu072. [DOI] [PubMed] [Google Scholar]
- 46.Alche J.D. A concise appraisal of lipid oxidation and lipoxidation in higher plants. Redox Biol. 2019:101136. doi: 10.1016/j.redox.2019.101136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mano J., Biswas M.S., Sugimoto K. Reactive Carbonyl Species: A Missing Link in ROS Signaling. Plants. 2019;8:391. doi: 10.3390/plants8100391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Belenky P., Ye J.D., Porter C.B., Cohen N.R., Lobritz M.A., Ferrante T., Jain S., Korry B.J., Schwarz E.G., Walker G.C., et al. Bactericidal Antibiotics Induce Toxic Metabolic Perturbations that Lead to Cellular Damage. Cell Rep. 2015;13:968–980. doi: 10.1016/j.celrep.2015.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vistoli G., De Maddis D., Cipak A., Zarkovic N., Carini M., Aldini G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): An overview of their mechanisms of formation. Free Radic Res. 2013;47:3–27. doi: 10.3109/10715762.2013.815348. [DOI] [PubMed] [Google Scholar]
- 50.Pizzimenti S., Ciamporcero E., Daga M., Pettazzoni P., Arcaro A., Cetrangolo G., Minelli R., Dianzani C., Lepore A., Gentile F., et al. Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front. Physiol. 2013;4:242. doi: 10.3389/fphys.2013.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oeste C.L., Pérez-Sala D. Modification of cysteine residues by cyclopentenone prostaglandins: Interplay with redox regulation of protein function. Mass Spectrom. Rev. 2014;33:110–125. doi: 10.1002/mas.21383. [DOI] [PubMed] [Google Scholar]
- 52.Elguero B., Gonilski Pacin D., Cardenas Figueroa C., Fuertes M., Arzt E. Modifications in the cellular proteome and their clinical application. Medicina. 2019;79:570–575. [PubMed] [Google Scholar]
- 53.Perez-Sala D., Domingues R. Lipoxidation targets: From basic mechanisms to pathophysiology. Redox Biol. 2019;23:101208. doi: 10.1016/j.redox.2019.101208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aluise C.D., Camarillo J.M., Shimozu Y., Galligan J.J., Rose K.L., Tallman K.A., Marnett L.J. Site-Specific, Intramolecular Cross-Linking of Pin1 Active Site Residues by the Lipid Electrophile 4-Oxo-2-nonenal. Chem. Res. Toxicol. 2015;28:817–827. doi: 10.1021/acs.chemrestox.5b00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Doorn J.A., Hurley T.D., Petersen D.R. Inhibition of human mitochondrial aldehyde dehydrogenase by 4-hydroxynon-2-enal and 4-oxonon-2-enal. Chem. Res. Toxicol. 2006;19:102–110. doi: 10.1021/tx0501839. [DOI] [PubMed] [Google Scholar]
- 56.Srivastava S.K., Ramana K.V., Chandra D., Srivastava S., Bhatnagar A. Regulation of aldose reductase and the polyol pathway activity by nitric oxide. Chem. Biol. Interact. 2003;143–144:333–340. doi: 10.1016/S0009-2797(02)00214-4. [DOI] [PubMed] [Google Scholar]
- 57.Del Corso A., Dal Monte M., Vilardo P.G., Cecconi I., Moschini R., Banditelli S., Cappiello M., Tsai L., Mura U. Site-specific inactivation of aldose reductase by 4-hydroxynonenal. Arch Biochem. Biophys. 1998;350:245–248. doi: 10.1006/abbi.1997.0488. [DOI] [PubMed] [Google Scholar]
- 58.Covey T.M., Edes K., Coombs G.S., Virshup D.M., Fitzpatrick F.A. Alkylation of the tumor suppressor PTEN activates Akt and beta-catenin signaling: A mechanism linking inflammation and oxidative stress with cancer. PLoS ONE. 2010;5:e13545. doi: 10.1371/journal.pone.0013545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shearn C.T., Smathers R.L., Stewart B.J., Fritz K.S., Galligan J.J., Hail N., Jr., Petersen D.R. Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) inhibition by 4-hydroxynonenal leads to increased Akt activation in hepatocytes. Mol. Pharm. 2011;79:941–952. doi: 10.1124/mol.110.069534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Di Domenico F., Tramutola A., Barone E., Lanzillotta C., Defever O., Arena A., Zuliani I., Foppoli C., Iavarone F., Vincenzoni F., et al. Restoration of aberrant mTOR signaling by intranasal rapamycin reduces oxidative damage: Focus on HNE-modified proteins in a mouse model of down syndrome. Redox Biol. 2019;23:101162. doi: 10.1016/j.redox.2019.101162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doyle K., Fitzpatrick F.A. Redox signaling, alkylation (carbonylation) of conserved cysteines inactivates class I histone deacetylases 1, 2, and 3 and antagonizes their transcriptional repressor function. J. Biol. Chem. 2010;285:17417–17424. doi: 10.1074/jbc.M109.089250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Igarashi K., Uemura T., Kashiwagi K. Acrolein toxicity at advanced age: Present and future. Amino. Acids. 2018;50:217–228. doi: 10.1007/s00726-017-2527-x. [DOI] [PubMed] [Google Scholar]
- 63.Moldogazieva N.T., Mokhosoev I.M., Mel’nikova T.I., Porozov Y.B., Terentiev A.A. Oxidative Stress and Advanced Lipoxidation and Glycation End Products (ALEs and AGEs) in Aging and Age-Related Diseases. Oxid. Med. Cell. Longev. 2019;2019:3085756. doi: 10.1155/2019/3085756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koharudin L.M., Liu H., Di Maio R., Kodali R.B., Graham S.H., Gronenborn A.M. Cyclopentenone prostaglandin-induced unfolding and aggregation of the Parkinson disease-associated UCH-L1. Proc. Natl. Acad. Sci. USA. 2010;107:6835–6840. doi: 10.1073/pnas.1002295107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sánchez-Gómez F.J., Díez-Dacal B., Pajares M.A., Llorca O., Pérez-Sala D. Cyclopentenone prostaglandins with dienone structure promote cross-linking of the chemoresistance-inducing enzyme Glutathione Transferase P1-1. Mol. Pharm. 2010;78:723–733. doi: 10.1124/mol.110.065391. [DOI] [PubMed] [Google Scholar]
- 66.Miyata T., Kurokawa K., Van Ypersele De Strihou C. Advanced glycation and lipoxidation end products: Role of reactive carbonyl compounds generated during carbohydrate and lipid metabolism. J. Am. Soc. Nephrol. 2000;11:1744–1752. doi: 10.1681/ASN.V1191744. [DOI] [PubMed] [Google Scholar]
- 67.Takasugi N., Hiraoka H., Nakahara K., Akiyama S., Fujikawa K., Nomura R., Furuichi M., Uehara T. The Emerging Role of Electrophiles as a Key Regulator for Endoplasmic Reticulum (ER) Stress. Int. J. Mol. Sci. 2019;20:1783. doi: 10.3390/ijms20071783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.West J.D., Marnett L.J. Alterations in gene expression induced by the lipid peroxidation product, 4-hydroxy-2-nonenal. Chem. Res. Toxicol. 2005;18:1642–1653. doi: 10.1021/tx050211n. [DOI] [PubMed] [Google Scholar]
- 69.Vladykovskaya E., Sithu S.D., Haberzettl P., Wickramasinghe N.S., Merchant M.L., Hill B.G., McCracken J., Agarwal A., Dougherty S., Gordon S.A., et al. Lipid peroxidation product 4-hydroxy-trans-2-nonenal causes endothelial activation by inducing endoplasmic reticulum stress. J. Biol. Chem. 2012;287:11398–11409. doi: 10.1074/jbc.M111.320416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haberzettl P., Hill B.G. Oxidized lipids activate autophagy in a JNK-dependent manner by stimulating the endoplasmic reticulum stress response. Redox Biol. 2013;1:56–64. doi: 10.1016/j.redox.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shringarpure R., Grune T., Sitte N., Davies K.J. 4-Hydroxynonenal-modified amyloid-beta peptide inhibits the proteasome: Possible importance in Alzheimer’s disease. Cell. Mol. Life Sci. 2000;57:1802–1809. doi: 10.1007/PL00000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Santra M., Dill K.A., de Graff A.M.R. Proteostasis collapse is a driver of cell aging and death. Proc. Natl. Acad. Sci. USA. 2019;116:22173–22178. doi: 10.1073/pnas.1906592116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gharbi S., Garzón B., Gayarre J., Timms J., Pérez-Sala D. Study of protein targets for covalent modification by the antitumoral and anti-inflammatory prostaglandin PGA1: Focus on vimentin. J. Mass Spectrom. 2007;42:1474–1484. doi: 10.1002/jms.1291. [DOI] [PubMed] [Google Scholar]
- 74.Stamatakis K., Sánchez-Gómez F.J., Pérez-Sala D. Identification of novel protein targets for modification by 15-deoxy-Δ12,14-prostaglandin J2 in mesangial cells reveals multiple interactions with the cytoskeleton. J. Am. Soc. Nephrol. 2006;17:89–98. doi: 10.1681/ASN.2005030329. [DOI] [PubMed] [Google Scholar]
- 75.Chavez J., Chung W.G., Miranda C.L., Singhal M., Stevens J.F., Maier C.S. Site-specific protein adducts of 4-hydroxy-2(E)-nonenal in human THP-1 monocytic cells: Protein carbonylation is diminished by ascorbic acid. Chem. Res. Toxicol. 2010;23:37–47. doi: 10.1021/tx9002462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Griesser E., Vemula V., Mónico A., Pérez-Sala D., Fedorova M. Dynamic posttranslational modifications of cytoskeletal proteins unveil hot spots under nitroxidative stress. BioRxiv. 2021:427971. doi: 10.1101/2021.01.24.427971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Viedma-Poyatos Á., Pablo Y.d., Pekny M., Pérez-Sala D. The cysteine residue of glial fibrillary acidic protein is a critical target for lipoxidation and required for efficient network organization. Free Rad. Biol. Med. 2018;120:380–394. doi: 10.1016/j.freeradbiomed.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 78.Aldini G., Dalle-Donne I., Vistoli G., Maffei Facino R., Carini M. Covalent modification of actin by 4-hydroxy-trans-2-nonenal (HNE): LC-ESI-MS/MS evidence for Cys374 Michael adduction. J. Mass Spectrom. 2005;40:946–954. doi: 10.1002/jms.872. [DOI] [PubMed] [Google Scholar]
- 79.Gayarre J., Sánchez D., Sánchez-Gómez F.J., Terrón C., Llorca O., Pérez-Sala D. Addition of electrophilic lipids to actin alters filament structure. Biochem. Biophys. Res. Commun. 2006;349:1387–1393. doi: 10.1016/j.bbrc.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 80.Aldini G., Carini M., Vistoli G., Shibata T., Kusano Y., Gamberoni L., Dalle-Donne I., Milzani A., Uchida K. Identification of Actin as a 15-Deoxy-Delta(12,14)-prostaglandin J(2) Target in Neuroblastoma Cells: Mass Spectrometric, Computational, and Functional Approaches To Investigate the Effect on Cytoskeletal Derangement. Biochemistry. 2007;46:2707–2718. doi: 10.1021/bi0618565. [DOI] [PubMed] [Google Scholar]
- 81.Dalle-Donne I., Carini M., Vistoli G., Gamberoni L., Giustarini D., Colombo R., Maffei Facino R., Rossi R., Milzani A., Aldini G. Actin Cys374 as a nucleophilic target of alpha,beta-unsaturated aldehydes. Free Radic. Biol. Med. 2007;42:583–598. doi: 10.1016/j.freeradbiomed.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 82.Díez-Dacal B., Gayarre J., Gharbi S., Timms J.F., Coderch C., Gago F., Pérez-Sala D. Identification of aldo-keto reductase AKR1B10 as a selective target for modification and inhibition by PGA1: Implications for anti-tumoral activity. Cancer Res. 2011;71:4161–4171. doi: 10.1158/0008-5472.CAN-10-3816. [DOI] [PubMed] [Google Scholar]
- 83.Reed T., Perluigi M., Sultana R., Pierce W.M., Klein J.B., Turner D.M., Coccia R., Markesbery W.R., Butterfield D.A. Redox proteomic identification of 4-hydroxy-2-nonenal-modified brain proteins in amnestic mild cognitive impairment: Insight into the role of lipid peroxidation in the progression and pathogenesis of Alzheimer’s disease. Neurobiol Dis. 2008;30:107–120. doi: 10.1016/j.nbd.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 84.Charles R.L., Burgoyne J.R., Mayr M., Weldon S.M., Hubner N., Dong H., Morisseau C., Hammock B.D., Landar A., Eaton P. Redox regulation of soluble epoxide hydrolase by 15-deoxy-delta-prostaglandin J2 controls coronary hypoxic vasodilation. Circ. Res. 2011;108:324–334. doi: 10.1161/CIRCRESAHA.110.235879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Camarillo J.M., Ullery J.C., Rose K.L., Marnett L.J. Electrophilic Modification of PKM2 by 4-Hydroxynonenal and 4-Oxononenal Results in Protein Cross-Linking and Kinase Inhibition. Chem. Res. Toxicol. 2017;30:635–641. doi: 10.1021/acs.chemrestox.6b00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aluise C.D., Rose K., Boiani M., Reyzer M.L., Manna J.D., Tallman K., Porter N.A., Marnett L.J. Peptidyl-prolyl cis/trans-isomerase A1 (Pin1) is a target for modification by lipid electrophiles. Chem. Res. Toxicol. 2013;26:270–279. doi: 10.1021/tx300449g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Garzón B., Gayarre J., Gharbi S., Díez-Dacal B., Sánchez-Gómez F.J., Timms J.F., Pérez-Sala D. A biotinylated analog of the anti-proliferative prostaglandin A1 allows assessment of PPAR-independent effects and identification of novel cellular targets for covalent modification. Chem. Biol. Interact. 2010;183:212–221. doi: 10.1016/j.cbi.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 88.Carbone D.L., Doorn J.A., Kiebler Z., Ickes B.R., Petersen D.R. Modification of heat shock protein 90 by 4-hydroxynonenal in a rat model of chronic alcoholic liver disease. J. Pharm. Exp. 2005;315:8–15. doi: 10.1124/jpet.105.088088. [DOI] [PubMed] [Google Scholar]
- 89.Carbone D.L., Doorn J.A., Kiebler Z., Sampey B.P., Petersen D.R. Inhibition of Hsp72-mediated protein refolding by 4-hydroxy-2-nonenal. Chem. Res. Toxicol. 2004;17:1459–1467. doi: 10.1021/tx049838g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schopfer F.J., Cole M.P., Groeger A.L., Chen C.S., Khoo N.K., Woodcock S.R., Golin-Bisello F., Motanya U.N., Li Y., Zhang J., et al. Covalent peroxisome proliferator-activated receptor gamma adduction by nitro-fatty acids: Selective ligand activity and anti-diabetic signaling actions. J. Biol. Chem. 2010;285:12321–12333. doi: 10.1074/jbc.M109.091512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shiraki T., Kamiya N., Shiki S., Kodama T.S., Kakizuka A., Jingami H. a,ß-Unsaturated Ketone is a Core Moiety of Natural Ligands for Covalent Binding to Peroxisome proliferator-activated receptor. J. Biol. Chem. 2005;280:14145–14153. doi: 10.1074/jbc.M500901200. [DOI] [PubMed] [Google Scholar]
- 92.Itoh T., Fairall L., Amin K., Inaba Y., Szanto A., Balint B.L., Nagy L., Yamamoto K., Schwabe J.W. Structural basis for the activation of PPARgamma by oxidized fatty acids. Nat. Struct. Mol. Biol. 2008;15:924–931. doi: 10.1038/nsmb.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim D.H., Kim E.H., Na H.K., Sun Y., Surh Y.J. 15-Deoxy-Delta(12,14)-prostaglandin J(2) stabilizes, but functionally inactivates p53 by binding to the cysteine 277 residue. Oncogene. 2010;29:2560–2576. doi: 10.1038/onc.2010.8. [DOI] [PubMed] [Google Scholar]
- 94.Cernuda-Morollón E., Pineda-Molina E., Cañada F.J., Pérez-Sala D. 15-Deoxy-Δ12,14-prostaglandin J2 inhibition of NF-κB DNA binding through covalent modification of the p50 subunit. J. Biol. Chem. 2001;276:35530–35536. doi: 10.1074/jbc.M104518200. [DOI] [PubMed] [Google Scholar]
- 95.Straus D.S., Pascual G., Li M., Welch J.S., Ricote M., Hsiang C.H., Sengchanthalangsy L.L., Ghosh G., Glass C.K. 15-deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in the NF- kappa B signaling pathway. Proc. Natl. Acad. Sci. USA. 2000;97:4844–4849. doi: 10.1073/pnas.97.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee E.J., Kim S.-J., Hahn Y.-I., Yoon H.-J., Hane B., Kim K., Lee S., Kim K.P., Suh Y.G., Na H.-K., et al. 15-Keto prostaglandin E2 suppresses STAT3 signaling and inhibits breast cancer cell growth and progression. Redox Biol. 2019;23:101175. doi: 10.1016/j.redox.2019.101175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pérez-Sala D., Cernuda-Morollón E., Cañada F.J. Molecular basis for the inhibition of AP-1 DNA binding by 15-deoxy-Δ12,14-prostaglandin J2. J. Biol. Chem. 2003;278:51251–51260. doi: 10.1074/jbc.M309409200. [DOI] [PubMed] [Google Scholar]
- 98.Kim H.J., Kim J.Y., Meng Z., Wang L.H., Liu F., Conrads T.P., Burke T.R., Veenstra T.D., Farrar W.L. 15-deoxy-Delta12,14-prostaglandin J2 inhibits transcriptional activity of estrogen receptor-alpha via covalent modification of DNA-binding domain. Cancer Res. 2007;67:2595–2602. doi: 10.1158/0008-5472.CAN-06-3043. [DOI] [PubMed] [Google Scholar]
- 99.Liu W., Akhand A.A., Kato M., Yokoyama I., Miyata T., Kurokawa K., Uchida K., Nakashima I. 4-hydroxynonenal triggers an epidermal growth factor receptor-linked signal pathway for growth inhibition. J. Cell Sci. 1999;112 Pt 14:2409–2417. doi: 10.1242/jcs.112.14.2409. [DOI] [PubMed] [Google Scholar]
- 100.Takahashi N., Mizuno Y., Kozai D., Yamamoto S., Kiyonaka S., Shibata T., Uchida K., Mori Y. Molecular characterization of TRPA1 channel activation by cysteine-reactive inflammatory mediators. Channels. 2008;2:287–298. doi: 10.4161/chan.2.4.6745. [DOI] [PubMed] [Google Scholar]
- 101.Tsujita T., Li L., Nakajima H., Iwamoto N., Nakajima-Takagi Y., Ohashi K., Kawakami K., Kumagai Y., Freeman B.A., Yamamoto M., et al. Nitro-fatty acids and cyclopentenone prostaglandins share strategies to activate the Keap1-Nrf2 system: A study using green fluorescent protein transgenic zebrafish. Genes Cells. 2011;16:46–57. doi: 10.1111/j.1365-2443.2010.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oh J.Y., Giles N., Landar A., Darley-Usmar V. Accumulation of 15-deoxy-delta(12,14)-prostaglandin J2 adduct formation with Keap1 over time: Effects on potency for intracellular antioxidant defence induction. Biochem. J. 2008;411:297–306. doi: 10.1042/BJ20071189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Suzuki T., Muramatsu A., Saito R., Iso T., Shibata T., Kuwata K., Kawaguchi S.I., Iwawaki T., Adachi S., Suda H., et al. Molecular Mechanism of Cellular Oxidative Stress Sensing by Keap1. Cell Rep. 2019;28:746–758. doi: 10.1016/j.celrep.2019.06.047. [DOI] [PubMed] [Google Scholar]
- 104.Kobayashi M., Li L., Iwamoto N., Nakajima-Takagi Y., Kaneko H., Nakayama Y., Eguchi M., Wada Y., Kumagai Y., Yamamoto M. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol. Cell. Biol. 2009;29:493–502. doi: 10.1128/MCB.01080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ji C., Kozak K.R., Marnett L.J. IkappaB kinase, a molecular target for inhibition by 4-hydroxy-2-nonenal. J. Biol. Chem. 2001;276:18223–18228. doi: 10.1074/jbc.M101266200. [DOI] [PubMed] [Google Scholar]
- 106.Rossi A., Kapahi P., Natoli G., Takahashi T., Chen Y., Karin M., Santoro M.G. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IκB kinase. Nature. 2000;403:103–108. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- 107.Oliva J.L., Pérez-Sala D., Castrillo A., Martínez N., Cañada F.J., Boscá L., Rojas J.M. The cyclopentenone 15-deoxy-Δ12,14-prostaglandin J2 binds to and activates H-Ras. Proc. Natl. Acad. Sci. USA. 2003;100:4772–4777. doi: 10.1073/pnas.0735842100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Renedo M., Gayarre J., García-Domínguez C.A., Pérez-Rodríguez A., Prieto A., Cañada F.J., Rojas J.M., Pérez-Sala D. Modification and activation of Ras proteins by electrophilic prostanoids with different structure are site-selective. Biochemistry. 2007;46:6607–6616. doi: 10.1021/bi602389p. [DOI] [PubMed] [Google Scholar]
- 109.Shearn C.T., Smathers R.L., Backos D.S., Reigan P., Orlicky D.J., Petersen D.R. Increased carbonylation of the lipid phosphatase PTEN contributes to Akt2 activation in a murine model of early alcohol-induced steatosis. Free Radic. Biol. Med. 2013;65:680–692. doi: 10.1016/j.freeradbiomed.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shearn C.T., Fritz K.S., Reigan P., Petersen D.R. Modification of Akt2 by 4-hydroxynonenal inhibits insulin-dependent Akt signaling in HepG2 cells. Biochemistry. 2011;50:3984–3996. doi: 10.1021/bi200029w. [DOI] [PubMed] [Google Scholar]
- 111.Caito S., Rajendrasozhan S., Cook S., Chung S., Yao H., Friedman A.E., Brookes P.S., Rahman I. SIRT1 is a redox-sensitive deacetylase that is post-translationally modified by oxidants and carbonyl stress. FASEB J. 2010;24:3145–3159. doi: 10.1096/fj.09-151308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Randall M.J., Haenen G.R., Bouwman F.G., van der Vliet A., Bast A. The tobacco smoke component acrolein induces glucocorticoid resistant gene expression via inhibition of histone deacetylase. Toxicol. Lett. 2016;240:43–49. doi: 10.1016/j.toxlet.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mishra N., Kar R., Singha P.K., Venkatachalam M.A., McEwen D.G., Saikumar P. Inhibition of mitochondrial division through covalent modification of Drp1 protein by 15 deoxy-Delta(12,14)-prostaglandin J2. Biochem. Biophys. Res. Commun. 2010;395:17–24. doi: 10.1016/j.bbrc.2010.03.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Isom A.L., Barnes S., Wilson L., Kirk M., Coward L., Darley-Usmar V. Modification of Cytochrome c by 4-hydroxy- 2-nonenal: Evidence for histidine, lysine, and arginine-aldehyde adducts. J. Am. Soc. Mass Spectrom. 2004;15:1136–1147. doi: 10.1016/j.jasms.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 115.Liu Q., Simpson D.C., Gronert S. Carbonylation of mitochondrial aconitase with 4-hydroxy-2-(E)-nonenal: Localization and relative reactivity of addition sites. Biochim. Biophys. Acta. 2013;1834:1144–1154. doi: 10.1016/j.bbapap.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yamaguchi S., Aldini G., Ito S., Morishita N., Shibata T., Vistoli G., Carini M., Uchida K. Delta(12)-Prostaglandin J(2) as a Product and Ligand of Human Serum Albumin: Formation of an Unusual Covalent Adduct at His146. J. Am. Chem. Soc. 2010;132:824–832. doi: 10.1021/ja908878n. [DOI] [PubMed] [Google Scholar]
- 117.Aldini G., Regazzoni L., Orioli M., Rimoldi I., Facino R.M., Carini M. A tandem MS precursor-ion scan approach to identify variable covalent modification of albumin Cys34: A new tool for studying vascular carbonylation. J. Mass Spectrom. 2008;43:1470–1481. doi: 10.1002/jms.1419. [DOI] [PubMed] [Google Scholar]
- 118.Ricote M., Li A.C., Willson T.M., Kelly C.J., Glass C.K. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 119.Li M., Pascual G., Glass C.K. Peroxisome proliferator-activated receptor gamma-dependent repression of the inducible nitric oxide synthase gene. Mol. Cell. Biol. 2000;20:4699–4707. doi: 10.1128/MCB.20.13.4699-4707.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Waku T., Shiraki T., Oyama T., Fujimoto Y., Maebara K., Kamiya N., Jingami H., Morikawa K. Structural insight into PPARgamma activation through covalent modification with endogenous fatty acids. J. Mol. Biol. 2009;385:188–199. doi: 10.1016/j.jmb.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 121.Zhang D.D. The Nrf2-Keap1-ARE signaling pathway: The regulation and dual function of Nrf2 in cancer. Antioxid. Redox Signal. 2010;13:1623–1626. doi: 10.1089/ars.2010.3301. [DOI] [PubMed] [Google Scholar]
- 122.Patinen T., Adinolfi S., Cortes C.C., Harkonen J., Jawahar Deen A., Levonen A.L. Regulation of stress signaling pathways by protein lipoxidation. Redox Biol. 2019:101114. doi: 10.1016/j.redox.2019.101114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mónico A., Duarte S., Pajares M.A., Pérez-Sala D. Vimentin disruption by lipoxidation and electrophiles: Role of the cysteine residue and filament dynamics. Redox Biol. 2019;23:101098. doi: 10.1016/j.redox.2019.101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mol M., Degani G., Coppa C., Baron G., Popolo L., Carini M., Aldini G., Vistoli G., Altomare A. Advanced lipoxidation end products (ALEs) as RAGE binders: Mass spectrometric and computational studies to explain the reasons why. Redox Biol. 2019;23:101083. doi: 10.1016/j.redox.2018.101083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oeste C.L., Díez-Dacal B., Bray F., García de Lacoba M., de la Torre B.G., Andreu D., Ruiz-Sánchez A.J., Pérez-Inestrosa E., García-Domínguez C.A., Rojas J.M., et al. The C-terminus of H-Ras as a target for the covalent binding of reactive compounds modulating Ras-dependent pathways. PLoS ONE. 2011;6:e15866. doi: 10.1371/journal.pone.0015866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hilliard M., Frohnert C., Spillner C., Marcone S., Nath A., Lampe T., Fitzgerald D., Kehlenbach R.H. The anti-inflammatory prostaglandin 15-Deoxy-{Delta}12,14 PGJ2 inhibits CRM1-dependent nuclear protein export. J. Biol. Chem. 2010;285:22202–22210. doi: 10.1074/jbc.M110.131821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jove M., Mota-Martorell N., Pradas I., Martin-Gari M., Ayala V., Pamplona R. The Advanced Lipoxidation End-Product Malondialdehyde-Lysine in Aging and Longevity. Antioxidants. 2020;9:1132. doi: 10.3390/antiox9111132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gesslbauer B., Kuerzl D., Valpatic N., Bochkov V.N. Unbiased Identification of Proteins Covalently Modified by Complex Mixtures of Peroxidized Lipids Using a Combination of Electrophoretic Mobility Band Shift with Mass Spectrometry. Antioxidants. 2018;7:116. doi: 10.3390/antiox7090116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Aldini G., Domingues M.R., Spickett C.M., Domingues P., Altomare A., Sánchez-Gómez F.J., Oeste C.L., Pérez-Sala D. Protein lipoxidation: Detection strategies and challenges. Redox Biol. 2015;5:253–266. doi: 10.1016/j.redox.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kansanen E., Jyrkkanen H.K., Levonen A.L. Activation of stress signaling pathways by electrophilic oxidized and nitrated lipids. Free Radic. Biol. Med. 2012;52:973–982. doi: 10.1016/j.freeradbiomed.2011.11.038. [DOI] [PubMed] [Google Scholar]
- 131.Chen Y., Liu Y., Hou X., Ye Z., Wang C. Quantitative and Site-Specific Chemoproteomic Profiling of Targets of Acrolein. Chem. Res. Toxicol. 2019;32:467–473. doi: 10.1021/acs.chemrestox.8b00343. [DOI] [PubMed] [Google Scholar]
- 132.Pamplona R., Borras C., Jove M., Pradas I., Ferrer I., Vina J. Redox lipidomics to better understand brain aging and function. Free Radic. Biol. Med. 2019;144:310–321. doi: 10.1016/j.freeradbiomed.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 133.Dong Y., Noda K., Murata M., Yoshida S., Saito W., Kanda A., Ishida S. Localization of Acrolein-Lysine Adduct in Fibrovascular Tissues of Proliferative Diabetic Retinopathy. Curr. Eye Res. 2017;42:111–117. doi: 10.3109/02713683.2016.1150491. [DOI] [PubMed] [Google Scholar]
- 134.Chavez J.D., Wu J., Bisson W., Maier C.S. Site-specific proteomic analysis of lipoxidation adducts in cardiac mitochondria reveals chemical diversity of 2-alkenal adduction. J. Proteom. 2011;74:2417–2429. doi: 10.1016/j.jprot.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Han B., Hare M., Wickramasekara S., Fang Y., Maier C.S. A comparative ‘bottom up’ proteomics strategy for the site-specific identification and quantification of protein modifications by electrophilic lipids. J. Proteom. 2012;75:5724–5733. doi: 10.1016/j.jprot.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Codreanu S.G., Ullery J.C., Zhu J., Tallman K.A., Beavers W.N., Porter N.A., Marnett L.J., Zhang B., Liebler D.C. Alkylation damage by lipid electrophiles targets functional protein systems. Mol Cell Proteom. 2014;13:849–859. doi: 10.1074/mcp.M113.032953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Campos-Pinto I., Mendez L., Schouten J., Wilkins J., Fedorova M., Pitt A.R., Davis P., Spickett C.M. Epitope mapping and characterization of 4-hydroxy-2-nonenal modified-human serum albumin using two different polyclonal antibodies. Free Radic. Biol. Med. 2019;144:234–244. doi: 10.1016/j.freeradbiomed.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 138.Ishii T., Ito S., Kumazawa S., Sakurai T., Yamaguchi S., Mori T., Nakayama T., Uchida K. Site-specific modification of positively-charged surfaces on human serum albumin by malondialdehyde. Biochem. Biophys. Res. Commun. 2008;371:28–32. doi: 10.1016/j.bbrc.2008.03.140. [DOI] [PubMed] [Google Scholar]
- 139.Tran T.N., Kosaraju M.G., Tamamizu-Kato S., Akintunde O., Zheng Y., Bielicki J.K., Pinkerton K., Uchida K., Lee Y.Y., Narayanaswami V. Acrolein modification impairs key functional features of rat apolipoprotein E: Identification of modified sites by mass spectrometry. Biochemistry. 2014;53:361–375. doi: 10.1021/bi401404u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Eliuk S.M., Renfrow M.B., Shonsey E.M., Barnes S., Kim H. Active site modifications of the brain isoform of creatine kinase by 4-hydroxy-2-nonenal correlate with reduced enzyme activity: Mapping of modified sites by Fourier transform-ion cyclotron resonance mass spectrometry. Chem. Res. Toxicol. 2007;20:1260–1268. doi: 10.1021/tx7000948. [DOI] [PubMed] [Google Scholar]
- 141.Vistoli G., Mantovani C., Gervasoni S., Pedretti A., Aldini G. Key factors regulating protein carbonylation by alpha,beta unsaturated carbonyls: A structural study based on a retrospective meta-analysis. Biophys. Chem. 2017;230:20–26. doi: 10.1016/j.bpc.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 142.Chen Y., Cong Y., Quan B., Lan T., Chu X., Ye Z., Hou X., Wang C. Chemoproteomic profiling of targets of lipid-derived electrophiles by bioorthogonal aminooxy probe. Redox Biol. 2017;12:712–718. doi: 10.1016/j.redox.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wall S.B., Oh J.Y., Mitchell L., Laube A.H., Campbell S.L., Renfrow M.B., Landar A. Rac1 modification by an electrophilic 15-deoxy Delta(12,14)-prostaglandin J2 analog. Redox Biol. 2015;4:346–354. doi: 10.1016/j.redox.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Roos G., Foloppe N., Messens J. Understanding the pK(a) of redox cysteines: The key role of hydrogen bonding. Antioxid. Redox Signal. 2013;18:94–127. doi: 10.1089/ars.2012.4521. [DOI] [PubMed] [Google Scholar]
- 145.Poole L.B. The basics of thiols and cysteines in redox biology and chemistry. Free Radic. Biol. Med. 2015;80:148–157. doi: 10.1016/j.freeradbiomed.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cai J., Bhatnagar A., Pierce W.M., Jr. Protein modification by acrolein: Formation and stability of cysteine adducts. Chem. Res. Toxicol. 2009;22:708–716. doi: 10.1021/tx800465m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fisher A.A., Labenski M.T., Malladi S., Gokhale V., Bowen M.E., Milleron R.S., Bratton S.B., Monks T.J., Lau S.S. Quinone electrophiles selectively adduct “electrophile binding motifs” within cytochrome c. Biochemistry. 2007;46:11090–11100. doi: 10.1021/bi700613w. [DOI] [PubMed] [Google Scholar]
- 148.Stewart M.D., Igumenova T.I. Reactive cysteine in the structural Zn(2+) site of the C1B domain from PKCalpha. Biochemistry. 2012;51:7263–7277. doi: 10.1021/bi300750w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gayarre J., Stamatakis K., Renedo M., Pérez-Sala D. Differential selectivity of protein modification by the cyclopentenone prostaglandins PGA1 and 15-deoxy-Δ12,14-PGJ2: Role of glutathione. FEBS Lett. 2005;579:5803–5808. doi: 10.1016/j.febslet.2005.09.069. [DOI] [PubMed] [Google Scholar]
- 150.Rajakariar R., Hilliard M., Lawrence T., Trivedi S., Colville-Nash P., Bellingan G., Fitzgerald D., Yaqoob M.M., Gilroy D.W. Hematopoietic prostaglandin D2 synthase controls the onset and resolution of acute inflammation through PGD2 and 15-deoxyDelta12 14 PGJ2. Proc. Natl. Acad. Sci. USA. 2007;104:20979–20984. doi: 10.1073/pnas.0707394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ong W.Y., Farooqui T., Kokotos G., Farooqui A.A. Synthetic and natural inhibitors of phospholipases A2: Their importance for understanding and treatment of neurological disorders. ACS Chem. Neurosci. 2015;6:814–831. doi: 10.1021/acschemneuro.5b00073. [DOI] [PubMed] [Google Scholar]
- 152.Hawkey C.J. Cyclooxygenase inhibition: Between the devil and the deep blue sea. Gut. 2002;50(Suppl. 3):III25–III30. doi: 10.1136/gut.50.suppl_3.iii25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Paumi C.M., Smitherman P.K., Townsend A.J., Morrow C.S. Glutathione S-Transferases (GSTs) inhibit transcriptional activation by the peroxisomal proliferator-activated receptor γ (PPARγ) ligand, 15-deoxy-Δ12,14prostaglandin J2 (15-d-PGJ2) Biochemistry. 2004;43:2345–2352. doi: 10.1021/bi035936+. [DOI] [PubMed] [Google Scholar]
- 154.Alary J., Gueraud F., Cravedi J.P. Fate of 4-hydroxynonenal in vivo: Disposition and metabolic pathways. Mol. Asp. Med. 2003;24:177–187. doi: 10.1016/S0098-2997(03)00012-8. [DOI] [PubMed] [Google Scholar]
- 155.Hayes J.D., Flanagan J.U., Jowsey I.R. GLUTATHIONE TRANSFERASES. Annu. Rev. Pharm. Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 156.Atsmon J., Freeman M.L., Meredith M.J., Sweetman B.J., Roberts L.J., II Conjugation of 9-deoxy-delta 9, delta 12(E)-prostaglandin D2 with intracellular glutathione and enhancement of its antiproliferative activity by glutathione depletion. Cancer Res. 1990;50:1879–1885. [PubMed] [Google Scholar]
- 157.Bogaards J.J., Venekamp J.C., van Bladeren P.J. Stereoselective conjugation of prostaglandin A2 and prostaglandin J2 with glutathione, catalyzed by the human glutathione S-transferases A1-1, A2-2, M1a-1a, and P1-1. Chem. Res. Toxicol. 1997;10:310–317. doi: 10.1021/tx9601770. [DOI] [PubMed] [Google Scholar]
- 158.Hashimoto K. Role of Soluble Epoxide Hydrolase in Metabolism of PUFAs in Psychiatric and Neurological Disorders. Front. Pharmacol. 2019;10:36. doi: 10.3389/fphar.2019.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Spite M., Baba S.P., Ahmed Y., Barski O.A., Nijhawan K., Petrash J.M., Bhatnagar A., Srivastava S. Substrate specificity and catalytic efficiency of aldo-keto reductases with phospholipid aldehydes. Biochem. J. 2007;405:95–105. doi: 10.1042/BJ20061743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shen Y., Zhong L., Johnson S., Cao D. Human aldo-keto reductases 1B1 and 1B10: A comparative study on their enzyme activity toward electrophilic carbonyl compounds. Chem. Biol. Interact. 2011;191:192–198. doi: 10.1016/j.cbi.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Aldini G., Granata P., Orioli M., Santaniello E., Carini M. Detoxification of 4-hydroxynonenal (HNE) in keratinocytes: Characterization of conjugated metabolites by liquid chromatography/electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 2003;38:1160–1168. doi: 10.1002/jms.533. [DOI] [PubMed] [Google Scholar]
- 162.Díez-Dacal B., Sánchez-Gómez F.J., Sánchez-Murcia P.A., Milackova I., Zimmerman T., Ballekova J., García-Martín E., Agúndez J.A.G., Gharbi S., Gago F., et al. Molecular interactions and implications of aldose reductase inhibition by PGA1 and clinically used prostaglandins. Mol. Pharm. 2016;89:42–52. doi: 10.1124/mol.115.100693. [DOI] [PubMed] [Google Scholar]
- 163.Sun R., Fu L., Liu K., Tian C., Yang Y., Tallman K.A., Porter N.A., Liebler D.C., Yang J. Chemoproteomics Reveals Chemical Diversity and Dynamics of 4-Oxo-2-nonenal Modifications in Cells. Mol. Cell. Proteom. 2017;16:1789–1800. doi: 10.1074/mcp.RA117.000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lin D., Lee H.G., Liu Q., Perry G., Smith M.A., Sayre L.M. 4-Oxo-2-nonenal is both more neurotoxic and more protein reactive than 4-hydroxy-2-nonenal. Chem. Res. Toxicol. 2005;18:1219–1231. doi: 10.1021/tx050080q. [DOI] [PubMed] [Google Scholar]
- 165.Randall M.J., Hristova M., van der Vliet A. Protein alkylation by the alpha,beta-unsaturated aldehyde acrolein. A reversible mechanism of electrophile signaling? FEBS Lett. 2013;587:3808–3814. doi: 10.1016/j.febslet.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cui Y., Li X., Lin J., Hao Q., Li X.D. Histone Ketoamide Adduction by 4-Oxo-2-nonenal Is a Reversible Posttranslational Modification Regulated by Sirt2. ACS Chem. Biol. 2017;12:47–51. doi: 10.1021/acschembio.6b00713. [DOI] [PubMed] [Google Scholar]
- 167.Jin J., He B., Zhang X., Lin H., Wang Y. SIRT2 Reverses 4-Oxononanoyl Lysine Modification on Histones. J. Am. Chem. Soc. 2016;138:12304–12307. doi: 10.1021/jacs.6b04977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Freeman B.A., O’Donnell V.B., Schopfer F.J. The discovery of nitro-fatty acids as products of metabolic and inflammatory reactions and mediators of adaptive cell signaling. Nitric Oxide. 2018;77:106–111. doi: 10.1016/j.niox.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Khoo N.K.H., Schopfer F.J. Nitrated fatty acids: From diet to disease. Curr. Opin. Physiol. 2019;9:67–72. doi: 10.1016/j.cophys.2019.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Aldini G., Vistoli G., Stefek M., Chondrogianni N., Grune T., Sereikaite J., Sadowska-Bartosz I., Bartosz G. Molecular strategies to prevent, inhibit, and degrade advanced glycoxidation and advanced lipoxidation end products. Free Radic. Res. 2013;47(Suppl. 1):93–137. doi: 10.3109/10715762.2013.792926. [DOI] [PubMed] [Google Scholar]
- 171.Hill B.G., Haberzettl P., Ahmed Y., Srivastava S., Bhatnagar A. Unsaturated lipid peroxidation-derived aldehydes activate autophagy in vascular smooth-muscle cells. Biochem. J. 2008;410:525–534. doi: 10.1042/BJ20071063. [DOI] [PubMed] [Google Scholar]
- 172.Díez-Dacal B., Pérez-Sala D. Anti-inflammatory prostanoids: Focus on the interactions between electrophile signalling and resolution of inflammation. Sci. World J. 2010;10:655–675. doi: 10.1100/tsw.2010.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nimse S.B., Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015;5:27986–28006. doi: 10.1039/C4RA13315C. [DOI] [Google Scholar]
- 174.Suzuki M., Mori M., Niwa T., Hirata R., Furuta K., Ishikawa T., Noyori R. Chemical implications for antitumor and antiviral prostaglandins: Reaction of Δ7-prostaglandin A1 and prostaglandin A1 methyl esters with thiols. J. Am. Chem. Soc. 1997;119:2376–2385. doi: 10.1021/ja9628359. [DOI] [Google Scholar]
- 175.Goto S., Ihara Y., Urata Y., Izumi S., Abe K., Koji T., Kondo T. Doxorubicin-induced DNA intercalation and scavenging by nuclear glutathione S-transferase pi. FASEB J. 2001;15:2702–2714. doi: 10.1096/fj.01-0376com. [DOI] [PubMed] [Google Scholar]
- 176.Soh Y., Goto S., Kitajima M., Moriyama S., Kotera K., Nakayama T., Nakajima H., Kondo T., Ishimaru T. Nuclear localisation of glutathione S-transferase pi is an evaluation factor for drug resistance in gynaecological cancers. Clin. Oncol. 2005;17:264–270. doi: 10.1016/j.clon.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 177.Kondo M., Oya-Ito T., Kumagai T., Osawa T., Uchida K. Cyclopentenone prostaglandins as potential inducers of intracellular oxidative stress. J. Biol. Chem. 2001;276:12076–12083. doi: 10.1074/jbc.M009630200. [DOI] [PubMed] [Google Scholar]
- 178.Roede J.R., Carbone D.L., Doorn J.A., Kirichenko O.V., Reigan P., Petersen D.R. In vitro and in silico characterization of peroxiredoxin 6 modified by 4-hydroxynonenal and 4-oxononenal. Chem. Res. Toxicol. 2008;21:2289–2299. doi: 10.1021/tx800244u. [DOI] [PubMed] [Google Scholar]
- 179.Go Y.M., Halvey P.J., Hansen J.M., Reed M., Pohl J., Jones D.P. Reactive aldehyde modification of thioredoxin-1 activates early steps of inflammation and cell adhesion. Am. J. Pathol. 2007;171:1670–1681. doi: 10.2353/ajpath.2007.070218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Shibata T., Yamada T., Ishii T., Kumazawa S., Nakamura H., Masutani H., Yodoi J., Uchida K. Thioredoxin as a molecular target of cyclopentenone prostaglandins. J. Biol. Chem. 2003;278:26046–26054. doi: 10.1074/jbc.M303690200. [DOI] [PubMed] [Google Scholar]
- 181.Moos P.J., Edes K., Cassidy P., Massuda E., Fitzpatrick F.A. Electrophilic Prostaglandins and Lipid Aldehydes Repress Redox- sensitive Transcription Factors p53 and Hypoxia-inducible Factor by Impairing the Selenoprotein Thioredoxin Reductase. J. Biol. Chem. 2003;278:745–750. doi: 10.1074/jbc.M211134200. [DOI] [PubMed] [Google Scholar]
- 182.Huang H.Y., Appel L.J., Croft K.D., Miller E.R., 3rd, Mori T.A., Puddey I.B. Effects of vitamin C and vitamin E on in vivo lipid peroxidation: Results of a randomized controlled trial. Am. J. Clin. Nutr. 2002;76:549–555. doi: 10.1093/ajcn/76.3.549. [DOI] [PubMed] [Google Scholar]
- 183.Kang Z., Li H., Li G., Yin D. Reaction of pyridoxamine with malondialdehyde: Mechanism of inhibition of formation of advanced lipoxidation end-products. Amino Acids. 2006;30:55–61. doi: 10.1007/s00726-005-0209-6. [DOI] [PubMed] [Google Scholar]
- 184.Metz T.O., Alderson N.L., Chachich M.E., Thorpe S.R., Baynes J.W. Pyridoxamine traps intermediates in lipid peroxidation reactions in vivo: Evidence on the role of lipids in chemical modification of protein and development of diabetic complications. J. Biol. Chem. 2003;278:42012–42019. doi: 10.1074/jbc.M304292200. [DOI] [PubMed] [Google Scholar]
- 185.Gianazza E., Brioschi M., Fernandez A.M., Banfi C. Lipoxidation in cardiovascular diseases. Redox Biol. 2019;23:101119. doi: 10.1016/j.redox.2019.101119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Maret W. The redox biology of redox-inert zinc ions. Free Radic. Biol. Med. 2019;134:311–326. doi: 10.1016/j.freeradbiomed.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 187.Pace N.J., Weerapana E. A competitive chemical-proteomic platform to identify zinc-binding cysteines. ACS Chem. Biol. 2014;9:258–265. doi: 10.1021/cb400622q. [DOI] [PubMed] [Google Scholar]
- 188.Pérez-Sala D., Oeste C.L., Martínez A.E., Garzón B., Carrasco M.J., Cañada F.J. Vimentin filament organization and stress sensing depend on its single cysteine residue and zinc binding. Nat. Commun. 2015;6:7287. doi: 10.1038/ncomms8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hao Q., Maret W. Aldehydes release zinc from proteins. A pathway from oxidative stress/lipid peroxidation to cellular functions of zinc. FEBS J. 2006;273:4300–4310. doi: 10.1111/j.1742-4658.2006.05428.x. [DOI] [PubMed] [Google Scholar]
- 190.Aldini G., Facino R.M., Beretta G., Carini M. Carnosine and related dipeptides as quenchers of reactive carbonyl species: From structural studies to therapeutic perspectives. Biofactors. 2005;24:77–87. doi: 10.1002/biof.5520240109. [DOI] [PubMed] [Google Scholar]
- 191.Anderson E.J., Vistoli G., Katunga L.A., Funai K., Regazzoni L., Monroe T.B., Gilardoni E., Cannizzaro L., Colzani M., De Maddis D., et al. A carnosine analog mitigates metabolic disorders of obesity by reducing carbonyl stress. J. Clin. Investig. 2018;128:5280–5293. doi: 10.1172/JCI94307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Davies M.J. The oxidative environment and protein damage. Biochim. Biophys. Acta. 2005;1703:93–109. doi: 10.1016/j.bbapap.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 193.Hagglund P., Mariotti M., Davies M.J. Identification and characterization of protein cross-links induced by oxidative reactions. Expert Rev. Proteom. 2018;15:665–681. doi: 10.1080/14789450.2018.1509710. [DOI] [PubMed] [Google Scholar]
- 194.Hawkins C.L., Davies M.J. Detection, identification, and quantification of oxidative protein modifications. J. Biol. Chem. 2019;294:19683–19708. doi: 10.1074/jbc.REV119.006217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lavergne S.N., Wang H., Callan H.E., Park B.K., Naisbitt D.J. “Danger” conditions increase sulfamethoxazole-protein adduct formation in human antigen-presenting cells. J. Pharm. Exp. 2009;331:372–381. doi: 10.1124/jpet.109.155374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Karimi M., Crossett B., Cordwell S.J., Pattison D.I., Davies M.J. Characterization of disulfide (cystine) oxidation by HOCl in a model peptide: Evidence for oxygen addition, disulfide bond cleavage and adduct formation with thiols. Free Radic. Biol. Med. 2020;154:62–74. doi: 10.1016/j.freeradbiomed.2020.04.023. [DOI] [PubMed] [Google Scholar]
- 197.Klatt P., Lamas S. Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur. J. Biochem. 2000;267:4928–4944. doi: 10.1046/j.1432-1327.2000.01601.x. [DOI] [PubMed] [Google Scholar]
- 198.Hill B.G., Bhatnagar A. Role of glutathiolation in preservation, restoration and regulation of protein function. IUBMB Life. 2007;59:21–26. doi: 10.1080/15216540701196944. [DOI] [PubMed] [Google Scholar]
- 199.Viedma-Poyatos A., Pajares M.A., Pérez-Sala D. Type III intermediate filaments as targets and effectors of electrophiles and oxidants. Redox Biol. 2020;36:101582. doi: 10.1016/j.redox.2020.101582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kaus-Drobek M., Mucke N., Szczepanowski R.H., Wedig T., Czarnocki-Cieciura M., Polakowska M., Herrmann H., Wyslouch-Cieszynska A., Dadlez M. Vimentin S-glutathionylation at Cys328 inhibits filament elongation and induces severing of mature filaments in vitro. FEBS J. 2020 doi: 10.1111/febs.15321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Duarte S., Melo T., Domingues R., Alché J.d.D., Pérez-Sala D. Insight into the cellular effects of nitrated phospholipids: Evidence for pleiotropic mechanisms of action. Free Rad. Biol. Med. 2019;144:192–202. doi: 10.1016/j.freeradbiomed.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 202.Burgoyne J.R., Eaton P. Oxidant sensing by protein kinases a and g enables integration of cell redox state with phosphoregulation. Sensors. 2010;10:2731–2751. doi: 10.3390/s100402731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Anderson M.E. Oxidant stress promotes disease by activating CaMKII. J. Mol. Cell. Cardiol. 2015;89:160–167. doi: 10.1016/j.yjmcc.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Shearn C.T., Backos D.S., Orlicky D.J., Smathers-McCullough R.L., Petersen D.R. Identification of 5’ AMP-activated kinase as a target of reactive aldehydes during chronic ingestion of high concentrations of ethanol. J. Biol. Chem. 2014;289:15449–15462. doi: 10.1074/jbc.M113.543942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Adler V., Yin Z., Fuchs S.Y., Benezra M., Rosario L., Tew K.D., Pincus M.R., Sardana M., Henderson C.J., Wolf C.R., et al. Regulation of JNK signaling by GSTp. EMBO J. 1999;18:1321–1334. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Thevenin A.F., Zony C.L., Bahnson B.J., Colman R.F. GST pi modulates JNK activity through a direct interaction with JNK substrate, ATF2. Protein Sci. A Publ. Protein Soc. 2011;20:834–848. doi: 10.1002/pro.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.De Luca A., Mei G., Rosato N., Nicolai E., Federici L., Palumbo C., Pastore A., Serra M., Caccuri A.M. The fine-tuning of TRAF2-GSTP1-1 interaction: Effect of ligand binding and in situ detection of the complex. Cell Death Dis. 2014;5:e1015. doi: 10.1038/cddis.2013.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Millán O., Rico D., Peinado H., Zarich N., Stamatakis K., Pérez-Sala D., Rojas J.M., Cano A., Boscá L. Potentiation of tumor formation by topic administration of 15-deoxy-Δ12,14-prostaglandin J2 in a model of skin carcinogenesis. Carcinogenesis. 2006;27:328–336. doi: 10.1093/carcin/bgi213. [DOI] [PubMed] [Google Scholar]
- 209.Rhee S.G., Bae Y.S., Lee S.R., Kwon J. Hydrogen peroxide: A key messenger that modulates protein phosphorylation through cysteine oxidation. Sci. Signal. 2000;2000:pe1. doi: 10.1126/stke.2000.53.pe1. [DOI] [PubMed] [Google Scholar]
- 210.Xu G.B., Guan P.P., Wang P. Prostaglandin A1 Decreases the Phosphorylation of Tau by Activating Protein Phosphatase 2A via a Michael Addition Mechanism at Cysteine 377. Mol. Neurobiol. 2020 doi: 10.1007/s12035-020-02174-z. [DOI] [PubMed] [Google Scholar]
- 211.Suh J., Kim D.H., Kim E.H., Park S.A., Park J.M., Jang J.H., Kim S.J., Na H.K., Kim N.D., Kim N.J., et al. 15-Deoxy-Delta(12,14)-prostaglandin J2 activates PI3K-Akt signaling in human breast cancer cells through covalent modification of the tumor suppressor PTEN at cysteine 136. Cancer Lett. 2018;424:30–45. doi: 10.1016/j.canlet.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 212.Ichikawa T., Zhang J., Chen K., Liu Y., Schopfer F.J., Baker P.R., Freeman B.A., Chen Y.E., Cui T. Nitroalkenes suppress lipopolysaccharide-induced signal transducer and activator of transcription signaling in macrophages: A critical role of mitogen-activated protein kinase phosphatase 1. Endocrinology. 2008;149:4086–4094. doi: 10.1210/en.2007-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Steeg P.S., Palmieri D., Ouatas T., Salerno M. Histidine kinases and histidine phosphorylated proteins in mammalian cell biology, signal transduction and cancer. Cancer Lett. 2003;190:1–12. doi: 10.1016/S0304-3835(02)00499-8. [DOI] [PubMed] [Google Scholar]
- 214.Fritz K.S., Galligan J.J., Smathers R.L., Roede J.R., Shearn C.T., Reigan P., Petersen D.R. 4-Hydroxynonenal inhibits SIRT3 via thiol-specific modification. Chem. Res. Toxicol. 2011;24:651–662. doi: 10.1021/tx100355a. [DOI] [PMC free article] [PubMed] [Google Scholar]