Supplemental digital content is available in the text.
Key Words: viloxazine, SPN-812, attention-deficit/hyperactivity disorder, lisdexamfetamine, pharmacokinetics
Abstract
Background
Viloxazine extended-release is a novel nonstimulant under investigation as a potential treatment for attention-deficit/hyperactivity disorder (ADHD). Given the potential for viloxazine extended-release to be co-administered with stimulant ADHD pharmacotherapies, this trial investigated the pharmacokinetics and safety of combination viloxazine extended-release + lisdexamfetamine dimesylate (lisdexamfetamine) versus viloxazine extended-release and lisdexamfetamine alone.
Methods
In this single-center, cross-over, open-label trial, healthy, non-ADHD adults received single oral doses of 700 mg viloxazine extended-release alone, 50 mg lisdexamfetamine alone, and a combination of viloxazine extended-release (700 mg) + lisdexamfetamine (50 mg), with blood samples collected over 4 days postadministration. The active drug in viloxazine extended-release (viloxazine) and primary metabolite of lisdexamfetamine (d-amphetamine) were measured using chromatographic tandem mass spectrometry. Safety assessments included adverse events, vital signs, echocardiograms, and clinical laboratory evaluations.
Results
Thirty-six adults were enrolled, and 34 completed the trial. The least squares geometric mean ratios are reported as [combination / single drug (90% confidence intervals)]. Viloxazine extended-release: Cmax = 95.96% (91.33–100.82), area under the concentration–time curve from 0 to the last measurable time (AUC0-t) = 99.19% (96.53–101.91), and area under the concentration–time curve from 0 to infinity (AUCinf) = 99.23% (96.61–101.93). Lisdexamfetamine: Cmax = 112.78% (109.93–115.71), AUC0-t = 109.64% (105.25–114.22), and AUCinf = 109.52% (105.19–114.03). All reported adverse events, except 1 (moderate vomiting), were mild in severity.
Conclusions
Co-administration of viloxazine extended-release and lisdexamfetamine did not impact the pharmacokinetics of viloxazine or d-amphetamine relative to administration of either drug alone. After single dose administration, the combination appeared to be safe and well tolerated.
Attention-deficit/hyperactivity disorder (ADHD), characterized by age-inappropriate and persistent aberrant behavioral patterns, including the core symptoms of inattention, hyperactivity, and impulsivity,1–3 has been diagnosed in approximately 6.1 million (9.4%) US children and adolescents.4 Although the reported prevalence in adults (18–44 years) is lower—estimated between 2.5% and 4.4%5–7—ADHD frequently persists as a lifelong disorder that may require new pharmacological treatment paradigms when others are no longer suitable.
Current Food and Drug Administration-approved ADHD pharmacotherapies are classified as either stimulants (eg, methylphenidate, lisdexamfetamine dimesylate [lisdexamfetamine], amphetamine) or nonstimulants (eg, atomoxetine, extended-release guanfacine, extended-release clonidine).8,9 Stimulant pharmacotherapy is generally used as first-line treatment, because stimulants are associated with rapid onset of effect and greater efficacy than nonstimulants in improving ADHD symptoms.10–12 However, stimulants may be contraindicated in patients with other conditions (eg, insomnia, anorexia, bipolar disorders, cardiovascular abnormalities such as hypertension or arrythmias),13–16 have a generally short (eg, 3–12 hours) duration of action,17 and the potential for abuse and diversion.18,19 Most frequently, clinicians prescribe nonstimulants or a combination of stimulant and nonstimulant therapy to treat ADHD when presented with signs and/or symptoms that preclude a stimulant-only treatment approach, such as an inability to optimize dosing because of adverse events (AEs), inadequate treatment response, or an insufficient duration of action.10–12
SPN-812 (viloxazine extended-release) has a novel, multimodal mechanism of action, with demonstrated activity at serotonin receptors and the norepinephrine transporter.20 The original, immediate-release viloxazine formulation was approved in Europe as an antidepressant in 1974 and marketed for 28 years, before being discontinued in the early 2000s for business reasons unrelated to safety or efficacy,21,22 resulting in a long history of clinical safety and tolerability.23–25 Viloxazine extended-release is currently under development as an extended release nonstimulant treatment for ADHD.24,26–29
A recent study30 of viloxazine demonstrated that viloxazine's primary metabolic route in humans is through 5-hydroxylation followed by glucuronidation. Based on in vitro data from this same study, the major enzyme contributing to the formation of 5-hydroxyviloxazine is CYP2D6, with minor involvement of CYP1A2, CYP2B6, CYP2C9, CYP2C19, and CYP3A4. The subsequent glucuronidation to 5-hydroyviloxazine glucuronide (5-HVLX-gluc) is mediated by UGT1A9 and 2B15.
Despite its long history of use in Europe, no studies (contemporary or historic) have been conducted to investigate potential drug-drug interactions (DDIs) with viloxazine and other treatments for ADHD. Therefore, the purpose of the current study was to determine whether the coadministration of viloxazine extended-release and a psychostimulant, such as lisdexamfetamine (a stimulant prodrug distributed under the brand name Vyvanse by Shire US, Inc. Lexington, MA) impacts the pharmacokinetics (PK) of viloxazine extended-release or lisdexamfetamine, compared with either drug administered alone. Using clinically relevant, one-time single doses (orally administered), the PK of a single dose of viloxazine extended-release co-administered with a single dose of lisdexamfetamine was assessed and compared with the PK of a single dose of viloxazine extended-release or lisdexamfetamine alone in healthy adults. Safety and tolerability were also evaluated.
METHODS
The trial conduct was reviewed and approved by IntegReview IRB (Austin, TX), and conducted in accordance with the Helsinki Declaration and the International Council for Harmonization (ICH) Note for Guidance on Good Clinical Practice. All participants provided written informed consent. The trial was conducted by Worldwide Clinical Trials (Worldwide; King of Prussia, PA).
Participants
Healthy adult male and female participants (n = 36), 18 to 55 years of age, were recruited for the trial. Inclusion criteria required participants to be nonsmokers, with a body mass index of 18 to 30 kg/m2 (inclusive), and women of childbearing potential had to be abstinent or use an acceptable birth control. Criteria for exclusion included a history or presence of significant diseases, a history of seizures, clinically significant safety laboratory or electrocardiogram (ECG) abnormalities, infection with human immunodeficiency virus or hepatitis B or C, alcohol or drug abuse, a need for prescription medication (other than topical or hormonal agents), use of drugs known to notably induce or inhibit hepatic drug metabolism, pregnancy or lactation, allergy to viloxazine extended-release or amphetamine-containing products, or any other qualifier that had the potential to interfere with participation in the trial as determined by the investigator.
Study Design
This was a single-center, randomized, cross-over, 3 treatment period, 6 sequence, open-label trial in healthy adult participants (n = 36). The study evaluated single doses of viloxazine extended-release alone, lisdexamfetamine alone, and the combination of viloxazine extended-release + lisdexamfetamine. Eligibility was confirmed within 28 days of screening, and patients began one continuous 13-day clinic residency for treatment. Patients received each of the 3 treatments in a counterbalanced sequence (Supplemental Materials, Fig. S1A, http://links.lww.com/JCP/A729), where blood for PK analysis was collected for 4 days after each treatment (Supplemental Materials, Fig. S1B, http://links.lww.com/JCP/A729). End of study procedures were completed after the last day of period 3 (study day 13) or before study discontinuation in the case of early withdrawal and discharge.
Treatments
Participants received 3 single-dose treatments: single dose viloxazine extended-release alone (700 mg), single dose lisdexamfetamine alone (50 mg), and the combination of single dose viloxazine extended-release (700 mg) + single dose lisdexamfetamine (50 mg). Viloxazine extended-release has been evaluated for treatment of ADHD at doses of 100, 200, and 400 mg in children (6–12 years)24,26,27 and 200, 400, and 600 mg in adolescents (12–17 years).28,29 The higher doses used in these studies correspond to approximately 700 mg after multiple-dose administration in healthy adults based on overall exposure extrapolation. Thus, this trial used 700 mg viloxazine extended-release in order to safely assess the potential for DDI at concentrations likely to be seen in children. A single dose of 50 mg lisdexamfetamine has been previously used to assess DDIs between d-amphetamine (the primary metabolite of lisdexamfetamine) and guanfacine in adults.31
Treatment was administered orally after an overnight fast of minimum 10 hours, and fasting continued for at least 4 hours after dosing. With the exception of 240 mL water at the time of dosing, fluid was restricted from 1 hour before to 1 hour after dosing to minimize absorption variability after oral dosing. Participants received all 3 treatments in 1 of 6 possible sequences (Supplemental Materials, Fig. S1A, http://links.lww.com/JCP/A729). Participants received a single dose of the assigned treatment on the morning of day 1 of each period, followed by a washout period of 4 days between doses (Supplemental Materials, Fig. S1B, http://links.lww.com/JCP/A729).
Sample Collection and Bioanalytical Methods
Blood samples were collected across the treatment period, beginning at time 0 (predose), and at 0.5, 1, 2, 3, 4, 5, 6, 8, 10, 12, 16, 20, 24, 30, 36, 48, 60, 72, 84, and 96 hours postdose. Plasma concentrations of viloxazine, 5-hydroxyviloxazine-glucuronide (5-HVLX-gluc, the active metabolite of viloxazine), and d-amphetamine (the active metabolite of lisdexamfetamine) were quantified using validated chromatographic tandem mass spectrometry as described below.
Viloxazine was extracted from 50 μL of human plasma by supported liquid extraction in a 96-well plate format, using viloxazine-d5 as an internal standard. Extracts were analyzed by ultra-performance liquid chromatography-tandem mass spectrometer (UPLC-MS/MS) using positive electrospray ionization and multiple reaction monitoring (precursor/product ion, m/z: 238/100 for viloxazine; 243/56 for viloxazine-d5). Data were acquired on a Waters Quattro Premier XE (Waters Corporation, Milford, MA) coupled with a Waters Acquity BEH C8 UPLC 1.7 μm particle, 2.1 × 50 mm column. Aqueous mobile phase used 10 mM ammonium bicarbonate (pH 9.0), and high organic mobile phase used acetonitrile, with gradient elution. The validated range was 0.0100 to 10.0 μg/mL, with a lower limit of quantitation (LLOQ) of 0.0100 μg/mL. The interrun and intrarun precisions (relative standard deviations) were 9% or less and 10% or less, respectively, whereas the intrarun accuracy (accuracy) was 97% to 107%.
5-HVLX-gluc was extracted from 100 μL of human plasma by mixed mode cation exchange solid phase extraction (SPE) in a 96 well plate format, using 5-HVLX-gluc-d5 as an internal standard. Extracts were analyzed by UPLC-MS/MS using positive electrospray ionization and multiple reaction monitoring (precursor/product ion, m/z: 430.1/254.1 for 5-HVLX-G, 435.2/259.1 for 5-HVLX-G-d5). Data were acquired on a Waters Quattro Premier XE or a Xevo TQ-S Micro coupled with a Restek Pinnacle DB Biphenyl 1.9 μm, 2.1 × 50 mm column (Restek Corporation, Bellefonte, PA). Aqueous mobile phase used 10 mM ammonium bicarbonate (pH 9.0), and high organic mobile phase used acetonitrile, with isocratic elution. The validated range was 0.00500 to 10.0 μg/mL, with a LLOQ of 0.00500 μg/mL. The interrun and intrarun precisions (relative standard deviations) were both 14% or less, whereas the intrarun accuracy was 94% to 115%.
d-amphetamine bioanalysis was performed by Worldwide, using a proprietary enantiospecific liquid chromatography-tandem mass spectrometer (LC-MS/MS) method validated for the quantitation of derivatized l-amphetamine and d-amphetamine as N-trifluoro acetyl-l-prolyl diastereomers of amphetamine. For this study, only d-amphetamine was extracted from 150 μL of human plasma by liquid-liquid extraction (LLE) followed by derivatization, using racemic dl-amphetamine-d5 as an internal standard. The extracted and derivatized samples are analyzed by high-performance LC-MS/MS (HPLC-MS/MS) using atmospheric pressure chemical ionization and multiple reaction monitoring (precursor/product ion, m/z: 329/91 for d-amphetamine, 334/96 for dl-amphetamine-d5). Data were acquired on a Sciex API 4000 (Sciex, Framingham, MA) coupled with a with Kinetex PFP, 2.6 micron, 100A, 30 × 3.0 mm HPLC column (Phenomenex Inc., Torrance, CA), using 1.0 M sodium carbonate in water aqueous phase and ethyl acetate/hexanes 4:1 organic phase. The organic phase was transferred and treated with an enantioselective derivatizing reagent (N-trifluoro acetyl-l-prolyl chloride/ethyl acetate/hexanes, 0.200:4.00:1.00), evaporated and reconstituted in acetonitrile/water 1:1. The validated range was 0.500 to 80.0 ng/mL, with a LLOQ of 0.500 ng/mL. The interrun and intrarun precisions (relative standard deviations) were 4.5% or less and 3.0% or less, respectively, whereas the intrarun accuracy was 93.6% to 110%.
Safety Monitoring and Assessments
Baseline (predose) measurements included medical history, physical examination, ECG recording, clinical laboratory tests (ie, serum chemistry, complete blood count, urinalysis), and the Columbia-Suicide Severity Rating Scale (C-SSRS) suicidality assessment. Vital signs assessed were diastolic/systolic blood pressure, pulse rate, respiratory rate, and temperature. Electrocardiograms were performed on day 1 of each treatment cycle at the expected time of the maximum measured plasma concentration (Tmax) of each treatment (7 hours postdose for viloxazine extended-release, 4 hours postdose for lisdexamfetamine, 2 ECGs collected for the combination treatment), and vital signs were assessed on day 1 of each cycle, 4 hours postdose. Adverse events were monitored over the course of treatment. Before discharge on treatment day 13 or upon study withdrawal, participants underwent a final ECG, physical examination, recording of vital signs, C-SSRS suicidality assessment, a blood draw and urine sample collection, and a final assessment of AEs.
Pharmacokinetics and Statistical Analyses
The PK analyses compared single doses of viloxazine extended-release alone and lisdexamfetamine alone to the combination of viloxazine extended-release with lisdexamfetamine. The primary outcome was the relative bioavailability of viloxazine and d-amphetamine (comparing the drug plasma exposure when administered alone relative to adjunct). The following PK parameters were derived using noncompartmental analyses: area under the concentration–time curve from 0 to the last measurable time (AUC0-t), area under the concentration–time curve from 0 to infinity (AUCinf), and maximum measured plasma concentration (Cmax). Secondary outcomes were the PK of viloxazine, d-amphetamine, and 5-HVLX-gluc using the following parameters: AUC0-t, AUCinf, Cmax, Tmax, and terminal phase elimination half-life (t1/2). Secondary outcomes also included safety and tolerability, as measured by reported AEs, ECGs, clinical laboratory tests, vital signs, physical examinations, and C-SSRS suicidality assessment.
The PK population (n = 34) included all participants with an adequate PK profile with no major protocol deviations that could impact PK data (eg, emesis). The bioavailability population (n = 33) included all participants from the PK population who completed Treatment C (i.e., the combination) and at least one other treatment period, with PK profiles sufficient to calculate AUCinf, Cmax, or AUC0-t for d-amphetamine or viloxazine (as appropriate) with no protocol deviations that could have impact PK data (eg, emesis). The safety population (n = 36) included all participants who received at least 1 dose of study medication.
The PK parameters and number of AEs related to treatment are presented using descriptive statistics. Plasma concentrations of viloxazine, 5-HVLX-gluc, and d-amphetamine that were below the limit of quantification were entered as zero in the descriptive statistics tables (Tables 1 and 2). Concentration-time data were summarized by subject, treatment, and analyte using descriptive statistics (n, arithmetic mean, standard deviation, median, minimum, maximum, and coefficient of variation (CV%)) at each scheduled collection time with actual PK sampling time. The relative bioavailability of viloxazine and d-amphetamine was evaluated using analysis of variance (ANOVA) on the log-transformed PK parameters Cmax, AUC0-t, and AUCinf. The model included treatment period and sequence as fixed effects, with subject nested within sequence as a random effect. The least squares mean (LSM) for each treatment group and the difference in the LSMs were calculated, then back-exponentiated to the original scale to obtain the 90% confidence intervals (CIs) for the geometric LSM ratios. The absence of an impact of combination treatment on the bioavailability of viloxazine or d-amphetamine was declared if the 90% CIs were fully contained within the predefined 80% to 125% CI limits for Cmax, AUC0-t, and AUCinf ratios. All PK analyses were conducted using Phoenix WinNonlin (version 6.3 or higher, Certara, L.P., Princeton, NJ); all statistical analyses and clinical data presentations were performed using SAS Version 9.3 or higher (SAS Institute, Inc., Cary, NC).
TABLE 1.
Summary of Viloxazine and d-Amphetamine Plasma Pharmacokinetic Parameters
| Viloxazine Plasma PK | d-Amphetamine Plasma PK | ||||
|---|---|---|---|---|---|
| Parameter | Viloxazine Extended-Release Alone (n = 34) | Combination (n = 34) | Parameter | Lisdexamfetamine Alone (n = 35) | Combination (n = 34) |
| Cmax, μg/mL | 4.98 ± 1.12 (22.58) | 4.76 ± 0.85 (17.76) | Cmax, ng/mL | 54.8 ± 7.84 (14.30) | 62.0 ± 10.3 (16.57) |
| AUC0-t, h*μg/mL | 103.5 ± 27.68 (26.75) | 103.7 ± 27.67 (26.69) | AUC0-t, h*ng/mL | 864.5 ± 156.6 (18.11) | 948.7 ± 168.1 (17.72) |
| AUCinf, h*μg/mL | 104.6 ± 27.79 (26.57) | 105.7 ± 27.31 (25.84) | AUCinf, h*ng/mL | 876.2 ± 156.8 (17.89) | 960.2 ± 169.3 (17.64) |
| Tmax, h | Median (range) | Tmax, h | Median (range) | ||
| 5.00 (3.00–10.00) | 5.00 (3.00–10.12) | 3.00 (2.00–5.03) | 3.54 (2.00–8.02) | ||
| t1/2, h | 7.01 ± 2.67 (38.17) | 6.28 ± 2.62 (41.76) | t1/2, h | 11.43 ± 1.94 (16.99) | 11.42 ± 1.96 (17.14) |
All data reported as mean ± SD (CV%) unless otherwise specified.
TABLE 2.
Summary of 5-HVLX-Gluc Plasma Pharmacokinetic Parameters
| Parameter | Viloxazine Extended-Release Alone (n = 34) | Combination (n = 34) |
|---|---|---|
| Cmax, μg/mL | 3.44 ± 0.73 (21.22) | 3.24 ± 0.63 (19.50) |
| AUC0-t, h*μg/mL | 70.30 ± 12.27 (17.46) | 66.60 ± 10.56 (15.86) |
| AUCinf, h*μg/mL | 70.44 ± 12.28 (17.43) | 66.72 ± 10.56 (15.82) |
| Tmax, h [median (range)] | 6.00 (5.00–12.00) | 5.50 (4.00–12.00) |
| t1/2, h | 6.86 ± 2.81 (40.97) | 6.62 ± 2.11 (31.84) |
| Metabolite-to-Parent (5-HVLX-gluc to Viloxazine) Ratios | ||
| Cmax | 0.395 ± 0.101 (25.52) | 0.384 ± 0.082 (21.29) |
| AUC0-t | 0.396 ± 0.111 (27.94) | 0.372 ± 0.089 (23.78) |
| AUCinf | 0.393 ± 0.109 (27.81) | 0.365 ± 0.084 (23.12) |
All data reported as mean ± SD (CV%) unless otherwise specified.
Metabolite-to-parent ratios are adjusted based on the molecular weights of viloxazine (237) and 5-HVLX-gluc (429).
RESULTS
Patient Demographics
Healthy adult participants (n = 36, 13 men, 23 women) were enrolled in the study and included in the safety population. Of these, 34 participants met the criteria for evaluation and were included in the PK population. Participants in the safety population were 35.6 ± 7.25 years (mean ± standard deviation [SD]; range, 19–50), 36.1% men, had a body mass index of 26.11 ± 2.55 (mean ± SD) kg/m2, and 55.6% of participants were Black or African American and 44.4% were White.
Three participants discontinued from the study early: one participant was discontinued because of an AE (vomiting) during period 1 (Combination treatment), and was disqualified entirely from the PK and bioavailability populations; one participant was discontinued after an AE (vomiting) during period 3 (viloxazine extended-release treatment), and thus was not included in the Bioavailability population for viloxazine, nor the PK population for viloxazine extended-release treatment; another participant was discontinued because of a house rule violation during period 2 (lisdexamfetamine treatment) and was not included in the bioavailability population, nor the PK population for combination treatment or lisdexamfetamine treatment after the 60-hour timepoint. All 36 participants enrolled in the study received at least one treatment and were included in the safety population; 35 participants had sufficient PK data to be included in the PK population; 34 participants met criteria for inclusion in the d-amphetamine bioavailability population, and 33 participants met the criteria for inclusion in the viloxazine/5-HVLX-gluc bioavailability population.
Pharmacokinetics and Bioavailability of Viloxazine, 5-HVLX-Gluc, and d-Amphetamine
The PK parameters for viloxazine and 5-HVLX-gluc are listed in Tables 1 and 2, and mean plasma concentrations over time profiles are shown in Figures 1 and 2, respectively. The visual comparison of plasma concentrations of viloxazine and 5-HVLX-gluc over time after a single dose of 700 mg viloxazine extended-release alone and after coadministration with 50 mg lisdexamfetamine showed similar profiles, suggesting no difference was observed, which was confirmed by statistical analysis where all 90% CIs were fully contained within the predetermined no-difference criteria of 80%–125% (Table 3).
FIGURE 1.
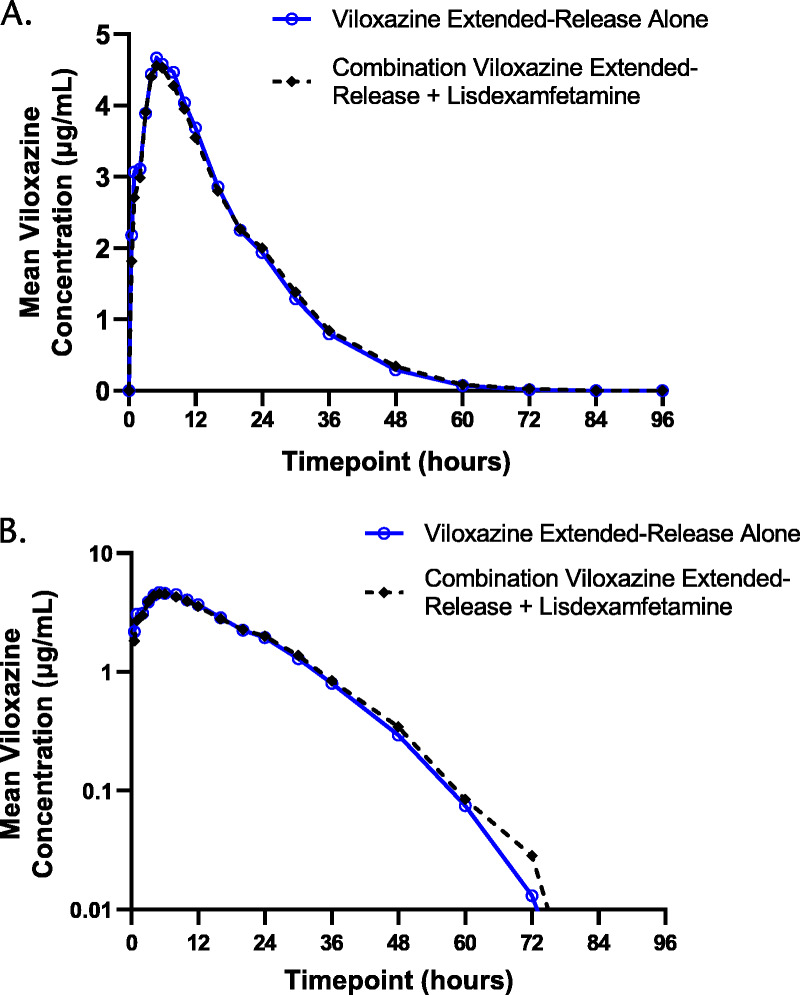
Viloxazine plasma concentration-time profiles after viloxazine extended-release alone and after combination viloxazine extended-release + lisdexamfetamine on linear (A) and semilogarithmic (B) scales.
FIGURE 2.
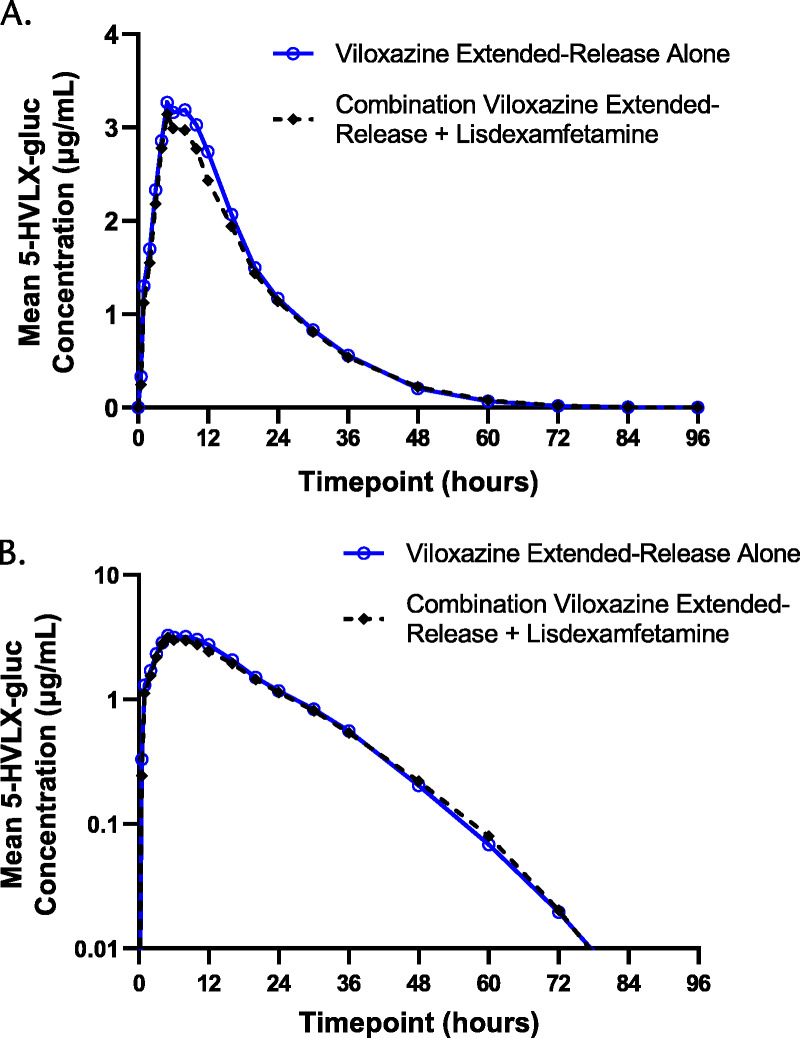
5-HVLX-gluc plasma concentration-time profiles after viloxazine extended-release alone and after combination viloxazine extended-release + lisdexamfetamine on linear (A) and semilogarithmic (B) scales.
TABLE 3.
Relative Bioavailability of Viloxazine and d-Amphetamine
| Geometric Mean* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Viloxazine | d-Amphetamine | ||||||||
| Viloxazine Extended-Release Alone | Combination | Difference† | Ratio‡ (%) | 90% CI | Lisdexamfetamine Alone | Combination | Difference† | Ratio‡ (%) | 90% CI | |
| Cmax | 4.91 | 4.71 | 0.20 | 95.96 | 91.33–100.82 | 54.16 | 61.09 | 6.92 | 112.78 | 109.93–115.71 |
| AUC0-t | 101.30 | 100.47 | 0.83 | 99.19 | 96.53–101.91 | 853.35 | 935.66 | 82.31 | 109.64 | 105.25–114.22 |
| AUCinf | 102.42 | 101.63 | 0.79 | 99.23 | 96.61–101.93 | 864.70 | 947.04 | 82.34 | 109.52 | 105.19–114.03 |
* Geometric mean based on LSM.
† Difference = PK parameter for (combination) − PK parameter for (viloxazine extended-release or lisdexamfetamine alone).
‡ Ratio, % = geometric mean for (combination)/geometric mean for (viloxazine extended-release or lisdexamfetamine alone).
The PK parameters for d-amphetamine are listed in Table 1, and mean plasma concentration-time curves are shown in Figure 3. As with viloxazine and 5-HVLX-gluc, the visual comparison of plasma concentrations of d-amphetamine over time after a single dose of 50 mg lisdexamfetamine alone and after coadministration with 700 mg viloxazine extended-release showed similar profiles, suggesting no drug interaction occurred, which was confirmed by statistical analysis where all 90% CIs were fully contained within the predetermined no-difference criteria of 80% to 125% (Table 3).
FIGURE 3.
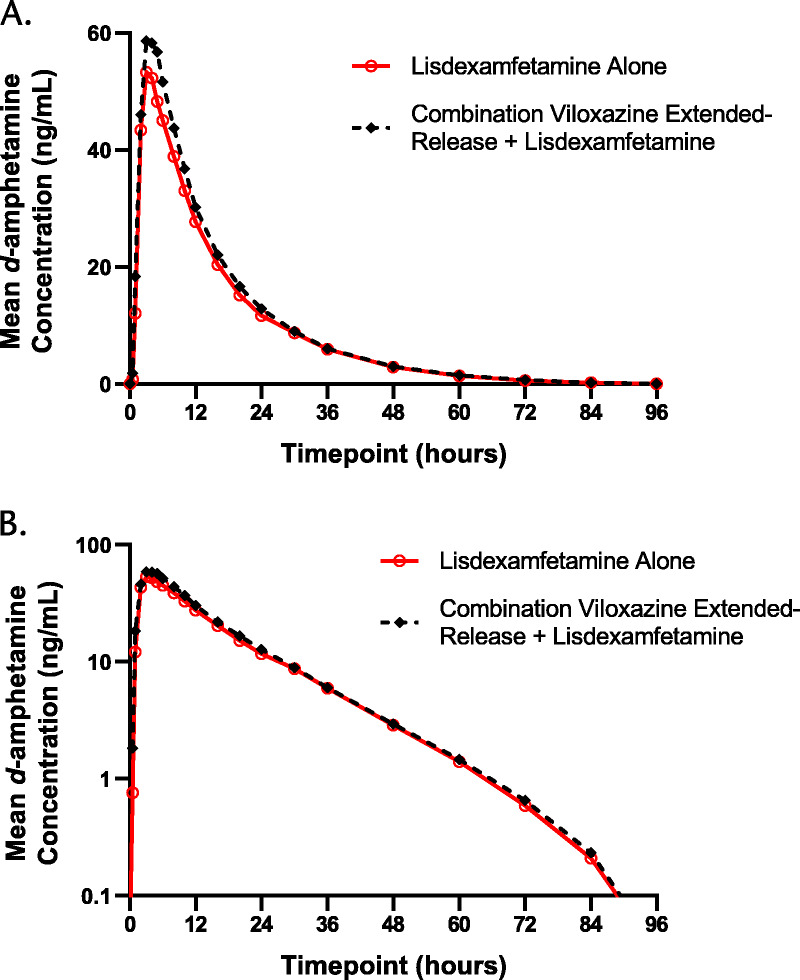
d-Amphetamine plasma concentration-time profiles after lisdexamfetamine alone and after combination viloxazine extended-release + lisdexamfetamine on linear (A) and semilogarithmic (B) scales.
The number of participants with detectable concentrations of viloxazine, 5-HLVX-gluc, and d-amphetamine in plasma is listed in Supplemental Materials, Table S1 (http://links.lww.com/JCP/A729). At 36 hours postdosing, all participants had measurable concentrations of all 3 analytes in their plasma. No participant had measurable concentrations of viloxazine at 96 hours, and fewer than 10% had detectable levels of 5-HLVX-gluc or d-amphetamine at that time point.
Safety
All participants that received at least one dose of study drug were included in the safety population (n = 36). For participants that discontinued the study early (n = 3), AEs for each individual treatments are reported only for the treatments received. Only 35 participants received viloxazine extended-release, 35 received lisdexamfetamine, and 35 received the combination treatment. Adverse events considered related to study treatment were reported by 36.1% (n = 13/36) of participants: 20.0% (n = 7/35) of participants after viloxazine extended-release alone, 22.9% (n = 8/35) of participants after lisdexamfetamine alone, and 31.4% (n = 11/35) when receiving the combination. All reported AEs were mild in severity with the exception of 1 case of vomiting after the combination treatment, which was moderate. No serious AEs were reported. The most common AEs related to study treatment (reported by 5% of participants or more) are listed in Table 4.
TABLE 4.
Treatment-Related AEs Reported by ≥5% of Healthy Adult Participants
| Viloxazine Extended-Release Alone (n = 35), n (%) | Lisdexamfetamine Alone (n = 35), n (%) | Combination (n = 35), n (%) | Overall (n = 36), n (%) | |
|---|---|---|---|---|
| Any AE | 7 (20.0) | 8 (22.9) | 11 (31.4) | 13 (36.1) |
| Nausea | 3 (8.6) | 0 | 5 (14.3) | 7 (19.4) |
| Dry mouth | 1 (2.9) | 1 (2.9) | 3 (8.6) | 4 (11.1) |
| Dizziness | 2 (5.7) | 1 (2.9) | 1 (2.9) | 4 (11.1) |
| Somnolence | 3 (8.6) | 0 | 0 | 3 (8.3) |
| Headache | 0 | 2 (5.7) | 2 (5.7) | 3 (8.3) |
| Feeling hot | 1 (2.9) | 1 (2.9) | 1 (2.9) | 3 (8.3) |
| Increased Energy | 0 | 3 (8.6) | 1 (2.9) | 3 (8.3) |
| Decreased appetite | 0 | 2 (5.7) | 1 (2.9) | 3 (8.3) |
| Head discomfort | 1 (2.9) | 1 (2.9) | 0 | 2 (5.6) |
| Vomiting | 1 (2.9) | 0 | 1 (2.9) | 2 (5.6) |
| Palpitations | 0 | 0 | 2 (5.7) | 2 (5.6) |
| Hot flush | 2 (5.7) | 1 (2.9) | 0 | 2 (5.6) |
There were no clinically significant abnormal results for clinical laboratory test results, vital signs (Supplemental file, Fig. S2, http://links.lww.com/JCP/A729), or physical examinations, and no increase in suicidality as measured by the C-SSRS at end of study. Two (5.6%) participants had normal ECGs at baseline, but had abnormal ECGs at end of study; all abnormal ECG results were determined to be not clinically significant by the Investigator. There were no marked differences in ECG results for participants after treatment with viloxazine extended-release or lisdexamfetamine alone versus the combination of viloxazine extended-release + lisdexamfetamine.
DISCUSSION
We report no impact of combination administration of single dose viloxazine extended-release + single dose lisdexamfetamine on the PK parameters of viloxazine, 5-HVLX-gluc, or d-amphetamine, relative to administration of either drug alone, as reflected in our primary endpoints Cmax, AUC0-t, and AUCinf. All 3 treatments appeared to be safe and generally well tolerated, with a relatively low incidence of AEs and the absence of any reported serious AEs. Importantly, in this small sample of healthy adult subjects, the combination treatment did not increase the severity of reported AEs, relative to either viloxazine extended-release or lisdexamfetamine alone.
Pharmacokinetic DDIs can predict significant changes in targeted clinical outcomes, and are thus strongly considered in the decision to co-prescribe ADHD or other medications. Lisdexamfetamine metabolism begins with PEPT1-mediated transport in the small intestine, where it is absorbed into portal circulation, and subsequently undergoes enzymatic hydrolysis of the parent molecule into the active metabolite d-amphetamine.32 Although lisdexamfetamine itself is not metabolized by CYP enzymes, its primary metabolite, d-amphetamine, is known to be metabolized by CYP2D6,33 though d-amphetamine's full metabolic profile remains to be elucidated. Notably, viloxazine extended-release metabolism does rely on the CYP system—specifically, the formation of the 5-HVLX-gluc metabolite is primarily mediated by CYP2D6 as a first step before its glucuronidation. Viloxazine extended-release also functions as a substrate for various CYP isoenzymes, most notably as a reversible inhibitor of CYP1A2 (IC50 = 0.269 μM).30 Despite this, the current study did not detect any significant impact of the combination treatment on any evaluated PK parameter of d-amphetamine or viloxazine metabolism.
Consistent with prior reports of relatively few serious DDIs with amphetamine,34,35 we did not detect any apparent increased risks to patient safety or tolerability because of the combination (versus viloxazine extended-release or lisdexamfetamine alone) as assessed by clinical laboratory results, vital signs, physical examinations, ECG analysis, or reported AEs. Notably, the robust, nontitrated single doses used in this study—although within the ranges used for clinical purposes—may not fully represent the PK profiles of these drugs when used chronically in the course of therapy. Despite this, our results support the hypothesis that viloxazine extended-release and lisdexamfetamine do not rely extensively on any shared metabolic pathway, and are thus likely to be generally well tolerated when administered in combination.
Clinicians frequently turn from mono- to combination pharmacotherapy for ADHD in stimulant partial or nonresponders (estimated to occur in 30% of patients36), patients experiencing dose-limiting side effects, and those with comorbid diagnoses, especially in patients with hyperactivity.11 Polypharmacy for ADHD frequently combines different drugs based on duration of action or primary drug type, such as medium- or long-acting stimulants co-administered with short-acting stimulants or nonstimulants, to most effectively address side effects, comorbidities, or, most important, partial response to monotherapy.10–12
Viloxazine extended-release is a novel nonstimulant whose efficacy and safety has recently been demonstrated in several phase 3 multicenter, randomized, double-blind, placebo-controlled trials of children (NCT0324753026 and NCT0324754327) and adolescents (NCT0324751728 and NCT0324755629) with ADHD. Although the potential clinical benefits of viloxazine extended-release combined with stimulant therapy (such as lisdexamfetamine) remain to be investigated, our data suggest that single doses of the combination do not show evidence of a DDI as measured by relative bioavailability of viloxazine or d-amphetamine, PK profiles, or safety measures. All 3 single-dose treatments in this study (viloxazine extended-release, lisdexamfetamine, and the combination) were safe and well tolerated, indicating this combination is unlikely to result in any clinically significant DDIs.
Supplementary Material
AUTHOR DISCLOSURE INFORMATION
This trial was fully sponsored by Supernus Pharmaceuticals, Inc. The protocol was designed by Supernus employees and the study was conducted by Worldwide Clinical Trials. Editorial support was provided by IMPRINT Science, New York, NY, and funded by Supernus Pharmaceuticals, Inc. N Fry, T Adewole, O Odebo, Z Wang, and A Nasser are employees of Supernus Pharmaceuticals, Inc. SL Faison was an employee of Supernus Pharmaceuticals, Inc. at the time of this work, and is now an employee of Certara Strategic Consulting. V Maletic is an employee of the University of South Carolina School of Medicine. He is a consultant for ACADIA Pharmaceuticals Inc.; Alfasigma USA, Inc.; Alkermes, Inc.; Allergan; Eisai-Purdue; Intra-Cellular Therapies; Janssen; H. Lundbeck A/S; Otsuka America Pharmaceutical, Inc.; Sage Pharmaceuticals; Sunovion Pharmaceuticals Inc.; Supernus Pharmaceuticals, Inc.; and Takeda Pharmaceutical Company Limited. He serves on the speakers bureau of ACADIA Pharmaceuticals Inc.; Alkermes, Inc.; Allergan; Ironshore; Intra-Cellular; Janssen; H. Lundbeck A/S; Otsuka America Pharmaceutical, Inc.; Sunovion Pharmaceuticals Inc.; and Takeda Pharmaceutical Company Limited; and his spouse serves on the speakers bureau of Otsuka America Pharmaceutical, Inc.
Data Sharing: The data are not available in a repository, but requests can be directed to anasser@supernus.com.
Footnotes
Previous Presentations: A poster was presented at the American Society for Clinical Psychopharmacology Annual Meeting, Scottsdale, AZ, May 28–31, 2019 and at the American Professional Society of ADHD and Related Disorders 2020 Annual Meeting, Washington, DC, January 17–19, 2020.
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.psychopharmacology.com).
Contributor Information
Shamia L. Faison, Email: shamiaf@umaryland.edu.
Nicholas Fry, Email: nfry@supernus.com.
Toyin Adewole, Email: tadewole@supernus.com.
Oyinkansola Odebo, Email: oodebo@supernus.com.
Zhao Wang, Email: zwang@supernus.com.
Vladimir Maletic, Email: vmaletic@icloud.com.
REFERENCES
- 1.Adesman AR. The diagnosis and management of attention-deficit/hyperactivity disorder in pediatric patients. Prim Care Companion J Clin Psychiatry. 2001;3:66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banaschewski T Becker K Dopfner M, et al. Attention-deficit/hyperactivity disorder: a current overview. Dtsch Arztebl Int. 2017;114:149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh A Yeh CJ Verma N, et al. Overview of attention deficit hyperactivity disorder in young children. Health Psychol Res. 2015;3:2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danielson ML Bitsko RH Ghandour RM, et al. Prevalence of parent-reported ADHD diagnosis and associated treatment among U.S. children and adolescents, 2016. J Clin Child Adolesc Psychol. 2018;47:199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kessler RC Adler L Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163:716–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polanczyk G de Lima MS Horta BL, et al. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942–948. [DOI] [PubMed] [Google Scholar]
- 7.Simon V Czobor P Balint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194:204–211. [DOI] [PubMed] [Google Scholar]
- 8.Briars L, Todd T. A review of pharmacological management of attention-deficit-hyperactivity disorder. J Pediatr Pharmacol Ther. 2016;21:192–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catala-Lopez F Hutton B Nunez-Beltran A, et al. The pharmacological and non-pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: a systematic review with network meta-analyses of randomised trials. PLoS One. 2017;12:e0180355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adler LA Reingold LS Morrill MS, et al. Combination pharmacotherapy for adult ADHD. Curr Psychiatry Rep. 2006;8:409–415. [DOI] [PubMed] [Google Scholar]
- 11.Molife C Bernauer MJ Farr AM, et al. Combination therapy patterns and predictors of ADHD in commercially insured and Medicaid populations. Postgrad Med. 2012;124:7–22. [DOI] [PubMed] [Google Scholar]
- 12.Wilens TE Spencer T Biederman J, et al. Combined pharmacotherapy: an emerging trend in pediatric psychopharmacology. J Am Acad Child Adolesc Psychiatry. 1995;34:110–112. [DOI] [PubMed] [Google Scholar]
- 13.Biederman J Wilens T Mick E, et al. Psychoactive substance use disorders in adults with attention deficit hyperactivity disorder (ADHD): effects of ADHD and psychiatric comorbidity. Am J Psychiatry. 1995;152:1652–1658. [DOI] [PubMed] [Google Scholar]
- 14.Wilens TE. Impact of ADHD and its treatment on substance abuse in adults. J Clin Psychiatry. 2004;65(suppl 3):38–45. [PubMed] [Google Scholar]
- 15.Soutullo CA DelBello MP Ochsner JE, et al. Severity of bipolarity in hospitalized manic adolescents with history of stimulant or antidepressant treatment. J Affect Disord. 2002;70:323–327. [DOI] [PubMed] [Google Scholar]
- 16.Corkum P Moldofsky H Hogg-Johnson S, et al. Sleep problems in children with attention-deficit/ hyperactivity disorder: impact of subtype, comorbidity, and stimulant medication. J Am Acad Child Adolesc Psychiatry. 1999;38:1285–1293. [DOI] [PubMed] [Google Scholar]
- 17.Nafees B Setyawan J Lloyd A, et al. Parent preferences regarding stimulant therapies for ADHD: a comparison across six European countries. Eur Child Adolesc Psychiatry. 2014;23:1189–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clemow DB, Walker DJ. The potential for misuse and abuse of medications in ADHD: a review. Postgrad Med. 2014;126:64–81. [DOI] [PubMed] [Google Scholar]
- 19.Wilens TE Adler LA Adams J, et al. Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature. J Am Acad Child Adolesc Psychiatry. 2008;47:21–31. [DOI] [PubMed] [Google Scholar]
- 20.Yu C Garcia-Olivares J Candler S, et al. New insights into the mechanism of action of viloxazine: serotonin and norepinephrine modulating properties. J Exp Pharmacol. 2020;12:285–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viloxazine, CID=5666. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Viloxazine. Accessed January 17, 2019.
- 22.Williams D. Antidepressants. In: Lemke TL Williams DA Roche VF, et al., eds. Foye's Principles of Medicinal Chemistry. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2013. [Google Scholar]
- 23.Bereen FJ. A new potential antidepressive. Lancet. 1973;301:379–380. [DOI] [PubMed] [Google Scholar]
- 24.Johnson JK Liranso T Saylor K, et al. A phase II double-blind, placebo-controlled, efficacy and safety study of SPN-812 (extended-release viloxazine) in children with ADHD. J Atten Disord. 2020;24:348–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinder RM Brogden RN Speight TM, et al. Viloxazine: a review of its pharmacological properties and therapeutic efficacy in depressive illness. Drugs. 1977;13:401–421. [DOI] [PubMed] [Google Scholar]
- 26.Nasser A Liranso T Adewole T, et al. A phase III, randomized, placebo-controlled trial to assess the efficacy and safety of once-daily SPN-812 (viloxazine extended-release) in the treatment of attention-deficit/hyperactivity disorder in school-age children. Clin Ther. 2020;42:1452–1466. [DOI] [PubMed] [Google Scholar]
- 27.Nasser A Liranso T Adewole T, et al. Once-Daily 200-mg and 400-mg SPN-812 in the Treatment of ADHD in School-Age Children: A Phase 3 Randomized Controlled Trial). Clin Ther. In press. [DOI] [PubMed]
- 28.Nasser A Hull JT Chowdhry F, et al. Extended-release viloxazine (SPN-812) 200 mg or 400 mg for the treatment of ADHD in adolescents: Topline results of a phase 3, randomized, double-blind, placebo-controlled study (P302). Presented at 32nd Annual Psych Congress. October 3–6, 2019, 2019; San Diego (CA). [Google Scholar]
- 29.Nasser A Liranso T Adewole T, et al. A Phase 3 Placebo-Controlled Trial of Once-Daily 400-mg and 600-mg SPN-812 (Viloxazine Extended-Release) in Adolescents with ADHD. Psychopharm Bull. 2021;51:1–22. [PMC free article] [PubMed] [Google Scholar]
- 30.Yu C. Metabolism and in vitro drug-drug interaction assessment of viloxazine. Xenobiotica. 2020;50:1285–1300. [DOI] [PubMed] [Google Scholar]
- 31.Roesch B Corcoran ME Fetterolf J, et al. Pharmacokinetics of coadministered guanfacine extended release and lisdexamfetamine dimesylate. Drugs R D. 2013;13:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pennick M. Absorption of lisdexamfetamine dimesylate and its enzymatic conversion to d-amphetamine. Neuropsychiatr Dis Treat. 2010;6:317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bach MV, Coutts RT, Baker GB. Involvement of CYP2D6 in the in vitro metabolism of amphetamine, two N-alkylamphetamines and their 4-methoxylated derivatives. Xenobiotica. 1999;29:719–732. [DOI] [PubMed] [Google Scholar]
- 34.Markowitz JS, Patrick KS. Pharmacokinetic and pharmacodynamic drug interactions in the treatment of attention-deficit hyperactivity disorder. Clin Pharmacokinet. 2001;40:753–772. [DOI] [PubMed] [Google Scholar]
- 35.Markowitz JS, Patrick KS. The clinical pharmacokinetics of amphetamines utilized in the treatment of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2017;27:678–689. [DOI] [PubMed] [Google Scholar]
- 36.Spencer T Biederman J Wilens T, et al. Pharmacotherapy of attention-deficit hyperactivity disorder across the life cycle. J Am Acad Child Adolesc Psychiatry. 1996;35:409–432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


