Abstract
Background
Heart rate variability (HRV) has been found reduced in patients with schizophrenia and depression. However, there is a lack of knowledge on how demographic, lifestyle, and pharmacological factors contribute to the reduction in HRV in these patients.
Methods
We recruited 37 patients with schizophrenia, 43 patients with unipolar depression, and 64 healthy controls. A combined chest-worn HRV and accelerometer device was used in an ambulatory measurement. Age, sex, anticholinergic burden of medication, nicotine use, body mass index, and ongoing physical activity were assessed in multiple regression models regarding their influence on HRV, measured as the standard deviation of all the RR intervals (SDNN).
Results
In the fully adjusted model, schizophrenia (β = −0.23, P = 0.019), depression (β = −0.18, P = 0.028), age (β = −0.34, P < 0.000), ongoing physical activity (β = −0.23, P = 0.001), and anticholinergic burden (β = −0.19, P = 0.025) influenced SDNN negatively. Sex, nicotine use, and BMI had negligible effects on SDNN.
Conclusions
We show for the first time that a quantified score of anticholinergic burden of medication has a negative relationship to HRV in patients with schizophrenia or depression, but that the diagnoses themselves still exhibit an effect on HRV.
Key Words: schizophrenia, depression, anticholinergics, antipsychotics, antidepressants
Heart rate variability (HRV) has, with some exceptions, repeatedly been found to be reduced in patients with schizophrenia and depression respectively.1–4 Heart rate variability is the variation in the time between two adjacent heart beats, and it reflects the functioning of the autonomic nervous system.5 Low HRV is regarded as an indicator of an unresponsive system, whereas high HRV is thought to reflect a more adaptive system, and both reduced and elevated HRV have been associated with various heart diseases.6 There are many ways to operationalize HRV, and they are all based on the RR interval, also called the interbeat interval (IBI). The RR interval is the elapsed time between two R-spikes, and it is measured in milliseconds. The standard deviation of all the RR intervals is called SDNN. SDNN is recommended as a standard HRV metric, especially in ambulatory settings where it might be difficult to isolate vagal activity.7,8 In more controlled settings, other HRV metrics, such as the root-mean-square of successive RR interval differences (RMSSD) and the frequency domain measure, high-frequency (HF) band HRV, can be used, and both are conceptualized as representing vagal activity.5,6 The HRV within the low-frequency (LF) band has been regarded as a mixture of both parasympathetic and sympathetic function. The LF/HF ratio is often termed the “sympathovagal balance” or “vagal tone.” This model, however, has been criticized for being overly simplified.8,9
There are several factors that influence HRV and potentially contribute to the observed lower levels in schizophrenia and depression. Both body mass index (BMI) and nicotine use have a modest negative influence on HRV.6,10 The effect of alcohol on HRV seems to be dose dependent: the more alcohol, the lower the vagal measures of HRV are. However, this has mainly been studied in heavy drinkers and patients with alcohol use disorders, and the effect on HRV in social drinkers is much less pronounced and thus only essential to account for in studies where the subjects have excessive alcohol consumption.11,12 A course of at least 4 weeks of aerobic exercise has been found to increase HRV.13 Also, a higher level of fitness seems to be associated with higher HRV,14,15 although some have found no such association.16 It is important to distinguish between a subject's general fitness level and the actual performed physical activity while recording HRV in an ambulatory setting. In such a setting, there are indications that everyday physical activities are not associated with alterations in HRV,17,18 but nonetheless, it has been recommended to account for ongoing physical activity.8,19 Regarding sex, women have been suggested to exhibit higher parasympathetic tone, although this has not been replicated in some studies.20,21 The effect of age on HRV has been thoroughly investigated: HRV declines with age.22
Anticholinergic drugs are known to significantly reduce HRV,2 and many of the drugs commonly used in schizophrenia and depression have substantial anticholinergic effects. Nevertheless, many former HRV studies have not accounted for the individual anticholinergic burden of the patients, even though there are validated instruments available for this purpose.23,24 To summarize, there are many possible factors influencing HRV in patients with schizophrenia and depression. Their contribution to individual differences in HRV has not yet been disentangled satisfactorily.
The aims of this study were to evaluate if there is a difference in HRV (measured as SDNN) between patients with schizophrenia, patients with depression, and healthy controls when adjusting for factors known to influence HRV, and to estimate the relative importance of these factors. Of specific interest for these patient groups is the influence of anticholinergic burden.
METHODS
Participants
Inclusion criteria were being 18 to 59 years old and having a diagnosis of either schizophrenia spectrum disorder or unipolar depression according to the International Statistical Classification of Diseases and Related Health Problems (ICD-10). This was confirmed through a Mini-International Neuropsychiatric Interview.25 Exclusion criteria were changes in medication in the last month, alcohol or illicit drug abuse, and insufficient Swedish command. Thirty-seven patients with a diagnosis of schizophrenia and 43 with a diagnosis of unipolar depression were included in the study. Patients were recruited from the outpatient clinic at Uppsala University Hospital, Sweden. Sixty-four healthy controls were recruited by word of mouth and advertisement at the hospital and the university and interviewed with the Mini-International Neuropsychiatric Interview to assure that they had no ongoing psychiatric disorders. All participants provided written informed consent. The study was approved by the Research Ethical Review Board in Uppsala (Dnr: 2014/286, 2015/018, 2015/362, and 2017/073) and conducted in accordance with the Helsinki declaration.
Clinical Assessment
Demographic and clinical characteristics (eg, medication status) were obtained by questionnaires and medical records. Body mass index was calculated (kg/m2) by weighing and measuring all subjects. Psychiatric symptoms were assessed by experienced and trained clinicians using a semistructured clinical interview with the 24-item, 7-point Brief Psychiatric Rating Scale (BPRS).26,27 The patients were also rated with the Clinical Global Impression–Severity, which is a 7-point Likert scale, ranging from 1 (normal) to 7 (among the most extremely ill patients).28
Heart Rate Variability
Heart rate was monitored using the two-lead heart rate recorder Firstbeat Bodyguard 2 (Firstbeat Technologies Ltd, Jyväskylä, Finland) attached across the chest for 24 hours with continuous sampling of RR intervals. Sampling rate was 1000 Hz with a resolution of 1 millisecond. The device was attached in the morning by a research nurse, and participants were instructed not to pay any attention to the device or change their normal everyday activities. Visual inspection of the entire 24-hour data revealed many artifacts, but there was a time window between 9:00 am and 1:00 pm during which all participants had at least 1 hour of relatively good data quality. This time window was also chosen in order not to have data from disparate parts of the day. A full 1-hour interval of data was extracted from this time window. The starting time for this 1-hour interval was randomly selected between subjects. Within this hour, the main outcome SDNN and the following HRV metrics were calculated: RMSSD, HF, LF, and LF/HF ratio. First, the data were visually inspected, and if deemed necessary, an artifact correction was made using the software's inbuilt algorithm. The artifact correction algorithm compares the length of each IBI to an average of the surrounding IBIs and then classifies IBIs that differ from a selected threshold as potential artifacts. The software Kubios HRV Standard (version 3.0.2) was used.
Anticholinergic Burden
To quantify anticholinergic burden we used the Anticholinergic Drug Scale (ADS).29 This scale divides drugs in 4 different categories where level 0 drugs have no known anticholinergic effects, level 1 drugs have potentially anticholinergic effects (based on receptor binding studies), level 2 implicates drugs that sometimes exhibit anticholinergic adverse events (most often at high doses), and level 3 drugs have marked anticholinergic effects. All the patient's drugs are scored separately and then summed to achieve the final score. This score is referred to as the anticholinergic burden of each participant in our study. We used a slightly modified version of the original scale. Medications prescribed as needed were not included in the total score. Because the dose-adjusted score was reported not to add any additional explanatory value when the ADS was developed, we chose not to adjust for dose. Some drugs that are not listed in the original scale were added and rated in accordance with our clinical experience and to fit the scores of other similar substances in the original scale (eg, benzodiazepines that were not included in the original scale were rated as 1 because other benzodiazepines in the original scale were rated as 1). These were the following (with our score in parenthesis): agomelatine (0), alimemazine (3), aripiprazole (0), atomoxetine (0), desogestrel (0), dexamphetamine (0), flupentixol (0), levomepromazine (2), melatonin (0), nitrazepam (1), paliperidone (0), paracetamol (0), paroxetine (0), pregabalin (0), propiomazine (2), vortioxetine (0), and zuclopenthixol (0). Finally, quetiapine was rated as 2 (instead of 0 in the original scale) because of its moderate anticholinergic activity, which may not have been evident when the original scale was developed.30–32
Ongoing Physical Activity During HRV Recording
Accelerometer data were monitored over 24 hours using the accelerometer in the same Firstbeat Bodyguard 2 that was attached across the chest. Data were sampled at 12.5 Hz. Sample size was set to 8 bits. Maximum values in all three directions (anteroposterior, mediolateral, and vertical) were reached at a force of 4G. Accelerometer data were extracted during the same hour interval as the HRV data for each subject. Raw acceleration data were plotted and visually inspected for missing or spurious data. Participants were excluded if any data were missing during the selected period or deemed artefactual. This resulted in that 8 participants were excluded (3 patients with schizophrenia, 2 patients with depression, and 3 healthy controls) because one axis was stuck on full acceleration (4G) during the selected period. The raw acceleration data were converted to a vector magnitude, and an algorithm derived from ActiGraph accelerometers was used to calculate activity counts using scripts in Matlab R2017b kindly provided by the authors.33 The mean activity counts per minute were used as a measure of ongoing physical activity.
Statistics
To assess differences between the groups, one-way analysis of variance (ANOVA) was used for continuous variables and χ2 for dichotomic variables. After having theorized the possible interactions between diagnosis, HRV, and covariates, we chose six covariates to control for age, sex, ongoing physical activity, nicotine use, BMI, and anticholinergic burden. These covariates were introduced successively in multiple regression models to follow their respective contribution of the effect on SDNN. We first performed a crude analysis with all variables separately inserted in a linear regression, with SDNN as the dependent variable. Second, we created a multiple regression model where we included the variables diagnosis (coded as dummy variables defining the presence of absence of schizophrenia or depression), age, sex, and ongoing physical activity (model 1). Third, we created a model that added nicotine use and BMI (model 2). Finally, we added anticholinergic burden of medication (model 3). In all regression analyses, we computed both P values and Bayes factors (using the JASP software package, JASP Team 2019, version 0.11.1), with default prior settings. The Bayes factor reported is “BF_inclusion,” which indicates the probability of the data under a model that includes the covariate compared with the same model without the covariate. A Bayes factor below 1 indicates evidence against an effect of that covariate, whereas a Bayes factor above 1 indicates evidence in favor of an effect of the current covariate. To handle the potential contribution of heart period on HRV, we performed the same regression analyses with an adjusted value of SDNN, according to earlier recommendations.6 The adjusted SDNN was calculated as the coefficient of variation of SDNN (cvSDNN) with the formula cvSDNN = 100*(SDNN/IBI). A Pearson correlation analysis was performed to investigate the correlations between SDNN and cvSDNN, assessing the extent to which the correction affected HRV. Pearson correlations were also used to assess relationship between artifact correction and all HRV metrics to rule out that artifact correction would influence the results. To assess consistency between different HRV metrics, we performed the regression analyses with the frequency domain measure HF (log transformed to achieve normal distribution) as the dependent variable. In all models, linearity assumptions were confirmed through visual inspection. Absence of influential outliers and multicollinearity were confirmed with Cook's distance and the variance inflation factor, respectively. The distribution of the residuals was inspected visually with PP plots, and the independency of the residual's values was tested with the Durbin-Watson statistic. The variance of the residuals revealed that there was no severe heteroscedasticity in the data.
RESULTS
Background Variables
There was a significant difference in age between the groups. There were also significant differences in sex, BMI, and anticholinergic burden, but not in nicotine use and ongoing physical activity. No significant differences were observed regarding alcohol or drug use. As expected, there were significant differences between the groups regarding clinical data such as symptom severity and medication. See Table 1 for all demographics.
TABLE 1.
Demographics
| Schizophrenia (n = 37) | Depression (n = 43) | Controls (n = 64) | P* | |
|---|---|---|---|---|
| Age, mean (SD), y | 40 (10) | 30 (9) | 31 (10) | <0.000 |
| Sex (males), n (%) | 27 (73%) | 22 (51%) | 27 (42%) | 0.011 |
| BMI, mean (SD), kg/m2 | 30 (8) | 26 (6) | 24 (5) | <0.000 |
| Supported housing, n (%) | 13 (35%) | 7 (16%) | 0 (0%)† | <0.000 |
| Graduated from high school, n (%) | 34 (92%) | 36 (84%) | 62 (97%) | 0.053 |
| Working/studying at least half-time, n (%) | 13 (35%) | 26 (60%) | 61 (95%) | <0.000 |
| Sheltered work program, n (%) | 15 (40%) | 1 (2%)† | 1 (2%)‡ | <0.000 |
| Nicotine use, n (%) | 15 (40%) | 15 (35%) | 14 (22%) | 0.111 |
| Coffee, day of assessment (cups), mean (SD) | 1.8 (1.4)† | 1.4 (1.3)† | 1.4 (1.3) | 0.209 |
| AUDIT total score, mean (SD) | 3.5 (5.4) | 4.4 (3.4) | 5.3 (3.0) | 0.071 |
| DUDIT total score, mean (SD) | 1.0 (2.0) | 1.0 (1.8) | 0.3 (1.7) | 0.109 |
| CGI-S, mean (SD) | 4.0 (1.0) | 4.9 (0.8) | n.a. | <0.000 |
| BPRS total score, mean (SD) | 44 (12) | 49 (7) | 27 (3) | <0.000 |
| BPRS positive symptoms subscale§, mean (SD) | 7 (4) | 3 (1.5) | 3 (0) | <0.000 |
| BPRS negative symptoms subscale§, mean (SD) | 8 (3) | 9 (3) | 3 (1) | <0.000 |
| MADRS-S total score, mean (SD) | 14 (6) | 30 (8) | 3 (3) | <0.000 |
| ADS score our version, mean (SD) | 3.0 (3.0) | 1.1 (1.5) | 0.0 (0.0) | <0.000 |
| ADS score original version, mean (SD) | 2.7 (2.5) | 1.7 (2.0) | 0.0 (0.1) | <0.000 |
| ADS score above 0, n (%) | 29 (78%) | 20 (47%) | 0 (0%) | <0.000 |
| Clozapine prescription, n (%) | 16 (43%) | 0 (0%) | 0 (0%) | <0.000 |
| TCA prescription, n (%) | 2 (5%) | 6 (14%) | 0 (0%) | 0.008 |
| ESRS total score, mean (SD) | 2.1 (2.6) | 0.6 (1.3) | 0 (0.1) | <0.000 |
| Positive chronotropic drug use∥, n (%) | 1 (3%) | 11 (26%) | 2 (3%) | <0.000 |
| Negative chronotropic drug use∥, n (%) | 5 (14%) | 6 (14%) | 0 (0%) | 0.009 |
| Antidiabetics¶, n (%) | 4 (11%) | 1 (3%) | 0 (0%) | 0.015 |
| Antihypertensives¶, n (%) | 2 (5%) | 0 (0%) | 1 (2%) | 0.223 |
| Ongoing physical activity, mean (SD), cpm | 265 (447)# | 232 (389)‡ | 267 (474)# | 0.919 |
*One-way ANOVA for continuous variables and χ2 for dichotomic variables.
†One missing.
‡Two missing.
§Positive symptoms subscale comprises items suspiciousness, hallucinations, and unusual thought content. Negative symptoms subscale comprises items blunted affect, emotional withdrawal, and motor retardation.
∥Positive chronotropic drugs included dexamphetamine, levothyroxine and methylphenidate. Negative chronotropic drugs included β-blockers, guanfacine, and thiamazole. Anticholinergics were not included as neither positive nor negative chronotropic drugs (eg, clozapine).
¶Antidiabetics included metformin, empagliflozin, sitagliptin, liraglutide, glipizide, and insulin. Antihypertensives included angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists (β-blockers were only coded as negative chronotropic drugs).
#Three missing.
AUDIT indicates Alcohol Use Disorders Identification Test; CGI-S, Clinical Global Impression–Severity; cpm, counts per minute; DUDIT, Drug Use Disorders Identification Test; ESRS, Extrapyramidal Symptom Rating Scale; MADRS-S, Montgomery Asberg Depression Rating Scale self-rating; TCA, tricyclic antidepressant agent.
Heart Rate Variability Metrics
There was a significant difference in IBI, HR, SDNN, and all other HRV metrics except LF/HF ratio between the groups. There was no significant difference in the percentage of beats removed during artifact correction between the groups. Moreover, there was no correlation between artifact correction and any of the HRV metrics (data not shown). See Table 2 for all HRV metrics. The main regression results are presented in Table 3. In the crude analysis, both patients with schizophrenia and depression had lower SDNN compared with controls. Other covariates related to SDNN were age, ongoing physical activity, BMI, and anticholinergic burden. After controlling for age, sex, and ongoing physical activity in model 1, both patient groups still had lower SDNN than controls. The findings of lower SDNN in patients with schizophrenia and depression compared with controls were consistent in all models. Controlling for nicotine use and BMI did not change the results substantially (model 2). Adding anticholinergic burden to the model lowered the effect of both diagnoses on SDNN, indicating that the effect was partially mediated by anticholinergic burden (model 3). In this fully adjusted model, patients with schizophrenia or depression still had statistically significant lower SDNN than the controls (9.7 milliseconds in schizophrenia and 7.1 milliseconds in depression). Age, ongoing physical activity, and anticholinergic burden also had a negative significant relationship with SDNN. There were similar results when running the same regression models using cvSDNN as the dependent variable (data not shown). When using the frequency domain logHF as the dependent variable, the effect of both diagnoses on HRV was no longer significant, but age, ongoing physical activity, and anticholinergic burden still exerted a statistically significant effect on HRV (Table 4).
TABLE 2.
HRV Metrics
| Schizophrenia (n = 37) | Depression (n = 43) | Controls (n = 64) | P* | |
|---|---|---|---|---|
| IBI, ms | 678 (105) | 723 (126) | 776 (142) | 0.001 |
| HR, bpm | 91 (13) | 86 (15) | 80 (16) | 0.004 |
| SDNN, ms | 26 (18) | 38 (17) | 47 (18) | <0.000 |
| cvSDNN | 3.7 (2.2) | 5.1 (1.8) | 5.9 (1.7) | <0.000 |
| RMSSD, ms | 21 (16) | 28 (17) | 39 (19) | <0.000 |
| logLF | 2.3 (0.8) | 2.9 (0.4) | 3.0 (0.4) | <0.000 |
| logHF | 1.9 (0.8) | 2.3 (0.5) | 2.6 (0.6) | <0.000 |
| LF/HF | 3.8 (2.8) | 4.0 (2.3) | 3.1 (1.9) | 0.165 |
| Artifacts corrected (% of beats removed) | 3.3 (7.3) | 1.1 (1.6) | 1.6 (2.6) | 0.054 |
All values presented as “mean (standard deviation)”.
*One-way ANOVA.
bpm indicates beats per minute; cv, coefficient of variance; HR, heart rate.
TABLE 3.
Regression Results (Dependent Variable SDNN)
| Crude* | Model 1† | Model 2† | Model 3† | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | β | P | BF | B | β | P | BF | B | β | P | BF | B | β | P | BF | |
| Diagnosis (ref: healthy) | ||||||||||||||||
| Schizophrenia | −20.72 | −0.50 | <0.000 | >1000 | −14.2 | −0.34 | <0.000 | >1000 | −14.4 | −0.34 | <0.000 | 639.5 | −9.74 | −0.23 | 0.019 | 8.4 |
| Depression | −9.20 | −0.25 | 0.008 | 4.9 | −9.33 | −0.24 | 0.003 | 106.1 | −9.29 | −0.23 | 0.004 | 56.2 | −7.14 | −0.18 | 0.028 | 8.2 |
| Age | −0.84 | −0.46 | <0.000 | >1000 | −0.62 | −0.35 | <0.000 | >1000 | −0.62 | −0.35 | 0.000 | >1000 | −0.59 | −0.34 | <0.000 | >1000 |
| Women (ref: men) | 3.60 | 0.09 | 0.264 | 0.3 | −2.30 | −0.06 | 0.392 | 1.2 | −2.55 | −0.07 | 0.355 | 0.8 | −2.39 | −0.07 | 0.380 | 0.8 |
| Ongoing physical activity | −0.01 | −0.26 | 0.002 | 12.6 | −0.01 | −0.25 | 0.001 | 184.7 | −0.01 | −0.25 | 0.001 | 105.5 | −0.01 | −0.23 | 0.001 | 58.5 |
| Nicotine use (ref: no use) | −6.38 | −0.15 | 0.067 | 0.8 | −1.65 | −0.04 | 0.577 | 0.7 | −1.68 | −0.04 | 0.565 | 0.8 | ||||
| BMI | −0.75 | −0.26 | 0.002 | 15.1 | 0.06 | 0.02 | 0.795 | 0.7 | 0.07 | 0.03 | 0.733 | 0.7 | ||||
| Anticholinergic burden | −3.83 | −0.42 | <0.000 | >1000 | −1.79 | −0.19 | 0.025 | 4.7 | ||||||||
*All analyses are bivariate.
†Multivariate analysis of all variables below and n = 136 in all analyses including ongoing physical activity due to 8 participants without acceleration data (see “Ongoing physical activity during HRV recording” section).
BF indicates Bayes factor.
TABLE 4.
Regression Results (Dependent Variable logHF)
| Crude* | Model 1† | Model 2† | Model 3† | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | β | P | BF | B | β | P | BF | B | β | P | BF | B | β | P | BF | |
| Diagnosis (ref: healthy) | ||||||||||||||||
| Schizophrenia | −0.76 | −0.51 | <0.000 | >1000 | −0.51 | −0.34 | 0.000 | >1000 | −0.51 | −0.34 | 0.000 | >1000 | −0.24 | −0.16 | 0.085 | 3.0 |
| Depression | −0.30 | −0.29 | 0.002 | 15.0 | −0.31 | −0.22 | 0.004 | 63.7 | −0.31 | −0.22 | 0.006 | 33.1 | −0.19 | −0.13 | 0.089 | 2.4 |
| Age | −0.03 | −0.47 | <0.000 | >1000 | −0.02 | −0.33 | 0.000 | >1000 | −0.02 | −0.33 | 0.000 | >1000 | −0.02 | −0.30 | <0.000 | >1000 |
| Women (ref: men) | 0.27 | 0.21 | 0.013 | 3.1 | 0.10 | 0.07 | 0.316 | 1.9 | 0.09 | 0.07 | 0.348 | 1.1 | 0.10 | 0.08 | 0.277 | 1.1 |
| Ongoing physical activity | <0.00 | −0.24 | 0.005 | 5.9 | <0.00 | −0.22 | 0.002 | 76.6 | <0.00 | −0.22 | 0.002 | 43.1 | <0.00 | −0.20 | 0.004 | 16.8 |
| Nicotine use (ref: no use) | −0.21 | −0.15 | 0.082 | 0.7 | −0.02 | −0.01 | 0.888 | 0.7 | −0.02 | −0.01 | 0.871 | 0.6 | ||||
| BMI | −0.03 | −0.29 | <0.000 | 64.9 | <0.00 | −0.01 | 0.895 | 0.8 | <0.00 | <0.00 | 0.992 | 0.7 | ||||
| Anticholinergic burden | −0.16 | −0.49 | <0.000 | >1000 | −0.10 | −0.31 | <0.000 | 114.3 | ||||||||
*All analyses are bivariate.
†Multivariate analysis of all variables below and n = 136 in all analyses including ongoing physical activity due to 8 participants without acceleration data (see “Ongoing physical activity during HRV recording” section).
BF indicates Bayes factor; logHF, logarithmic high frequency.
Correlations
To follow up on the finding of anticholinergic burden being important for HRV, we performed correlations between anticholinergic burden and SDNN in each group separately. We found a significant correlation in patients with schizophrenia (r = −0.36, P = 0.031) and a borderline significant correlation in patients with depression (r = −0.26, P = 0.091) (Table 5). There was a significant correlation between our version of the ADS score and the original version (r = 0.803, P = 0.000). The correlation between SDNN and cvSDNN was high (r = 0.930, P = 0.000).
TABLE 5.
Correlations Between SDNN and Anticholinergic Burden
| Anticholinergic Burden | ||
|---|---|---|
| Pearson r | P | |
| Whole sample (n = 144) | −0.42 | <0.000 |
| Schizophrenia (n = 37) | −0.36 | 0.031 |
| Depression (n = 43) | −0.26 | 0.091 |
| Controls (n = 64) | n.a. | n.a. |
DISCUSSION
To the best of our knowledge, this is the first study showing that a quantified score of anticholinergic burden exhibits a contribution to the reduced HRV in patients with schizophrenia and depression. Nevertheless, even after adjusting for potential confounders, schizophrenia and depression still explained an additional part of the variance in HRV. Our results indicate that there is a reduction in HRV intrinsic to the respective condition, which is in line with former studies.19,34
The effect of age on HRV was the most pronounced throughout all analyses. This confirms previous findings and iterates the importance of taking age into account in HRV studies.22,35
It has been known for a long time that drugs with a pronounced anticholinergic profile lower HRV.36 Some recent studies have also successfully differentiated the effects of different anticholinergic profiles.30,37,38 However, there are still claims that antipsychotics as a group does not lower HRV, which is hard to state without assessing the respective anticholinergic action of each drug.39 We believe that it is of great importance not to regard antipsychotics as a homogeneous group of drugs, but rather assess their actual receptor profiles depending on the focus of the study.
It is unlikely that the result of anticholinergic burden being important for HRV is only reflecting actual group differences in anticholinergic burden and SDNN. There was a significant correlation between anticholinergic burden and SDNN not only in the whole sample but also within the schizophrenia group. In the depression group, there was a correlation in the same direction as in the whole sample, although this finding was only borderline significant. This might be due to the depression group not having enough anticholinergic burden to produce a detectable effect in HRV and the use of medication with anticholinergic effects was quite low in this group. There might be a dose-dependent effect of anticholinergic burden where HRV is affected only if the vagal activity is inhibited to a certain extent. Inspecting the scatter plot (Fig. 1) lends support to that; if this is the case, such a threshold would be around an ADS score of 4. A recent study focusing on cognitive impairment in patients with schizophrenia used an ADS score of 3 or more as a cutoff to define a high ADS group.40
FIGURE 1.
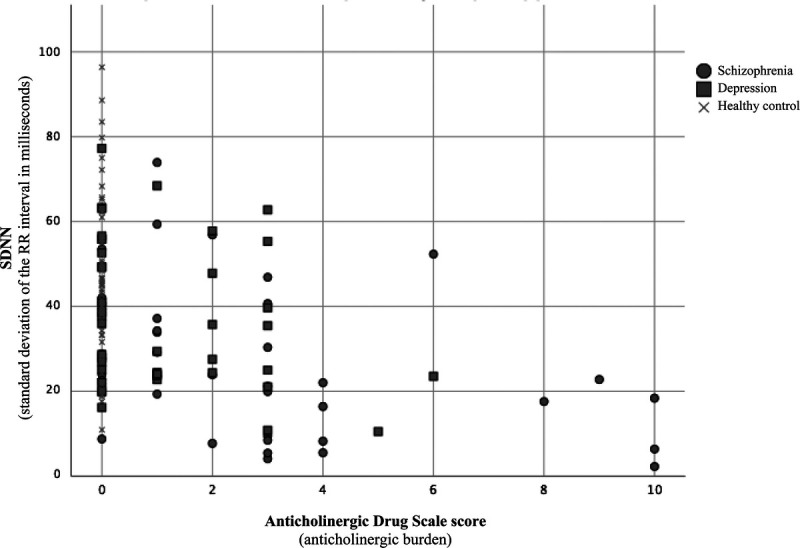
Scatterplot of SDNN and anticholinergic burden.
It is interesting that when analyzing a more vagal measure (logHF), anticholinergic burden seems to be an even stronger predictor of HRV than when using SDNN. This is in line with the theory, given that anticholinergic drugs affect vagal activity. However, some recommend not to estimate vagal activity during ambulatory recordings.8
We found that ongoing physical activity had a significant relationship to HRV. This might be mediated by the increasing heart rate that accompanies a higher intensity of physical activity. Our results differ somewhat from earlier findings. In a study of 45 healthy controls, there were no correlations between ongoing physical activity (measured as metabolic equivalents with an accelerometer) and any time or frequency domain measure of HRV.17 Another study of 49 participants used self-reported measures of ongoing physical activity and found no correlation with RMSSD.18 Although it is hard to compare our results with these earlier findings, it is an interesting novel finding that will need to be followed up in more controlled settings.
Limitations
Although we assessed many covariates in this study, there is always potential unmeasured residual confounding.
The groups are not ideally matched for age or sex. This reflects the difficulties in recruiting patients with different diagnoses to the same study (schizophrenia being more common among men and depression among women). In order not to lose valuable patient data, we instead chose to include age and sex as variables in the regression model.
We did not assess psychiatric comorbidity in this study. Yet, we believe that we capture the majority of this potential effect of such comorbidity by including the anticholinergic effect of all of the patients' prescribed drugs.
The optimal way of calculating HRV metrics is by a raw electrocardiogram (ECG) signal.35 The device we used does not provide an ECG, but there are many other groups that reliably have used various devices for assessing HRV, including the device we used is this study.34,41 The main issue with not using an ECG is that the software cannot distinguish between ectopic and ordinary heartbeats to the same extent. However, this would occur in all groups. The artifact correction between the groups in our study was only borderline nonsignificant, being slightly higher in the schizophrenia group. This could be a potential problem because stronger artifact correction in general reduces HRV. Nonetheless, there was no correlation between the amount of artifact correction and any HRV measure, assuring us that different levels of artifact correction did not influence the results.
We chose to use HRV data from a 1-hour interval, during which the participants performed everyday activities. Most HRV studies collect data in laboratory settings. Our choice might therefore reduce comparability between studies. However, we wanted to assess HRV in an ambulatory setting and also take account the physical activity performed.
We have assessed the anticholinergic burden of the patients from their prescribed medication. Because compliance is not always 100%, a more precise, yet demanding method would have been to assess the serum activity of anticholinergic compounds. However, the ADS was originally validated by examining its relation to serum anticholinergic activity. An even more precise method would have been to assess the actual receptor binding of each patient or at least to consider the potency of the different compounds.24 It would also be of interest to know at which level of serum anticholinergic activity the maximum occupancy is achieved. No anticholinergic rating scale will possibly be able to perfectly reflect the actual occupancy of the muscarinic receptors, but it is nevertheless of interest to implement existing scales in research and clinical practice. More knowledge on the anticholinergic properties of different compounds42 will probably aid in developing newer versions of the ADS, and such attempts have already been made.43
Inspection of the accelerometer data revealed that many of the participants were not performing much physical activity during the HRV recording. This has probably reduced the amount of variance in the sample. However, it is important to remember that we only wanted to account for the potential influence of ongoing movement during the actual recording, not assess the subjects' overall fitness level or performed physical activity over time. This would nonetheless have been of interest and could potentially have diminished the group differences observed in our study. It is recommended that future studies also account for general fitness level in some way. Eight participants did not have acceleration data. Excluding ongoing physical activity during HRV recording as a covariate from the regression analyses did not change the coefficients or significance of any of the other covariates (data not shown).
CONCLUSIONS
A contributor to the observed reduction in HRV in patients with schizophrenia or depression is the anticholinergic burden of medication. However, there is an intrinsic effect of these diagnoses on HRV even after controlling for important confounders.
ACKNOWLEDGMENTS
Apart from funders and coauthors, we would like to acknowledge Viktor Elgemark and Björn Nilsson for valuable help and comments on the ADS.
J.B. and R.B. collected the HRV, accelerometer, and clinical data. J.B. also extracted the HRV data and prepared it for analysis. J.P. extracted the accelerometer data and prepared it for analysis. J.B. and J.P. performed the statistical analyses. E.O. contributed with methodology regarding the HRV data and statistical analyses. H.I. contributed with methodology and interpretation of the accelerometer data. J.B. drafted the first version of the manuscript. All authors contributed to the interpretation of the data, revised the manuscript critically for important intellectual content, and approved the final version.
AUTHOR DISCLOSURE INFORMATION
The study was funded by unrestricted research grants from the Märta and Nicke Nasvell Foundation, Selander Foundation, Söderström-Königska Foundation, and Linné Foundation. R.B. was supported by the Swedish Research Council Grant 2016-02362 and the Gullstrand Research Fellow grant from Uppsala County Council. J.P. was supported by The Swedish Brain Foundation. J.B. was granted conference attendance from Anna Maria Lundin's scholarship committee.
The authors declare no conflicts of interest.
Footnotes
Jonas Persson and Robert Bodén contributed equally to this work.
Contributor Information
Erik Olsson, Email: erik.olsson@kbh.uu.se.
Helena Igelström, Email: helena.igelstrom@neuro.uu.se.
Jonas Persson, Email: jonas.persson@neuro.uu.se.
Robert Bodén, Email: robert.boden@neuro.uu.se.
REFERENCES
- 1.Montaquila JM, Trachik BJ, Bedwell JS. Heart rate variability and vagal tone in schizophrenia: a review. J Psychiatr Res. 2015;69:57–66. [DOI] [PubMed] [Google Scholar]
- 2.Alvares GA Quintana DS Hickie IB, et al. Autonomic nervous system dysfunction in psychiatric disorders and the impact of psychotropic medications: a systematic review and meta-analysis. J Psychiatry Neurosci. 2016;41:89–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bar KJ. Cardiac autonomic dysfunction in patients with schizophrenia and their healthy relatives—a small review. Front Neurol. 2015;6:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kemp AH Quintana DS Gray MA, et al. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol Psychiatry. 2010;67:1067–1074. [DOI] [PubMed] [Google Scholar]
- 5.Malik M. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- 6.de Geus EJC Gianaros PJ Brindle RC, et al. Should heart rate variability be “corrected” for heart rate? Biological, quantitative, and interpretive considerations. Psychophysiology. 2019;56:e13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sassi R Cerutti S Lombardi F, et al. Advances in heart rate variability signal analysis: joint position statement by the e-cardiology ESC Working Group and the European Heart Rhythm Association co-endorsed by the Asia Pacific Heart Rhythm Society. Europace. 2015;17:1341–1353. [DOI] [PubMed] [Google Scholar]
- 8.Laborde S, Mosley E, Thayer JF. Heart rate variability and cardiac vagal tone in psychophysiological research—recommendations for experiment planning, data analysis, and data reporting. Front Psychol. 2017;8:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Penttila J Syvalahti E Hinkka S, et al. The effects of amitriptyline, citalopram and reboxetine on autonomic nervous system. A randomised placebo-controlled study on healthy volunteers. Psychopharmacology (Berl). 2001;154:343–349. [DOI] [PubMed] [Google Scholar]
- 10.Fagard RH, Pardaens K, Staessen JA. Influence of demographic, anthropometric and lifestyle characteristics on heart rate and its variability in the population. J Hypertens. 1999;17:1589–1599. [DOI] [PubMed] [Google Scholar]
- 11.Cheng YC, Huang YC, Huang WL. Heart rate variability as a potential biomarker for alcohol use disorders: a systematic review and meta-analysis. Drug Alcohol Depend. 2019;204:107502. [DOI] [PubMed] [Google Scholar]
- 12.Ralevski E, Petrakis I, Altemus M. Heart rate variability in alcohol use: a review. Pharmacol Biochem Behav. 2019;176:83–92. [DOI] [PubMed] [Google Scholar]
- 13.Sandercock GR, Bromley PD, Brodie DA. Effects of exercise on heart rate variability: inferences from meta-analysis. Med Sci Sports Exerc. 2005;37:433–439. [DOI] [PubMed] [Google Scholar]
- 14.Rennie KL Hemingway H Kumari M, et al. Effects of moderate and vigorous physical activity on heart rate variability in a British study of civil servants. Am J Epidemiol. 2003;158:135–143. [DOI] [PubMed] [Google Scholar]
- 15.Buchheit M Simon C Charloux A, et al. Heart rate variability and intensity of habitual physical activity in middle-aged persons. Med Sci Sports Exerc. 2005;37:1530–1534. [DOI] [PubMed] [Google Scholar]
- 16.Fagard RH. A population-based study on the determinants of heart rate and heart rate variability in the frequency domain. Verh K Acad Geneeskd Belg. 2001;63:57–89; discussion 90-51. [PubMed] [Google Scholar]
- 17.Hautala AJ Karjalainen J Kiviniemi AM, et al. Physical activity and heart rate variability measured simultaneously during waking hours. Am J Physiol Heart Circ Physiol. 2010;298:H874–H880. [DOI] [PubMed] [Google Scholar]
- 18.Sperry SH Kwapil TR Eddington KM, et al. Psychopathology, everyday behaviors, and autonomic activity in daily life: an ambulatory impedance cardiography study of depression, anxiety, and hypomanic traits. Int J Psychophysiol. 2018;129:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rottenberg J. Cardiac vagal control in depression: a critical analysis. Biol Psychol. 2007;74:200–211. [DOI] [PubMed] [Google Scholar]
- 20.Ryan SM Goldberger AL Pincus SM, et al. Gender- and age-related differences in heart rate dynamics: are women more complex than men? J Am Coll Cardiol. 1994;24:1700–1707. [DOI] [PubMed] [Google Scholar]
- 21.Antelmi I de Paula RS Shinzato AR, et al. Influence of age, gender, body mass index, and functional capacity on heart rate variability in a cohort of subjects without heart disease. Am J Cardiol. 2004;93:381–385. [DOI] [PubMed] [Google Scholar]
- 22.Umetani K Singer DH McCraty R, et al. Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J Am Coll Cardiol. 1998;31:593–601. [DOI] [PubMed] [Google Scholar]
- 23.Welsh TJ van der Wardt V Ojo G, et al. Anticholinergic drug burden tools/scales and adverse outcomes in different clinical settings: a systematic review of reviews. Drugs Aging. 2018;35:523–538. [DOI] [PubMed] [Google Scholar]
- 24.Lozano-Ortega G Johnston KM Cheung A, et al. A review of published anticholinergic scales and measures and their applicability in database analyses. Arch Gerontol Geriatr. 2020;87:103885. [DOI] [PubMed] [Google Scholar]
- 25.Sheehan DV Lecrubier Y Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33. [PubMed] [Google Scholar]
- 26.Ventura J Green MF Shaner A, et al. Training and quality assurance with the Brief Psychiatric Rating Scale: ‘The drift busters’. Int J Methods Psychiatr Res. 1993;3:221–244. [Google Scholar]
- 27.Shafer A, Dazzi F, Ventura J. Factor structure of the Brief Psychiatric Rating Scale–Expanded (BPRS-E) in a large hospitalized sample. J Psychiatr Res. 2017;93:79–86. [DOI] [PubMed] [Google Scholar]
- 28.Guy W. ECDEU Assessment Manual for Psychopharmacology Publication. Washington DC, USA: Department of Health, Education and Welfare; 1976. [Google Scholar]
- 29.Carnahan RM Lund BC Perry PJ, et al. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46:1481–1486. [DOI] [PubMed] [Google Scholar]
- 30.Huang WL Chang LR Kuo TB, et al. Impact of antipsychotics and anticholinergics on autonomic modulation in patients with schizophrenia. J Clin Psychopharmacol. 2013;33:170–177. [DOI] [PubMed] [Google Scholar]
- 31.Correll CU. From receptor pharmacology to improved outcomes: individualising the selection, dosing, and switching of antipsychotics. Eur Psychiatry. 2010;25:S12–S21. [DOI] [PubMed] [Google Scholar]
- 32.Hattori S Kishida I Suda A, et al. Effects of four atypical antipsychotics on autonomic nervous system activity in schizophrenia. Schizophr Res. 2018;193:134–138. [DOI] [PubMed] [Google Scholar]
- 33.Brond JC, Andersen LB, Arvidsson D. Generating ActiGraph counts from raw acceleration recorded by an alternative monitor. Med Sci Sports Exerc. 2017;49:2351–2360. [DOI] [PubMed] [Google Scholar]
- 34.Quintana DS Westlye LT Kaufmann T, et al. Reduced heart rate variability in schizophrenia and bipolar disorder compared to healthy controls. Acta Psychiatr Scand. 2016;133:44–52. [DOI] [PubMed] [Google Scholar]
- 35.Quintana DS, Alvares GA, Heathers JA. Guidelines for reporting articles on psychiatry and heart rate variability (GRAPH): recommendations to advance research communication. Transl Psychiatry. 2016;6:e803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zahn TP, Pickar D. Autonomic effects of clozapine in schizophrenia: comparison with placebo and fluphenazine. Biol Psychiatry. 1993;34:3–12. [DOI] [PubMed] [Google Scholar]
- 37.Huang WL Liao SC Kuo TB, et al. The effects of antidepressants and quetiapine on heart rate variability. Pharmacopsychiatry. 2016;49:191–198. [DOI] [PubMed] [Google Scholar]
- 38.Cacciotti-Saija C Quintana DS Alvares GA, et al. Reduced heart rate variability in a treatment-seeking early psychosis sample. Psychiatry Res. 2018;269:293–300. [DOI] [PubMed] [Google Scholar]
- 39.Clamor A Lincoln TM Thayer JF, et al. Resting vagal activity in schizophrenia: meta-analysis of heart rate variability as a potential endophenotype. Br J Psychiatry. 2016;208:9–16. [DOI] [PubMed] [Google Scholar]
- 40.Kim SJ Jung D Shim JC, et al. The effect of anticholinergic burden on cognitive and daily living functions in patients with schizophrenia. Asian J Psychiatr. 2019;46:111–117. [DOI] [PubMed] [Google Scholar]
- 41.Fohr T Pietila J Helander E, et al. Physical activity, body mass index and heart rate variability-based stress and recovery in 16 275 Finnish employees: a cross-sectional study. BMC Public Health. 2016;16:701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chew ML Mulsant BH Pollock BG, et al. Anticholinergic activity of 107 medications commonly used by older adults. J Am Geriatr Soc. 2008;56:1333–1341. [DOI] [PubMed] [Google Scholar]
- 43.Eum S Hill SK Rubin LH, et al. Cognitive burden of anticholinergic medications in psychotic disorders. Schizophr Res. 2017;190:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]


