Abstract
Tubulointerstitial accumulation of matrix proteins in human kidney biopsies is the best predictor of renal survival. In this issue of the JCI, Yen-Ting Chen et al. elegantly show that an endoplasmic reticulum resident protein, thioredoxin domain containing 5 (TXNDC5), is a key mediator of experimental kidney fibrosis. The researchers used knockout or conditional knockout animals to reduce Txndc5 expression, which reduced the accumulation of fibrous tissue in three models of chronic kidney disease (CKD), including unilateral ureteral obstruction, unilateral ischemia reperfusion injury, and folic acid nephropathy. More importantly, the studies demonstrate that the activated fibroblasts are almost exclusively responsible for producing matrix proteins. The study also showed that reducing Txndc5 in mice after tubulointerstitial fibrosis (TIF) was established mitigated the fibrosis. These experiments have obvious clinical importance but warrant caution because a key question remains unanswered. The impact of reducing TXNDC5 on renal function itself, the very heart of CKD, demands further exploration.
The TXNDC5 pathway and tubulointerstitial fibrosis
There is ample documentation from human biopsies that the best predictor of renal survival, whether in the background of glomerular disease or toxic injury to the kidney, is the amount of tubulointerstitial accumulation of matrix proteins including collagens I, III, IV, and fibronectin. The accumulation of such products and the often-associated replacement of normal parenchyma is termed tubulointerstitial fibrosis (TIF). There is great interest in understanding the pathogenesis of TIF, since the close association of TIF with progression suggests that targeting the pathway may provide effective therapeutic interventions. The paper by Yen-Ting Chen et al. (1) in this issue of the JCI is impressive in providing a possible target as it so clearly establishes a pathway unique to the fibroblast.
The group has identified an endoplasmic reticulum resident protein, thioredoxin domain containing 5 (TXNDC5), as a key mediator of experimental kidney fibrosis. Yen-Ting Chen et al. (1) used an impressive array of techniques that were used previously to identify TXNDC5 as a mediator of cardiac fibrosis (2). After confirming TXNDC5 upregulation in renal biopsy specimens, the authors embarked on a series of experiments with the goal of establishing its primacy in the development of fibrosis in three models of renal fibrosis: unilateral ureteral obstruction (UUO), unilateral ischemia-reperfusion injury (uIRI), and folic acid (FA) administration. Using reporter mice, they unequivocally show that the protein is specifically upregulated in collagen-secreting fibroblasts in fibrotic mice. In vitro, TXNDC5 was required for TGF-β1–provoked fibrogenic responses in human kidney fibroblasts (HKFs) and its overexpression in these cells promoted increased proliferation and collagen production. In unravelling the pathway responsible for these fibrogenic effects, the authors revealed that TXNDC5 is transcriptionally controlled by an ATF6-dependent ER stress pathway. Notably, the ATF6-dependent ER stress pathway is necessary for TGF-β1 to induce fibrosis. These experiments dissected, step-by-step, the intermediate progression that leads to posttranslational stabilization, upregulation of the type 1 TGF-β receptor, and its display on the surface of the fibroblast. Finally, in a key experiment the researchers deleted Txndc5 after fibrosis was established in the UUO mice, which mitigated fibrosis development. Thus, these experiments identify that the pathway is not only restricted to the fibroblast and no other cells in the kidney, but also that reducing fibrosis, even at a time when it is well established, is possible. This latter point has substantial clinical implications, as TIF has already been established by the time biopsies are performed.
Challenging the exclusive focus on fibrosis regulation
There are many controversies that surround the notion that TIF is responsible for the progressive decline in renal function, as the data in humans are associative rather than causal. But there is reason to doubt the association as causative. This is particularly so at the experimental level, where researchers can examine serial samples in well-delineated time points during the development of CKD. Thus, CKD can develop in the absence of fibrosis (3–5) and fibrosis can develop without evidence of progressive decline in renal function (6). So too can mitigation of fibrosis occur without improving renal function (7). Even more compelling is evidence that fibrosis is necessary for healing (8) and can resolve in the natural course of toxic injury, including the folic acid model that was studied in Yen-Ting Chen et al. (1). Given the specificity of the intervention studied here being restricted to the fibroblast, TXNDC5 is an ideal mediator to study the isolated effect on fibrosis and its effect on function. We anxiously await such a test from the authors.
An alternative focus for study
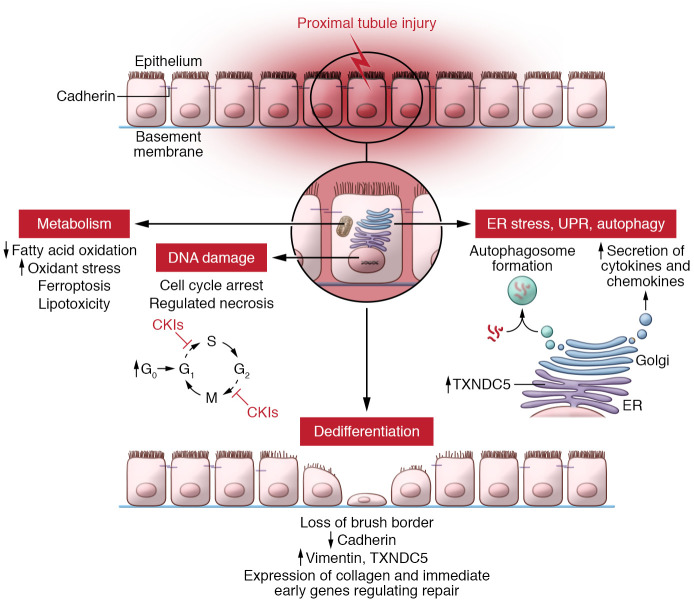
Evidence for an alternate hypothesis for the progressive nature of renal injury that places the proximal tubule at the center of the problem has been critically reviewed by Leslie Gerwin (9) and is compelling (Figure 1). Injured but surviving proximal tubules assume a partially dedifferentiated phenotype driven by cell-cycle arrest. These dedifferentiated proximal tubules express proinflammatory and profibrotic factors that engage many cell types, such as fibroblasts, within the kidney, as well as attract potentially damaging bone marrow–derived cells. These bone marrow cells also assume a senescence phenotype, eventually reducing their numbers and possibly reducing renal mass, a characteristic of chronic kidney disease. No single gene or pathway seems to underpin this response (3). We posit that justifying further study requires documenting the functional impact of such interdiction.
Figure 1. Multicompartmental model of proximal tubule injury underlying chronic kidney disease.
The proximal tubule responds to chronic injury with adaptive and maladaptive outcome. Normally, proximal tubule cells are quiescent with low rates of entry in the cell cycle (G0). When injured, proximal tubule cells enter the cell cycle at higher rates but fail to progress to mitosis. Damage to DNA and the endoplasmic reticulum provokes cyclin kinase inhibitors (CKIs) to block proximal tubule cells at several stages of the cell cycle (10). Injured proximal tubule cells dedifferentiate as they lose their brush border, have decreased E-cadherin expression and increased vimentin expression, and lose transport proteins. These dedifferentiated cells are the source of many genes underpinning repair or activating neighboring cells, including the immediate early genes, such as the mitogen activated kinases that could either aide or impair repair. Yen-Ting Chen et al. showed that fibroblasts from fibrotic mouse kidneys upregulated TXNDC5, which was controlled by the ATF6-dependent ER stress pathway (1). Chronic injury alters the proximal tubule cell metabolism by inhibiting mitochondrial fatty acid oxidation, generating superoxide, increasing potentially damaging triglycerides, and provoking ferroptotic cell death. Injured proximal tubule cells also undergo the unfolded protein response and autophagy, which arrests the cell cycle and promotes senescence. Each injured compartment may provoke secretion of inflammatory mediators that may attract or activate particular cells to amplify or repair the injury. The balance between adaptive and maladaptive pathways depends on the particular insult as well as its duration and severity.
Version 1. 03/01/2021
Electronic publication
Footnotes
Conflict of interest: The author has declared that no conflict of interest exists.
Copyright: © 2021, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2021;131(5):e144803. https://doi.org/10.1172/JCI144803.
References
- 1.Chen YT, et al. Endoplasmic reticulum protein TXNDC5 promotes renal fibrosis by enforcing TGF-β signaling in kidney fibroblasts. J Clin Invest. 2021;131(5):e143645. doi: 10.1172/JCI143645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sin YC, et al. Endoplasmic reticulum protein TXNDC5 augments myocardial fibrosis by facilitating extracellular matrix protein folding and redox-sensitive cardiac fibroblast activation. Circ Res. 2018;122(8):1052–1068. doi: 10.1161/CIRCRESAHA.117.312130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torres R, et al. Three-dimensional morphology by multiphoton microscopy with clearing in a model of cisplatin-induced CKD. J Am Soc Nephrol. 2016;27(4):1102–1112. doi: 10.1681/ASN.2015010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christov M, et al. Inducible podocyte-specific deletion of CTCF drives progressive kidney disease and bone abnormalities. JCI Insight. 2018;3(4):95091. doi: 10.1172/jci.insight.95091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao W, et al. Contrast-enhanced ultrasound for assessing renal perfusion impairment and predicting acute kidney injury to chronic kidney disease progression. Antioxid Redox Signal. 2017;27(17):1397–1411. doi: 10.1089/ars.2017.7006. [DOI] [PubMed] [Google Scholar]
- 6.Yazdani S, et al. Targeting tubulointerstitial remodeling in proteinuric nephropathy in rats. Dis Model Mech. 2015;8(8):919–930. doi: 10.1242/dmm.018580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ninichuk V, et al. Multipotent mesenchymal stem cells reduce interstitial fibrosis but do not delay progression of chronic kidney disease in collagen4A3-deficient mice. Kidney Int. 2006;70(1):121–129. doi: 10.1038/sj.ki.5001521. [DOI] [PubMed] [Google Scholar]
- 8.Zhong J, et al. A perspective on chronic kidney disease progression. Am J Physiol Renal Physiol. 2017;312(3):F375–F384. doi: 10.1152/ajprenal.00266.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gewin LS. Renal fibrosis: primacy of the proximal tubule. Matrix Biol. 2018;68–69:248–262. doi: 10.1016/j.matbio.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Megyesi J, et al. The lack of a functional p21 gene ameliorates progression to chronic renal failure. Proc Natl Acad Sci. 1999;96(19):10830–10835. doi: 10.1073/pnas.96.19.10830. [DOI] [PMC free article] [PubMed] [Google Scholar]