Abstract
Innate lymphoid cells (ILCs) are enriched at barrier surfaces, including the gastrointestinal tract. While most studies have focused on the balance between pathogenic group 1 ILCs (ILC1s) and protective ILC3s in maintaining gut homeostasis and during chronic intestinal inflammation, such as Crohn’s disease (CD), less is known regarding ILC2s. Using an established murine model of CD-like ileitis, i.e., the SAMP1/YitFc (SAMP) mouse strain, we showed that ILC2s, compared with ILC1s and ILC3s, were increased within draining mesenteric lymph nodes and ilea of SAMP versus AKR (parental control) mice early, during the onset of disease. Gut-derived ILC2s from CD patients versus healthy controls were also increased and expanded, similarly to ILC1s, in greater proportion compared with ILC3s. Importantly, we report that the intracellular bacteria–sensing protein, nucleotide-binding oligomerization domaining–containing protein 2, encoded by Nod2, the first and strongest susceptibility gene identified for CD, promoted ILC2 expansion, which was dramatically reduced in SAMP mice lacking NOD2 and in SAMP mice raised under germ-free conditions. Furthermore, these effects occurred through a mechanism involving the IL-33/ST2 ligand-receptor pair. Collectively, our results indicate a functional link between NOD2 and ILC2s, regulated by the IL-33/ST2 axis, that mechanistically may contribute to early events leading to CD pathogenesis.
Keywords: Gastroenterology, Immunology
Keywords: Cytokines, Inflammatory bowel disease, Innate immunity
Introduction
Innate lymphoid cells (ILCs) are a diverse family of developmentally related immune cells that are heterogeneous in their tissue location, cytokine secretion, and effector functions. The term ILC has been widely used since 2010, with subsets formally proposed in 2013 (1), based on transcription factors and cytokine profiles regulating their development and function. Three distinct ILC groups have been described, representing innate counterparts functionally mirroring CD4+ Th cell subsets; additionally, NK and lymphoid tissue inducer (LTi) cells have since been designated ILCs, thereby denoting the 5 different ILC subsets (2). ILC1s express IFN-γ and depend on the transcription factor T-bet, but not eomesodermin; ILC2s produce IL-5 and IL-13, and are dependent on GATA-binding protein-3 (GATA3); and ILC3s utilize retinoic acid receptor–related orphan receptor γt (RORγt) to drive production of IL-22, but also IL-17, and are further divided into subsets based on expression of the natural cytotoxicity receptors NKp46 and NKp44. Although much has been discovered regarding ILC phenotype and function (reviewed in ref. 3), investigation has also been hampered by their often low frequency in primary and secondary lymphoid tissues, even in disease settings, and difficulty in targeted depletion and/or deletion of specific subsets due to ambiguity in identifying markers among other types of immune cells, as well as between ILC subgroups themselves. Nonetheless, it is well accepted that ILCs are particularly abundant within the gut and studies from the last decade highlight various functions that are important at the intestinal barrier (4), both in maintaining homeostasis and in promoting chronic inflammation, such as in inflammatory bowel disease (IBD).
IBD, including its 2 idiopathic forms, Crohn’s disease (CD) and ulcerative colitis (UC), encompasses a family of chronic inflammatory disorders of the GI tract that results from dysregulated immune responses to typically harmless commensal flora in genetically susceptible hosts. Overall, studies reporting the contribution of ILCs to the development of IBD have been relatively sparse (summarized in Supplemental Table 1; supplemental material available online with this article; https://doi.org/10.1172/JCI140624DS1), yet emerging dogma suggests a general increase in pathogenic ILC1s concomitant with a decrease in IL-22–producing ILC3s, which are important in maintaining epithelial barrier function (reviewed in ref. 5). Specifically, under homeostatic conditions, ILC3s constitute the main ILC population within the gut, but during active inflammation, IFN-γ–producing ILC1s and IL-17–producing ILC3s increase, while IL-22–producing ILC3s decrease. This redistribution is associated with more severe disease, particularly in CD that generally has been more studied than UC (Supplemental Table 1); similar trends have been observed using various models of intestinal inflammation (reviewed in ref. 4). Conversely, ILC2s, although best known to contribute to inflammatory disorders of the lung, such as allergy and asthma, and extensively studied in intestinal helminth infection, are enigmatic in regards to their role in IBD, and investigation, to date, has been limited compared with that focused on ILC1s and ILC3s (Supplemental Table 1).
To mechanistically address the role of ILC2s in IBD, we investigated a cohort of CD patients and utilized ileitis-prone SAMP1/YitFc (SAMP) mice that spontaneously develop a progressive, chronic intestinal inflammation displaying similarities to CD, including disease location, histologic features, and response to standard therapies (reviewed in ref. 6). Genetically, SAMP mice share many susceptibility loci identified to be important in IBD, and similar to patients, disease in SAMP mice is multifactorial, relying on the interactions of several, versus a single, gene products (6). Importantly, SAMP mice provide an excellent system to evaluate the onset and natural course of disease that is more difficult to study in colitis models that are chemically induced or genetically manipulated, or in IBD patients, who are most often studied when disease is already active.
Results and Discussion
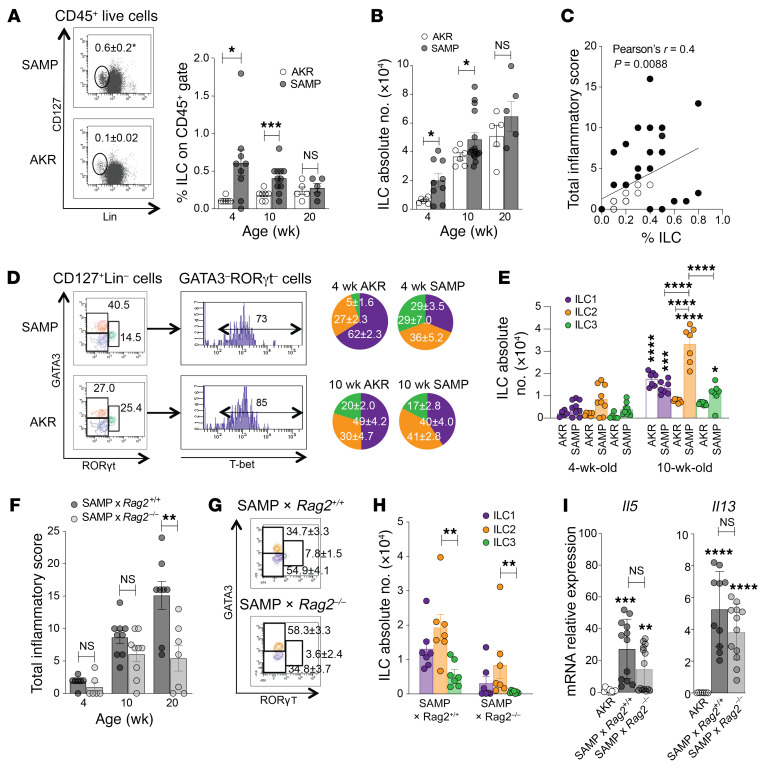
We evaluated total ILC abundance in draining mediastinal lymph nodes (MLNs) of SAMP mice prior to histologic evidence of disease (4 weeks), early in the development of active inflammation (10 weeks), and during established, chronic inflammation (20 weeks; Supplemental Figure 1, A and B). Compared with AKR controls, total ILCs in SAMP mice were increased, with no significant difference by 20 weeks (Figure 1, A and B), suggesting that the primary disease-contributing role for ILCs likely occurs during the early stages of ileitis. In fact, while total numbers increased as disease became more severe, total ILC frequency diminished within the CD45+ population (Figure 1A), as expansion of pathogenic T and B lymphocytes dominate during chronic gut inflammation (7). In further support of this concept, the correlation of ILC frequency with ileal inflammation was greater when disease was less severe, but overall poor as inflammation progressed (Figure 1C), suggesting replacement of ILCs with adaptive immune cells as disease/inflammation worsened, as reported by others (8).
Figure 1. Increased ILC2s during early disease in ileitis-prone SAMP mice that persists in the absence of adaptive immunity.
(A) Gating strategy to detect total ILCs, identified as CD127+ and lineage– (Lin–) cells within the live CD45+ population (4-week-old mice, left) in draining MLNs of SAMP vs. AKR (control) mice, expressed as percentages (right) and (B) absolute numbers (n = 5–11). *P ≤ 0.05, ***P ≤ 0.001 by 2-tailed, unpaired Student’s t test. (C) Correlation by Pearson’s r between ILC frequency and disease severity ian SAMP and AKR mice (purple; n = 28). (D) ILC subsets, defined by GATA3+ (ILC2, orange), RORγt+ (ILC3, green), and GATA3–RORγt–T-bet+ (ILC1, purple) within the CD45+CD127+Lin– population (representative plots from 10-week-old mice, left), expressed as percentages (right) and (E) absolute numbers (n = 7–9). *P ≤ 0.05, ***P ≤ 0.001, ****P ≤ 0.0001 vs. 4-week-old mice or as indicated by the horizontal bars, by 2-way ANOVA with Tukey’s multiple-comparison post hoc test. (F) Ileitis severity in SAMP × Rag2–/– vs. WT (SAMP × Rag2+/+) mice (n = 7–10), with ILC (G) percentages (10-week-old mice shown) and (H) absolute numbers (n = 7). (I) Th2 cytokine expression, shown as fold-difference vs. AKR, which was set arbitrarily as 1 (n = 7–14). **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001 by 2-tailed, unpaired Student’s t test. Experiments were performed at least in duplicate.
We therefore focused on characterizing ILC subsets during the early stages of SAMP ileitis and show that, while ILC frequencies were relatively equal (Figure 1D) and absolute numbers very low (Figure 1E) among the 3 subsets prior to disease onset (4 weeks), ILC1s and ILC3s, but to a greater extent ILC2s, proliferated and represented the predominant subset in SAMP mice that expanded in number (by almost 4-fold) as inflammation progressed and significantly increased, with no differences in ILC1s and ILC3s versus age-matched AKR (Figure 1E). Interestingly, although ILC plasticity, specifically ILC3s and ILC2s differentiating into ILC1s, has been reported during intestinal inflammation, including CD (9, 10), no difference in the presence of ILC2s was observed in 20-week-old SAMP mice (Supplemental Figure 1C) compared with 10-week-old mice, suggesting that conversion of ILC2s to ILC1s does not likely have a significant impact on the development of SAMP ileitis, although this phenomenon cannot be ruled out.
To isolate the contribution of ILC2s in the absence of robust adaptive immune responses, we utilized SAMP mice deficient in T and B lymphocytes (SAMP × Rag2–/–), and found that, while disease severity was substantially greater in older SAMP × Rag2+/+ (WT) versus SAMP × Rag2–/–, these mice still displayed significant ileitis at 10 weeks that was equal to age-matched WT and native SAMP mice (Figure 1F and Supplemental Figure 1D), indicating that an intact mucosal innate immune system is adequate to initiate SAMP ileitis. Importantly, ILC2s, as well as ILC1s, were greatly increased at the expense of ILC3s, even in SAMP × Rag2–/– mice (Figure 1, G and H), supporting the concept that ILC1 expansion plays a pathogenic role in IBD (Supplemental Table 1), but also indicating that ILC2s are important. Furthermore, we confirmed that Il5 and Il13 were considerably upregulated in WT SAMP versus AKR (11, 12) but persisted in SAMP × Rag2–/– mice (Figure 1I), suggesting that ILC2s, and not CD4+ lymphocytes, are the primary source of these Th2 cytokines early, during active inflammation.
We next investigated potential, early triggers of ILC2 expansion and function in SAMP mice. ILC2s were originally described as expressing IL-33R/ST2 and respond to IL-33 by potent proliferation and production of Th2 cytokines (13, 14). However, although we (15) and others reported a strong association between increased IL-33 and IBD, mechanistic studies using various colitis models reveal dichotomous pathogenic versus protection functions. In general, colitis models that entail acute challenge, whether infectious or chemical, of healthy, immunocompetent mice support a protective role for IL-33 (16), which may be different from other models possessing genetic and/or immunologic abnormalities that predispose to chronic intestinal inflammation, such as SAMP mice (12, 15), and similar to that observed in IBD patients.
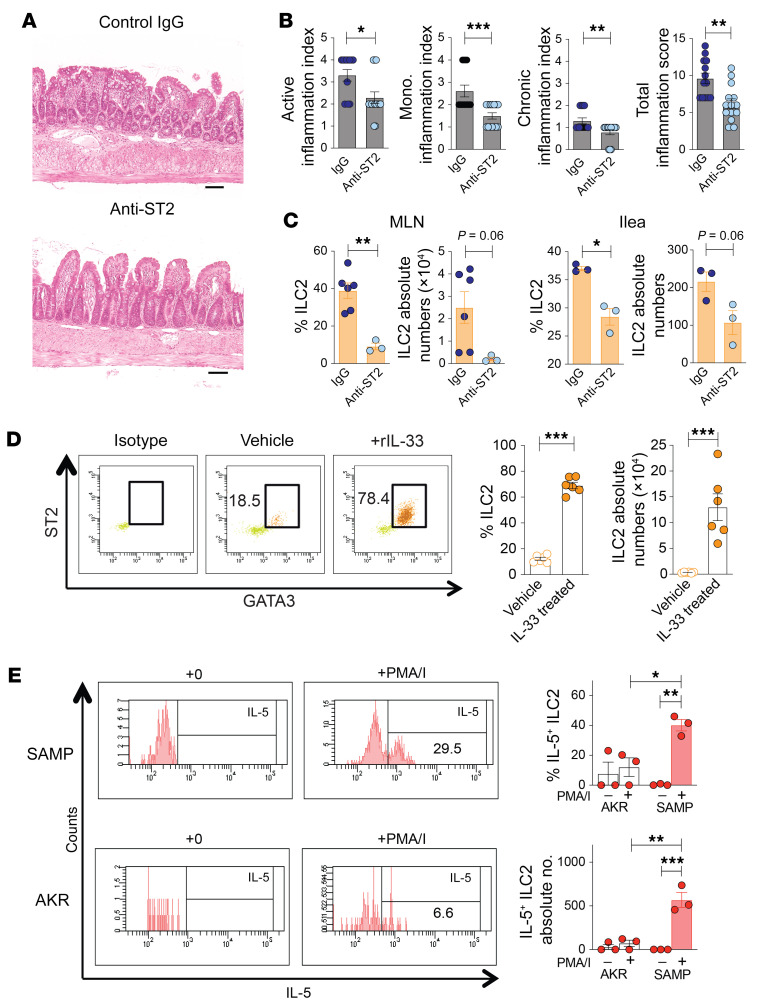
In fact, we confirmed that blockade of IL-33 signaling significantly reduced SAMP ileitis (Figure 2, A and B, and ref. 12), and MLN- and ileum-derived ILC2s, compared with controls (Figure 2C). To exclude the confounding effects of inflammation and test IL-33’s direct impact on in vivo ILC2 expansion, we exogenously administered rIL-33 to healthy AKR mice, and observe dramatic increases in ILC2 frequency (by 5.6-fold) and absolute numbers (by 39.1-fold) versus vehicle-treated controls (Figure 2D). Functionally, ex vivo activation of ILC2s effectively boosted the frequency and absolute numbers of IL-5–producing ILC2s from SAMP versus AKR mice (Figure 2E). Together, these data indicate IL-33–dependent regulation of ILC2s in ileitis-prone SAMP, with increased IL-5–producing ILC2s compared with AKR.
Figure 2. Blockade of IL-33 signaling reduces ILC2 expansion and function, and protects from ileitis.
(A) Representative H&E-stained histologic images (scale bars: 100 μm), (B) disease severity, and (C) ILC2s in SAMP mice after anti-ST2 vs. control IgG (n = 13–14). (D) Representative dot plots (left) and frequency/absolute numbers (right) of MLN-derived ST2+GATA3+ ILC2s in healthy AKR mice after exogenous rIL-33 vs. vehicle (n = 6). (E) Representative histograms (left) and IL-5–expressing ILC2s (right) after ex vivo stimulation with and without PMA/ionomycin (I) in the presence of brefeldin A (n = 3). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 by 2-tailed, unpaired Student’s t test (B–D) or 2-way ANOVA with Tukey’s multiple-comparison post hoc test (E). Experiments were performed in triplicate.
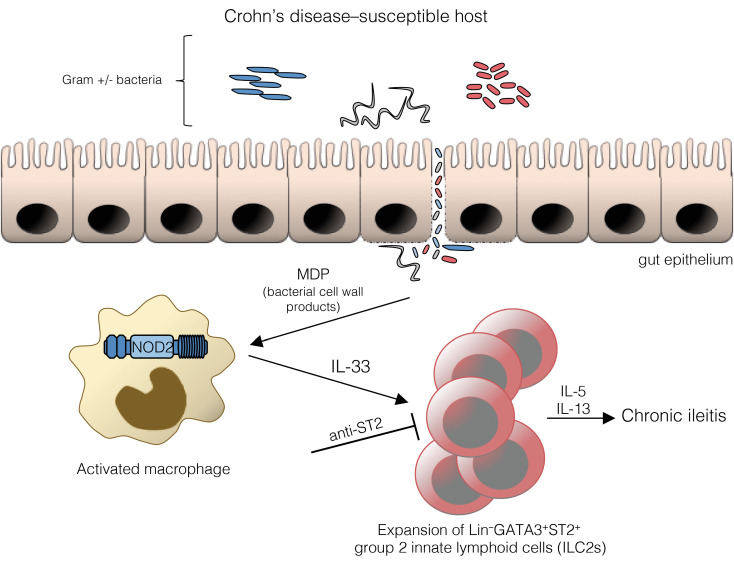
Notably, prior studies from our group showed requirement of the gut microbiome for IL-33 expression and that NOD2 plays an important role in the development of SAMP ileitis (12, 17, 18). NOD2 detects muramyl dipeptide (MDP), a peptidoglycan by-product found in cell walls of both gram-positive and gram-negative bacteria. Importantly, mutations in NOD2/CARD15/IBD1 are highly associated with CD susceptibility, representing an increased risk factor for ileum-specific disease (19, 20). However, precisely how aberrant NOD2 leads to chronic intestinal inflammation characterizing CD remains controversial and at present, is still not fully understood.
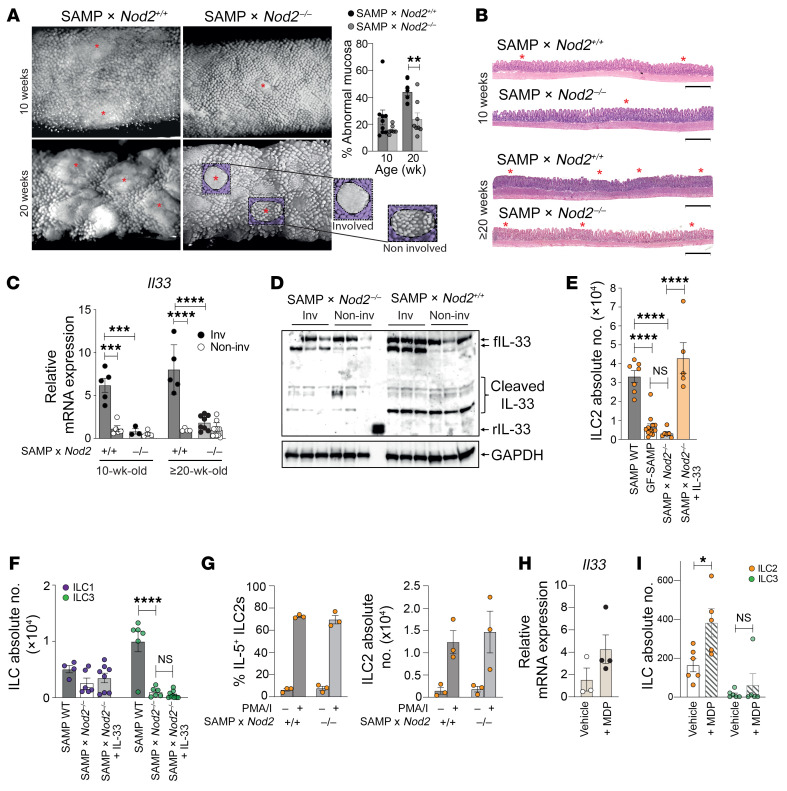
Disease in SAMP mice is strongly linked to the presence of cobblestone lesions, which are defined structural areas within the gut mucosa containing accumulations of inflammatory infiltrates and flattened villi that are also found in CD patients (21). Stereomicroscopy (SM) of ilea from SAMP lacking Nod2 (SAMP × Nod2–/–) revealed fewer cobblestone lesions compared with WT (SAMP × Nod2+/+), which became even more evident as disease progressed (Figure 3A). The presence of abnormal mucosa was noticeably reduced in SAMP × Nod2–/– mice (Figure 3A), which is consistent with histological evidence of decreased villous blunting, restoration of epithelial architecture, and less immune cell infiltration compared with WT (Figure 3B). Harvest of cobblestone (involved) and noninvolved areas from both 10- and ≥20-week-old mice revealed increased Il33 in the involved ilea from WT, which was decreased in SAMP × Nod2–/– mice and reached baseline levels equivalent to those in noninvolved areas, independent of mouse strain (Figure 3C). IL-33 protein reflected similar trends, showing that, different from involved ilea of WT in which bioactive, full-length, as well as cleaved IL-33 isoforms were robustly detected, decreased levels were found in SAMP × Nod2–/– mice (Figure 3D). Collectively, these data provide evidence that NOD2 regulates IL-33 expression and contributes to SAMP ileitis.
Figure 3. Nod2 regulates IL-33–mediated ILC2 expansion and function during SAMP ileitis.
(A) Representative 3D stereomicroscopy (SM) images of SAMP × Nod2–/– and WT (SAMP × Nod2+/+) ilea, highlighting cobblestone lesions (red asterisks), with SM scores and (B) representative H&E-stained histologic images (n = 7–9). Scale bars: 500 μm. (C) Relative Il33 expression, with noninvolved WT set arbitrarily as 1 (n = 4–11), and (D) representative Western blots showing bioactive, full-length (f), and cleaved IL-33 in 10-week-old SAMP × Nod2–/– vs. WT ilea (n = 3). (E and F) ILCs in 10-week-old WT, GF-SAMP (E only), and SAMP × Nod2–/– with and without rIL-33 (n = 6–12). (G) IL-5–expressing ILC2s after ex vivo activation with and without PMA/ionomycin (I) in the presence of brefeldin A (n = 3). (H) Relative Il33 expression, with vehicle-treated set arbitrarily as 1, and (I) ileal ILC2s/ILC3s in MDP- vs. vehicle-treated GF-SAMP mice (n = 5–6). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001 by 2-tailed, unpaired Student’s t test (A and G–I), and 1-way (E and F) or 2-way ANOVA (C) with Tukey’s multiple-comparison post hoc test. Experiments were performed at least in duplicate.
To extend these studies, we compared the presence of ILC2s in SAMP × Nod2–/– versus WT and SAMP mice raised under germ-free conditions (GF-SAMP), and found that ILC2s in SAMP × Nod2–/– were dramatically decreased compared with WT, reaching levels found in GF-SAMP, but could be reversed upon exogenous rIL-33 administration (Figure 3E), indicating that presence of the gut microbiome, and sensitization to NOD2, are necessary to induce pathogenic IL-33–dependent ILC2 expansion. Although NOD2 deficiency in SAMP mice did not affect total numbers of ileal ILC1s, a decrease in ILC3s was also observed, but did not change in response to rIL-33 (Figure 3F), indicating specificity for IL-33–dependent ILC2 expansion. Interestingly, after ex vivo activation, IL-5 production from ILC2s of SAMP × Nod2–/– mice was robust and similar to WT (Figure 3F), suggesting that ILC2s may not inherently express NOD2 and that other NOD2-responsive cell population(s) are likely directly affecting ILC2 function. Together, these findings are in line with a recent study reporting that ileum-specific macrophages are responsible for NOD2 sensing and selective activation of IL-2–producing ILC3s that depends on IL-1β (22), which may also be the case during SAMP ileitis, but also activation of IL-5–producing ILC2s that depends on IL-33. In fact, transcriptional regulation of IL-33 by NOD2 has been reported in vitro, in a mouse macrophage cell line (23).
Therefore, to determine if specific NOD2 stimulation expands ILC2s in vivo, GF-SAMP mice were administered exogenous MDP, as previously described (17). Compared with vehicle, MDP increased Il33 (Figure 3H), and while ILC3s were virtually absent in GF-SAMP ilea and did not significantly change after MDP treatment, total ILC2 numbers significantly increased (Figure 3I), confirming NOD2-dependent sensing in SAMP, likely from other non-ILC innate cells (e.g., macrophages) that results in expansion of pathogenic gut mucosal ILC2s, but not ILC3s. These data are somewhat in contrast to what has been reported in other (chemically induced) colitis models in which MDP appears to have protective effects; however, ILCs were not evaluated in these studies, which employed healthy, immunocompetent mice that, unlike SAMP, can mount effective immune responses when challenged with MDP (24). In fact, although ILCs were not assessed, prior studies from our group show that dysregulated immune responses to MDP predispose SAMP mice raised in specific pathogen–free (SPF) conditions to ileitis, and that this effect is conferred by the hematopoietic compartment (17). Different from GF-SAMP used herein, SPF-SAMP mice were evaluated and we found that isolated macrophages were blunted in their innate cytokine response to MDP (although IL-33 was not measured; ref. 17), which may be due to early tolerance by exposure to gut microbes that may also be essential for ILC3 development and its associated protective function (22), posing the possibility that NOD2-specific sensing affecting ILC2s influences early susceptibility to SAMP ileitis.
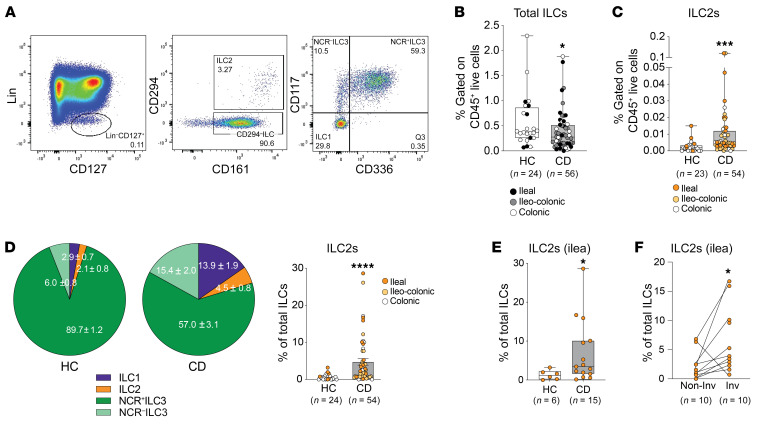
To determine the translational relevance of our findings, we measured ILC2s in gut mucosal biopsies from CD patients and found a general decrease in total ILC frequency, but increased ILC2s versus healthy controls (Figure 4, A–D), similar to SAMP mice with established disease (Supplemental Figure 1C). Furthermore, while natural cytotoxicity receptor–positive (NCR+) ILC3s constituted the overwhelming majority of ILCs in healthy controls, ILC2s, as well as ILC1s, underwent the greatest percentage expansion compared with total ILC3s in CD versus controls (Figure 4D), similarly to disease progression in SAMP mice (Figure 1, D and E). Also similarly to SAMP mice, ILC2 frequency was increased in ileum-specific CD (Figure 4E) and in ileum-matched inflamed (vs. noninflamed) lesions from individual CD patients (Figure 4F). These data are in line with Forkel et al., who reported an overall increase in ILC1s and ILC2s in established CD (and UC), and that decreased NKp44+ (an NCR, also known as CD336) ILC3s and increased ILC1s, ILC2s, and NKp44– ILC3s correlate with disease severity (25). Differently from the aforementioned study, however, we propose that ILC2s, and not ILC1s, may be important during early CD. Future studies are warranted to phenotypically, as well as functionally, characterize ILC subsets in treatment-naive CD patients, perhaps within early-disease cohorts.
Figure 4. Increased ILC2s in CD patients versus healthy controls.
(A) Gating strategy to detect total ILCs (Lin–CD161+CD127+ in live CD45+ population), ILC2s (CD294+), NCR+ ILC3s (CD294–CD117+CD336+), NCR– ILC3s (CD294–CD117+CD336–), and ILC1s (CD294–CD117–CD336–) in gut mucosal biopsies from CD (shown) vs. healthy controls (HC), with frequencies of (B) total ILCs and (C) ILC2s by disease location, displayed as box plots (25th, 50th, and 75th quantiles shown). (D) Percentages of ILC subsets in total ILC population, highlighting differences in ILC2s between CD vs. HC, and ILC2s in ileum-specific CD, compared with (E) HC and (F) in noninvolved vs. involved lesions from individual CD patients. *P ≤ 0.05, ***P ≤ 0.001, ****P ≤ 0.0001 by 2-tailed unpaired Mann-Whitney test (B–E) or 2-tailed paired t test (F).
Finally, while our study identifies a role for normal NOD2 signaling leading to early ileal inflammation, it remains to be seen whether NOD2/CARD15/IBD1 functional variants impact ILC2s and lead to the development of CD. Preliminary findings interrogating the possible correlation between ILC2 frequency and carriage of one or more NOD2 variants in a limited number of CD patients revealed negative results (Supplemental Figure 2); however, the possibility exists that future studies, again, evaluating newly diagnosed, treatment-naive CD patients, with a much larger sampling size, may uncover different results.
In summary, our findings highlight a critical role for NOD2-mediated signaling in driving pathogenic IL-33–dependent inflammatory responses, and identify an important role for NOD2 in regulating ILC2s, particularly during the early stages of CD-like ileitis. These studies lay the foundation for potential early intervention, targeting the NOD2/IL-33/ILC2 axis, in patients with IBD.
Methods
Experimental mice.
AKR (The Jackson Laboratory) and SAMP mice (6) were propagated/maintained at Case Western Reserve University and provided through core services supported by the Animal and Mouse Models Cores of the NIH, P01 DK091222 and P30 DK097948, respectively.
Patient samples.
Intestinal mucosal biopsies were collected from patients with confirmed diagnoses of CD and noninflamed controls (Supplemental Tables 2, 3, and 5), with single-cell suspensions prepared and analyzed by flow cytometry, as described in the supplemental material.
In vivo studies.
All animal experiments were conducted as previously described (12, 17), with full description in the supplemental material.
Tissue harvest, SM-assisted microdissection, and histologic assessment.
Mice were euthanized and ileal tissues harvested and processed (12, 15, 22). Single-cell suspensions from MLNs and ilea were prepared for flow cytometry or ex vivo functional assays, as described in the supplemental material.
Western blots and qPCR.
Ileal tissues were processed to detect either IL-33 protein by Western blotting or Th2 cytokine gene expression by qPCR, calculated using the change-in-cycling threshold (Ct) method and normalized to Gapdh (12, 15).
Statistics.
Data were analyzed using Prism 5 (GraphPad Software). Selection of appropriate statistical tests was based on variance and underlying distribution of data. Differences among and between individual groups were compared as indicated in each figure legend, with P ≤ 0.05 considered significant.
Study approval.
Animal procedures were approved by Case Western Reserve University’s IACUC (number 2015-0179). Human studies were approved using a prospective protocol by the UZ Leuven Ethical Committee Review Board (S53684); written informed consent was received from participants prior to inclusion in study.
Author contributions
CDS and KAB designed/performed experiments, analyzed/interpreted results, and wrote the manuscript (shared first authorship determined randomly). BC, DC, NR, and ARP performed experiments and analyzed/interpreted results. HLW, CLC, and PGD performed experiments. FC and SV analyzed/interpreted results and provided reagents. TTP designed experiments, analyzed/interpreted results, provided reagents, and wrote the manuscript.
Supplementary Material
Acknowledgments
This work was supported by NIH grants DK091222 (to TTP), DK042191 (to TTP and FC), DK055812 (to FC), and DK097948 (Cleveland Digestive Diseases Research Core Center: Wei Xin/histologic analysis, Danian Che/technical support); and Crohn’s & Colitis Foundation grants RFA-410354 (to KAB), CDA-581292 (to CDS), and SRFA-534789 (to CLC). PGD and CLC were supported by Eli & Edythe Broad Foundation SRFAs from the American Gastroenterological Association. We acknowledge Bram and Sare Verstockt for data analyses.
Version 1. 01/14/2021
In-Press Preview
Version 2. 03/01/2021
Electronic publication
Footnotes
DC’s present address is: MRC Human Immunology Unit, MRC Weatherall Institute of Molecular Medicine, University of Oxford, Oxford, United Kingdom.
PGD’s present address is: University of Michigan School of Medicine, Ann Arbor, Michigan, USA.
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2021, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2021;131(5):e140624.https://doi.org/10.1172/JCI140624.
Contributor Information
Carlo De Salvo, Email: cxd198@case.edu.
Kristine-Ann Buela, Email: kgb21@case.edu.
Brecht Creyns, Email: brecht.creyns@kuleuven.be.
Daniele Corridoni, Email: daniele.corridoni@ndm.ox.ac.uk.
Nitish Rana, Email: nxr186@case.edu.
Hannah L. Wargo, Email: hlh48@case.edu.
Peter G. Delaney, Email: petergde@med.umich.edu.
Alexander Rodriguez-Palacios, Email: axr503@case.edu.
Fabio Cominelli, Email: fabio.cominelli@uhhospitals.org.
Séverine Vermeire, Email: severine.vermeire@uzleuven.be.
Theresa T. Pizarro, Email: theresa.pizarro@case.edu.
References
- 1.Spits H, et al. Innate lymphoid cells—a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13(2):145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 2.Vivier E, et al. Innate lymphoid cells: 10 years on. Cell. 2018;174(5):1054–1066. doi: 10.1016/j.cell.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Bal SM, et al. Plasticity of innate lymphoid cell subsets. Nat Rev Immunol. 2020;20(9):552–565. doi: 10.1038/s41577-020-0282-9. [DOI] [PubMed] [Google Scholar]
- 4.Geremia A, Arancibia-Carcamo CV. Innate lymphoid cells in intestinal inflammation. Front Immunol. 2017;8:1296. doi: 10.3389/fimmu.2017.01296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castellanos JG, Longman RS. The balance of power: innate lymphoid cells in tissue inflammation and repair. J Clin Invest. 2019;129(7):2640–2650. doi: 10.1172/JCI124617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pizarro TT, et al. SAMP1/YitFc mouse strain: a spontaneous model of Crohn’s disease-like ileitis. Inflamm Bowel Dis. 2011;17(12):2566–2584. doi: 10.1002/ibd.21638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olson TS, et al. Expanded B cell population blocks regulatory T cells and exacerbates ileitis in a murine model of Crohn disease. J Clin Invest. 2004;114(3):389–398. doi: 10.1172/JCI20855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mao K, et al. Innate and adaptive lymphocytes sequentially shape the gut microbiota and lipid metabolism. Nature. 2018;554(7691):255–259. doi: 10.1038/nature25437. [DOI] [PubMed] [Google Scholar]
- 9.Bernink JH, et al. Interleukin-12 and -23 control plasticity of CD127(+) group 1 and group 3 innate lymphoid cells in the intestinal lamina propria. Immunity. 2015;43(1):146–160. doi: 10.1016/j.immuni.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 10.Lim AI, et al. IL-12 drives functional plasticity of human group 2 innate lymphoid cells. J Exp Med. 2016;213(4):569–583. doi: 10.1084/jem.20151750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bamias G, et al. Proinflammatory effects of TH2 cytokines in a murine model of chronic small intestinal inflammation. Gastroenterology. 2005;128(3):654–666. doi: 10.1053/j.gastro.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 12.De Salvo C, et al. IL-33 drives eosinophil infiltration and pathogenic type 2 helper T-cell immune responses leading to chronic experimental ileitis. Am J Pathol. 2016;186(4):885–898. doi: 10.1016/j.ajpath.2015.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moro K, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463(7280):540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 14.Neill DR, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464(7293):1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pastorelli L, et al. Epithelial-derived IL-33 and its receptor ST2 are dysregulated in ulcerative colitis and in experimental Th1/Th2 driven enteritis. Proc Natl Acad Sci U S A. 2010;107(17):8017–8022. doi: 10.1073/pnas.0912678107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopetuso LR, et al. IL-33 promotes recovery from acute colitis by inducing miR-320 to stimulate epithelial restitution and repair. Proc Natl Acad Sci U S A. 2018;115(40):E9362–E9370. doi: 10.1073/pnas.1803613115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corridoni D, et al. Dysregulated NOD2 predisposes SAMP1/YitFc mice to chronic intestinal inflammation. Proc Natl Acad Sci U S A. 2013;110(42):16999–17004. doi: 10.1073/pnas.1311657110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corridoni D, et al. Genetic deletion of the bacterial sensor NOD2 improves murine Crohn’s disease-like ileitis independent of functional dysbiosis. Mucosal Immunol. 2017;10(4):971–982. doi: 10.1038/mi.2016.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hugot JP, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411(6837):599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 20.Ogura Y, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411(6837):603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez-Palacios A, et al. Stereomicroscopic 3D-pattern profiling of murine and human intestinal inflammation reveals unique structural phenotypes. Nat Commun. 2015;6:7577. doi: 10.1038/ncomms8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou L, et al. Innate lymphoid cells support regulatory T cells in the intestine through interleukin-2. Nature. 2019;568(7752):405–409. doi: 10.1038/s41586-019-1082-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Z, et al. Mutated major histocompatibility complex class II transactivator up-regulates interleukin-33-dependent differentiation of Th2 subset through Nod2 binding for NLR (NOD-like receptor) signaling initiation. J Biol Chem. 2012;287(13):9972–9981. doi: 10.1074/jbc.M111.288498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe T, et al. Muramyl dipeptide activation of nucleotide-binding oligomerization domain 2 protects mice from experimental colitis. J Clin Invest. 2008;118(2):545–559. doi: 10.1172/JCI33145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forkel M, et al. Distinct alterations in the composition of mucosal innate lymphoid cells in newly diagnosed and established crohn’s disease and ulcerative colitis. J Crohns Colitis. 2019;13(1):67–78. doi: 10.1093/ecco-jcc/jjy119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.