Abstract
Background
North America is facing an unprecedented public health crisis of opioid-related morbidity and mortality, increasingly as a result of the introduction of illicitly manufactured fentanyl into the street drug market. Although the treatment of opioid use disorder (OUD) is a key element in the response to the opioid overdose epidemic, currently available pharmacotherapies (e.g., methadone, buprenorphine) may not be acceptable to or effective in all patients. Available evidence suggests that slow-release oral morphine (SROM) has similar efficacy rates as methadone with respect to promoting abstinence, and with improvements in a number of patient-reported outcomes among persons using heroin. However, little is known about the relative effectiveness and acceptability of SROM compared to methadone in the context of fentanyl use. This study aims to address this research gap.
Methods
pRESTO is a 24-week, open-label, two arm, non-inferiority, randomized controlled trial comparing SROM versus methadone for the treatment of OUD. Participants will be 298 clinically stable, non-pregnant adults with OUD, recruited from outpatient clinics in Vancouver, Canada, where the majority of the illicit opioids are contaminated with fentanyl. The primary outcome is suppression of illicit opioid use, measured by bi-weekly urine drug screens. Secondary outcomes include: treatment retention, medication safety, overdose events, treatment satisfaction, psychological functioning, changes in drug-related problems, changes in quality of life, opioid cravings, other substance use, and cost-effectiveness.
Discussion
pRESTO will be among the first studies to evaluate treatment options for individuals primarily using synthetic street opioids, providing important evidence to guide treatment strategies for this population.
Keywords: Slow-release oral morphine, Methadone, Opioid use disorder, Randomized clinical trial, Fentanyl, Overdose
1. INTRODUCTION
North America is facing an unprecedented public health crisis of opioid-related morbidity and mortality. It is estimated that over 2.5 million Americans have an opioid use disorder (OUD), and that over 47,000 died from an opioid-related overdose in 2018 [1]. Although precise estimates for Canada do not exist, available data suggest that as many as 0.5–1 million Canadians may suffer problematic opioid use, and that there were more than 4500 opioid-related deaths in 2018 in Canada, an increase of over 45% from 2016 [2]. Of note, opioid-related overdose deaths are now one of the leading causes of injury and death in North America [3,4]. While the current opioid crisis is largely attributable to the misuse of prescription opioids, in recent years there has been a dramatic rise in overdose deaths from illicitly manufactured fentanyl (IMF) and related analogs [5,6]. For example, among the more than 47,000 overdose deaths in the United States (U.S.) in 2018, approximately two thirds involved IMF. Some jurisdictions in Canada are experiencing similar rising overdose epidemics, as demonstrated by the over 1500 illicit drug overdose deaths in 2018 in British Columbia (BC; 31 deaths per 100,000 individuals), a 300% increase from 2015, with IMF involved in over 80% of the cases [5].
While the causes of the opioid-related overdose crisis in North America are multifactorial, one of the major drivers is untreated OUD. Indeed, despite the well-established benefits of medications for OUD (MOUD), such as buprenorphine/naloxone or methadone in reducing opioid-related morbidity and mortality [[7], [8], [9], [10]], major individual- and structural-level barriers to uptake of this medication and engagement in OUD care persist [11]. This unmet treatment gap has resulted in substantial numbers of individuals with untreated OUD who continue to be at risk of death and other adverse health outcomes [11].
Due to its relative safety profile [10], buprenorphine/naloxone has emerged as the preferred first-line treatment option in Canada and the U.S. [12,13]. However, buprenorphine/naloxone may not be appropriate for all patients, particularly for individuals with social instability who are at high risk of attrition [14,15]. Although methadone may offer an alternative treatment option in these cases, its safety and toxicity profile (e.g., increased cardiovascular and overdose risk) [16,17], as well as the high potential for drug-drug interactions [18] and patient reported concerns with its side effects [19], further limits its broader use. In addition, while extended-release naltrexone is also available in the U.S., the efficacy of this medication for people with more severe OUD, including those who have not benefitted from oral MOUD is unknown [20].
Comparative effectiveness research that can aid in the identification of viable new therapies that can better account for patients’ perceptions, needs, and factors shaping satisfaction with treatment will be critical to improving retention in treatment as well as health and social outcomes [21–23]. In this regard, a number of small studies have explored the potential of slow release oral morphine (SROM) versus methadone, and provided preliminary evidence suggesting similar efficacy rates in suppressing illicit opioid use and retention in treatment [24–27]. In addition, some of these preliminary studies have suggested that SROM may have a superior safety profile (e.g., shorter mean QTc interval) and perform better than methadone in improving patient-reported outcomes, including mental health, alleviation of cravings and withdrawal symptoms, and treatment satisfaction [24–26, 28–30]. Based on this growing evidence base, SROM is increasingly and successfully used in several European countries [31] and Canadian provinces (off-label) [13,32]. However, as identified in three systematic reviews on the topic [25–27], available clinical studies have a number of important limitations including that, among the only four available randomized trials [24,25], these were relatively small, and none involved individuals using illicit fentanyl. Likewise, there are no clinical trials evaluating methadone or other MOUD for people with OUD using fentanyl, and thus questions remain as to how these medications perform in this population, particularly given fentanyl’s higher potency compared to other opioids.
To better inform clinical practice in the context of the escalating and evolving opioid epidemic in North America, this study will assess the relative effectiveness, safety, and acceptability of SROM as MOUD compared to methadone in real world outpatient settings. The present manuscript describes the study design of this randomized effectiveness trial, in accordance with recommendations from SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) Statement [33].
2. METHODS
2.1. Study design
2.1.1. Overview
“Repurposing Slow-Release Oral Morphine as a New Oral Alternative for the Treatment of Opioid Use Disorder” (pRESTO) is a 24-week, open-label, two arm, non-inferiority, randomized controlled trial with a parallel design comparing the effectiveness of SROM to methadone in outpatient substance use treatment settings among individuals with OUD (Fig. 1).
Figure 1.
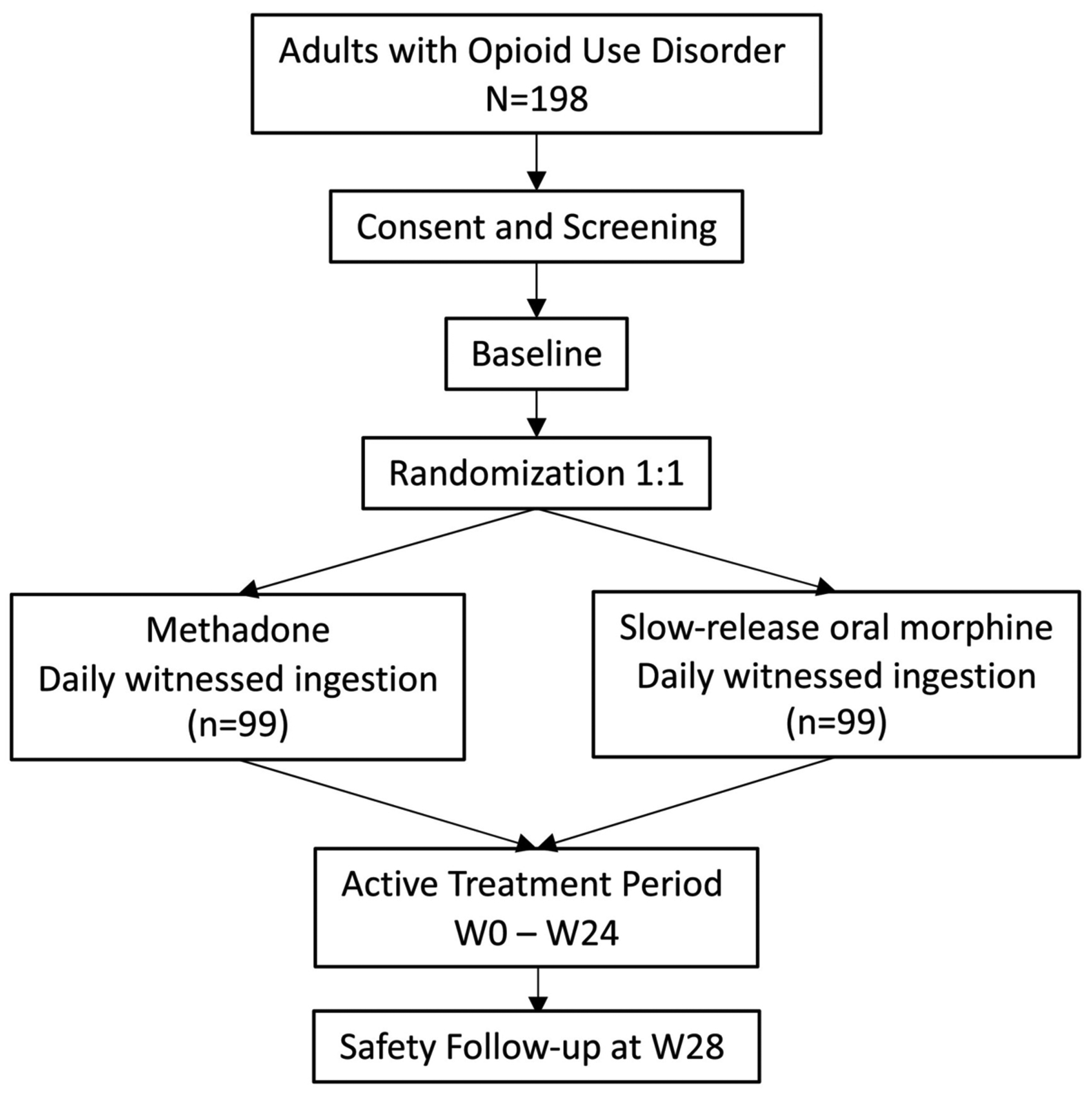
Study schema
Interested individuals will undergo an initial pre-screening assessment to determine general eligibility, and potentially eligible candidates will then be invited to complete the informed consent process. Once written consent is voluntarily given, participants will undergo screening assessments to confirm eligibility. Eligible and consenting participants will be randomized as soon as possible, on a 1:1 ratio to either: a) Methadone supplied as part of standard of care or b) Slow Release Oral Morphine. Both medications will be dispensed via daily witnessed ingestion in designated community pharmacies. Randomization will be stratified by use of medication to manage withdrawal symptoms before treatment can be initiated, using a permuted block design, with blocks of varying sizes.
Once treatment is initiated, participants will be followed for 24 weeks, with research visits every 2 weeks. At the end of the 24-week active treatment period, the research team will ensure that all participants are transitioned to community addiction care with the least possible disruption. A follow-up visit will be conducted 28 weeks after randomization to assess short-term safety after the end of the study, and to determine current engagement in addiction care. Long-term follow up will include confidential data linkages with provincial health administrative databases to monitor health care utilization and outcomes among enrolled participants up to 36 months after randomization.
2.1.2. Study objectives
The primary objective is to compare the relative effectiveness of SROM versus methadone in suppressing illicit opioid use among individuals with OUD. Secondary objectives are to compare other key indicators of treatment success between the two arms, including: retention, safety, overdose events, treatment satisfaction, psychological functioning, quality of life, cravings, other substance use, and cost-effectiveness.
2.1.3. Study sites
In Canada, MOUD are typically prescribed in the context of primary care, and dispensed through community-based pharmacies [34]. In line with this low-threshold model of OUD care, the study will be implemented at outpatient clinics in the lower mainland area of British Columbia. Two sites have initially been selected: (1) the Columbia Street Community Clinic, a comprehensive primary care clinic run by Portland Hotel Society Community Services Society, a non-profit organization located in Vancouver’s Downtown Eastside neighborhood, where most fentanyl use is concentrated, and (2) the Rapid Access Addiction Clinic at St. Paul’s Hospital, an addiction outpatient clinic co-located within an inner-city hospital in downtown Vancouver. Participants will be able to choose from three community pharmacies located in close proximity to the clinical sites to receive their daily witnessed dose of study drug.
2.1.4. Study population
To ensure diversity and representativeness of the population with OUD and to examine the relative effectiveness of each model of care in different health system environments, recruitment will be done through a number of venues, including primary care clinics, emergency departments, overdose preventions sites and other community venues commonly visited by the target population.
In addition, to maximize representativeness of the broader population with OUD across Canada who may benefit from alternative forms of oral MOUD, this trial has broad eligibility criteria, which are presented in Table 1. In brief, participants will be non-pregnant clinically stable adults with OUD who are not currently on a stable dose of MOUD and are interested in and eligible for treatment with either methadone or SROM as per the British Columbia and Canadian guidelines for the management of OUD [12,13]. Given high rates of contamination of the street drug supply with IMF in BC (>90% for opioids), we expect that most of our study population will be intentionally or unintentionally exposed to fentanyl [35].
Table 1.
pRESTO eligibility criteria.
| Inclusion criteria |
|
| Exclusion criteria |
|
OUD, opioid use disorder. MOUD, medication for opioid use disorder. SROM, slow-release oral morphine. MAOI, monoamine oxidase inhibitor.
2.2. Study interventions
Dosing and administration of both study drugs will be in line with British Columbia and Canadian guidelines for the management of OUD [12,13]. Typically, this involves administration via once-daily directly witnessed ingestion in community pharmacies. Take-home doses may be allowed for stable patients for short periods of time (e.g., weekends, holidays), at the discretion of the study physician. For the purposes of this trial, we have selected three community pharmacies with experience providing MOUD and located in close proximity to the selected clinic sites and patient catchment areas.
2.2.1. Slow-release oral morphine
The use of SROM for the treatment of OUD is off-label in Canada, and is decided on a case by case basis. According to existing provincial and national guidelines, common starting doses are 30 to 60 mg per day [12,13]. Based on expert-led approach, the maximum SROM dose is 200 mg on the first day. Doses are increased by 50–100 mg every 1–2 days until the patient achieves a stable daily dosage (e.g., control of withdrawal symptoms and cravings, without opioid toxicity). Most patients will achieve stabilization with doses between 800 and 1200 mg/day; however, higher doses may be needed for some patients.
2.2.2. Methadone
In Canada, methadone is the recommended pharmacotherapy for individuals with poor response, side effects or contraindications to buprenorphine/naloxone. The starting dose of methadone is based on the level of opioid tolerance, ranging from 5 to 10 mg/day to 30 mg/day [12,13]. Doses are then slowly titrated by 5–10 mg every 3–5 days until a stable dose is achieved. Most patients achieve stabilization with daily doses of 60–120 mg.
2.2.3. Medical management
Participants in both arms will receive medical management through trained study physicians, and as per regular standard practice. Typically, these visits include education on OUD and MOUD, monitoring of efficacy, side effects, and adherence to the assigned MOUD, as well as potential dose adjustments, review of use of opioids and other substances, as well as referrals to other health and social services, as needed and appropriate [36].
2.3. Assessments
The schedule of visits and assessments is presented in Table 2. Screening and baseline assessments gather information on participant socio-demographics, medical, psychiatric, overdose and addiction treatment history, patterns of substance use, health status, motivations for treatment, utilization of health services, and urine drug tests (UDT). In addition, pregnancy and birth control are assessed at screening/baseline and every 4 weeks thereafter for female participants of childbearing potential. Baseline assessments will be conducted immediately before randomization and treatment initiation; there will be only one baseline measurement (as opposed to averaging multiple measurements).
Table 2.
Schedule of study procedures and assessments.
| Assessment | Frequency |
|---|---|
| General | |
| Informed Consent Form | SCR |
| Demographics | SCR |
| Inclusion/Exclusion criteria | SCR |
| Locator Form | SCR, then at each study visit |
| Randomization | BSL |
| Safety and medical assessments | |
| DSM-5 Diagnostic Criteria for OUD | SCR |
| Medical and Psychiatric History | SCR |
| Targeted Physical Exam & Vital Signs | SCR, EOT |
| Pregnancy and Birth Control Assessment | SCR, BSL, W 4, 8, 12, 16, 20, EOT |
| Concomitant medications | SCR, BSL, then W 1 to 24 |
| Adverse Events and Serious Adverse Events | W 1 to 24 |
| Non-fatal Overdose Events | BSL, W 12, EOT |
| Efficacy and acceptability assessments | |
| Urine Drug Test (UDT) | BSL, then at each study visit (i.e., every 2 weeks) |
| Assigned MOUD - Pharmacy Abstraction | W 1 to 24 |
| Medical Satisfaction Questionnaire | W 4, 12, EOT |
| PROMIS Short Forms for Anxiety and Depression | BSL, W 4, 12, EOT |
| ASI-Self Report Form | BSL and EOT |
| Visual Analog Scale for opioid craving | BSL, W 2, 4, 8, 12, 16, 20, EOT |
| EuroQol-5D-5 L | BSL, W 4, 8, 12, 16, 20, EOT |
| Non-study Medical and Other Services form | BSL, W 4, 8, 12, 16, 20, EOT |
| Criminal and Legal Activities form | BSL, W 4, 8, 12, 16, 20, EOT |
| Timeline Follow Back | BSL, W 4, 8, 12, 16, 20, EOT |
| Motivations and expectations form | BSL |
| Health Data Linkages | BSL, M12, M24, M36 |
| Treatment | |
| Assigned MOUD-Dosing | BSL, then W 1 to 24 |
| Medical Management | BSL, then as clinically needed |
SCR, Screening. BSL, Baseline. EOT, End of Treatment. W, week. M, month. OUD, opioid use disorder. MOUD, medication for opioid use disorder.
2.4. Outcomes
2.4.1. Primary outcome measure
The primary outcome measure is suppression of illicit opioid use (including fentanyl), measured by the overall percentage of opioid-free UDT (excluding the assigned MOUD and its metabolites) from weeks 2 to 24 of the trial. UDT will be collected at baseline, and every 2 weeks for the 24-week active treatment period. Missing urine samples will be considered positive for opioids.
2.4.2. Secondary outcome measures
Secondary outcome measures include: (1) retention on the assigned intervention, defined as having both a) an active prescription for the assigned MOUD, and b) a positive UDT result for the assigned MOUD at week 24; (2) safety measured by adverse and serious adverse events; (3) overdose events; (4) treatment satisfaction assessed by the Medication Satisfaction Questionnaire (MSQ) [37]; (5) psychological functioning assessed by PROMIS short form measures for anxiety and depression [38]; (6) drug-related problems, evaluated by the the Addiction Severity Index (ASI) Self-Report Form [39,40]; (7) health-related quality of life assessed by the EQ-5D-5 L [41,42]; (8) opioid craving, measured using a visual analog scale, and (9) use of other substances measured by a combination of UDT results and self-report using the Timeline Follow-Back (TLFB) instrument [43,44]. The schedule of secondary outcome assessments is shown in Table 2.
2.4.3. Long-term outcome measures
Long-term outcomes, including engagement in MOUD, health care utilization and mortality up to 36 months post randomization will be assessed through confidential data linkages with the provincial, centralized health administrative data system, which incorporates databases for prescription drug dispensations, inpatient and outpatient care, and vital statistics.
2.5. Sample size and power calculation
The sample size calculation for the primary outcome is based on testing for non-inferiority in a parallel trial. Being consistent with the existing literature [45], we will compute the proportion of opioid-free UDT for each participant, and the mean of these proportions per treatment group will be derived for the comparison. The methadone arm will have an expected mean of 50% opioid-free UDT test results during the 24-week intervention period [46–51]. Following FDA guidance [52], and based on consultations with addiction medicine experts (i.e., what would be the largest loss in efficacy in suppressing opioid use that would be clinically acceptable, especially if SROM shows superiority in key secondary outcomes) and literature review [24], the non-inferiority margin was set conservatively at 10%. The choice of this margin reflects our willingness to accept a small decrease in effectiveness in suppressing opioid use in return for increased safety, tolerability and other improvements in patient-reported outcomes. Based on the literature, the standard deviation is conservatively expected to be 25% [24]. Given the above assumptions and using a recommended one-sided significance level of 2.5%, a power of 80% [53], and a 1:1 allocation ratio, a total of 198 participants (99 per arm) will be required. Once half of the participants have completed the 24-week study period, following FDA guidance [54], the DSMB will conduct a blinded interim analysis of aggregate data to assess the accuracy of the variance parameter, and whether a sample size re-estimation may be required.
2.6. Statistical analyses
All analyses will adhere to recommendations of the Extension of the CONSORT 2010 Statement for the reporting of non-inferiority and equivalence randomized trials [55], under the intention-to-treat (ITT) principle. A “switch equals failure” approach will be used, where UDTs from participants who discontinue their assigned medication for any reason are considered positive for opioids from that time forward [56]. We will also conduct a per-protocol (PP) analysis that will comprise participants who receive at least one dose of the assigned medication, and analysis adjusted to actual treatment received.
2.6.1. Primary outcome
For each treatment arm, the overall mean and 95% confidence interval of the individual proportion of opioid-free UDT will be calculated. Non-inferiority will be established if the lower limit of the two-sided 95% confidence interval for the mean difference between the SROM and methadone (TAU) arms is greater than −10%. For the primary analysis linear regression will be used, adjusting only for the stratification factor (i.e., use of withdrawal medications). In sensitivity analyses, we will build multivariable models to adjust for known relevant confounders (e.g., gender, Indigenous ancestry) and other covariates that show imbalance across arms (i.e., association with the outcome at p < 0.1 levels in bivariate analyses), using a previously utilized stepwise procedure [57]. We will also conduct exploratory analyses using multivariable models to: (1) investigate potential demographic and clinical factors (e.g., participants’ treatment preferences) predicting success of each treatment intervention; and (2) assess the possibility of differential treatment effects across subgroups (e.g., exposure to fentanyl, gender, ethnicity) by examining the interaction terms between treatment assignment and subgroup characteristics.
2.6.2. Secondary outcomes
Secondary outcome analyses will use “superiority” hypotheses, where two-sided tests will be performed with a significance level of 5%. For the assessment of secondary outcomes involving repeated measures, generalized linear mixed-effects modeling (GLMM) with random intercepts will be utilized to account for the repeated measurements, adjusting for known relevant confounders and covariates that show imbalance across arms [58].
2.6.3. Missing data
Regarding the handling of missing data, consistent with international standards in substance use disorder trials [59], missing UDT will be considered positive for illicit opioids, and thus there will be no missing data for the primary outcome. In addition, and recognizing that dropout from treatment typically reflects a failure of that particular model of care to engage patients in long-term care, a “switch equals failure” approach will be used, where participants who discontinue their assigned medication for any reason (including switches to the other arm) are classified as failures, and UDT from this time point forward will be considered positive for opioids [60,61]. This approach will allow a better evaluation of the overall effectiveness of each model of OAT in real-world treatment conditions (including efficacy, tolerability, safety profile, and patients’ preferences), enhancing the external validity of the study results.
For secondary outcomes, there may be missing data due to missed visits or study dropouts. Our statistical approach (GLMM) assumes that missing data are missing at random. Sensitivity analyses will also be conducted to fully understand the impact of missing data on conclusions, and to examine the stability of our assumption of missing data as random. Multiple imputation techniques will be utilized to handle missing values, and the analysis results based on multiple imputation will be reported [62].
2.6.4. Health economic analysis
We will conduct a comprehensive economic evaluation of SROM compared to methadone, from the healthcare-sector, provincial-policymaker, and societal perspectives. The evaluation will follow well-established guidelines for conducting health-economic analyses alongside clinical trials [63,64]. The healthcare sector perspective includes all medical costs incurred on behalf of the patient, regardless of who is responsible for paying them. The provincial-policymaker perspective includes all costs necessary to inform resource allocation decisions for provincial agencies and the public; therefore, it will include all publicly-funded healthcare costs, and other costs relevant to the province, such as direct costs to the criminal justice system. The societal perspective includes all costs from the healthcare sector perspective, as well as other costs important to the public, such as the direct and indirect (e.g., pain and suffering) costs of crime, lost workplace productivity, etc.
First, we will conduct a microcosting analysis to estimate the costs associated with the implementation of SROM, as well as the costs associated with the day-to-day management of the intervention, using a tailored version of the Drug Abuse Treatment Cost Analysis Program (DATCAP) instrument [65,66]. Healthcare service utilization will be captured using medical records, and through self-report using the Non-study Medical and Other Services (NMOS) form. The NMOS has been used extensively in previous research with similar populations [67–70]. Two measures of effectiveness will be included in the cost-effectiveness analysis. The primary effectiveness measure will be the quality-adjusted life-year (QALY), a measure that combines a person’s health-related quality-of-life and the amount of time spent in that health state [64]. The secondary measure of effectiveness will be the abstinent year, measured as the predicted proportion of the year that the participant was abstinent from opioids.
All measures of cost and effectiveness will be obtained using longitudinal multivariable regression, in order to control for potentially confounding factors relevant to the economic analysis that are unbalanced between arms at any given time point. All monetary values will be adjusted for inflation, and measures obtained beyond 12 months of baseline will be discounted for time preference [63,64]. Nonparametric bootstrapping techniques will be used to estimate standard errors and to generate an acceptability curve, which will display the probability that SROM is cost-effective, relative to methadone, at various value thresholds. Finally, sensitivity analyses will be conducted to account for uncertainty in parameter inputs, such as prices.
2.7. Data safety and monitoring
An independent Data and Safety Monitoring Board (DSMB) reviewed the final draft of the protocol and recommended final revisions. The DSMB will also monitor accumulating trial data on a regular basis to ensure participant’s safety and adequate trial performance. In addition, an appointed medical monitor will oversee and evaluate all adverse events.
2.8. Approvals and registration
pRESTO has received approvals from the Health Canada Therapeutic Products Directorate and Office of Controlled Substances, and the University of British Columbia/ Providence Health Care Research Ethics Board. The study is registered on Clinicaltrials.gov (NCT03948464).
3. DISCUSSION
There is an urgent need for science-driven solutions with potential for rapid scalability to address the rising opioid epidemic in North America. This is true particularly in the context of the recent escalation of fentanyl use and other highly potent fentanyl derivatives. Alongside continued efforts to expand access to and capacity of methadone- and buprenorphine-based treatment programs, alternative treatment options are needed to improve access to treatment while better addressing the evolving needs of individuals with OUD and optimizing treatment outcomes in this population.
In this context, the rigorous evaluation of SROM as an alternative form of oral treatment for individuals with OUD is particularly timely and relevant. Indeed, the need for alternative evidence-based oral MOUD has consistently emerged as a key issue reported by patient groups, health care providers and other key stakeholders in Canada, the U.S. and elsewhere [31, 71–75]. The goal of this study is to evaluate the relative effectiveness, safety and acceptability of SROM vs. methadone for the treatment of OUD. In doing so, the proposed study aims to address some of the more urgent clinical questions in the context of the worsening opioid epidemic in Canada. First, to confirm previous encouraging preliminary results on the potential of SROM as an effective, safe and acceptable oral form of MOUD. Second, to provide critical and novel information on the clinical utility of SROM-based treatment as a potential alternative treatment option for OUD. Third, to evaluate treatment approaches for the growing and difficult-to-treat population of people who use or are exposed to fentanyl in North America.
Given the lack of clinical studies evaluating different MOUD for individuals exposed to novel synthetic opioids, and the limited evidence to guide strategies for individuals intolerant or not responding to first-line MOUD, the proposed study offers a timely and unique opportunity to address these research gaps, with high potential to inform patient-centered approaches and stepped care strategies in the management of OUD. Importantly, given that SROM is already approved in Canada for pain management, if results from this study confirm its clinical utility as MOUD it may present significant potential to rapidly increase access to an additional form of evidence-based MOUD.
Supplementary Material
Acknowledgements
The authors thank the study participants for their contributions to the research, as well as current and past researchers and staff. We would specifically like to thank Robyn Tkatch, Mithra Bahrami, Stephen Juwono and Victor Chiang for their research and administrative assistance. Mayne Pharma provided Kadian® capsules in kind.
Funding
This work was supported by the Canadian Institutes of Health Research (CIHR, F17-03454) and Vancouver Foundation (UNR18-0005). MES and NF are supported by Michael Smith Foundation for Health Research (MSFHR)/St Paul’s Foundation Scholar Awards. PB is supported by a MSFHR fellowship award. EW is supported by the CIHR Canada Research Chairs program. HD is supported by a CIHR doctoral award. SMM is supported by the National Institute on Drug Abuse (P30DA040500).
Footnotes
Declaration of Competing Interest
SMM reports having consulted for/advised Sandoz Inc. on an economic model unrelated to the submitted work. All other authors declare no conflict of interests.
Appendix A. Supplementary data
REFERENCES
- [1].Ahmad F, Escobedo L, Rossen L, Spencer M, Warner M, Sutton P, Provisional Drug Overdose Death Counts, https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm, (2019).
- [2].Special Advisory Committee on the Epidemic of Opioid Overdoses, National Report: Apparent Opioid-Related Deaths in Canada (January 2016 to March 2019), Web Based Report https://health-infobase.canada.ca/datalab/national-surveillance-opioid-mortality.html, (September 2019) Accessed September 26 2019.
- [3].King NB, Fraser V, Boikos C, Richardson R, Harper S, Determinants of increased opioid-related mortality in the United States and Canada, 1990–2013: a systematic review, Am. J. Public Health 104 (8) (2014) e32–e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rudd RA, Seth P, David F, Scholl L, Increases in drug and opioid-involved overdose deaths - United States, 2010–2015, MMWR Morb. Mortal. Wkly Rep 65 (5051) (2016) 1445–1452. [DOI] [PubMed] [Google Scholar]
- [5].BC Coroners Service, Illicit Drug Overdose Deaths in BC (January 1 2009–January 31, 2019), (March 2019).
- [6].Gomes T, Greaves S, Martins D, Bandola D, Tadrous M, Singh S, Juurlink D, Mamdani M, Paterson M, Ebejer M, May D, Quercia J, Latest Trends in Opioid-Related Deaths in Ontario: 1991 to 2015, Ontario Drug Policy Research Network, Toronto, ON, Canada, April 2017. [Google Scholar]
- [7].Degenhardt L, Bucello C, Mathers B, Briegleb C, Ali H, Hickman M, McLaren J, Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies, Addiction 106 (1) (2011) 32–51. [DOI] [PubMed] [Google Scholar]
- [8].MacArthur GJ, Minozzi S, Martin N, Vickerman P, Deren S, Bruneau J, Degenhardt L, Hickman M, Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis, BMJ 345 (2012) e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, Ferri M, Pastor-Barriuso R, Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies, BMJ 357 (2017) j1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Connery HS, Medication-assisted treatment of opioid use disorder: review of the evidence and future directions, Harv. Rev. Psychiatr 23 (2) (2015) 63–75. [DOI] [PubMed] [Google Scholar]
- [11].Sharma A, Kelly SM, Mitchell SG, Gryczynski J, O’Grady KE, Schwartz RP, Update on barriers to pharmacotherapy for opioid use disorders, Curr. Psychiatr. Rep 19 (6) (2017) 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].BCCSU A Guideline for the Clinical Management of Opioid Use Disorder, British Columbia Centre on Substance Use (BCCSU), Vancouver, BC, Canada, 2017. [Google Scholar]
- [13].Bruneau J, Ahamad K, Goyer ME, Poulin G, Selby P, Fischer B, Wild TC, Wood E, Misuse C.C.R.I.i.S., Management of opioid use disorders: a national clinical practice guideline, CMAJ 190 (9) (2018) E247–E257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Timko C, Schultz NR, Cucciare MA, Vittorio L, Garrison-Diehn C, Retention in medication-assisted treatment for opiate dependence: a systematic review, J. Addict. Dis 35 (1) (2016) 22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Proctor SL, Copeland AL, Kopak AM, Herschman PL, Polukhina N, A naturalistic comparison of the effectiveness of methadone and two sublingual formulations of buprenorphine on maintenance treatment outcomes: findings from a retrospective multisite study, Exp. Clin. Psychopharmacol 22 (5) (2014) 424–433. [DOI] [PubMed] [Google Scholar]
- [16].Chou R, Weimer MB, Dana T, Methadone overdose and cardiac arrhythmia potential: findings from a review of the evidence for an American Pain Society and College on Problems of Drug Dependence clinical practice guideline, J. Pain 15 (4) (2014) 338–365. [DOI] [PubMed] [Google Scholar]
- [17].Marteau D, McDonald R, Patel K, The relative risk of fatal poisoning by methadone or buprenorphine within the wider population of England and Wales, BMJ Open 5 (5) (2015) e007629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].McCance-Katz EF, Sullivan LE, Nallani S, Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review, Am. J. Addict 19 (1) (2010) 4–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yee A, Loh HS, Hisham Hashim HM, Ng CG, The prevalence of sexual dysfunction among male patients on methadone and buprenorphine treatments: a meta-analysis study, J. Sex. Med 11 (1) (2014) 22–32. [DOI] [PubMed] [Google Scholar]
- [20].Jarvis BP, Holtyn AF, Subramaniam S, Tompkins DA, Oga EA, Bigelow GE, Silverman K, Extended-release injectable naltrexone for opioid use disorder: a systematic review, Addiction 113 (7) (2018) 1188–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Marchand KI, Oviedo-Joekes E, Guh D, Brissette S, Marsh DC, Schechter MT, Client satisfaction among participants in a randomized trial comparing oral methadone and injectable diacetylmorphine for long-term opioid-dependency, BMC Health Serv. Res 11 (2011) 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kelly SM, O’Grady KE, Brown BS, Mitchell SG, Schwartz RP, The role of patient satisfaction in methadone treatment, Am. J. Drug Alcohol Abuse 36 (3) (2010) 150–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Trujols J, Iraurgi I, Oviedo-Joekes E, Guardia-Olmos J, A critical analysis of user satisfaction surveys in addiction services: opioid maintenance treatment as a representative case study, Patient Prefer Adherence 8 (2014) 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Beck T, Haasen C, Verthein U, Walcher S, Schuler C, Backmund M, Ruckes C, Reimer J, Maintenance treatment for opioid dependence with slow-release oral morphine: a randomized cross-over, non-inferiority study versus methadone, Addiction 109 (4) (2014) 617–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ferri M, Minozzi S, Bo A, Amato L, Slow-release oral morphine as maintenance therapy for opioid dependence, Cochrane Database Syst. Rev 6 (2013) CD009879. [DOI] [PubMed] [Google Scholar]
- [26].Jegu J, Gallini A, Soler P, Montastruc JL, Lapeyre-Mestre M, Slow-release oral morphine for opioid maintenance treatment: a systematic review, Br. J. Clin. Pharmacol 71 (6) (2011) 832–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Klimas J, Gorfinkel L, Giacomuzzi SM, Ruckes C, Socias ME, Fairbairn N, Wood E, Slow release oral morphine versus methadone for the treatment of opioid use disorder, BMJ Open 9 (4) (2019) e025799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Falcato L, Beck T, Reimer J, Verthein U, Self-reported cravings for heroin and cocaine during maintenance treatment with slow-release oral morphine compared with methadone A randomized, crossover clinical trial, J. Clin. Psychopharmacol 35 (2) (2015) 150–157. [DOI] [PubMed] [Google Scholar]
- [29].Hammig R, Kohler W, Bonorden-Kleij K, Weber B, Lebentrau K, Berthel T, Babic-Hohnjec L, Vollmert C, Hopner D, Gholami N, Verthein U, Haasen C, Reimer J, Ruckes C, Safety and tolerability of slow-release oral morphine versus methadone in the treatment of opioid dependence, J. Subst. Abus. Treat 47 (4) (2014) 275–281. [DOI] [PubMed] [Google Scholar]
- [30].Verthein U, Beck T, Haasen C, Reimer J, Mental symptoms and drug use in maintenance treatment with slow-release Oral morphine compared to methadone: results of a randomized crossover study, Eur. Addict. Res 21 (2) (2015) 97–104. [DOI] [PubMed] [Google Scholar]
- [31].Bond AJ, Reed KD, Beavan P, Strang J, After the randomised injectable opiate treatment trial: post-trial investigation of slow-release oral morphine as an alter-native opiate maintenance medication, Drug Alcohol Rev 31 (4) (2012) 492–498. [DOI] [PubMed] [Google Scholar]
- [32].Socias ME, Wood E, Evaluating slow-release Oral morphine to narrow the treatment gap for opioid use disorders, Ann. Intern. Med 168 (2) (2018) 141–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chan AW, Tetzlaff JM, Gotzsche PC, Altman DG, Mann H, Berlin JA, Dickersin K, Hrobjartsson A, Schulz KF, Parulekar WR, Krleza-Jeric K, Laupacis A, Moher D, SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials, BMJ 346 (2013) e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nosyk B, Anglin MD, Brissette S, Kerr T, Marsh DC, Schackman BR, Wood E, Montaner JSG, A call for evidence-based medical treatment of opioid dependence in the United States and Canada, Health Aff. 32 (8) (2013) 1462–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tupper KW, McCrae K, Garber I, Lysyshyn M, Wood E, Initial results of a drug checking pilot program to detect fentanyl adulteration in a Canadian setting, Drug Alcohol Depend. 190 (2018) 242–245. [DOI] [PubMed] [Google Scholar]
- [36].Fiellin DA, Pantalon MV, Chawarski MC, Moore BA, Sullivan LE, O’Connor PG, Schottenfeld RS, Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence, N. Engl. J. Med 355 (4) (2006) 365–374. [DOI] [PubMed] [Google Scholar]
- [37].Vernon MK, Revicki DA, Awad AG, Dirani R, Panish J, Canuso CM, Grinspan A, Mannix S, Kalali AH, Psychometric evaluation of the Medication Satisfaction Questionnaire (MSQ) to assess satisfaction with antipsychotic medication among schizophrenia patients, Schizophr. Res 118 (1–3) (2010) 271–278. [DOI] [PubMed] [Google Scholar]
- [38].Johnston KL, Lawrence SM, Dodds NE, Yu L, Daley DC, Pilkonis PA, Evaluating PROMIS(R) instruments and methods for patient-centered outcomes research: patient and provider voices in a substance use treatment setting, Qual. Life Res 25 (3) (2016) 615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Rosen CS, Henson BR, Finney JW, Moos RH, Consistency of self-administered and interview-based Addiction Severity Index composite scores, Addiction 95 (3) (2000) 419–425. [DOI] [PubMed] [Google Scholar]
- [40].Cacciola JS, Mclellan AT, Alterman AI, Mulvaney FD, A comparison of a self-administered ASI with the standard ASI interview, problems of drug dependence, 1997, Proceedings of the 59th Annual Scientific Meeting, The College on Problems of Drug Dependence, Inc, 1998NIH Publication no. 98 ± 4305. [Google Scholar]
- [41].Brooks R, EuroQol: the current state of play, Health Policy 37 (1) (1996) 53–72. [DOI] [PubMed] [Google Scholar]
- [42].Nosyk B, Sun H, Guh DP, Oviedo-Joekes E, Marsh DC, Brissette S, Schechter MT, Anis AH, The quality of eight health status measures were com-pared for chronic opioid dependence, J. Clin. Epidemiol 63 (10) (2010) 1132–1144. [DOI] [PubMed] [Google Scholar]
- [43].Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P, The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties, J. Consult. Clin. Psychol 68 (1) (2000) 134–144. [DOI] [PubMed] [Google Scholar]
- [44].Robinson SM, Sobell LC, Sobell MB, Leo GI, Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use, Psychol. Addict. Behav 28 (1) (2014) 154–162. [DOI] [PubMed] [Google Scholar]
- [45].Mattick RP, Breen C, Kimber J, Davoli M, Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence, Cochrane Database Syst. Rev 2 (2014) CD002207. [DOI] [PubMed] [Google Scholar]
- [46].Mattick RP, Ali R, White JM, O’Brien S, Wolk S, Danz C, Buprenorphine versus methadone maintenance therapy: a randomized double-blind trial with 405 opioid-dependent patients, Addiction 98 (4) (2003) 441–452. [DOI] [PubMed] [Google Scholar]
- [47].Soyka M, Zingg C, Koller G, Kuefner H, Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: results from a randomized study, Int. J. Neuropsychopharmacol 11 (5) (2008) 641–653. [DOI] [PubMed] [Google Scholar]
- [48].Petitjean S, Stohler R, Déglon JJ, Livoti S, Waldvogel D, Uehlinger C, Ladewig D, Double-blind randomized trial of buprenorphine and methadone in opiate dependence, Drug Alcohol Depend 62 (1) (2001) 97–104. [DOI] [PubMed] [Google Scholar]
- [49].Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE, A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence, N. Engl. J. Med 343 (18) (2000) 1290–1297. [DOI] [PubMed] [Google Scholar]
- [50].Schottenfeld RS, Chawarski MC, Pakes JR, Pantalon MV, Carroll KM, Kosten TR, Methadone versus buprenorphine with contingency management or performance feedback for cocaine and opioid dependence, Am. J. Psychiatry 162 (2) (2005) 340–349. [DOI] [PubMed] [Google Scholar]
- [51].Mattick RP, Breen C, Kimber J, Davoli M, Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence, Cochrane Database Syst. Rev 3 (2009) CD002209. [DOI] [PubMed] [Google Scholar]
- [52].Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), Non-Inferiority Clinical Trials to Establish Effectiveness, Guidance for Industry, Food and Drug Administration, U.S. Department of Health and Human Services, November 2016.
- [53].Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG, Group C, Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement, JAMA 308 (24) (2012) 2594–2604. [DOI] [PubMed] [Google Scholar]
- [54].Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), Guidance for Industry, Adaptive Design Clinical Trials for Drugs and Biologics, Food and Drug Administration, U.S. Department of Health and Human Services, February 2010.
- [55].Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG, Group C, Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement, Jama 308 (24) (2012) 2594–2604. [DOI] [PubMed] [Google Scholar]
- [56].Morden JP, Lambert PC, Latimer N, Abrams KR, Wailoo AJ, Assessing methods for dealing with treatment switching in randomised controlled trials: a simulation study, BMC Med. Res. Methodol 11 (2011) 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Maldonado G, Greenland S, Simulation study of confounder-selection strategies, Am. J. Epidemiol 138 (11) (1993) 923–936. [DOI] [PubMed] [Google Scholar]
- [58].Brown H, Prescott R, Applied Mixed Models in Medicine, 2 ed., John Wiley & Sons, Ltd., West Sussex, 2006. [Google Scholar]
- [59].Mattick RP, Breen C, Kimber J, Davoli M, Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence, Cochrane Database Syst. Rev 2 (2014). [Google Scholar]
- [60].Morden JP, Lambert PC, Latimer N, Abrams KR, Wailoo AJ, Assessing methods for dealing with treatment switching in randomised controlled trials: a simulation study, BMC Med. Res. Methodol 11 (4) (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].H. A, D. R, Discordant conclusions from HIV clinical trials–an evaluation of efficacy endpoints, Antiviral Ther 10 (3) (2005) 367–374. [PubMed] [Google Scholar]
- [62].Johnson DR, Young R, Toward best practices in analyzing datasets with missing data: comparisons and recommendations, J. Marriage Fam 73 (5) (2011) 926–945. [Google Scholar]
- [63].Glick HA, Doshi JA, Sonnad SS, Polsky D, Economic Evaluation in Clinical Trials, Oxford University Press, 2014. [Google Scholar]
- [64].Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG, Cost-Effectiveness in Health and Medicine, 2nd ed., Oxford University Press, New York, NY, 2017. [Google Scholar]
- [65].French MT, Drug Abuse Treatment Cost Analysis Program (DATCAP): User’s Manual, University of Miami, Florida, 2003. [Google Scholar]
- [66].French MT, Dunlap LJ, Zarkin GA, McGeary KA, McLellan AT, A structured instrument for estimating the economic cost of drug abuse treatment: The Drug Abuse Treatment Cost Analysis Program (DATCAP), J. Subst. Abus. Treat 14 (5) (1997) 445–455. [DOI] [PubMed] [Google Scholar]
- [67].Murphy SM, Campbell AN, Ghitza UE, Kyle TL, Bailey GL, Nunes EV, Polsky D, Cost-effectiveness of an internet-delivered treatment for substance abuse: data from a multisite randomized controlled trial, Drug Alcohol Depen. 161 (2016) 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Murphy SM, Polsky D, Lee JD, Friedmann PD, Kinlock TW, Nunes EV, Bonnie RJ, Gordon M, Chen DT, Boney TY, Cost-effectiveness of extended release naltrexone to prevent relapse among criminal-justice-involved persons with a history of opioid use disorder, Addiction 112 (8) (2017) 1440–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Polsky D, Glick HA, Yang J, Subramaniam GA, Poole SA, Woody GE, Cost-effectiveness of extended buprenorphine–naloxone treatment for opioid-dependent youth: data from a randomized trial, Addiction 105 (9) (2010) 1616–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Murphy SM, McCollister KE, Leff JA, Yang X, Jeng PJ, Lee JD, Nunes EV, Novo P, Rotrosen J, Schackman BR, Cost-effectiveness of buprenorphine-naloxone versus extended-release naltrexone to prevent opioid relapse, Ann. Intern. Med 170 (2019) 90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Socias ME, Wood E, McNeil R, Kerr T, Dong H, Shoveller J, Montaner J, Milloy MJ, Unintended impacts of regulatory changes to British Columbia Methadone Maintenance Program on addiction and HIV-related outcomes: an interrupted time series analysis, Int. J. Drug Policy 45 (2017) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].McNeil R, Kerr T, Anderson S, Maher L, Keewatin C, Milloy MJ, Wood E, Small W, Negotiating structural vulnerability following regulatory changes to a provincial methadone program in Vancouver, Canada: A qualitative study, Soc. Sci. Med 133 (2015) 168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Oviedo-Joekes E, Guh D, Marchand K, Marsh DC, Lock K, Brissette S, Anis AH, Schechter MT, Differential long-term outcomes for voluntary and involuntary transition from injection to oral opioid maintenance treatment, Subst. Abuse Treat Prev. Policy 9 (2014) 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Oviedo-Joekes E, Marchand K, Lock K, Chettiar J, Marsh DC, Brissette S, Anis AH, Schechter MT, A chance to stop and breathe: participants’ experiences in the North American Opiate Medication Initiative clinical trial, Addict. Sci. Clin. Pract 9 (2014) 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].BC Overdose Action Exchange II, BC Centre for Disease Control, Vancouver, BC: (August 2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


