Abstract
Evidence supports no link between maternal smoking in pregnancy and autism spectrum disorder (autism) overall. To address remaining questions about the unexplained heterogeneity between study results and the possibility of risk for specific autism sub-phenotypes, we conducted a whole-population cohort study in Denmark. We followed births 1991 – 2011 (1,294,906 persons, including 993,301 siblings in 728,271 families), from 1 year of age until an autism diagnosis (13,547), death, emigration, or December 31, 2012. Autism, with and without attention deficit hyperactivity disorder (ADHD) and with and without intellectual disability (ID) were based on ICD-8 and ICD-10 codes from Danish national health registers, including 3,319 autism+ADHD, 10,228 autism-no ADHD, 2,205 autism+ID, and 11,342 autism-no ID. We estimated hazard ratios (HR) and 95% confidence intervals (95% CI) between any maternal smoking (from birth records) and autism (or sub-phenotypes) using survival models with robust standard errors, stratifying by birth year and adjusting for child sex, parity, and parental age, education, income, and psychiatric history. To additionally address confounding using family designs, we constructed a maternal cluster model (adjusting for the smoking proportion within the family), and a stratified sibling model. Associations with maternal smoking and autism were elevated in conventional analyses (HR of 1.17 [1.13–1.22]) but attenuated in the maternal cluster (0.98 [0.88–1.09]) and sibling (0.86[0.64–1.15]) models. Similarly, risks of autism sub-phenotypes with maternal smoking were attenuated in the family-based models. Together these results support that smoking in pregnancy is not linked with autism or select autism comorbid sub-phenotypes after accounting for familial confounding.
Keywords: Tobacco, Maternal smoking, Autism, Autism spectrum disorder, Intellectual disability, Attention Deficit Hyperactivity Disorder, Neurodevelopment, Confounding, Family-based designs
Lay Summary:
Smoking during pregnancy has many harmful impacts, which may include harming the baby’s developing brain. However, in a study of thousands of families in Denmark, it does not appear that smoking in pregnancy leads to autism or autism in combination with intellectual problems or attention deficits, once you account for the way smoking patterns and developmental disabilities run in families.
Introduction
It is widely accepted that exposure to maternal smoking during pregnancy has deleterious effects on health outcomes in children, including stillbirth (Flenady et al., 2011), lowered birth weight (Jaddoe et al., 2008), and obesity (Gorog et al., 2011). In utero tobacco exposures have also been linked to neurodevelopmental disorders, including cognitive decrements and externalizing disorders such as attention deficit disorders (Obel et al., 2009; Thapar et al., 2003; Zhu et al., 2014). Animal and human studies have suggested that maternal smoking can disrupt neurodevelopment via effects on maturing neurotransmitter systems and brain architecture, and constituents of tobacco smoke exhibit a wide range of oxidative stress and other toxicities that may negatively impact development (Slotkin, 2004; Soothill, Morafa, Ayida, & Rodeck, 1996). Despite these findings, debates continue with regard to whether observed associations between maternal smoking and neurodevelopmental disorders are causal (D’Onofrio, Lahey, Turkheimer, & Lichtenstein, 2013; Langley, Heron, Smith, & Thapar, 2012; Skoglund, Chen, D’Onofrio, Lichtenstein, & Larsson, 2014; Thapar & Rutter, 2009).
This difficulty in establishing a causal link is in part because maternal smoking is known to be associated with numerous social and social-class related factors (teenage motherhood, lower maternal education, increased single motherhood) that influence childhood outcomes (Ellingson, Rickert, Lichtenstein, Långström, & D’Onofrio, 2012; Gilman, Gardener, & Buka, 2008; Rai et al., 2012). In addition, genes associated with the likelihood of women smoking in pregnancy may also affect childhood outcomes through maternal-child genetic inheritance (Agrawal et al., 2008; Chang, Lichtenstein, & Larsson, 2012). Thus, studies utilizing quasi-experimental designs (such as siblings with discordant exposures) have been undertaken in an attempt to control for unmeasured genetic and demographic confounding (D’Onofrio et al., 2013; Knopik, 2009). These studies have generally demonstrated attenuation of previously observed maternal smoking-neurodevelopment associations (Gustavson et al., 2017; S. M. Meier et al., 2017; Sandra M. Meier, Mors, & Parner, 2017; Obel et al., 2009; Skoglund et al., 2014).
Autism spectrum disorder (autism) is a neurodevelopmental disorder that may plausibly be influenced by maternal smoking in pregnancy. Studies of this potential link have reported risk (Hultman, Sparén, & Cnattingius, 2002; Visser et al., 2012), null (Lee et al., 2011; Tran et al., 2013), and even borderline protective associations (Burstyn, Sithole, & Zwaigenbaum, 2010; Kalkbrenner et al., 2012). When combined in three meta-analyses (each with a slightly different set of included primary studies), overall risk estimates of maternal smoking in pregnancy were null, with pooled odds ratios and 95% confidence intervals of: 1.02 (0.93, 1.12) (Rosen, Lee, Lee, Yang, & Burstyn, 2015), 1.02 (0.93, 1.13) (Tang, Wang, Gong, & Wang, 2015), and 1.16 (0.97, 1.40) (Jung, Lee, McKee, & Picciotto, 2017). The heterogeneity between results of each individual study was determined to be high, and while partly explained by controlling for social class confounding and the quality of the studies, additional sources of heterogeneity remained unaccounted for (Jung et al., 2017; Rosen et al., 2015; Tang et al., 2015). Importantly, these analyses were not able to assess whether impacts of maternal smoking may differ among case subgroups. Studies of autism etiology are known to be complicated by evidence that autism represents a spectrum of phenotypes (e.g. with varying levels of severity and co-occurring conditions) that may result from distinct causal pathways. For example, the possibility remains that maternal smoking may exert risk for autism without co-occurring intellectual disability, and this hypothesis has some data support (Kalkbrenner et al., 2012; Kalkbrenner, Schmidt, & Penlesky, 2014; Lee et al., 2011). Differences in risk between maternal smoking and autism co-occurring with attention deficit hyperactivity disorder (ADHD) has not previously been explored. Furthermore, publications of maternal smoking and autism have not accounted for shared inheritance or psychiatric liability, via family-based or other designs.
A greater understanding of the role of maternal smoking in autism is relevant because this exposure is potentially preventable and has biologic relevance and suspected links with other neurodevelopmental disorders. We examined whether maternal smoking in pregnancy increased the risk of diagnosis with autism in a large, whole-population cohort study. Our design included the features identified as important in a prior meta-analyses: prenatal reporting of tobacco use and control for social class and psychiatric history (Rosen et al., 2015). To more fully account for family-clustered confounders and shared inheritance, we have additionally conducted sibling-based analyses. Furthermore, we have explored whether risk differs by autism with and without other co-occurring neurodevelopmental disorders.
Methods
Data sources
This whole-population cohort study was conducted using linked data from the Danish Civil Registration System (Pedersen, 2011), the Danish Psychiatric Central Register (Mors, Perto, & Mortensen, 2011), the Danish National Hospital Registry (Lynge, Sandegaard, & Rebolj, 2011), the Danish Medical Birth Register (Bliddal, Broe, Pottegård, Olsen, & Langhoff-Roos, 2018) (Knudsen & Olsen, 1998), the Danish Education Registers (Jensen & Rasmussen, 2011), and the Registers on Personal Income and Transfer Payments (Baadsgaard & Quitzau, 2011). Registry linkage is accurate because all residents of Denmark, including immigrants, have a unique personal identification number that is used in all national registers. The Danish Civil Registration System includes information to gender, date of birth, parental information and vital status (continuously updated) of all persons living in Denmark (Pedersen, Gøtzsche, Møller, & Mortensen, 2006). The Danish Psychiatric Central Register includes data on all people admitted to a psychiatric hospital for assessment, treatment, or both, since 1970 and people who had appointments with psychiatric outpatient services from 1995 onwards (Mors et al., 2011). In the Danish National Hospital Registry all inpatient treatments at non-psychiatric facilities are recorded from 1977 onwards, whereas outpatient and emergency room contacts are also recorded from 1995 onwards (Andersen, Madsen, Jørgensen, Mellemkjoer, & Olsen, 1999). Diagnoses are based on the International Classification of Diseases, eighth version (ICD-8) (before 1994), and tenth revision (ICD-10) (1994 onwards). The Danish Medical Birth Registry provides data on antenatal and delivery care services and health of newborns (Knudsen & Olsen, 1998). The Danish Education Registers contain individual-level information on educational enrolment status, completed levels of education, and exams (Jensen & Rasmussen, 2011). The Income Statistics Register includes information on salaries, entrepreneurial income, taxes, public transfer payments, capital income, private pension contributions, and payouts (Baadsgaard & Quitzau, 2011).
Study population
We identified all persons born in Denmark between Jan 1, 1991 and December 31, 2011 with complete linkage available for both parents (N=1,499,191). After the exclusion of persons with missing values on maternal smoking status (N=136,887), death, emigration or diagnosis of autism before 1 year of age or before 1992 (N=67,398), the study population included 1,294,906 persons covering 993,301 siblings nested within 728,271 families. Individuals were followed from the age of 1 until the diagnosis of interest, death, emigration, or December 31, 2012, whichever occurred first. The study was approved by the Danish Data Protection Agency. The investigators were blind to the identity of participants, and as the study did not result in any contact with the participants, no written informed consent was required.
Psychiatric Diagnoses
We identified all persons diagnosed with autism spectrum disorders (ICD-8 codes: 299.00, 299.01, 299.02 and 299.03; ICD-10 codes: F84.0, F84.1, F84.5, F84.8, and F84.9). We identified parental history of any psychiatric diagnosis (F00-F99) prior to the child’s birth and reported co-occurring attention deficit hyperactivity disorder (ADHD) (ICD-8 codes: 308.01; ICD-10 codes: F90) and intellectual disability (ID) (ICD-8 codes: 310.00 – 315.01; ICD-10 codes: F70 - F79.99) in persons with autism.
Maternal Smoking in Pregnancy
Information on maternal smoking in pregnancy was available from 1991 onward, as reported at the first antenatal visit derived from the Danish Medical Birth Register. All women are asked by the midwife at their first antenatal visit whether they had ever smoked in the present pregnancy. For analyses, we focused on a dichotomous variable, comparing women who ever smoked in pregnancy (at the first visit to the midwife stated that they were current smokers or reported to have stopped smoking during the first trimester at the beginning of the second trimester) versus women who at the visit to the midwife stated that they had never smoked in the present pregnancy. We combined information on stopping smoking in pregnancy with current smokers because of evidence that self-report of quitting smoking in pregnancy is unreliable (England et al., 2007).
Statistical Analyses
We used Cox proportional survival analysis to estimate the magnitude of the associations between maternal smoking in pregnancy and offspring autism. All models calculated Hazard Ratios (HRs) for time to autism diagnosis across different lengths of follow-up across the cohort, and used robust standard errors for the 95% confidence intervals (CIs) to account for familial clustering. All statistical analyses were conducted in SAS software version 9.3 (SAS Institute, Cary, NC, USA). We included all persons regardless of missing data, by treating missing education as its own category (3–4%, see Table 1) and by assuming that other missing data reflected absence; assuming parity of 1 (< 0.01%) and low/no income (< 0.03% for maternal and < 0.35% paternal).
Table 1.
Characteristics of Danish Persons Born 1991–2012 by Maternal Smoking in Pregnancy and Diagnosis of Autism Spectrum Disorder
| Maternal Smoking in Pregnancy | Autism Spectrum Disorder Diagnosis | |||
|---|---|---|---|---|
| Yes N = 278 981 | No N = 1 015 925 | Yes N = 13 547 | No N = 1 281 359 | |
| Gender | ||||
| Female | 135 820 (48.68%) | 495 371 (48.76%) | 2 906 (21.45%) | 628 285 (49.03%) |
| Male | 143 161 (51.32%) | 520 554 (51.24%) | 10 641 (78.55%) | 653 074 (50.97%) |
| Parity | ||||
| 1 | 120 314 (43.13%) | 445 581 (43.86%) | 6 755 (49.86%) | 559 140 (43.64%) |
| 2 | 99 816 (35.78%) | 386 856 (38.08%) | 4 706 (34.74%) | 481 966 (37.61%) |
| 3 | 42 413 (15.20%) | 138 663 (13.65%) | 1 564 (11.54%) | 179 512 (14.01%) |
| 4 | 12 050 (4.32%) | 31 836 (3.13%) | 375 (2.77%) | 43 511 (3.40%) |
| >=5 | 4 388 (1.57%) | 12 989 (1.28%) | 147 (1.09%) | 17 230 (1.34%) |
| Birth Year | ||||
| 1991–1994 | 58 380 (20.93%) | 122 142 (12.02%) | 3’271 (24.15%) | 242 578 (18.93%) |
| 1995–1998 | 69 573 (24.94%) | 186 422 (18.35%) | 4’068 (30.03%) | 248’425 (19.39%) |
| 1999–2002 | 56 283 (20.17%) | 192 812 (18.98%) | 3 549 (26.20%) | 245’066 (19.13%) |
| 2003–2006 | 45 211 (16.21%) | 201 994 (19.88%) | 2 143 (15.82%) | 245’933 (19.19%) |
| 2007–2011 | 49 534 (17.76%) | 312 555 (30.77%) | 525 (3.88%) | 299’357 (23.36%) |
| Paternal Age (years) | ||||
| <=20 | 6 110 (2.19%) | 6 468 (0.64%) | 154 (1.14%) | 12 424 (0.97%) |
| 21–25 | 42 234 (15.14%) | 79 663 (7.84%) | 1 472 (10.87%) | 120 425 (9.40%) |
| 26–30 | 88 828 (31.84%) | 310 814 (30.59%) | 4 235 (31.26%) | 395 407 (30.86%) |
| 31–35 | 67 259 (24.11%) | 298 999 (29.43%) | 4 192 (30.94%) | 434 363 (33.90%) |
| >35 | 74 550 (26.72%) | 319 981 (31.50%) | 3 494 (25.79%) | 318 740 (24.88%) |
| Maternal Age (years) | ||||
| <=20 | 16 940 (6.07%) | 22 109 (2.18%) | 507 (3.74%) | 38 542 (3.01%) |
| 21–25 | 67 230 (24.10%) | 155 083 (15.27%) | 2 752 (20.31%) | 219 561 (17.14%) |
| 26–30 | 99 754 (35.76%) | 401 281 (39.50%) | 5 037 (37.18%) | 495 998 (38.71%) |
| 31–35 | 58 485 (20.96%) | 275 064 (27.08%) | 3 732 (27.55%) | 383 874 (29.96%) |
| >35 | 36 572 (13.11%) | 162 388 (15.98%) | 1 519 (11.21%) | 143 384 (11.19%) |
| Paternal Psychiatric Illness | ||||
| No | 242 131 (86.79%) | 949 186 (93.43%) | 11 715 (86.48%) | 1 179 602 (92.06%) |
| Yes | 36 850 (13.21%) | 66 739 (6.57%) | 1 832 (13.52%) | 101 757 (7.94%) |
| Maternal Psychiatric Illness | ||||
| No | 228 575 (81.93%) | 927 026 (91.35%) | 11 048 (81.55%) | 1 144 553 (89.32%) |
| Yes | 50 406 (18.07%) | 88 899 (8.65%) | 2 499 (18.45%) | 136 806 (10.68%) |
| Paternal Income | ||||
| 1. Quintile | 76 676 (27.48%) | 185 289 (18.24%) | 3 562 (26.29%) | 258 403 (20.17%) |
| 2. Quintile | 74 269 (26.62%) | 183 902 (18.10%) | 3 366 (24.85%) | 254 805 (19.89%) |
| 3. Quintile | 58 947 (21.13%) | 199 373 (19.62%) | 2 877 (21.24%) | 255 443 (19.94%) |
| 4. Qunitile | 42 778 (15.33%) | 215 372 (21.20%) | 2 125 (15.69%) | 256 025 (19.98%) |
| 5. Quintile | 26 311 (9.43%) | 231 989 (22.84%) | 1 617 (11.94%) | 256 683 (20.03%) |
| Maternal Income | ||||
| 1. Quintile | 70 155 (25.15%) | 189 035 (18.61%) | 3 582 (26.44%) | 255 608 (19.95%) |
| 2. Quintile | 82 004 (29.39%) | 176 836 (17.41%) | 3 451 (25.47%) | 255 389 (19.93%) |
| 3. Quintile | 61 129 (21.91%) | 197 909 (19.48%) | 2 749 (20.29%) | 256 289 (19.82%) |
| 4. Qunitile | 43 333 (15.53%) | 215 607 (21.22%) | 2 341 (17.28%) | 256 599 (20.03%) |
| 5. Quintile | 22 360 (8.01%) | 236 538 (23.28%) | 1 424 (10.51%) | 257 474 (20.09%) |
| Paternal Education | ||||
| Unknown | 10 415 (3.73%) | 39 194 (3.86%) | 492 (3.63%) | 49 117 (3.83%) |
| Elementary School | 971 (0.35%) | 4 744 (0.47%) | 42 (0.31%) | 5 673 (0.44%) |
| Secondary School | 234 911 (84.20%) | 651 211 (64.10%) | 9 797 (72.32%) | 876 325 (68.39%) |
| Tertiary education | 8 966 (3.21%) | 62 075 (6.11%) | 688 (5.08%) | 70 353 (5.49%) |
| University | 23 718 (8.50%) | 258 701 (25.46%) | 2 528 (18.66%) | 279 891 (21.84%) |
| Maternal Education | ||||
| Unknown | 6 263 (2.24%) | 35 368 (3.48%) | 318 (2.35%) | 41 313 (3.22%) |
| Elementary School | 1 153 (0.41%) | 8 667 (0.85%) | 67 (0.49%) | 9 753 (0.76%) |
| Secondary School | 233 763 (83.78%) | 587 808 (57.86%) | 9 623 (71.03%) | 811 921 (63.36%) |
| Tertiary education | 6 023 (2.16%) | 49 990 (4.92%) | 512 (3.78%) | 55 501 (4.33%) |
| University | 31 806 (11.40%) | 334 092 (32.89%) | 3 027 (22.34%) | 362 871 (28.32%) |
| Autism Spectrum Disorder | 3 882 (1.39%) | 9 665 (0.95%) | 13 547 | 0 |
We used three different modeling approaches to account for confounding influences: 1) a conventional adjusted model to estimate risk at the population level including terms such as parental education and psychiatric history, 2) a maternal cluster model adjusting for the family mean of maternal smoking in pregnancy, and 3) a discordant sibling model. We assumed that the family-based models would produce more valid estimates than the conventional, population-level model, because of the ability to account for unmeasured confounding at the family level. We included two common types of family-based models in case results were sensitive to approach.
In the conventional adjusted model, to account for temporal trends, we stratified the proportional hazards model by year of birth. We additionally included terms for sex, parity (1, 2, 3, 4, or ≥ 5), and both maternal and paternal variables for: age at childbirth (≤20, 20–25, 25–30, 30–35, ≥ 35 years), psychiatric history (yes or no), highest education at time of birth (unknown, elementary school, secondary school, tertiary education, and university), and income at time of birth (annual brutto income in quintiles).
For the maternal cluster model, we followed the suggestion of Begg & Parides in their model number 3, using a between and within-type model to disentangle familial and individual level effects of maternal smoking, treating the between-family measure as a confounder of the within-family measure of interest (Begg & Parides, 2003). To do so we further adjusted models for the familial mean exposure to maternal smoking and centered the individual smoking variable by subtracting the family mean. We included families with one person (offspring) as recommended because they contribute to the between-family estimates which are adjustment terms in our design (Begg & Parides, 2003). Because we treated maternal smoking as a dichotomous variable, the family mean is the proportion of siblings in the family for whom the mother smoked in pregnancy. In families consisting of one offspring, the individual deviation from the family mean will be 0. Since the individual measurement is replaced by its deviation from the familial-level mean, this new version of the individual smoking variable represents how much larger, or smaller, the individual measurement is compared to the proportion of siblings within the family who were exposed to maternal smoking in pregnancy. We interpreted the parameter on the individual smoking variable (the within family effect), assuming that adjusting for the between-family effect removed unmeasured confounding shared by siblings (Begg & Parides, 2003; Sjölander, Frisell, & Oberg, 2012).
For the discordant sibling model we applied stratified Cox regression models with a separate stratum for each set of maternal siblings, akin to conditional logistic regression, restricted to sets of maternal siblings nested in nuclear families (993,301 persons from 426,665 families), excluding persons without siblings in the datasets (N=301,605). Sibling comparisons adjust for all unmeasured factors that are constant within the nuclear family. The stratified Cox regression models using sibling data were adjusted for the same covariates as in the conventional models. To assess whether any differences in results were due to limiting the sample to sibling sets, we also performed the conventional analysis and maternal cluster model among the multiple-child families (restricted cohort).
We conducted exploratory analysis of autism sub-phenotypes using the same modelling approaches but with different dependent variables. We defined the sub-phenotypes as persons with autism with and without co-occurring attention deficit/hyperactivity disorder (ADHD) or intellectual disabilities.
Results
Of the 1,294,906 persons included in the study cohort, we censored 27,916 (2.2%) due to emigration (25,255) or loss to follow-up (496) or the competing risk of death (2,165). Among the 1,294,906 persons followed from 1992 to 2012, 278,981 (21.5%) had a report of maternal smoking in pregnancy and 13,547 persons were diagnosed with autism during the 12,973,139 person-years at risk. For 9% of persons, the mother started or stopped smoking across pregnancies (full detail in Supporting Information Table 1). The sample restricted to maternal sibling sets (59% of the familes) included 10,068 persons diagnosed with autism. Of these families, 48,297 families had siblings discordant for maternal smoking in pregnancy, among whom 582 families also had a child with an autism diagnosis.
Maternal smoking in pregnancy was more common among persons born in earlier birth years, to younger mothers and fathers, to parents with psychiatric illness and with lower income and education (Table 1). Persons with autism were more likely to be male, firstborn, and born in earlier years. Their parents were not substantially older than the population as a whole, but they were more likely to have psychiatric illness and lower income, and were less likely to have a university degree.
The risk of autism diagnosis was null in unadjusted models but elevated with maternal smoking in conventional adjusted models, with hazard ratios (HR) and 95% confidence limits of 1.17 (1.13–1.22) in the entire cohort (Table 2). The negative confounding removed was largely attributable to birth year, as expected given increasing autism diagnoses alongside decreasing maternal smoking temporal trends (results not shown). Findings were almost identical for the restricted cohort (multi-child families), with null unadjusted results and elevated conventional adjusted results of: 1.19 (1.14–1.25), suggesting similar underlying smoking-autism risk across single and multiple-child families. This association was attenuated in the maternal cluster model after accounting for family maternal smoking: for all families: 0.98 (0.88–1.09) and the maternal-sibling cohort: 0.98 (0.87–1.09), and also in the discordant sibling model: 0.86 (0.64–1.15).
Table 2.
Hazard Ratios between Maternal Smoking in Pregnancy and Autism Spectrum Disorder among Danish Persons Born 1991–2012
| Sample | Model | HR (95% CI) 1 |
|---|---|---|
|
Entire Cohort 1 294 906 Persons 728 271 Families 13 547 with autism1 |
Unadjusted | 0.99 (0.95–1.03) |
| Conventional Adjusted2 | 1.17 (1.13–1.22) | |
| Maternal Cluster Model3 | 0.98 (0.88–1.09) | |
|
Restricted Cohort 993 301 Persons 426 665 Maternal Sibling Sets 10 068 with autism1 |
Unadjusted | 1.00 (0.96–1.05) |
| Conventional Adjusted2 | 1.19 (1.14–1.25) | |
| Maternal Cluster Model3 | 0.98 (0.87–1.09) | |
| Discordant Sibling Model4 | 0.64–1.15) | |
HR=hazard ratio, CI=confidence interval.
Proportional hazards model stratified by year of birth, also adjusted for sex, parity, maternal and paternal age at birth, maternal and paternal psychiatric family history, maternal and paternal income, maternal and paternal education, with robust standard errors.
Maternal cluster model is the adjusted model, centering smoking by, and adjusting for, the family mean of maternal smoking in pregnancy.
Discordant sibling model is the adjusted model with the addition of stratification by maternal sibling sets.
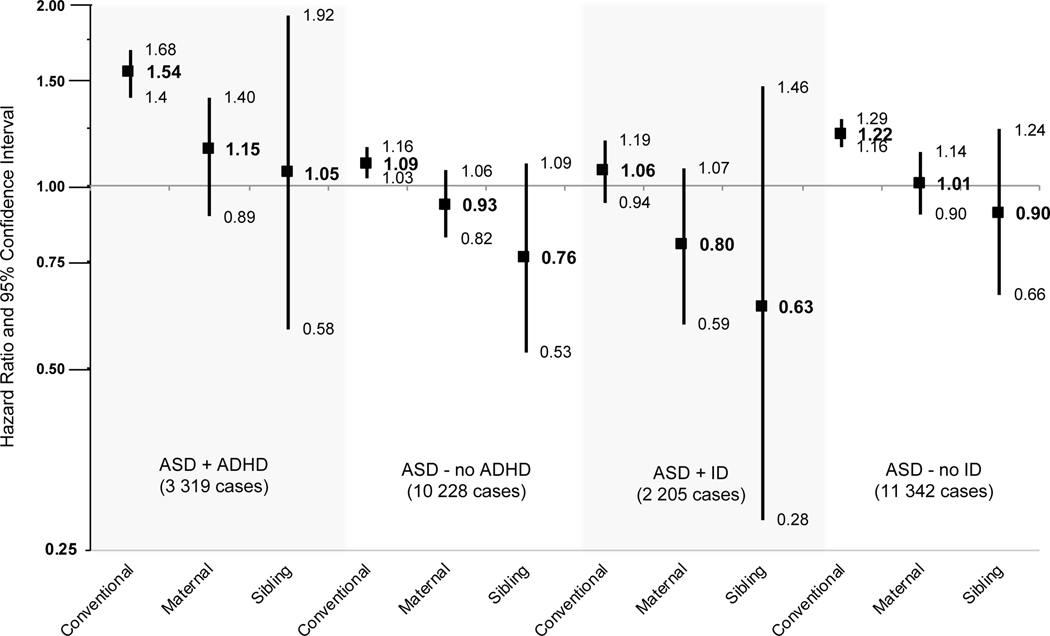
In the sub-phenotype analyses considering autism with or without a co-occurring diagnosis of ADHD, the population-level associations in the conventional adjusted models showed elevated risk with maternal smoking in pregnancy, and this association was stronger for autism+ADHD: 1.54 (1.40–1.68) compared to autism- no ADHD: 1.09 (1.03–1.16) (Table 3 and Figure 1). All of the family-based estimates (maternal cluster and discordant sibling estimates) for autism+ADHD and autism-no ADHD were substantially attenuated and did not support a risk relationship.
Table 3.
Hazard Ratios between Maternal Smoking in Pregnancy and Autism Spectrum Disorder with and without Co-occurring Intellectual Disability and Attention Deficit Hyperactivity Disorder among Danish Persons Born 1991–2012
| Sample | Model |
Hazard Ratio (95% Confidence Interval) |
|||
|---|---|---|---|---|---|
|
Co-occurring ADHD1 |
Co-occurring ID1 |
||||
| Autism+ADHD1 (N=3 319) |
Autism-no ADHD1 (N=10 228) |
Autism+ID1 (N=2 205) |
Autism-no ID1 (N=11 342) |
||
|
Entire Cohort 1 294 906 Persons 728 271 Families |
Conventional Adjusted2 | 1.54 (1.40–1.68) | 1.09 (1.03–1.16) | 1.06 (0.94–1.19) | 1.22 (1.16–1.29) |
| Maternal Cluster Model3 | 1.15 (0.89–1.40) | 0.93 (0.82–1.06) | 0.80 (0.59–1.07) | 1.01 (0.90–1.14) | |
| Autism+ADHD1 (N=2 502) |
Autism- no ADHD1 (N=7 566) |
Autism+ID1 (N=1 606) |
Autism- no ID1 (N=8 462) |
||
|
Restricted Cohort 993 301 Persons 426 665 Maternal Sibling Sets |
Discordant Sibling Model4 | 1.05 (0.58–1.92) | 0.76 (0.53–1.09) | 0.63 (0.28–1.46) | 0.90 (0.66–1.24) |
ADHD = attention deficit hyperactivity disorder, ID = intellectual disability.
Proportional hazards model stratified by year of birth, also adjusted for sex, parity, maternal and paternal age at birth, maternal and paternal psychiatric family history, maternal and paternal income, maternal and paternal education, with robust standard errors.
Maternal cluster model is the adjusted model with the addition of adjustment for the family mean of maternal smoking in pregnancy.
Discordant sibling model is the adjusted model with the addition of stratification by maternal sibling sets.
Figure 1.
Hazard ratios and 95% confidence intervals of maternal smoking in pregnancy and autism spectrum disorder with and without co-occurring intellectual disabilities (ID) and attention deficit hyperactivity disorder (ADHD), arising from three statistical designs: a conventional proportional hazards model stratified by year of birth, and adjusting for sex, parity, maternal and paternal age at birth, maternal and paternal psychiatric family history, maternal and paternal income, maternal and paternal education; a maternal cluster model including all covariates from the conventional model with the addition of adjustment for the family mean of maternal smoking in pregnancy; a discordant sibling model including all covariates from the conventional model with the addition of stratification by maternal sibling sets.
Considering sub-phenotypes of autism defined by the presence or absence of a co-occurring ID diagnosis, the risk associated with maternal smoking was slightly higher for autism without co-occurring ID (autism- no ID) compared to autism with ID (autism+ID), across all models, although the majority of confidence limits included the null (Table 3 and Figure 1). Only in the conventional adjusted model was the risk elevated above sampling error for maternal smoking and autism- no ID: 1.22 (1.16–1.29). Similar to the associations evaluating all persons with autism, the associations in autism with or without ID were reduced in magnitude following adjustment in the maternal cluster models, and reduced more so in the discordant sibling model.
Discussion
We examined the association between maternal smoking in pregnancy and autism to clarify whether this exposure poses risk independent of family social class or other shared family factors, especially among autism sub-phenotypes. Our design included a large, whole-population sample and information on maternal smoking well before the psychiatric diagnoses. Correlations between this self-report of maternal smoking and biomarkers of smoking-related methylation in an overlapping sample supports the validity of this measure (Hannon et al., 2018). While we excluded 9% of the original sample missing information on smoking status, these exclusions were largely due to a slow phase-in of smoking reporting in the early years of the study; in later years smoking information was 97% complete, see Table 5 in (Bliddal et al., 2018). Advancing beyond previous studies, we used siblings nested within families to account for such unmeasured family factors using two statistical approaches and did not find maternal smoking to exert significant risk on autism.
Autism represents a spectrum of phenotypes and it is hypothesized that specific causal influences may differ across the sub-phenotypes, for example only contributing to the risk for autism in combination with another neurodevelopmental disorder. We evaluated whether the impact of maternal smoking would differ when case groups were defined using information on co-occurring ADHD and ID. Autism susceptibility factors, generally, have been shown to differ for autism with and without comorbid intellectual disabilities (Polyak, Kubina, & Girirajan, 2015; Robinson et al., 2014; Sanders et al., 2015). While we observed a larger magnitude of risk across all models for autism- no ID compared to autism+ID (a pattern that is consistent with the prior literature: (Kalkbrenner et al., 2012; Lee et al., 2011), our results after accounting for family factors do not support that maternal smoking in pregnancy is a prominent risk factor for either of these groups. Similarly, we found that maternal smoking in pregnancy did not exert risk on autism with or without co-occurring ADHD after accounting for shared family factors.
Whether variation in risk magnitude across autism sub-phenotypic groups reflects an influence of maternal smoking on a discrete aspect of nervous system development, only observable in a narrowly defined sub-phenotype, cannot be fully resolved in these data. Such patterns could also be influenced by sampling error or different degrees of residual confounding across these sub-phenotypes.
A strength of our approach is in the use of 3 statistical designs, each adjusting for confounding through a different strategy. Confounding bias may be especially large for studies of maternal smoking in pregnancy and child neurodevelopment. Genetic liability for both smoking behaviors and neurodevelopmental impairment are strong and may involve neurotransmitter systems, thereby potentially linking the mother’s smoking with her child’s neurodevelopment without involving the direct toxic impacts of tobacco constituents. Other non-genetic aspects of a family, such as education and parenting, may also link maternal smoking and impaired neurodevelopment. Family-based designs offer a strategy to account for these factors beyond the reliance on measurement and statistical adjustment. We note that the confounding removed by conventional adjustment (where adjusted estimates were higher) was in a different direction than the confounding removed in the discordant sibling and maternal cluster models (where adjusted estimates were attenuated), suggesting that these different models addressed different types of confounding structure. We show substantial attenuation of risk due to maternal smoking on neurodevelopment after accounting for presumed unmeasured confounding at the family level, and others have also shown this effect (Gustavson et al., 2017; S. M. Meier et al., 2017; Sandra M. Meier et al., 2017; Obel et al., 2009; Skoglund et al., 2014).
Yet these sibling designs have some limitations, especially that results are largely driven by the families for whom the mother’s smoking behavior changed between pregnancies (26%; see Supporting Information Table 2). It is reasonable to imagine that this behavioral change may be accompanied by other changes. Discordant sibling analyses may inadvertently open the possibility for bias due to these hypothesized changes or other unshared factors across siblings (Frisell, Öberg, Kuja-Halkola, & Sjölander, 2012), factors which may be highly relevant in the development of autism (Schendel & Parner, 2016). Sibling designs may also incur bias by adjusting for shared mediators (Sjölander & Zetterqvist, 2017). Furthermore, the sibling stratified results would be expected to have decreased precision compared to the maternal cluster (between-within) model, as has been empirically shown (Sjölander, Lichtenstein, Larsson, & Pawitan, 2013), and observed here. The maternal cluster model of Begg and Parides adjusts for the mean maternal smoking pattern among siblings, assuming that by so doing, the confounding influences driving smoking behavior for the mother are reduced, to allow the individual’s exposure to be separately statistically resolved (Begg & Parides, 2003). Results from these maternal cluster models preserved statistical precision but may exhibit additional biases, such as the possibility of induced bias from opening a back-door confounding path from unmeasured non-shared confounders (Sjölander et al., 2012). Additionally, there is the possibility of inducing bias by adjusting for measured confounders that differ among siblings (e.g. birth year, parental age), as we have done (Sjölander et al., 2012) (Frisell, Öberg, Kuja-Halkola, & Sjölander, 2012). An important feature of the maternal cluster model is that single-child families are included and contribute to parameters through adjustment for the family mean. Inclusion of all families may increase the generalizability of findings over a discordant sibling model that only includes multiple-child families. In our sample we do not see evidence for differences that would suggest problems with generalizability or bias due to including or excluding single-child families (Table 2 and Supporting Information Table 3).
Overall, these family-based designs bring strengths in accounting for unmeasured confounding that may be strong in these associations. Yet alternate explanations for the observed attenuation include inflated bias from measurement error, unmeasured confounding influences not shared by siblings, and the influence of a small proportion of the sample for whom smoking behavior changed between pregnancies.
Another strength included the ability to examine the possibility of maternal smoking risk differences by autism sub-phenotypes, a deficit which was noted in the recent meta-analysis of this question by Rosen et al (Rosen et al., 2015). Our study had the needed information on multiple psychiatric diagnoses and large number of persons with co-occurring diagnoses to enhance statistical precision even for the smallest of the sub-phenotype groups. Additional strengths include the large number of persons representing an entire-country birth cohort without attrition, and the prospective measures of smoking prior to birth and well before the autism diagnosis, a feature also found important to results in the Rosen et al. meta-analysis (Rosen et al., 2015).
Our study focused on direct maternal smoking in pregnancy and results cannot be generalized to other routes of tobacco exposure, which may exert different degrees of risk through different pathophysiological mechanisms or timing. For example, father’s tobacco use prior to conception is capable of causing de novo germ cell mutations (Beal, Yauk, & Marchetti, 2017), and de novo mutations have been linked to autism (Sanders et al., 2015). Another exposure not addressed here is second-hand tobacco exposure to the mother during pregnancy, which could exert risk via methylation changes (Christensen et al., 2017; Reynolds et al., 2017). In line with this hypothesis, the role of father’s smoking at the population level (a presumed proxy of second-hand tobacco exposure) was shown to modify the association with maternal smoking in pregnancy on autism risk in a meta-regression (Jung et al., 2017). Furthermore, we could not consider grandparent tobacco use. Grandparent exposure may be biologically relevant because the gametes destined to become the offspring in this study were developing during the mother’s fetal development, and this hypothesis is supported by a report that maternal grandmother smoking, specifically, may increase autism risk (Golding et al., 2017). Our maternal cluster and discordant sibling designs control for maternal influences, and by doing so, may adjust for those exposures incurred when the mother was in utero. Lastly, these results cannot be extrapolated to second-hand smoke to the child postnatally.
In conclusion, these results suggest that maternal smoking in pregnancy is not a direct risk factor for autism independent of family-level factors. Nevertheless, maternal smoking in pregnancy exhibits a wealth of deleterious health impacts on the developing fetus and women should not be deterred from following existing public health and medical recommendations to not smoke in pregnancy (or any time).
Supplementary Material
Acknowledgments
Funding
Research reported in this publication was supported by the National Institute Of Environmental Health Sciences of the National Institutes of Health under Award Number R01ES026993. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support came from an unrestricted grant from the Lundbeck Foundation (iPSYCH).
Footnotes
Conflict Statement
The authors declare no financial or other conflicts or interests impacting this work.
References
- Agrawal A, Knopik VS, Pergadia ML, Waldron M, Bucholz KK, Martin NG, … Madden PAF (2008). Correlates of cigarette smoking during pregnancy and its genetic and environmental overlap with nicotine dependence. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 10(4), 567–578. 10.1080/14622200801978672 [DOI] [PubMed] [Google Scholar]
- Andersen TF, Madsen M, Jørgensen J, Mellemkjoer L, & Olsen JH (1999). The Danish National Hospital Register. A valuable source of data for modern health sciences. Danish Medical Bulletin, 46(3), 263–268. [PubMed] [Google Scholar]
- Baadsgaard M, & Quitzau J. (2011). Danish registers on personal income and transfer payments. Scandinavian Journal of Public Health, 39(7 Suppl), 103–105. 10.1177/1403494811405098 [DOI] [PubMed] [Google Scholar]
- Beal MA, Yauk CL, & Marchetti F. (2017). From sperm to offspring: Assessing the heritable genetic consequences of paternal smoking and potential public health impacts. Mutation Research/Reviews in Mutation Research, 773, 26–50. 10.1016/j.mrrev.2017.04.001 [DOI] [PubMed] [Google Scholar]
- Begg MD, & Parides MK (2003). Separation of individual-level and cluster-level covariate effects in regression analysis of correlated data. Statistics in Medicine, 22(16), 2591–2602. 10.1002/sim.1524 [DOI] [PubMed] [Google Scholar]
- Bliddal M, Broe A, Pottegård A, Olsen J, & Langhoff-Roos J. (2018). The Danish Medical Birth Register. European Journal of Epidemiology, 33(1), 27–36. 10.1007/s10654-018-0356-1 [DOI] [PubMed] [Google Scholar]
- Burstyn I, Sithole F, & Zwaigenbaum L. (2010). Autism spectrum disorders, maternal characteristics and obstetric complications among singletons born in Alberta, Canada. Chronic Diseases in Canada, 30, 125–134. (20946713). [PubMed] [Google Scholar]
- Chang Z, Lichtenstein P, & Larsson H. (2012). The effects of childhood ADHD symptoms on early-onset substance use: a Swedish twin study. Journal of Abnormal Child Psychology, 40(3), 425–435. 10.1007/s10802-011-9575-6 [DOI] [PubMed] [Google Scholar]
- Christensen S, Jaffar Z, Cole E, Porter V, Ferrini M, Postma B, … Cho YH (2017). Prenatal environmental tobacco smoke exposure increases allergic asthma risk with methylation changes in mice. Environmental and Molecular Mutagenesis, 58(6), 423–433. 10.1002/em.22097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio BM, Lahey BB, Turkheimer E, & Lichtenstein P. (2013). Critical need for family-based, quasi-experimental designs in integrating genetic and social science research. American Journal of Public Health, 103 Suppl 1, S46–55. 10.2105/AJPH.2013.301252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingson JM, Rickert ME, Lichtenstein P, Långström N, & D’Onofrio BM (2012). Disentangling the relationships between maternal smoking during pregnancy and co-occurring risk factors. Psychological Medicine, 42(7), 1547–1557. 10.1017/S0033291711002534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- England LJ, Grauman A, Qian C, Wilkins DG, Schisterman EF, Yu KF, & Levine RJ (2007). Misclassification of maternal smoking status and its effects on an epidemiologic study of pregnancy outcomes. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 9(10), 1005–1013. 10.1080/14622200701491255 [DOI] [PubMed] [Google Scholar]
- Flenady V, Koopmans L, Middleton P, Frøen JF, Smith GC, Gibbons K, … Ezzati M. (2011). Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet (London, England), 377(9774), 1331–1340. 10.1016/S0140-6736(10)62233-7 [DOI] [PubMed] [Google Scholar]
- Frisell T, Öberg S, Kuja-Halkola R, & Sjölander A. (2012). Sibling comparison designs: bias from non-shared confounders and measurement error. Epidemiology (Cambridge, Mass.), 23(5), 713–720. 10.1097/EDE.0b013e31825fa230 [DOI] [PubMed] [Google Scholar]
- Gilman SE, Gardener H, & Buka SL (2008). Maternal smoking during pregnancy and children’s cognitive and physical development: a causal risk factor? American Journal of Epidemiology, 168(5), 522–531. 10.1093/aje/kwn175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding J, Ellis G, Gregory S, Birmingham K, Iles-Caven Y, Rai D, & Pembrey M. (2017). Grand-maternal smoking in pregnancy and grandchild’s autistic traits and diagnosed autism. Scientific Reports, 7, 46179. 10.1038/srep46179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorog K, Pattenden S, Antova T, Niciu E, Rudnai P, Scholtens S, … Houthuijs D. (2011). Maternal smoking during pregnancy and childhood obesity: results from the CESAR Study. Maternal and Child Health Journal, 15(7), 985–992. 10.1007/s10995-009-0543-5 [DOI] [PubMed] [Google Scholar]
- Gustavson K, Ystrom E, Stoltenberg C, Susser E, Surén P, Magnus P, … Reichborn-Kjennerud T. (2017). Smoking in Pregnancy and Child ADHD. Pediatrics, 139(2), e20162509. 10.1542/peds.2016-2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon E, Schendel D, Ladd-Acosta C, Grove J, Hansen CS, Andrews SV, … Mill J. (2018). Elevated polygenic burden for autism is associated with differential DNA methylation at birth. Genome Medicine, 10, 19. 10.1186/s13073-018-0527-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultman CM, Sparén P, & Cnattingius S. (2002). Perinatal risk factors for infantile autism. Epidemiology, 13(4), 417–423. [DOI] [PubMed] [Google Scholar]
- Jaddoe VWV, Troe E-JWM, Hofman A, Mackenbach JP, Moll HA, Steegers EAP, & Witteman JCM (2008). Active and passive maternal smoking during pregnancy and the risks of low birthweight and preterm birth: the Generation R Study. Paediatric and Perinatal Epidemiology, 22(2), 162–171. 10.1111/j.1365-3016.2007.00916.x [DOI] [PubMed] [Google Scholar]
- Jensen VM, & Rasmussen AW (2011). Danish Education Registers. Scandinavian Journal of Public Health, 39(7 Suppl), 91–94. 10.1177/1403494810394715 [DOI] [PubMed] [Google Scholar]
- Jung Y, Lee AM, McKee SA, & Picciotto MR (2017). Maternal smoking and autism spectrum disorder: meta-analysis with population smoking metrics as moderators. Scientific Reports, 7, 4315. 10.1038/s41598-017-04413-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkbrenner AE, Braun JM, Durkin MS, Maenner MJ, Cunniff C, Lee L-C, … Daniels JL (2012). Maternal smoking during pregnancy and the prevalence of autism spectrum disorders, using data from the autism and developmental disabilities monitoring network. Environmental Health Perspectives, 120(7), 1042–1048. 10.1289/ehp.1104556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkbrenner AE, Schmidt RJ, & Penlesky AC (2014). Environmental chemical exposures and autism spectrum disorders: a review of the epidemiological evidence. Current Problems in Pediatric and Adolescent Health Care, 44(10), 277–318. 10.1016/j.cppeds.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik VS (2009). Maternal smoking during pregnancy and child outcomes: real or spurious effect? Developmental Neuropsychology, 34(1), 1–36. 10.1080/87565640802564366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen LB, & Olsen J. (1998). The Danish Medical Birth Registry. Danish Medical Bulletin, 45(3), 320–323. [PubMed] [Google Scholar]
- Langley K, Heron J, Smith GD, & Thapar A. (2012). Maternal and Paternal Smoking During Pregnancy and Risk of ADHD Symptoms in Offspring: Testing for Intrauterine Effects. American Journal of Epidemiology, 176(3), 261–268. 10.1093/aje/kwr510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BK, Gardner RM, Dal H, Svensson A, Galanti MR, Rai D, … Magnusson C. (2011). Brief report: maternal smoking during pregnancy and autism spectrum disorders. Journal of Autism and Developmental Disorders, 42(9), 2000–2005. 10.1007/s10803-011-1425-4 [DOI] [PubMed] [Google Scholar]
- Lynge E, Sandegaard JL, & Rebolj M. (2011). The Danish National Patient Register. Scandinavian Journal of Public Health, 39(7 Suppl), 30–33. 10.1177/1403494811401482 [DOI] [PubMed] [Google Scholar]
- Meier SM, Plessen KJ, Verhulst F, Mors O, Mortensen PB, Pedersen CB, & Agerbo E. (2017). Familial confounding of the association between maternal smoking during pregnancy and internalizing disorders in offspring. Psychological Medicine, 1–10. 10.1017/S0033291716003627 [DOI] [PubMed] [Google Scholar]
- Meier Sandra M., Mors O, & Parner E. (2017). Familial Confounding of the Association Between Maternal Smoking During Pregnancy and Schizophrenia. The American Journal of Psychiatry, 174(2), 187. 10.1176/appi.ajp.2016.16080898 [DOI] [PubMed] [Google Scholar]
- Mors O, Perto GP, & Mortensen PB (2011). The Danish Psychiatric Central Research Register. Scandinavian Journal of Public Health, 39(7 Suppl), 54–57. 10.1177/1403494810395825 [DOI] [PubMed] [Google Scholar]
- Obel C, Linnet KM, Henriksen TB, Rodriguez A, Järvelin MR, Kotimaa A, … Olsen J. (2009). Smoking during pregnancy and hyperactivity-inattention in the offspring--comparing results from three Nordic cohorts. International Journal of Epidemiology, 38(3), 698–705. 10.1093/ije/dym290 [DOI] [PubMed] [Google Scholar]
- Pedersen CB (2011). The Danish Civil Registration System. Scandinavian Journal of Public Health, 39(7 Suppl), 22–25. 10.1177/1403494810387965 [DOI] [PubMed] [Google Scholar]
- Pedersen CB, Gøtzsche H, Møller JO, & Mortensen PB (2006). The Danish Civil Registration System. A cohort of eight million persons. Danish Medical Bulletin, 53(4), 441–449. [PubMed] [Google Scholar]
- Polyak A, Kubina RM, & Girirajan S. (2015). Comorbidity of intellectual disability confounds ascertainment of autism: implications for genetic diagnosis. American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics: The Official Publication of the International Society of Psychiatric Genetics, 168(7), 600–608. 10.1002/ajmg.b.32338 [DOI] [PubMed] [Google Scholar]
- Rai D, Lewis G, Lundberg M, Araya R, Svensson A, Dalman C, … Magnusson C. (2012). Parental socioeconomic status and risk of offspring autism spectrum disorders in a Swedish population-based study. Journal of the American Academy of Child and Adolescent Psychiatry, 51(5), 467–476.e6. 10.1016/j.jaac.2012.02.012 [DOI] [PubMed] [Google Scholar]
- Reynolds LM, Magid HS, Chi GC, Lohman K, Barr RG, Kaufman JD, … Liu Y. (2017). Secondhand Tobacco Smoke Exposure Associations With DNA Methylation of the Aryl Hydrocarbon Receptor Repressor. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco, 19(4), 442–451. 10.1093/ntr/ntw219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson EB, Samocha KE, Kosmicki JA, McGrath L, Neale BM, Perlis RH, & Daly MJ (2014). Autism spectrum disorder severity reflects the average contribution of de novo and familial influences. Proceedings of the National Academy of Sciences of the United States of America, 111(42), 15161–15165. 10.1073/pnas.1409204111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen BN, Lee BK, Lee NL, Yang Y, & Burstyn I. (2015). Maternal Smoking and Autism Spectrum Disorder: A Meta-analysis. Journal of Autism and Developmental Disorders, 45(6), 1689–1698. 10.1007/s10803-014-2327-z [DOI] [PubMed] [Google Scholar]
- Sanders SJ, He X, Willsey AJ, Ercan-Sencicek AG, Samocha KE, Cicek AE, … State MW (2015). Insights into Autism Spectrum Disorder Genomic Architecture and Biology from 71 Risk Loci. Neuron, 87(6), 1215–1233. 10.1016/j.neuron.2015.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendel DE, & Parner E. (2016). Sibling Comparisons and Confounding in Autism Epidemiological Studies. JAMA Psychiatry, 73(3), 302–303. 10.1001/jamapsychiatry.2015.2661 [DOI] [PubMed] [Google Scholar]
- Sjölander A, Frisell T, & Oberg A. (2012). Causal Interpretation of Between-Within Models for Twin Research. Epidemiologic Methods, 1. 10.1515/2161-962X.1015 [DOI] [Google Scholar]
- Sjölander A, Lichtenstein P, Larsson H, & Pawitan Y. (2013). Between–within models for survival analysis. Statistics in Medicine, 32(18), 3067–3076. 10.1002/sim.5767 [DOI] [PubMed] [Google Scholar]
- Sjölander A, & Zetterqvist J. (2017). Confounders, Mediators, or Colliders: What Types of Shared Covariates Does a Sibling Comparison Design Control For? Epidemiology (Cambridge, Mass.), 28(4), 540–547. 10.1097/EDE.0000000000000649 [DOI] [PubMed] [Google Scholar]
- Skoglund C, Chen Q, D’Onofrio BM, Lichtenstein P, & Larsson H. (2014). Familial confounding of the association between maternal smoking during pregnancy and ADHD in offspring. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 55(1), 61–68. 10.1111/jcpp.12124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA (2004). Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicology and Applied Pharmacology, 198, 132–151. 10.1016/j.taap.2003.06.001 [DOI] [PubMed] [Google Scholar]
- Soothill PW, Morafa W, Ayida GA, & Rodeck CH (1996). Maternal smoking and fetal carboxyhaemoglobin and blood gas levels. British Journal of Obstetrics and Gynaecology, 103, 78–82. (8608103). [DOI] [PubMed] [Google Scholar]
- Tang S, Wang Y, Gong X, & Wang G. (2015). A Meta-Analysis of Maternal Smoking during Pregnancy and Autism Spectrum Disorder Risk in Offspring. International Journal of Environmental Research and Public Health, 12(9), 10418–10431. 10.3390/ijerph120910418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A, Fowler T, Rice F, Scourfield J, van den Bree M, Thomas H, … Hay D. (2003). Maternal smoking during pregnancy and attention deficit hyperactivity disorder symptoms in offspring. The American Journal of Psychiatry, 160(11), 1985–1989. 10.1176/appi.ajp.160.11.1985 [DOI] [PubMed] [Google Scholar]
- Thapar A, & Rutter M. (2009). Do prenatal risk factors cause psychiatric disorder? Be wary of causal claims. The British Journal of Psychiatry: The Journal of Mental Science, 195(2), 100–101. 10.1192/bjp.bp.109.062828 [DOI] [PubMed] [Google Scholar]
- Tran PL, Lehti V, Lampi KM, Helenius H, Suominen A, Gissler M, … Sourander A. (2013). Smoking during Pregnancy and Risk of Autism Spectrum Disorder in a Finnish National Birth Cohort: Smoking during pregnancy and risk of Autism. Paediatric and Perinatal Epidemiology, 27(3), 266–274. 10.1111/ppe.12043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser JC, Rommelse N, Vink L, Schrieken M, Oosterling IJ, Gaag RJ, & Buitelaar JK (2012). Narrowly versus broadly defined autism spectrum disorders: differences in pre- and perinatal risk factors. Journal of Autism and Developmental Disorders, 43(7), 1505–1516. 10.1007/s10803-012-1678-6 [DOI] [PubMed] [Google Scholar]
- Zhu JL, Olsen J, Liew Z, Li J, Niclasen J, & Obel C. (2014). Parental smoking during pregnancy and ADHD in children: the Danish national birth cohort. Pediatrics, 134(2), e382–388. 10.1542/peds.2014-0213 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.