Abstract
Context: Virtual and augmented imagery are emerging technologies with potential to reduce the severity and impact of neuropathic pain in people with spinal cord injury (SCI).
Objective: We aimed to identify and discuss studies using virtual and augmented reality applications for the management of neuropathic pain in people with spinal cord injury.
Methods (data sources, data extraction): A systematic literature search was conducted using PRISMA scoping review guidelines. Articles were searched in PubMed, Embase and Web of Science databases using search terms relating to SCI, virtual and augmented reality and neuropathic pain. With no strong evidence for visual imagery in the treatment of pain in SCI patients, we selected exploratory, feasibility and more rigorous methodologies such as randomized controlled trials and case-control studies. We only selected studies evaluating the effects of visual imagery on neuropathic pain at or below the spinal cord injury level.
Results: Of 60 articles located, we included nine articles involving 207 participants. All studies were exploratory using head-mounted devices or 3D and 2D screens with virtual walking or limb movement imagery. Outcomes included pain sensitivity, motor function and body ownership. Eight of the nine studies reported significant reductions in neuropathic pain intensity. However, given small sample sizes in all studies, results may be unreliable.
Conclusion: Although the number of studies and individual sample sizes are small, these initial findings are promising. Given the limited options available for the effective treatment of neuropathic SCI pain and early evidence of efficacy, they provide valuable incentive for further research.
Keywords: Spinal cord injury, Virtual reality, Neuropathic pain, Visual illusion
Introduction
Spinal cord injury (SCI) is a life-changing event that causes not only a debilitating loss of sensorimotor function, but also unremitting neuropathic pain in many patients. Although our understanding of neuropathic pain in these instances is largely derived from preclinical studies, neuropathic pain below and at the level of spinal injury is thought to be due to exaggerated neuronal responses to stimuli below normal activation thresholds.1 This hyperexcitability results from altered expression of various receptors, ion channels, glial cell stimulation and decreased endogenous inhibitory neurons in the dorsal horn.2 The mechanisms of neuropathic pain below the neurological level of SCI are less clear. Current theories for explaining the mechanisms underlying neuropathic pain in people with these conditions include neurophysiological changes at a supraspinal level such as neuroplasticity with reorganization of the primary somatosensory cortex.3–5 As well as changes in cortical organization, neuropathic pain is also associated with functional changes in both cortex and thalamus. Animal studies show that neuropathic pain due to SCI is associated with abnormal patterns of firing in the thalamus.6,7 It is not surprising then that currently available pharmacological treatments are only of partial benefit.8,9 Much of the difficulty in obtaining satisfactory relief is due to these complex mechanisms involving multiple levels of the neuroaxis. These include functional and structural changes in somatotopic regions of the central nervous system and psychological factors such as perceptions which influence neuropathic pain.4,10
Given the lack of benefit provided by current treatments, there is need to find alternative therapeutic approaches such as the use of virtual reality (VR) devices. Technological developments in virtual imagery, including VR, offer an alternative approach towards the treatment of several medical and psychological conditions. These techniques show promise in improving symptoms and reducing pain in both the short and long term. VR is a simulated construction of a 3D environment using computer technology.11 While initial VR systems used computer screen technology, current VR systems include head-mounted devices (HMD) with 3D-enabled glasses, sensory input devices, headphones for noise-cancelling, sound and music, head and/or body-tracking sensors and additional input devices such as joysticks and data gloves.12 Together this multisystem setup forms a realistic multisensory experience. Because of these features, over the previous decade, VR technology has been taken from entertainment businesses to clinical medicine. Here, researchers and clinicians have investigated and used VR technologies for physical rehabilitation, pain management, psychiatric treatment as well as surgical training and anatomical education.12
The processes underlying the effect of VR on pain have been broadly divided into two main types: distraction and neuroplasticity.13 These processes that are believed to contribute to the analgesic effect of VR have quite different modes of action. Distraction refers to the short-term diversion of attention away from pain towards an alternative stimulus. In this context, VR may act directly and indirectly to reduce pain by “hijacking” of attention, emotion and memory as well as visual, auditory and touch senses.14 Neuroplasticity is the other putative process contributing to the effects of VR and refers to long-term structural changes in neuron populations. This may occur negatively due to a stroke, or positively following long-term practice of a skill such as playing a musical instrument. In the context of VR, it may involve interactive real-time simulations of scenes or activities, leading to neuroplastic alterations in sensory and motor brain regions.15
Assuming that below-level neuropathic pain in SCI is considered to have a strong contribution from supraspinal mechanisms, it may be that this type of SCI pain would benefit most from VR or other types of visual imagery. Thus, based on findings showing changes in cortical reorganization and function, several studies have investigated the use of imagery and visualization and its effect on neuropathic pain in people with SCI.4,16 Although results from such studies show promise, the use of imagery and visualization are not reliable. The development of more immersive VR has led to the recent use of this technology to impact and potentially modify central pain pathways. Here, there is strong evidence for the benefits of VR in the treatment of injurious and procedural acute pain,17 and more recently for chronic pain disorders such as low back pain and fibromyalgia.18,19 However, research into the use of VR in people with neuropathic pain, especially below-level SCI pain is currently still in its infancy. Augmented reality (AR) technologies are also becoming available. Here, a handful of studies have examined its benefit with amputee patients with phantom limb pain (PLP)20 and most recently with neuropathic upper limb pain.21 Consequently, continued research in these areas using VR and AR has the potential to improve the physical and emotional wellbeing of people with SCI as well as their overall quality of life.
Thus, the purpose of this scoping review is to first, search and identify studies using both immersive and non-immersive VR and AR applications investigating both long and short-term treatment effects, specifically for the management of neuropathic pain in people with SCI. Second, to review and discuss advantages and limitations of current VR technologies in SCI clinical settings in order to determine directions for future studies.
Methods
This scoping review was conducted using a search strategy framework defined by the recently published PRISMA guidelines for scoping reviews.22 PubMed, Embase and Web of Science databases were searched in May 2018 for articles published or in press up to the end of April 2018 using the keywords shown in Table 1. Study selection criteria were; publication in full and English and non-English articles (to reduce cultural and language bias). Abstracts of all articles were read and those showing the use of VR and AR in adults with SCI and neuropathic pain were read in full. We accepted articles where:
At least half the sample included para/tetraplegic and complete/incomplete SCI patients
Use any form of VR and AR therapy
Included quantitative outcome measures assessing the effects of VR and AR on pain intensity at or below the SCI level
Non-peer reviewed journals were not accepted for this scoping review
Single case studies were not accepted for this scoping review
Table 1. Keywords used in the location of articles investigating the use of visual illusion and virtual or augmented reality to reduce pain in people with spinal cord injury.
| PubMed | Web of Science | EMBASE |
|---|---|---|
| Keyword limits | Keyword limits | Keyword limits |
| Advanced search – “Title/Abstract” Boolean search – “AND” |
Basic search – “Topic” Boolean search “AND” |
Multifield search – “Abstract” Boolean search “AND” Exclude Medline Journals |
| Keywords | ||
| ||
Due to the novelty of this type of research and the lack of strong evidence relating to the use of VR and AR in the treatment of pain in SCI patients, we selected exploratory, feasibility and pilot studies in addition to more rigorous methodologies such as randomized controlled trials and case-control studies.
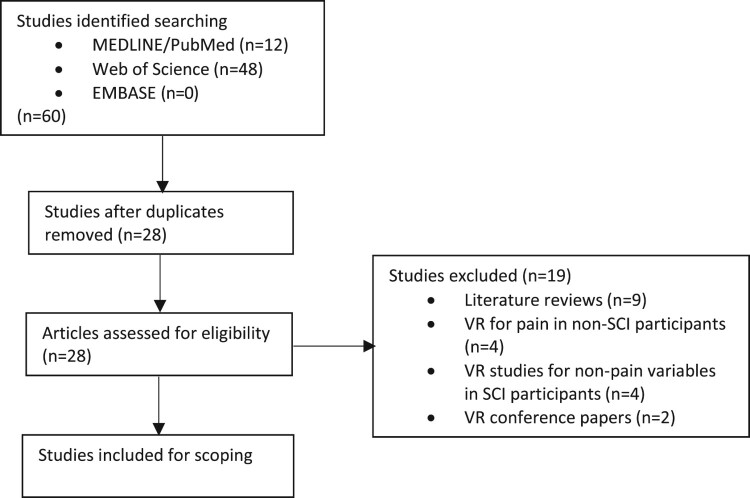
Using the above search protocols, we located 60 articles relating to the use of VR or AR in people with SCI pain (Fig. 1). After removing duplicates (n-32), literature reviews (n-9), VR developmental studies in healthy samples (n-2), VR for pain in non-SCI patients (n-2), VR for only motor skills in SCI patients (n-2), VR for mindfulness training in SCI patients (n-1), VR for body ownership only in SCI patients (n-1), VR and cortical activation in SCI patients (n-1) and a cross-sectional attitudes study (n-1), we identified nine articles of interest (Table 2). We found no studies investigating the use of AR for pain relief in SCI patients. All selected studies used virtual walking or limb movement applications in samples including para/tetraplegic and complete/incomplete SCI. Outcomes related not only to the effects of VR on pain intensity but also other sensory factors such as touch, proprioception, and body ownership in addition to alterations in motor function. All accepted studies investigated changes in sensory, motor and psychological function. All but one study report reductions in neuropathic pain intensity.
Figure 1.
Flow diagram showing the identification, eligibility and inclusion of articles for review.
Table 2. Characteristics of studies assessing the effects of VR on neuropathic pain in people with spinal cord injury. Abbreviations: BPI, Brief Pain Inventory; HMD, head-mounted device; NP, neuropathic pain; NRS, Numerical Rating Scale; QST, quantitative sensory testing; SCI, spinal cord injury; tDCS, transcranial direct current stimulation; TENS, transcutaneous electrical nerve stimulation; VR, virtual reality; VAS, Visual Analogue Scale.
| First author | Date | Objectives | Study type | Sample | Modality (VR/AR) | Interface | VR treatment application | Dose | Key Findings |
|---|---|---|---|---|---|---|---|---|---|
| Pozeg P | 2017 | Changes in body ownership and neuropathic pain | Case-control |
|
VR | HMD |
|
2×2 repeated measure (60 s) |
|
| Donati A | 2016 | Explore long-term motor and sensory effects of brain-machine interfaces in SCI patients with paraplegia | Exploratory single cohort | 8 chronic SCI (paraplegia) patients |
|
Robotic device HMD |
Virtual legs and arm movement | 12 months (663 h – neuro-rehab training) | Neurological improvements in
|
| Jordan M | 2016 | Investigate effects of VR walking on below-level SCI pain compared to at level SCI pain | Single cohort (randomized selection from larger sample) | 15 of 35 SCI | VR | 3D monitor |
|
20-minute video |
|
| Roosink M | 2016 | Assess immediate effect of interactive virtual feedback and motor imagery after SCI | Exploratory Single cohort | 9 SCI | VR | 3D screen | Interactive and static virtual walking (forward and backward) in a virtual scene | X 2 sessions (90 min each) 1 week apart |
|
| Villiger M | 2013 | Assess effect of VR-augmented movement on motor function and neuropathic pain | Single cohort | 14 Incomplete SCI | VR | 3D monitor | Foot and leg movement (foot and hand sensors) using 4 VR movement exercises | 16–20 sessions (45 min), 4–5 times / week for 4 weeks |
|
| Moseley L | 2007 | Investigate effect of visual illusion for the treatment of neuropathic SCI pain | Exploratory single cohort | 5 SCI (paraplegia) | VR | 2D screen |
|
|
|
| Kumru H | 2013 | Assess whether pain relief with tDCS + VR interventions is accompanied by changes in thermal QST scores | Case-control |
|
VR | 2D screen |
|
|
|
| Soler M | 2010 | Evaluate analgesic effect of tDCS + VR interventions applied in isolation or in combination | Single cohort | 40 SCI patients with NP | 2D screen |
|
|
|
|
| Özkul C | 2014 | Compare effects of VR and TENS on pain intensity, quality and functional capacity | Single cohort | 24 SCI patients with NP | 2D screen |
|
|
|
Results
Effectiveness of VR modalities for SCI-related neuropathic pain
Head-mounted devices
Two studies used HMD units.23,24 Most recently, in a randomized repeated measures case-control study, Pozeg and colleagues examined changes in body ownership and neuropathic pain in paraplegic patients. Using both immersive virtual leg and body illusions in combination with visual-tactile stimulation on the patient's back and the participant's virtual leg, the authors showed a significant neuropathic pain reduction when stimulated simultaneously (P = 0.04).24 In the second, more complex study, Donati and co-workers explored the effects of a long-term neurorehabilitation program for eight people with complete paraplegia that combined immersive VR training, visual-tactile feedback and walking with robotic lower limb exoskeleton.23 Following 12 months of training, all participants showed reductions in neuropathic pain intensity (P-0.01, fine/crude touch (P-0.05) and proprioception across multiple dermatomes. Encouragingly, participants also recovered voluntary motor control in muscles below SCI levels (P-0.01) to the extent that half of the sample were reclassified as incomplete paraplegia. As with Pozeg's study, but in a long-term rehabilitation setting, visual stimulation of a body part within a VR environment is hypothesized to most influence sensory improvement.
Computer monitors and screens
The remaining seven studies used either 3D monitors,25,26 a large 3D screen27 or a large 2D screen.16,28–30 All seven studies primarily investigated the effects of virtual walking on neuropathic pain at and/or below the level of SCI. Jordan and Richardson examined the effects of virtual walking on neuropathic pain in 15 tetraplegic and paraplegic SCI patients compared to virtual wheeling.25 The greatest decreases in pain, regardless of location were found using virtual walking compared to virtual wheeling (P = 0.03). Given the associations between below level neuropathic SCI pain and altered spinal and supraspinal mechanisms,31,32 the authors hypothesized that below level pain would respond to VR interventions more positively than at level pain. However, although not significant, reductions in pain severity occurred in people with at level neuropathic pain (P = 0.08). Although surprising, these results do suggest that neuropathic pain in any location may be due to altered supraspinal mechanisms as well as additional spinal and peripheral mechanisms.33
Moseley investigated the effects of virtual walking (patient's body aligned in a mirror above a film of a lower body walking), guided imagery (psychologist-run script guiding patients through a “pain-free” scene) and watching a film (animated comedy) on four paraplegic SCI patients.16 Results showed that greatest decreases in pain were observed with virtual walking (mean 42mm reduction on 0–100 visual analogue scale) (VAS) compared to 18mm reduction for guided imagery and only 4mm reduction for watching a film.16 Importantly, in the second part of this study, the clinical applicability of virtual walking was examined where participants performed this intervention for 10 min over 15 consecutive days. Here, the mean decrease in pain over this time-period was 53mm, but more significantly, this pain relief continued where at three-month follow-up, pain-relief continued with a mean VAS reduction of 43mm.
Alternatively, Özkul and colleagues compared the effect of Moseley's virtual imagery protocols with transcutaneous electrical nerve stimulation (TENS) on neuropathic pain intensity in SCI patients with neuropathic pain.29 In this randomized cross-over trial, ten 15-minute sessions were applied for two weeks, separated by a one-week washout period. Although decreases in neuropathic pain occurred immediately after both applications, only TENS showed significant decreases in pain after two weeks (P = 0.04). Interestingly however, virtual imagery application use showed significant improvements in specific neuropathic pain symptoms such as “hot” (P = 0.05), “sharp” (P = 0.02) and “unpleasant” (P = 0.03), in addition to improvements in functional capacity (P = 0.04) compared to TENS.
Roosink and colleagues hypothesized that motor imagery immediately after virtual walking would be more vivid and require less mental effort. In nine SCI patients, using both forward and backward virtual walking, the authors showed that motor imagery vividness and speed were significantly higher compared to static walking scenes.27 However, in contrast with other studies, there was no effect on neuropathic pain either at or below the lesion level. Although these results are surprising, it may be that motor imagery takes time to practice and that the effort and possible frustration in visualising forward and backward walking may negate the positive effect of virtual walking on neuropathic pain. It may also be realistic to assume that therapeutic motor imagery takes time and training: for example, chronic regional pain syndrome studies show motor imagery is a practice that involves mental training, which in turn promotes changes in cortical activity and as such reorganization in these brain regions.34,35 However, studies additionally show that patients with SCI have more difficulty in imagining movements compared to healthy controls, thus underlining a lack of immediate effect and the requirement for training.36,37
Effectiveness of virtual modalities in combination with other non-pharmacological therapies
Two studies using 2D screens investigated the combined effects of VI and other non-pharmacological treatments. Both examined virtual walking plus transcranial direct current stimulation (tDCS). First, in a sham-controlled, double-blind parallel group study with 39 SCI patients, Soler and colleagues showed that over ten 20-minute treatment sessions over two weeks, the combination of virtual walking (Moseley's methods described above) and tDCS reduced neuropathic pain intensity significantly more than any single intervention (P = 0.008). At the 12-week follow-up, only the patients in the combined treatment group showed continued significant reductions in pain intensity (P = 0.05). More recently, the same research group used the same VI and tDCS protocols in 18 SCI patients with neuropathic pain, 20 SCI patients without neuropathic pain and 14 healthy controls. They further tested whether pain relief was accompanied by changes in experimental evoked heat potentials and quantitative thermal testing.28 Again, in ten 20-minute treatment sessions over two weeks, SCI patients reporting nearly 50% reductions in neuropathic pain after receiving tDCS and VI also showed significantly greater reductions in evoked heat potentials (P < 0.0001) compared to SCI (no pain) and healthy controls.
Villiger and colleagues examined the effect of VR-augmented training of observed and performed leg movement on limb function and neuropathic pain in 14 established incomplete SCI patients.26 With VR systems using limb sensors, participants performed foot and leg movements, balance, strength and mobility exercises augmented by four different VR games. After between 16 and 20 forty-five-minute sessions over four weeks, results showed significant decreases in pain intensity (P-0.004) and unpleasantness (P = 0.004) as well as improvements in locomotion (P-0.005) and balance (P = 0.005). These findings remained stable for up to 16 weeks after the study. Concerning pain outcomes, these results again show that in addition to continued pain relief after treatment, pain relief may also increase over the course of training, suggesting again the possibility of cortical reorganization.
Short-term versus long-term effects of VR on neuropathic pain
Short-term effects of VR on neuropathic SCI pain
Results of studies measuring immediate effects of VR for reducing neuropathic pain in SCI patients are unconvincing. In this review, only Pozeg and colleagues show significant decreases in pain severity when applying VR alone (Table 2). Again, methodologies between these studies differed. Pozeg and colleagues used virtual leg illusion together with actual and virtual tactile stimuli, Jordan and Richardson used interactive virtual walking and wheeling on a 3D monitor, while Roosink and co-workers used a walking avatar on a large 3D screen.
However, differences in results may be due primarily to the type of VR application used to create visual illusion. By way of illustration, Pozeg used a fully immersive head-mounted display compared to Jordan25 and Roosink27 who used 3D screen applications. Thus, fully-immersive VR may provide greater immediate distraction from neuropathic pain. The use of immersive VR has been further investigated in chronic pain and phantom limb patients. This is supported by the findings of studies using immersive VR in other chronic pain conditions such as phantom limb pain. For example, Jones and colleagues investigated the impact of fully-immersive and interactive VR fantasy landscapes on 30 chronic pain patients. After one session, participants reported significant reductions in pain severity (P = 0.001).18 Equally, studies examining the use of immersive VR applications in PLP patients also show significant pain relief after both single and multiple sessions.38–40
Long and mid-term effects of VR on neuropathic SCI pain
The greatest reductions in neuropathic pain intensity were shown in four studies examining mid-to-long-term VR effects, where training over a longer time-period shows greater reductions in neuropathic pain intensity (Table 2). In three studies, VR was used in combination with another treatment modality, namely tDCS (two weeks)28,30 and exoskeleton muscle training (one year),23 while one study used VR alone.26 Concerning the former studies, the effect of VR combined with tDCS over multiple sessions show greatest reductions in neuropathic pain. Concerning the Soler study, this improvement remained 12 weeks after treatment.
Alternatively, Donati and colleagues with their use of an immersive VR avatar (arm and leg movement) together with a lower-limb exoskeleton show significant decreases in below-level neuropathic pain over a one-year period (663 h).23 Although sampling and VR applications were not comparable, Villiger and co-workers26 and Moseley16 showed increasing reductions in neuropathic pain over four and three weeks of VR training respectively. More encouragingly, follow-up data from both studies showed continued improvement at three and four months after treatment, suggesting long-term neuroplastic changes in central pain pathways.23,41 Combined findings from the long and mid-term studies in this review suggest that continued exposure to VR training over weeks or longer initiates cortical functional plasticity that in turn may be associated with decreases in neuropathic pain. Indeed, Donati and colleagues using longitudinal EEG recordings over one year of neurorehabilitation showed both the emergence and subsequent activation in the leg representation region of somatosensory and motor cortices.23
Discussion
Findings from our scoping review of nine studies suggest that VR is an effective analgesic intervention for at and below-level neuropathic SCI pain with short, medium and long-term effects especially in combination with tDCS. However, although these results are promising, the use of VR in SCI settings remains exploratory. As such, more rigorous investigations are needed not only to verify these experimental findings in clinical settings but additionally to determine differences in neurophysiological mechanisms between short and long-term VR and AR use. Different VR techniques show different findings and implications for potential use as discussed below.
Visual display units
Head mounted displays
Positive results were obtained with the use of HMDs and they have been shown to benefit people with other chronic pain conditions such as fibromyalgia. For example, Jones and colleagues showed significant decreases in pain during a short 5-minute session (P < 0.001) invoking a slow interactive journey through virtual landscapes that included hills, snow scenes, caves and animals.18 Again, using a single VR session (15 min), Wiederhold and colleagues exposed six chronic pain patients to calming environments such as beaches, mountains and forests with both relaxing music and natural sound effects (wind, swaying branches etc.).42 Here, all participants reported a reduction in pain intensity with significance ranging from P < 0.05 to P < 0.001 in reported outcomes). In another exploratory study, Jin and co-workers showed that when people with chronic pain play an interactive VR game (Cryoslide® – sliding in an ice world), they again reported significant decreases during the experience (P < 0.001).43 It should be noted that although significant pain relief occurred in these single session studies during VR immersion, these improvements did not continue after interventions. It is also interesting to note significant differences in HMD VR protocols between SCI and chronic pain participant studies. SCI studies use interactive virtual limb illusion and movement, while the chronic pain studies use more ambient environments that include both visual and auditory stimuli. Additionally, observation of these exploratory data suggests there may be differences in pain relief between these different immersive VR protocols. However, larger, more randomized trials are needed to address these observations.
Computer monitors and screens
Seven of the nine located studies used 3D and 2D computer monitors or screens, two of which use VR in combination with tDCS. Although the heterogeneity of methodologies between studies makes it difficult to compare different these VR approaches, all but one study27 show borderline reductions pain intensity with stand-alone VR (P = 0.08 to P = 0.05), compared to combined treatments (P < 0.005 to P = 0.004). More convincingly, these studies show more significant reductions in pain unpleasantness,26 increased mobility26 and increased muscle strength (incomplete SCI).27 These findings are similar to studies investigating the effects of screen-projected VR on people with chronic pain. For example, Garcia-Palacios and colleagues exposed fibromyalgia patients to three 20-minute VR sessions using a screen projection. Although there were no significant decreases in pain intensity, there were significant increases in perceived quality of life and reduced impact of fibromyalgia.44 Although reductions in pain with these techniques alone are not large, current evidence suggests that the immersiveness of an experience is an important contributing factor to outcomes.45 Therefore, more immersive experiences using HMD may be more effective in reducing pain than 2D and 3D screens.
Short-term VR use (distraction)
As described in the Introduction, VR can be broadly divided into two main types based on presumed mechanisms and it may be helpful to discuss the potential of VR according to these types which are thought to be quite different. The ability of short-term VR use to decrease pain severity has mostly been attributed to active distraction where attention towards pain is diverted towards an alternative stimulus.46 Although distractive methods have been used for many years to reduce pain and enhance mood, immersive VR is considered to be a more effective form of distraction.
These immediate effects of distraction type VR may be due to short-term cellular changes accompanied by temporary alterations in excitability of neuron populations in brain regions associated with pain modulation.15 For example, Bantick and colleagues showed that when healthy participants are distracted during painful thermal stimulation, they report decreases in pain intensity. Simultaneously, functional imaging using fMRI showed decreases in activation of the thalamus, insula and cognitive anterior cingulate cortex (ACC) and increase inactivity in the affective ACC and orbitofrontal cortex were observed.47 Later, Valet and co-workers showed similar results using distraction task during positron emission tomography and fMRI. However, in addition to increases in ACC and orbitofrontal cortex activity, they further showed activity increases in the periaqueductal grey (PAG) and posterior thalamus, suggesting a “top-down” influence of the cingulo-frontal cortex on pain modulation via the PAG during distraction.48 These findings strongly suggest that uncontrolled attention to “bottom-up” influences of pain may be modulated in a “top-down” manner by voluntary goal-directed attention. Thus, VR, an arguably more potent form of distraction should hypothetically activate these supraspinal regions to decrease the perception of pain in people with SCI. The studies identified in this review were not convincing, but this may be due to the relative lack of immersion of the VR techniques used in the studies.
Long-term VR use (neuroplasticity)
A key mechanism thought to contribute to the onset and maintenance of SCI neuropathic pain is neuroplasticity.31 Here, neuroplasticity is defined as the ability of the nervous system to respond to intrinsic and extrinsic stimuli by reorganizing its structure, function and connections.49 SCI is shown to be associated with structural atrophic changes within the brain and spinal cord, most notably reduced grey matter volumes in the ACC, insula, somatosensory cortex and the thalamus.31 Recently, Jutzeler and colleagues found that in SCI patients, the presence of neuropathic pain was associated with smaller cord area, increased grey matter in the ACC, primary motor cortex and decreased grey matter in the primary somatosensory cortex.31 Interestingly, they also found that the level of neuropathic pain below the level of spinal cord lesion related to greater grey matter volumes in the primary motor cortex.
Modalities including VR are now known to provide short-term pain relief due to altered cortical excitability (seconds to hours).50 However, longer-term reductions in neuropathic pain intensity (weeks to years) due to SCI are scarce. VR may be capable of promoting neuroplastic changes which occur over time that may allow for complete or partial recovery of both sensory and motor function. One of the SCI studies examining longer term effects used tDCS which is believed to induce alterations in neuronal membrane potentials due to changes in extracellular ion concentrations.51 Indeed, tDCS alone provides analgesic benefits both during and after stimulation where effects are thought to be due to alterations in local glutamate concentrations and μ-opioid receptor activity.52 This, in conjunction with VR shown to enhance corticospinal excitability and intracortical inhibition, may combine to produce a significant synergistic analgesic effect.28,30
To date, long-term changes in brain activity have only been investigated among stroke patients using physical tasks to increase motor function. For example, studies show that therapy-related improvements in hand function correlate with increases in fMRI activity in the pre-motor and secondary somatosensory cortices contralateral to the affected hand.53,54 Concerning the use of VR in stroke rehabilitation, a recent Cochrane review identified 72 studies involving 2470 post-stroke patients.55 The authors found that VR and interactive gaming was not more beneficial than conventional therapy but was beneficial when used as an adjunct to usual care. Interestingly, they also found that severity of impairment, time since onset or type of device were not strong influencers to outcomes. However, they further suggested that higher doses over longer time periods were preferable, suggesting that increased time practicing with VR applications may increase the likelihood of more long term neuroplastic changes in sensory and motor brain regions.
Conclusions and future directions
Current treatments for SCI neuropathic pain have major limitations. For example, both short and long-acting opioids have been commonly prescribed in an attempt to control pain after SCI, regardless of risk and lack of benefit.56 Additionally, although tricyclic, antiepileptic and other centrally acting analgesic medications show efficacy in the management of SCI neuropathic pain, the occurrence of side-effects, especially with high doses are common.8 Consequently, alternative and/or adjunct therapeutic options are required for most SCI patients.46 Here, VR applications may provide a non-pharmacological option where less medication is required to control neuropathic pain.
Despite recent awareness of VR and its short-term benefits for people with pain following a SCI, its long-term effect on pain in this and other populations shows much potential but requires further investigation. Of the studies we located for this scoping review, six of nine studies included multiple VR sessions over several weeks. Only one of these studies investigated the long-term effects (12 months) of immersive VR on neuropathic pain, this in combination with brain machine interfaces.23 These findings together with studies using VR and tDCS suggest that the longer the course of a combined treatment, the greater the reduction in pain intensity. However, the long-term use of VR as a stand-alone treatment is yet to be determined.
Despite showing promise, the statistical power of all studies was limited with no investigation having more than 40 enrolled SCI participants and outcomes were variable. Although evidence is limited, the reason for the difference in outcomes may be due to differences in influence on underlying mechanisms. For example, short-term distraction-type VR may offer immediate pain relief due to fast recruitment of brain neuronal networks that activate inhibitory circuits. On the other hand, neuroplasticity-type VR which is applied over weeks or months may result in pain relief due to more long term neuroplastic changes in sensorimotor regions of the brain.
Studies investigating the use of VR and AR in other neuropathic pain conditions such as PLP, show similar positive findings for decreases in pain intensity and provide further support for the potential of these technologies in providing pain relief. Likewise, these studies are also varied in approach and under-powered, with most being confined to case studies and case series reports.57–59 However, given the increasing availability and accessibility of affordable commercial VR technologies that have increased levels of realism and immersion, there are greater opportunities to develop randomized studies addressing the long-term benefits of VR and AR in larger samples of people with pain following a SCI. Additionally, reduced costs, increased flexibility and the ability to customize VR environments mean that VR may have numerous applications for not only SCI patients, but also many other chronic medical conditions. Here, current evidence46,60,61 further suggests that the quality, the level of immersion and number of different senses provided by VR applications are associated with the measured quantity of pain relief; all of which must be considered when developing future SCI neuropathic pain VR studies.
Disclaimer statements
Conflicts of interest None.
Funding The authors received no specific funding for this review.
References
- 1.Hadjipavlou G, Cortese AM, Ramaswamy B.. Spinal cord injury and chronic pain. BJA Educ 2016;16(8):264–8. doi: 10.1093/bjaed/mkv073 [DOI] [Google Scholar]
- 2.Laumet G, Chen S-R, Pan H-L.. Nmda receptors and signaling in chronic neuropathic pain. In: Hashimoto K, (ed.) The NMDA Receptors. Cham: Springer International Publishing; 2017. p. 103–19. [Google Scholar]
- 3.Anderson-Barnes VC, McAuliffe C, Swanberg KM, Tsao JW.. Phantom limb pain – a phenomenon of proprioceptive memory? Med Hypotheses 2009;73(4):555–8. doi: 10.1016/j.mehy.2009.05.038 [DOI] [PubMed] [Google Scholar]
- 4.Wrigley PJ, Press SR, Gustin SM, Macefield VG, Gandevia SC, Cousins MJ, et al. Neuropathic pain and primary somatosensory cortex reorganization following spinal cord injury. Pain. 2009;141(1):52–9. doi: 10.1016/j.pain.2008.10.007 [DOI] [PubMed] [Google Scholar]
- 5.Moxon KA, Oliviero A, Aguilar J, Foffani G.. Cortical reorganization after spinal cord injury: always for good? Neurosci 2014;283:78–94. doi: 10.1016/j.neuroscience.2014.06.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerke MB, Duggan AW, Xu L, Siddall PJ.. Thalamic neuronal activity in rats with mechanical allodynia following contusive spinal cord injury. Neurosci 2003;117(3):715–22. doi: 10.1016/S0306-4522(02)00961-2 [DOI] [PubMed] [Google Scholar]
- 7.Jones EG, Pons TP.. Thalamic and brainstem contributions to large-scale plasticity of primate somatosensory cortex. Sci 1998;282(5391):1121–5. doi: 10.1126/science.282.5391.1121 [DOI] [PubMed] [Google Scholar]
- 8.Hagen EM, Rekand T.. Management of neuropathic pain associated with spinal cord injury. Pain Ther 2015;4(1):51–65. doi: 10.1007/s40122-015-0033-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teasell RW, Mehta S, Aubut J-AL, Foulon B, Wolfe DL, Hsieh JTC, et al. A systematic review of pharmacologic treatments of pain after spinal cord injury. Arch Phys Med Rehabil 2010;91(5):816–31. doi: 10.1016/j.apmr.2010.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig A, Tran Y, Siddall P, Wijesuriya N, Lovas J, Bartrop R, et al. Developing a model of associations between chronic pain, depressive mood, chronic fatigue, and self-efficacy in people with spinal cord injury. J Pain 2013;14(9):911–20. doi: 10.1016/j.jpain.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 11.Pourmand A, Davis S, Marchak A, Whiteside T, Sikka N.. Virtual reality as a clinical tool for pain management. Curr Pain Headache Rep 2018;22(8):53. doi: 10.1007/s11916-018-0708-2 [DOI] [PubMed] [Google Scholar]
- 12.Vespa A, Giulietti MV, Spatuzzi R, Fabbietti P, Meloni C, Gattafoni P, et al. Validation of brief multidimensional spirituality/religiousness inventory (BMMRS) in Italian adult participants and in participants with medical diseases. J Relig Health 2017;56(3):907–15. doi: 10.1007/s10943-016-0285-9 [DOI] [PubMed] [Google Scholar]
- 13.Gupta A, Scott K, Dukewich M.. Innovative technology using virtual reality in the treatment of pain: does it reduce pain via distraction, or is there more to it? Pain Med 2018;19(1):151–9. doi: 10.1093/pm/pnx109 [DOI] [PubMed] [Google Scholar]
- 14.Mahrer NE, Gold JI.. The use of virtual reality for pain control: a review. Curr Pain Headache Rep 2009;13(2):100–9. doi: 10.1007/s11916-009-0019-8 [DOI] [PubMed] [Google Scholar]
- 15.Cheung K, Tunik E, Adamovich S, Boyd L.. Neuroplasticity and virtual reality. In: Weiss P, Keshner EA, Levin MF, (eds.) Virtual Reality for Physical and Motor Rehabilitation. 2. New York: Springer Sciences; 2014:14–6. [Google Scholar]
- 16.Moseley LG. Using visual illusion to reduce at-level neuropathic pain in paraplegia. Pain 2007;130(3):294–8. doi: 10.1016/j.pain.2007.01.007 [DOI] [PubMed] [Google Scholar]
- 17.Hoffman HG, Patterson DR, Seibel E, Soltani M, Jewett-Leahy L, Sharar SR.. Virtual reality pain control during burn wound debridement in the hydrotank. Clin J Pain 2008;24(4):299–304. doi: 10.1097/AJP.0b013e318164d2cc [DOI] [PubMed] [Google Scholar]
- 18.Jones T, Moore T, Choo J.. The impact of virtual reality on chronic pain. PLoS One 2016;11(12):e0167523. doi: 10.1371/journal.pone.0167523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garrett B, Taverner T, McDade P.. Virtual reality as an adjunct home therapy in chronic pain management: an exploratory study. JMIR Med Inform 2017;5(2):e11. doi: 10.2196/medinform.7271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ortiz-Catalan M, Guðmundsdóttir RA, Kristoffersen MB, Zepeda-Echavarria A, Caine-Winterberger K, Kulbacka-Ortiz K, et al. Phantom motor execution facilitated by machine learning and augmented reality as treatment for phantom limb pain: a single group, clinical trial in patients with chronic intractable phantom limb pain. Lancet 2016;388(10062):2885–94. doi: 10.1016/S0140-6736(16)31598-7 [DOI] [PubMed] [Google Scholar]
- 21.Mouraux D, Brassinne E, Sobczak S, Nonclercq A, Warzee N, Sizer PS, et al. 3D augmented reality mirror visual feedback therapy applied to the treatment of persistent, unilateral upper extremity neuropathic pain: a preliminary study. J Man Manip Ther 2017;25(3):137–43. doi: 10.1080/10669817.2016.1176726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. Prisma extension for scoping reviews (prisma-scr): checklist and explanation. Ann Intern Med 2018;169(7):467–73. doi: 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 23.Donati ARC, Shokur S, Morya E, Campos DSF, Moioli RC, Gitti CM, et al. Long-term training with a brain-machine interface-based gait protocol induces partial neurological recovery in paraplegic patients. Sci Rep 2016;6. doi: 10.1038/srep30383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pozeg P, Palluel E, Ronchi R, Solca M, Al-Khodairy AW, Jordan X, et al. Virtual reality improves embodiment and neuropathic pain caused by spinal cord injury. Neurol 2017;89(18):1894–903. doi: 10.1212/WNL.0000000000004585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jordan M, Richardson EJ.. Effects of virtual walking treatment on spinal cord injury-related neuropathic pain: pilot results and trends related to location of pain and at-level neuronal hypersensitivity. Am J Phys Med Rehabil 2016;95(5):390–6. doi: 10.1097/PHM.0000000000000417 [DOI] [PubMed] [Google Scholar]
- 26.Villiger M, Bohli D, Kiper D, Pyk P, Spillmann J, Meilick B, et al. Virtual reality-augmented neurorehabilitation improves motor function and reduces neuropathic pain in patients with incomplete spinal cord injury. Neurorehabil Neural Repair 2013;27(8):675–83. doi: 10.1177/1545968313490999 [DOI] [PubMed] [Google Scholar]
- 27.Roosink M, Robitaille N, Jackson PL, Bouyer LJ, Mercier C.. Interactive virtual feedback improves gait motor imagery after spinal cord injury: an exploratory study. Restor Neurol Neurosci 2016;34(2):227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumru H, Soler D, Vidal J, Navarro X, Tormos JM, Pascual-Leone A, et al. The effects of transcranial direct current stimulation with visual illusion in neuropathic pain due to spinal cord injury: an evoked potentials and quantitative thermal testing study. Eur J Pain 2013;17(1):55–66. doi: 10.1002/j.1532-2149.2012.00167.x [DOI] [PubMed] [Google Scholar]
- 29.Ozkul C, Kilinc M, Yildirim SA, Topcuoglu EY, Akyuz M.. Effects of visual illusion and transcutaneous electrical nerve stimulation on neuropathic pain in patients with spinal cord injury: a randomised controlled cross-over trial. J Back Musculoskelet Rehabil 2015;28(4):709–19. doi: 10.3233/BMR-140573 [DOI] [PubMed] [Google Scholar]
- 30.Soler MD, Kumru H, Pelayo R, Vidal J, Tormos JM, Fregni F, et al. Effectiveness of transcranial direct current stimulation and visual illusion on neuropathic pain in spinal cord injury. Brain 2010;133(9):2565–77. doi: 10.1093/brain/awq184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jutzeler CR, Huber E, Callaghan MF, Luechinger R, Curt A, Kramer JLK, et al. Association of pain and CNS structural changes after spinal cord injury. Sci Rep 2016;6:18534. doi: 10.1038/srep18534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gustin SM, Wrigley PJ, Siddall PJ, Henderson LA.. Brain anatomy changes associated with persistent neuropathic pain following spinal cord injury. Cereb Cortex 2010;20(6):1409–19. doi: 10.1093/cercor/bhp205 [DOI] [PubMed] [Google Scholar]
- 33.Meacham K, Shepherd A, Mohapatra DP, Haroutounian S.. Neuropathic pain: central vs. peripheral mechanisms. Curr Pain Headache Rep 2017;21(6):28. doi: 10.1007/s11916-017-0629-5 [DOI] [PubMed] [Google Scholar]
- 34.Walz AD, Usichenko T, Moseley GL, Lotze M.. Graded motor imagery and the impact on pain processing in a case of CRPS. Clin J Pain 2013;29(3):276–9. doi: 10.1097/AJP.0b013e318250f4e8 [DOI] [PubMed] [Google Scholar]
- 35.de Souza NS, Martins ACG, Bastos VHdV, Orsini M, Leite MAA, Teixeira S, et al. Motor imagery and its effect on complex regional pain syndrome: an integrative review. Neurol Int 2015;7(3):5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michele S, M. AS, Polona P, Renato A, Valentina M.. Motor imagery in spinal cord injured people is modulated by somatotopic coding, perspective taking, and post-lesional chronic pain. J Neuropsychol 2017;11(3):305–26. doi: 10.1111/jnp.12098 [DOI] [PubMed] [Google Scholar]
- 37.Thomschewski A, Ströhlein A, Langthaler PB, Schmid E, Potthoff J, Höller P, et al. Imagine there is no plegia. Mental motor imagery difficulties in patients with traumatic spinal cord injury. Front Neurosci 2017;11:689. doi: 10.3389/fnins.2017.00689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osumi M, Ichinose A, Sumitani M, Wake N, Sano Y, Yozu A, et al. Restoring movement representation and alleviating phantom limb pain through short-term neurorehabilitation with a virtual reality system. Eur J Pain 2017;21(1):140–7. doi: 10.1002/ejp.910 [DOI] [PubMed] [Google Scholar]
- 39.Sano Y, Ichinose A, Wake N, Osumi M, Sumitani M, Kumagaya S, et al., editors. Reliability of phantom pain relief in neurorehabilitation using a multimodal virtual reality system. 2015 37th annual international conference of the IEEE engineering in medicine and biology society (EMBC); 2015 25–29 Aug. 2015. [DOI] [PubMed]
- 40.Wake N, Sano Y, Oya R, Sumitani M, Kumagaya S, Kuniyoshi Y, editors. Multimodal virtual reality platform for the rehabilitation of phantom limb pain. 2015 7th international IEEE/EMBS conference on neural engineering (NER); 2015 22–24 April 2015.
- 41.Cheung KL, Tunik E, Adamovich SV, Boyd LA.. Neuroplasticity and Virtual Reality 2014. p. 5–24.
- 42.Wiederhold B, Gao K, Sulea C, Wiederhold M.. Virtual reality as a distraction technique in chronic pain patients. Cyberpsychol Behav Soc Netw 2014;17(6):346–52. doi: 10.1089/cyber.2014.0207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin W CA, Gromala D, Shaw C, Squire PA.. Virtual reality game for chronic pain management: a randomized, controlled clinical study. Stud Health Technol Informatics 2016(220):154–60. [PubMed] [Google Scholar]
- 44.Garcia-Palacios A, Herrero R, Vizcaíno Y, Belmonte MA, Castilla D, Molinari G, et al. Integrating virtual reality with activity management for the treatment of fibromyalgia: acceptability and preliminary efficacy. Clin J Pain 2015;31(6):564–72. doi: 10.1097/AJP.0000000000000196 [DOI] [PubMed] [Google Scholar]
- 45.Shahrbanian S, Ma X, Aghaei N, Korner-Bitensky N, Moshiri K, Simmonds MJ.. Use of virtual reality (immersive vs. non immersive) for pain management in children and adults: a systematic review of evidence from randomized controlled trials Eur J Exp Biol 2012;2(5):1408–22. [Google Scholar]
- 46.Gupta A, Scott K, Dukewich M.. Innovative technology using virtual reality in the treatment of pain: does It reduce pain via distraction, or is there more to it? Pain Med (Malden, Mass) 2018;19(1):151–9. doi: 10.1093/pm/pnx109 [DOI] [PubMed] [Google Scholar]
- 47.Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I.. Imaging how attention modulates pain in humans using functional MRI. Brain 2002;125(2):310–9. doi: 10.1093/brain/awf022 [DOI] [PubMed] [Google Scholar]
- 48.Valet M, Sprenger T, Boecker H, Willoch F, Rummeny E, Conrad B, et al. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain—an fMRI analysis. Pain 2004;109(3):399–408. doi: 10.1016/j.pain.2004.02.033 [DOI] [PubMed] [Google Scholar]
- 49.Cramer SC, Sur M, Dobkin BH, O’Brien C, Sanger TD, Trojanowski JQ, et al. Harnessing neuroplasticity for clinical applications. Brain 2011;134(6):1591–609. doi: 10.1093/brain/awr039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bassolino M, Franza M, Bello Ruiz J, Pinardi M, Schmidlin T, Stephan MA, et al. Non-invasive brain stimulation of motor cortex induces embodiment when integrated with virtual reality feedback. Eur J Neurosci 2018;47(7):790–9. doi: 10.1111/ejn.13871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nitsche MA, Paulus W.. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurol 2001;57(10):1899–901. doi: 10.1212/WNL.57.10.1899 [DOI] [PubMed] [Google Scholar]
- 52.Hunter MA, Coffman BA, Gasparovic C, Calhoun VD, Trumbo MC, Clark VP.. Baseline effects of transcranial direct current stimulation on glutamatergic neurotransmission and large-scale network connectivity. Brain Res 2015;1594:92–107. doi: 10.1016/j.brainres.2014.09.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johansen-Berg H, Dawes H, Guy C, Smith SM, Wade DT, Matthews PM.. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain 2002;125(12):2731–42. doi: 10.1093/brain/awf282 [DOI] [PubMed] [Google Scholar]
- 54.Askim T, Indredavik B, Vangberg T, Håberg A.. Motor network changes associated with successful motor skill relearning after acute ischemic stroke: a longitudinal functional magnetic resonance imaging study. Neurorehabil Neural Repair 2008;23(3):295–304. doi: 10.1177/1545968308322840 [DOI] [PubMed] [Google Scholar]
- 55.Laver KE, Lange B, George S, Deutsch JE, Saposnik G, Crotty M.. Virtual reality for stroke rehabilitation. Cochrane Database Syst Rev 2017(11). doi: 10.1002/14651858.CD008349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hand BN, Krause JS, Simpson KN.. Dose and duration of opioid use in propensity score–matched, privately insured opioid users with and without spinal cord injury. Arch Phys Med Rehabil 2018;99(5):855–61. doi: 10.1016/j.apmr.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dunn J, Yeo E, Moghaddampour P, Chau B, Humbert S.. Virtual and augmented reality in the treatment of phantom limb pain: a literature review. Neuro Rehabil 2017;40(4):595–601. [DOI] [PubMed] [Google Scholar]
- 58.Chau B, Phelan I, Ta P, Humbert S, Hata J, Tran D.. Immersive virtual reality therapy with myoelectric control for treatment-resistant phantom limb pain: case report. Innov Clin Neurosci 2017;14(7-8):3–7. [PMC free article] [PubMed] [Google Scholar]
- 59.Mouraux D, Brassinne E, Sobczak S, Nonclercq A, Warzée N, Sizer PS, et al. 3D augmented reality mirror visual feedback therapy applied to the treatment of persistent, unilateral upper extremity neuropathic pain: a preliminary study. J Man Manip Ther 2017;25(3):137–43. doi: 10.1080/10669817.2016.1176726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoffman HG, Seibel EJ, Richards TL, Furness TA, Patterson DR, Sharar SR.. Virtual reality helmet display quality influences the magnitude of virtual reality analgesia. J Pain 2006;7(11):843–50. doi: 10.1016/j.jpain.2006.04.006 [DOI] [PubMed] [Google Scholar]
- 61.Johnson S, Coxon M.. Sound can enhance the analgesic effect of virtual reality. R Soc Open Sci 2016;3(3):150567. doi: 10.1098/rsos.150567 [DOI] [PMC free article] [PubMed] [Google Scholar]