Abstract
Context/objective: Manipulation of the microbiome is an emerging approach to promote health. We conducted a Phase Ia safety study of a single bladder instillation of probiotics in asymptomatic patients with neuropathic bladder to determine the tolerability and safety of a single Lactobacillus instillation.
Design: Phase Ia safety study.
Setting: Outpatient rehabilitation clinic at a rehabilitation hospital (adults) and urology clinic at a free-standing children’s hospital (children).
Participants: Ten patients with neuropathic bladder were included: five children with spina bifida and five adults with spinal cord injury.
Interventions: A single Lactobacillus rhamnosus GG (Culturelle, 20 billion live organisms) instillation.
Outcome measures: After the instillation, participants self-monitored symptoms using the Urinary Symptoms Questionnaire for People with Neuropathic Bladder using Intermittent Catheterization daily for one week. Repeat urinalysis, urine culture, and 16S bacterial rRNA-based microbiome analyses were performed 7–10 days after instillation.
Results: Probiotic instillation was well-tolerated. One child had upper respiratory tract symptoms during the trial, and two had transient cloudy urine. No adults reported any symptoms following instillation. Lactobacillus did not grow on culture post-instillation. There were differences in beta diversity of the urine microbiome in children vs. adults with neuropathic bladder (P < 0.0156). Lactobacillus was present in the pre-instillation urinary microbiomes all of the adults and 4 out of 5 of the pediatric subjects, and identified in 4 out of 5 of both the adult and pediatric subjects’ post-instillation urinary microbiomes.
Conclusion: Intravesical instillation of Culturelle probiotic may be safe and well-tolerated in patients with neuropathic bladder.
Keywords: Microbiome, Urinary tract infection, Neuropathic bladder, Probiotics, Safety study
Introduction
The importance of the microbiome, the culture-independent bacterial milieu growing in and on our bodies, is becoming increasingly recognized in human health and disease.1 The urinary microbiome is hypothesized to be a factor in the pathogenesis of urinary tract infections (UTI), especially in patients with recurrent UTIs, such as those with neuropathic bladders. Indeed, the urinary microbiome in patients with neuropathic bladder is different than non-neuropathic bladders and contains more uropathogens than is seen in those with non-neuropathic bladders.2,3 Comparatively, the urinary microbiome of individuals with normally functioning bladders have a higher proportion of Lactobacillus.2 Limited data demonstrates that host-detrimental changes in the urinary microbiome, a state referred to as dysbiosis, occur in the setting of UTI. This suggests that microbiome-modulating therapies, such as probiotics, may have utility in correcting dysbiosis and decreasing the number of UTIs in patients with neuropathic bladders. While the use of oral probiotics is increasingly wide-spread, oral probiotics have not been shown to be effective in preventing recurrent UTIs.4 As UTIs are a local, rather than systemic, infection, instillation of probiotics directly into the bladder may be more likely to correct the dysbiosis and prevent UTIs than oral administration. However, there is no data on the safety or efficacy of intravesical administration of probiotics. Therefore, we conducted an FDA-approved phase 1 safety study of a single intravesical administration of Lactobacillus probiotics in healthy adults and children with neuropathic bladder. We sought to determine the tolerability and safety of a single instillation, whether the instilled Lactobacillus persists in the urine determined by cultivation and 16S rRNA gene high-throughput sequencing, and if children differ from adults with regards to these endpoints.
Materials and methods
Patients
Pediatric patients with spina bifida (SB) and adults with spinal cord injury (SCI) were enrolled in this study. Patients were eligible for participation if they met the following criteria: neuropathic bladder managed with clean intermittent catheterization, living in the community (i.e. not within a long-term care facility), and presence of SCI for over one year for adults. We excluded patients with: (1) genitourinary pathology beyond neuropathic bladder; (2) instillation of other intravesical agents; (3) psychological or psychiatric conditions influencing the ability to follow instructions; (4) participation in a confounding study; (5) pregnant or breastfeeding women; (6) immunodeficiencies; active or chronic serious infections; (7) cancer/autoimmune disorders; (8) allergy to any component in the probiotic product; (9) a change in neurologic status in the previous 2 weeks; (10) antibiotic use in the previous 2 weeks; (11) sensitivity to ampicillin or tetracycline; and (12) UTI within the previous 2 weeks (as defined by Infectious Diseases Society of America CAUTI Guidelines).5 Pediatric patients also had to be between 6 and 18 years of age in order to be eligible. This work was conducted with formal approval by the Institutional Review Board at both sites, with informed consent provided by all participants or parents / legal guardians for pediatric patients.
Intervention
At the time of enrollment, subjects completed a 12-question survey on urinary symptoms. Following survey completion, a urine sample was collected at home using a new catheter for urinalysis, urine culture, and bacterial 16S rRNA gene-based analyses. Study personnel then trained subjects on bladder instillation technique, and subjects instilled Lactobacillus rhamnosus GG (ATCC 53103, Culturelle GG, 20 billion live organisms) into their bladders with study personnel supervising at the study site. This strain was chosen for use in this work as it is readily available and commercially accessible, allowing for rapid translation into clinical practice. Pediatric patients received smaller volumes of instillate based on their anticipated bladder capacity, which was 10% of their anticipated capacity. Following instillation, subjects were observed in the clinic for 30 min to assess for adverse reactions. Participants then self-monitored symptoms using the Urinary Symptoms Questionnaire for People With Neuropathic Bladder using Intermittent Catheterization (USQNB-IC),6 a 29-item instrument with face- and content-validity in these populations, to assess patient-reported outcomes regarding urinary symptoms daily for one week. Participants were instructed to contact study personnel for any urinary symptoms. Participants returned to the clinic 7–10 days after instillation for repeat urinalysis, urine culture, and microbiome analyses. Urinalysis and urine culture were performed by Quest Diagnostics (Chantilly, VA). Urinalysis was completed utilizing standard clinical microbiology semiquantitative chemical testing using commercial disposable test strips, and urine culture was performed using standard laboratory techniques.
Sample preparation and DNA isolation
Urinary bacteria were pelleted using low-speed centrifugation, washed with phosphate buffered saline (PBS: 10 mM Na2HPO4, 1.8 mM KH2PO4, 137 mM NaCl and 2.7 mM KCl) and stored at −80°C. Depending on the size of the pelleted material, genomic DNA was isolated either with the DNeasy Kit (Qiagen) using manufacturer’s protocol for Gram-negative bacteria or with the QIAmp DNA Micro Kit (Qiagen) using manufacturer’s protocol for DNA isolation from urine. Purified DNA was quantified using NanoDrop spectrophotometer (Thermo Fisher Scientific). Fractions of human and bacterial DNA in each sample were determined using Femto Human and Femto Bacterial DNA quantification kits (Zymo Research) according to the manufacturer’s instructions. DNA isolation using PBS as the starting material was used as a negative control.
16S rRNA gene amplification and high-throughput sequencing
V4 regions of 16S rRNA genes were amplified using primers 5’-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGGTGCCAGCMGCCGCGGTAA-3’ and 5’-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTWTCTAAT-3’ and the following reagent concentrations: 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 1.5 mM MgCl2, 200 µM of each dNTP, 2 µM of each primer, 1% glycerol, 0.3 U AccuPrime Taq polymerase (Thermo Fisher Scientific) and 25 ng of template DNA in 20 µl total volume. Amplification conditions were 2 min at 95°C initial denaturation followed by 30 cycles of 20 s denaturation at 95°C, 15 s annealing at 55°C and a 5-min extension at 72°C, and a 5-min final extension at 72°C. Amplification reaction without template DNA was used as a negative control. Amplification products were purified with the AMPure XP system (Beckman Coulter) and their size was verified with the DNA 1000 Kit (Agilent). Indexing and pooling of amplification products were carried out according to Illumina’s 16S Metagenomic Sequencing Library Preparation protocol. Negative control samples were indexed and included in the library preparation. The resulting library was sequenced using Illumina MiSeq Reagent Kit v2 (500 cycles), which produced 250 bases paired-end reads.
Statistical analysis
Raw FASTQ files were processed in Mothur v1.35.1.7 Default settings were used to minimize sequencing errors.8 Clean sequences were aligned to the SILVA128-based bacterial reference alignment at http://www.mothur.org. Chimeras were removed using uchime,9 and non-chimeric sequences were classified using a naïve Bayesian classifier.10 Sequences were clustered into Operational Taxonomic Units (OTUs) at the 0.03 threshold (species level). OTU sequence representatives and taxonomy were imported (BIOM format) into QIIME for subsequent analyses.11 Samples were subsampled (rarefaction analysis) to the smallest sample size to remove the effect of sample size bias on community composition. Trees for phylogenetic diversity calculations were constructed using FastTree and midpoint rooting.12 Taxonomic alpha diversity was estimated as the number of observed OTUs, Chao1 and Shannon indices. Phylogenetic alpha diversity was calculated by Faith’s phylogenetic diversity index.13 Phylogenetic beta diversity Unifrac metrics (unweighted and weighted unifrac) were calculated between pairs of samples. The dissimilarity between samples was explored using principal coordinates analysis (PCoA) and both Unifrac distances. Linear mixed-effects (LME) models analysis was applied to both alpha diversity indices and taxa (genera and phyla) proportions (response) while accounting for non-independence of subjects, subject’s baseline levels (random effect), predictors (e.g. pyuria) and confounders (e.g. age, sex). Beta diversity Unifrac indices were compared using permutational multivariate analysis of variance (adonis) as implemented in the vegan R package.14 Significance was determined through 10,000 permutations. Sample pairs were compared using the Fisher exact test. Bonferroni or Benjamini-Hochberg FDR multiple test correction methods were applied. All analyses were performed in mothur, QIIME and RStudio.15
Results
Patients
Five children with SB and five adults with SCI were included in this study. Adult patients had a mean age of 35 years, were all male, and were, on average, 4.1 years post-SCI. Two patients had a cervical injury, two had a thoracic injury, and one had a lumbar injury, all of which were incomplete. Pediatric patients had a mean age of 8.4 years and were 80% male (Table 1).
Table 1. Patient demographics.
| Adults (n = 5) | Pediatrics (n = 5) | |
|---|---|---|
| Age (years) | 35.4 (12.7) | 8.4 (2.3) |
| Male (n (%)) | 5 (100) | 4 (80) |
| Level of spinal cord injury | ||
| Cervical (n (%)) | 2 (40) | – |
| Thoracic (n (%)) | 2 (40) | – |
| Lumbar (n (%)) | 1 (20) | – |
| Year post injury | 4.1 (3.6) | – |
| Myelomeningocele level | ||
| L4 | – | 1 (20) |
| L5-S1 | 4 (80) | |
Note: Data presented as: mean (SD) unless otherwise specified.
Clinical symptoms
Lactobacillus instillations were well-tolerated by all patients, with no immediate adverse events. In the pediatric group, one child developed upper respiratory symptoms following instillation, and two children reported transient cloudy and malodorous urine that self-resolved during the week following instillation. Neither of these children received antibiotics for these symptoms. No adults reported urinary symptoms in the week following probiotic instillation.
Urinalysis and urine culture
Seven of the ten patients had a decrease in urinary pH following probiotic instillation (mean change (95% confidence interval): −0.45 (−0.99, 0.01) and one patient had no change in pH. No children had positive nitrites on their pre-instillation urinalysis whereas four of the five children had positive nitrites following the instillation. Two adults had nitrites present on their pre-instillation urinalysis, and one had nitrites following instillation. One pediatric patient and three adults had pyuria present prior to instillation, and four pediatric patients and two adults had pyuria after the instillation (Table 2).
Table 2. Pre- and post-instillation urinalysis and urine culture results.
| Pre-instillation | Post-instillation | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | Nitrites | Leukocyte esterase | Urine WBCS | Culture result, colony count (CFU/mL) | Culture result, organism | pH | Nitrites | Leukocyte esterase | Urine WBCS | Culture result, colony count (CFU/mL) | Culture result, organism | |
| Children | 7.0 | Absent | 1+ | 0–5 | 1,000-10,000 | E. coli | 6.0 | Present | 2+ | 20–40 | >100,000 | E. coli |
| 7.5 | Absent | 0 | 0–5 | No Growth | – | 6.0 | Present | 1+ | 10–20 | >100,000 | E. coli | |
| 6.0 | Absent | 2+ | 10–20 | 10,000-50,000 | E. coli | 7.0 | Present | 1+ | 6–10 | >100,000 | E. coli | |
| 6.5 | Absent | 0 | 0–5 | >100,000 | E. coli | 6.0 | Present | 1+ | 10–20 | >100,000 | E. coli | |
| 6.5 | Absent | 0 | 0–5 | No growth | – | 7.0 | Absent | 0 | 0–5 | No growth | – | |
| Adult | 6.0 | Absent | 0 | 1–2 | No growth | – | 5.5 | Absent | 0 | 0–5 | No growth | – |
| 6.0 | Present | 1+ | 10–20 | >100,000 | E. coli | 6.0 | Present | 2+ | 10–20 | >100,000 | E. coli | |
| 7.5 | Absent | 2+ | 0–5 | >100,000 | Pseudomonas | 7.0 | Absent | 2+ | 0–5 | >100,000 | Pseudomonas | |
| 7.0 | Absent | 2+ | 6–10 | >100,000 | E. coli | 6.0 | Absent | 1+ | 6–10 | >100,000 | E. coli | |
| 7.0 | Absent | 2+ | 6–10 | >100,000 | E. coli | 6.0 | Absent | Trace | 0–5 | >100,000 | E. coli | |
WBCs: white blood cells; CFU: colony-forming units.
There were no changes in results from the pre-instillation urine culture to the post-instillation urine culture in any adult patients. Of the five pediatric patients, two had no changes between the pre- and post-instillation urine cultures, two had an increased colony count of the same bacteria in the post-instillation culture compared to the pre-instillation culture, and one patient had a negative pre-instillation urine culture, and a positive urine culture post-instillation (Table 2).
Microbiome
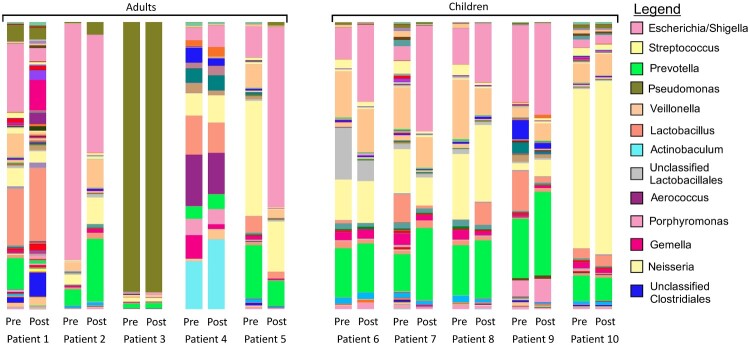
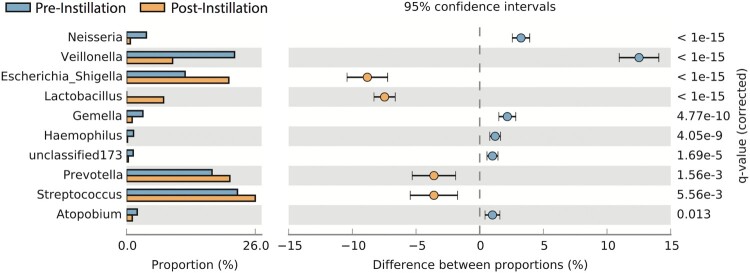
There was a significant difference in beta diversity (inter-sample) of the combined (i.e. both pre- and post-instillation) urinary microbiomes in children versus adults (P = 0.0156) (Fig. 1). There were no differences in any of the alpha diversity (intra-sample) indices (i.e. OTU richness, Shannon diversity, phylogenic diversity, Chao 1 richness) between the pre- and post-instillation microbiomes. Lactobacillus was present in the urinary microbiomes of all adult patients, and four out of five pediatric patients pre-instillation. Following instillation, Lactobacillus was present in four of the five adult patients, and four of the five pediatric patients. Three of the four pediatric patients had persistent Lactobacillus in both their pre- and post-instillation microbiomes, while one pediatric patient only had Lactobacillus present following the instillation. In the one pediatric patient that had Lactobacillus that was present only in the post-instillation urinary microbiome, it was not the predominant organism: there were higher proportions of Streptococcus, Prevotella, Escherichia, and Veillonella (Fig. 2). The largest mean change in proportion of bacteria in all patients between pre- and post-instillation was Escherichia, although there was significant intra-patient variability. In pediatric patients, those patients with changes in their urine culture also had an increase in the proportion of Escherichia between their pre- and post- instillation urinary microbiomes. While the majority of bacteria identified were present in both the pre- and post-instillation microbiomes, nine out of ten patients had significant changes in the proportion of specific bacteria between the pre- and post- microbiomes (Table 3).
Figure 1.
Microbial diversity in each of the 10 patients both pre- and post-instillation of Lactobacillus. Beta diversity is significantly different between all adult samples (both pre- and post-instillation) and all pediatric samples. (P = 0.0156). The legend includes the 13 most common bacteria: the entire list of bacteria identified can be found in the supplementary material.
Figure 2.
Significant differences (Fisher exact test) in the relative abundance of bacterial genera in the urinary microbiome of a single pediatric patient between pre (first bar)-and post-instillation (second bar). This is the only patient in the cohort to have a new presence of Lactobacillus following the instillation.
Table 3. Bacteria with significant changes in proportions between pre- and post-instillation microbiomes.
| Bacteria (n = patients with significant change) | Percent of total subjects with significant increase | Percent of total subjects with significant decrease |
|---|---|---|
| Escherichia (n = 8) | 40 | 40 |
| Prevotella (n = 7) | 60 | 10 |
| Lactobacillus (n = 7) | 30 | 40 |
| Streptococcus (n = 6) | 40 | 20 |
| Veillonella (n = 6) | 40 | 20 |
| Acinetobacter (n = 3) | 20 | 10 |
| Neisseria (n = 3) | 0 | 30 |
| Corynebacterium (n = 2) | 20 | 0 |
| Pseudomonas (n = 2) | 0 | 20 |
| Campylobacter (n = 2) | 20 | 0 |
| Haemophilus (n = 2) | 0 | 20 |
| Gemella (n = 2) | 0 | 20 |
Note: Thirteen additional microbes were significantly changed in a single patient. N in Bacteria column refers to total number of patients with a significant change in that bacteria, all percentages are out of the total population of n = 10.
Discussion
In this work, we show that one dose of intravesical L. rhamnosus GG is well-tolerated, without any adverse events or change in urinary symptoms, suggesting that intravesical instillation is a safe route of administration. The only urinary symptoms reported following probiotic instillation were isolated new onset cloudy and malodorous urine in two pediatric patients, which were not considered to be suggestive of a UTI per the Infectious Disease Society of America’s guideline on catheter-associated UTIs. Therefore, no intervention was suggested.16 The lack of other urinary symptoms suggests that this method of administration of Lactobacillus is well-tolerated. Indeed, previous in vitro work with L. rhamnosus GR-1 demonstrated a lack of urothelial cytotoxicity.17 Further, 7–10 days following the single instillation, there was no change in alpha diversity, although the majority of patients had significant changes in the proportions of bacteria within their own microbiomes.
No urinary symptoms developed as a result of the intravesical administration of Lactobacillus. However, there was a change in asymptomatic bacteriuria in the pediatric patients: Three of the five pediatric patients demonstrated an increase in E. coli growth on urine culture at 7–10 days post-instillation. Asymptomatic bacteriuria and pyuria, which are common in these children and frequently persist for weeks in the absence of symptoms, are not associated with either increased risk of developing a UTI or renal scarring.18,19 As there were not any corresponding clinical symptoms, these positive cultures are not UTIs, but rather asymptomatic bacteriuria that is representative of the underlying bacterial colonization of the urinary tract. Given the clean technique used and the frequency with which these children are catheterized at baseline, it is unlikely that this increase in E. coli growth on urine culture is due to inadvertent instillation of E. coli. Rather, this relative increase in E. coli could potentially be a result of bacterial displacement from urothelial cells. Limited data suggest that various strains of Lactobacillus have the ability to displace bacteria adhered to vaginal epithelial cells.20 E. coli colonization of the bladder is mediated though urothelial binding. Therefore, it is possible that the increase in E. coli cultured from urine following Lactobacillus instillation is a result of bacterial displacement from the uroepithelial cells, a hypothesis that is further supported by the relative increase in Escherichia in the post-instillation urinary microbiomes. However, none of the adult patients had a change in urine culture results following probiotic instillation, suggesting that an alternative explanation for the increase in E. coli bacteriuria following instillation is likely.
There was an overall decrease in the mean urine pH on the post-instillation urinalysis compared to the pre-instillation urinalysis, with 7 of the 10 patients demonstrating a lower pH following instillation. L. rhamnosus GG, like most strains of Lactobacillus, produces acids that result in a lowering of environmental pH.21 Further, the antimicrobial properties of L. rhamnosus GG function in a pH-dependent manner.21 Therefore, changes in urinary pH may serve as a proxy for the increase in such bacteria in the urinary microbiome. However, there are several other bacterial species frequently found within the urinary microbiome that also produce acid, suggesting that a lower pH cannot be solely attributed to the presence of L. rhamnosus GG. It is possible that the single instillation of L. rhamnosus GG was associated with a microbiome shift, allowing for the growth of other acid-producing bacteria. For example, six of the 10 patients had increased proportions of Streptococcus, another lactic-acid producing bacteria. This concept of microbial community shift has been demonstrated to occur in a pH-dependent manner in other settings,22 providing support for the presence in a community shift in the urinary microbiome concordant with a change in pH. However, our results are preliminary, and further work with recurrent instillation of Lactobacillus is needed to fully investigate this hypothesis.
The lack of side effects and the tolerability of intravesical administration of Lactobacillus make this route of administration a viable method of UTI prophylaxis. In addition to this favorable safety profile, Lactobacillus is a good candidate for UTI prophylaxis given its impact on uropathogens through its ability to regulate uropathogenic growth.20 However, there are more than 80 species of Lactobacillus, each with varying effects on uropathogens.4 L. rhamnosus GG, the strain used in this work, has in vitro activity against strains of uropathogenic E. coli,23 suggesting that it is potentially effective in preventing UTIs. Despite this, neither of the two trials examining the utility of L. rhamnosus GG in preventing UTIs demonstrated a significant reduction in episodes of UTI.24,25 However, in these prior studies, the probiotics were administered either orally or vaginally in women with recurrent UTI, both routes of administration that do not lead to bladder colonization. Thus, assuming that L. rhamnosus GG can only exert antibacterial properties locally, oral and vaginal administration of this probiotic may preclude potential therapeutic effects. While intravesical L. casei has demonstrated efficacy in treating chronic UTIs in mice,26 to our knowledge, ours is the first human trial of intravesical Lactobacilli rhamnosus GG.
There are several limitations to this work, including the small number of patients in this work. Other limitations include the different etiologies of neuropathic bladder in the adults and children, the inability to make comparisons between patients with flaccid and spastic bladders, and that all patients are from a single geographic region. Future work will focus on a more homogenous patient population. We cannot verify that the Lactobacillus which appeared in the urine of some patients post-instillation was L. rhamnosus GG due to an inability of our methodologies to detect specific bacterial strains. Although this study was designed to test the safety and tolerability of a single dose of intravesical dose of Lactobacillus, and therefore not designed to the study longitudinal changes in the urinary microbiome as a result of intravesical probiotic use, we are unable to comment on the long-term effect of Lactobacillus on the urinary microbiome.
Conclusion
In this pilot study, a single dose of intravesical L. rhamnosus GG was well-tolerated. Given these results, a larger trial has been initiated to evaluate the safety, tolerability, efficacy and usability of a self-management protocol for pre-infectious urinary symptoms using instilled intravesical Lactobacillus.
Supplementary Material
Acknowledgements
The content of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Funding Statement
This work was funded by Patient-Centered Outcomes Research Institute (PCORI) [Grant Number AD-1310-08215]. This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development [Grant Number K12 HD001399]; National Center for Advancing Translational Sciences [Grant Number UL1TR001876]; National Center for Advancing Translational Sciences [Grant Number KL2TR001877]; National Institutes of Health [Grant Number K12HL119994]; National Institutes of Health (US) [Grant Number RO1: KO113504].
Disclaimer statements
Contributors None.
Conflicts of interest Drs Caldovic, Pohl, and Groah have submitted a patent for the intravesical use of Lactobacillus.
References
- 1.Cho I, Blaser MJ.. The human microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13(4):260–70. doi: 10.1038/nrg3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Groah SL, Pérez-Losada M, Caldovic L, Ljungberg IH, Sprague BM, Castro-Nallar E, et al. Redefining healthy urine: a cross-sectional exploratory metagenomic study of people with and without bladder dysfunction. J Urol. 2016;196(2):579–87. doi: 10.1016/j.juro.2016.01.088 [DOI] [PubMed] [Google Scholar]
- 3.Fouts DE, Pieper R, Szpakowski S, Pohl H, Knoblach S, Suh M-J, et al. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J Transl Med 2012;10(1):174. [Internet] [cited 2017 January 31]. Available from http://www.ncbi.nlm.nih.gov/pubmed/22929533 doi: 10.1186/1479-5876-10-174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrons R, Tassone D.. Use of Lactobacillus probiotics for bacterial genitourinary infections in women: a review. Clin Ther. 2008;30(3):453–68. doi: 10.1016/j.clinthera.2008.03.013 [DOI] [PubMed] [Google Scholar]
- 5.Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis 2010;50(5):625–63. [Internet] [cited 2016 April 25]. Available from http://www.ncbi.nlm.nih.gov/pubmed/20175247 doi: 10.1086/650482 [DOI] [PubMed] [Google Scholar]
- 6.Tractenberg R, Groah SL, Rounds AK, Ljungberg IH, Schladen MM.. Preliminary validation of a Urinary Symptom Questionnaire for individuals with Neuropathic Bladder using Intermittent Catheterization (USQNB-IC): a patient-centered patient reported outcome. PLoS One. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 2009;75(23):7537–41. [Internet] [cited 2017 October 9]. Available from http://aem.asm.org/cgi/doi/10.1128/AEM.01541-09 doi: 10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schloss PD, Gevers D, Westcott SL. Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. Gilbert JA, editor. PLoS One 2011;6(12):e27310. [Internet] [cited 2017 October 9]. Available from http://dx.plos.org/10.1371/journal.pone.0027310. [DOI] [PMC free article] [PubMed]
- 9.Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R.. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011;27(16):2194–200. [Internet] [cited 2017 October 9]. Available from https://academic.oup.com/bioinformatics/article-lookup/doi/10.1093/bioinformatics/btr381 doi: 10.1093/bioinformatics/btr381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Q, Garrity GM, Tiedje JM, Cole JR.. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 2007;73(16):5261–7. [Internet] [cited 2017 October 9]. Available from http://aem.asm.org/cgi/doi/10.1128/AEM.00062-07 doi: 10.1128/AEM.00062-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–6. [Internet] [cited 2017 October 9]. Available from http://www.nature.com/doifinder/10.1038/nmeth.f.303 doi: 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Price MN, Dehal PS, Arkin AP. FastTree 2 – Approximately Maximum-Likelihood Trees for Large Alignments. Poon AFY, editor. PLoS One. 2010;5(3):e9490. [Internet] [cited 2017 February 4]. Available from http://www.ncbi.nlm.nih.gov/pubmed/20224823. [DOI] [PMC free article] [PubMed]
- 13.Faith D. Conservation evaluation and phylogenetic diversity. Biol Conserv 1992;61(1):1–10. [Internet] [cited 2017 October 9]. Available from http://www.sciencedirect.com/science/article/pii/0006320792912013.
- 14.Dixon P. VEGAN, a package of R functions for community ecology. http://dx.doi.org/101658/1100-9233(2003)014[0927:VAPORF]20CO;2 [Internet]. 2009 [cited 2017 October 9]. Available from http://www.bioone.org/doi/abs/10.1658/1100-9233(2003)014%5B0927:VAPORF%5D2.0.CO;2.
- 15.RStudio Team Rs . Integrated Development for R. Boston, MA: RStudio, Inc; 2015.
- 16.Hooton TM, Bradley SF, Cardenas DD, Colgan R, Geerlings SE, Rice JC, et al. Diagnosis, prevention, and treatment of catheter-associated urinary tract infection in adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin Infect Dis 2010;50(5):625–63. [Internet] [cited 2017 January 26]. Available from https://academic.oup.com/cid/article-lookup/doi/10.1086/650482 doi: 10.1086/650482 [DOI] [PubMed] [Google Scholar]
- 17.Karlsson M, Scherbak N, Reid G, Jass J.. Lactobacillus rhamnosis GR-1 ehances NF-kappaB activation in Escherichia coli-stimulated urinary bladder cels through TLR4. BMC Microbiol 2012;12(15). [Internet] [cited 2017 October 3]. Available from http://doi.wiley.com/10.1046/j.1365-2958.2001.02361.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schlager TA, Dilks S, Trudell J, Whittam TS, Hendley JO.. Bacteriuria in children with neurogenic bladder treated with intermittent catheterization: natural history. J Pediatr 1995;126(3):490–96. Available from http://www.ncbi.nlm.nih.gov/pubmed/7869216 doi: 10.1016/S0022-3476(95)70477-9 [DOI] [PubMed] [Google Scholar]
- 19.Ottolini MC, Shaer CM, Rushton HG, Majd M, Gonzales EC, Patel KM.. Relationship of asymptomatic bacteriuria and renal scarring in children with neuropathic bladders who are practicing clean intermittent catheterization. J Pediatr 1995;127(3):368–72. [Internet] [cited 2016 February 5]. Available from http://www.sciencedirect.com/science/article/pii/S002234769570065X doi: 10.1016/S0022-3476(95)70065-X [DOI] [PubMed] [Google Scholar]
- 20.Osset J, Bartolomé RM, García E, Andreu A.. Assessment of the capacity of Lactobacillus to inhibit the growth of uropathogens and block their adhesion to vaginal epithelial cells. J Infect Dis 2001;183(3):485–91. [Internet] [cited 2017 October 9]. Available from https://academic.oup.com/jid/article-lookup/doi/10.1086/318070 doi: 10.1086/318070 [DOI] [PubMed] [Google Scholar]
- 21.Silva M, Jacobus N V., Deneke C, Gorbach SL.. Antimicrobial substance from a human Lactobacillus strain. Antimicrob Agents Chemother. 1987;31(8):1231–3. doi: 10.1128/AAC.31.8.1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou J, Zhang R, Liu F, Yong X, Wu X, Zheng T, et al. Biogas production and microbial community shift through neutral pH control during the anaerobic digestion of pig manure. Bioresour Technol 2016;217:44–9. [Internet] [cited 2018 March 1]. Available from http://linkinghub.elsevier.com/retrieve/pii/S0960852416302140 doi: 10.1016/j.biortech.2016.02.077 [DOI] [PubMed] [Google Scholar]
- 23.Hutt P, Shchepetova J, Loivukene K, Kullisaar T, Mikelsaar M.. Antagonistic activity of probiotic lactobacilli and bifidobacteria against entero- and uropathogens. J Appl Microbiol 2006;100(6):1324–32. [Internet] [cited 2017 October 3]. Available from http://doi.wiley.com/10.1111/j.1365-2672.2006.02857.x doi: 10.1111/j.1365-2672.2006.02857.x [DOI] [PubMed] [Google Scholar]
- 24.Kontiokari T, Sundqvist K, Nuutinen M, Pokka T, Koskela M, Uhari M.. Randomised trial of cranberry-lingonberry juice and Lactobacillus GG drink for the prevention of urinary tract infections in women. Br Med J 2001;322(7302):1571.. [Internet] [cited 2017 October 3]. Available from http://www.ncbi.nlm.nih.gov/pubmed/11431298 doi: 10.1136/bmj.322.7302.1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baerheim A, Larsen E, Digranes A.. Vaginal application of lactobacilli in the prophylaxis of recurrent lower urinary tract infection in women. Scand J Prim Health Care 1994;12(4):239–243. [Internet] [cited 2017 October 3]. Available from http://www.ncbi.nlm.nih.gov/pubmed/7863140 doi: 10.3109/02813439409029247 [DOI] [PubMed] [Google Scholar]
- 26.Asahara T, Nomoto K, Watanuki M.. Antimicrobial activity of intraurethrally administered probiotic Lactobacillus casei in a murine model of Escherichia coli urinary tract infection. Antimicrob Agents Chemother. 2001;45(6):1751–1760. doi: 10.1128/AAC.45.6.1751-1760.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.