Abstract
Context
Allium cepa L. (Liliaceae), known as onion, is consumed throughout the world. Onion and its derivatives including saponins, aglycones, quercetin, cepaenes, flavonoids, organosulfurs, and phenolic compounds, showed various pharmacological properties and therapeutic effects.
Objective
Anti-inflammatory, antioxidant, and immunomodulatory effects of A. cepa and its main constituents, along with the underlying molecular mechanisms are presented.
Methods
Databases including, Web of Knowledge, Medline/PubMed, Scopus, and Google Scholar were checked for articles published between 1996 and the end of July 2020, using the key-words Allium cepa, quercetin, anti-inflammatory, antioxidant and immunomodulatory.
Results
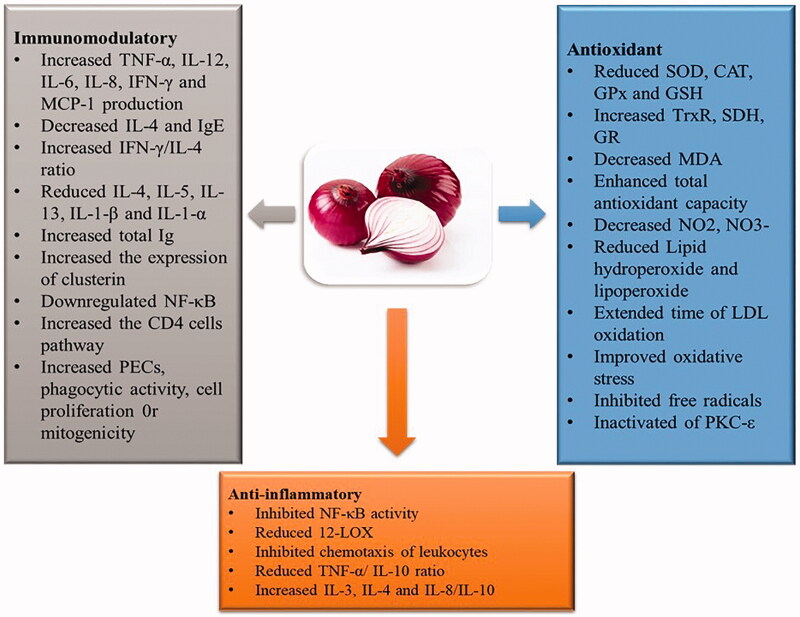
A. cepa and its constituents mainly quercetin showed anti-inflammatory effects mediated via reduction of total and differential WBC counts, inhibition of chemotaxis of polymorphonuclear leukocytes, COX, and LOX pathways and prevented formation of leukotrienes and thromboxanes, prostaglandin E2 (PGE2) as onVCAM-1, NF-κB, MARK,d STAT-1, JNK, p38 and osteoclastogenesis. A. cepa and its derivatives showed antioxidant effect by decreasing lipid peroxidation, NAD(P)H, MDA, NO, LPO and eNOS but enhancing antioxidants such as SOD, CAT, GSH, GPx, GSPO, TrxR, SDH, GST and GR activities and thiol level. Immunomodulatory effects of the plant and quercetin was also shown by reduction of Th2 cytokines, IL-4, IL-5, and IL-13 as well as IL-6, IL-8, IL-10, IL-1β and TNF-α and IgE levels, but increased CD4 cells, IFN-γ level and IFN-γ/IL4 ratio (Th1/Th2 balance).
Conclusions
The effect of onion and its constituents on oxidative stress, inflammatory and immune system were shown indicating their therapeutic value in treatment of various diseases associated with oxidative stress, inflammation, and immune-dysregulation.
Keywords: Onion, flavonoids, phenolic, inflammation, oxidative stress, quercetin
Introduction
Allium cepa L. (Liliaceae) is commonly known as onion. The Liliaceae family includes over 250 genera and 3700 species (Nasri et al. 2012; Akash et al. 2014; Bisen and Emerald 2016). Onion, one of the oldest cultivated plants (Lanzotti 2006), originated from central Asia (Benkeblia 2004; Albishi et al. 2013) and is currently cultivated all over the world, particularly in zones with moderate climates (Nasri et al. 2012; Bisen and Emerald 2016). A. cepa is characterised by its colour (yellow, red, or white), and taste (sweet or non-sweet) (Benkeblia 2004; Albishi et al. 2013). It is consumed fresh in powder form, as an essential oil (Corea et al. 2005; Takahashi and Shibamoto 2008), and as a spice to enhance food flavour due to its odour and taste (Bouba et al. 2014).
A. cepa contains several constituents (Benmalek et al. 2013) and shows various pharmacological properties. The oldest usage of A. cepa was reported from ancient Egypt where it was used because of its antimicrobial, anti-inflammatory, and other healing properties (Dorsch et al. 1988). From ancient times, it has also been recognised as an effective treatment for stomach diseases, throat infection, and hepatitis (Akash et al. 2014). In Chinese medicine, A. cepa tea is used against fever, headache, cholera, dysentery, common cold, and arthritis (Corzo-Martínez et al. 2007). This plant was also used as an antifungal (Lanzotti 2006), anticancer, anti-inflammatory (Elberry et al. 2014), antioxidant, antispasmodic (Albishi et al. 2013; Benmalek et al. 2013), antimicrobial, antimutagenic (Shri and Bora 2008), antidiabetic (Ali et al. 2000; El-Aasr et al. 2010; Nasri et al. 2012), antiplatelet (Galmarini et al. 2001), and anti-asthmatic agent (Takahashi and Shibamoto 2008). Moreover, A. cepa showed antimicrobial, antithrombotic, antitumor, anti-hyperlipidaemic, anti-arthritic, anti-hyperglycemic anticarcinogenic properties (Upadhyay 2017).
Long-term consumption of A. cepa produced a preventive effect on the incidence of vascular and heart diseases, neurodegenerative disorders and cataract formation (Albishi et al. 2013). Other pharmacological properties shown for this plant include improvement of kidney function (Bisen and Emerald 2016), and anthelmintic, aphrodisiac, carminative, emmenagogue, and expectorant activities; also, it has beneficial effects on dysmenorrhoea, vertigo, fainting, migraine, wounds, scars, keloids, pain and swelling after bee or wasp stings, and is used for treatment of bruises, earache, jaundice, and pimples (Shri and Bora 2008).
A. cepa and its constituents have been extensively studied and several original and review articles were published on their pharmacological effects; the present article is an updated review of the studies that examined anti-inflammatory, antioxidants and immunomodulatory effects of A. cepa and its main constituents including flavonoid and phenolic compounds.
Methods
The scientific databases, Web of Knowledge, Medline/PubMed, Scopus, and Google Scholar were searched to find studies on anti-inflammatory, antioxidants and immunomodulatory effects of A. cepa and its main constituents, published from 1996 until the end of July 2020. The following key words were used: ‘Allium cepa’, ‘onion’, ‘flavonoid’, ‘quercetin’, ‘organosulfur’, ‘saponin’, ‘phenolic compounds’, ‘anti-inflammatory’, ‘antioxidant’ and ‘immunomodulatory’.
Chemical constituents
A. cepa contains water, carbohydrates, proteins, lectin, fats, vitamins, minerals, flavonoids, and organosulfur, and phenolic compounds (Benmalek et al. 2013). Interactions among the genotype, environment and agronomic practices influence the quality of onion constituents (Agnieszka et al. 2017).
Also, onion is a pool of free amino acids including aspartate (Asp), glutamate (Glu), asparagine (Asn), serine (Ser), glutamine (Gln), histidine (His), glycine (Gly), threonine (Thr), arginine (Arg), alanine (Ala), tyrosine (Tyr), methionine (Met), valine (Val), tryptophan (Trp), phenylalanine (Phe), isoleucine (Ile), leucine (Leu) and lysine (Lys) which supply the nitrogen content of onion and produce the characteristic taste called 'umami' (Hansen 2001). Importantly, these amino acids in onion are potential fingerprints of geographical origin (Ianni et al. 2018). The amount of chemicals in onion varies based on the variety, geographical location and storage factors. Some varieties including yellow, red, and pink onions have high amounts of quercetin compared to white varieties. The highest level of flavonoids such as quercetin is observed in the dry skin and thus, peeling may significantly decrease these components and affect health benefits of onion. In addition, various cooking methods can affect flavonoid content of onion. Boiling significantly reduces flavonoids, microwaving has less effect on flavonoids content while frying results in the greatest loss (Hedges and Lister 2007).
There are several types of flavonoids present in the A. cepa in scales and disc of onion bulbs (Wiczkowski et al. 2008; Slimestad et al. 2007; Rodríguez Galdón et al. 2008; Ko et al. 2011; Shi et al. 2016; Rodrigues et al. 2017). More than 80% of the total content of flavonoids exists in the outer scales of A. cepa which is a result of exposure to sunlight. Free form of phenolic constituents is predominant in yellow and white onion skins, red onion flesh, sprouted flesh and green shoot (Albishi et al. 2013). Different types of onion significantly vary in terms of polyphenols. Yellow onion has the highest levels of flavonoids, 11-time higher than that of white onion. Red onion contains significant amounts of anthocyanin and has 10% flavonoid content (Yang et al. 2004; Slimestad et al. 2007).
Two groups of chemicals that are abundant in onions and have health benefits to humans, include flavonoids and alkenyl cysteine sulphoxides (Griffiths et al. 2002). About 31 unique proteins in the lower epidermis (LE) and upper epidermis (UE) of the scales of onion bulb were identified which involved in pigment synthesis, stress response, and cell division. Differences in chalcone-flavanone isomerase and flavone O-methyltransferase 1-like in LE of the onion scale, were shown to be responsible for red and yellow colours of onions. Also, differences in UDP-arabinopyranose mutase 1-like protein and β-1,3-glucanase in the LE, may be related to the differences in cell sizes in LE and UE of red and yellow onions (Wu et al. 2016). It was also shown that onions contain allylsulfides and flavonoids including quercetin which exert antioxidative activities and could reduce hepatocytes apoptosis; also, onion contains steroid saponins and sapogenins, and β-chlorogenin which is a characteristic steroid sapogenin. The other constituents of A. cepa are organosulfur compounds, including DATS, diallyl disulphide (DADS), ajoene, and sallylmercaptocysteine (SAMC), with cell cycle arrest effect in cancer cells (Upadhyay 2017).
Total polyphenols are 444.3–1591 mg/kg in garlic (A. sativum L.), chives (A. schoenoprasum L.), ramson (A. ursinum L.) and red, yellow and white onion (A. cepa L.) which decline in the order of chives > red onion > garlic > yellow onion > ramson > white onion (Lenková et al. 2016). Phytochemical analysis of A. cepa L. and A. cornutum using high-performance liquid chromatography (HPLC), showed that two quercetin conjugates, (1) and (2), account for about 80% of the total flavonol content in both onions (Fredotović et al. 2017).
The highest concentrations of quercetin, quercetin 3-β-d-glucoside, luteolin, and kaempferol in cv. ‘NHRDF Red’ (11,885.025 mg/kg), ‘Hissar-2′. (1432.875 mg/kg), ‘Pusa Riddhi’ (1669.925 mg/kg) and ‘Bhima Shakti’ (709.975 mg/kg), were found in dry skin of 15 Indian cultivars onion when their flavonoid concentration, total phenolic content (TPC) and total flavonoid content (TFC) were examined. In addition, cv. ‘NHRDF Red’ had the highest while cv ‘Bhima Shubhra’ had the lowest TPC and TFC content (Sagar et al. 2020). Detailed constituents of A. cepa are shown in Table 1.
Table 1.
The major constituents of Allium cepa.
| Major compounds | Minor compounds | Reference. |
|---|---|---|
| Water | Nile and Park (2014) | |
| Proteins | Nile and Park (2014), Ashwini and Sathishkumar (2014) | |
| Carbohydrates | Inulin, fructooligosaccharides, isorhamnetin-4-glucoside, galactose, glucose and mannose | Nile and Park (2014) |
| Vegetal hormone Lectin |
Glycoquinine | Corzo-Martínez et al. (2007) |
| Steroids | Catechol, protocatechnic acid, thiocyanate and thiopropiono aldehyde | Ashwini and Sathishkumar (2014), Nile and Park (2014), Benmalek et al. (2013) |
| Phytoestrogens | Coumestrol, zearalenol, isoflavones and humulone | Ashwini and Sathishkumar (2014), Nile and Park (2014), Benmalek et al. (2013) |
| Vitamins | A, B complex, C and E | Ashwini and Sathishkumar (2014), Nile and Park (2014), Benmalek et al. (2013) |
| Minerals | Selenium, phosphorus, iron, calcium and chromium | Nile and Park (2014), Ashwini and Sathishkumar (2014) |
| Flavonoids | Quercetin, apigenin, rutin, myricetin, kaempferol, catechin, resveratrol, epigallocatechol-3-gallate, luteolin and genistein Quercetin aglycone, quercetin diglucoside, quercetin 4-glucoside and isorhamnetin monoglycoside or kaempferol monoglycoside |
Ashwini and Sathishkumar (2014); Lanzotti (2006), Benmalek et al. (2013), Corzo-Martínez et al. (2007), Yamamoto and Yasuoka (2010), Nile and Park (2014), Rhodes and Price (1996), Wiczkowski et al. (2008), Shi et al. (2016), Rodríguez Galdón et al. (2008), Ko et al. (2011), Rodrigues et al. (2017), Slimestad et al. (2007) |
| Organosulfuric compounds | Thiosulphinates, cepaenes, cysteine, S-methyl cysteine sulfoxide, diallyl disulphide, allyl methyl sulphide, allyl propyl disulphide, gamma-L-glutamyl-trans-S-1-propenyl-L-cysteine sulfoxide, S-propenyl cysteine sulfoxide, S-alk(en)yl cysteine sulfoxides, S-allyl cysteine sulfoxide | Ashwini and Sathishkumar (2014), Lanzotti (2006), Nile and Park (2014), Benmalek et al. (2013) |
| Allicin | Diallyl disulphide, diallyl trisulphide and ajoene | Ashwini and Sathishkumarn (2014), Lanzotti (2006), Nile and Park (2014), Corzo-Martínez et al. (2007), Benmalek et al. (2013) |
| Phenolic compounds | Phenolics, phenolic acids, anthocyanins and hydroxycinnamic acid | Lanzotti (2006), Nile and Park (2014) |
| Lipophilic antioxidants | Dialkyl disulphides, aglycones, anthocyanin, saponins and fistulosin (octadecyl 3-hydroxyindole) | Ashwini and Sathishkumar (2014), Lanzotti (2006), Takahashi and Shibamoto (2008), Benmalek et al. (2013), Ernst and Feldheim (2000), Corzo-Martínez et al. (2007), Dorsch et al. (1990), Yamamoto and Yasuoka (2010), Nile and Park (2014), Griffiths et al. (2002), Augusti (1996), Rhodes and Price (1996), Khaki et al. (2009), Kuhnau (1976), Arjmandi et al. (1996) |
Anti-inflammatory effects of A. cepa
Inflammation is a defensive reaction of the body to eliminate damaging factors and re-establish domestic homeostasis. In the process of inflammation, blood flow increases in the damaged site due to the release of vasodilatory agents, capillary permeability enhances, and migration of white blood cells to the inflamed site is augmented; these lead to emergence of classic symptoms of inflammation namely, redness, warmth, swelling, pain, and in some cases, stiffness, and ultimately, damage to the affected area (O'Byrne and Dalgleish 2001). These changes are induced by cytokines and other inflammatory mediators (Dalgleish and O'Byrne 2002). Cytokines are classified into two major categories: pro-inflammatory and anti-inflammatory cytokines. Several cytokines including interleukin (IL)-1, tumour necrosis factor-alpha (TNF-α), IL-6 and IL-8, and chemokines such as granulocyte colony stimulating factor (G-CSF) and granulocyte-macrophage colony stimulating factor (GM-CSF) play a key role in acute inflammatory reactions (Rothwell et al. 1996). Several studies showed alleviative effects of onion and its active ingredients on inflammation and suggested that Allium plants are effective in treating inflammatory disorders at lower costs with limited side effects, in comparison to chemical drugs (Ali et al. 2000), as detailed below.
Anti-inflammatory effects of the plant
Administration of aqueous extract of bulb of the red onion (EAC; 150 and 300 mg/kg) decreased eosinophil and lymphocyte counts in the blood and bronchoalveolar lavage fluid (BALF) in a Wistar rat model of asthma (Dawud et al. 2016). Anti-inflammatory and antibacterial effects of A. cepa were shown in previous studies and it was mentioned that this plant was extensively used for cure of catarrhal diseases, flu, angina, catarrh, cough, and prostatic hypertrophy (Kumar et al. 2010). The methanol extract A. cepa (50, 250 and 500 µg/mL) also showed a protective effect on neuroinflammation in lipopolysaccharide (LPS)-treated BV-2 microglial cells by reducing of pro-inflammatory cytokines TNF-α, IL-6, and IL-1-β (Jakaria et al. 2019).
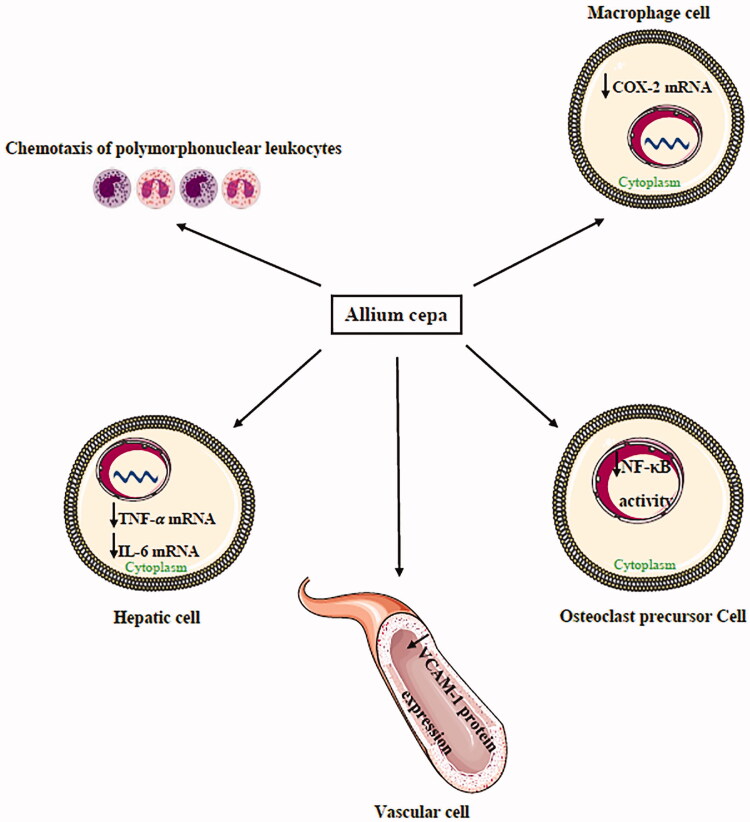
It was observed that A. cepa skin extracts decreased cyclooxygenase-2 (COX-2) mRNA level in J774A.1 mouse macrophage cells treated with LPS (Albishi et al. 2013). In another study, intra-gastric intubation of juice of fresh onion bulbs at a daily dose of 1 mL sngiotensin converting enzyme (CE) for 14 days, inhibited COX and lipoxygenase (LOX) pathways and prevented formation of leukotrienes and thromboxanes in a male Sprague-Dawley rat model of doxorubicin-induced cardiotoxicity (Alpsoy et al. 2013)Aqueous extract of A. cepa also attenuated vascular inflammation by decreasing protein expression of vascular cell adhesion protein 1 (VCAM-1) (Alpsoy et al. 2013).
The bulb extract of A. cepa (35, 70, and 140 mg/kg/day for 21 days) significantly reduced total WBC and lung inflammatory cells such as neutrophil, eosinophil and monocyte counts, but led to a significant increase in lymphocytes counts in asthmatic Wistar rats (Ghorani et al. 2018) and the extract resulted in relaxation of tracheal smooth muscle (Memarzia et al. 2019). Also, the extract of onion reduced lung inflammatory cytokines such as IL-4, 5, and 13 and T helper 2 in BALF of asthmatic animals. These findings suggested therapeutic potential of A. cepa in the treatment of allergic disorders such as asthma (Oliveira, Campos et al. 2015). Chloroform extracts of yellow onion bulbs (0.3, 1, 3, 10 and 30 µM) inhibited M2 receptor in human monocyte-derived macrophages and suppressed the expression of CD163 which is involved in inflammation processes (El-Aasr et al. 2010). Methanol extract of the outer scales and edible parts of A. cepa bulb (100 and 200 mg/kg) showed protective effects on the infarct volume after cerebral ischaemia as reflected by a marked decrease in inflammation cascade in Swiss albino mice (Shri and Bora 2008). Onion peel hot water extract (0.1, 1, 10, 50, 100 µg/mL) administered to BALB/c mice, produced considerable anti-inflammatory activities as it could suppress the production of pro-inflammatory cytokines IL-6, TNF-α, and IL-1-β (Kang et al. 2015). It was shown that fresh onion juice (10 mg/kg) inhibited both acute and chronic pain and significantly decreased hind-paw thickness in male albino mice (Nasri et al. 2012). Steam distillate from freeze-dried A. cepa (methanol and water) attenuated 15-lipoxygenase type I activity which is an inflammatory mediator (Takahashi and Shibamoto 2008). Alcohol, chloroform and water extract of bulbs of A. cepa (300 mg/kg) showed wound healing activities in Wister albino rats which indicated the effect of the plant on initial phases of wound formation as an acute inflammatory process (Shenoy et al. 2009).
The anti-inflammatory properties of the plant against carrageenan-induced paw edoema in rats, were also investigated. Fresh onion juice was able to significantly decrease hind-paw thickness and demonstrated better results compared to the standard treatment, diclofenac (10 mg/kg). The anti-inflammatory effect of onion was attributed to it potential in preventing the formation of leukotrienes and thromboxanes via inhibition of COX and LOX pathways (Alpsoy et al. 2013).
Anti-inflammatory effects of the constituents of A. cepa
It was reported that anti-inflammatory properties of Allium species are due to the presence of effective compounds such as tannin, flavonoids, anthocyanin, saponin, etc. (Aathira et al. 2020). A. cepa contains various flavonoids which can help in treatment of oxidative stress-mediated diseases, as well as inflammation, and thermal and mechanical hyperalgesia associated diseases (Vazhappilly et al. 2019).
Thiosulfinates and cepaenes found in A. cepa, can inhibit production of arachidonic acid as well as its downstream pro-inflammatory prostaglandins and leukotrienes (Wilson and Demmig-Adams 2007). Thiosulfinates and cepaenes (100 µM) exerted anti-inflammatory properties mediated through inhibition of chemotaxis of human polymorphonuclear leukocytes. It was also shown that cepaenes inhibit COX and LOX enzymes (Dorsch et al. 1990).
Quercetin, a well-known constituent of A. cepa showed several biological activities including reduction of swelling (inflammation), lung tightness and cholesterol and sugar levels in the blood (Hashemzaei et al. 2017; Aathira et al. 2020).
Quercetin (0, 0.1, 1, 10, 25 and 50 µM) significantly suppressed nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) induced by receptor activator of NF-κΒ ligand (RANKL) in MC3T3-E1 preosteoblastic cell line (Yamaguchi and Weitzmann 2011), (Figure 1). Quercetin also acts as a potent antioxidant and anti-inflammatory agent. This compound decreased the production of inflammatory cytokines such as IL-1α, IL-4, and TNF-α and inhibited the proliferation and activity of lymphocytes. In addition, quercetin reduced TNF-α/IL-10 and IL-8/IL-10 ratios in animal and human studies (Lanzotti 2006; Rivera et al. 2008; Boots et al. 2011). Umoh et al. (2019) showed that red onion is able to decrease inflammation by inhibition of NF-κB, MARK and STAT-1 possibly by its effective components such as quercetin.
Figure 1.
The anti-inflammatory effects of Allium cepa in different cells.: Decrease. COX-2: cyclooxygenase-2, IL-6: interleukin-6, NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells, TNF-α: tumour necrosis factor-alpha, VCAM-1: vascular cell adhesion protein 1.
Vazquez-Prieto et al. (2015) exanimated the effects of 6-week treatment with dietary catechin, quercetin, and a mixture of both, on tumour necrosis factor alpha (TNF-α) and adipose inflammation induced by high-fructose consumption, in Wistar rats. Catechin, quercetin, and their combination at the dose of 20 mg/kg/day, improved pro-inflammatory cytokines expression such as mitogen-activated protein kinase (MCP-1), resistin, and adipose tissue inflammation. In addition, catechin, quercetin, and their combination reduced the activation of the mitogen-activated kinases (MAPKs), JNK and p38. Also, catechin, quercetin, and their combination prevented downregulation of PPAR-γ.
Treatment with quercetin (0.1%) for 8 weeks, suppressed hepatic expression of TNF-α and IL-6 and decreased inflammation in high-fat diet/streptozotocin (STZ)-induced diabetic male Sprague-Dawley rats (Jung et al. 2011). It was also reported that quercetin was more effective in inhibiting inflammation processes compared to onion itself (Simin et al. 2013). In vitro studies showed that A. cepa extract and quercetin down-regulated NF-κB level and inhibited osteoclastogenesis in inflammatory conditions induced by LPS (Oliveira, Figueiredo et al. 2015). The anti-inflammatory activities of quercetin were attributed to its inhibitory effects on production of eicosanoids like thromboxane B2 (TXB2), prostaglandin E2 (PGE2) and 12 (S)-hydroxy-(5Z,8Z,10E,14Z)-eicosatetraenoic acid (12-HHT) which are inflammatory mediators derived from arachidonic acid (AA) (Lesjak et al. 2018). A. cepa (10, 100 and 1000 µM) and quercetin (3.5, 7.5 and 15 µM) also reduced the level of inflammatory cytokines such as IL-4, −5 and −13 in the BALF in a murine model of asthma (Oliveira, Campos et al. 2015). Some reports showed that allyl methyl disulphide (100 µM) from garlic had an anti-inflammatory effect on human colon cancer cell lines HT-29 and Caco-2 by increasing IL-8/IP-10 formation and suppressing IL-8 mRNA level in intestinal epithelial cells. Active butanolic fraction of ethanol extract of dried and grounded onion, quercetin and luteolin showed anti-inflammatory properties comparable to ibuprofen in rat peritoneal mast cells, and anti-edematogenic effect in the early phase of carrageenan-induced paw edoema (Zhang et al. 2015).
According to Simin et al. (2013), phenolic compounds in methanol extract of Allium flavum subsp. flavum or small yellow onion (71 and 81 µg/mL), including p-coumaric, caffeic, p-hydroxybenzoic, vanillic, protocatechuic and syringic acid, rutin, quercetin-3-O-glucoside and kaempferol-3-O-glucoside, expressed a high inhibitory potential for COX-1 and 12-lipoxygenase (12-LOX) activity. The methanol extract inhibited cell growth of cervix epithelioid carcinoma and colon adenocarcinoma cells. Phenolic compounds in soluble extracts of pearl onion skin at concentrations as low as 5 µg/mL, showed an antioxidant, anti-inflammatory and DNA scission inhibitory activity and inhibition of COX-2 expression and LDL cholesterol oxidation (Albishi et al. 2013).
Pre-treatment with the phenolic-rich extract of red onion peels (100 and 500 mg/kg) against oxidative stress induced by carbon tetrachloride (CCl4) free radicals in rat liver and kidney, ameliorated tissue levels of malondialdehyde (MDA) and significantly reduced the net carrageenan-induced edoema in the paw of rats (Ahmed et al. 2017).
The results of the above studies indicated the effect of A. cepa and its constituents such polyphenolics and flavonoids mainly quercetin, in inflammatory disorders of cardiovascular, gastrointestinal, neuronal respiratory and urogenital systems. The anti-inflammatory effects of the plant and its constituents were mediated via modulation of different inflammatory cells and mediators. Reduction of total WBC, neutrophils and eosinophil counts and inhibition of chemotaxis of human polymorphonuclear leukocytes, were reported in this context. The plant and its constituents showed inhibitory effects on COX and LOX pathways and prevented formation of leukotrienes and thromboxanes (such as TXB2), prostaglandin E2 (PGE2) and 12-HHT. Inhibitory effects of A. cepa and its constituents onVCAM-1, NF-κB, MARK,d STAT-1, JNK, p38 and osteoclastogenesis as well as downregulation of PPAR-γ were also shown. The results also indicated that the anti-inflammatory effects of the plant is due to its constituents mainly quercetin. The anti-inflammatory effects of A. cepa and its constituents are shown in Table 2.
Table 2.
The anti-inflammatory effects of Allium cepa and its constituents.
| Preparations | Doses | Model of study | Effects | Reference |
|---|---|---|---|---|
| Onion juice |
10 mg/kg | Sprague-Dawley rat pain model | Inhibition of pain induced by inflammation | Nasri et al. (2012) |
| Methanolic Ext, outer scales and bulb | 100 mg/kg and 200 mg/kg | Cerebral ischaemia model in Swiss albino mice | Reduction of infarct volume | Shri and Bora (2008) |
| methanol Ext, A. flavum | 0.5-12 mg/mL | Human cell lines | Reduction of COX-1 and 12-LOX | Simin et al. (2013) |
| Allicin | 5–20 mg/mL to >100 mM | Intestinal epithelial cells | Reduction of inhibition of TNF-α and IL-1β | Zhang et al. (2015) |
| Allium cepa Ext | 1 mL/d for 14 days | Doxorubicin-induced cardiotoxicity Sprague-Dawley model |
Inhibited COX and LOX pathways | Alpsoy et al. (2013) |
| Allium cepa Ext | 35, 70 and 140 mg/kg/d, 21 days | Asthma model in Wistar rat | Reduced total WBC and lung inflammatory cells | Marefati et al. (2018) |
| Aqueous peel Ext quercetin | 0.5 or 1% of OPE | STZ-induced diabetic Sprague-Dawley rats | Suppressed hepatic expressions of TNF-α and IL-6 | Jung et al. (2011) |
| synthetic thiosulfinates, cepaene | 0.1-100 microM | Human granulocytes chemotaxy | Inhibition of chemotaxis of leukocytes | Dorsch et al. (1990) |
| Quercetin | 15 mg/d | Human study | Reduces the TNF-α/IL-10 ratio | Boots et al. (2011) |
| Quercetin | 2 or 10 mg/kg/day, Gavage, 10 weeks |
Zucker Rat | Reduction TNF-α | Rivera et al. (2008) |
| Quercetin | 2.5 and 5 µM | LPS-Induced Osteoclastogenesis in murine macrophage cells | Decreased NF-κB, increased IL-3 and IL-4 | Oliveira, Figueiredo et al. (2015) |
| Quercetin | 30 mg/kg | Asthma murine model | Reduced IL4,5 and 13 in BALF | Oliveira, Campos et al. (2015) |
| Allyl methyl disulphide | 0.5 and 1 mL/100 g bw/day | Human intestinal epithelial HT-29 cells | increase of IL-8/IP-10 suppression of the IL-8 mRNA |
Zhang et al. (2015) |
| Phenolic compounds | 0.125 and 0.5 mg/mL | J774A.1 mouse macrophage cells | Decreased COX-2 mRNA | Albishi et al. (2013) |
| Allicin | 5–20 mg/mL to >100 mM | Intestinal epithelial HT-29 and Caco-2 cells | Reduction of TNF-α and IL-1β | Zhang et al. (2015) |
| Synthetic thiosulfinates, and cepaene | 0.1-100 microM | Human granulocytes chemotaxy | Inhibition of chemotaxis of leukocytes | Dorsch et al. (1990) |
Ref.: References, NF-κB: Nuclear Factor beta, COX: Cyclooxygenase, LOX: lipoxygenase, TNF-α: Tumour necrosis factoralpha, IL-1β: Interleukin 1 beta, bulf: bronco lung fluid, WBC: White blood cell, d: Day, Ext: Extract. COX: Cyclooxygenase, TNF-α: Tumour necrosis factoralpha, IL: Interleukin, Bulf: bronco lung fluid, d: Day.
Antioxidant effects of A. cepa
Oxidative stress is characterised by over production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) (Karimian et al. 2012). These free radicals, mainly nitric oxide, superoxide anion, hydroxyl radical and hydrogen peroxide, can cause oxidative damages to nucleic acids, proteins, and lipids. Thus, excess production of free radicals under pro-inflammatory conditions may initiate various diseases (Kim et al. 2012; Rezaee et al. 2014; Ghorani et al. 2018). Natural antioxidants are compounds that can delay or inhibit oxidative reactions by scavenging free radicals. The most important of these compounds are phenolic acids, polyphenols, flavonoids, alkaloids and terpenoids (Del Bano et al. 2006; Amidi et al. 2012; Kim IS et al. 2012). Therefore, suppression of oxidative stress could be achieved by using potential sources of natural antioxidants such as medicinal plants (Zarei et al. 2013; Parhiz et al. 2015; Boskabady et al. 2019; Hashemzaei et al. 2020). Essential oils derived from these plants, are rich sources of antioxidant components with different biological activities (Hasani-Ranjbar et al. 2009). A. cepa contains high levels of phenolic compounds mainly flavonoids, which have antioxidant properties besides other pharmacological effects such as antibiotic, antidiabetic, anti-atherogenic and anticancer activities (Helen et al. 2000; Liguori et al. 2017). Flavones, flavanones, flavonols, isoflavones, flavanonols, chalcones, and anthocyanins which are subclasses of flavonoids and flavonols, are the most abundant flavonoids in A. cepa (Liguori et al. 2017). Several studies reported the antioxidant activities of A. cepa and its constituents and introduced the plant as a potential source of natural antioxidants (Razavi and Kenari 2016; Roldán et al. 2008; Ola-Mudathir and Maduagwu 2014). Alterations in oxidant/antioxidant markers including lipid peroxidation (LPO), glutathione (GSH), superoxide dismutase (SOD), catalase (CAT), and MDA were observed by studies that investigated the effects of A. cepa and its constituents (Dadkhah et al. 2014).
Antioxidant effects of the plant
Several studies examined the antioxidant capacity of onion essential oils and extracts using different methods including diphenyl-1-picrylhydrazyl (DPPH), β-carotene bleaching assays, azinobis (3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS), oxygen radical absorbance capacity (ORAC) and Trolox equivalent antioxidant capacity (TEAC); findings confirmed remarkable antioxidant activity for the plant (Benkeblia 2005; Kim et al. 2010; Santas et al. 2010; Ye et al. 2013; Lisanti et al. 2016; Fredotović et al. 2017).
Razavi and Kenari (2016) reported the relationship between phenolic content and antioxidant activity of red onion peel extract using two methods of inhibition of free radical DPPH and β-carotene-linoleate bleaching assay. The results revealed that onion extract has a great antioxidant capacity and exhibited a significant relation between phenolic content and antioxidant activity of the plant. Furthermore, Lee et al. (2012) showed that Sprague-Dawley rats treated with red onion diet containing 5% red onion flesh for 4 weeks, exerted an elevation in the plasma SOD and glutathione peroxidase (GPx) activity.
Similarly, the antioxidant activity of onion peels extracts was assessed by DPPH radical scavenging activity. The results suggested that onion peel extracts have a remarkable antioxidant activity (Joung and Jung 2014).
In another study, the potency of methanol extract of A. cepa to scavenge free radicals, was examined by DPPH and ORAC methods which confirmed the extract’s phenolic compounds antioxidant effects (Fredotović et al. 2017). The radical scavenging and antioxidant activities of extracts of skin and edible part of A. cepa, were investigated by Škerget et al. (2009). Results showed a robust radical scavenging potential for the onion skin pure acetone extract, while the highest antioxidant activity was found for the onion skin extracted by 35 and 60% acetone and 60% ethanol. Also, a low antioxidant activity was seen for onion edible part in these experiments. Santas and colleagues (2010) reported the antioxidant properties of three different Spanish A. cepa cultivars including white skinned onion ‘Fuentes de Ebro’ white skinned onion ‘Calçot de Valls’ and yellow skinned onion ‘Grano de Oro’. Ethyl acetate sub-fraction contained the highest number of flavonoids and the TEAC was 74.86, 24.59 and 4.55 mol Trolox/g for ‘Grano de Oro,’ ‘Fuentes de Ebro’ and ‘Calçot de Valls,’ respectively. A potent antioxidant potential was also reported for the bulb methanol extracts of three A. cepa cultivars including ‘Pusa Red’ (red), ‘Pusa White Round’ (white) and ‘Arka Pitamber’ (yellow) (Santas et al. 2010) and a strong antioxidant activity was shown for red onion ‘N-530 from India (Singh et al. 2009). Benmalek and co-workers (2013) assessed the radical scavenging activity of A. cepa and showed an IC50 value of 2.91 × 10−5 mg/mL for the outer layer of red onion.
Ren et al. (2017) measured the in vitro antioxidant activity of two onion varieties, Hyskin and Red Baron grown in a six-year field study. The effects of conventional, organic and mixed cultivation practices on phytochemical composition and antioxidant activity were also verified.
Gawlik-Dziki et al. (2013) measured the antioxidant potential of bread enriched with A. cepa skin. The food supplement was prepared by drying onions (‘Wolska’) in an oven at 50 °C followed by powdering the plant material using a laboratory mill. In these experiments, the flour used in the formula of control bread (wheat bread flour 600 g, type 750) was replaced with onion skin at 1, 2, 3, 4, and 5% levels. Bio-accessibility and bio-availability were determined in vitro using a human gastrointestinal tract model. Breads were then enriched with 80% methanol extract and antioxidant activity was measured in terms of antiradical activity, potential to suppress lipid peroxidation, metal chelating activity and ferric reducing power. The antioxidant activity of onion-supplemented bread was significantly higher than that seen for the control. Also, Helen et al. (2000) mentioned that 100 mg/kg onion oil given for 21 days is a potent antioxidant against oxidative injury caused by nicotine in Sprague-Dawley rats and its antioxidant activity was comparable to that of vitamin E.
In vitro antioxidant activity of methanol and aqueous extracts of A. cepa was compared using various methods such as DPPH and superoxide scavenging activity. The results showed that both extracts had antioxidant activity but this capacity was higher for the methanol extract of onion (Kaur et al. 2016). Various in vitro studies showed the presence of higher levels of antioxidants in oil and extracts of A. cepa using DPPH radical scavenging activity and other methods. In vivo evidence also confirmed potential antioxidant activity of the plant in different animal models (Votto et al. 2010; Cheng et al. 2013; Ye et al. 2013; Soto et al. 2015; Lenková et al. 2016; Shrestha et al. 2016; Ola-Mudathir et al. 2018).
In a Sprague-Dawley rat model, the antioxidant effect of A. cepa oil on nicotine-induced damages was compared to vitamin E. The results showed that 100 mg/kg/day onion oil given for 21 days led to significant increases in antioxidants (SOD, CAT and GSH) levels suggesting that A. cepa is an effective antioxidant against oxidative damage induced by nicotine (Helen et al. 2000).
Enhancement of antioxidant parameters such as SOD, CAT, thioredoxin reductase (TrxR), sorbitol dehydrogenase (SDH), and glutathione reductase (GR) and decrease of LPO were observed in the liver of mice infected with Schistosoma mansoni after treating with onion powder (2 g/100 g bw/day), (Mantawy et al. 2012). The protective effect of onion extract against doxorubicin-induced hepatotoxicity in rats, was also demonstrated. Doxorubicin, a chemotherapeutic agent, produces cardiotoxicity (Georgiadis et al. 2020) and hepatotoxicity via production of free oxygen radicals. However, significant reductions of MDA level, and increased levels of SOD, GSH and GPx were observed in Sprague-Dawley rats after treatment with 1 mL/day aqueous extract of onion, for 14 days (Mete et al. 2016). Similarly, aqueous extract of A. cepa (100 and 300 mg/kg/day) caused hepato-protective effects by improvement of antioxidant parameters such as SOD, CAT, GPx, GSH and MDA in alloxan-induced diabetic rabbits (Ogunmodede et al. 2011).
Cadmium, as a nephrotoxic agent, causes kidney damage through induction of oxidative stress. Preventive effect of A. cepa aqueous extract (1 mL for 8 weeks) against cadmium-induced renal dysfunction in a Wistar rats, was evaluated and the results showed significant improvements in plasma and tissue levels of SOD, CAT and MDA (Ige et al. 2009). Another study also showed that treatment of cadmium-intoxicated Wistar rats with aqueous extract of A. cepa (0.5 and 1 mL onion/100 g bw/day) for 7 days, led to a significant and dose-dependent restoration of renal oxidant (lipid peroxidation and glutathione-S transferase)/antioxidant (SOD, CAT and GSH) parameters (Suru 2008). The protective effects of methanol extract of A. cepa on cyanide-induced renal toxicity, were assessed in Wistar rats. Significant increases in antioxidant enzymes (SOD, CAT and GSH) and a significant reduction of MDA and LPO in the kidney, were observed in rats treated for 14 days with 600 mg/kg/day onion extract (Ola-Mudathir and Maduagwu 2014).
Administration of onion aqueous extract (0.5 and 1 mL onion/100 g bw/day) for 6 weeks, caused marked increases in hepatic and renal levels of GSH, GST, SOD and CAT, but significant reductions in MDA level in Wistar rats (Suru and Ugwu 2015).
In an in vivo study, the antioxidant effect of A. cepa juice (3 mL/day) on Wistar rat testis tissue and seminiferous tubules affected by Escherichia coli, was evaluated and the results showed significant increases in total antioxidant capacity after treatment of animals with A. cepa juice. Thus, this plant showed protective effects against E. coli infection-induced oxidative stress (Shahverdi et al. 2017). A. cepa aqueous extract (0.5 and 1 mL onion/100 g bw/day for 7 days) against cadmium-induced damage in prostate glands of Wistar rats produced significant improvements in oxidant/antioxidant status. These results suggested a chemoprotective capacity for this plant against biochemical alterations induced by cadmium in the prostate glands (Ola-Mudathir and Suru 2015). Protective effects of various doses (0.5 and 1.0 mL/100 g bw/day for one week) of onion aqueous extracts, were also indicated on sperm and testicular oxidative damage induced by cadmium in Wistar rats, which were mediated through reduction of LPO and MDA as well as improved antioxidant parameters (Ola-Mudathir et al. 2008).
The effects of A. cepa on the levels of oxidants and antioxidant markers in the BALF of ovalbumin-sensitised Wistar rats, were evaluated. Treatment with A. cepa juice (0.175, 0.35, or 0.7 mg/mL) significantly reduced oxidant markers such as nitrogen dioxide (NO2), nitrate (NO3–), and MDA but increased the levels of SOD and CAT in sensitised Wistar rats (Marefati et al. 2018).
Treatment of STZ-induced diabetic Wistar rats with aqueous extract of onion (0.4 g/mL/day) resulted in reduction of lipid hydroperoxide and lipoperoxide concentrations but did not alter GPx (Campos et al. 2003). Also, the level of free radicals was diminished in plasma and tissues of alloxan-induced diabetic rats after administration of onion extract (El-Demerdash et al. 2005) which was in agreement with previous studies (Baynes and Thorpe 1999; Kumari and Augusti 2002). Campos and co-workers (2003) examined the effects of the consumption of onion extract (40 g/100 mL for 30 days) in STZ-induced diabetic Wistar rats. It was shown that onion intake reduced SOD activity and prevented the increment of lipid hydroperoxide and lipoperoxide concentrations in treated diabetic rats. The antioxidant potential of the ethanol extract and fractions of aerial parts of A. cepa was examined by Baragob et al. (2015), in in vitro and in vivo studies. In vitro experiments used DPPH and NO radical scavenging methods, whereas the in vivo effects on antioxidant enzyme were investigated in the erythrocytes and pancreas of normal and STZ-induced diabetic albino rats. Before treatment, compared to diabetic groups, normal groups had higher levels of SOD, CAT, GSH but lower LPO level, while administration of A. cepa ethanol extract (200 mg/kg/day for 21 days) and its chloroform fraction, significantly augmented the levels of SOD, CAT and GSH and declined LPO level to near normal levels, in the diabetic groups.
Vazquez-Prieto et al. (2011) indicated anti-inflammatory and antioxidant effect of onion so that oral administration of onion extract (400 mg/kg/day for 8 weeks) in fructose-fed Wistar rats led to attenuation of lipid peroxidation and NAD(P)H oxidase activity and decreased heart endothelial nitric oxide synthase (eNOS) activity that are related to oxidative stress. Also, they found that the vascular inflammation was decreased through reduction of VCAM-1 expression.
In addition, the effects of processing technologies and storage conditions on antioxidant capacity of onions were investigated. Siddiq et al. (2013) showed that usage of mild-heat (50 and 60 °C) treatment for processing fresh-cut onions, did not affect the antioxidant activity (as assessed by ABTS and DPPH analysis) and the colour of fresh-cut onions.
The oxygen radical scavenging capacity of A. cepa was also reported (Kim et al. 2010). Pulp and skin of onion were extracted using distilled water and 95% ethyl alcohol. The highest ORAC value and total phenolic content were detected for the ethyl alcohol extract of onion skin. Lee et al. (2014) evaluated the antioxidant properties of four different extracts of A. cepa peels. Plant material was extracted by hot ethanol (60 °C), and hot water (80 °C) and by means of subcritical water extraction at 110 and 165 °C. The ethanol onion peel extract showed a better DPPH radical scavenging activity and a higher antioxidant activity compared to the other samples as determined by ferric thiocyanate assay.
Also, the antioxidant properties of the essential oil of A. cepa extracted by supercritical CO2 extraction were assayed by ABTS assay. The essential oil showed antioxidant (IC50 0.67 mg/mL), using DPPH test (IC50 0.63 mg/mL) and metal chelating assay (IC50 0.51 mg/mL) (Ye et al. 2013).
In a clinical study, administration of 100 mL of onion juice daily for 8 weeks to subjects with mild hypercholesterolaemia, increased total antioxidant capacity and extended time of LDL oxidation (Lu et al. 2015) which was supported by other studies (Jain et al. 2018). Similarly, onion juice consumption (100 mL/day) for 8 weeks by healthy subjects, significantly improved total antioxidant capacity and levels of various antioxidants such as GSH and GR. Also, the levels of free radicals significantly reduced after treatment with the A. cepa (Law et al. 2016).
Antioxidant effects of the constituents of A. cepa
Antioxidant effects of various constituents of A. cepa mainly quercetin, were shown in several studies. Treatment of hyperuricemic Wistar rats with 5 mg/kg quercetin for 14 days, led to a significant improvement in oxidative stress (Haidari et al. 2008). Pre-treatment of cortical neuronal cells derived from mouse embryos with quercetin (1-10 μM) for 30 min, protected cells from oxidative stress suggesting antioxidant effects of quercetin (Lee and Jung 2016).
It was also reported that oral administration of quercetin and piperine (100 mg/kg each) combination once a daily for 7 days, increased antioxidant and induced hepatoprotective effects against oxidative stress induced by paracetamol, in Wistar rats (Mehta et al. 2012). Quercetin oxidation metabolite, 2-(3,4-dihydroxybenzoyl)-2,4,6-trihydroxy-3(2H)-benzo-furanone (BZF), showed antioxidant effects at very low nanomolar concentrations (0.03 nM), in its pure form or as part of a plant extract. BZF protected human colonic adenocarcinoma cell line (Caco2 cells) against indomethacin-induced damage (Fuentes et al. 2020).
The antioxidant effects of active compounds extracted from medicinal herbs including monotropein from Morinda officinalis How (Rubiaceae), astragalin (kaempferol 3-O-glucoside) from Cuscuta chinensis Lamark (Convolvulaceae) and spiraeoside from the outer scales of A. cepa (200 mg/kg, oral) on varicocele-induced Sprague-Dawley rats significantly improved parameters of oxidative stress such as MDA, SOD and GPx (Karna et al. 2019).
Treatment of hepatitis induced by tetrachloromethane in Wistar rats, with dihydroquercetin (100 mg/kg, oral) for 4 days prior to the first administration of CCl4 and during the subsequent 14 days, indicated antioxidant effects of this agent (Teselkin et al. 2000).
Enhanced antioxidant capacity was shown for quercetin and quercetin + quercetin monoglycosides in the serum of Wistar rats fed with high-fat diets (Grzelak-Błaszczyk et al. 2018). Polysaccharide from A. cepa such as HBSS, CHSS, DASS and CASS, showed ABTS radical scavenging activity, DPPH radical scavenging activity, iron (Fe2+) chelating activity, and superoxide anion radical scavenging activity in a dose-dependent manner at concentrations of 0.5-2.0 mg/mL, and CHSS had the highest antioxidant action in vitro (Ma et al. 2018).
Free radical-scavenging activity of quercetin-3′-O-β-d-glucoside isolated from methanol extract of dried skin of A. cepa, was evaluated by ORAC assay and results showed that this component could be used as an antioxidant agent (Arung et al. 2011). Antioxidant properties, including OH radical scavenging effect of quercetin, isorhamnetin-3-glucoside, dipropyl disulphide and dipropyl sulphide extracted from methanol extract of A. cepa, were also demonstrated (Teshika et al. 2019).
Ouyang et al. (2018) showed DPPH radical-scavenging, FRAP radical scavenging, and OH radical scavenging activities of polyphenols from onion with IC50 values of 43.24, 560.61, and 12.97 μg/mL, respectively. In addition, these polyphenols significantly inhibited xanthine oxidase activity with an IC50 of 17.36 μg/mL. Insani et al. (2016) showed a positive correlation between total antioxidant activity and content of polyphenols particularly quercetin, suggesting that phenolic compounds have a major role in antioxidant properties of this plant. In another study, assessment of relationship between bioactive compounds content and antioxidant activities of 6 Allium vegetable species, revealed that chive onion had the highest antioxidant activity compared to others. A significant positive correlation between phenolic content and antioxidant activity was also shown (Beretta et al. 2017). Antioxidant effects of skin of 15 Indian cultivars onion showed the maximum antioxidant capacity for cv. ‘NHRDF Red’ whereas the least capacity was obtained for cv. ‘Bhima Shubhra’ (Sagar et al. 2020).
Together, the available literature indicated protective effects of A. cepa extract, fractions and its constituents especially quercetin, against several diseases associated with oxidative stress and lipid peroxidation in various body organs using different methods such as DPPH, ABTS, ORAC and TEAC. Treatment with A. cepa and its derivative mainly quercetin, decreased lipid peroxidation and NAD(P)H, MDA, NO, LPO and eNOS but enhanced antioxidant parameters such as total antioxidant capacity as well as SOD, CAT, GSH, GPx, GSPO, TrxR, SDH, GST and GR activities and thiol level. Also, free radical-scavenging activity such as OH radical scavenging effect was shown for the plant and its constituents. Therefore, onion and its component such as quercetin, may be used as an antioxidant agent in treatment of disorders associated with oxidative stress. In addition, the results showed that quercetin could be responsible for the antioxidant effects of the plant. Antioxidant effects of A. cepa and its constituents are summarised in Table 3.
Table 3.
The antioxidant effects of Allium cepa and its constituents.
| Preparations | Doses | Model of study | Effects | Reference |
|---|---|---|---|---|
| Essential oil | 100 mg/kg/day, gavage | Nicotine-induced damages in Sprague–Dawley rats | Reduced LPO, increased SOD, CAT and GSH |
Helen et al. (2000) |
| Onion powder | 2 g/100 g body weigh/day, orally | Murine infected with Schistosoma mansoni | Reduced LPO, increased SOD, TrxR, SDH, GR |
Mantawy et al. (2012) |
| Aqueous | 1mL/day, orally | Doxorubicin-induced hepatotoxicity in Sprague-Dawley rats |
Reduced MDA, increased SOD, GSH and GPx | Mete et al. (2016) |
| Aqueous | 100 and 300 mg/kg/day, orally | Alloxan-induced diabetic rabbits | Increased SOD, CAT, GPx, GSH, reduced MDA content | Ogunmodede et al. (2011) |
| Aqueous | 1 mL/day, orally | Cadmium-induced renal dysfunction | Improveed plasma and tissue levels of SOD, CAT and MDA | Ige et al. (2009) |
| Aqueous | 0.5 and 1 mL/100 g bw/day, gavage | Cadmium-induced nephrotoxicity in Wistar rats |
Reduced LPO and GST, increased SOD, CAT and GSH | Suru (2008) |
| Methanolic | 600 mg/kg/day, orally | Cyanide-induced renal toxicity in Wistar rats | Reduced LPO and MDA, increased SOD, CAT and GSH | Ola-Mudathir and Maduagwu (2014) |
| Aqueous | 0.5 and 1 mL/100 g bw/day, gavage | Endogenous hepatic and renal antioxidant status in Wistar rats | Decreased MDA, increased GSH, GST, SOD and CAT | Suru and Ugwu (2015) |
| Onion juice | 3 mL/day, gavage | Escherichia coli induced testis and seminiferous tubules damage in Wistar rats | Enhanced total antioxidant capacity | Shahverdi et al. (2017) |
| Aqueous | 0.5 and 1 mL/100 g bw/day, gavage | Cadmium-induced prostate glands damage in Wistar rats | Reduced GST, increased SOD, CAT and GSH | Suru and Ugwu (2015) |
| Aqueous | 0.5 and 1 mL/100 g bw/day, gavage | Cadmium-induced sperm and testicular damage in Wistar rats | Reduced LPO, GST and MDA, increased SOD, CAT and GSH | Ola-Mudathir et al. (2008) |
| Onion juice | 0.175, 0.35, or 0.7 mg/mL in drinking water | Wistar rat model of asthma | Decreased NO2, NO3–, MDA, elevated SOD and CAT | Marefati et al. (2018) |
| Aqueous | 0.4 g/ mL/day, gavage | STZ-induced diabetic rats Wistar rats | Reduced lipid hydroperoxide and lipoperoxide | Campos et al. (2003) |
| Onion juice | 100 mL/day, orally | Subjects with mild hypercholesterolaemia | Increased total antioxidant capacity and extended time of LDL oxidation | Jain et al. (2018), Lu et al. (2015) |
| Onion juice | 100 mL/day, orally | Health subjects | Improved total antioxidant capacity, GSH and GR | Law et al. (2016) |
| Quercetin | 5 mg/kg | Hyperuricemic Wistar rats | Improved oxidative stress | Haidari et al. (2008) |
| Quercetin | 1–10 μM | Cortical neuronal cells | Protected cells from oxidative stress by inactivation of protein kinase C-ε | Lee and Jung (2016) |
| Quercetin | 100 mg/kg, orally | Paracetamol induced oxidative stress in Wistar rats | Inhibited free radicals | Mehta et al. (2012) |
| Quercetin oxidation metabolite (BZF) | 0.03 nanomolar | Indomethacin-induced damage in human Caco2 cells | Protected Caco2 cells against damage by antioxidant effect | Fuentes et al. (2020) |
| MAS | 200 mg/kg, gavage | Varicocele-induced Sprague-Dawley rats | Improved parameters of oxidative stress such as MDA, SOD and GPx | Karna et al. (2019) |
| Dihydroquercetin | 100 mg/kg, orally | CCl4- induced hepatitis in rat model | Hepatoprotective effect | Teselkin et al. (2000) |
| Polysaccharides | 0.5-2.0 mg/mL | In vitro study | ABTS radical scavenging activity, DPPH radical scavenging activity, iron (Fe2+) chelating activity, and superoxide anion radical scavenging activity | Ma et al. (2018) |
STZ: streptozocin; LPO: lipid peroxidation; SOD: superoxide dismutase; CAT: catalase; GSH: glutathione; TrxR: thioredoxin reductase; SDH: sorbitol dehydrogenase; GR: glutathione reductase; MDA: malondialdehyde; GPx: glutathione peroxidase; GST: glutathione S-transferase; NO2, nitrogen dioxide, NO3–: nitrate; BZF: 2-(3,4-dihydroxybenzoyl)-2,4,6-trihydroxy-3(2H)-benzo-furanone; Caco2: colonic adenocarcinoma cell line; MAS: MAS: monotropein, Astragalin (kaempferol 3-O-glucoside) and spiraeoside; ABTS: azinobis (3-ethyl-benzothiazolin-6-sulfonic acid); DPPH: diphenyl-1-picrylhydrazyl.
Immunomodulatory effects of A. cepa
Immunomodulation is the process of moderating an immune response by administration of a chemical. The modulatory effects of several medicinal plants on cytokines and eventually, on the immune system were shown to be mediated through stimulation or suppression of various components of the immune system including the innate and adaptive immune responses (Spelman et al. 2006); immunomodulatory properties of A. cepa and its constituents have been widely evaluated by several studies (Spelman et al. 2006).
Immunomodulatory effects of the plant
Several in vitro and in vivo studies reported the immunomodulatory effects of A. cepa in different diseases. In ovalbumin-sensitised Wistar rats, A. cepa aqueous extract (0.175, 0.35, and 0.7 mg/mL, oral) caused reductions in IL-4 and IgE levels, and increased the level of IFN-γ and IFN-γ/IL4 ratio (Th1/Th2 balance) indicating its stimulatory effect on Th1 but inhibitory effect on Th2 activity (Marefati et al. 2018).
An in vitro study on cultured spleen cells stimulated with pokeweed (PWM) from Blomia tropicalis-sensitised BALB/c mice, demonstrated that A. cepa methanol extract (10, 100 and 1000 μg/mL) inhibited the production of Th2 cytokines, IL-4, IL-5, and IL-13, and IgE (Oliveira, Campos et al. 2015). Also, oral administration of methanol extract of A. cepa (100 and 1000 mg/kg) attenuated the levels of IL-4, IL-5, IL-13, and IgE in BALF of a murine model of Blomia tropicalis-induced asthma (Oliveira, Campos et al. 2015). Zinc oxide nanoparticles (ZnO-NPs) synthesised from the extract of A. cepa (15 µg/mL) in UVB radiation-mediated inflammation in human epidermal keratinocytes (HaCaT cells), showed decreased levels such of IL-6, IL-10 and TNF-α (Wu et al. 2019).
Dietary administration of A. cepa (20 g/kg, oral) for 12 weeks, significantly increased weight gain, haematocrit, and total Ig in brown-marbled grouper fish compared to the control group (Apines-Amar et al. 2012). A. cepa scales ethanol extract (75, 150, and 300 mg/kg/day, oral) for 30 days, increased the tissue levels of IL-6, IL-8, and TNF-α and the expression of clusterin, while showing no effect on TGF-ΒR1 in Wistar rats with experimentally induced atypical prostatic hyperplasia (Elberry et al. 2014). In a similar study, ethanol extract of A. cepa (0.1, 1, 10, 50, and 100 μg/mL) in RAW264.7 cells, inhibited the secretion of IL-6, TNF-α, and IL-1β and the expression of COX-2, iNOS, NF-κB, and MAPKs in a dose-dependent manner (Ahn et al. 2015).
The effects of ethanol extract of A. cepa (100, 500, and 1000 μg/mL) on osteoclastogenesis under LPS-induced inflammatory conditions, were examined in RAW264.7 cells. Findings showed that A. cepa reduced the production of IL-6 and IL-1α, increased the production of IL-3 and IL-4, and down regulated NF-κB pathway (Oliveira, Figueiredo et al. 2015).
Stimulatory effects of ethanol extract of A. cepa (0.8–409.6 μg/mL) on lymphocyte response to a mitogen and IL-2 and IFN-γ gene expressions in white leghorn chickens, were evaluated (Hanieh et al. 2012). Oral administration of aqueous extract of A. cepa (250, 500 and 750 mg/kg) significantly increased CD4 cells in Wistar rats, indicating immunostimulatory potential of A. cepa (Mirabeau and Samson 2012).
Administration of A. cepa aqueous extract (0.1 mL/100 g bw, oral) for 7 weeks to female BALB/c mice with breast cancer, caused reductions in IL-4 and increases in IFN-γ level and IFN-γ/IL4 ratio (Th1/Th2 balance) indicating stimulatory effects of A. cepa on Th1 but inhibitory effects on Th2 activity (Karishchi and Bidaran 2018).
A. cepa (10 and 30 g/kg, oral) was administrated to white leghorn chickens immunised with Newcastle disease virus (NDV), sheep red blood cells (SRBC) and Brucella abortus (BA) which induced a dose-dependent increase in antibody titres higher than control, indicating stimulatory effect of A. cepa on humoral immune responses (Hanieh et al. 2010). Topical administration of two outer shells including the skin of A. cepa aqueous extract (20 and 40 µL,) five times a week for three consecutive weeks from day 21 to day 41 to BALB/c mice with allergic rhinitis, reduced allergic symptoms, eosinophil infiltration of nasal turbinate mucosa, and OVA-specific IgE levels. In addition, levels of IL-4, IL-5, IL-10, IL-13 and IFN-γ decreased in groups treated with onion extract (Seo et al. 2019).
Effects of ethanol extract of A. cepa (100 µg/mL) on LPS-induced inflammatory responses, were examined in RAW 264.7 cells and results showed reduced secretion of IL-6, TNF-α, and IL-1-β and NO production in a dose-dependent manner (Ahn et al. 2015). Methanol extract of A. cepa (50, 250, and 500 µg/mL) in LPS-induced BV-2 microglial cells (N27-A cells), reduced pro-inflammatory cytokines TNF-α, IL-6, and IL-1-β (Jakaria et al. 2019).
Immunomodulatory effects of the constituents of A. cepa
Immunomodulatory effects of A. cepa constituents were also shown in various studies. Quercetin (3.5, 7.5, 15 μg/mL) inhibited production of Th2 cytokines, including IL-4, IL-5, IL-13, and IgE in cultured spleen cells stimulated with pokeweed (PWM) from Blomia tropicalis-sensitised BALB/c mice (Oliveira, Campos et al. 2015).
The effect of quercetin (1.25, 2.5 and 5 μM) on LPS-induced osteoclastogenesis in RAW264.7 cells, showed that quercetin reduced IL-6 and IL-1α, but increased IL-3 and IL-4 and down regulated NF-κB pathway (Oliveira, Figueiredo et al. 2015). The immunoprotective properties of A. cepa agglutinin (ACA) in normal and cyclophosphamide-induced immunosuppressed Wistar rats were demonstrated. ACA (1, 10, and 100 μg, intraperitoneal) increased TNF-α, IL-10, COX-2, IgG and IgA levels in serum and improved immune parameters such as cells of myeloid origin (RBC, WBC, and Hb), body weight, splenic index and thymic index in the spleen and thymus (Kumar and Venkatesh 2016).
The immunomodulatory activity of ACA (0.01, 0.1, 1 and 10 μg/well) was assessed in RAW264.7 cell and rat peritoneal macrophages. Results showed that ACA induced pro-inflammatory cytokines such as TNF-α and IL-12 and enhanced the proliferation of murine thymocytes and the expression of IFN-γ and IL-2; however, ACA caused no effect on proliferation of B cell-enriched rat splenocytes (Prasanna and Venkatesh 2015).
Onion fructo-oligosaccharides (FOS; 0.5, 5, 50 and 250 µg/mL) enhanced phagocytic activity in peritoneal exudates cells (PECs) in Wistar rats and cell proliferation or mitogenicity of splenocytes and thymocytes in BALB/c mice (Kumar et al. 2015). Lectin as an effective constituent of A. cepa also showed remarkable immunoprotective effects and elevated the levels of proinflammatory COX-2 and nitric oxide and expression of immunoregulatory cytokines TNF-α, IL-2, IL-12 and IFN-γ (Prasanna and Venkatesh 2015; Kumar and Venkatesh 2016).
Total phenol content (TLC) of Toscana (red onion) bulb extract were tested on immunological cells such as T helper cells (CD4+ cells), cytotoxic T lymphocytes (CD8+ cells), T regulatory cells (CD25high CD4+ cells), and natural killer cells/monocytes (CD16+ cells). The results showed that TLC increased the frequency of antitumor/anti-infection NK CD16+ immune cells (Lisanti et al. 2016). In addition, polyphenols extracted from lyophilised A. cepa (100 mg/mL) inhibited cancer cell growth by induction of caspase-dependent apoptosis through suppressing caspase 8 and 9 and up-regulation of TNF-related apoptosis-inducing ligand (TRAIL) receptor DR5 and down-regulation of the cellular inhibitor of apoptosis 1 (cIAP-1). Also, polyphenols inhibited phosphatidylinositol 3-kinase (PI3K)/Akt signalling pathway in human leukemic cells and U937 cells (Han et al. 2013).
The reviewed in vitro and in vivo studies showed the modulatory effects of A. cepa and its constituents on the immune system in various immune dysregulatory disorders. The plant and its components mainly quercetin, reduced Th2 cytokines, IL-4, IL-5, and IL-13 as well as IL-6, IL-8, IL-10, IL-1β and TNF-α and IgE levels, but increased CD4 cells, IFN-γ level and IFN-γ/IL4 ratio (Th1/Th2 balance), indicating their stimulatory effect on Th1 but inhibitory effect on Th2 activity in inflammatory disorders such as asthma and breast cancer. However, under inflammatory conditions such as osteoclastogenesis induced by LPS in RAW264.7 cells, A. cepa and quercetin reduced IL-6 and IL-1α production, but increased IL-3 and IL-4 levels. In animal models of allergic rhinitis, the plant reduced allergic symptoms, eosinophil infiltration of nasal turbinate mucosa, and OVA-specific IgE as well as IL-4, IL-5, IL-10, IL-13 and IFN-γ levels. Therefore, the varying types of immunomodulatory effects of A. cepa and its constituents were observed in different immune dysregulaton disorders. The above studies showed that A. cepa and its constituents especially quercetin, are potential immunomodulatory therapeutic candidates for treatment of disorders with immune dysregulation. Table 4 illustrates immunomodulatory effects of A. cepa and its constituents. Based on the described studies, quercetin contributes to immunomodulatory effect of the plant.
Table 4.
The immunomodulatory effects of Allium cepa and its constituents.
| Preparations | Dose | Study models | Effects | Ref. |
|---|---|---|---|---|
| A. fistulosom | 2.5, 5 and 10 mg/400 mL /mouse 125–1000 µg/mL |
Murine macrophage cell line RAW264.7 | Increased TNF-α, IL-12, IFN-γ production, phagocytosis, NK cell activities, increased TNF-α, IL-12, and MCP-1 production | Ueda et al. (2013) |
| AE | 0.175, 0.35, and 0.7 mg/mL, orally | OVA-sensitised Wistar rat | Decreased IL-4 and IgE, increased IFN-γ and IFN-γ/IL-4 ratio | Marefati et al. (2018) |
| AE | 20 and 40 μL, orally | OVA-sensitised BALB/c mice | Reduced the levels of IL-4, IL-5, IL-10, IL-13 and IFN-γ | Seo et al. (2019) |
| 100 µg/ mL | Murine macrophage cell line RAW264.7 | Ahn et al. (2015) | ||
| EE | LPS-induced inflammatory markers in BV-2 microglial cells | Reduced NO production and IL-6, TNF-α, and IL-1β secretion | ||
| ME | 50, 250, and 500 µg/mL | Testosterone induced atypical prostatic hyperplasia in Wistar rats | Reduced TNF-α, IL-6, and IL-1β | Jakaria et al. (2019) |
| Allium cepa | 75, 150, and 300 mg/kg | Reduced tissue expressions of IL-6, IL-8, TNF-α and, IGF-1 | Elberry et al. (2014) | |
| ME | 10, 100 and 1000 μg/ml | PWM-stimulated splenocytes from Bt-sensitised BALB/c mice | Reduced IL-4, IL-5, IL-13, and IgE | Oliveira, Figueiredo et al. (2015) |
| ME | 100 and 1000 mg/kg, orally | Bt-sensitised BALB/c mice | Reduced the levels of IL-4, IL-5, IL-13, and IgE in BALF | Oliveira, Campos et al. (2015) |
| Allium cepa | 20 g/kg, orally | Brown-marbled grouper fish | Increased weight gain, haematocrit, and total Ig | Apines-Amar et al. (2012) |
| EE | 75, 150, and 300 mg/kg/day, orally | Testosterone-induced APH in Wistar rat | Increased IL-6, IL-8, and TNF-α and the expression of clusterin | Elberry et al. (2014) |
| EE | 0.1, 1, 10, 50, and 100 μg/mL | Murine macrophage cell line RAW264.7 | Inhibited IL-6, TNF-a, and IL-1ß secretion and COX-2, iNOS, NF-κB, and MAPKs expression | Ahn et al. (2015) |
| ME | 100, 500, and 1000µg/mL | Murine macrophage cell line RAW264.7 | Reduced IL-6 and IL-1α production, increased IL-3 and IL-4 production, Downregulated NF-κB pathway | Oliveira, Campos et al. (2015) |
| EE | 0.8–409.6 μg/mL | Lymphocyte isolated white leghorn chickens | Lymphocyte response to a mitogen Stimulation and IL-2 and IFN-γ gene expressions | Hanieh et al. (2012) |
| AE | 250, 500 and 750 mg/kg, orally | Wistar rat | Increased the CD4 cells | Mirabeau and Samson (2012) |
| AE | 0.1 mL /100 gBW, orally | Breast cancer-induced by cell line 4T1 | Reduced IL-4, increased IFN-γ | Karishchi and Bidaran (2018) |
| Allium cepa | 10 and 30 g/kg, orally | Chickens immunised with NDV, SRBC and BA vaccines | Improvement of efficacy of vaccines | Hanieh et al. (2010) |
| Quercetin | 3.5, 7.5, 15 μg/mL | PWM-stimulated splenocytes from Bt-sensitised BALB/c mice | Reduced IL-4, IL-5, IL-13, and IgE | Oliveira, Figueiredo et al. (2015) |
| Quercetin | 30 mg/kg, orally | Bt-sensitised BALB/c mice | Reduced the levels of IL-4, IL-5, IL-13, and IgE in BALF | Oliveira, Figueiredo et al. (2015) |
| Quercetin | 1.25, 2.5 and 5 µM | Murine macrophage cell line RAW264.7 | Reduced IL-6 and IL-1α production, increased IL-3 and IL-4 production, Downregulated NF-κB pathway | Oliveira, Campos et al. (2015) |
| ACA | 1, 10, and 100 μg, i.p. | CP- immunosuppressed Wistar rats | Increased serum TNF–α, IL-10, COX–2, IgG and IgA, improved immune parameters in spleen and thymus | Kumar and Venkatesh (2016) |
| ACA | 0.01, 0.1, 1 and 10 μg/well | Murine macrophage cell line RAW264.7 | Stimulated TNF-α and IL-12 production, enhanced murine thymocytes proliferation and IFN-γ and IL-2 expression | Prasanna and Venkatesh (2015) |
| FOS | 0.5, 5, 50 and 250 µg/mL | Splenocytes and thymocytes | Increased PECs phagocytic activity, cell proliferation or mitogenicity | Kumar et al. (2015) |
Ref.: References; AE: aqueous extract; ME: methanolic extract; EE: ethanoic extract; ME: methanolic extract; CE: chloroformic extract; PWM: pokeweed; Bt: Blomia tropicalis; CP: cyclophosphamide; APH: atypical prostatic hyperplasia; FOS: onion fructo-oligosaccharides; PECs: peritoneal exudates cells; ACA: Allium cepa agglutinin; SRBC: sheep red blood cells; NDV: newcastle disease virus; SRBC: sheep red blood cells; BA: brucella abortus.
This review highlighted anti-inflammatory, antioxidant, and immunomodulatory effects of A. cepa and its major constituents, mediated via mechanisms that are depicted in Figure 2.
Figure 2.
A summary of the possible mechanisms of onions.
Conclusions
This review discussed the effects of A. cepa (onion) and its constituents on inflammation, oxidative stress and immune disorders as shown by in vitro and in vivo studies.
A. cepa and its components showed antidotal effects in different pathological conditions induced by various chemicals/toxins. Together, the plant and its components showed anti-inflammatory and immunomodulatory effects mediated by modulation of innate (neutrophils, macrophage, and NK cells) and acquired immunity components (inflammatory and anti-inflammatory cytokines, B cells, and Th1/Th2 balance).
Antioxidant effects of A. cepa and its constituents were mediated through stabilisation of cellular membranes, ROS scavenging, and decrement of unsaturated membrane lipids peroxidation. Therefore, A. cepa and its constituents could be of therapeutic value in disorders such as aging, anti-inflammatory, and wound healing processes where radical scavenging activity can be of therapeutic value.
Although the exact molecular mechanisms underlying such effects are not fully understood yet, most of pharmacological activities of A. cepa are related to the presence of bioactive compounds such as quercetin. Further clinical studies are needed to evaluate the effect of the plant and its constituents on conditions induced by inflammation, oxidative stress and immune-dysregulation. In addition, scientific information on toxicity or safety of onion is lacking and requires further studies.
Funding Statement
This work was supported by Mashhad University of Medical Sciences, Mashhad, Iran.
Disclosure statement
The authors declare no conflict of interest in the present article.
References
- Aathira K, Monisha L, Nandhini S, Gomathi V, Subhashini S, Sakthivel R.. 2020. Studies on phytochemical and antibacterial activity of ethanolic extracts of Allium cepa L., Mentha arensis L., and Mirabilis jalapa L. Our Herit. 68:3423–3430. [Google Scholar]
- Agnieszka S, Robert P, Del VL, Silvano S, Gianluca C.. 2017. Interactions among genotype, environment and agronomic practices on production and quality of storage onion (Allium cepa L.)–A review. J Hortic Sci Biotech. 44:21–42. [Google Scholar]
- Ahmed AF, Al-Yousef HM, Al-Qahtani JH, Al-Said MS.. 2017. A hepatonephro-protective phenolic-rich extract from red onion (Allium cepa L.) peels. Pak J Pharm Sci. 3:1971–1979. [PubMed] [Google Scholar]
- Ahn N-K, Kang B-K, Kim K-B-W-R, Kim M-J, Bae N-Y, Park J-H, Park S-H, Ahn D-H.. 2015. Anti-inflammatory effect of ethanol extract from onion (Allium cepa L.) peel on lipopolysaccharide-induced inflammatory responses in RAW 264.7 cells and mice ears. J Korean Soc Food Sci Nutr. 44(11):1612–1620. [Google Scholar]
- Akash MSH, Rehman K, Chen S.. 2014. Spice plant Allium cepa: dietary supplement for treatment of type 2 diabetes mellitus. Nutrition. 30(10):1128–1137. [DOI] [PubMed] [Google Scholar]
- Albishi T, John JA, Al-Khalifa AS, Shahidi F.. 2013. Antioxidant, anti-inflammatory and DNA scission inhibitory activities of phenolic compounds in selected onion and potato varieties. J Funct Foods. 5(2):930–939. [Google Scholar]
- Ali M, Thomson M, Afzal M.. 2000. Garlic and onions: their effect on eicosanoid metabolism and its clinical relevance. Prostaglandins Leukot Essent Fatty Acids. 62(2):55–73. [DOI] [PubMed] [Google Scholar]
- Alpsoy S, Aktas C, Uygur R, Topcu B, Kanter M, Erboga M, Karakaya O, Gedikbasi A.. 2013. Antioxidant and anti-apoptotic effects of onion (Allium cepa) extract on doxorubicin-induced cardiotoxicity in rats. J Appl Toxicol. 33(3):202–208. [DOI] [PubMed] [Google Scholar]
- Amidi S, Mojab F, Bayandori Moghaddam A, Tabib K, Kobarfard F.. 2012. A simple electrochemical method for the rapid estimation of antioxidant potentials of some selected medicinal plants. Iran J Pharm Res. 11(1):117–121. [PMC free article] [PubMed] [Google Scholar]
- Apines-Amar MJS, Amar EC, Faisan JP Jr, Pakingking RV Jr, Satoh S.. 2012. Dietary onion and ginger enhance growth, hemato-immunological responses, and disease resistance in brown-marbled grouper. Epinephelus Fuscoguttatus. AACL Bioflux. 5:231–239. [Google Scholar]
- Arjmandi BH, Alekel L, Hollis BW, Amin D, Stacewicz-Sapuntzakis M, Guo P, Kukreja SC.. 1996. Dietary soybean protein prevents bone loss in an ovariectomized rat model of osteoporosis. J Nutr. 126(1):161–167. [DOI] [PubMed] [Google Scholar]
- Arung ET, Furuta S, Ishikawa H, Tanaka H, Shimizu K, Kondo R.. 2011. Melanin biosynthesis inhibitory and antioxidant activities of quercetin-3'-O-beta-D-glucoside isolated from Allium cepa. Z Naturforsch C J Biosci. 66(5-6):209–214. [DOI] [PubMed] [Google Scholar]
- Ashwini M, Sathishkumar R.. 2014. Onion (Allium cepa)-Ethnomedicinal and therapeutic properties. In: Handbook of Medicinal plants and their Bioactive compounds. p. 27–34. [Google Scholar]
- Augusti KT. 1996. Therapeutic values of onion (Allium cepa L.) and garlic (Allium sativum L.). Indian J Exp Biol. 34(7):634–640. [PubMed] [Google Scholar]
- Baragob AE, Al-Wabel NA, Ahmed NA, Babiker M, Abdalkarim AS, Elboshra M.. 2015. Study to investigate the pancreatic regeneration and evaluation of the antidiabetic and antihyperlipidemic potential of aerial parts of Allium cepa. Biochem Biotechnol Res. 3:19–29. [Google Scholar]
- Baynes JW, Thorpe SR.. 1999. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 48(1):1–9. [DOI] [PubMed] [Google Scholar]
- Benkeblia N. 2004. Antimicrobial activity of essential oil extracts of various onions (Allium cepa) and garlic (Allium sativum). LWT – Food Sci Technol. 37(2):263–268. [Google Scholar]
- Benkeblia N. 2005. Free-radical scavenging capacity and antioxidant properties of some selected onions (Allium cepa L.) and garlic (Allium sativum L.) extracts. Braz Arch Biol Technol. 48(5):753–759. [Google Scholar]
- Benmalek Y, Yahia OA, Belkebir A, Fardeau M-L.. 2013. Anti-microbial and antioxidant activities of Illicium verum, Crataegus oxyacantha ssp monogyna and Allium cepa red and white varieties. Bioeng. 4:244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beretta HV, Bannoud F, Insani M, Berli F, Hirschegger P, Galmarini CR, Cavagnaro PF.. 2017. Relationships between bioactive compound content and the antiplatelet and antioxidant activities of six Allium vegetable species. Food Technol Biotech. 55:266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisen PS, Emerald M.. 2016. Nutritional and therapeutic potential of garlic and onion (Allium sp. CNF. 12(3):190–199. [Google Scholar]
- Boots AW, Drent M, de Boer VC, Bast A, Haenen GR.. 2011. Quercetin reduces markers of oxidative stress and inflammation in sarcoidosis. Clin Nutr. 30(4):506–512. [DOI] [PubMed] [Google Scholar]
- Boskabady MH, Kaveh M, Shakeri K, Roshan NM, Rezaee R.. 2019. Hydro-ethanolic extract of Portulaca oleracea ameliorates total and differential WBC, lung pathology and oxidative biomarkers in asthmatic rats. Iran J Pharm Res. 18(4):1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouba AA, Njintang NY, Nkouam GB, Mang YD, El-Sayed Mehanni AH, Scher J, Montet D, Mbofung CM.. 2014. Desorption isotherms, net isosteric heat and the effect of temperature and water activity on the antioxidant activity of two varieties of onion (Allium cepa L.). Int J Food Sci Technol. 49(2):444–452. [Google Scholar]
- Campos K, Diniz Y, Cataneo A, Faine L, Alves M, Novelli E.. 2003. Hypoglycaemic and antioxidant effects of onion, Allium cepa: dietary onion addition, antioxidant activity and hypoglycaemic effects on diabetic rats. Int J Food Sci Nutr. 54(3):241–246. [DOI] [PubMed] [Google Scholar]
- Cheng A, Chen X, Jin Q, Wang W, Shi J, Liu Y.. 2013. Comparison of phenolic content and antioxidant capacity of red and yellow onions. Czech J Food Sci. 31(No. 5):501–508. [Google Scholar]
- Corea G, Fattorusso E, Lanzotti V, Capasso R, Izzo AA.. 2005. Antispasmodic saponins from bulbs of red onion, Allium cepa L. var. Tropea. J Agric Food Chem. 53(4):935–940. [DOI] [PubMed] [Google Scholar]
- Corzo-Martínez M, Corzo N, Villamiel M.. 2007. Biological properties of onions and garlic. Trends Food Sci Technol. 18(12):609–625. [Google Scholar]
- Dadkhah A, Fatemi F, Malayeri MRM, Rasooli A.. 2014. Cancer chemopreventive effect of dietary Zataria multiflora essential oils. Turk J Biol. 38:930–939. [Google Scholar]
- Dalgleish AG, O'Byrne KJ.. 2002. Chronic immune activation and inflammation in the pathogenesis of AIDS and cancer. Adv Cancer Res. 84:231–276. [DOI] [PubMed] [Google Scholar]
- Dawud F, Dubo A, Yusuf N, Umar I.. 2016. Effects of aqueous extract of Allium cepa (red onion) on ovalbumin induced allergic asthma in Wistar rats. Bayero J Pure App Sci. 9(2):95–101. [Google Scholar]
- Del Bano M, Castillo J, Benavente-Garcia O, Lorente J, Martín-Gil R, Acevedo C, Alcaraz M.. 2006. Radioprotective-antimutagenic effects of rosemary phenolics against chromosomal damage induced in human lymphocytes by gamma-rays. J Agric Food Chem. 54(6):2064–2068. [DOI] [PubMed] [Google Scholar]
- Dorsch W, Schneider E, Bayer T, Breu W, Wagner H.. 1990. Anti-inflammatory effects of onions: inhibition of chemotaxis of human polymorphonuclear leukocytes by thiosulfinates and cepaenes. Int Arch Allergy Appl Immunol. 92(1):39–42. [DOI] [PubMed] [Google Scholar]
- Dorsch W, Wagner H, Bayer T, Fessler B, Hein G, Ring J, Scheftner P, Sieber W, Strasser T, Weiss E.. 1988. Anti-asthmatic effects of onions. Alk(en)ylsulfinothioic acid alk(en)yl-esters inhibit histamine release, leukotriene and thromboxane biosynthesis in vitro and counteract PAF and allergen-induced bronchial obstruction in vivo. Biochem Pharmacol. 37(23):4479–4486. [DOI] [PubMed] [Google Scholar]
- El-Aasr M, Fujiwara Y, Takeya M, Ikeda T, Tsukamoto S, Ono M, Nakano D, Okawa M, Kinjo J, Yoshimitsu H, et al. . 2010. Onionin A from Allium cepa inhibits macrophage activation. J Nat Prod. 73(7):1306–1308. [DOI] [PubMed] [Google Scholar]
- Elberry AA, Mufti S, Al-Maghrabi J, Abdel Sattar E, Ghareib SA, Mosli HA, Gabr SA.. 2014. Immunomodulatory effect of red onion (Allium cepa Linn) scale extract on experimentally induced atypical prostatic hyperplasia in Wistar rats. Mediators Inflamm. 2014:640746–640746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Demerdash FM, Yousef MI, El-Naga NA.. 2005. Biochemical study on the hypoglycemic effects of onion and garlic in alloxan-induced diabetic rats. Food Chem Toxicol. 43(1):57–63. [DOI] [PubMed] [Google Scholar]
- Ernst M, Feldheim W. 2000. Fructans in higher plants and in human nutrition. J Appl Botany. 74(1/2):5–9. [Google Scholar]
- Fredotović Ž, Šprung M, Soldo B, Ljubenkov I, Budić-Leto I, Bilušić T, Čikeš-Čulić V, Puizina J.. 2017. Chemical composition and biological activity of Allium cepa L. and Allium× cornutum (Clementi ex Visiani 1842) methanolic extracts. Molecules. 22(3):448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes J, Arias-Santé MF, Atala E, Pastene E, Kogan MJ, Speisky H.. 2020. Low nanomolar concentrations of a quercetin oxidation product, which naturally occurs in onion peel, protect cells against oxidative damage. Food Chem. 11:126–166. [DOI] [PubMed] [Google Scholar]
- Galmarini CR, Goldman IL, Havey MJ.. 2001. Genetic analyses of correlated solids, flavor, and health-enhancing traits in onion (Allium cepa L.). Mol Genet Genomics. 265(3):543–551. [DOI] [PubMed] [Google Scholar]
- Gawlik-Dziki U, Świeca M, Dziki D, Baraniak B, Tomiło J, Czyż J.. 2013. Quality and antioxidant properties of breads enriched with dry onion (Allium cepa L.) skin. Food Chem. 138(2-3):1621–1628. [DOI] [PubMed] [Google Scholar]
- Georgiadis N, Tsarouhas K, Rezaee R, Nepka H, Kass GE, Dorne JL, Stagkos D, Toutouzas K, Spandidos DA, Kouretas D, et al. . 2020. What is considered cardiotoxicity of anthracyclines in animal studies. Oncol Rep. 44(3):798–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorani V, Marefati N, Shakeri F, Rezaee R, Boskabady M, Boskabady MH.. 2018. The effects of Allium cepa extract on tracheal responsiveness, lung inflammatory cells and phospholipase A2 level in asthmatic rats. Iran J Allergy Asthma Immunol. 17:221–231. [PubMed] [Google Scholar]
- Griffiths G, Trueman L, Crowther T, Thomas B, Smith B.. 2002. Onions-a global benefit to health. Phytother Res. 16(7):603–615. [DOI] [PubMed] [Google Scholar]
- Grzelak-Błaszczyk K, Milala J, Kosmala M, Kołodziejczyk K, Sójka M, Czarnecki A, Klewicki R, Juśkiewicz J, Fotschki B, Jurgoński A.. 2018. Onion quercetin monoglycosides alter microbial activity and increase antioxidant capacity. J Nutr Biochem. 56:81–88. [DOI] [PubMed] [Google Scholar]
- Haidari F, Rashidi MR, Eshraghian MR, Mahboob SA, Shahi MM, Keshavarz SA.. 2008. Hypouricemic and antioxidant activities of Allium cepa Lilliaceae and quercetin in normal and hyperuricemic rats. Saudi Med J. 29(11):1573–1579. [PubMed] [Google Scholar]
- Han MH, Lee WS, Jung JH, Jeong J-H, Park C, Kim HJ, Kim GSup, Jung J-M, Kwon TK, Kim G-Y, et al. . 2013. Polyphenols isolated from Allium cepa L. induces apoptosis by suppressing IAP-1 through inhibiting PI3K/Akt signaling pathways in human leukemic cells. Food Chem Toxicol. 62:382–389. [DOI] [PubMed] [Google Scholar]
- Hanieh H, Narabara K, Piao M, Gerile C, Abe A, Kondo Y.. 2010. Modulatory effects of two levels of dietary Alliums on immune response and certain immunological variables, following immunization, in White Leghorn chickens. Anim Sci J. 81(6):673–680. [DOI] [PubMed] [Google Scholar]
- Hanieh H, Narabara K, Tanaka Y, Gu Z, Abe A, Kondo Y.. 2012. Immunomodulatory effects of Alliums and Ipomoea batata extracts on lymphocytes and macrophages functions in White Leghorn chickens: in vitro study. Anim Sci J. 83(1):68–76. [DOI] [PubMed] [Google Scholar]
- Hansen SL. 2001. Content of free amino acids in onion (Allium cepa L.) as influenced by the stage of development at harvest and long-term storage. Acta Agr Scand B-S P. 51(2):77–83. [Google Scholar]
- Hasani-Ranjbar S, Larijani B, Abdollahi M.. 2009. A systematic review of the potential herbal sources of future drugs effective in oxidant-related diseases. Inflamm Allergy Drug Targets. 8(1):2–10. [DOI] [PubMed] [Google Scholar]
- Hashemzaei M, Far AD, Yari A, Heravi RE, Tabrizian K, Taghdisi SM, Sadegh SE, Tsarouhas K, Kouretas D, Tzanakakis G, et al. . 2017. Anticancer and apoptosis-inducing effects of quercetin in vitro and in vivo. Oncol Rep. 138(2):819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemzaei M, Mamoulakis C, Tsarouhas K, Georgiadis G, Lazopoulos G, Tsatsakis A, Asrami ES, Rezaee R.. 2020. Crocin: a fighter against inflammation and pain. Food Chem Toxicol. 143:111521. [DOI] [PubMed] [Google Scholar]
- Hedges L, Lister C.. 2007. The nutritional attributes of Allium species. Crop and food research confidential report. 1814. New Zealand Institute for Crop & Food Research Limited. [Google Scholar]
- Helen A, Krishnakumar K, Vijayammal P, Augusti K.. 2000. Antioxidant effect of onion oil (Allium cepa Linn) on the damages induced by nicotine in rats as compared to alpha-tocopherol. Toxicol Lett. 116(1-2):61–68. [DOI] [PubMed] [Google Scholar]
- Ianni F, Lisanti A, Marinozzi M, Camaioni E, Pucciarini L, Massoli A, Sardella R, Concezzi L, Natalini B.. 2018. Amino acid content in onions as potential fingerprints of geographical origin: the case of rossa da inverno sel. Rojo Duro. Molecules. 23(6):1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ige S, Salawu E, Olaleye S, Adeeyo O, Badmus J, Adeleke A.. 2009. Onion (Allium cepa) extract prevents cadmium induced renal dysfunction. Indian J Nephrol. 19(4):140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insani EM, Cavagnaro P, Salomon VM, Langman LE, Sance M, Pazos AA, Carrari F, Filippini O, Vignera L, Galmarini CR.. 2016. Variation for health-enhancing compounds and traits in onion (Allium cepa L.) germplasm. FNS. 07(07):577–591. [Google Scholar]
- Jain S, Buttar HS, Chintameneni M, Kaur G.. 2018. Prevention of cardiovascular diseases with anti-inflammatory and anti- oxidant nutraceuticals and herbal products: an overview of pre-clinical and clinical studies. Recent Pat Inflamm Allergy Drug Discov. 12(2):145–157. [DOI] [PubMed] [Google Scholar]
- Jakaria M, Azam S, Cho D-Y, Haque M, Kim I-S, Choi D-K.. 2019. The methanol extract of Allium cepa L. protects inflammatory markers in LPS-induced BV-2 microglial cells and upregulates the antiapoptotic gene and antioxidant enzymes in N27-A cells. Antioxidants. 8(9):348–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joung EM, Jung KH.. 2014. Antioxidant activity of onion (Allium cepa L.) peel extracts obtained as onion byproducts. Korean J Food Sci Tech. 46(3):364–368. [Google Scholar]
- Jung JY, Lim Y, Moon MS, Kim JY, Kwon O.. 2011. Onion peel extracts ameliorate hyperglycemia and insulin resistance in high fat diet/streptozotocin-induced diabetic rats. Nutr Metab (Lond). 8(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B-K, Kim K-B-W-R, Ahn N-K, Choi Y-U, Kim M-j, Bark S-W, Pak W-M, Kim B-R, Park J-H, Bae N-Y, et al. . 2015. Anti-inflammatory effect of onion (Allium cepa) peel hot water extract in vitro and in vivo. Ksbb J. 30(4):148–154. [Google Scholar]
- Karimian P, Kavoosi G, Saharkhiz MJ.. 2012. Antioxidant, nitric oxide scavenging and malondialdehyde scavenging activities of essential oils from different chemotypes of Zataria multiflora. Nat Prod Res. 26(22):2144–2147. [DOI] [PubMed] [Google Scholar]
- Karishchi KP, Bidaran S.. 2018. Study of Allium cepa effect to inhibit the growth of tumor cells in BALB/c mice breast cancer model. JSUMS. 25:63–71. [Google Scholar]
- Karna KK, Choi BR, You JH, Shin YS, Cui WS, Lee SW, Kim JH, Kim CY, Kim HK, Park JK.. 2019. The ameliorative effect of monotropein, astragalin, and spiraeoside on oxidative stress, endoplasmic reticulum stress, and mitochondrial signaling pathway in varicocelized rats. BMC Complement Altern Med. 19(1):333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G, Gupta V, Christopher AF, Bansal P.. 2016. Antioxidant potential of most commonly used vegetable-onion (Allium cepa L.). JOCAMR. 1(1):1–5. [Google Scholar]
- Khaki A, Fathi AF, Nouri M, Khaki AA, Ozanci CC, Ghafari NM, Hamadeh M.. 2009. The effects of Ginger on spermatogenesis and sperm parameters of rat. IJRM. 7(1):7. [Google Scholar]
- Kim M-H, Jo S-H, Jang H-D, Lee MS, Kwon Y-I.. 2010. Antioxidant activity and α-glucosidase inhibitory potential of onion (Allium cepa L.) extracts. Food Sci Biotechnol. 19(1):159–164. [Google Scholar]
- Kim IS, Yang M, Goo TH, Jo C, Ahn DU, Park JH, Lee OH, Kang SN.. 2012. Radical scavenging-linked antioxidant activities of commonly used herbs and spices in Korea. Int J Food Sci Nutr. 63(5):603–609. [DOI] [PubMed] [Google Scholar]
- Ko MJ, Cheigh CI, Cho SW, Chung MS.. 2011. Subcritical water extraction of flavonol quercetin from onion skin. J Food Eng. 102(4):327–333. [Google Scholar]
- Kuhnau J. 1976. The flavonoids. A class of semi-essential food components: their role in human nutrition. World Rev Nutr Diet. 24:117–191. [PubMed] [Google Scholar]
- Kumar KS, Bhowmik D, Chiranjib B, Tiwari P.. 2010. Allium cepa: a traditional medicinal herb and its health benefits. Carbohydr Res. 2:283–291. [Google Scholar]
- Kumar VP, Prashanth KH, Venkatesh Y.. 2015. Structural analyses and immunomodulatory properties of fructo-oligosaccharides from onion (Allium cepa). Carbohydr Polym. 117:115–122. [DOI] [PubMed] [Google Scholar]
- Kumar VP, Venkatesh YP.. 2016. Alleviation of cyclophosphamide-induced immunosuppression in Wistar rats by onion lectin (Allium cepa agglutinin). J Ethnopharmacol. 186:280–288. [DOI] [PubMed] [Google Scholar]
- Kumari K, Augusti K.. 2002. Antidiabetic and antioxidant effects of S-methyl cysteine sulfoxide isolated from onions (Allium cepa Linn) as compared to standard drugs in alloxan diabetic rats. NISCAIR. 40:1005–1009. [PubMed] [Google Scholar]
- Lanzotti V. 2006. The analysis of onion and garlic. J Chromatogr A. 1112(1–2):3–22. [DOI] [PubMed] [Google Scholar]
- Law YY, Chiu HF, Lee HH, Shen YC, Venkatakrishnan K, Wang CK.. 2016. Consumption of onion juice modulates oxidative stress and attenuates the risk of bone disorders in middle-aged and post-menopausal healthy subjects. Food Funct. 7(2):902–912. [DOI] [PubMed] [Google Scholar]
- Lee BK, Jung Y-S.. 2016. Allium cepa extract and quercetin protect neuronal cells from oxidative stress via PKC-ε inactivation/ERK1/2 activation. Oxid Med Cell Longev. 2016:2495624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Jung J-H, Kim H-S.. 2012. Assessment of red onion on antioxidant activity in rat. Food Chem Toxicol. 50(11):3912–3919. [DOI] [PubMed] [Google Scholar]
- Lee KA, Kim K-T, Kim HJ, Chung M-S, Chang P-S, Park H, Pai H-D.. 2014. Antioxidant activities of onion (Allium cepa L.) peel extracts produced by ethanol, hot water, and subcritical water extraction. Food Sci Biotechnol. 23(2):615–621. [Google Scholar]
- Lenková M, Bystrická J, TóTh T, Hrstková M.. 2016. Evaluation and comparison of the content of total polyphenols and antioxidant activity of selected species of the genus Allium. J Cent Eur Agric. 17(4):1119–1133. [Google Scholar]
- Lesjak M, Beara I, Simin N, Pintać D, Majkić T, Bekvalac K, Orčić D, Mimica-Dukić N.. 2018. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J Funct Foods. 40:68–75. [Google Scholar]
- Liguori L, Califano R, Albanese D, Raimo F, Crescitelli A, Di Matteo M.. 2017. Chemical composition and antioxidant properties of five white onion (Allium cepa L.) landraces. J Food Qual. 2017:1–9. [Google Scholar]
- Lisanti A, Formica V, Ianni F, Albertini B, Marinozzi M, Sardella R, Natalini B.. 2016. Antioxidant activity of phenolic extracts from different cultivars of Italian onion (Allium cepa) and relative human immune cell proliferative induction. Pharm Biol. 54(5):799–806. [DOI] [PubMed] [Google Scholar]
- Lu T-M, Chiu H-F, Shen Y-C, Chung C-C, Venkatakrishnan K, Wang C-K.. 2015. Hypocholesterolemic efficacy of quercetin rich onion juice in healthy mild hypercholesterolemic adults: a pilot study. Plant Foods Hum Nutr. 70(4):395–400. [DOI] [PubMed] [Google Scholar]
- Ma Y-L, Zhu D-Y, Thakur K, Wang C-H, Wang H, Ren Y-F, Zhang J-G, Wei Z-J.. 2018. Antioxidant and antibacterial evaluation of polysaccharides sequentially extracted from onion (Allium cepa L.). Int J Biol Macromol. 111:92–101. [DOI] [PubMed] [Google Scholar]
- Mantawy M, Aly H, Zayed N, Fahmy Z.. 2012. Antioxidant and schistosomicidal effect of Allium sativum and Allium cepa against Schistosoma mansoni different stages. Eur Rev Med Pharmacol Sci. 16:69–80. [PubMed] [Google Scholar]
- Marefati N, Eftekhar N, Kaveh M, Boskabadi J, Beheshti F, Boskabady M.. 2018. The effect of Allium cepa extract on lung oxidant, antioxidant, and immunological biomarkers in ovalbumin-sensitized rats. Med Princ Pract. 27(2):122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta A, Kaur G, Chintamane M.. 2012. Piperine and quercetin enhances antioxidant and hepatoprotective effect of curcumin in paracetamol induced oxidative stress. International J of Pharmacology. 8(2):101–107. [Google Scholar]
- Memarzia A, Amin F, Saadat S, Jalali M, Ghasemi Z, Boskabady MH.. 2019. The contribution of beta-2 adrenergic, muscarinic and histamine (H1) receptors, calcium and potassium channels and cyclooxygenase pathway in the relaxant effect of Allium cepa L. on the tracheal smooth muscle. J Ethnopharmacol. 241:112012. [DOI] [PubMed] [Google Scholar]
- Mete R, Oran M, Topcu B, Oznur M, Seber ES, Gedikbasi A, Yetisyigit T.. 2016. Protective effects of onion (Allium cepa) extract against doxorubicin-induced hepatotoxicity in rats. Toxicol Ind Health. 32(3):551–557. [DOI] [PubMed] [Google Scholar]
- Mirabeau TY, Samson ES.. 2012. Effect of Allium cepa and Allium sativum on some immunological cells in rats. Afr J Tradit Complement Altern Med. 9(3):374–379. [PMC free article] [PubMed] [Google Scholar]
- Nasri S, Anoush M, Khatami N.. 2012. Evaluation of analgesic and anti-inflammatory effects of fresh onion juice in experimental animals. Afr J Tradit Complement Altern Med. 6:1679–1684. [Google Scholar]
- Nile SH, Park SW.. 2014. Total, soluble, and insoluble dietary fibre contents of wild growing edible mushrooms. Czech J Food Sci. 32(No. 3):302–307. [Google Scholar]
- O'Byrne KJ, Dalgleish AG.. 2001. Chronic immune activation and inflammation as the cause of malignancy. Br J Cancer. 85(4):473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogunmodede O, Saalu L, Ogunlade B, Akunna G, Oyewopo A.. 2011. An evaluation of the hypoglycemic, antioxidant and hepatoprotective potentials of onion (Allium cepa L.) on alloxan-induced diabetic rabbits. Inter J of Pharmacology. 8(1):21–29. [Google Scholar]
- Ola-Mudathir F, Suru S.. 2015. Onion and garlic extracts as potential antidotes for cadmium‐induced biochemical alterations in prostate glands of rats. Andrologia. 47(9):1075–1082. [DOI] [PubMed] [Google Scholar]
- Ola-Mudathir FK, Abdul-Wahab A, Moshood AI, Obuotor EM.. 2018. Comparative evaluation of antioxidant properties of methanol extracts of Allium cepa bulb, Allium cepa bulb peels and Allium fistulosum. Kragujevac J Sci. 40:131–141. [Google Scholar]
- Ola-Mudathir K, Maduagwu E.. 2014. Antioxidant effects of methanol extract of Allium cepa Linn on cyanide-induced renal toxicity in male Wistar rats. Niger J Physiol Sci. 29(2):147–151. [PubMed] [Google Scholar]
- Ola-Mudathir KF, Suru SM, Fafunso MA, Obioha UE, Faremi TY.. 2008. Protective roles of onion and garlic extracts on cadmium-induced changes in sperm characteristics and testicular oxidative damage in rats. Food Chem Toxicol. 46(12):3604–3611. [DOI] [PubMed] [Google Scholar]
- Oliveira T, Campos KM, Cerqueira-Lima AT, Carneiro TCB, da Silva Velozo E, Melo ICAR, Figueiredo EA, de Jesus Oliveira E, de Vasconcelos DFSA, Pontes-de-Carvalho LC.. 2015. Potential therapeutic effect of Allium cepa and quercetin in a murine model of Blomia tropicalis induced asthma. Daru. 23:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira T, Figueiredo CA, Brito C, Stavroullakis A, Ferreira AC, Nogueira-Filho G, Prakki A.. 2015. Allium cepa and quercetin inhibit RANKL/porphyromonas gingivalis LPS-induced osteoclastogenesis by downregulating NF-κB signaling pathway. Evid Based Complement Altern Med. 2015:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ouyang H, Hou K, Peng W, Liu Z, Deng H.. 2018. Antioxidant and xanthine oxidase inhibitory activities of total polyphenols from onion. Saudi J Biol Sci. 25(7):1509–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parhiz H, Roohbakhsh A, Soltani F, Rezaee R, Iranshahi M.. 2015. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: an updated review of their molecular mechanisms and experimental models. Phytother Res. 29(3):323–331. [DOI] [PubMed] [Google Scholar]
- Prasanna VK, Venkatesh YP.. 2015. Characterization of onion lectin (Allium cepa agglutinin) as an immunomodulatory protein inducing Th1-type immune response in vitro. Int Immunopharmacol. 26(2):304–313. [DOI] [PubMed] [Google Scholar]
- Razavi R, Kenari RE.. 2016. Antioxidant activity of red onion (Allium cepa) peel extract produced by maceration, ultrasonic assisted and supercritical extraction techniques. In: 24th Iranian Food Science and Technology Congress. [Google Scholar]
- Ren F, Reilly K, Kerry JP, Gaffney M, Hossain M, Rai DK.. 2017. Higher antioxidant activity, total flavonols, and specific quercetin glucosides in two different onion (Allium cepa L.) varieties grown under organic production: results from a 6-year field study. J Agric Food Chem. 65(25):5122–5132. [DOI] [PubMed] [Google Scholar]
- Rezaee R, Behravan E, Behravan J, Soltani F, Naderi Y, Emami B, Iranshahi M.. 2014. Antigenotoxic activities of the natural dietary coumarins umbelliferone, herniarin and 7-isopentenyloxy coumarin on human lymphocytes exposed to oxidative stress. Drug Chem Toxicol. 37(2):144–148. [DOI] [PubMed] [Google Scholar]
- Rhodes MJ, Price KR.. 1996. Analytical problems in the study of flavonoid compounds in onions. Food Chem. 57(1):113–117. [Google Scholar]
- Rivera L, Morón R, Sánchez M, Zarzuelo A, Galisteo M.. 2008. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity (Silver Spring). 16(9):2081–2087. [DOI] [PubMed] [Google Scholar]
- Rodrigues AS, Almeida DP, Simal-Gándara J, Pérez-Gregorio MR.. 2017. Onions: a source of flavonoids. Flavonoids: From Biosynthesis Hum Health. 23:439. [Google Scholar]
- Rodríguez Galdón B, Rodríguez Rodríguez EM, Díaz Romero C.. 2008. Flavonoids in onion cultivars (Allium cepa L.). J Food Sci. 73:599–605. [DOI] [PubMed] [Google Scholar]
- Roldán E, Sánchez-Moreno C, de Ancos B, Cano MP.. 2008. Characterisation of onion (Allium cepa L.) by-products as food ingredients with antioxidant and antibrowning properties. Food Chem. 108(3):907–916. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Luheshi G, Toulmond S.. 1996. Cytokines and their receptors in the central nervous system: physiology, pharmacology, and pathology. Pharmacol Ther. 69(2):85–95. [DOI] [PubMed] [Google Scholar]
- Sagar NA, Pareek S, Gonzalez-Aguilar GA.. 2020. Quantification of flavonoids, total phenols and antioxidant properties of onion skin: a comparative study of fifteen Indian cultivars. J Food Sci Technol. 57(7):2423–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santas J, Almajano MP, Carbó R.. 2010. Antimicrobial and antioxidant activity of crude onion (Allium cepa L.) extracts. Int J Food Sci Technol. 45(2):403–409. [Google Scholar]
- Seo MY, Kim KR, Lee JJ, Ryu G, Lee SH, Hong SD, Dhong H-J, Baek C-H, Chung S-K, Kim HY.. 2019. Therapeutic effect of topical administration of red onion extract in a murine model of allergic rhinitis. Sci Rep. 9(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahverdi S, Khaki A, Jafari B.. 2017. Investigating the antioxidant effect of Allium cepa after exposure to Escherichia coli on biochemical factors, the blood antioxidants, and testis tissue in rats. Crescent J Med Biol Sci. 4:32–38. [Google Scholar]
- Shenoy C, Patil M, Kumar R, Patil S.. 2009. Preliminary phytochemical investigation and wound heal activity of Allium cepa Linn (Liliaceae). Int J Pharm Pharm Sci. 2:117–167. [Google Scholar]
- Shi GQ, Jing Y, Jiang L, Sheng-Nan L, Han-Xue S, Wen-En Z, Yan-Qi L.. 2016. Isolation of flavonoids from onion skins and their effects on K562 cell viability. Bangladesh J Pharmacol. 11:18–S25. [Google Scholar]
- Shrestha DK, Sapkota H, Baidya P, Basnet S. 2016. Antioxidant and antibacterial activities of Allium sativum and Allium cepa L. Bull Pharm Res. 6(2):50–55. [Google Scholar]
- Shri R, Bora KS.. 2008. Neuroprotective effect of methanolic extracts of Allium cepa on ischemia and reperfusion-induced cerebral injury. Fitoterapia. 79(2):86–96. [DOI] [PubMed] [Google Scholar]
- Siddiq M, Roidoung S, Sogi D, Dolan K.. 2013. Total phenolics, antioxidant properties and quality of fresh-cut onions (Allium cepa L.) treated with mild-heat. Food Chem. 136(2):803–806. [DOI] [PubMed] [Google Scholar]
- Simin N, Orcic D, Cetojevic-Simin D, Mimica-Dukic N, Anackov G, Beara I, Mitic-Culafic D, Bozin B.. 2013. Phenolic profile, antioxidant, anti-inflammatory and cytotoxic activities of small yellow onion (Allium flavum L. subsp. flavum, Alliaceae). LWT- Food Sci Technol. 54(1):139–146. [Google Scholar]
- Singh BN, Singh BR, Singh RL, Prakash D, Singh DP, Sarma BK, Upadhyay G, Singh HB.. 2009. Polyphenolics from various extracts/fractions of red onion (Allium cepa) peel with potent antioxidant and antimutagenic activities. Food Chem Toxicol. 47(6):1161–1167. [DOI] [PubMed] [Google Scholar]
- Škerget M, Majhenič L, Bezjak M, Knez Ž.. 2009. Antioxidant, radical scavenging and antimicrobial activities of red onion (Allium cepa L) skin and edible part extracts. Chem Biochem Eng Q. 23:435–444. [Google Scholar]
- Slimestad R, Fossen T, Vågen IM.. 2007. Onions: a source of unique dietary flavonoids. J Agric Food Chem. 55(25):10067–10080. [DOI] [PubMed] [Google Scholar]
- Soto VC, González RE, Sance MM, Galmarini CR.. 2015. Organosulfur and phenolic content of garlic (Allium sativum L.) and onion (Allium cepa L.) and its relationship with antioxidant activity. VII Int Sympos Edible Alliaceae. 1143(1143):277–290. [Google Scholar]
- Spelman K, Burns J, Nichols D, Winters N, Ottersberg S, Tenborg M.. 2006. Modulation of cytokine expression by traditional medicines: a review of herbal immunomodulators. Altern Med Rev. 11(2):128–151. [PubMed] [Google Scholar]
- Suru SM. 2008. Onion and garlic extracts lessen cadmium-induced nephrotoxicity in rats. Biometals. 21(6):623–633. [DOI] [PubMed] [Google Scholar]
- Suru SM, Ugwu CE.. 2015. Comparative assessment of onion and garlic extracts on endogenous hepatic and renal antioxidant status in rat. J Basic Clin Physiol Pharmacol. 26:347–354. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Shibamoto T.. 2008. Chemical compositions and antioxidant/anti-inflammatory activities of steam distillate from freeze-dried onion (Allium cepa L.) sprout. J Agric Food Chem. 56(22):10462–10467. [DOI] [PubMed] [Google Scholar]
- Teselkin YO, Babenkova IV, Kolhir VK, Baginskaya AI, Tjukavkina NA, Kolesnik YA, Selivanova IA, Eichholz AA.. 2000. Dihydroquercetin as a means of antioxidative defence in rats with tetrachloromethane hepatitis. Phytother Res. 14(3):160–162. [DOI] [PubMed] [Google Scholar]
- Teshika JD, Zakariyyah AM, Zaynab T, Zengin G, Rengasamy KR, Pandian SK, Fawzi MM.. 2019. Traditional and modern uses of onion bulb (Allium cepa): a systematic review. Crit Rev Food Sci Nutr. 59:39–70. [DOI] [PubMed] [Google Scholar]
- Ueda H, Takeuchi A, Wako T.. 2013. Activation of immune responses in mice by an oral administration of bunching onion (Allium fistulosum) mucus. Biosci Biotechnol Biochem. 77(9):1809–1813. [DOI] [PubMed] [Google Scholar]
- Umoh U, Umoh R, Enema O, Ekpo E, Ajibesin K, Eseyin O.. 2019. Anti-inflammatory constituents of plants: a review. JCHPR. 11:74–85. [Google Scholar]
- Upadhyay RK. 2017. Nutritional and therapeutic potential of Allium vegetables. J Nutr Ther. 6(1):18–37. [Google Scholar]
- Vazhappilly CG, Ansari SA, Al-Jaleeli R, Al-Azawi AM, Ramadan WS, Menon V, Hodeify R, Siddiqui SS, Merheb M, Matar R, et al. . 2019. Role of flavonoids in thrombotic, cardiovascular, and inflammatory diseases. Inflammopharmacol. 27(5):863–869. [DOI] [PubMed] [Google Scholar]
- Vazquez-Prieto MA, Bettaieb A, Rodriguez Lanzi C, Soto VC, Perdicaro DJ, Galmarini CR, Haj FG, Miatello RM, Oteiza PI.. 2015. Catechin and quercetin attenuate adipose inflammation in fructose-fed rats and 3T3-L1 adipocytes . Mol Nutr Food Res. 59(4):622–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Prieto MA, Rodriguez Lanzi C, Lembo C, Galmarini CR, Miatello RM.. 2011. Garlic and onion attenuates vascular inflammation and oxidative stress in fructose-fed rats. J Nutr Metab. 2011:475216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votto APS, Domingues BS, de Souza MM, da Silva Júnior FMR, Caldas SS, Filgueira DMVB, Clementin RM, Primel EG, Vallochi AL, Furlong EB, et al. . 2010. Toxicity mechanisms of onion (Allium cepa) extracts and compounds in multidrug resistant erythroleukemic cell line. Biol Res. 43(4):429–437. [PubMed] [Google Scholar]
- Wiczkowski W, Romaszko J, Bucinski A, Szawara-Nowak D, Honke J, Zielinski H, Piskula MK.. 2008. Quercetin from shallots (Allium cepa L. var. aggregatum) is more bioavailable than its glucosides. J Nutr. 138(5):885–888. [DOI] [PubMed] [Google Scholar]
- Wilson EA, Demmig-Adams B.. 2007. Antioxidant, anti-inflammatory, and antimicrobial properties of garlic and onions. Nutr Food Sci. 37(3):178–183. [Google Scholar]
- Wu F, Chen Y, Li G, Zhu D, Wang L, Wang J.. 2019. Zinc oxide nanoparticles synthesized from Allium cepa prevents UVB radiation mediated inflammation in human epidermal keratinocytes (HaCaT cells). Artif Cells Nanomed Biotechnol. 47(1):3548–3558. [DOI] [PubMed] [Google Scholar]
- Wu S, Ning F, Wu X, Wang W.. 2016. Proteomic characterization of differential abundant proteins accumulated between lower and upper epidermises of fleshy scales in onion (Allium cepa L.) bulbs. PLoS One. 11(12):e0168959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Weitzmann MN.. 2011. Quercetin, a potent suppressor of NF-κB and Smad activation in osteoblasts. Int J Mol Med. 28(4):521–525. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Yasuoka A.. 2010. Welsh onion attenuates hyperlipidemia in rats fed on high-fat high-sucrose diet. Biosci Biotechnol Biochem. 74(2):402–404. [DOI] [PubMed] [Google Scholar]
- Yang J, Meyers KJ, van der Heide J, Liu RH.. 2004. Varietal differences in phenolic content and antioxidant and antiproliferative activities of onions. J Agric Food Chem. 52(22):6787–6793. [DOI] [PubMed] [Google Scholar]
- Ye CL, Dai DH, Hu WL.. 2013. Antimicrobial and antioxidant activities of the essential oil from onion (Allium cepa). Food Cont. 30(1):48–53. [Google Scholar]
- Zarei H, Rezaee R, Behravan E, Soltani F, Mosaffa F, Iranshahi M, Behravan J.. 2013. Diversin, from Ferula diversivittata protects human lymphocytes against oxidative stress induced by H2O2. Nat Prod Res. 27(11):1016–1019. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang Y, Zhang F, Wang K, Liu G, Yang M, Luan Y, Zhao Z, Zhang J, Cao X, et al. . 2015. Allyl methyl disulfide inhibits IL-8 and IP-10 secretion in intestinal epithelial cells via the NF-кB signaling pathway. Int Immunopharmacol. 27(1):156–163. [DOI] [PubMed] [Google Scholar]