Abstract
Objective: The study was aimed to investigate whether the neuroprotective role of curcumin is associated with regulation of autophagy.
Methods: Rat spinal cord injury (SCI) models were established according to Allen’s weight-drop trauma method. Curcumin was administered 30 min after the contusion and continued weekly. At 3, 7, 14, 21, and 28 days after SCI, functional recovery was evaluated using the Basso, Beattie and Bresnahan (BBB) scoring and the oblique plate test, following which, spinal cord tissues were obtained. Histological changes and apoptosis were then measured with H&E staining and TUNEL assay. Glia activation, inflammatory infiltration, inflammatory factor release, and myelination were observed through immunohistochemical (IHC) staining and ELISA. Autophagy and Akt activation were detected by western blotting. After autophagy was inhibited by injection of chloroquine, TUNEL, inflammatory factor release, myelin basic protein (MBP) IHC staining and functional recovery evaluation were performed again.
Results: Curcumin treatment promoted functional recovery after SCI and reduced neuron apoptosis, improved spinal cord integrity, recovery, and re-myelination, and suppressed the inflammatory response. Autophagy was enhanced and Akt/mTOR pathway was inhibited by curcumin. Autophagy inhibition partially eliminated the protective effect of curcumin on SCI.
Conclusion: Curcumin may exert its therapeutic effect on SCI through the enhancement of autophagy, in which, inhibition of the Akt/mTOR signaling pathway may be also involved.
Keywords: Curcumin, Spinal cord injury, Autophagy, Akt, mTOR
Introduction
Spinal cord injury (SCI) is a major cause of death and long-term disability worldwide.1,2 Patients with SCI usually suffer enormous physical and emotional pain as injuries lead to long-term sensorimotor, cognitive, and psychiatric changes.2 Over the years, a number of animal models have been established and developed to investigate the mechanisms and the functional consequences of SCI as well as for the evaluation of potential therapeutic interventions.3,4 It is known that trauma causes both direct tissue damage, which is considered to be the primary injury, and biochemical changes that lead to progressive cell loss, referred to as secondary injury.2,5
Basal levels of autophagy are essential for homeostasis in terminally differentiated cells like neurons. However, pathologically increased autophagy has been reported as a contributor to cell death.6 When essential autophagy genes were knocked out, mice developed severe neurodegeneration, and the underlying mechanism of which was proposed to be cell injury and death caused by inhibited autophagy flux.6,7 In experimental studies, combining an autophagy inhibitor with chemotherapy augments therapeutic benefits and helps overcomes drug resistance in cancer.8,9 Clinical trials using the late stage autophagy inhibitor chloroquine or its derivative hydroxychloroquine showed that patients have safely gone through the combinatory treatment of chloroquine-hydroxychloroquine with standard chemotherapies and exhibited prolonged survival in certain types of cancer.10 Therefore, chloroquine has been used as an autophagy inhibitor in basic experiments in the laboratory. Impaired autophagy flux has been associated with many neurodegenerative disorders, such as Alzheimer’s, Parkinson’s, and Huntington’s disease.11 Emerging data suggest that autophagy flux may be either increased or decreased after SCI depending on the location and severity of the injury.12,13 Thus, it is not yet known whether autophagy plays a beneficial or detrimental role after injury. However, it is clear that restoration and augmentation of autophagy flux improve functional recovery after injury by increasing cell survival, suggesting that the autophagy pathway is a potential therapeutic target for treatment of SCI.13
Curcumin, an active component of Curcuma longa, was initially reported having antibacterial function in 1949.14 Studies have shown that issues with fast metabolism, low bioavailability, and low chemical stability have been overcome in the clinical application of curcumin and its analogs for the treatment of several inflammatory conditions in cancer,15 cardiovascular disease,16 and central nervous system disorder/trauma.17 Additional reports have demonstrated the positive influence of curcumin for treatment of SCI and revealed that curcumin contributes to neurological recovery.18 However, to the best of our knowledge, the therapeutic role of curcumin in SCI with respect to autophagy, autophagy flux, and related signaling pathways remains unreported. Thus, in the present study, we investigated the treatment of SCI using curcumin in a rat model and its influence on autophagy induction and related signaling pathways.
Materials and methods
Rat SCI model and treatments
A total of 160 male SD (Sprague Dawley) rats (Eight weeks old, weighing 235 ± 15 g), provided by Kunming University of Science and Technology, were used in this study. This study was approved by the Ethics Committee of Kunming University of Science and Technology (ethics approval no.: 2015FB095). Rats were housed with free access to water and food in a room conditioned with 12 h light–dark cycles. The rats were initially divided into two types: sham operated rats (n = 30), and SCI rats (n = 100). In sham rats, laminectomy was performed without SCI, and they were treated with 98% normal saline (NS) solution (VNS:Vwater = 98/2). SCI rats were established according to a modified Allen’s weight-drop trauma method.19 Briefly, the rats were anesthetized with intraperitoneal injection of chloral hydrate (300 mg/kg) and operated under aseptic conditions. The skin was cut, the lamina was exposed, and a laminectomy was performed to expose the spinal cord at T9–T11. The exposed spinal cord segment was subjected to the subsequent SCI induced by dropping a 10-g iron body with a diameter of 10 mm from a height of 20 mm onto the spinal cord surface. Three days after the surgery, behavioral symptoms were evaluated. Successful SCI exhibits following characteristics in the rats: formation of tail sway reflex, spinal cord ischemia, flicking of legs, and the appearance of paralysis. These 100 SCI rats were subsequently subjected to injection with dimethyl sulfoxide (DMSO) (n = 50) and curcumin (n = 50). Curcumin (Sigma-Aldrich, Santa Clara, CA, USA) was dissolved in 2.0% DMSO with 98.0% normal saline solution. The group treated with the vehicle (2.0% DMSO with 98.0% normal saline solution; percutaneous, intramuscular injection near the site of injury within 30 min following the contusion) was designated as DMSO group, serving as a negative control.
In order to find out the role of autophagy in the protective effect of curcumin on SCI, we established 30 more SCI rat models using the same method mentioned above. The SCI rats received either: DMSO (n = 10), curcumin (Cur, n = 10), or curcumin combined with chloroquine (Cur + CQ, n = 10). These treatments were administered every week via percutaneous, intramuscular injection near the site of injury within 30 min following the contusion and continued weekly for 3 weeks (DMSO: 1 ml/kg BW (body weight); curcumin: 60 mg/ml/kg BW in DMSO;20,21 Cur + CQ: 60 mg/ml/kg curcumin in DMSO + 50 mg/kg chloroquine).22 Rats in the sham group received DMSO injection within 10 min after laminectomy.
Evaluation of functional recovery
Locomotor activity was assessed using Basso, Beattie and Bresnahan (BBB) scoring in accordance with a previous report23 and functional tests were conducted three days and weekly after the treatment (time points of 3, 7, 14, 21, 28 days). Hind-limb movements were observed by two independent, blinded examiners as the rats were forced towards the edges of an open field.23 Rats that exhibited any functional recovery within 72 h of injury were excluded from the study.
In addition, Rivlin’s oblique plate test was performed to evaluate each rat’s ability to grip and maintain posture as previously described.24 The action of each rat was measured three times by placing it in the middle of an oblique plate. In each trial, the plate was periodically lifted by 5°, and the maximum angle was recorded as the angle at which the rat could maintain its position for five seconds without sliding down.
Hematoxylin–eosin (H&E) staining and immunohistochemistry
The rats were euthanized using an overdose of 2% pentobarbital sodium (120 mg/kg; intraperitoneal injection) when the corresponding treatment was finished. Tissue segments containing the lesion were paraffin embedded. Tissues were cut into 5-μm-thick sections, which were taken 2 mm rostral to the epicenter of the injury, and mounted. Parts of them were fixed in 10% formalin for 2 days and used for histopathological analysis, and other parts were stored in liquid nitrogen, which were intended for western blotting.
For histopathological observation, the sections were subjected to routine H&E staining. Simply, the paraffin sections spinal cord were dewaxed with xylene and an alcohol series. Slides were placed in hematoxylin for 5 min, followed by a dip in 2% acid alcohol and then in lithium carbonate. The slides were dipped in eosin for 2 min and then dehydrated with ethanol before xylene. The area of preserved tissue and the cavity in spinal cord were measured with ImageJ.
Immunohistochemical staining was performed after deparaffinization. Intrinsic peroxidase activity was blocked by incubation in 5% H2O2 in PBS solution for 20 min. Sections were incubated overnight at 4°C with anti-rat primary antibody against myelin basic protein (MBP, 1:500; Cell Signaling, Boston, USA), Iba-1 (1:1000; Wako Pure Chemical Industries, Osaka, Japan), and GFAP (1:200; Abcam, Cambridge, UK). Sections were then incubated with conjugated secondary antibody (Cell Signaling, Boston, USA) for 1 h at 37°C. Sections were rinsed with PBS and mounted. Immunostaining was examined with an Olympus BX60 microscope, and the results were semi-quantified by Image Pro-Plus.
Neun & TUNEL (TdT mediated dUTP nick end labeling) double staining
Neuronal death was assessed by double staining of NeuN and TUNEL, using a commercially available kit (Merck, Darmstadt, Germany). The sections were dewaxed, dehydrated, and rinsed for the TUNEL technique. The sections were permeabilized and subjected to antigen retrieval using proteinase K. Endogenous peroxidase was blocked using a methanol solution and hydrogen peroxide (3% H2O2). Prior to TUNEL staining, sections were washed once with PBS and subsequently incubated overnight at 4°C with monoclonal mouse anti-rat NeuN (1:200; Millipore, MA, USA) diluted in carrier solution I (1% goat serum, 1% BSA, and 0.3% Triton X-100 in PBS). The percentage of dead neuron cells = TUNEL+/NeuN+ cells/DAPI+ cells.
Wet/dry weight ratio assay
Spinal cord water content was determined as previously described25 using the wet/dry weight method. Spinal cord samples were taken 48 h after SCI. Spinal cords were removed, and 1.5 cm-size spinal tissues were obtained at the edge of the injury site. The tissues were weighed, heated at 110°C for 24 h, and re-weighed to obtain the dry weight. The percentage of water content was calculated using the following equation: (wet weight – dry weight)/wet weight × 100.
ELISA analysis
Tissue samples were homogenized twice after adding stainless steel beads and Triton X-100 (2 mL, 0.1%) using a Bullet Blender (Next Advance, NY, USA). Homogenates were incubated at room temperature for 1 h and centrifuged for 15 min at 150 × g and 0°C. Equal amounts of each supernatant (200 μL) were transferred to a microwell plate prior to addition of 800 μL PBS. A commercially available ELISA kit (Immunalysis Corporation, Pomona, CA, USA) was used to evaluate the release of inflammatory cytokines, TNF-α, IL-6, and IL-1β in accordance with the manufacturer’s instructions.
Western blot analysis
Rats were decapitated 4 h, 3 and 7 days after the treatment and sham group. The spinal cord from the rats in each group was removed and stored at −80°C. Western blotting was conducted by researchers blinded to the groups. Spinal cord tissues were homogenized and total protein was extracted using RIPA lysis buffer. After assessment of protein concentration, 40 μg of total protein was subjected to SDS-PAGE separation and transferred to PVDF membrane (Millipore, MA, USA). The membrane was then blocked with 5% non-fat milk at room temperature. The primary antibody (Abcam, Cambridge, UK) against caspase 3 (1: 500), LC3B (1:1,000), p62 (1:1,500), p-Akt (1:1,000), Akt (1:500), p-mTOR (1:2,000), or mTOR (1:2,000) was diluted with TBS at 1:2000 and membranes were incubated with the primary antibody at 4°C overnight. The secondary antibody was added and incubated for 45 min at room temperature and visualized with the enhanced chemiluminescence (ECL) technique. Imaging data were analyzed using the ImageJ (NIH, MD, USA) software using the expression level of GAPDH as an internal reference.
Statistical analysis
The data are presented as means ± standard deviations (SD) and were analyzed using SPSS version 16.0 (IBM, Chicago, IL, USA). Intergroup data were compared using analysis of variance (ANOVA). A P-value of less than 0.05 was regarded as statistically significant.
Results
Curcumin reduces damage to tissue structure, decreases neuronal apoptosis, and improves functional recovery after SCI
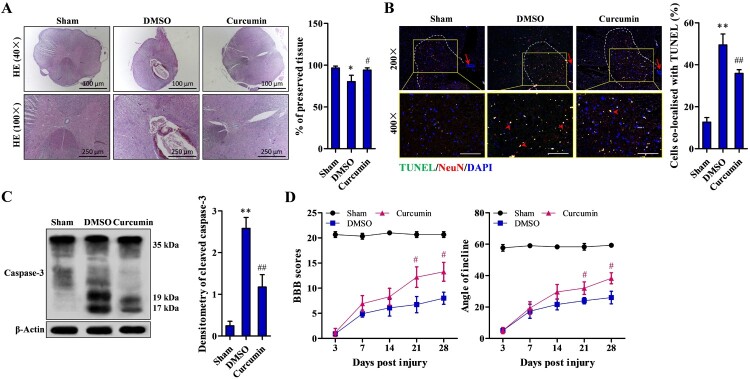
Histological observation of the injured spinal cords treated with DMSO exhibited a central cavity with severe focal necrosis, widespread edema, diffuse hemorrhage and severe focal necrosis in both gray-matter and white-matter and tissue loss (P < 0.05, Fig. 1A). Curcumin treatment resulted in a notable amelioration of necrosis and considerable tissue preservation after 28 days of treatment (P < 0.05, Fig. 1A). TUNEL staining revealed significantly more neuronal death in injured spinal cords treated with DMSO compared to the sham control group (P < 0.01). A significant reduction in the percentage of apoptotic neurons was seen in the curcumin treatment group compared to the DMSO group (P < 0.01) (Fig. 1B). Cleaved caspase-3 could be barely found in the DMSO group, compared to the which, sham control group showed significantly increased caspase-3 cleavage (P < 0.05). But the cleavage in the curcumin treatment group was significantly lower compared to the DMSO group (P < 0.01) (Fig. 1C). Hind limb locomotion function was assessed with BBB scoring. As shown in Fig. 1D, the BBB score was significantly higher in the curcumin group compared to the DMSO group 21 days after SCI and was even higher after 28 days (P < 0.05).
Figure 1.
Decreased damage to tissue structure and improved functional recovery due to 28-day curcumin treatment after SCI. (A) Representative images of H&E staining of the injury site after SCI; 100× magnified pictures were taken from 40× photos. (B) Neuron apoptosis in left ventral horn (within the dotted lines) detected by TUNEL staining; apoptotic neurons were co-localized with TUNEL (arrow head); scale bar: 200 μm; arrow: central canal. (C) Caspase-3 activity examined by western blotting. (D) Functional recovery assessed by BBB scoring and the oblique plate test. Sham (n = 10), DMSO (n = 10), curcumin (n = 10) treatment group. Each experiment was conducted in triplicate. *P < 0.05, **P < 0.01 vs. sham controls; #P < 0.05, ##P < 0.01 vs. DMSO.
Curcumin decreases gliosis, inflammation response, and improves myelination after SCI
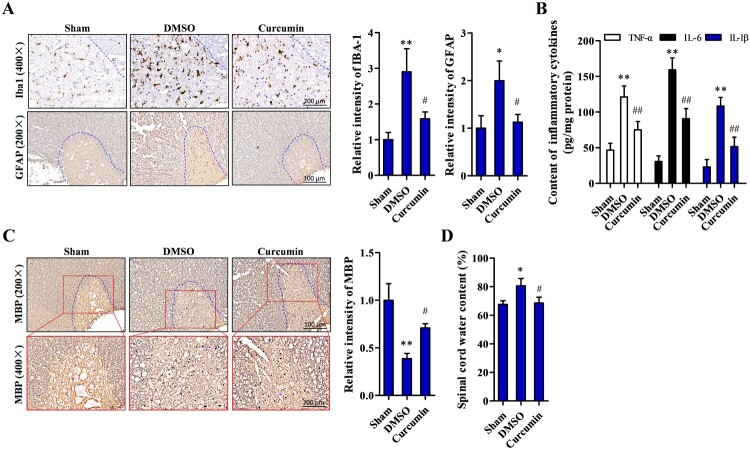
Our histopathological examination revealed that Iba-1 signal intensity in the DMSO group was approximately 3-fold higher than in the sham control group (P < 0.05) and more than 1-fold lower in the curcumin treatment group compared to the DMSO group (P < 0.05). Likewise, the percentage of GFAP-positive cells was significantly increased in the DMSO group and significantly decreased in the curcumin treatment group (both P < 0.05) (Fig. 2A). To determine the effect of curcumin on the inflammatory response, ELISAs were performed to determine inflammatory cytokine concentrations. TNF-α, IL-6, and IL-1β were significantly higher in the DMSO group (P < 0.01) compared to the sham group, and their levels were remarkably reduced (P < 0.01) with curcumin treatment compared to DMSO alone (Fig. 2B). In addition, myelination of nerves was determined by examining the expression level of myelin basic protein (MBP) by immunohistochemical staining analysis. MBP was lower (P < 0.01) in the DMSO group than the sham controls, but significantly higher (P < 0.05) in the curcumin treatment group compared to the DMSO group (Fig. 2C). The effect of curcumin on spinal cord edema was assessed with the wet/dry ratio method. The results showed that the spinal cord water content was higher in the DMSO group compared to the sham controls (P < 0.05) but was significantly lower in the curcumin treatment group compared to the DMSO group (P < 0.05) (Fig. 2D).
Figure 2.
Decreased gliosis and inflammation, and improved myelination by curcumin treatment after SCI. (A) Examination of astrocytes and gliosis level by their representative markers Iba-1 and GFAP in left ventral horn (within the dotted lines) using immunohistochemistry. (B) Detection of inflammatory cytokine TNF-α, IL-6, and IL-1β by ELISA kit. (C) Myelination status observed by immunohistochemical staining of MBP at the site of injured tissue. (D) Spinal edema determined by wet/dry weight ratio assay. Sham (n = 15), DMSO (n = 20), curcumin (n = 20) treatment group. Each experiment was conducted in triplicate. *P < 0.05, **P < 0.01 vs. sham controls; #P < 0.05, ##P < 0.01 vs DMSO.
Curcumin promotes autophagy flux and downregulates Akt activation after SCI
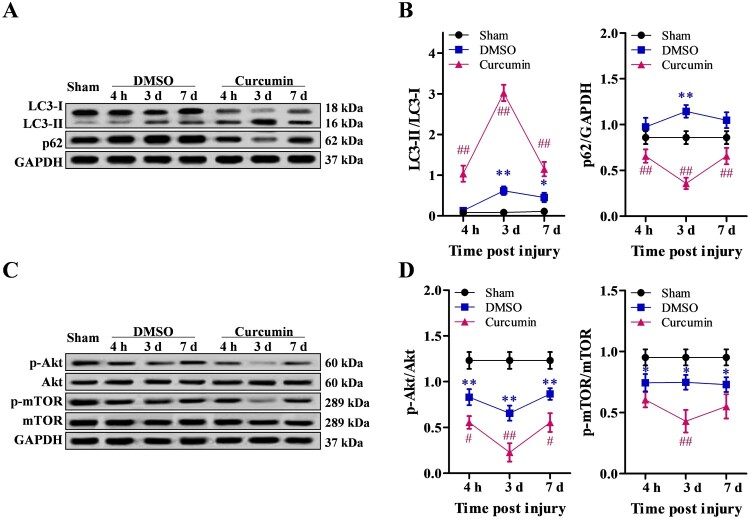
To examine the induction of autophagy after SCI, we assessed the levels of autophagy protein LC3 (MAP1LC3B) in the injured spinal cord by western blotting. Conversion of LC3-I to LC3-II is an essential marker of autophagosome formation and accumulation. With curcumin treatment, LC3-II/LC-I ratio was significantly increased 4 h after injury compared to DMSO alone (P < 0.01) and reached peak expression on day 3, followed by a reduction on day 7 after injury. On the other hand, the expression of p62 (normalized to GAPDH) was lower in the curcumin treatment group compared to the DMSO group (P < 0.01), and was higher in the DMSO group compared to the sham controls (P < 0.05) (Fig. 3A, B). Because autophagy flux is often associated with Akt/mTOR pathway,26,27 we examined the expression of Akt and mTOR molecules by western blot analysis. The results showed that both Akt and mTOR expression were significantly lower in the DMSO group compared to the sham controls (P < 0.05), and were further decreased in the curcumin treatment group compared to the DMSO group (P < 0.05) (Fig. 3C, D).
Figure 3.
Autophagy flux promoted by curcumin treatment after SCI through the Akt/mTOR signaling pathway. (A, B) Autophagy flux detected 4 h, 3, and 7 days after mechanical injury by western blot analysis. (C, D) Activation of the Akt/mTOR signaling pathway examined 4 h, 3, and 7 days after treatment by western blot analysis. Sham (n = 5), DMSO (n = 30), curcumin (n = 30) treatment group. Each experiment was conducted in triplicate. *P < 0.05, **P < 0.01, vs. sham control; #P < 0.05, ##P < 0.01, vs. DMSO.
Inhibition of autophagy partly abolishes the protective effect of curcumin on spinal cord injury
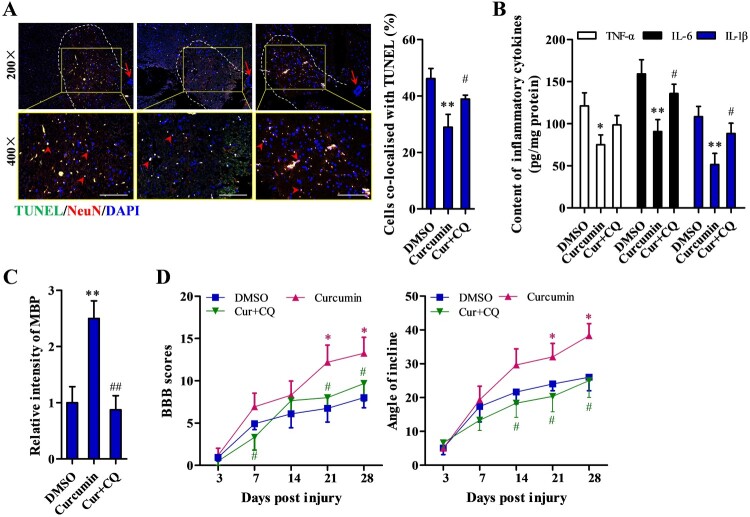
We examined the consequences of inhibiting autophagy on the protective effect of curcumin after spinal cord injury by combined treatment with curcumin and chloroquine (CQ, autophagy pathway inhibitor). The percentage of apoptotic cells was significantly increased with combined treatment (Cur + CQ) compared to curcumin treatment alone (P < 0.05) (Fig. 4A). In addition, the concentrations of inflammatory cytokines, TNF-α, IL-6, and IL-1β, were increased with combined treatment compared to curcumin treatment only (P < 0.05) (Fig. 4B). Immunohistochemical staining revealed an elevated number of MBP positive cells with curcumin treatment that was reduced by addition of chloroquine (P < 0.05) (Fig. 4C). In functional recovery analysis, the significantly increased BBB score and angle of incline after curcumin treatment were markedly blocked by CQ (Curcumin + CQ v.s. Curcumin, P < 0.05) 21 and 28 days after SCI (Fig. 4D).
Figure 4.
Abrogation of curcumin’s protective effect by inhibition of autophagy. (A) Nerve cell apoptosis detected by TUNEL staining and apoptotic neurons in left ventral horn (within the dotted lines) were co-locolized with TUNEL (arrow head); scale bar: 200 μm. (B) Detection of inflammatory cytokine TNF-α, IL-6, and IL-1β by ELISA detection kit. (C) Myelination status measured by immunohistochemical staining of MBP at the site of injured tissue. (D) Functional recovery assessed by BBB scoring and angle of incline analysis. DMSO (n = 10), curcumin (n = 10), and curcumin + CQ treatment group (n = 10). Each experiment was conducted in triplicate. *P < 0.05, **P < 0.01 vs. DMSO; #P < 0.05, ##P < 0.01 vs. curcumin.
Discussion
This study demonstrates that the active compound curcumin enhances functional recovery after SCI as evidenced by higher locomotor ratings, amelioration of tissue damage, and reduced neuronal death. Histopathological investigation revealed that glial scar formation and inflammatory responses were reduced and that spinal myelination was improved after curcumin treatment. In addition, curcumin promoted the induction of autophagy flux after SCI and inhibited the activation of the Akt/mTOR signaling pathway. To further explore the effect of curcumin on induction of autophagy flux, we inhibited the autophagy pathway. The results showed that autophagy inhibition partially abrogates the protective effect of curcumin after SCI as evidenced by increased tissue damage, neuronal death rate, and inflammatory cytokines, and reduced BBB score.
SCI causes the cell death of neurons, astrocytes, and oligodendrocytes and leads to massive loss of sensory and motor skills beneath the site of injury.28,29 In addition, SCI provokes an inflammatory response30 which contributes to activation of resident microglia cells31 and demyelination.32 In our work, overproduction of microglia and astrocytes was detected by examining the expression levels of Iba-1 and GFAP in SCI rats and this elevation was significantly reduced with curcumin treatment. Furthermore, inflammatory cytokine concentrations, TNF-α, IL-6, and IL-1β, were significantly higher in the DMSO group compared to the sham group, and their levels were remarkably reduced with curcumin treatment. Considering the pathophysiology of SCI that incudes neuronal death, reactive gliosis, axon demyelination, and syringomyelia around the lesion, alleviating secondary damage is a crucial element in the treatment of SCI.33 Curcumin improves motor function recovery and reduces spinal cord edema by inhibiting the JAK/STAT signaling pathway.34 In addition, it promotes spinal cord repair through inhibition of glial scar formation and inflammation.35 Our results demonstrated that myelination of nerves (biomarker: myelin basic protein) was lower in the DMSO group than the sham controls, but significantly higher in the curcumin treatment group compared to the DMSO group. Along with the therapeutic function to promote nerve myelination, our result also exhibited that curcumin could reduce spinal cord edema. These results may suggest that curcumin could be a potential candidate for treating loss of sensory and motor skills caused by SCI.
Since induction of autophagy flux could be beneficial for functional recovery after SCI, in this study we further investigated whether curcumin regulates the autophagy pathway after SCI in rats. In previous literature, nicotinate-curcumin, one of the curcumin derivatives, was reported to decrease lipid accumulation by restoring autophagy flux, suggesting therapeutic potential to reverse atherosclerosis.36 In a study of transthyretin (TTR)-related amyloidosis disease, which is characterized by extracellular deposition of aggregates and associated with autophagy impairment, curcumin was suggested as an alternative therapeutic agent due to its potential role in modulating autophagy.37 Interestingly, in malignant mesothelioma cell lines, curcumin was reported to block autophagy and activate apoptosis.38 It has also been reported that curcumin induces autophagy through inhibition of the Akt/mTOR pathway in Alzheimer’s disease,39 malignant glioma,40 and human leukemia.41 In agreement, our results demonstrated that the therapeutic role of curcumin in spinal cord injury was associated with elicitation of autophagy through inhibition of the Akt/mTOR pathway.
Increasing literatures reported that blocking of autophagic flux in neurons results from lysosome defects or the failure in fusion between atophagosomes and lysosomes, and leads to the development of some CNS diseases.42 Upstream of mTOR, the survival PI3K/AKT pathway modulates mTOR activity that is also altered in neurodegenerative diseases of Alzheimer and Parkinson. Suppression of the PI3K/AKT/mTOR pathway promotes autophagy and activation of PI3K/AKT/mTOR inhibits authophagy.27 Rapamycin specifically inhibits mTOR pathway and increases the formation of autophagosomes, and therefore stimulates the process of autophagy.43 Hence, this agent was commonly used as an autophagic agonist. On the other hand, the behavior improvement and mitigation of histological destruction strongly supports the benefit of autophagy in SCI.12 However, rampamycin tend to overstimulate autophagy and may lead to detrimental outcomes. As a natural component curcumin may play a safer role in suppressed mTOR induced autophagy. Our results suggest that, when autophagy was inhibited, therapeutic role of curcumin was diminished as evidenced by increased level of inflammatory cytokines, reduced neuron myelination, decreased BBB score. Discovery indicates that curcumin is a direct inhibitor of mTOR, which may remove the association of Raptor with mTOR at low concentration and suppress the binding of Rictor with mTOR at a high concentration,44 but it is not supported by molecular insight into the regulation. Therefore, a much more detailed mechanism about the inhibition of curcumin in mTOR signaling pathway should be further studied.
In addition, we noticed that the autophagy flux reached a peak point followed by a decrease 7 days post injury/treatment. Given that the time-dependent accumulation of cellular damage is the general cause of aging, it is easy to infer that a gradual, age-related decline in autophagic activity may contribute to this result. However, this speculation needs further experiment for confirmation. Along with the decline in autophagy flux 7 days post treatment, Akt/mTOR expression was also decreased at the same time point. As to how this result occurs, we could not have any clue yet. Our next experiment has already been launched to find an explanation for such phenomenon.
In summary, the present study demonstrates that curcumin promotes motor function recovery and reduces neuronal apoptosis. In addition, curcumin may exert its neuroprotective effect on SCI through the modulation of autophagy and inhibiting Akt/mTOR signaling.
Disclaimer statements
Conflicts of interest The authors declare that they have no conflict of interest.
Funding Statement
This study was supported by the National Natural Science Foundation of China (NSFC, Grant No.: 81860240), the Development Program of Kunming University of Science and Technology (sponsored by Yunnan Provincial Department of Science and Technology; Grant No.: KKSY201560051), and Yunnan Provincial Science and Technology Department-Kunming Medical University Joint Special Project (sponsored by Yunnan Provincial Department of Science and Technology; Grant No.: 2015FB095).
References
- 1.Dumont RJ, Okonkwo DO, Verma S, Hurlbert RJ, Boulos PT, Ellegala DB, et al. Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol. 2001;24(5):254–264. doi: 10.1097/00002826-200109000-00002 [DOI] [PubMed] [Google Scholar]
- 2.Werner C, Engelhard K.. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99(1):4–9. doi: 10.1093/bja/aem131 [DOI] [PubMed] [Google Scholar]
- 3.Cernak I. Animal models of head trauma. NeuroRx . 2005;2(3):410–422. doi: 10.1602/neurorx.2.3.410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loane DJ, Faden AI.. Neuroprotection for traumatic brain injury: translational challenges and emerging therapeutic strategies. Trends Pharmacol Sci. 2010;31(12):596–604. doi: 10.1016/j.tips.2010.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beattie MS, Hermann GE, Rogers RC, Bresnahan JC.. Cell death in models of spinal cord injury. Prog Brain Res. 2002;137:37–47. doi: 10.1016/S0079-6123(02)37006-7 [DOI] [PubMed] [Google Scholar]
- 6.Nixon RA. Autophagy in neurodegenerative disease: friend, foe or turncoat? Trends Neurosci. 2006;29(9):528–535. doi: 10.1016/j.tins.2006.07.003 [DOI] [PubMed] [Google Scholar]
- 7.Bove J, Martinez-Vicente M, Vila M.. Fighting neurodegeneration with rapamycin: mechanistic insights. Nat Rev Neurosci. 2011;12(8):437–452. doi: 10.1038/nrn3068 [DOI] [PubMed] [Google Scholar]
- 8.Chittaranjan S, Bortnik S, Dragowska WH, Xu J, Abeysundara N, Leung A, et al. Autophagy inhibition augments the anticancer effects of epirubicin treatment in anthracycline-sensitive and -resistant triple-negative breast cancer. Clin Cancer Res. 2014;20(12):3159–3173. doi: 10.1158/1078-0432.CCR-13-2060 [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Wu GS. . Role of autophagy in cisplatin resistance in ovarian cancer cells. J Biol Chem. 2014;289(24):17163–17173. doi: 10.1074/jbc.M114.558288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogl DT, Stadtmauer EA, Tan KS, Heitjan DF, Davis LE, Pontiggia L, et al. Combined autophagy and proteasome inhibition: a phase 1 trial of hydroxychloroquine and bortezomib in patients with relapsed/refractory myeloma. Autophagy. 2014;10(8):1380–1390. doi: 10.4161/auto.29264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizushima N, Levine B, Cuervo AM, Klionsky DJ.. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069–1075. doi: 10.1038/nature06639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou K, Sansur CA, Xu H, Jia X.. The Temporal Pattern, flux, and function of autophagy in spinal cord injury. Int J Mol Sci. 2017;18(2):466. doi: 10.3390/ijms18020466 doi: 10.3390/ijms18020466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipinski MM, Wu J, Faden AI, Sarkar C.. Function and mechanisms of autophagy in Brain and spinal cord trauma. Antioxid Redox Signal. 2015;23(6):565–577. doi: 10.1089/ars.2015.6306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schraufstatter E, Bernt H.. Antibacterial action of curcumin and related compounds. Nature. 1949;164(4167):456. doi: 10.1038/164456a0 [DOI] [PubMed] [Google Scholar]
- 15.Liang B, Liu Z, Cao Y, Zhu C, Zuo Y, Huang L, et al. MC37, a new mono-carbonyl curcumin analog, induces G2/M cell cycle arrest and mitochondria-mediated apoptosis in human colorectal cancer cells. Eur J Pharmacol 2017;796:139–148. doi: 10.1016/j.ejphar.2016.12.030 [DOI] [PubMed] [Google Scholar]
- 16.Jiang S, Han J, Li T, Xin Z, Ma Z, Di W, et al. Curcumin as a potential protective compound against cardiac diseases. Pharmacol Res 2017;119:373–383. doi: 10.1016/j.phrs.2017.03.001 [DOI] [PubMed] [Google Scholar]
- 17.Ullah F, Liang A, Rangel A, Gyengesi E, Niedermayer G.. High bioavailability curcumin: an anti-inflammatory and neurosupportive bioactive nutrient for neurodegenerative diseases characterized by chronic neuroinflammation. Arch Toxicol. 2017;91(4):1623–1634. doi: 10.1007/s00204-017-1939-4 [DOI] [PubMed] [Google Scholar]
- 18.Yao M, Yang L, Wang J, Sun YL, Dun RL, Wang YJ, et al. Neurological recovery and antioxidant effects of curcumin for spinal cord injury in the rat: a network meta-analysis and systematic review. J Neurotrauma. 2015;32(6):381–391. doi: 10.1089/neu.2014.3520 [DOI] [PubMed] [Google Scholar]
- 19.Hu JZ, Long H, Wu TD, Zhou Y, Lu HB.. The effect of estrogen-related receptor alpha on the regulation of angiogenesis after spinal cord injury. Neuroscience. 2015;290:570–580. doi: 10.1016/j.neuroscience.2015.01.067 [DOI] [PubMed] [Google Scholar]
- 20.Ormond DR, Peng H, Zeman R, Das K, Murali R, Jhanwar-Uniyal M.. Recovery from spinal cord injury using naturally occurring antiinflammatory compound curcumin: laboratory investigation. J Neurosurg Spine. 2012;16(5):497–503. doi: 10.3171/2012.1.SPINE11769 [DOI] [PubMed] [Google Scholar]
- 21.Zhu X, Li Q, Chang R, Yang D, Song Z, Guo Q, et al. Curcumin Alleviates Neuropathic pain by inhibiting p300/CBP Histone Acetyltransferase activity-Regulated expression of BDNF and Cox-2 in a Rat model. Plos One 2014;9(3):e91303. doi: 10.1371/journal.pone.0091303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ormond DR, Shannon C, Oppenheim J, Zeman R, Das K, Murali R, et al. Stem cell therapy and curcumin synergistically enhance recovery from spinal cord injury. PLoS One 2014;9(2):e88916. doi: 10.1371/journal.pone.0088916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basso DM, Beattie MS, Bresnahan JC.. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139(2):244–256. doi: 10.1006/exnr.1996.0098 [DOI] [PubMed] [Google Scholar]
- 24.Dong Y, Miao L, Hei L, Lin L, Ding H.. Neuroprotective effects and impact on caspase-12 expression of tauroursodeoxycholic acid after acute spinal cord injury in rats. Int J Clin Exp Pathol. 2015;8(12):15871–15878. [PMC free article] [PubMed] [Google Scholar]
- 25.Jin W, Kong J, Lu T, Wang H, Ni H, Wu J, et al. Erythropoietin prevents secondary brain injury induced by cortical lesion in mice: possible involvement of Nrf2 signaling pathway. Ann Clin Lab Sci. 2011;41(1):25–32. [PubMed] [Google Scholar]
- 26.Saiki S, Sasazawa Y, Imamichi Y, Kawajiri S, Fujimaki T, Tanida I, et al. Caffeine induces apoptosis by enhancement of autophagy via PI3 K/Akt/mTOR/p70S6 K inhibition. Autophagy. 2011;7(2):176–187. doi: 10.4161/auto.7.2.14074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heras-Sandoval D, Perez-Rojas JM, Hernandez-Damian J, Pedraza-Chaverri J.. The role of PI3 K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal. 2014;26(12):2694–2701. doi: 10.1016/j.cellsig.2014.08.019 [DOI] [PubMed] [Google Scholar]
- 28.Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21(10):1371–1383. doi: 10.1089/neu.2004.21.1371 [DOI] [PubMed] [Google Scholar]
- 29.Thuret S, Moon LD, Gage FH.. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7(8):628–643. doi: 10.1038/nrn1955 [DOI] [PubMed] [Google Scholar]
- 30.Ren Y, Young W.. Managing inflammation after spinal cord injury through manipulation of macrophage function. Neural Plast. 2013;2013:945034. doi: 10.1155/2013/945034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim YS, Joh TH.. Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson's disease. Exp Mol Med. 2006;38(4):333–347. doi: 10.1038/emm.2006.40 [DOI] [PubMed] [Google Scholar]
- 32.Totoiu MO, Keirstead HS.. Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurol. 2005;486(4):373–383. doi: 10.1002/cne.20517 [DOI] [PubMed] [Google Scholar]
- 33.Lin J, Huo X, Liu X.. mTOR signaling pathway: A potential target of curcumin in the treatment of spinal cord injury. Biomed Res Int. 2017;2017:1634801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zu J, Wang Y, Xu G, Zhuang J, Gong H, Yan J.. Curcumin improves the recovery of motor function and reduces spinal cord edema in a rat acute spinal cord injury model by inhibiting the JAK/STAT signaling pathway. Acta Histochem. 2014;116(8):1331–1336. doi: 10.1016/j.acthis.2014.08.004 [DOI] [PubMed] [Google Scholar]
- 35.Wang YF, Zu JN, Li J, Chen C, Xi CY, Yan JL.. Curcumin promotes the spinal cord repair via inhibition of glial scar formation and inflammation. Neurosci Lett. 2014;560:51–56. doi: 10.1016/j.neulet.2013.11.050 [DOI] [PubMed] [Google Scholar]
- 36.Gu HF, Li HZ, Tang YL, Tang XQ, Zheng XL, Liao DF.. Nicotinate-Curcumin Impedes Foam cell formation from THP-1 cells through restoring autophagy flux. PLoS One. 2016;11(4):e0154820. doi: 10.1371/journal.pone.0154820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teixeira CA, Almeida Mdo R, Saraiva MJ.. Impairment of autophagy by TTR V30M aggregates: in vivo reversal by TUDCA and curcumin. Clin Sci (Lond). 2016;130(18):1665–1675. doi: 10.1042/CS20160075 [DOI] [PubMed] [Google Scholar]
- 38.Masuelli L, Benvenuto M, Di Stefano E, Mattera R, Fantini M, De Feudis G, et al. Curcumin blocks autophagy and activates apoptosis of malignant mesothelioma cell lines and increases the survival of mice intraperitoneally transplanted with a malignant mesothelioma cell line. Oncotarget. 2017;8(21):34405–34422. doi: 10.18632/oncotarget.14907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang C, Zhang X, Teng Z, Zhang T, Li Y.. Downregulation of PI3 K/Akt/mTOR signaling pathway in curcumin-induced autophagy in APP/PS1 double transgenic mice. Eur J Pharmacol. 2014;740:312–320. doi: 10.1016/j.ejphar.2014.06.051 [DOI] [PubMed] [Google Scholar]
- 40.Aoki H, Takada Y, Kondo S, Sawaya R, Aggarwal BB, Kondo Y.. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: role of Akt and extracellular signal-regulated kinase signaling pathways. Mol Pharmacol. 2007;72(1):29–39. doi: 10.1124/mol.106.033167 [DOI] [PubMed] [Google Scholar]
- 41.Wu JC, Lai CS, Badmaev V, Nagabhushanam K, Ho CT, Pan MH.. Tetrahydrocurcumin, a major metabolite of curcumin, induced autophagic cell death through coordinative modulation of PI3 K/Akt-mTOR and MAPK signaling pathways in human leukemia HL-60 cells. Mol Nutr Food Res. 2011;55(11):1646–1654. doi: 10.1002/mnfr.201100454 [DOI] [PubMed] [Google Scholar]
- 42.Nikoletopoulou V, Papandreou ME, Tavernarakis N.. Autophagy in the physiology and pathology of the central nervous system. Cell Death Differ. 2015;22(3):398–407. doi: 10.1038/cdd.2014.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang P, Hou H, Zhang L, Lan X, Mao Z, Liu D, et al. Autophagy reduces neuronal damage and promotes locomotor recovery via inhibition of apoptosis after spinal cord injury in rats. Mol Neurobiol. 2014;49(1):276–287. doi: 10.1007/s12035-013-8518-3 [DOI] [PubMed] [Google Scholar]
- 44.Beevers CS, Chen L, Liu L, Luo Y, Webster NJ, Huang S.. Curcumin disrupts the Mammalian target of rapamycin-raptor complex. Cancer Res. 2009;69(3):1000–1008. doi: 10.1158/0008-5472.CAN-08-2367 [DOI] [PMC free article] [PubMed] [Google Scholar]