Abstract
The humerus is the second most common long bone for metastatic tumours. These lesions result in weakened bone architecture and increased fracture risk with patients suffering pain, loss of function and diminished quality of life, often when life expectancy is short. Fractures or impending fractures require surgical stabilisation to relieve pain and restore function for the remainder of the patient’s life without the need for further surgery. Conventional management of these lesions in the humerus is intramedullary nailing, however there are issues with this technique, particularly regarding rigidity of fixation. Advances in contoured locking plates have led to the development of different stabilisation techniques. The preferred technique in our regional oncology unit is curettage of the tumour and plating, augmented with cement to fill the defect and restore the structural morphology. In this case series we evaluate the survivorship of the construct and the clinical outcomes in patients who had an established or prospective pathological humeral fracture treated with curettage and cement augmented plating, since 2010. We identified 19 patients; 17 had metastasis and 2 myeloma of whom 15 had established fractures and four impending. The mean age at surgery was 69 years (51–86), and mean time since surgery 3.2 years. Overall mean follow up time was 20 months with 14 patients deceased and 5 surviving. There was 100% survivorship of the construct with no mechanical failures and no re-operations. There were no post-operative wound complications. Excellent early pain control was achieved in 18 patients with one experiencing pain controlled by analgesia. Function was assessed using Toronto Extremity Salvage Score (TESS) and was satisfactory; mean 79/100 (range 72–85). Cement augmented plating for pathological humerus fractures is a suitable alternative to intramedullary nailing and addresses several of the concerns with that technique. It provides immediate rigidity and allows early unrestricted function.
Keywords: Humerus, Fracture, Cement, Plating, Tumour
1. Introduction
Pathological lesions of bone, when not mesenchymal in origin, often include myeloma, lymphoma, and most commonly metastatic carcinoma. After the lungs and the liver, the skeleton is the next most common site of metastasis.1 The vertebrae, pelvis, ribs, and femur are more frequently involved in metastases than the humerus.1 Nevertheless, metastasis to the long bones is usually reflective of advanced disease states, and of these, the humerus is second only to the femur in developing a metastatic tumour.1, 2, 3, 4, 5 The most common primary lesions to metastasise to bone are breast, prostate, lung, renal and thyroid, while in myeloma, bone involvement is ubiquitous.6 Neoplastic lesions will progressively destroy the bone, creating lytic and sclerotic zones within cancellous or cortical bone. This usually culminates, particularly in the case of lytic lesions, in a weakened structural integrity making the bone more prone to fracture.7 (Fig. 1).
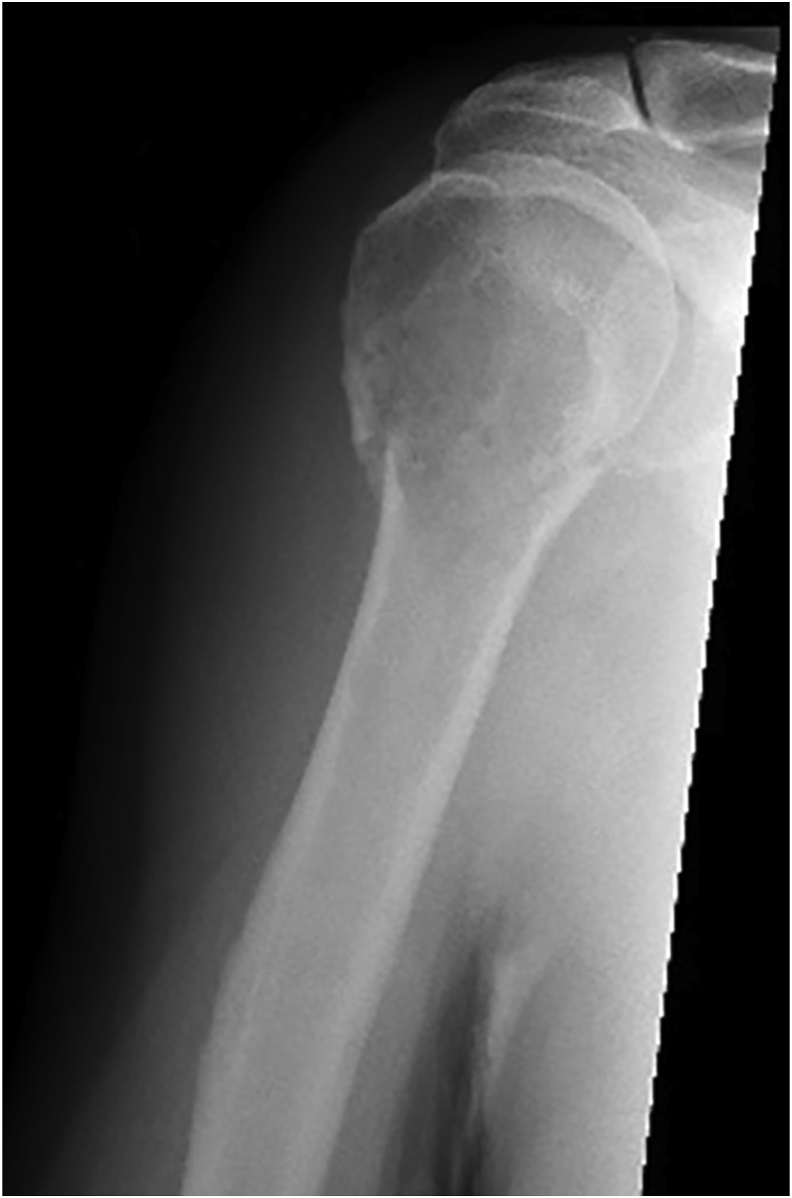
Fig. 1.
Radiograph showing pathological fracture of proximal humerus.
Established and impending pathological fractures are associated with pain and loss of function of the involved limb, leading to an overall diminished quality of life.6 Conservative management of these fractures of long bones is usually not recommended, with patients potentially consigned to functional deficiency and chronic pain.4,8,9 Despite surgical advances, both prospective and established pathological humeral fractures continue to be a surgical challenge.10 The aims of stabilising such fractures are to provide good pain relief and immediate rigidity of the humerus to achieve unrestricted restoration of shoulder function.10, 11, 12 The surgical construct should last for the remainder of the patient’s life, without the need for further surgery.
The conventional technique of choice to stabilise a pathological humeral fracture is intramedullary nailing.12 This method allows a large section of the humerus to be stabilised with minimal invasion.4 However, the technique has several disadvantages; rotator cuff damage with chronic shoulder pain and weakness, nail protrusion at the shoulder, and the risk of unstable fixation especially in poor quality bone.11 In the context of tumour related fractures, nailing will not reduce the local tumour burden, therefore local disease can progress rapidly.13
In an attempt to overcome some of the disadvantages of intramedullary nailing, a technique of internal fixation using a plate, with cement augmentation has been described.4,10,14, 15, 16 In the situation of a fracture secondary to metastatic tumour, Frassica and Frassica4 described open intralesional curettage of the tumour prior to cement augmented plating. Bone cement is injected into the resulting defect and within the intramedullary canal prior to plate application. This provides immediate rigidity and allows unrestricted restoration of function soon after the operation. Indeed, biomechanical studies have shown this construct to be superior to other fixation options in achieving the goal of immediate stability.17 With extensive curettage, local tumour burden is reduced which in turn could reduce the risk of local disease progression. This technique also carries lower risk of damage to the rotator cuff than with nailing.12 Given the potential advantages and with initial good clinical outcomes in other studies,4,14, 15, 16 curettage and cement augmented plating has emerged as a viable alternative to intramedullary nailing for pathological fractures. This has been the standard practice for managing patients with pathological humerus fractures in our regional musculoskeletal oncology unit in the recent past.
The aim of the study was to evaluate the survivorship of the cement plate construct, surgical complications, and functional outcome in patients who had an established or prospective pathological humeral fracture treated with curettage and cement augmented plating at our unit.
2. Methods
Data was recorded prospectively for all eligible patients in a regional musculoskeletal oncology unit from 2010 to 2019. We identified those who had cement augmented plate fixation of a pathological humerus fracture. This included established and impending fractures related to metastatic and haematological neoplasms. Decisions on operative management, particularly in the case of impending fractures were made by an experienced musculoskeletal surgical oncologist in conjunction with medical oncology, the patient, and their families, rather than a scoring system with thresholds.
The clinical records of the patients identified for this study were reviewed up to and including the last clinical note at the end of the study. Mortality and date of death were recorded. Patients who were still alive were contacted at the end of the study by telephone and consultation carried out to obtain information on pain and function assessed using the Toronto Extremity Salvage Score (TESS).18
The operative technique was standardised across three different musculoskeletal oncology surgeons. A deltopectoral approach (Henry’s) was used, the tumour was debulked at the fracture site and access gained to the intramedullary canal of the humerus. The canal was then curetted immediately proximal and/or distal to remove tumour mass and reduce local disease burden. In bones with an impending fracture, tumour was debulked via a cavity through the lesion to create access, but no fracture was created. In the case of prostate metastases, sclerotic bone was not debulked. Antibiotic loaded polymethylmethacrylate bone cement was introduced into the canal along its length proximally and distally. The cement used in the procedure provides the humerus with immediate rigidity, and acts as an anchor for the plate screws. A standard contoured humeral locking plate was then applied to the bone using a combination of locking and non-locking screws (Fig. 2). Closure was standard for the procedure with absorbable subcuticular suture used to close the skin.
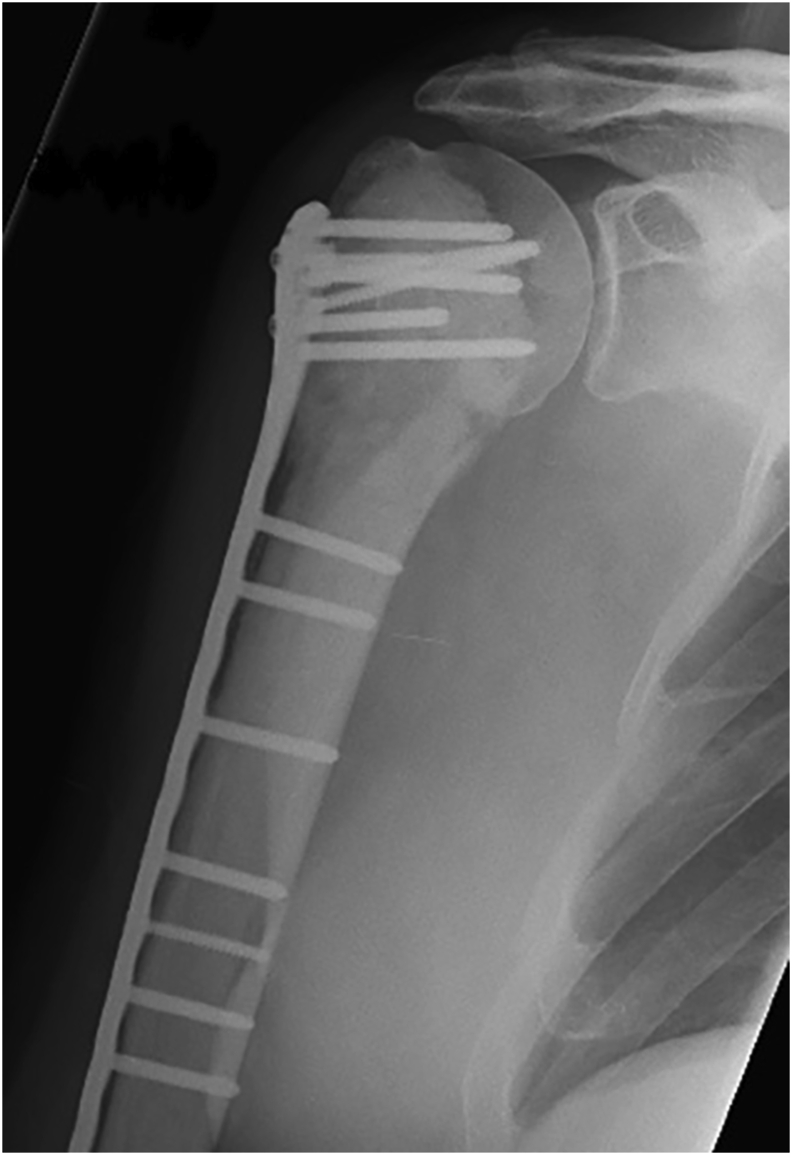
Fig. 2.
Radiograph showing pathological fracture of proximal humerus stabilised by cement augmented plating.
Following surgery, patients used a sling for 2–3 weeks to immobilise the shoulder during the period for wound healing. Once the wound was healed, the patients could mobilise with unrestricted active movement as tolerated. No formal or standardised physiotherapy protocol was used and patients were referred to their relevant oncology team for ongoing treatment, including radiotherapy and chemotherapy as appropriate for their primary pathology and prognosis.
The primary outcome measure was survivorship of the cement plate construct until the end of follow-up or during the lifespan of patient. This was assessed clinically and by plain radiographs to evaluate the position of the hardware, and any need for revision. It was assumed that if a patient had neither been re-referred to the unit with symptoms or had a subsequent radiograph demonstrating implant failure, then there was no failure of their reconstruction.
Secondary outcome measures included evaluation of early or delayed surgery related complications, patient reported pain relief, and functional recovery.
Functional outcome was assessed using the Toronto Extremity Salvage Score (TESS).
Statistical analysis was performed using SPSS (Chicago, Illinois) with descriptive statistics used to compare the data.
3. Results
A total of 19 patients were identified from the database who met the inclusion criteria. They underwent curettage and cement plating of the humerus between 2010 and 2019. Of the 19 patients, 17 (89%) had a metastatic lesion, and 2 (11%) had a myelomatous lesion. At the time of surgery, the mean age of the patients in the cohort was 69 years (51–86) and 11 (58%) of the patients were female. An established pathological fracture was found in 15 (79%) while four patients had a metastasis in the humerus with an impending fracture.
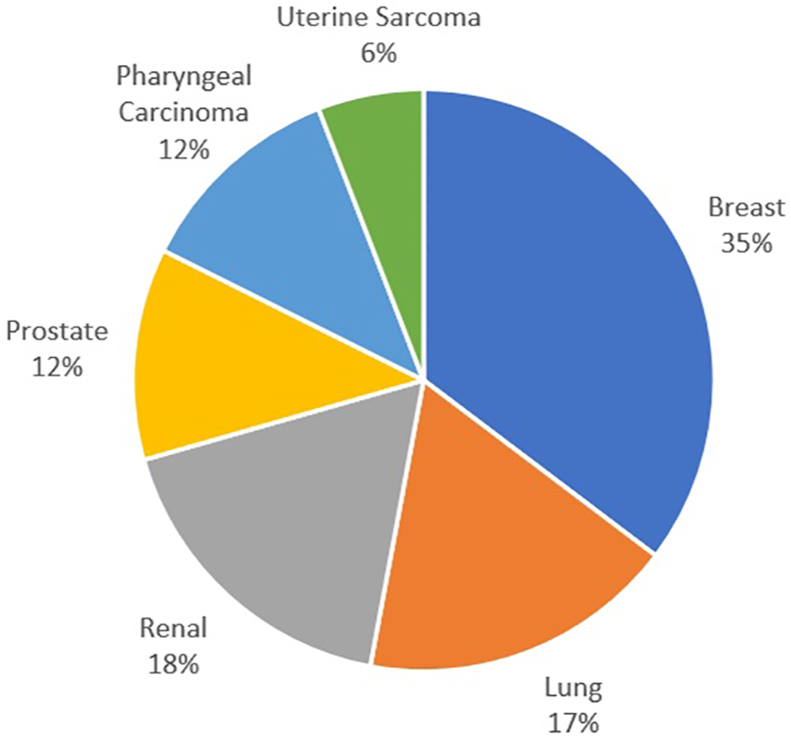
In 6 (32%) of the patients the humeral lesion was a metastatic breast carcinoma. A metastatic renal cell carcinoma was found in 3 (16%) patients, as was carcinoma of the lung. The remaining patients had metastatic disease from the prostate, pharynx or uterus (Fig. 3). The location of the lesion was proximal metaphyseal in 14 (74%), diaphyseal in 3 (16%) and distal metaphyseal in 2 (10%) of cases.
Fig. 3.
Graph showing the primary site of the metastatic lesions.
The mean follow-up time was 20 months, median 8 months, and range 2–78 months. By the end of the study, 14 (74%) of the patients had died and the mean time between surgery and mortality was 17 months (range 2–78 months). In all the patients who died, no humeral fixation failures were noted at any point. Therefore, 100% of the reconstructions survived beyond the lifespan of the patient (Table 1).
Table 1.
Summary of outcomes in patients treated with cement augmented plating.
| Outcome | Value |
|---|---|
| Implant Survival | 100% |
| Mean follow up time | 20 months |
| Wound complication rate | 0% |
| Complete pain control | 95% |
| Mean TESS Score | 79% |
In the surviving 5 (26%) patients, no revision surgery was required, and radiographs showed well sited implants with satisfactory fixation. At the end of the study, the mean survival time of the living patients and their constructs was 2.5 years. The radiograph of all patients between the surgery and the end of follow-up showed satisfactory construct position with no evidence of failure.
At post-operative review, no patients showed any symptoms or signs of wound problems. Pain was completely relieved in 18 (95%) patients and one patient complained of pain beyond the expected time, but this pain was well controlled by simple oral analgesia.
Limited range of glenohumeral movement was documented in 4 (33%) patients. Of these 4 patients, 1 had restricted abduction by the end of follow-up and the other 3 had some degree of terminal restriction of shoulder abduction and rotation. These 3 patients recovered functional range of movements by the end of follow-up.
TESS scores were obtained from 7 patients in this cohort. The mean time from surgery until TESS scoring was 23 months (range 2 months–6 years). The mean TESS score was 79% (range 72–85).
Neurological complications were experienced by 2 patients. One patient had transient motor weakness in the radial and ulnar nerve distributions 3 months following curettage, cementoplasty and plating for metastatic breast cancer. This weakness fully resolved a month later. The other patient suffered wrist drop nearly 3 years following surgery due to local progression of metastatic lung adenocarcinoma.
The post-operative oncology treatment was determined by the specialist medical team with the cohort split almost equally, a quarter receiving local radiotherapy, chemotherapy, both therapies or none. There were no observed differences in outcomes between groups for post-operative therapy.
4. Discussion
The objective of this study was to measure construct survival alongside clinical and functional outcomes. The results show that the cement plating construct out survived all the patients who died, and there was no requirement for revision in the living patients. There were no wound problems noted for any patients and pain and function was improved considerably by the procedure.
Metastatic carcinoma and myeloma are the most common examples of malignant pathological lesions affecting bone.12 Bone pain is often one of the first presenting symptoms in patients who are found to have this diagnosis. The aetiology of the pain includes structural weakness, irritation of periosteum, tumour cells producing prostaglandins, nerve compression, or a combination of the these.6,19,20 In addition to pain, metastases can cause functional deficiencies and pathological fracture.6 Aside from the oncological consequences of bony metastases, these lesions in isolation can cause a reduction in the patients’ quality of life, often when life expectancy is short. The main treatment for prospective and established fractures is surgical stabilisation to relieve pain and maximise functional outcome.8, 9, 10, 11, 12,21, 22, 23 Whilst the prognosis for most patients with bone metastases is poor, this should not be considered a pre-terminal event. For example, the median survival of patients with prostate cancer and bony metastases is 40 months and the mean 5 year survival rate is 25%.24 Therefore, treatment of the skeletal disease should provide symptom relief and be robust enough to last for the remainder of the patients’ life.
The method of fixation of the fracture depends on several factors including the location, size, and the quality of the surrounding bone.1 Humeral bone loss from pathological fracture poses a surgical challenge which will affect the reconstructive technique used and the potential outcome.10 The glenohumeral joint is principally a non-weight-bearing joint and is the most mobile in the body.10,25 As a result, the proximal humerus is subject to extremes of bending and rotational forces from its various muscle insertions.1 Preserving the function of the rotator cuff as much as reasonably possible helps to maximise post-operative function.1 Since the shoulder is not primarily subject to weight bearing, using a load-bearing construct such an intramedullary nail is not as important as in the femur for example.10
Initial biomechanical testing comparing plating with nailing favoured nailing in an impending fracture model,26 however a more recent study comparing several fixation techniques demonstrated that cement augmented plating is superior, with the highest torque and energy to failure with torsional forces.17
Intramedullary nailing typically involves smaller incisions, so one may expect a lower risk of wound problems and nerve injury than open plate fixation. Nevertheless, no post-operative wound problems were experienced in any cases in this study. This is despite curettage and plating technique requiring a significantly larger incision. One patient in our group did suffer motor weakness of the radial and ulnar nerves post-operatively but this was transient, being fully resolved by the end of follow-up. In contrast to curettage, nailing does not reduce disease burden in the humerus. Indeed, as the nail passes through the canal it may transport some tumour to a distal site, resulting in multifocal tumour spread. It is well documented that intramedullary nailing also has a higher risk of embolisation of fat and tumour, with potentially life endangering consequences.10
The Toronto group12 showed that cementation of a non-locking plate provides immediate, absolute rigidity providing prompt pain relief and unrestricted restoration of function without the need for bony union. Fig. 4, Fig. 5 show radiographs illustrating the application of this technique for treating lesions in different regions of the humerus. This method protects rotator cuff function, in contrast to intramedullary nailing, where there is disruption of the rotator cuff, often resulting in weakness and stiffness of the shoulder. In a non-pathological cohort, Lim et al.27 showed that significant complications occurred in 19% of patients treated with intra-medullary nailing of diaphyseal humerus fractures, including non-union, shoulder or elbow impairment and radial nerve palsy. In contrast, Niall et al.28 reported a 96% success rate in a similar cohort treated with plating. A randomised trial comparing plating with nailing of non-pathological humeral shaft fractures found plating to be the best treatment with a lower rate of complications.29 Additionally, no long-term complications were experienced in our patient cohort and only one patient had pain beyond the immediate post-operative period, but good control was reported by using simple oral analgesia.
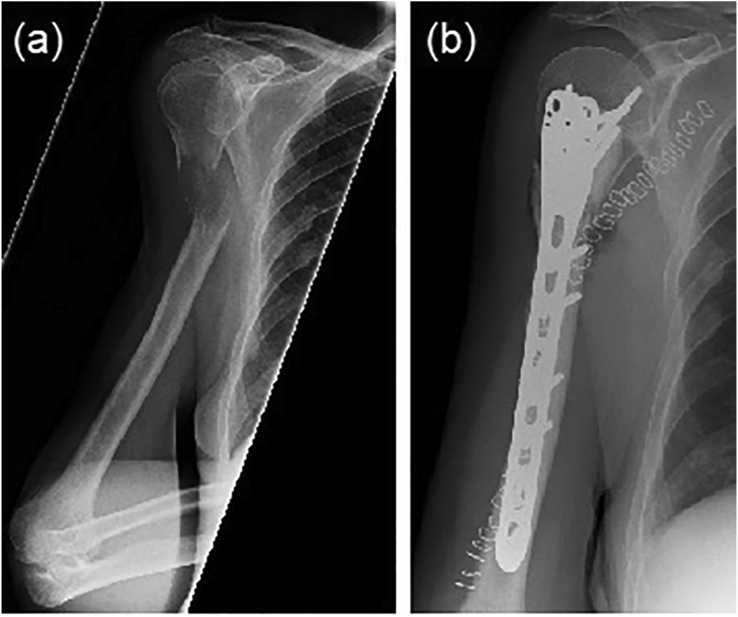
Fig. 4.
Radiographs showing an established pathological fracture of meta-diaphyseal region of humerus (a) pre-operatively, and (b) stabilised by cement augmented plating.
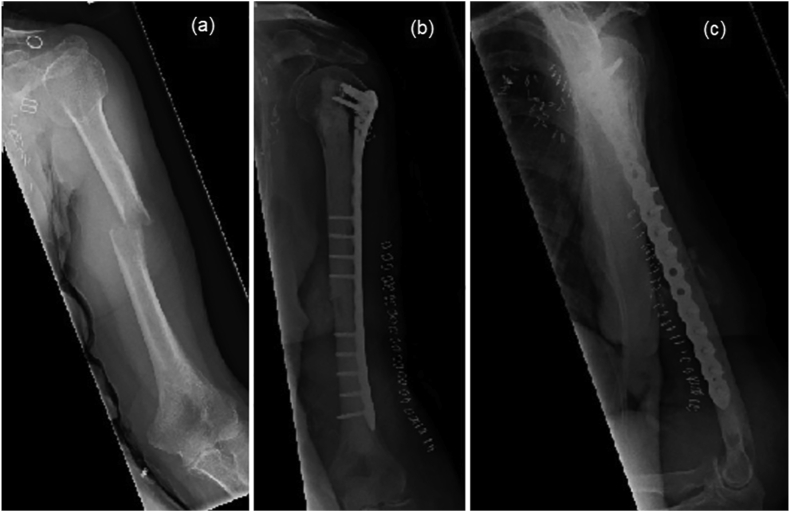
Fig. 5.
Radiographs showing an established pathological fracture of humeral diaphysis (a) pre-operatively, (b) antero-posterior view following cement augmented plating, and (c) lateral view following cement augmented plating.
Treatments such as chemotherapy and radiotherapy have improved the prognosis of patients with metastatic disease.12 Nevertheless, pathological fractures still happen and the incidence of these fractures is expected to increase with an aging population.12 In this series, the most common neoplasm was metastatic breast carcinoma accounting for 32% of cases. This is in keeping with other case series of humeral metastases where breast carcinoma is the most common primary tumour origin.30,31
The primary outcome measure in our study was survivorship of the fixation in terms of duration compared to the life span of the patient. There were no mechanical failures observed meaning the construct survived the patients who died and was still functioning in the living patients. Spencer et al.30 reported no revisions from 35 intramedullary nailing cases, but reported a mortality of 94% at the end of the period studied with a mean survival of 7 months. Such a short mean survival limited the potential for construct failure and could explain the absence of revisions in their study. The mean survival time of the 26% of patients who survived in this cohort was significantly longer at over 2 years.
Secondary outcome measures included consideration of wound healing, pain relief and preserved shoulder function. The surgical wounds of all patients healed well and no permanent, surgery related neurological damage was noted in any of the patients.
There were no observed differences in outcomes for patients receiving different adjuvant chemo/radiotherapy regimes. Radiotherapy was able commence 2–3 weeks post-operatively once the wound had healed, a potential disadvantage over nailing where minimal incisions permit commencement after a few days.
The TESS is routinely used as an assessment tool for function after limb salvage surgery in musculoskeletal tumours and is validated to allow a patient to score their own perceptions of activity limitations.18 It was developed as a measure of physical function, comprising restrictions in mobility, personal care and in carrying out activities of daily life. In this study the mean TESS score was 79%, suggesting that the average patient consider themselves to be limited in their ability to perform activities of daily living by approximately 20%. TESS scoring has been used to evaluate functional outcome in other areas of musculoskeletal oncology, however this is the first study to record TESS scores in a cohort of patients who underwent curettage and cement plating for a pathological humeral fracture. The score of 79% compares well to scores published in similar studies, indicating good functional outcomes following this technique.32
There was no incidence of patients returning with local disease recurrence or peri-prosthetic fracture, potentially indicating that the tumour debulking part of the surgery was successful, although we acknowledge that this is multifactorial with post-operative oncology treatment an important factor. We acknowledge that this study has other limitations, being a retrospective study on a small cohort of patients. There was no ability to compare factors such as operative time, blood loss and time taken to start adjuvant therapy with those of nailing.
Our results support the findings of the innovative institutions that curettage and cement augmented plating is a suitable alternative to intramedullary nailing for treating pathological fractures of the humerus.10,12 The technique provides immediate fixation and stability to give good pain control and allow early unrestricted movement. Furthermore, we have demonstrated excellent survivorship over a longer period than previously described and excellent patient reported functional outcomes.
In conclusion, we suggest that our findings add evidence to the theory that cement augmented plating, rather than intramedullary nailing should be considered as the primary treatment for pathological humerus fractures. To confirm this, a well-designed randomised controlled trial comparing cement augmented plating with intramedullary nail fixation would be required.
Contributions
WW was involved in study design, data collection, analysis, writing and editing manuscript, AP data collection and manuscript drafting, HF data collection, SG manuscript review, AM conceptualisation and manuscript review.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors have no conflicts of interest to declare.
References
- 1.Gobezie R.P.B., Ready J. Pathologic humerus fractures. Journal at Harvard Medical School. 2003;5:126–129. [Google Scholar]
- 2.Colyer R.A. Surgical stabilization of pathological neoplastic fractures. Curr Probl Canc. 1986;10(3):117–168. doi: 10.1016/s0147-0272(86)80005-8. [DOI] [PubMed] [Google Scholar]
- 3.Frassica F.J., Frassica D.A. Evaluation and treatment of metastases to the humerus. Clin Orthop Relat Res. 2003;415:S212–S218. doi: 10.1097/01.blo.0000093052.96273.a7. [DOI] [PubMed] [Google Scholar]
- 4.Frassica F.J., Frassica D.A. Metastatic bone disease of the humerus. J Am Acad Orthop Surg. 2003;11(4):282–288. doi: 10.5435/00124635-200307000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Sarahrudi K., Wolf H., Funovics P., Pajenda G., Hausmann J.T., Vécsei V. Surgical treatment of pathological fractures of the shaft of the humerus. J Trauma Inj Infect Crit Care. 2009;66(3):789–794. doi: 10.1097/TA.0b013e3181692132. [DOI] [PubMed] [Google Scholar]
- 6.Coleman R.E. Skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1588–1594. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1588::aid-cncr9>3.3.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Pretell J., Rodriguez J., Blanco D., Zafra A., Resines C. Treatment of pathological humeral shaft fractures with intramedullary nailing. A retrospective study. Int Orthop. 2009;34(4):559–563. doi: 10.1007/s00264-009-0833-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flemming J.E., Beals R.K. Clinical Orthopaedics and Related Research; 1986. Pathologic Fracture of the Humerus; pp. 258–260. [PubMed] [Google Scholar]
- 9.Lancaster J.M., Koman L.A., Gristina A.G. Pathologic fractures of the humerus. South Med J. 1988;81(1):52–55. doi: 10.1097/00007611-198801000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Siegel H.J., Lopez-Ben R., Mann J.P., Ponce B.A. Pathological fractures of the proximal humerus treated with a proximal humeral locking plate and bone cement. The Journal of Bone and Joint Surgery British. 2010;92-B(5):707–712. doi: 10.1302/0301-620X.92B5.23246. [DOI] [PubMed] [Google Scholar]
- 11.Harrington K.D. Orthopedic surgical management of skeletal complications of malignancy. Cancer. 1997;80(8 Suppl):1614–1627. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1614::aid-cncr12>3.3.co;2-0. [DOI] [PubMed] [Google Scholar]
- 12.Weiss K.R., Bhumbra R., Biau D.J. Fixation of pathological humeral fractures by the cemented plate technique. The Journal of Bone and Joint Surgery British. 2011;93-B(8):1093–1097. doi: 10.1302/0301-620X.93B8.26194. [DOI] [PubMed] [Google Scholar]
- 13.Vail T.P., Harrelson J.M. Treatment of pathologic fracture of the humerus. Clin Orthop Relat Res. 1991;(268):197–202. [PubMed] [Google Scholar]
- 14.Dijkstra S., Stapert J., Boxma H., Wiggers T. Treatment of pathological fractures of the humeral shaft due to bone metastases: a comparison of intramedullary locking nail and plate osteosynthesis with adjunctive bone cement. Eur J Surg Oncol. 1996;22(6):621–626. doi: 10.1016/s0748-7983(96)92450-6. [DOI] [PubMed] [Google Scholar]
- 15.Lewallen R.P., Pritchard D.J., Sim F.H. Treatment of pathologic fractures or impending fractures of the humerus with rush rods and methylmethacrylate. Clin Orthop Relat Res. 1982;(166):193–198. &NA. [PubMed] [Google Scholar]
- 16.Sim F.H., Pritchard D.J. Metastatic disease in the upper extremity. Clin Orthop Relat Res. 1982 NA;(169):83???94. [PubMed] [Google Scholar]
- 17.Al-Jahwari A., Schemitsch E.H., Wunder J.S., Ferguson P.C., Zdero R. The biomechanical effect of torsion on humeral shaft repair techniques for completed pathological fractures. J Biomech Eng. 2012;134(2) doi: 10.1115/1.4005696. 024501. [DOI] [PubMed] [Google Scholar]
- 18.Davis A.M., Wright J.G., Williams J.I., Bombardier C., Griffin A., Bell R.S. Development of a measure of physical function for patients with bone and soft tissue sarcoma. Qual Life Res. 1996;5(5):508–516. doi: 10.1007/BF00540024. [DOI] [PubMed] [Google Scholar]
- 19.Rosen L., Harland S.J., Oosterlinck W. Broad clinical activity of zoledronic acid in osteolytic to osteoblastic bone lesions in patients with a broad range of solid tumors. Am J Clin Oncol. 2002;25:S19–S24. doi: 10.1097/00000421-200212001-00004. [DOI] [PubMed] [Google Scholar]
- 20.Theriault R.L., Hortobagyi G.N. Bone metastasis in breast cancer. Anti Canc Drugs. 1992;3(5):455–462. doi: 10.1097/00001813-199210000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Fidler M. Prophylactic internal fixation of secondary neoplastic deposits in long bones. Br Med J. 1973;1(5849):341–343. doi: 10.1136/bmj.1.5849.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frassica F.J., Gitelis S., Sim F.H. Metastatic bone disease: general principles, pathophysiology, evaluation, and biopsy. Instr Course Lect. 1992;41:293–300. [PubMed] [Google Scholar]
- 23.Redmond B.J., Biermann J.S., Blasier R.B. Interlocking intramedullary nailing of pathological fractures of the shaft of the humerus∗. J Bone Joint Surg. 1996;78(6):891–896. doi: 10.2106/00004623-199606000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Peng B., Yang C., He J. Radiotherapy cannot prolong overall survival of young prostate cancer patients with bone metastases. J Transl Med. 2016;14:102. doi: 10.1186/s12967-016-0868-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khmelnitskaya E., Lamont L.E., Taylor S.A., Lorich D.G., Dines D.M., Dines J.S. Evaluation and management of proximal humerus fractures. Advances in Orthopedics. 2012;2012:1–10. doi: 10.1155/2012/861598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Damron T.A., Rock M.G., Choudhury S.N., Grabowski J.J., An K.N. Biomechanical analysis of prophylactic fixation for middle third humeral impending pathologic fractures. Clin Orthop Relat Res. 1999;(363):240–248. [PubMed] [Google Scholar]
- 27.Lin J., Shen P.W., Hou S.M. Complications of locked nailing in humeral shaft fractures. J Trauma. 2003;54(5):943–949. doi: 10.1097/01.TA.0000032252.57947.47. [DOI] [PubMed] [Google Scholar]
- 28.Niall D.M., O’Mahony J., McElwain J.P. Plating of humeral shaft fractures; has the pendulum swung back? Injury. 2004;35(6):580–586. doi: 10.1016/j.injury.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 29.McCormack R.G., Brien D., Buckley R.E., McKee M.D., Powell J., Schemitsch E.H. Fixation of fractures of the shaft of the humerus by dynamic compression plate or intramedullary nail. A prospective, randomised trial. J Bone Joint Surg Br. 2000;82(3):336–339. doi: 10.1302/0301-620x.82b3.9675. [DOI] [PubMed] [Google Scholar]
- 30.Spencer S.J., Holt G., Clarke J.V., Mohammed A., Leach W.J., Roberts J.L.B. Locked intramedullary nailing of symptomatic metastases in the humerus. The Journal of Bone and Joint Surgery British. 2010;92-B(1):142–145. doi: 10.1302/0301-620X.92B1.22399. [DOI] [PubMed] [Google Scholar]
- 31.Thai D.M., Kitagawa Y., Choong P.F. Outcome of surgical management of bony metastases to the humerus and shoulder girdle: a retrospective analysis of 93 patients. Int Semin Surg Oncol. 2006;3:5. doi: 10.1186/1477-7800-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zore Z., Filipović Zore I., Matejcić A., Kamal M., Arslani N., Knezović Zlatarić D. Surgical treatment of pathologic fractures in patients with metastatic tumors. Coll Antropol. 2009;33(4):1383–1386. [PubMed] [Google Scholar]