Abstract
Diabetic foot infection is a preventable complication of diabetes mellitus. It is an essential component of diabetic foot disease, which is characterised by a triad of neuropathy, ischaemia and infection. These factors may lead to foot ulceration, sepsis and amputation resulting in increased morbidity and poor quality of life. Confirming or excluding infection can be difficult especially when routine laboratory tests and plain radiographs are inconclusive. Early diagnosis and localization of diabetic foot infection is extremely important to institute timely, appropriate therapy. Structural imaging using computed tomography and magnetic resonance imaging all have individual applications towards the diagnostic workup of this condition but have their own limitations. Scintigraphic detection is based on physiochemical changes and hence provides a functional evaluation of bone pathology.
We describe the evolution of functional nuclear medicine imaging including immunoscintigraphy in diabetic foot infection and highlight current applications of physiological 18-Fluoro-deoxyglucose positron emission tomography (18-FDG-PET) and computed tomography (18-FDG-PET/CT) in such patients.
18-FDG-PET/CT is a promising modality for imaging diabetic foot infection. Future studies will allow standardisation of technological details and options of 18-FDG-PET/CT interpretation in diabetic foot infection.
Keywords: Musculoskeletal infection, Osteomyelitis, Diabetic foot, Infection imaging, Immunoscintigraphy, PET/CT, 18F-FDG-PET, 18F-FDG PET/CT
1. Introduction
Diabetic foot disease (DFD) involves a spectrum of conditions and is defined as infection, ulceration or destruction of tissues associated with neuropathy and/or peripheral artery disease in the lower extremities of a person with (a history of) diabetes mellitus (DM).1 Diabetic foot infection (DFI) is a critical component of DFD.
Foot complications are common in patients with DM. The estimated lifetime risk of developing foot ulcers in diabetic patients is about 15–25%. Diabetic foot ulcers are the leading cause of non-traumatic lower extremity amputation.2,3
Diabetic foot care accounts for a substantial proportion of healthcare expenditure in England, with a significant impact on the National Health Service (NHS). About £ 800 million was spent on DFD care in 2014–2015 and is expected to grow further.4
DFI can range from a superficial infection (cellulitis) to one that penetrates deeper into the bone (osteomyelitis). Delays in treatment can result in impaired healing, infection, hospitalization, minor and major nontraumatic lower limb amputations and mortality.5 Hence early diagnosis and effective treatment are essential for the prevention of amputation. Planned therapeutic strategy is based on a multidisciplinary and multifactorial approach.6,7
DFI is diagnosed through clinical history and examination supplemented by radiological and biopsy findings. The definitive diagnosis of bone infection is made by bone biopsy or culture of an organism from pus or tissue samples.8,9
Radiological imaging forms a crucial step in the diagnostic workup of DFI.10 Among the diagnostic methods currently used, radionuclide scanning can be helpful in the early diagnosis of DFI and is a supportive imaging modality for patients with suspected foot infections as in other musculoskeletal conditions.11
We describe the application of physiological 18-Fluoro-deoxyglucose positron emission tomography (18-FDG-PET/CT) in DFD and its role in evaluating DFI.
2. Structural imaging in diabetic foot infection
2.1. Plain radiography
Plain radiographs (x-rays) tend to be the first investigation of choice in the diagnosis of bone infections along with evaluation of inflammatory parameters. Although crucial, they are often inconclusive, non-specific and sometimes misleading, especially in the early stages. Changes do not occur for one to several weeks and early x-rays may be normal. In later stages of the infection, x-rays may identify bone destruction, periosteal reaction and new bone formation.
2.2. Complementary imaging
In DFI, supplementary imaging such as Ultrasonography (USG), Computed Tomography (CT) scan or Magnetic Resonance Imaging (MRI) have individual value in diagnosis including guiding biopsy and treatment.
USG offers a non-invasive, operator-dependent evaluation of musculoskeletal infection. However, its use is limited in diagnosing bone infection in diabetes. It detects soft tissue abnormalities around the bone, but the sonic beam does not cross the bone cortex and therefore may not identify osteomyelitis.
CT scan helps localize the exact location and extent of abscess and bone involvement but involves significant radiation exposure.
MR imaging has the highest sensitivity for detection of osteomyelitis and soft tissue infections and provides high spatial contrast resolution. It has high diagnostic accuracy but is often not helpful in patients with claustrophobia, metallic implants, aneurysm clips, pacemakers or prosthetic joints. It also has limitations in differentiating between osteomyelitis and acute Charcot neuroarthropathy.10,12,13 These modalities are also constrained in their ability to detect and characterize acute or chronic post-operative or post-traumatic bone infection.14
3. Evolving models of functional nuclear medicine imaging in diabetic foot infection
Nuclear medicine imaging in diabetic foot infection has evolved over the years and is useful for patients with suspected orthopaedic and DFI (Table 1). It has earned its place in the diagnostic work-up of DFI where supplementary imaging modalities have limited or equivocal applications. Radionuclide imaging uses applied physiology in which tracers (radiopharmaceuticals) accumulate in inflamed and infected tissues especially in leukocytes or granulocytes due to increased blood flow and enhanced vascular permeability according to the principle of chemotaxis. This functional imaging process is utilised for diagnostic work-up in a patient with suspected DFI.
Table 1.
Evolution and characteristics of common nuclear medicine modalities in diagnosis of musculoskeletal infection.
| Technique | Radio pharmaceutical | Mechanism | Advantages | Disadvantages | |
|---|---|---|---|---|---|
| 1 | Three-phase bone scan | 99mTc-MDP | Localization in sites of leucocytes accumulated in infective foci by diapedesis and chemotaxis | Readily available Inexpensive High sensitivity 99mTc provides good quality images |
Low specificity |
| 2 | Gallium | 67Gallium citrate | Ga-67 citrate circulates in plasma bound to transferrin. Its ferric ion-like properties allow it to bind to lactoferrin released from dying leukocytes and bacterial siderophores at site of infection | Easy to prepare Low toxicity Detects low grade infection |
Time consuming, Delayed imaging High radiation dose |
| 3 | WBC scan | 11Indium oxime99mTc hexamethyl propyleneamine oxime | White blood cell labelling at site of infection | High target to background Ratio | Time-consuming preparation Complex and expensive radiolabelling In vitro labelling required |
| 4 | Immunoscintigraphy | Antigen binding antigranulocyte monoclonal antibody fragment, e.g. 99mTc Sulesomab Or Polyclonal human immunoglobulin G, e.g.99mTc-HIG |
Migration of circulating antibody-labelled granulocytes to the site of infection |
99mTc provides good quality images In-vivo technique Readymade kit, Ease of preparation Good sensitivity and specificity |
Expensive Availability issues May be restricted to tertiary centres. Incompatibility, immune related reactions |
| 5 | Positron emission imaging | 18F-FDG | Uptake in metabolically active cells e.g.18F-deoxyglucose | Physiological imaging Fusion Anatomical imaging with CT scan |
Availability and cost may restrict it to tertiary centre use |
Abbreviations: WBC= White blood cell; CT= Computerised Tomography; 18F-FDG = radio-active Fluoro-deoxy glucose.
3.1. Traditional scintigraphy
The primary skeletal scintigraphy technique using plain 99mTc- labelled methylene diphosphonate (99mTc-MDP) is a highly sensitive method for detecting bone infection, but lacks specificity to differentiate between infection, fracture, heterotrophic ossification, Charcot’s neuropathy and arthritis components of DFD.15 Plain bone scans rely on the property of orthopaedic lesions to excite a local osteoblastic response and increase in vascularity. A three-phase bone scan is the basic examination which detects sites of increased bone turnover with high sensitivity of more than 90%. Typically the scintigraphic appearance of osteomyelitis is associated with increase in uptake in all three phases (blood flow/angiographic, blood pool and delayed phases), especially the third. A normal 99mTc-diphosphonate three phase bone scan excludes chronic osteomyelitis with a very high certainty. However, if any other cause of bone remodelling, such as fracture or Charcot’s osteoneuropathy complicating the diagnosis of infection are present, the sensitivity remains high but the specificity reduces markedly. To imorove specificity, additional more specific scintigraphic techniques are required.15
3.2. Gallium-67 scanning increases specificity but has an accuracy rate of only 70%.11,15,16
White blood cell (WBC) scintigraphy with either Indium- 111 oxine or 99mTc- hexamethylpropyleneamine oxime is more specific than triple-phase 99mTc-MDP bone scan and may be useful when magnetic resonance imaging is not available or is contraindicated. These techniques, however, have been quoted to have variable specificity. Additionally, they are complex, expensive and require in vitro labelling of the WBC with potential for pathogen contamination or mixing of blood samples amongst patients. In-vitro processes also involve biological hazards for medical personnel. Sometimes physiological bone marrow (BM) expansion secondary to chronic inflammation can result in a lower specificity of traditional Indium- 111 oxine or 99mTc- hexamethylpropyleneamine oxime WBC scintigraphy. This may make it difficult to differentiate from Charcot osteoneuroarthropathy and osteomyelitis of DFI. An additional bone marrow scintigraphy (BMS) using nano colloids is suggested in such a situation. In the bone marrow; both radiopharmaceuticals accumulate, but the WBC accumulate more in infective foci. Consequently if the images of these two modalities are congruent (match), the diagnosis of Charcot osteoneuroarthropathy is the most probable whilst if there is mismatch of the imaging modalities (i.e. positive at WBC scintigraphy and negative at colloids scintigraphy/BMS), the diagnosis of osteomyelitis may be made.17,18
3.3. Immunoscintigraphy
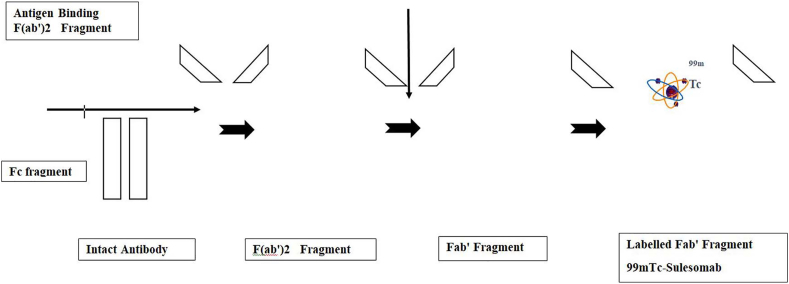
To improve the specificity of traditional scintigraphy, antibodies produced during infection can be targeted by using antibodies labelled with radiopharmaceuticals. This is called immunoscintigraphy. Infection imaging using 99mTc labelled anti-granulocyte monoclonal antibody Fab fragment (99mTc Sulesomab) has shown promising results in the evaluation of osteomyelitis, prosthetic joint infections and DFI 15,19, 20, 21, 22, 23 (Fig. 1). Unlike autologous leukocyte techniques in the imaging of infection, immunoscintigraphy does not require isolation of white blood cells ex vivo for tagging. In-vivo tagging avoids the chances of misadministration and biological hazard to healthcare professionals. However, the lack of widespread availability and high cost of 99mTc Sulesomab restricts the use of immunoscintigraphy to specialised centres. Other potential disadvantages include the rare possibilty of lowered accuracy in identifying the foci of infection and incompatibility reactions.24
Fig. 1.
Preparation of 99mTc-Sulesomab radiotracer for immunoscintigraphy.
4. Physiological nuclear medicine imaging in diabetic foot infection
Physiological imaging with 18-Fluoro-deoxyglucose positron emission tomography (18-FDG-PET/CT) in DFI has gained popularity over the last few years following guidelines published in 2013 by the European Association of Nuclear Medicine (EANM) and the Society of Nuclear Medicine and Molecular Imaging (SNMMI).25
4.1. Mechanism and physiological basis of 18F-FDG imaging
Positron emission tomography (PET) uses small amounts of radioactive materials called radiotracers or radiopharmaceuticals, a special camera and a computer to evaluate organ and tissue functions. The material accumulates around your body where it releases a small amount of energy in the form of gamma rays. Special cameras detect this energy and, with the help of a computer, create images that provide details about the structure and function of organs and tissues.
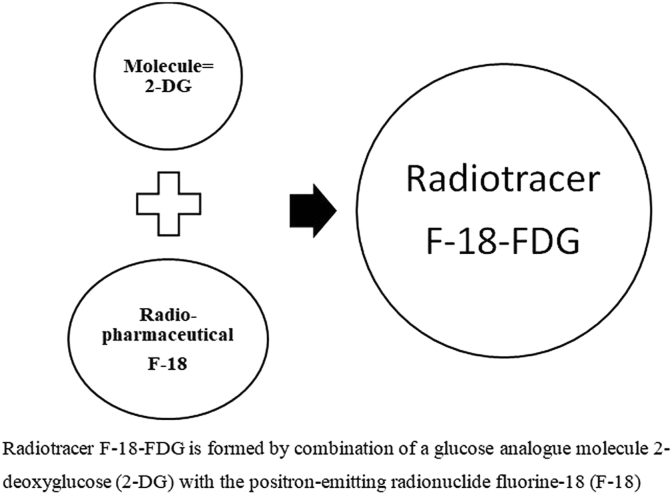
Increased expression and metabolism of glucose by infected cells is the physiological basis used in this functional nuclear medicine modality. An analogue of glucose called 2-deoxyglucose is tagged with radio-active Fluorine-18. This forms a radiolabelled pharmaceutical called 2-fluoro-2-deoxyglucose (FDG) and when given to a patient, the body assumes it is glucose. This is taken up by the metabolically active cells. The chemical, 18F-FDG is an important source of photons suitable for imaging (Fig. 2). This positron emission or beta plus decay (β+ decay) from the cells with increased uptake of the 18F-FDG by tumour and inflammatory cells is captured by gamma cameras to produce a pattern of accumulation in the body, thus plays an important role in the diagnostic work-up of musculoskeletal infection.26 Positron emission tomography (PET) with 18F-FDG, thus provides valuable functional information based on increased glucose uptake by infected cells and depicts metabolic abnormalities before morphological alterations occur.27
Fig. 2.
Preparation of 18F-FDG radiotracer for Positron Emission Tomography scan.
FDG-PET is thus an exciting future imaging modality but lacks structural detail. To improve the structural clarity, FDG-PET is combined with CT to provide an Hybrid or Fusion imaging modality.
PET-CT - image fusion or co-registration i.e. superimposing of nuclear medicine images with CT provides further details about the structure and function of organs and tissues. These views allow multiplanar correlation and can assist in interpreting information from two different examinations into one image, resulting in more precise information and accurate diagnoses. Therefore, 18F-FDG positron emission tomography/computed tomography (18F-FDG -PET/CT) provides a combination of functional and anatomical localization, which is crucial for treatment planning.
4.2. Advantages and applications in diabetic foot infection
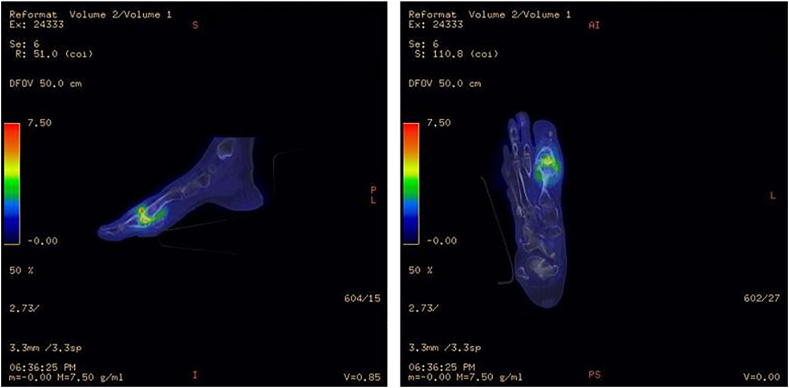
18F-FDG PET/CT nuclear medicine imaging has several advantages which can be applied in DFI evaluation.27, 28, 29 It is a non-invasive, 3-dimensional imaging modality and is not hampered by metallic artifacts in contrast to MRI/CT scan. It allows precise anatomical localization of infection in small structures of the distal foot. The increased uptake can accurately differentiate between osteomyelitis and soft-tissue infection30 (Fig. 3). Since its uptake is by inflammatory cells at the site of infection and does not rely on just leukocyte migration itself, 18F-FDG PET/CT imaging is less affected by prior antibiotic use. It allows differentiaton between infected and non-infected neuropathic osteoarthropathy and hence its advantage in the setting of complicated DFD with Charcot’s neuroarthropathy.31
Fig. 3.
Illustrative case of 18-Fluoro-deoxyglucose positron emission tomography (18-FDG-PET/CT) imaging in diabetic foot infection.
5. Patient outcomes from clinical studies and undergoing 18F-FDG PET/CT nuclear medicine imaging for the diabetic foot infection
The following section highlights some recent studies of 18F-FDG PET/CT nuclear medicine imaging, interventions, and outcomes in patients with a suspected diabetic foot infection and their comparison with complementary imaging modalities.
5.1. In detecting osteomyelitis related to diabetic foot
A Systematic review and meta-analysis of published data of diagnostic accuracy of 18F-FDG PET/CT imaging suggest a high specificity when combined with complementary modalities such as MRI.32 Kagna et al. have reported excellent diagnostic accuracy with good sensitivity, specificity, positive and negative predictive values and 18F-FDG PET/CT imaging accuracy in diabetic foot infection. They found that 18F-FDG PET/CT imaging allows a correct distinction between osteomyelitis and soft-tissue infection.33
5.2. In detecting osteomyelitis in patients with associated Charcot’s neuropathy
Osteomyelitis caused by DFI can be reliably diagnosed by using an MRI, FDG PET/CT and Single-photon emission computed tomography (SPECT). There is no clear reason identified in the literature to favour one test over the other in terms of diagnostic accuracy. 18F-FDG PET/CT imaging provides high specificity for the diagnosis of osteomyelitis in complicated DFD and allows characterisation of underlying Charcot’s neuropathy. 18F-FDG PET/CT imaging also provides an earlier diagnosis of DFI and directs focused use of antibiotics.34
5.3. Comparison with WBC scintigraphy
Familiari et al. suggest that WBC scintigraphy performs better than 18F-FDG PET/CT sequential imaging for the diagnosis of osteomyelitis in the diabetic foot albeit with the disadvantages associated with in-vitro WBC labelling, risk of contamination and longer evaluation time for individual patients.35 They found that although blood cell scintigraphy appears to be the most reliable imaging modality for differentiating osteomyelitis, soft tissue infection, and Charcot in patients with suspected DFI, both FDG and WBC have significantly higher specificity than MRI.28
5.4. Comparison with MRI
It is recognised that MRI is likely the first preferred complementary imaging modality for a DFI after plain radiographs.36 However, in situations when MRI is not possible or contraindicated, 18F-FDG PET/CT imaging appears to provide encouraging results with diagnostic accuracy.36,37 In a recent comparative study Diez et al. have found that 18F-FDG PET/CT has the highest accuracy for differentiating diabetic foot osteomyelitis from Charcot neuro-osteoarthropathy.38
6. Conclusion
The evolving concepts of functional nuclear medicine imaging have highlighted the role of scintigraphy in diagnosis of musculoskeletal infection. Early diagnosis of osteo-articular infections is the key to successful therapy and prevention of complications. This is especially relevant in DFI to prevent associated morbidity and prevent progression of the disease. Nuclear medicine imaging is an essential tool in the diagnostic work-up of DFI. Fluorine-18 (F-18) fluorodeoxyglucose-positron emission tomography (FDG-PET) is a promising modality for imaging DFI and DFD. It allows early diagnosis and targeted therapy in such conditions along with multi-disciplinary management of DFD. Though current studies suggest variable diagnostic accuracy of 18F-FDG PET/CT nuclear medicine imaging in DFI, authors have acknowledged these to be preliminary results. Current evidence suggests more studies need to be undertaken towards standardisation of technological details and options of interpretation in DFI.
Author statements
KPI involved in Conceptualization, literature search, review, and editing. MM and VK involved in literature search, writing, editing, drafting. SV involved in editing, revision, and supervision of the manuscript. All authors have read and agreed on the final draft submitted.
Conflicts of interest
The authors declare No conflict of interest.
Disclosure
None.
Funding of the study
No funding was involved in this study.
Contributor Information
Karthikeyan P. Iyengar, Email: kartikp31@hotmail.com.
Vijay K. Jain, Email: drvijayortho@gmail.com.
Muyed Kamal Awadalla Mohamed, Email: m.mohamed9@nhs.net.
Raju Vaishya, Email: raju.vaishya@gmail.com.
Sobhan Vinjamuri, Email: sobhan.vinjamuri@gmail.com.
References
- 1.The International Working Group on the Diabetic Foot (IWGDF) Editorial Board. IWGDF Definitions and Criteria. 2019. https://iwgdfguidelines.org/definitions-criteria/ Available at: [Google Scholar]
- 2.Reiber G.E., Vileikyte L., Boyko E.J. Causal pathways for incident lower-extremity ulcers in patients with diabetes from two settings. Diabetes Care. 1999 Jan;22(1):157–162. doi: 10.2337/diacare.22.1.157. [DOI] [PubMed] [Google Scholar]
- 3.Ferreira L., Carvalho A., Carvalho R. Short-term predictors of amputation in patients with diabetic foot ulcers. Diabetes Metab Syndr. 2018 Nov;12(6):875–879. doi: 10.1016/j.dsx.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Kerr M., Barron E., Chadwick P. The cost of diabetic foot ulcers and amputations to the National Health Service in England. Diabet Med. 2019 Aug;36(8):995–1002. doi: 10.1111/dme.13973. [DOI] [PubMed] [Google Scholar]
- 5.Petersen B.J., Bus S.A., Rothenberg G.M., Linders D.R., Lavery L.A., Armstrong D.G. Recurrence rates suggest delayed identification of plantar ulceration for patients in diabetic foot remission. BMJ Open Diabetes Res Care. 2020 Sep;8(1) doi: 10.1136/bmjdrc-2020-001697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institute for Health and Care Excellence . NICE Guideline; 2019. Diabetic Foot Problems: Prevention and Management; p. NG19.https://www.nice.org.uk/guidance/ng19https://www.nice.org.uk/guidance/ng19 [PubMed] [Google Scholar]
- 7.Wang A., Lv G., Cheng X. Guidelines on multidisciplinary approaches for the prevention and management of diabetic foot disease (2020 edition) Burns Trauma. 2020 Jul 6;8 doi: 10.1093/burnst/tkaa017. tkaa017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindbloom B.J., James E.R., McGarvey W.C. Osteomyelitis of the foot and ankle: diagnosis, epidemiology, and treatment. Foot Ankle Clin. 2014 Sep;19(3):569–588. doi: 10.1016/j.fcl.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Game F.L. Osteomyelitis in the diabetic foot: diagnosis and management. Med Clin. 2013 Sep;97(5):947–956. doi: 10.1016/j.mcna.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Llewellyn A., Kraft J., Holton C., Harden M., Simmonds M. Imaging for detection of osteomyelitis in people with diabetic foot ulcers: a systematic review and meta-analysis. Eur J Radiol. 2020 Oct;131:109215. doi: 10.1016/j.ejrad.2020.109215. [DOI] [PubMed] [Google Scholar]
- 11.Palestro C.J. Radionuclide imaging of musculoskeletal infection: a review. J Nucl Med. 2016 Sep;57(9):1406–1412. doi: 10.2967/jnumed.115.157297. [DOI] [PubMed] [Google Scholar]
- 12.Senneville É., Lipsky B.A., Abbas Z.G. Diagnosis of infection in the foot in diabetes: a systematic review. Diabetes Metab Res Rev. 2020 Mar;36(suppl 1) doi: 10.1002/dmrr.3281. [DOI] [PubMed] [Google Scholar]
- 13.Tan P.L., Teh J. MRI of the diabetic foot: differentiation of infection from neuropathic change. Br J Radiol. 2007 Nov;80(959):939–948. doi: 10.1259/bjr/30036666. [DOI] [PubMed] [Google Scholar]
- 14.Seabold J.E., Nepola J.V. Imaging techniques for evaluation of postoperative orthopaedic infections. Q J Nucl Med. 1999;43:21–28. [PubMed] [Google Scholar]
- 15.Palestro C.J., Love C. Radionuclide imaging of musculoskeletal infection: conventional agents. Semin Muscoskel Radiol. 2007 Dec;11(4):335–352. doi: 10.1055/s-2008-1060336. [DOI] [PubMed] [Google Scholar]
- 16.Delcourt A., Huglo D., Prangere T. Comparison between Leukoscan (Sulesomab) and Gallium-67 for the diagnosis of osteomyelitis in the diabetic foot. Diabetes Metab. 2005 Apr;31(2):125–133. doi: 10.1016/s1262-3636(07)70178-7. [DOI] [PubMed] [Google Scholar]
- 17.Palestro C.J., Love C. Nuclear medicine and diabetic foot infections. Semin Nucl Med. 2009 Jan;39(1):52–65. doi: 10.1053/j.semnuclmed.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Sella E.J., Grosser D.M. Imaging modalities of the diabetic foot. Clin Podiatr Med Surg. 2003 Oct;20(4):729–740. doi: 10.1016/S0891-8422(03)00070-3. [DOI] [PubMed] [Google Scholar]
- 19.Ryan P.J. Leukoscan for orthopaedic imaging in clinical practice. Nucl Med Commun. 2002;23:707–714. doi: 10.1097/00006231-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Iyengar K.P., Vinjamuri S. Role of 99mTc Sulesomab in the diagnosis of prosthetic joint infections. Nucl Med Commun. 2005 Jun;26(6):489–496. doi: 10.1097/00006231-200506000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Vicente A.G., Almoguera M., Alonso J.C. Diagnosis of orthopedic infection in clinical practice using Tc-99m sulesomab (antigranulocyte monoclonal antibody fragment Fab’2) Clin Nucl Med. 2004;29(12):781–785. doi: 10.1097/00003072-200412000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Harwood S.J., Valdivia S., Hung G.L., Quenzer R.W. Use of Sulesomab, a radiolabelled antibody fragment, to detect osteomyelitis in diabetic patients with foot ulcers by leukoscintigraphy. Clin Infect Dis. 1999;28(6):1200–1205. doi: 10.1086/514791. [DOI] [PubMed] [Google Scholar]
- 23.Palestro C.J., Caprioli R., Love C. Rapid diagnosis of pedal osteomyelitis in diabetics with a technetium-99m-labeled monoclonal antigranulocyte antibody. J Foot Ankle Surg. 2003;42(1):2–8. doi: 10.1053/jfas.2003.50002. [DOI] [PubMed] [Google Scholar]
- 24.Loessel C., Mai A., Starke M., Vogt D., Stichling M., Willy C. Value of antigranulocyte scintigraphy with Tc-99m-sulesomab in diagnosing combat-related infections of the musculoskeletal system. J Roy Army Med Corps. 2019 Feb 20 doi: 10.1136/jramc-2019-001172. jramc-2019-001172. Epub ahead of print. PMID: 30787111. [DOI] [PubMed] [Google Scholar]
- 25.Jamar F., Buscombe J., Chiti A. EANM/SNMMI guideline for 18F-FDG use in inflammation and infection. J Nucl Med. 2013;54:647–658. doi: 10.2967/jnumed.112.112524. [DOI] [PubMed] [Google Scholar]
- 26.Peterson T.E., Manning H.C. Molecular imaging: 18F FDG PET and a whole lot more. J Nucl Med Technol. 2009;37:151–161. doi: 10.2967/jnmt.109.062729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palestro C.J. FDG-PET in musculoskeletal infections. Semin Nucl Med. 2013 Sep;43(5):367–376. doi: 10.1053/j.semnuclmed.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Lauri C., Leone A., Cavallini M., Signore A., Giurato L., Uccioli L. Diabetic foot infections: the diagnostic challenges. J Clin Med. 2020 Jun 8;9(6):1779. doi: 10.3390/jcm9061779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruiz-Bedoya C.A., Gordon O., Mota F. Molecular imaging of diabetic foot infections: new tools for old questions. Int J Mol Sci. 2019 Nov 28;20(23):5984. doi: 10.3390/ijms20235984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keidar Z., Militianu D., Melamed E., Bar-Shalom R., Israel O. The diabetic foot: initial experience with 18F-FDG PET/CT. J Nucl Med. 2005 Mar;46(3):444–449. [PubMed] [Google Scholar]
- 31.Basu S., Chryssikos T., Houseni M. Potential role of FDG PET in the setting of diabetic neuro-osteoarthropathy: can it differentiate uncomplicated Charcot’s neuroarthropathy from osteomyelitis and soft-tissue infection? Nucl Med Commun. 2007 Jun;28(6):465–472. doi: 10.1097/MNM.0b013e328174447f. [DOI] [PubMed] [Google Scholar]
- 32.Treglia G., Sadeghi R., Annunziata S. Diagnostic performance of Fluorine-18-Fluorodeoxyglucose positron emission tomography for the diagnosis of osteomyelitis related to diabetic foot: a systematic review and a meta-analysis. Foot. 2013 Dec;23(4):140–148. doi: 10.1016/j.foot.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Kagna O., Srour S., Melamed E., Militianu D., Keidar Z. FDG PET/CT imaging in the diagnosis of osteomyelitis in the diabetic foot. Eur J Nucl Med Mol Imag. 2012 Oct;39(10):1545–1550. doi: 10.1007/s00259-012-2183-z. [DOI] [PubMed] [Google Scholar]
- 34.Rastogi A., Bhattacharya A., Prakash M. Utility of PET/CT with fluorine-18-fluorodeoxyglucose-labeled autologous leukocytes for diagnosing diabetic foot osteomyelitis in patients with Charcot’s neuroarthropathy. Nucl Med Commun. 2016 Dec;37(12):1253–1259. doi: 10.1097/MNM.0000000000000603. [DOI] [PubMed] [Google Scholar]
- 35.Familiari D., Glaudemans A.W., Vitale V. Can sequential 18F-FDG PET/CT replace WBC imaging in the diabetic foot? J Nucl Med. 2011 Jul;52(7):1012–1019. doi: 10.2967/jnumed.110.082222. [DOI] [PubMed] [Google Scholar]
- 36.Nawaz A., Torigian D.A., Siegelman E.S., Basu S., Chryssikos T., Alavi A. Diagnostic performance of FDG-PET, MRI, and plain film radiography (PFR) for the diagnosis of osteomyelitis in the diabetic foot. Mol Imag Biol. 2010 Jun;12(3):335–342. doi: 10.1007/s11307-009-0268-2. [DOI] [PubMed] [Google Scholar]
- 37.Lauri C., Tamminga M., Glaudemans A.W.J.M. Detection of osteomyelitis in the diabetic foot by imaging techniques: a systematic review and meta-analysis comparing MRI, white blood cell scintigraphy, and FDG-PET. Diabetes Care. 2017 Aug;40(8):1111–1120. doi: 10.2337/dc17-0532. [DOI] [PubMed] [Google Scholar]
- 38.Diez A.I.G., Fuster D., Morata L. Comparison of the diagnostic accuracy of diffusion-weighted and dynamic contrast-enhanced MRI with 18F-FDG PET/CT to differentiate osteomyelitis from Charcot neuro-osteoarthropathy in diabetic foot. Eur J Radiol. 2020 Nov;132:109299. doi: 10.1016/j.ejrad.2020.109299. [DOI] [PubMed] [Google Scholar]