Abstract
Introduction
Intra-articular corticosteroid (CSI) or hyaluronic acid (HAI) injections alleviate symptoms of osteoarthritis in patients who may be candidates for total hip or total knee arthroplasty (THA/TKA). However, their effect on time to total joint arthroplasty (TJA) and complications remains uncertain. We sought to evaluate (1) delay in time to surgery for patients receiving injections prior to THA/TKA (2) incidence of patients that receive injections, (3) type and number of injections, and (4) compare complication rates between patients with and without injections.
Methods
We retrospectively reviewed 3340 consecutive TJA (1770 THA and 1570 TKA). Patients were divided into two cohorts depending if they received preoperative intra-articular injection or not. We identified dates of first clinic presentation and index surgery, injection type, total administered, and 90-day complications, including periprosthetic joint infection.
Results
150/1770 THA and 192/1570 TKA patients received injections (8.5%vs.12.2%,p = 0.0004). Time from first presentation to clinic to TJA was significantly greater in patients receiving injections [12.4 ± 11 months vs.7.3 ± 10.7,p < 0.001 for THA; 20.0 ± 17.4 months vs.11.6 ± 15.4,p < 0.001 for TKA]. This delay in time was greater in TKA versus THA (8.4 months vs.5.1,p < 0.001). TKA patients had a higher incidence of receiving HAI versus THA patients (9%vs.0.6%,p < 0.0001). There were no differences in overall complication profiles (p = 0.19 for THA, p = 0.3 for TKA).
Conclusion
Injections are associated with an increased time to TJA by a statistically significant amount, however its clinical significance is debatable. Injections are safe if administered at least three months preoperatively. If patients present with appropriate surgical indications and are ready, we do not recommend intra-articular injections to delay surgery.
Keywords: Total joint arthroplasty, Corticosteroids, Hyaluronic acid, Intra-articular injection, Complications
1. Introduction
Intra-articular injections of the hip or knee joint are useful for both diagnostic and therapeutic purposes in the management of moderate to end-stage osteoarthritis (OA).1, 2, 3, 4, 5, 6, 7, 8, 9 When used in conjunction with a local anesthetic, these injections can be used as a diagnostic tool to distinguish between intra-articular sources of pain and extra-articular sources, such as those originating from the spine or surrounding soft tissue structures.1,3, 4, 5,7,9 Corticosteroid injections (CSI) and hyaluronic acid injections (HAI) are widely used to alleviate pain and inflammation in patients who are not ready or suitable to undergo total hip arthroplasty (THA) or total knee arthroplasty (TKA).1,7,10, 11, 12, 13, 14, 15, 16
The use of intra-articular injections, however, is not without complications or controversy. Intra-articular CSI has been shown to increase patient risk for several local complications including skin and fat atrophy, tendon rupture, exacerbation of pain, septic arthritis, and, periprosthetic joint infection (PJI).1,2,17,18 Some studies have demonstrated the association between intra-articular CSI preoperatively and PJI after THA, noting an increased risk of infection when THA is performed within 3 months of injection.18, 19, 20, 21 HAI has been shown in some studies to be no more effective at relieving pain compared to placebo or CSI in patients with hip or knee OA.22, 23, 24 Despite these concerns, intra-articular injections still remain an attractive treatment option for patients who may need to have their total joint arthroplasty (TJA) delayed for medical or social reasons.13,14,30
Even though intra-articular CSI and HAI are used extensively by primary care physicians, physiatrists, rheumatologists, and orthopedic surgeons alike for preoperative pain management in patients with mild, moderate, and end-stage OA, there are surprisingly few studies that discuss their role in offsetting time to TJA. Currently, there is only one study published from France that found that having at least one HAI increased the mean time to TKA by an average of 7 months compared to patients who received CSI alone during the 7.5 year time from diagnosis of knee OA.31 It also remains unclear the percentage of patients undergoing TJA who receive preoperative injections and at what time period prior to surgery they are receiving them. Previous studies have reported that patients receive hip injections between 6.2 months and 1.5 years prior to surgery.19,30,32, 33, 34 However, a common limitation in these existing data is the low number of patients studied. Furthermore, it remains unknown as to which patients are more likely to receive injections prior to TJA. More importantly, there are currently no studies that compare these measures between patients undergoing THA and TKA.
The primary objective of this study was to compare the time from clinic presentation to TJA between patients who did and did not receive injections and to determine whether the delay in time to surgery for patients receiving injections differed between THA and TKA. The secondary objectives were (1) to evaluate the incidence of patients that receive injections prior to THA and TKA, (2) to determine the type and number of injections patients received prior to THA and TKA, (3) to identify predictive factors for patients receiving injections versus patients who do not, and (4) to compare 90-day complication rates after THA or TKA between patients who did and did not receive injections.
2. Methods
After receiving approval from our institutional review board, a retrospective review of prospectively collected data was conducted to identify all consecutive patients who underwent primary TJA at a single academic medical institution from January 2018 to December 2018 using Current Procedure Terminology (CPT) code 27,130 for primary THA and 27,447 for primary TKA. We then identified through manual review of the electronic medical record (EMR) the date the patient was first diagnosed with symptomatic moderate-to-severe OA in orthopedic clinic (diagnosed by history, physical examination, and plain radiography). Any previous patient clinical encounters with non-orthopedic surgeons prior to their first orthopedic clinical encounter were not documented in our study. We also identified if patients received a preoperative intra-articular injection, the injection type (CSI vs. HAI) and number of injections received, date of the injections, date of index arthroplasty procedure, and any cause of complications after TJA that required readmissions, emergency department (ED) visits, or reoperations within a 90-day postoperative period. All patients included were followed for a minimum of 90 days post-TJA to ensure we captured all 90-day complications. Patients were excluded if they underwent non-elective TJA (i.e. trauma or revision surgery) or had <2 weeks of reportable data in the EMR. We have only included patients who had a 2-week minimum pre-operative EMR data to account for the time it takes for a patient to schedule surgery and come in for a pre-admission clinic assessment. The primary outcome, which looks at the role of injection therapy in offsetting time to TJA once the patient presented to the orthopedic clinic, was measured by calculating the difference between the date of first presentation to the orthopedic clinic and the date of index procedure. De-identified patient demographic data including age, body mass index (BMI), American Society of Anesthesiologists (ASA) score, unadjusted and age-adjusted Charlson Comorbidity Index (CCI; which encompasses patient comorbidities such as diabetes mellitus, rheumatoid diseases, cardiac and renal conditions, and cancer), gender, race, and smoking status were also collected.
Groups were separated based on whether they received an intra-articular injection at the hip or knee joint prior to TJA (exposure) or not (control). Injection types identified in this study were either CSI or HAI. Methylprednisolone acetate (40 mg or 80 mg), triamcinolone acetonide (10 mg, 40 mg, 60 mg, or 80 mg), or dexamethasone (4 mg or 10 mg) injections were classified as CSI therapy. Sodium hyaluronate (10 mg/mL, 30 mg/mL, 16 mg/2 mL, or 48 mg/6 mL) or cross-linked hyaluronate acid (30 mg/3 mL or 88 mg/4 mL) were classified as HAI therapy. Local anesthetics utilized in combination with the injections included lidocaine or ropivacaine. The type of injection, dose of medications, and local anesthetic used were all based on surgeon preference. All preoperative hip injections at our institution were performed using ultrasound or fluoroscopy-guided technique and administered by either a radiologist specialized in musculoskeletal (MSK) imaging (40% of THA cases), interventional radiologist (46%), orthopedic surgeon (3%), non-surgical sports medicine physician (2%), or pain interventionist (9%). All preoperative knee injections were performed with or without ultrasound-guided technique and administered by either an orthopedic surgeon (86% of TKA cases), rheumatologist (7%), pain interventionist (4%), physical medicine and rehabilitation specialist (2%), or a radiologist specialized in MSK imaging (1%). Frequency of CSI administration was dependent on patient and provider preference. The majority of HAI injections were administered in a series of three shots with each injection spread one week apart.
2.1. Statistical analysis
Descriptive statistics was reported as means ± standard deviations for continuous variables and counts (%) for categorical data. Independent, two-tailed t-tests were performed to compare the means of continuous data, such as patient demographic data, while chi-squared tests were used for categorical data, including revision rates. A multivariable regression analysis was used to compare the infection rates between the two groups to account for any confounding variables. These findings were reported as an odds ratio (OR) with an associated 95% confidence interval (CI). All statistical analyses were performed using SPSS version 25 (IBM Corporation, Armonk, New York). A cutoff p-value ≤ 0.05 was considered to be statistically significant.
3. Results
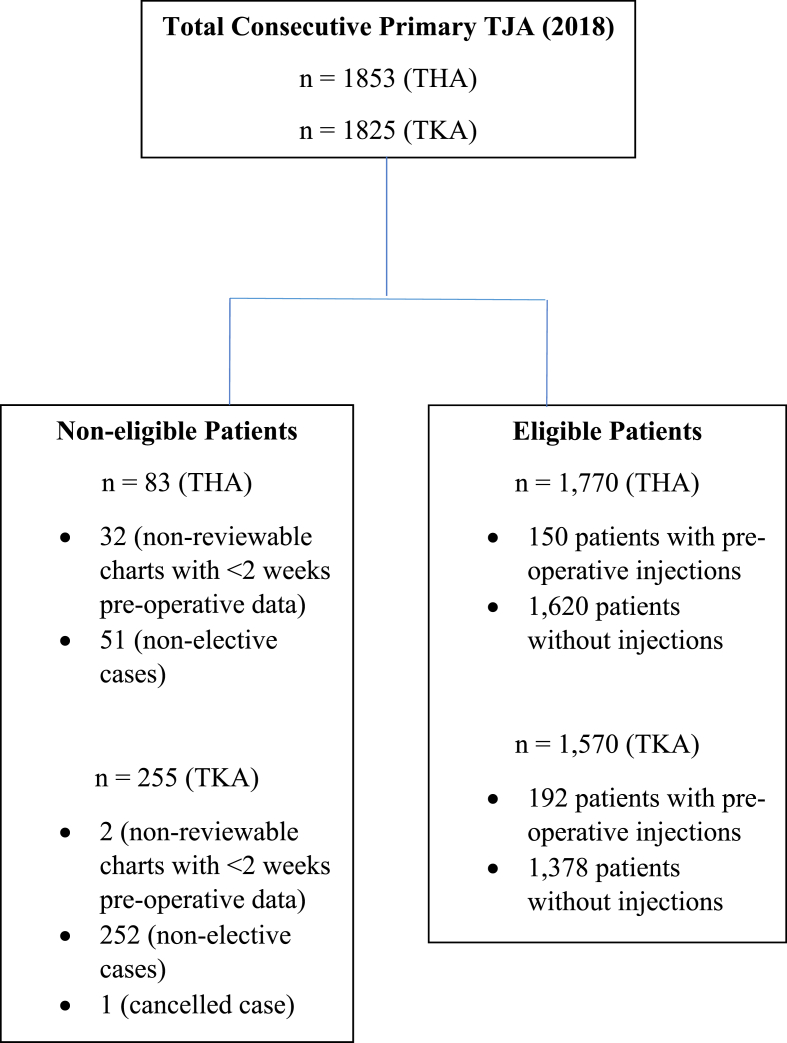
A total of 3340 consecutive primary TJA cases (1770 THA and 1570 TKA) were identified and deemed eligible for this study (Fig. 1). 150 out of 1770 patients who underwent THA received preoperative CSI or HAI while 192 out of 1570 patients who underwent TKA received preoperative CSI or HAI (8.5% incidence THA vs 12.2% incidence TKA, p < 0.0004). There were no demographic differences in age, BMI, ASA score, race, or gender between the exposure and control groups in either THA or TKA cohorts (Table 1). The time from first presentation to clinic to THA or TKA was significantly greater in patients receiving preoperative injections than in patients who did not receive injection therapy. In patients who eventually underwent THA, patients who received hip injections had their THA completed by an average of 12.4 ± 11 months (range: 0.7–58) versus 7.3 ± 10.7 months (range: 0.5–83.9) in patients who did not receive hip injections (p < 0.001). In patients who eventually underwent TKA, patients who received knee injections had their TKA completed by an average of 20.0 ± 17.4 months (range: 0.8–88) versus 11.6 ± 15.4 months (range: 0.5–90) in patients who did not receive knee injections (p < 0.001) (Table 2). This delay in time from first clinic presentation to TJA for patients receiving injections was greater in TKA patients compared to THA patients (8.4 months vs. 5.1 months, p < 0.001). These results remained consistent between TKA and THA patients receiving intra-articular injections from orthopedic surgeons alone [20.7 months (range: 1–88.1) vs. 7.3 months (range: 3.3–17.9), p = 0.004]. There was no difference in time from first clinic presentation to surgery when comparing orthopedic surgeon administered injections to non-surgeon administered injections in either TKA [20.7 months (range: 1–88.1) vs. 15.8 months (range: 0.76–62.1), p = 0.17] or THA [7.3 months (range: 3.3–17.9) vs. 12.6 months (range: 0.72–58), p = 0.13].
Fig. 1.
Patient inclusion/exclusion Criteria.
Table 1.
TJA patient demographic data.
| Total Hip Arthroplasty Cohort (Total n = 1770) | |||
|---|---|---|---|
| Variables | Injection (n = 150) | Control (n = 1620) | p-valuea |
| Age | 63.8 ± 11.5 | 63.9 ± 11.4 | 0.96 |
| BMI | 29.5 ± 5.4 | 29.1 ± 5.9 | 0.33 |
| ASA score | 0.1 | ||
| I | 9 (6%) | 137 (8%) | |
| II | 88 (59%) | 987 (61%) | |
| III | 50 (33%) | 480 (30%) | |
| IV | 3 (2%) | 16 (1%) | |
| CCI | |||
| Unadjusted | 0.82 ± 1.6 | 0.43 ± 1.1 | 0.005 |
| Age-adjusted | 0.94 ± 2.0 | 0.56 ± 1.6 | 0.036 |
| Gender | 0.07 | ||
| Male | 53 (35%) | 698 (43%) | |
| Female | 97 (65%) | 921 (57%) | |
| Race | 0.23 | ||
| White | 118 (78%) | 1215 (75%) | |
| African American | 19 (13%) | 186 (12%) | |
| Other (including Asian) | 13 (9%) | 216 (13%) | |
| Smoking Status | 0.67 | ||
| Current | 11 (8%) | 123 (7%) | |
| Former | 62 (41%) | 604 (37%) | |
| Never | 77 (51%) | 886 (55%) | |
| Unknown |
0 (0%) |
7 (1%) |
|
| Total Knee Arthroplasty Cohort (Total n = 1570) | |||
| Variables |
Injection (n = 192) |
Control (n = 1378) |
p-valuea |
| Age | 67.04 ± 8.6 | 66.9 ± 9.7 | 0.82 |
| BMI | 32.3 ± 5.9 | 32.3 ± 6.3 | 0.99 |
| ASA score | 0.29 | ||
| I | 6 (3%) | 32 (2%) | |
| II | 111 (58%) | 729 (53%) | |
| III | 74 (38%) | 591 (43%) | |
| IV | 1 (1%) | 26 (2%) | |
| CCI | 0.12 0.83 |
||
| Unadjusted | 0.7 ± 1.3 | 0.54 ± 1.2 | |
| Age-adjusted | 0.67 ± 1.7 | 0.7 ± 1.7 | |
| Gender | 0.39 | ||
| Male | 56 (29%) | 444 (32%) | |
| Female | 136 (71%) | 934 (68%) | |
| Race | 0.14 | ||
| White | 112 (58%) | 736 (53%) | |
| African American | 40 (21%) | 262 (19%) | |
| Other (including Asian) | 40 (21%) | 380 (28%) | |
| Smoking Status | 0.001 | ||
| Current | 4 (2%) | 91 (6%) | |
| Former | 90 (47%) | 469 (34%) | |
| Never | 98 (51%) | 813 (59%) | |
| Unknown | 0 (0%) | 5 (1%) | |
p-values derived from unpaired t-tests for continuous variables or chi-squared tests for categorical data.
Table 2.
Average Time from First Presentation to Clinic to Surgery in TJA patients with or without preoperative injections (in months).
| Total Hip Arthroplasty Cohort (Total n = 1770) | |||
|---|---|---|---|
| Variables | Injection (n = 150) | Control (n = 1620) | p-valuea |
| Time from First Presentation to Clinic to Surgery |
12.4 ± 11 (range: 0.7–58 months) |
7.3 ± 10.7 (range: 0.5–83.9 months) |
<0.001 |
| Total Knee Arthroplasty Cohort (Total n = 1570) | |||
| Variables |
Injection (n = 192) |
Control (n = 1378) |
p-valuea |
| Time from First Presentation to Clinic to Surgery | 20 ± 17.4 (range: 0.8–88 months) | 11.6 ± 15.4 (range: 0.5–90 months) | <0.001 |
p-values derived from unpaired t-tests for continuous variables or chi-squared tests for categorical data.
Of the 150 patients who received injections prior to THA, 148 patients received CSI, 1 patient received HAI, and 1 other patient received a combination of both CSI and HAI (i.e. 1 CSI and 1 HAI). Of the 192 patients who received injections prior to TKA, 23 patients received CSI, 141 received HAI, and 28 received a combination of both CSI and HAI. The breakdown of number and type of injections in the THA and TKA cohorts is further detailed in Table 3. TKA patients had a higher incidence of receiving HAI compared to THA patients (9% vs. 0.6%, p < 0.0001).
Table 3.
TJA patient totals by injection type.
| Injection Type | Total THA Patients (n = 1770) | Total TKA Patients (n = 1570) |
|---|---|---|
| Corticosteroids (CSI) | ||
| 1 | 106 | 16 |
| 2 | 31 | 3 |
| 3 | 7 | 2 |
| 4 | 2 | 2 |
| 5+ | 2 | 0 |
| Total | 148 | 23 |
| Hyaluronic acid (HAI) | ||
| 1 | 1 | 62 |
| 2 | 0 | 31 |
| 3 | 0 | 18 |
| 4 | 0 | 9 |
| 5+ | 0 | 21 |
| Total | 1 | 141 |
| Combined CSI/HAI | ||
| 1–3 CSI & 1–3 HAI | 1 | 16 |
| 4–6 CSI & 1–3 HAI | 0 | 2 |
| 7 + CSI & 1–3 HAI | 0 | 0 |
| 1–3 CSI & 4–6 HAI | 0 | 2 |
| 4–6 CSI & 4–6 HAI | 0 | 4 |
| 4–6 CSI & 7 + HAI | 0 | 1 |
| 7 + CSI & 4–6 HAI | 0 | 2 |
| 7 + CSI & 7 + HAI | 0 | 1 |
| Total | 1 | 28 |
| None | 1620 | 1378 |
No risk factors assessed, such as demographic variables, were different between the injection group or control group for TKA (Table 1). For TKA, patients in the injection group had a significantly higher proportion of former smokers than patients in the control group (47% vs. 34%, p = 0.001). For THA, patients in the injection group had significantly higher CCI than patients in the control group (0.82 ± 1.6 vs. 0.43 ± 1.1 for unadjusted CCI, p = 0.005; and 0.94 ± 2.0 vs. 0.56 ± 1.6 for age-adjusted CCI, p = 0.036). There were no differences in 90-day postoperative reoperation rates or overall complication profiles, including PJI, between patients who did or did not receive injection (p = 0.19 for THA, p = 0.3 for TKA). A multivariate regression analysis found no significant differences in infection rates between the two study groups even after adjusting the model for potential confounding variables (Table 5). There was, however, a significant difference found in dislocation rates between the two groups, with higher rates seen in patients with injections than patients without injections prior to undergoing THA (1.3% vs. 0.25%, p = 0.03) (Table 4). The time from latest intra-articular injection to TJA was greater in TKA patients (13.5 months, range: 0.7–58) compared to THA patients (8.1 months, range: 1.3–58) (p < 0.0001). These results remained consistent between TKA and THA in patients receiving injections from orthopedic surgeons alone [14.01 months (range: 1.6–76.4) vs. 3.7 months (range: 1.4–4.9 months), p < 0.0001]. When comparing orthopedic surgeon administered injections to non-surgeon administered injections, TKA patients received their latest intra-articular injections at a greater time prior to surgery [14.01 months (range: 1.6–76.4) vs. 10.1 months (range: 1.9–23), p = 0.03] than THA patients receiving their latest intra-articular injections [3.7 months (range: 1.4–4.9 months) vs. 8.3 months (range: 1.3–58), p = 0.0002].
Table 5.
Multivariate logistic regression for infection rates.
| Total Hip Arthroplasty Cohort (Total n = 1770) | ||||
|---|---|---|---|---|
| Outcome | Unadjusted Odds Ratio (95% CI)a | p-value | Adjusted Odds Ratio (95% CI)b | p-value |
| Infection |
0.367 (0.77, 1.745) |
0.208 |
0.192 (0.034, 1.077) |
0.061 |
| Total Knee Arthroplasty Cohort (Total n = 1570) | ||||
| Outcome |
Unadjusted Odds Ratio (95% CI)a |
p-value |
Adjusted Odds Ratio (95% CI)b |
p-value |
| Infection |
8.24 × 106 (0, --) |
0.994 |
8.41 × 106 (0, --) |
0.995 |
| Total Joint Arthroplasty Cohort (Total n = 3340) | ||||
| Outcome |
Unadjusted Odds Ratio (95% CI)a |
p-value |
Adjusted Odds Ratio (95% CI)b |
p-value |
| Infection | 0.855 (0.195, 3.754) | 0.835 | 0.761 (0.177, 3.550) | 0.761 |
Unadjusted model only studies the association between infection rate and injection use.
Adjusted model studies the association between infection rate, injection use, and demographic variables (age, BMI, ASA score, CCI, gender, race, and smoking status).
Table 4.
90-Day revision and complication rates following TJA.
| Total Hip Arthroplasty Cohort (Total n = 1770) | |||||
|---|---|---|---|---|---|
| Variable | Exp (n = 150) | Mean Time to Follow-Up (in months) | Control (n = 1620) | Mean Time to Follow-Up (in months) | p-value∗ |
| Aseptic Loosening | 0 (0%) | – | 0 (0%) | – | – |
| Dislocation | 2 (1.3%) | 0.76 (range: 0.6 to 0.89) | 4 (0.25%) | 0.6 (range: 0.36 to 0.95) | 0.03 |
| Liner Exchange | 0 (0%) | – | 1 (0.06%) | 0.92 | 0.76 |
| Deep Tissue Infection (PJI) | 2 (1.3%) | 0.51 (range: 0.43 to 0.59) | 8 (0.5%) | 1.48 (range: 0.56 to 2.6) | 0.19 |
| Trauma (Peri-prosthetic fracture) | 1 (0.7%) | 1.9 | 5 (0.3%) | 0.96 (range: 0.2 to 2.5) | 0.47 |
| Wound Complications (Drainage) | 0 (0%) | – | 3 (0.2%) | 0.5 (range: 0.43 to 0.62) | 0.6 |
| DVT | 0 (0%) | – | 1 (0.06%) | 0.3 | 0.76 |
| Pain (ED visit) | 0 (0%) | – | 1 (0.06%) | 1.5 | 0.76 |
| All-cause complication ratea |
5 (3.3%) |
0.89 (range: 0.43 to 1.9) |
24 (1.5%) |
1.0 (range: 0.2 to 2.6) |
0.09 |
| Total Knee Arthroplasty Cohort (Total n = 1570) | |||||
|
Variable |
Exp (n = 192) |
Mean Time to Follow-Up (in months) |
Control (n = 1378) |
Mean Time to Follow-Up (in months) |
p-value∗ |
| Manipulation Under Anesthesia±Lysis of Adhesions | 5 (2.6%) | 2.1 (range: 1.6 to 2.7) | 16 (1.2%) | 2.3 (range: 1.6 to 2.9) | 0.1 |
| Aseptic Loosening | 0 (0%) | – | 1 (0.07%) | 2.1 | 0.7 |
| Liner Exchange | 0 (0%) | – | 1 (0.07%) | 1.6 | 0.7 |
| Deep Infection (i.e. PJI) | 0 (0%) | – | 7 (0.5%) | 1.1 (range: 0.7 to 1.6) | 0.3 |
| Superficial Infection (e.g. abscess) | 0 (0%) | – | 2 (0.1%) | 0.74 (range: 0.66 to 0.82) | 0.6 |
| Trauma (i.e. periprosthetic fracture) | 0 (0%) | – | 4 (0.3%) | 1.4 (range: 0.7 to 2.7) | 0.5 |
| Wound Complications (e.g. dehiscence, drainage) | 0 (0%) | – | 8 (0.6%) | 1.6 (range: 0.4 to 2.3) | 0.29 |
| All-cause complicationsa | 5 (2.6%) | 2.1 (range: 1.6 to 2.7) | 39 (2.8%) | 1.7 (range: 0.4 to 2.9) | 0.86 |
All-cause complications are calculated from the total complicated events for each cohort.
4. Discussion
Intra-articular CSI and HAI have long been employed as a form of conservative therapy to alleviate symptoms and improve functional disability in patients with hip or knee OA.1,7,10, 11, 12, 13, 14,30 To our knowledge, our study is the first to evaluate the association of intra-articular injections with the increase in time to eventual TJA and to compare this time with patients who did not receive injections. We are also the first to compare characteristics of injection use between patients with hip and knee OA who have underwent THA or TKA. Our findings show that the impact of intra-articular injections in delaying time to TJA is statistically significant, and that this impact is of greater significance in patients undergoing TKA than THA.
In our study, the effect of injections was an average delay of 5.1 months (12.4 months vs 7.3 months, p < 0.001) for patients who eventually underwent THA, and an average delay of 8.4 months (20.0 months vs 11.6 months, p < 0.001) for patients who eventually underwent TKA. To our knowledge, only one study is published from France that discusses the role of intra-articular injections in delaying TJA, and their study focused specifically on the impact of HAI exclusively in patients with knee OA.31 Delbarre et al. reviewed 14,782 patients over a 7-year period who were treated for knee OA (9476 patients were treated with HAI and 5306 patients were treated with CSI). Of this total population, 1662 patients had TKA (1296 received preoperative HAI while 366 received preoperative CSI). The authors found that the mean time from diagnosis to TKA in patients receiving HAI was significantly higher than patients who received CSI by an average difference of 1.7 months at one year after diagnosis of OA and 7 months at 7.5 years after diagnosis (p < 0.0001). Their study included patients who did or did not receive injections and who did or did not undergo TKA. Using a Kaplan-Meier survivorship analysis they found that at all time points studied, mean survival time to TKA was significantly higher in patients treated with HAI. A sub-analysis comparing time to surgery between preoperative HAI use and CSI use in our TKA population suggested that patients receiving HAI prior to eventual TKA delayed their surgery by 5.8 months greater than patients who received CSI prior to TKA. These findings, however, were not statistically significant [19.9 ± 18.3 months (range: 0.76–88.1) for HAI vs. 14.1 ± 14.3 months (range: 1.9–66.2) for CSI, p = 0.10].
Interestingly, the delay to TJA in patients undergoing injections with hip OA and knee OA in our study differed by 3.3 months with significantly greater delay to arthroplasty seen in patients receiving knee injections than hip injections (8.4 vs. 5.1 months, p < 0.001). The reason behind the difference in response to injections in patients with hip and knee OA may be due to several factors. One reason could be due to anatomic factors relating to the ability to reliably deliver injections in the intraarticular space of the hip versus the knee. Since it is inherently more difficult to access the intraarticular hip joint, most physicians rely on either ultrasound or fluoroscopic guidance to guide the injection procedure.24, 25, 26, 27, 28 Despite a 100% rate of image guidance for hip injections in our study, it is possible that the provider mistargeted the joint space when administering the injection, leading to lower efficacy of hip injections compared to knee injections, and therefore an earlier time to TJA.
Other reasons for the difference in time to surgery between the two injection groups could be due to the differences in presentation, progression, and rates of arthroplasty between hip and knee OA. Our results show that regardless of administration of injections or not, patients with hip OA have surgery sooner after presenting to clinic than patients with knee OA. Dabare et al. performed a 6-year observational study of 247 consecutive patients with hip (n = 80) and knee (n = 167) OA comparing the natural progression of OA between the two groups. Similar to our study, the authors found that patients with hip OA were more likely to require earlier TJA from time to diagnosis than patients with knee OA.35 They hypothesized that additional anatomic factors may contribute to a delayed presentation of patients with hip OA with more advanced disease than patients with knee OA, such as the inherent increased stability of the ball and socket hip joint surrounded by significant soft tissue stabilizers. This stability, they concluded, would allow the disease to progress to later stages in which the need for TJA becomes more imminent, and intra-articular injections are unable to provide adequate long-lasting relief and comfort. Finally, patients with symptoms of hip OA might be missed in the early stages of the disease if they are misdiagnosed with other causes of pain to the lower limb, including spine pathology, nerve compression, and muscular injury among other causes. Therefore, patients presenting with hip pain may undergo unnecessary work up and treatment plans prior to reaching a definitive diagnosis, at which point the stage of hip OA would have progressed significantly. These factors all may explain the potential superior efficacy of intra-articular injections in delaying TJA in patients with knee OA than in patients with hip OA, as evidenced by our study results.
While the results of delayed time to surgery from injection therapy were statistically significant for both the hip and knee groups, the clinical significance of a delay by 5–8 months is debatable. It is well established that the efficacy of HAI and CSI is thought to be most beneficial in the short term.1,11, 12, 13, 14, 15,36, 37, 38 Lai et al. studied a cohort of 82 patients who received steroid injections to the hip joint and found that close to 70% of their patients received less than or equal to 2 weeks of pain relief following injection therapy.12 Furthermore, the authors determined that nearly 50% of patients who had steroid injections underwent THA or hip resurfacing within 2 years after initial injection. Another study from the United Kingdom found that 70% of patients underwent THA within 2 years after therapeutic hip injection.36 We believe that the use of intra-articular injections at our institution provides comfort and allows patients who will inevitably need surgery to cope with their symptoms for the time being. Intra-articular injections bridge the gap between initial presentation and diagnosis to surgery to allow the patient more time to prepare and make arrangements for their elective surgery. Therefore, while surgical intervention remains the gold standard treatment for moderate-to-severe OA and should not be delayed in appropriately indicated patients who are ready for surgery, the results of our study support the use of intra-articular injections in delaying time to THA and TKA for a short-term period to allow patients to prepare mentally, medically, and from a social support standpoint.
Our study revealed a relatively low but not negligible incidence of injections administered in patients undergoing THA or TKA at our institution. 150 out of 1770 total patients undergoing THA in 2018 at our institution received intra-articular hip injections prior to surgery (8.5%). The majority of patients who received intra-articular injections to the hip prior to undergoing surgery were more likely to receive CSI alone (98%) than HAI alone (1%) or a combination of both (1%). On the other hand, 192 of 1570 total patients undergoing TKA in 2018 at our institution received preoperative intra-articular knee injections (12.2%). The majority of patients who received intra-articular injections to the knee prior to surgery were more likely to receive HAI alone (73%) than CSI alone (12%) or a combination of both (15%). Neither patients who had THA or TKA at our institution received platelet-rich plasma (PRP) prior to surgery. These findings highlight a few important considerations. First, our results show that intra-articular injections at our institution were less frequently used in the hip than in the knee, which is consistent with previous reports that attribute this phenomenon to the technical challenge and difficulty in accessing the hip joint.25, 26, 27, 28, 29 Second, significantly more patients undergoing TKA received HAI than THA patients (9% vs 0.6%, p < 0.0001). According to the American Academy of Orthopedic Surgeons (AAOS) recommendations for treatment of symptomatic hip and knee OA, the current guidelines have actually recommended against the use of HAI.39 These recommendations are based on the results of previous meta-analyses that assessed the meaningfully important difference (MID) in pain measurement outcomes such as the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain, function, and stiffness subscales or the visual analogue scale (VAS) for pain intensity.40 These studies found that the effect of using HAIs were less than 0.5 MID units, which demonstrate the low likelihood that an appreciable number of patients with hip or knee OA would achieve clinically important benefits in pain outcomes. Aside from the use of HAI, the current guidelines interestingly recommend use of CSI in hips but remain inconclusive and cannot recommend for or against the use of CSI or PRP for patients with symptomatic OA of the knee due to the lack of compelling evidence.39,41 The findings from our study suggest that while injection therapy and especially HAI may not be recommended by clinical practice guidelines for treatment of symptomatic OA due to questionable efficacy, in real-life clinical practice injections including HAI for the knee are not infrequently utilized in efforts to provide patients with some form of localized treatment and pain relief while buying time to surgical intervention.
In an attempt to identify patient predictive factors for patients receiving preoperative intra-articular hip or knee injections, we compared patient demographic data between patients receiving injections versus patients who did not. Our results found no statistically significant differences between the two cohorts in age, BMI, ASA score, gender, and race. In regards to smoking status, a greater proportion of former smokers who eventually undergo TKA received knee injections. While there were no differences in CCI between the control and injection group in the TKA cohort, we found that patients receiving injections prior to THA had significantly higher CCI than patients who do not receive them. These findings suggest that less healthy patients who eventually undergo THA are more likely to receive hip injections to delay surgery than healthier patients. To our knowledge, there are few studies that comment on predictive factors for patients who are more likely to receive injections. Delbarre et al. found that patients with knee OA who received HAI and underwent TKA were approximately 2 years younger at initial diagnosis and 1.4 years younger at the time of surgery than TKA patients receiving CSI.31 More importantly, when adjusting for covariates in a survival analysis using a Cox proportional hazards model, the authors found that women, younger patients, and patients with significant comorbidities (measured by a high CCI and increased number of hospitalizations) had a lower hazard ratio for TKA (all p-values<0.05). Further study is needed with larger sample sizes to determine whether these factors may be true predictors for patients receiving hip or knee injections or not.
Last, there were no differences in any reoperation or overall complication rates, including risk of PJI at 90-days following TJA, between patients receiving injections and those not receiving injections. Previous studies have demonstrated the association between intra-articular injections and PJI after TJA.17, 18, 19, 20, 21,30,32,33,41, 42, 43 The literature regarding the association between pre-operative intra-articular steroid injections to the hip and risk of PJI after THA have initially been conflicting.18 Earlier studies reported no increased risk of superficial or deep infection following CSI or HAI,17,30,32,33,41, 42, 43 while other studies have argued the exact opposite.18, 19, 20, 21 However, these studies report many variations and inconsistencies in the timing of injection administration to surgery, which in some cases were more than a year preoperatively.42 One of the most cited studies examining PJI risk in patients undergoing preoperative intraarticular hip injection by Werner et al. studied a cohort of 34,597 THA cases and demonstrated a significant increase in PJI in patients who underwent intra-articular hip injection within 3 months prior to THA.18 Our study found only 2 patients who had PJI following TJA, both of whom received preoperative steroid hip injections approximately 90 days prior their THA (one patient received their injection 90 days prior to surgery and the other patient received their injection 92 days prior). There were no infections found in our TKA cohort. As the average time from latest injections to surgery reported in our study were administered well before 3 months prior to TJA (8.1 months prior to THA and 13.5 months prior to TKA on average), it would follow that the infection rates detected in our review would be too small to determine any significant difference. The only significant finding of the study was the higher rate of dislocation in the injection group vs the control group (1.3% vs. 0.25%, p = 0.03). While infection may be one of the causes for early dislocation, we feel that these findings were unrelated to the preoperative hip injections. Upon further chart review, it was revealed that both patients experienced a posterior dislocation upon getting up from a seated position within one month following their THA. Both patients were operated on by the same surgeon who performed a posterolateral approach, which is known to be associated with higher dislocation rates.44 Further research is required to detail the post-operative complication rates in patients receiving hip injections as the current body of literature only reports these events using low-level evidence such as case reports.1,2,17
4.1. Limitations
The current study has several limitations. First, because our data was collected via retrospective chart review, it is susceptible to selection bias and unmeasured confounding variables that may influence the results of our study. For instance, patients are likely to receive injections if they want to delay their surgery for personal or medical reasons. Also, patients who receive injections are likely to have their surgery delayed due to the risk of infection. Perhaps the biggest confounder resulting from this potential selection bias is the degree of arthritis that patients presented with in the injection and non-injection cohorts. Without randomization, it is possible that patients in the non-injection cohort for both hips and knees had more advanced disease leading to earlier time to surgery. To minimize this limitation, we reviewed a large sample size of thousands of patients in one calendar year of consecutive TJA cases and found no difference in patient characteristics between the injection and no-injection groups, suggesting overall equivalent populations in the two cohorts. Another limitation that our study poses is that without access to outside electronic medical records, we are unable to fully assess total complications after surgery or if patients had injections performed elsewhere either before or after presentation to our clinics. While this would mean that our reported incidence may underrepresent the true incidence of administered injections, the complication profile from injection therapy reported in our study was still low. Furthermore, we performed a thorough manual chart review for both documented clinical notes and dispensed medication lists for each patient to determine the total number of injections received at our institution and to see if patients were presenting to outside providers. Our registered nurse clinical care coordinators call patients frequently postoperatively and our manual chart review of the data helps ensure thoroughness with these possible limitations. In doing so, we believe, to the best of our knowledge, the totals reported in this study are the most representative of our study population. Another plausible limitation that our study poses is that we included a variety of several different injection types and doses since there is no standardized injection protocol implemented at our institution. This would make it difficult to assess the efficacy of a single injection type or dose in delaying TJA, however, we feel the heterogeneity of injection administration practices at our institution reflects real world practice more closely and as such that our findings are more generalizable. Finally, we acknowledge that our study may be underpowered to show if injections made a true difference in some complication rates to see significance, as this was a secondary endpoint of the study. As we made no hypothesis regarding the results of our study, our findings could be incidental possibly by overuse of statistical testing. Thus, larger studies are needed to confirm these limitations.
5. Conclusion
The results of our study show that intra-articular corticosteroid and hyaluronic acid injections delay time to THA and TKA by a statistically significant amount. This delay is 5.1 months for THA patients and 8.4 months for TKA patients; as such, it is debatable whether this delay in time to surgery is clinically significant. The overall incidence of intraarticular injections before TJA was low but not negligible and patients with increased comorbidities were more likely to receive hip injections prior to THA. More importantly, there was no significant increase in overall complication rates including PJI for patients undergoing injections prior to TJA. Therefore, we recommend that while injections may be associated with a modest increase in time to surgery and are safe to administer when scheduled at least 3 months before THA or TKA, there is little reason to delay TJA in appropriately indicated and optimized patients with symptomatic hip or knee OA.
References
- 1.Chandrasekaran S., Lodhia P., Suarez-Ahedo C., Vemula S.P., Martin T.J., Domb B.G. Symposium: evidence for the use of intra-articular cortisone or hyaluronic acid injection in the hip. J Hip Preserv Surg. 2016;3 doi: 10.1093/JHPS/HNV020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cole B.J., Schumacher H.R. Injectable corticosteroids in modern practice. J Am Acad Orthop Surg. 2005;13:37–46. doi: 10.5435/00124635-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Crawford R.W., Gie G.A., Ling R.S., Murray D.W. Diagnostic value of intra-articular anaesthetic in primary osteoarthritis of the hip. J Bone Joint Surg Br. 1998;80:279–281. doi: 10.1302/0301-620x.80b2.8299. [DOI] [PubMed] [Google Scholar]
- 4.Kleiner J.B., Thorne R.P., Curd J.G. The value of bupivicaine hip injection in the differentiation of coxarthrosis from lower extremity neuropathy. J Rheumatol. 1991;18:422–427. [PubMed] [Google Scholar]
- 5.Faraj A.A., Kumaraguru P., Kosygan K. Intra-articular bupivacaine hip injection in differentiation of coxarthrosis from referred thigh pain: a 10 year study. Acta Orthop Belg. 2003;69:518–521. [PubMed] [Google Scholar]
- 6.Harvey W.F., Hunter D.J. The role of analgesics and intra-articular injections in disease management. Rheum Dis Clin N Am. 2008;34:777–788. doi: 10.1016/j.rdc.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Creamer P. Intra-articular corticosteroid treatment in osteoarthritis. Curr Opin Rheumatol. 1999;11:417–421. doi: 10.1097/00002281-199909000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Plant M.J., Borg A.A., Dziedzic K., Saklatvala J., Dawes P.T. Radiographic patterns and response to corticosteroid hip injection. Ann Rheum Dis. 1997;56:476–480. doi: 10.1136/ard.56.8.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deshmukh A.J., Thakur R.R., Goyal A., Klein D.A., Ranawat A.S., Rodriguez J.A. Accuracy of diagnostic injection in differentiating source of atypical hip pain. J Arthroplasty. 2010;25:129–133. doi: 10.1016/j.arth.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Scott D.L. Guidelines for the diagnosis, investigation and management of osteoarthritis of the hip and knee. Report of a joint working group of the British society for rheumatology and the research unit of the royal college of physicians. J R Coll Physicians Lond. 1993;27:391–396. [PMC free article] [PubMed] [Google Scholar]
- 11.Creamer P. Intra-articular corticosteroid injections in osteoarthritis: do they work and if so, how? Ann Rheum Dis. 1997;56:634–636. doi: 10.1136/ard.56.11.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai W.C., Arshi A., Wang D. Efficacy of intraarticular corticosteroid hip injections for osteoarthritis and subsequent surgery. Skeletal Radiol. 2018;47:1635–1640. doi: 10.1007/s00256-018-3052-z. [DOI] [PubMed] [Google Scholar]
- 13.Flanagan J., Casale F.F., Thomas T.L., Desai K.B. Intra-articular injection for pain relief in patients awaiting hip replacement. Ann R Coll Surg Engl. 1988;70:156–157. [PMC free article] [PubMed] [Google Scholar]
- 14.Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Arthritis Rheum. 2000;43 doi: 10.1002/1529-0131. 1905–15. [DOI] [PubMed] [Google Scholar]
- 15.Qvistgaard E., Christensen R., Torp-Pedersen S., Bliddal H. Intra-articular treatment of hip osteoarthritis: a randomized trial of hyaluronic acid, corticosteroid, and isotonic saline. Osteoarthritis Cartilage. 2006;14:163–170. doi: 10.1016/j.joca.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Spitzer A.I., Bockow B.I., Brander V.A. Hylan G-F 20 improves hip osteoarthritis: a prospective, randomized study. Physiother Sport. 2010 doi: 10.3810/psm.2010.06.1781. [DOI] [PubMed] [Google Scholar]
- 17.Meermans G., Corten K., Simon J.P. Is the infection rate in primary THA increased after steroid injection? Clin Orthop Relat Res. 2012;470:3213–3219. doi: 10.1007/s11999-012-2390-8. Springer New York LLC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Werner B.C., Cancienne J.M., Browne J.A. The timing of total hip arthroplasty after intraarticular hip injection affects postoperative infection risk. J Arthroplasty. 2016;31:820–823. doi: 10.1016/j.arth.2015.08.032. [DOI] [PubMed] [Google Scholar]
- 19.Kaspar S., de V de Beer J. Infection in hip arthroplasty after previous injection of steroid. J Bone Joint Surg Br. 2005;87-B doi: 10.1302/0301-620X.87B4.15546. 454–7. [DOI] [PubMed] [Google Scholar]
- 20.McIntosh A.L., Hanssen A.D., Wenger D.E., Osmon D.R. Recent intraarticular steroid injection may increase infection rates in primary THA. Clin Orthop Relat Res. 2006;451:50–54. doi: 10.1097/01.blo.0000229318.51254.79. Lippincott Williams and Wilkins. [DOI] [PubMed] [Google Scholar]
- 21.Ravi B., Escott B.G., Wasserstein D. Intraarticular hip injection and early revision surgery following total hip arthroplasty: a retrospective cohort study. Arthritis Rheum. 2015;67:162–168. doi: 10.1002/art.38886. [DOI] [PubMed] [Google Scholar]
- 22.van der Weegen W., Wullems J.A., Bos E., Noten H., van Drumpt R.A.M. No difference between intra-articular injection of hyaluronic acid and placebo for mild to moderate knee osteoarthritis: a randomized, controlled, double-blind trial. J Arthroplasty. 2015;30:754–757. doi: 10.1016/j.arth.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Tammachote N., Kanitnate S., Yakumpor T., Panichkul P. Intra-articular, single-shot hylan G-F 20 hyaluronic acid injection compared with corticosteroid in knee osteoarthritis:a double-blind, randomized controlled trial. J Bone Jt Surg - Am. 2016;98:885–892. doi: 10.2106/JBJS.15.00544. [DOI] [PubMed] [Google Scholar]
- 24.Atchia I., Kane D., Reed M.R., Isaacs J.D., Birrell F. Efficacy of a single ultrasound-guided injection for the treatment of hiposteoarthritis. Ann Rheum Dis. 2011 doi: 10.1136/ard.2009.127183. [DOI] [PubMed] [Google Scholar]
- 25.Micu M.C., Bogdan G.D., Fodor D. Steroid injection for hip osteoarthritis: efficacy under ultrasound guidance. Rheumatology. 2010;49:1490–1494. doi: 10.1093/rheumatology/keq030. [DOI] [PubMed] [Google Scholar]
- 26.Lynch T.S., Oshlag B.L., Bottiglieri T.S., Desai N.N. Ultrasound-guided hip injections. J Am Acad Orthop Surg. 2019;27:e451–e461. doi: 10.5435/JAAOS-D-17-00908. [DOI] [PubMed] [Google Scholar]
- 27.Mathews J., Alshameeri Z., Loveday D., Khanduja V. The role of fluoroscopically guided intra-articular hip injections in potential candidates for hip arthroscopy: experience at a UK tertiary referral center over 34 months. Arthrosc J Arthrosc Relat Surg. 2014;30:153–155. doi: 10.1016/j.arthro.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 28.Kullenberg B., Runesson R., Tuvhag R., Olsson C., Resch S. Intraarticular corticosteroid injection: pain relief in osteoarthritis of the hip? J Rheumatol. 2004;31:2265–2268. [PubMed] [Google Scholar]
- 29.Lambert R.G.W., Hutchings E.J., Grace M.G.A., Jhangri G.S., Conner-Spady B., Maksymowych W.P. Steroid injection for osteoarthritis of the hip: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2007;56:2278–2287. doi: 10.1002/art.22739. [DOI] [PubMed] [Google Scholar]
- 30.Sreekumar R., Venkiteswaran R., Raut V. Infection in primary hip arthroplasty after previous steroid infiltration. Int Orthop. 2007;31:125–128. doi: 10.1007/s00264-006-0152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delbarre A., Amor B., Bardoulat I., Tetafort A., Pelletier-Fleury N. Do intra-articular hyaluronic acid injections delay total knee replacement in patients with osteoarthritis – a Cox model analysis. PloS One. 2017;12 doi: 10.1371/journal.pone.0187227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chitre A.R., Fehily M.J., Bamford D.J. Total hip replacement after intra-articular injection of local anaesthetic and steroid. J Bone Jt Surg - Ser B. 2007;89:166–168. doi: 10.1302/0301-620X.89B2.18428. [DOI] [PubMed] [Google Scholar]
- 33.Sankar B., Seneviratne S., Radha S., Rajeev A., Banaszkiewicz P. Safety of total hip replacement following an intra-articular steroid hip injection--an audit. Acta Orthop Belg. 2012;78:183–186. [PubMed] [Google Scholar]
- 34.Hess S.R., O’Connell R.S., Bednarz C.P., Waligora A.C., Golladay G.J., Jiranek W.A. Association of rapidly destructive osteoarthritis of the hip with intra-articular steroid injections. Arthroplast Today. 2018;4:205–209. doi: 10.1016/j.artd.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dabare C., Le Marshall K., Leung A., Page C.J., Choong P.F., Lim K.K. Differences in presentation, progression and rates of arthroplasty between hip and knee osteoarthritis: observations from an osteoarthritis cohort study-a clear role for conservative management. Int J Rheum Dis. 2017;20:1350–1360. doi: 10.1111/1756-185X.13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reidy M., Cousins G., Finlayson D. Corticosteroid injection of the arthritic hip: what is the indication? Scot Med J. 2015;60:29–31. doi: 10.1177/0036933014563237. [DOI] [PubMed] [Google Scholar]
- 37.McCabe P.S., Maricar N., Parkes M.J., Felson D.T., O’Neill T.W. The efficacy of intra-articular steroids in hip osteoarthritis: a systematic review. Osteoarthritis Cartilage. 2016;24:1509–1517. doi: 10.1016/j.joca.2016.04.018. [DOI] [PubMed] [Google Scholar]
- 38.Berg P., Olsson U. Intra-articular injection of non-animal stabilised hyaluronic acid (NASHA) for osteoarthritis of the hip: a pilot study. Clin Exp Rheumatol. 2004 [PubMed] [Google Scholar]
- 39.Treatment of Osteoarthritis of the Knee, 2 Nd Edition SUMMARY of RECOMMENDATIONS. n.D.
- 40.Guyatt G.H., Thorlund K., Oxman A.D. GRADE guidelines: 13. Preparing Summary of Findings tables and evidence profiles - continuous outcomes. J Clin Epidemiol. 2013;66:173–183. doi: 10.1016/j.jclinepi.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Migliore A., Tormenta S., Laganà B. Safety of intra-articular hip injection of hyaluronic acid products by ultrasound guidance: an open study from ANTIAGE register. Eur Rev Med Pharmacol Sci. 2013 [PubMed] [Google Scholar]
- 42.Charalambous C.P., Prodromidis A.D., Kwaees T.A. Do intra-articular steroid injections increase infection rates in subsequent arthroplasty? A systematic review and meta-analysis of comparative studies. J Arthroplasty. 2014;29:2175–2180. doi: 10.1016/j.arth.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 43.Migliore A., Tormenta S., Martin Martin L.S. Safety profile of 185 ultrasound-guided intra-articular injections for treatment of rheumatic diseases of the hip. Reumatismo. 2011;56 doi: 10.4081/reumatismo.2004.104. [DOI] [PubMed] [Google Scholar]
- 44.Masonis J.L., Bourne R.B. Surgical approach, abductor function, and total hip arthroplasty dislocation. Clin Orthop Relat Res. 2002:46–53. doi: 10.1097/00003086-200212000-00006. Lippincott Williams and Wilkins. [DOI] [PubMed] [Google Scholar]