Abstract
An integral aspect of innate immunity is the ability to detect foreign molecules of viral origin to initiate antiviral signaling via pattern recognition receptors (PRRs). One such receptor is the RNA helicase retinoic acid inducible gene 1 (RIG-I), which detects and is activated by 5’triphosphate uncapped double stranded RNA (dsRNA) as well as the cytoplasmic viral mimic dsRNA polyI:C. Once activated, RIG-I’s CARD domains oligomerize and initiate downstream signaling via mitochondrial antiviral signaling protein (MAVS), ultimately inducing interferon (IFN) production. Another dsRNA binding protein PACT, originally identified as the cellular protein activator of dsRNA-activated protein kinase (PKR), is known to enhance RIG-I signaling in response to polyI:C treatment, in part by stimulating RIG-I’s ATPase and helicase activities. TAR-RNA-binding protein (TRBP), which is about 45% homologous to PACT, inhibits PKR signaling by binding to PKR as well as by sequestration of its’ activators, dsRNA and PACT. Despite the extensive homology and similar structure of PACT and TRBP, the role of TRBP has not been explored much in RIG-I signaling. This work focuses on the effect of TRBP on RIG-I signaling and IFN production. Our results indicate that TRBP acts as an inhibitor of RIG-I signaling in a PACT- and PKR-independent manner. Surprisingly, this inhibition is independent of TRBP’s post-translational modifications that are important for other signaling functions of TRBP, but TRBP’s dsRNA-binding ability is essential. Our work has major implications on viral susceptibility, disease progression, and antiviral immunity as it demonstrates the regulatory interplay between PACT and TRBP IFN production.
Keywords: RIG-I, interferon, PACT, TRBP, dsRNA, RLRs, virus sensor
Introduction
The ability of the host cell to discriminate between self, nonpathogenic and non-self, pathogenic molecules is a key determinant of the effectiveness of innate immunity [1]. Detection of pathogenic molecules is performed by pattern recognition receptors (PRRs), which specialize in detection of pathogen-associated molecular patterns (PAMPs) or microbe-associated molecular patterns (MAMPs) [2, 3]. After detection of foreign molecular patterns, PRRs initiate signaling pathways associated with innate immunity [1]. RIG-I (retinoic acid inducible gene 1), a cytoplasmic PRR, is activated by long double stranded (ds) RNAs, highly structured RNAs, 5’ mono-, di-, or tri-phosphorylated dsRNAs, polyU/UC rich RNAs, or by 3’ monophosphorylated RNAs [4]. Once bound to a non-self RNA molecule, RIG-I initiates downstream signaling by triggering oligomerization of the caspase activation and recruitment domains or CARDs. Once oligomerized, RIG-I’s CARD domains in turn oligomerize with CARD domains in Mitochondrial Anti-Viral Signaling Protein (MAVS) (also known as IPS-1, VISA, and Cardif) and activate downstream signaling [2, 5]. After MAVS activation, the transcription factors IRF-3 and NF-κB are activated, which induce type 1 interferon (IFN) genes [2, 6]. The induced IFNs then initiate anti-viral signaling responses in both autocrine and paracrine manner [7, 8] by inducing additional gene products [9].
PACT, a dsRNA-binding protein first identified as the endogenous protein activator of dsRNA-activated protein kinase PKR [10], has been reported also to function as an endogenous activator of RIG-I [11]. PACT activates RIG-I even in the absence of dsRNA, but the presence of both PACT and dsRNA further enhances RIG-I activation, to amplify IFN production. PACT was reported to directly interact with RIG-I through RIG-I’s C-terminal regulatory domain (CTD/RD) and increase RIG-I’s ATPase activity [11]. PACT also enhances RIG-I as well as melanoma differentiation-associated gene 5 (MDA5) mediated interferon production via its direct interaction with laboratory of genetics and physiology 2 (LGP2) [12]. The best evidence in support for PACT’s positive actions on RIG-I mediated interferon production is that numerous virally encoded inhibitors of PACT have been studied to date [13–17]. PACT contains two evolutionarily conserved dsRNA-binding motifs (dsRBMs), also found in the proteins PKR and TRBP [10]. These motifs are essential for binding to dsRNAs as well as for the ability to form homo- and heteromeric interactions with other proteins that possess similar dsRBMs [18–20].
TRBP (TAR RNA-binding protein, named for its ability to bind HIV TAR RNA) has been implicated in a multitude of signaling pathways. TRBP aids HIV replication in infected cells [21–24], regulates dicer activity during RNA interference (RNAi) in all cells [25–27], and inhibits PKR during cellular stress and viral infections [18, 19, 28–30]. The interest in investigating TRBP’s effect on RIG-I activity stems from the fact that PACT and TRBP are about 45% homologous and have been implicated in the same cellular pathways, performing overlapping functions in some instances [31–34] and exerting opposing effects in other instances [35, 36]. Previous work has shown that TRBP and PACT have opposite effects on PKR signaling during non-viral cellular stresses [18, 19, 37, 38]. This makes it interesting to investigate the effect of TRBP on RIG-I signaling, especially since it functions as a negative regulator of PKR, another major PRR and a viral restriction factor.
In this study, we examined if TRBP has a positive role similar to PACT, an inhibitory role opposite of PACT, or exerts no effect on RIG-I signaling. Our results establish that TRBP inhibits RIG-I signaling and thus uncovers key differences in PACT and TRBP mediated RIG-I regulation. These results demonstrate for the first time, a pathway in addition to the PKR pathway that is involved in innate immunity and is regulated in an opposite manner by these two dsRNA-binding proteins.
Experimental Procedures
Reagents and Cell lines
HEK293Ts, PACT −/− MEFs (gift from Dr. Ganes Sen), and PKR −/− MEFs (gift from Bryan Williams) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum and antibiotics penicillin/streptomycin. Low molecular weight (LMW) Polyinosinic-polycytidylic (PolyI:C, 0.2–1 kb) and 5’ppp dsRNA (short dsRNA with 5’ triphosphate, 19-mer) were purchased from Invivogen. The expression plasmid flag-RIG-I/pEFbos+ [39] was purchased from Addgene (plasmid #52877). IFN-β–luc [40], was a kind gift from Dr. Richard Randall and pEGFP-C1-hIRF3 [41], a kind gift from Dr. Chris Basler.
Generation of PACT and TRBP mutants
All TRBP and PACT AA and DD point mutations were generated using mutagenic primers for PCR amplification to change specific codons. The primer sequences were as follows:
wt TRBP FOR:
5’GCTCTAGACATATGGAAATGCTGGCCGCCAACCCAGGC 3’
wt TRBP REV:
5’GGATCCTCACTTGCTGCCTGCCATGC 3’
K59A TRBP REV:
5’GACTGCTCCCCCCTTTGAGGTGTTTGAGGGCCACCTCAGCTGCCTTGTGCTTGGCTGCCTTCGCGCTGGGGCCCTGC 3’
K189A TRBP FOR:
5’CGAATTCACCATGACCTGTCGAGTGGAGCGTTTCATTGAGATTGGGAGTGGCACTTCCGCAAAATTGGCAAAGC 3’
S131A TRBP FOR:
5’CGCCATGGAACTGCAGCCCCCTGTCGCCCCTCAGC 3’
S121D TRBP REV:
5’GTTCCATGGCGGGGTCCCTGGTTAGGACTACAGATGGAACTGGGG 3’
S121A TRBP REV:
5’GTTCCATGGCGGGGGCCCTGGTTAGGACTACAGATGGAACTGGGG 3’
S131D TRBP FOR:
5’CGCCATGGAACTGCAGCCCCCTGTCGACCCTCAGC 3’
S262D, S265D TRBP REV:
5’CGGAGCTCACTGAGGACACGGCAGCAGGCAGGGCCCAGGGCACCCAGGTCGCCCAGGTCGCAACTG 3’
S262A, S265A TRBP REV:
5’CGGAGCTCACTGAGGACACGGCAGCAGGCAGGGCCCAGGGCACCCAGGGCGCCCAGGGCGACCCTG 3’
AA PACT and DD PACT were cloned as described previously [19]. Briefly, the PCR products were sub-cloned into pGEMT-Easy vector (Promega). Once the sequence and correct mutation was verified, we generated full length mutant ORF in pBSIIKS+ by a three-piece ligation with the remaining wt sequence (TRBP phosphorylation mutants made from two mutant products and one wt product ligated into pBSIIKS+). Full length sequences are amino-terminally flag-tagged. PACT dsRNA binding mutants K84A and K189A were described before [20] and were generated using GeneEditor in vitro Site-Directed Mutagenesis System from Promega using mutagenic primers.
ATPase activity assay
HEK293T cells were transfected with flag-RIG-I/pEFbos+ for 24–48hrs. Cells were harvested, washed twice with 1X PBS, then lysed in 100μl lysis buffer (20mM Tris-HCl pH 7.5, 150mM NaCl, 1mM EDTA, 1mM DTT, 20% glycerol, 1% Triton-X, 0.2mM PMSF, 100u/mL aprotonin, 1:100 phosphatase inhibitor cocktail [Sigma]), lysate was then transferred to flag-conjugated agarose beads. After 2 hours of IP, lysate was washed three times with lysis buffer, then eluted with 3X flag peptide (Sigma). 20μl of eluted flag-RIG-I was then mixed with polyI:C, recombinant PACT, or recombinant TRBP as indicated. After 5 minutes incubation, mixtures were further incubated in Activity Buffer (500μM ATP, 8.3ηM [γ−32p] ATP, 50mM Tris-acetate (pH 6.0), 5mM dithiothreitol (DTT), and 1.5mM MgCl2) and reaction incubated at 37°C for 30 minutes. 10% of total reaction was then spotted onto TLC PEI Cellulose F plates (Millipore) and resolved in a buffer containing 1M formic acid and 0.5M LiCl. Resulting TLC plate was then scanned using a phosphorimager (Typhoon FLA7000).
Recombinant Proteins
The protein coding regions (PACT or TRBP) were sub-cloned into pET15b (Novagen) to generate in-frame fusion of PACT/TRBP ORF to the histidine tag. The recombinant proteins were expressed and purified as described [10, 19]. It is important to note that both hexahistidine-tagged TRBP and PACT were purified under denaturing conditions with 6M Urea during affinity purification on Ni-agarose beads followed by a stepwise renaturation after purification, thus eliminating the presence of carry-over RNA.
Transfections
Transfections of reporters, proteins, and polyI:C was done using Effectene (Qiagen) transfection reagent. 5’ppp dsRNA was transfected using 5’ppp dsRNA-lyovec purchased from Invivogen. dsRNA treatment was for 16 hours overnight, starting at 24 hours after reporter and protein vectors were transfected.
Luciferase assays
Luciferase activity was determined using the Dual-Luciferase Reporter Assay System from Promega. Luciferase readings were normalized to Renilla expressed from pRL null. For all luciferase experiments, 200ng of IFN-β–luc [40],1ng of pRL-null, 50ng of RIG-I/pEFbos+, 50ng of PACT/pcDNA3.1-, and 50ng of TRBP/pcDNA3.1- (unless otherwise stated) were transfected into HEK293Ts, PACT −/− MEFs, and PKR −/− MEFs. TRBP and PACT concentrations are listed for each experiment. HEK293T cells were harvested 16 hours after dsRNA treatment (50ng of polyI:C and 1μg/ml 5’ppp dsRNA were used for indicated experiments. Each sample was washed twice with 1X PBS then lysed in 200μl of 1X Passive Lysis Buffer for 5 min. Lysates were spun at 13.2 k for 5 minutes and 15μl of each lysate was transferred to a new tube. 25μl of both Luciferase and Stop and Glo-Renilla reagents (Promega) were used for readings. Enzymatic activity was measured using a luminometer. Each test was done in triplicate and repeated 2 times. For each test, firefly luciferase numbers were first normalized to Renilla luciferase numbers to normalize for transfection efficiency. Then, RIG-I + dsRNA stimulation was set as 100% and all samples within that set were compared to that.
dsRNA-binding assays
The in vitro translated, 35S-labeled PACT and TRBP proteins were synthesized using the TNT-T7 coupled reticulocyte lysate system from Promega and the dsRNA-binding activity was measured by using the previously well-established poly(I)-poly(C)-agarose binding assay [10, 42]. 4 μl of in vitro translation products were diluted with 25 μl of binding buffer (20 mM Tris, pH 7.5, 0.3 M NaCl, 5 mM MgCl2, 1 mM DTT, 0.1 mM PMSF, 0.5% IGEPAL, 10% glycerol) and incubated with 25 μl of poly(I)-poly(C)-agarose beads at 30° C for 30 min. The beads were washed 4 times with 500 μl of binding buffer and the bound proteins were analyzed by SDS-PAGE and fluorography. Several controls were included, which are not shown in the figure. For competition assay with soluble ssRNA or dsRNA, 1 μg of poly(C) or poly(I)-poly(C) was incubated with the proteins for 15 min at 30° C before the addition of poly(I)-poly(C)-agarose beads. To ascertain specific interaction between PACT and TRBP proteins and poly(I)-poly(C)-agarose beads, in vitro translated, 35S-labeled firefly luciferase protein was assayed for binding to the poly(I)- poly(C)-agarose beads using same conditions and no binding was detected. The poly(I)·poly(C)- agarose binding was quantified on Typhoon FLA7000 by analyzing the band intensities in T and B lanes. The percentage of PACT proteins bound to poly(I)·poly(C)-agarose was calculated from these values (% binding = 100 X band intensity in B lane/band intensity in T lane), and was plotted as bar graphs.
Quantifications
All radioactive TLC and SDS-PAGE gel scans (Typhoon FLA7000) were quantified using GE Life Sciences ImageQuant TL software. Each experiment was normalized to internal controls and repeated 3 times.
IRF3 nuclear translocation assay
To analyze the subcellular localization of IRF-3, HEK293 cells were transfected using Effectene with 0.250 μg of pEGFP-C1-hIRF3 ([41], a kind gift from Dr. Chris Basler), which expresses from a human cytomegalovirus promoter a human IRF-3-GFP fusion protein, and 0.250 μg of either empty vector pcDNA3.1- or TRBP/pcDNA3.1- expression plasmid. Translocation of IRF-3 was induced by treatment of cells with polyI.polyC at 24h post transfection. 2 h after dsRNA stimulation, the cells were fixed with 4% paraformaldehyde and stained with DAPI nuclear stain. Samples were examined under an EVOS fluorescence microscope and the percentage of cells with nuclear GFP-IRF-3 localization was then determined by examining about 300 green fluorescent cells per sample.
Results
TRBP is a robust inhibitor of RIG-I signaling
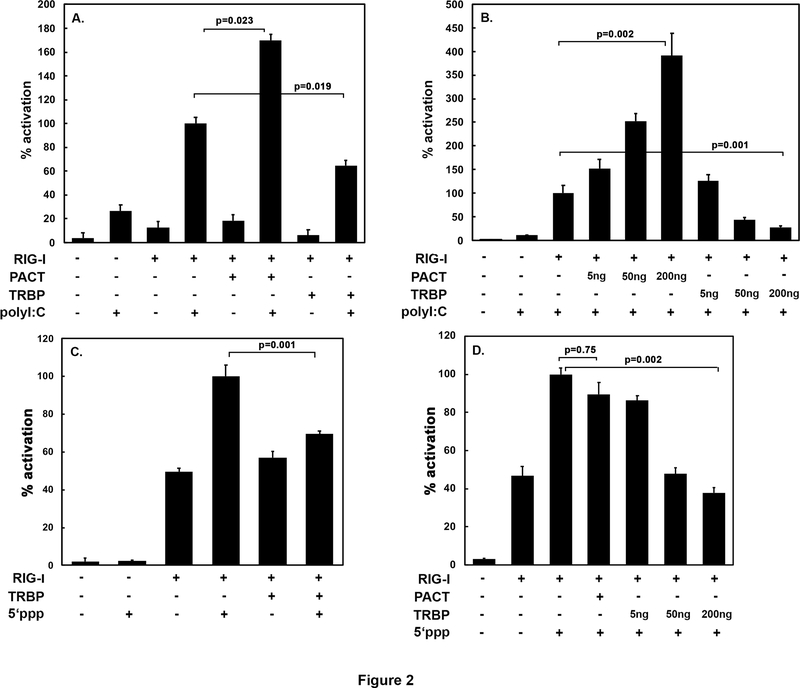
The domain structure of proteins involved in this study is schematically depicted in Figure 1. PACT, TRBP, and PKR possess the characteristic evolutionarily conserved dsRNA-binding motifs (dsRBMs) that also mediate protein-protein interactions [19, 20, 37, 43–47]. The interactions between the proteins that possess dsRBMs regulates many signaling pathways and biological processes [48–50]. RIG-I has two copies of CARD (caspase activation and recruitment domains) near the amino terminus, a central helicase domain, and a carboxy terminal regulatory domain [11]. In earlier studies PACT was shown to interact directly with RIG-I via the CTD and augment its ATPase activity. PACT is an established activator of RIG-I signaling that culminates in IFN-β induction at transcriptional level [11], and as PACT and TRBP have opposite effects on PKR signaling [35], we examined the effect of TRBP on RIG-I induced activation of IFN-β production in HEK293T cells. We used a reporter construct plasmid in which firefly luciferase expression was controlled by IFN-β promoter. Various RIG-I, PACT and TRBP expression constructs were tested for their effect on inducing transcription from IFN-β promoter in response to viral mimics polyI:C or 5’ ppp-dsRNA. As seen in Figure 2 A, PACT augments RIG-I mediated IFN-β production in response to polyI:C in agreement with what has been previously documented [11]. In contrast to this, TRBP inhibits RIG-I mediated IFN-β production in response to polyI:C. It is also interesting that TRBP inhibits RIG-I signaling even in the absence of polyI:C. Previously, LGP2 was reported to act as an inhibitor of MDA5 mediated IFN-β production at high concentrations but act as an activator at lower concentrations [51]. In order to examine any possible concentration-dependent effects of TRBP on RIG-I signaling, a dose response curve was performed (Figure 2 B). The results exhibit a dose-dependent increase in IFN-β production with PACT, and a dose-dependent decrease in IFN-β production with TRBP. It has been shown previously that PACT can enhance RIG-I signaling in the absence of any RNA activator as well as in response to polyI:C, but shows no additional enhancement in response to RIG-I’s best-characterized activator, 5’ppp-dsRNA [11]. We wanted to examine TRBP’s effect on RIG-I signaling in the presence of 5’ppp-dsRNA. As seen in Figure 2 C, TRBP does inhibit RIG-I signaling in response to 5’ppp-dsRNA, and indicates inhibition of RIG-I signaling by TRBP regardless of the activator. A dose response curve with increasing concentrations of TRBP in the presence of 5’ppp-dsRNA shows a concentration-dependent inhibition of RIG-I signaling (Figure 2 D). These results establish that TRBP inhibits RIG-I mediated IFN-β production in response to various RIG-I ligands. In agreement with previous reports, PACT showed no effect on 5’ ppp-dsRNA mediated RIG-I signaling.
Figure 1: Domain structure of PKR, PACT, TRBP, and RIG-I.
M1 and M2: the evolutionarily conserved dsRNA-binding motifs (dsRBMs) present in PKR, PACT, and TRBP also mediate protein-protein interactions. PBM: PACT Binding Motif present in PKR. M3 domain of PACT is essential for PKR activation. M3 (aka medipal domain) of TRBP mediates TRBP’s interactions with Merlin, Dicer, and PACT. Blue Arrows indicate known sites of phosphorylation on each protein. CARD: Caspase activation and recruitment domain, site of oligomerization with CARD domains in other proteins. DExD/H Helicase: helicase domain in RIG-I with inherent ATPase activity. CTD/RD: C-terminal domain and regulatory domain of RIG-I that also is an interaction site of PACT. I-VI: conserved helicase motifs in RIG-I.
Figure 2: TRBP inhibits RIG-I mediated IFN production in response to low molecular weight (LMW) polyI:C and 5’ppp dsRNA. (A and B) IFN-β induction after polyI:C treatment.
HEK293Ts were transfected with 200 ng IFN-β-Luc reporter, 50 ng RIG-I expression construct, and either 50 ng of PACT or TRBP (for A) or 5 ng, 50 ng, or 200 ng (for B) expression construct as indicated, then treated with 50 ng/ml polyI:C 24 h after transfection. 1 ng of pRLnull plasmid was co-transfected for normalization of transfections. Cell extracts were assayed for dual luciferase activity 8 h after polyI:C treatment. The p-values based on 3 independent experiments with each sample in triplicates are shown. (C) IFN-β induction after 5’ppp dsRNA treatment. HEK293Ts were transfected with 200 ng IFN-β-Luc reporter, 50 ng RIG-I expression construct, and 50 ng of TRBP expression constructs as indicated, then treated with 1 μg/ml 5’ppp dsRNA 24 h post-transfection. I ng of pRLnull plasmid was co-transfected for normalization of transfections. Cell extracts were assayed for dual luciferase activity 8 h after 5’ppp dsRNA treatment. The p-values based on 3 independent experiments with each sample in triplicates are shown. (D) IFN-β induction after 5’ppp dsRNA treatment. HEK293Ts were transfected with 200 ng IFN-β-Luc reporter, 50 ng RIG-I expression construct, and either 50 ng of PACT expression construct or 5 ng, 50 ng, 200 ng of TRBP expression constructs as indicated, then treated with 1 μg/ml 5’ppp dsRNA 24 h post-transfection. 1 ng of pRLnull plasmid was co-transfected for normalization of transfections. Cell extracts were assayed for dual luciferase activity 8 h after 5’ppp dsRNA treatment. The p-values based on 3 independent experiments with each sample in triplicates are shown.
TRBP-Mediated inhibition of RIG-I is independent of PACT as well as PKR
Previous work has shown that TRBP interacts directly with both PACT as well as PKR to inhibit PKR’s kinase activity [36]. Since TRBP, PACT, and PKR interact with each other to influence PKR activation and signaling during cellular stress and viral infections [19, 20, 30, 37], we next examined if TRBP’s inhibition of RIG-I signaling was mediated via its inhibitory actions on PACT or PKR. In addition, PKR has been shown to enhance IFN production during viral infections [52, 53], thus making it plausible that TRBP may act via inhibition of PKR either directly or by interaction with PACT. To determine if TRBP could inhibit RIG-I in the absence of PACT, we performed experiments similar to the ones shown in Figure 2 in PACT −/− MEFs. As seen in Figure 3 A, in the absence of PACT, TRBP is still able to inhibit RIG-I signaling in untreated as well as polyI:C treated cells. Similarly, the role of PKR in TRBP mediated inhibition of RIG-I signaling was addressed by using PKR −/−MEFs. As seen in Figure 3 B, TRBP is able to inhibit RIG-I signaling in response to polyI:C treatment in the absence of PKR. These results indicate that TRBP-mediated inhibition of RIG-I is independent of PKR and PACT and TRBP directly inhibits RIG-I induced signaling pathways.
Figure 3: TRBP-mediated inhibition of RIG-I does not require PACT or PKR. A. PACT is not required for TRBP inhibition of RIG-I signaling.
PACT −/− MEFs were transfected with 200 ng IFN-β-Luc reporter, 50 ng RIG-I expression construct, and 50 ng of either PACT or TRBP expression constructs as indicated, then treated with 50 ng/ml polyI:C 24 h post-transfection. 1 ng of pRLnull plasmid was co-transfected for normalization of transfections. Cell extracts were assayed 8 h after treatments for dual luciferase activity. The p-values based on 2 independent experiments with each sample in triplicates are shown. B. PKR is not required for TRBP inhibition of RIG-I signaling. PKR −/− MEFs were transfected with 200 ng IFN-β-Luc reporter, 50 ng RIG-I expression construct, and 50 ng of either PACT or TRBP expression constructs as indicated, then treated with 50 ng/ml polyI:C 24 h post-transfection. 1 ng of pRLnull plasmid was co-transfected for normalization of transfections. Cell extracts were assayed 8 h after treatments for dual luciferase activity. The p-values based on 2 independent experiments with each sample in triplicates are shown.
PACT as well as TRBP’s phosphorylation state does not influence RIG-I mediated signaling
Phosphorylation of PACT and TRBP in response to cellular stress influences their interactions with various binding partners including PKR and result in significant changes in signaling outcomes [19, 30, 37, 38, 54–56]. Thus, it is of interest to test if PACT and TRBP’s phosphorylation status changes their effects on RIG-I mediated signaling. PACT has two known phosphorylation sites that are involved in signaling in response to oxidative and endoplasmic reticulum (ER) stress; serine 246 (constitutive) and serine 287 (stress induced) [19, 37, 54]. To address the effect of PACT phosphorylation on RIG-I signaling, both serine 246 and 287 were mutated either to alanine to mimic unphosphorylated PACT (inactive state for PKR signaling) or to aspartic acid to mimic phosphorylated PACT (active state for PKR signaling) (Figure 4 A). As seen in Figure 4 B, neither the phospho-defective AA, or phospho-mimic DD mutants of PACT showed any difference in the ability to enhance RIG-I signaling in comparison to wt PACT. These results indicate that the sites that are known to be phosphorylated during oxidative and endoplasmic reticulum stress have no influence in regulation of PACT mediated enhancement of RIG-I signaling.
Figure 4: Phosphorylation status of PACT and TRBP does not influence RIG-I mediated signaling. A. Schematic of phosphorylation sites on PACT and TRBP.
Residues previously determined to be phosphorylated (S246 and S287 in PACT and S142, S152, S283, and S286 in TRBP) and established to be important for PKR signaling were mutated from serine to alanine (phospho-defective) or aspartic acid (phospho-mimic). B. Phospho-defective or phospho-mimic PACT mutations have no effect on RIG-I signaling. HEK293Ts were transfected with 200 ng IFN-β-Luc reporter, 50 ng RIG-I expression construct, and 50 ng of expression constructs for either wt PACT or phosphorylation mutants AA or DD as indicated, then treated with 50 ng/ml polyI:C 24 h post-transfection. 1 ng of pRLnull plasmid was co-transfected for normalization of transfections. Cell extracts were assayed 8 h after treatments for dual luciferase activity. The p-values based on 3 independent experiments with each sample in triplicates are shown. C. Phospho-defective or phospho-mimic TRBP mutations have no effect on RIG-I signaling. HEK293Ts were transfected with 200 ng IFN-β-Luc reporter, 50 ng RIG-I expression construct, and 50 ng of expression constructs of wt TRBP or phosphorylation mutants 4A or 4D of TRBP as indicated, then treated with 50 ng/ml polyI:C 24 h post-transfection. 1 ng of pRLnull plasmid was co-transfected for normalization of transfections. Cell extracts were assayed for dual luciferase activity 8 h after treatments. The p-values based on 3 independent experiments with each sample in triplicates are shown.
Similarly, phosphorylation of TRBP has been linked to changes in its ability to inhibit PKR during metabolic stress as well as to stabilize miRNA-generating complexes [30, 38, 55]. Phosphorylated TRBP interacts with PKR more efficiently and this higher affinity interaction is crucial for a timely inhibition of PKR activity at late time points after oxidative stress for a protecting the cells from apoptosis [30]. Four phosphorylation sites in TRBP (serines 142, 152, 283, and 286) are targets of mitogen-activated protein kinase (MAPK) Erk and JNK, and are also of functional significance during RNAi and metabolic stress and changes in phosphorylation state of TRBP can also alter TRBP’s stability in cells [38, 55]. These findings underscore the importance of phosphorylation in determining TRBP’s activity and suggest a possible role of TRBP phosphorylation in its ability to inhibit RIG-I signaling. In order to test the role of TRBP phosphorylation on RIG-I signaling, we mutated known all known phosphorylated serines to alanines to mimic unphosphorylated state (4A) or aspartic acids to mimic a phosphorylated state (4D) (Figure 4 A). In Figure 4 C, a comparison of the ability of wt TRBP, 4A, and 4D to inhibit RIG-I signaling is shown. Neither mutant 4A nor mutant 4D exhibited a significantly different inhibitory activity towards RIG-I signaling. This indicates that while TRBP phosphorylation status has been shown to play a crucial role during PKR signaling and RNAi, it appears to have no significant effect on the ability of TRBP to inhibit RIG-I signaling. This demonstrates that TRBP activity may be regulated differently in response to various stress signals, a feature it shares with PACT.
TRBP’s but not PACT’s dsRNA-binding ability influences RIG-I signaling
Being a cytoplasmic PRR that detects non-self dsRNA in cells to initiate anti-viral signaling, RIG-I’s dsRNA-binding activity is critical for its ability to identify non-self RNA molecules [3, 57]. Both PACT and TRBP have two conserved dsRBMs that can bind to dsRNA [22, 33, 36, 58, 59], and thus they could potentially affect RIG-I activity via their ability to sequester such RNAs. PACT and TRBP have opposite effects on PKR activity [35], another non-traditional PRR for dsRNA, and TRBP sequesters dsRNA to keep it from activating PKR during HIV infection [23, 60]. This leads to the question if PACT or TRBP’s ability to bind dsRNA could affect its ability to activate or inhibit RIG-I signaling, as PACT activation of RIG-I signaling could be via dsRNA recruitment and TRBP inhibition through sequestration of dsRNA away from RIG-I. To test this, known PACT mutants that are defective in dsRNA-binding [20] and TRBP mutants that would be defective in dsRNA-binding based on the knowledge of similar mutations in conserved domains of PKR and data from study of TRBP’s triple point mutants [59, 61] were tested (Figure 5 A). The dsRNA-binding activities of these point mutants was tested and compared to wt PACT and wt TRBP dsRNA-binding ability to measure the effect of point mutations (Figure 5 B and C). Both single mutations in PACT as well as TRBP showed significantly less dsRNA-binding as compared to wt proteins (Figure 5 B and C). After verification of a reduction in dsRNA-binding activities, both PACT and TRBP mutants’ ability to alter RIG-I signaling was assessed (Figure 5 D and E). Surprisingly, mutants of PACT lacking the ability to bind dsRNA were still able to activate RIG-I similar to wt PACT (Figure 5 D). This indicates that dsRNA-binding of PACT is not required for its ability to enhance RIG-I signaling. Single point mutations in TRBP’s dsRNA-binding domains were not as effective at abolishing dsRNA-binding as those in PACT, but were able to reduce dsRNA-binding by about 45% (Figure 5 B and C). Though dsRNA-binding was not completely compromised, a decrease in ability to inhibit RIG-I signaling was quite evident (Figure 5 E). This indicates a potential role of TRBP of in dsRNA sequestration away from RIG-I, thereby blocking RIG-I’s activation by dsRNA.
Figure 5: dsRNA binding ability of TRBP but not that of PACT influences RIG-I induced IFN production. A. Schematic of dsRNA-binding mutations in M1 and M2 domains of PACT and TRBP.
Residues mutated from lysine to alanine based on previously established research on residues important for dsRNA-binding. B. dsRNA-binding assay. dsRNA-binding ability of wt and mutant PACT and TRBP was measured by polyI:C-agarose binding assay with in vitro translated 35S-labeled proteins. T, total input; B, protein bound to polyI:C agarose. C. Quantification of data in 5 B. Radioactivity in both total input and bound protein bands was quantified, and % bound was calculated by (bound protein/total input) *100. The error bars represent standard deviation calculated from 3 experiments. The p-values are as indicated. D. dsRNA-binding ability of PACT does not affect RIG-I signaling. HEK293Ts were transfected with IFN-β-Luc reporter, RIG-I expression construct, and 50ng of expression construct for either wt PACT or dsRNA-binding mutants K84A or K177A as indicated, then treated with polyI:C 24 h after transfection. pRLnull plasmid was co-transfected for normalization of transfections. Cell extracts were assayed for dual luciferase activity 8 h after treatments. The p-values calculated from 2 independent experiments with each sample in triplicates are indicated. E. dsRNA-binding ability of TRBP is essential for inhibition of RIG-I signaling. HEK293Ts were transfected with IFN-β-Luc reporter, RIG-I expression construct, and 50ng of expression construct of either wt TRBP or dsRNA-binding mutants K59A or K189A as indicated, then treated with polyI:C 24 h after transfection. pRLnull plasmid was co-transfected for normalization of transfections. Cell extracts were assayed for dual luciferase activity 8 h after treatments. The p-values calculated from 2 independent experiments with each sample in triplicates are indicated.
Neither PACT nor TRBP affect RIG-I’s ATPase activity in the presence of a dsRNA activator
To investigate TRBP’s effect on RIG-I’s ATPase activity, an in vitro ATPase activity assay was performed with purified RIG-I in the presence of polyI:C and either recombinant PACT or TRBP (Figure 6). First, a polyI:C dose response curve was performed to identify the lowest concentration of polyI:C necessary to activate RIG-I and to allow for an increase or decrease in ATPase activity to be visualized (data not shown). We determined that both 1 ng and 10 ng of polyI:C showed good activation of immunoprecipitated RIG-I at sufficient levels, and an additional activation by increasing amounts of polyI:C could still be seen at both these concentrations so we judged that inhibition by TRBP could also be visualized at 10 ng amount of polyI:C. Using 10 ng of polyI:C as activator, we tested the effect of either recombinant PACT or TRBP for changes in ATPase activity. As seen in Figure 6 A and B, neither PACT nor TRBP affected RIG-I’s ATPase activity. To ensure that this lack of effect was not a result of using recombinant proteins produced in bacteria, this experiment was repeated with PACT and TRBP immunoprecipitated from mammalian cells and identical results were seen (data not shown). These results indicate that TRBP inhibits RIG-I signaling without affecting RIG-I’s ATPase activity.
Figure 6: Neither PACT nor TRBP affect RIG-I’s ATPase activity in the presence of a dsRNA. A. Effect of PACT and TRBP on RIG-I’s ATPase activity.
RIG-I immunoprecipitated from HEK293T cells was activated by incubation with 10 ng polyI:C, and varying amounts of recombinant PACT or TRBP were added as indicated to measure changes in ATPase activity as determined by ATP hydrolysis. Both TRBP and PACT were purified under denaturing conditions with 6M Urea during affinity purification on Ni-agarose beads and renatured after purification, thus eliminating any carry-over RNA. Their ability to interact with PKR and affect its kinase activity was also confirmed to ensure that the proteins retained their biological activities. B. Quantification of data in 6 A. Radioactivity in the free phosphate and ATP spots was quantified, and samples were first normalized by dividing free phosphate by total (free phosphate plus ATP). To calculate relative fold activation, these normalized numbers were compared to no polyI:C control values, which were as considered as 1. The experiment was repeated 4 times and the p-values are as represented.
TRBP blocks nuclear translocation of IRF-3 after dsRNA stimulation
In order to further investigate the mechanism by which TRBP inhibits RIG-I induced IFN production, we studied if the activation of transcription factor IFN regulatory factor 3 (IRF-3), which is essential for IFN production [62], is inhibited by TRBP. IRF-3 is a constitutively expressed transcription factor and in the absence of any stimulatory signal, the inactive IRF-3 shuttles between the nucleus and cytoplasm and is predominantly cytoplasmic. However, following its activation by phosphorylation, IRF-3 dimerizes and accumulates in the nucleus, where it functions as a transcription factor [63–65] for inducing IFN-β gene transcription. The carboxy-terminal serine/threonine phosphorylation of IRF-3 promotes its dimerization, the ability to bind DNA in a sequence-specific manner, and its interaction with the histone acetyltransferases CBP and p300 [63, 65, 66].
Thus, we tested the effect of TRBP on the nuclear accumulation of IRF-3 by using GFP-IRF-3 fusion protein, which has been used previously to study the nuclear accumulation of IRF-3 [63, 64, 67, 68]. HEK293 cells were co-transfected with GFP-IRF-3 expression plasmid and either the empty vector (pcDNA3.1-) or a TRBP expression plasmid (flag-TRBP/pcDNA3.1-). As seen in Fig. 7, in the absence of dsRNA stimulation, GFP-IRF3 is localized predominantly in the cytoplasm of most cells (panels a-c and g-i). Upon stimulation with dsRNA, GFP-IRF3 is translocated to the nucleus in most cells (panels d-f) that have unaltered endogenous levels of TRBP. In contrast to this, a majority of the cells overexpressing TRBP (panels j-l) showed exclusively cytoplasmic GFP-IRF-3 localization following stimulation with dsRNA. In order to quantify the inhibition of IRF-3 nuclear translocation by TRBP, we counted the percentage of GFP positive cells showing nuclear translocation. As seen in Fig 7 B, about 3% of cells show nuclear localized GFP-IRF3 without dsRNA stimulation both in the absence and presence of TRBP overexpression. Upon dsRNA stimulation, in the absence of TRBP overexpression, about 97% of cells show nuclear localization of GFP-IRF3 whereas only 23% of TRBP overexpressing cells show GFP-IRF3 nuclear localization. This demonstrates that TRBP inhibits nuclear translocation of IRF3 in response to dsRNA and likely explains the observed inhibition of IFN-β induction by TRBP.
Figure 7: TRBP inhibits 5’ppp-dsRNA induced IRF3 nuclear translocation. A. Fluorescence microscopy.
HEK293T cells were plated on cover slips and were co-transfected with 100 ng of pEGFPC1-IRF3 and 400 ng of pcDNA 3.1- or 250 ng of Flag-TRBP/pcDNA 3.1- + 150 ng pcDNA 3.1-. IRF3 activity in the transfected cells was either left unstimulated or stimulated with 5’ppp dsRNA 24 hours post-transfection. 2 hours after the addition of 5’ppp dsRNA, the cells were fixed and mounted in Vectashield® Mounting Media with DAPI nuclear stain. Representative fluorescent micrographs are shown, panels a-c: GFP-IRF3 + EV, unstimulated control; panels d-f: GFP-IRF3 + EV stimulated with 5’ppp-dsRNA; panels g-i: GFP-IRF3 +TRBP, unstimulated control, and panels j-l: GFP-IRF3 +TRBP, stimulated with 5’ppp-dsRNA. B. Quantification of data in 7 A. About 300 fluorescent cells in the samples were counted to classify the IRF3 as nuclear or cytoplasmic localized and the percentage of cells with nuclear localization of IRF3 was calculated as (number of cells with fluorescent nuclei/total fluorescent cells *100). The experiment was repeated 2 times and the p-values are as represented.
Discussion
In this study, we investigated the effect of the dsRNA-binding protein TRBP on RIG-I induced IFN production in response to dsRNA. Our results establish that TRBP inhibits RIG-I signaling in response to both polyI:C and 5’ppp dsRNA viral mimics. TRBP’s actions on RIG-I are opposite to the actions of PACT, which enhances RIG-I signaling in response to polyI:C but not in response to 5’ppp dsRNA [11]. TRBP inhibition of RIG-I is independent of PACT or PKR, indicating that TRBP affects RIG-I pathway directly and not by inhibiting PACT from activating RIG-I or via inhibiting PKR activity. Phosphorylation state of neither PACT nor TRBP showed any effect on RIG-I signaling, which is noteworthy as changes in these phosphorylation sites are essential for changes in signaling through PKR [19, 30, 37, 38, 54–56]. This could indicate that these proteins’ activities are constitutive and that post-translational modifications are unable to affect their activities, or that previously unidentified phosphorylation sites or other post-translation modifications may be necessary to change PACT and TRBP activity in response to RNA signals. No additional phosphorylation sites or additional post-translational modifications in TRBP are currently known. However, it is interesting that FTSJ3, a cellular 2’-O-methylatransferase, interacts with TRBP during HIV infection and modifies the viral RNA to reduce its ability to induce IFN induction, thus helping the virus to evade innate immune recognition [69]. It remains unexplored if similar mechanisms could operate on RNAs other than HIV-1 RNA through TRBP to downregulate IFN induction and can be examined in future.
Interestingly, dsRNA-binding ability of PACT was unimportant for activation of RIG-I signaling during polyI:C treatment, but TRBP’s dsRNA-binding activity seems essential for RIG-I inhibition at least in part. Inhibition of signaling via sequestration of dsRNA by TRBP has been previously observed for PKR signaling during viral infections and also for RNAi pathways [24, 70]. Since the dsRNA-binding mutants of TRBP we tested were not able to eliminate dsRNA-binding completely, it is yet to be determined if dsRNA sequestration can account for all of TRBP’s inhibitory capacity. Based on a study by Yamashita et al., a mutant carrying three substitution mutations in each of the two dsRBMs lost its ability to bind short interfering RNAs (SiRNAs) [59]. Binding to longer dsRNAs was not tested in this study, but it would be informative to test its effect on RIG-I signaling if it is defective in binding to long dsRNAs. While it may be surprising that dsRNA-binding is not necessary for PACT’s enhancement of RIG-I dependent signaling, it has been shown previously that LGP2 which acts as an endogenous activator of MDA5 also does not have a requirement for dsRNA-binding for its activity [39]. This suggests an alternative form of RIG-I activation by PACT independent of its dsRNA binding. It has been demonstrated previously that PACT forms homodimers readily within stressed cells, and this has been shown to aid in PKR activation [37, 56, 71]. During viral stress, PACT oligomerization may aid in RIG-I’s CARD domain oligomerization. RIG-I CARD domain oligomerization has been shown to be either induced by ubiquitination induced oligomerization or through a ubiquitin independent, filament mediated oligomerization [5, 72]. Previously, this RIG-I CARD filament has been shown to be induced near dsRNA ends, implicating proximity-induced oligomerization and this proximity-induced oligomerization could be recapitulated through an artificial fusion protein, which could enhance anti-viral signaling [5, 72]. This leads to the possibility that multimers of PACT may localize RIG-I CARD domains within close enough proximity to induce oligomerization. It was previously reported that PACT and TRBP exhibit different preferences and affinities for binding to their interacting partners; PACT preferred to form homodimers over binding to dsRNA while TRBP preferred to bind to dsRNA over forming homodimers [73]. Thus, TRBP by being predominantly monomeric could hinder RIG-I oligomerization and PACT may enhance it, but extensive further studies would be required to test this possibility. A dramatic increase in homodimer formation of PACT during non-viral cellular stress has been demonstrated to be heavily influenced by phosphorylation of PACT at serines 246 and 287 [19, 37, 54, 56], which we have demonstrated here is unable to affect RIG-I signaling. This raises an interesting possibility that additional sites of phosphorylation or other modifications on PACT in response to dsRNA could trigger such oligomerizations. RAX, a murine homolog of PACT is phosphorylated on serine 18 in response to serum deprivation, and is essential for PKR activation and apoptosis [74]. Because the region containing serine 18 is identical for RAX and PACT, it is a likely phosphorylation site in PACT that can be examined in future for its effect in IFN induction.
We also demonstrated that TRBP does not inhibit RIG-I’s ATPase activity in the presence of polyI:C activator in vitro. This distinguishes TRBP’s mode of action on RIG-I signaling pathway compared to that of PACT, as it was previously reported that PACT mediated enhancement of RIG-I signaling occurred mainly via PACT directly binding to RIG-I and stimulating RIG-I’s ATPase activity in the absence of a dsRNA [11]. In our experiments we did not observe any enhancement of RIG-I’s ATPase activity by PACT, which could possibly be attributed to differences in the use of recombinant RIG-I and immunoprecipitated RIG-I. Our data was obtained using RIG-I immunoprecipitated from mammalian cells whereas Kok et al. used recombinant RIG-I in their studies [11]. It is noteworthy that TRBP inhibits nuclear localization of IRF3 without directly inhibiting RIG-I’s ATPase activity. Previous work with RIG-I has linked RIG-I’s helicase and ATPase activity with initiation of downstream signaling, though it has been shown that ATPase activity alone is not sufficient for initiation of signaling [39]. As TRBP inhibits IRF3 nuclear localization in the absence of a direct effect on the ATPase activity of RIG-I, it most likely inhibits a step downstream of RIG-I oligomerization. A schematic model is presented in Figure 8, which depicts the known activities of PACT and dsRNA in activating RIG-I signaling and the additional novel aspect of RIG-I regulation by TRBP partially through dsRNA sequestration. Tight regulation of innate immunity is crucial during viral infections and is essential for host survival, while aberrant activation of innate immunity can lead to excessive inflammation and IFN production leading to various pathologies [75]. Anomalous production of IFN leads to a group of disorders termed interferonopathies, highlighting the importance of negative regulation of these pathways when not under a pathogenic threat [75]. Various types of Interferonopathies have been linked with mutations in RLR and dsRNA binding proteins, which lead to aberrant IFN production and development of disease [75, 76]. PACT activation and TRBP inhibition of RIG-I signaling may help to maintain the delicate balance of IFN production so the host can mount a rapid anti-viral response, and ascertain that the IFN response is not sustained once the pathogen threat is cleared. Based on our data, a lack of TRBP or mutations in TRBP may also lead to increased steady state levels of IFN, though no such mutations have yet been identified although it is certainly possible that in the absence of virus infection, RIG-I is in a basal inactive state and negative regulation is not essential. It would be informative to study in future if in the absence of TRBP, a virus infection can trigger and excessive or prolonged interferon response in a mouse model [77]. The regulatory interplay between these two dsRNA-binding proteins helps to maintain homeostasis during times of cellular stress and during recovery. Further research can address which step downstream of RIG-I is inhibited by TRBP as well as if TRBP can inhibit other PRRs during viral stress.
Figure 8: Regulation of RIG-I activation by dsRNA-binding proteins PACT and TRBP.
During most viral infections, dsRNA is produced as either a replicative intermediate or a by-product of viral replication. This dsRNA is sensed by RIG-I, which is one of the cytoplasmic PRRs. RIG-I can be activated by both dsRNA and by a dsRNA-binding protein PACT. TRBP, another cytoplasmic dsRNA-binding protein, inhibits RIG-I signaling and IRF3 nuclear localization independently of PACT and at least in part though dsRNA sequestration.
The ability of TRBP to influence IFN production has been investigated before with conflicting data. In agreement with our studies, Ling et al. reported that TRBP negatively regulates IFN-β production by targeting MAVS [78]. Their results indicated the TRBP inhibits IFN production during SeV infection by inhibition of interaction between MAVS and RIG-I as well as by weakening the interaction between MAVS and TRAF3 and also by promoting proteosomal RIG-I degradation. In contrast to this, Komuro et al reported that TRBP activates LGP2/MDA5-dependent IFN-β promoter activity induced by Cardiovirus infection [79]. Their work demonstrated a direct interaction of TRBP with LGP2 and overexpressed TRBP could increase Cardiovirus-triggered interferon promoter activity when LGP2 and MDA5 are both co-overexpressed. Exactly how TRBP contributed to Cardiovirus-triggered immune responses via a direct association with LGP2 was not further investigated in this study. In addition to these studies that examined the effect of TRBP on IFN production, TRBP and its interaction with LGP2 has also been shown to affect the RNAi pathway during virus infections by Takahashi et al. [80]. LGP2 was reported to bind to the dsRNA binding sites of TRBP, resulting in inhibition of pre-miRNA binding and recruitment and was reported to repress only specific TRBP-bound miRNA activities by competitively interacting with TRBP, resulting in selective regulation of gene expression. Similarly, the same group also reported that RIG-I, but not MDA5, interacted with TRBP indirectly through LGP2 to function as an RNAi modulator [81]. Furthermore, during SeV infection, LGP2 interaction with TRBP resulted in an inhibition of maturation of the TRBP-bound microRNAs and gene expression profiling in this study revealed that apoptosis regulatory genes were upregulated during SeV infection directly or indirectly through the repression of a typical TRBP-bound miRNA, miR-106b [82]. Taken together, these studies reveal that TRBP influences the outcome of virus infection by influencing both IFN production as well as RNAi pathways.
Our study establishes that similar to the opposite effects of PACT and TRBP on PKR activity, these two very homologous proteins also have opposite effects on RIG-I induced IFN production. The opposite actions of PACT and TRBP on PKR activity are mediated by the carboxy-terminal third copy of the dsRBM motif [35]. It remains to be investigated in future if the same region also exerts inhibitory effect on RIG-I mediated IFN induction. TRBP is known to exert pro-viral actions during HIV replication via inhibition of PKR activity [23, 24]. It is also worth investigating if TRBP works in a pro-viral manner by inhibition of IFN induction during HIV infections in addition to inhibition of PKR. Our studies presented here open many interesting possibilities that can be addressed in future, potentially leading to valuable insights into regulation of innate immunity.
Data Availability
Data sharing is not applicable to the paper, because all experimental procedures and data encompassing this work are included in this manuscript.
Acknowledgements
This work was supported in part by a pilot grant to RCP from the Center for Targeted Therapeutics at University of South Carolina supported by NIH COBRE grant IP20GM109091. The funders had no role in study, design, data collection and interpretation, or the decision to submit the work for publication.
The authors would like to thank Dr. Curt Horvath (Northwestern University) for his advice on RIG-I activity assays. We would also like to thank Drs. Ganes Sen (Cleveland Clinic) and Bryan Williams (Hudson Institute of Medical Research) for PACT and PKR null MEFs respectively and Drs. Richard Randall (University of St. Andrews) and Chris Basler (Georgia State University) for IFN-β-luc and pEGFP-C1-hIRF3 plasmids respectively.
Footnotes
Conflict of Interest
The authors declare that they have no conflicts of interest with the contents of this article.
References
- 1.Ahmad S and Hur S (2015) Helicases in Antiviral Immunity: Dual Properties as Sensors and Effectors. Trends Biochem Sci. 40, 576–585. 10.1016/j.tibs.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schlee M (2013) Master sensors of pathogenic RNA - RIG-I like receptors. Immunobiology. 218, 1322–1335. 10.1016/j.imbio.2013.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber F (2015) The catcher in the RIG-I. Cytokine. 76, 38–41. 10.1016/j.cyto.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 4.Rehwinkel J and Gack MU (2020) RIG-I-like receptors: their regulation and roles in RNA sensing. Nat Rev Immunol. 20, 537–551. 10.1038/s41577-020-0288-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu B and Hur S (2015) How RIG-I like receptors activate MAVS. Curr Opin Virol. 12, 91–98. 10.1016/j.coviro.2015.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seth RB, Sun L, Ea CK and Chen ZJ (2005) Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 122, 669–682. 10.1016/j.cell.2005.08.012 [DOI] [PubMed] [Google Scholar]
- 7.Lazear HM, Schoggins JW and Diamond MS (2019) Shared and Distinct Functions of Type I and Type III Interferons. Immunity. 50, 907–923. 10.1016/j.immuni.2019.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoggins JW (2014) Interferon-stimulated genes: roles in viral pathogenesis. Curr Opin Virol. 6, 40–46. 10.1016/j.coviro.2014.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schoggins JW (2019) Interferon-Stimulated Genes: What Do They All Do? Annu Rev Virol. 6, 567–584. 10.1146/annurev-virology-092818-015756 [DOI] [PubMed] [Google Scholar]
- 10.Patel RC and Sen GC (1998) PACT, a protein activator of the interferon-induced protein kinase, PKR. Embo j. 17, 4379–4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kok KH, Lui PY, Ng MH, Siu KL, Au SW and Jin DY (2011) The double-stranded RNA-binding protein PACT functions as a cellular activator of RIG-I to facilitate innate antiviral response. Cell Host Microbe. 9, 299–309. 10.1016/j.chom.2011.03.007 [DOI] [PubMed] [Google Scholar]
- 12.Sanchez David RY, Combredet C, Najburg V, Millot GA, Beauclair G, Schwikowski B, et al. (2019) LGP2 binds to PACT to regulate RIG-I- and MDA5-mediated antiviral responses. Sci Signal. 12, eaar3993. 10.1126/scisignal.aar3993 [DOI] [PubMed] [Google Scholar]
- 13.Brisse M and Ly H (2017) Viral inhibitions of PACT-induced RIG-I activation. Oncotarget. 8, 60725–60726. 10.18632/oncotarget.18928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, Fang P, Wang M, Peng Q, Ren J, Wang D, et al. (2019) Porcine deltacoronavirus nucleocapsid protein antagonizes IFN-beta production by impairing dsRNA and PACT binding to RIG-I. Virus Genes. 55, 520–531. 10.1007/s11262-019-01673-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams CG, Gibbons JS, Keiffer TR, Luthra P, Edwards MR and Basler CF (2020) Impact of Měnglà Virus Proteins on Human and Bat Innate Immune Pathways. J Virol. 94, e00191–00120. 10.1128/jvi.00191-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan CP, Yuen CK, Cheung PH, Fung SY, Lui PY, Chen H, et al. (2018) Antiviral activity of double-stranded RNA-binding protein PACT against influenza A virus mediated via suppression of viral RNA polymerase. Faseb j. 32, 4380–4393. 10.1096/fj.201701361R [DOI] [PubMed] [Google Scholar]
- 17.Shao J, Huang Q, Liu X, Di D, Liang Y and Ly H (2018) Arenaviral Nucleoproteins Suppress PACT-Induced Augmentation of RIG-I Function To Inhibit Type I Interferon Production. J Virol. 92, e00482–00418. 10.1128/jvi.00482-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daher A, Laraki G, Singh M, Melendez-Pena CE, Bannwarth S, Peters AH, et al. (2009) TRBP control of PACT-induced phosphorylation of protein kinase R is reversed by stress. Mol Cell Biol. 29, 254–265. 10.1128/MCB.01030-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh M, Castillo D, Patel CV and Patel RC (2011) Stress-induced phosphorylation of PACT reduces its interaction with TRBP and leads to PKR activation. Biochemistry. 50, 4550–4560. 10.1021/bi200104h [DOI] [PubMed] [Google Scholar]
- 20.Chukwurah E, Willingham V, Singh M, Castillo-Azofeifa D and Patel RC (2018) Contribution of the two dsRBM motifs to the double-stranded RNA binding and protein interactions of PACT. J Cell Biochem. 119, 3598–3607. 10.1002/jcb.26561 [DOI] [PubMed] [Google Scholar]
- 21.Gatignol A, Buckler-White A, Berkhout B and Jeang KT (1991) Characterization of a human TAR RNA-binding protein that activates the HIV-1 LTR. Science. 251, 1597–1600. [DOI] [PubMed] [Google Scholar]
- 22.Burugu S, Daher A, Meurs EF and Gatignol A (2014) HIV-1 translation and its regulation by cellular factors PKR and PACT. Virus Res. 193, 65–77. 10.1016/j.virusres.2014.07.014 [DOI] [PubMed] [Google Scholar]
- 23.Clerzius G, Gelinas JF and Gatignol A (2011) Multiple levels of PKR inhibition during HIV-1 replication. Rev Med Virol. 21, 42–53. 10.1002/rmv.674 [DOI] [PubMed] [Google Scholar]
- 24.Gatignol A, Laine S and Clerzius G (2005) Dual role of TRBP in HIV replication and RNA interference: viral diversion of a cellular pathway or evasion from antiviral immunity? Retrovirology. 2, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chendrimada TP, Gregory RI, Kumaraswamy E, Norman J, Cooch N, Nishikura K, et al. (2005) TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature. 436, 740–744.nature03868 [pii] 10.1038/nature03868 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haase AD, Jaskiewicz L, Zhang H, Laine S, Sack R, Gatignol A, et al. (2005) TRBP, a regulator of cellular PKR and HIV-1 virus expression, interacts with Dicer and functions in RNA silencing. EMBO Rep. 6, 961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee HY, Zhou K, Smith AM, Noland CL and Doudna JA (2013) Differential roles of human Dicer-binding proteins TRBP and PACT in small RNA processing. Nucleic Acids Res. 41, 6568–6576. 10.1093/nar/gkt361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorin D, Bonnet MC, Bannwarth S, Gatignol A, Meurs EF and Vaquero C (2003) The TAR RNA-binding protein, TRBP, stimulates the expression of TAR-containing RNAs in vitro and in vivo independently of its ability to inhibit the dsRNA-dependent kinase PKR. J Biol Chem. 278, 4440–4448. [DOI] [PubMed] [Google Scholar]
- 29.Park H, Davies MV, Langland JO, Chang HW, Nam YS, Tartaglia J, et al. (1994) TAR RNA-binding protein is an inhibitor of the interferon-induced protein kinase PKR. Proc Natl Acad Sci U S A. 91, 4713–4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chukwurah E and Patel RC (2018) Stress-induced TRBP phosphorylation enhances its interaction with PKR to regulate cellular survival. Sci Rep. 8, 1020. 10.1038/s41598-018-19360-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koscianska E, Starega-Roslan J and Krzyzosiak WJ (2011) The role of Dicer protein partners in the processing of microRNA precursors. PLoS One. 6, e28548. 10.1371/journal.pone.0028548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson RC, Tambe A, Kidwell MA, Noland CL, Schneider CP and Doudna JA (2015) Dicer-TRBP Complex Formation Ensures Accurate Mammalian MicroRNA Biogenesis. Mol Cell. 57, 397–407. 10.1016/j.molcel.2014.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heyam A, Lagos D and Plevin M (2015) Dissecting the roles of TRBP and PACT in double-stranded RNA recognition and processing of noncoding RNAs. Wiley Interdiscip Rev RNA. 6, 271–289. 10.1002/wrna.1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kok KH, Ng MH, Ching YP and Jin DY (2007) Human TRBP and PACT directly interact with each other and associate with dicer to facilitate the production of small interfering RNA. J Biol Chem. 282, 17649–17657. [DOI] [PubMed] [Google Scholar]
- 35.Gupta V, Huang X and Patel RC (2003) The carboxy-terminal, M3 motifs of PACT and TRBP have opposite effects on PKR activity. Virology. 315, 283–291. [DOI] [PubMed] [Google Scholar]
- 36.Daniels SM and Gatignol A (2012) The multiple functions of TRBP, at the hub of cell responses to viruses, stress, and cancer. Microbiol Mol Biol Rev. 76, 652–666. 10.1128/mmbr.00012-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh M and Patel RC (2012) Increased interaction between PACT molecules in response to stress signals is required for PKR activation. J Cell Biochem. 113, 2754–2764. 10.1002/jcb.24152 [DOI] [PubMed] [Google Scholar]
- 38.Nakamura T, Kunz RC, Zhang C, Kimura T, Yuan CL, Baccaro B, et al. (2015) A critical role for PKR complexes with TRBP in Immunometabolic regulation and eIF2alpha phosphorylation in obesity. Cell Rep. 11, 295–307. 10.1016/j.celrep.2015.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bamming D and Horvath CM (2009) Regulation of signal transduction by enzymatically inactive antiviral RNA helicase proteins MDA5, RIG-I, and LGP2. J Biol Chem. 284, 9700–9712. 10.1074/jbc.M807365200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.King P and Goodbourn S (1992) A method for sequence-specific deletion mutagenesis. Nucleic Acids Res. 20, 1039–1044. 10.1093/nar/20.5.1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Basler CF, Mikulasova A, Martinez-Sobrido L, Paragas J, Mühlberger E, Bray M, et al. (2003) The Ebola virus VP35 protein inhibits activation of interferon regulatory factor 3. J Virol. 77, 7945–7956. 10.1128/jvi.77.14.7945-7956.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel RC and Sen GC (1992) Identification of the double-stranded RNA-binding domain of the human interferon-inducible protein kinase. J Biol Chem. 267, 7671–7676. [PubMed] [Google Scholar]
- 43.Gatignol A, Buckler C and Jeang KT (1993) Relatedness of an RNA-binding motif in human immunodeficiency virus type 1 TAR RNA-binding protein TRBP to human P1/dsI kinase and Drosophila staufen. Mol Cell Biol. 13, 2193–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel RC, Stanton P, McMillan NM, Williams BR and Sen GC (1995) The interferon-inducible double-stranded RNA-activated protein kinase self-associates in vitro and in vivo. Proc Natl Acad Sci U S A. 92, 8283–8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel RC, Vestal DJ, Xu Z, Bandyopadhyay S, Guo W, Erme SM, et al. (1999) DRBP76, a double-stranded RNA-binding nuclear protein, is phosphorylated by the interferon-induced protein kinase, PKR. J Biol Chem. 274, 20432–20437. [DOI] [PubMed] [Google Scholar]
- 46.Chang KY and Ramos A (2005) The double-stranded RNA-binding motif, a versatile macromolecular docking platform. Febs j. 272, 2109–2117. [DOI] [PubMed] [Google Scholar]
- 47.Fierro-Monti I and Mathews MB (2000) Proteins binding to duplexed RNA: one motif, multiple functions. Trends Biochem Sci. 25, 241–246. [DOI] [PubMed] [Google Scholar]
- 48.Tian B, Bevilacqua PC, Diegelman-Parente A and Mathews MB (2004) The double-stranded-RNA-binding motif: interference and much more. Nat Rev Mol Cell Biol. 5, 1013–1023. 10.1038/nrm1528 [DOI] [PubMed] [Google Scholar]
- 49.Tian B and Mathews MB (2001) Functional characterization of and cooperation between the double-stranded RNA-binding motifs of the protein kinase PKR. J Biol Chem. 276, 9936–9944. [DOI] [PubMed] [Google Scholar]
- 50.Saunders LR and Barber GN (2003) The dsRNA binding protein family: critical roles, diverse cellular functions. Faseb j. 17, 961–983. [DOI] [PubMed] [Google Scholar]
- 51.Bruns AM, Leser GP, Lamb RA and Horvath CM (2014) The innate immune sensor LGP2 activates antiviral signaling by regulating MDA5-RNA interaction and filament assembly. Mol Cell. 55, 771–781. 10.1016/j.molcel.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taghavi N and Samuel CE (2012) Protein kinase PKR catalytic activity is required for the PKR-dependent activation of mitogen-activated protein kinases and amplification of interferon beta induction following virus infection. Virology. 427, 208–216. 10.1016/j.virol.2012.01.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McAllister CS, Taghavi N and Samuel CE (2012) Protein kinase PKR amplification of interferon β induction occurs through initiation factor eIF-2α-mediated translational control. J Biol Chem. 287, 36384–36392. 10.1074/jbc.M112.390039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peters GA, Li S and Sen GC (2006) Phosphorylation of specific serine residues in the PKR activation domain of PACT is essential for its ability to mediate apoptosis. J Biol Chem. 281, 35129–35136. [DOI] [PubMed] [Google Scholar]
- 55.Paroo Z, Ye X, Chen S and Liu Q (2009) Phosphorylation of the human microRNA-generating complex mediates MAPK/Erk signaling. Cell. 139, 112–122.S0092–8674(09)00791–0 [pii] 10.1016/j.cell.2009.06.044 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vaughn LS, Bragg DC, Sharma N, Camargos S, Cardoso F and Patel RC (2015) Altered Activation of Protein Kinase PKR and Enhanced Apoptosis in Dystonia Cells Carrying a Mutation in PKR Activator Protein PACT. J Biol Chem. 290, 22543–22557. 10.1074/jbc.M115.669408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feng M, Ding Z, Xu L, Kong L, Wang W, Jiao S, et al. (2013) Structural and biochemical studies of RIG-I antiviral signaling. Protein Cell. 4, 142–154. 10.1007/s13238-012-2088-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laraki G, Clerzius G, Daher A, Melendez-Pena C, Daniels S and Gatignol A (2008) Interactions between the double-stranded RNA-binding proteins TRBP and PACT define the Medipal domain that mediates protein-protein interactions. RNA Biol. 5, 92–103.6069 [pii] [DOI] [PubMed] [Google Scholar]
- 59.Yamashita S, Nagata T, Kawazoe M, Takemoto C, Kigawa T, Guntert P, et al. (2011) Structures of the first and second double-stranded RNA-binding domains of human TAR RNA-binding protein. Protein Sci. 20, 118–130. 10.1002/pro.543 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Daher A, Longuet M, Dorin D, Bois F, Segeral E, Bannwarth S, et al. (2001) Two dimerization domains in the trans-activation response RNA-binding protein (TRBP) individually reverse the protein kinase R inhibition of HIV-1 long terminal repeat expression. J Biol Chem. 276, 33899–33905. [DOI] [PubMed] [Google Scholar]
- 61.Patel RC, Stanton P and Sen GC (1996) Specific mutations near the amino terminus of double-stranded RNA-dependent protein kinase (PKR) differentially affect its double-stranded RNA binding and dimerization properties. J Biol Chem. 271, 25657–25663. [DOI] [PubMed] [Google Scholar]
- 62.Hiscott J (2007) Triggering the innate antiviral response through IRF-3 activation. J Biol Chem. 282, 15325–15329. 10.1074/jbc.R700002200 [DOI] [PubMed] [Google Scholar]
- 63.Kumar KP, McBride KM, Weaver BK, Dingwall C and Reich NC (2000) Regulated nuclear-cytoplasmic localization of interferon regulatory factor 3, a subunit of double-stranded RNA-activated factor 1. Mol Cell Biol. 20, 4159–4168. 10.1128/mcb.20.11.4159-4168.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin R, Heylbroeck C, Pitha PM and Hiscott J (1998) Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol Cell Biol. 18, 2986–2996. 10.1128/mcb.18.5.2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yoneyama M, Suhara W, Fukuhara Y, Fukuda M, Nishida E and Fujita T (1998) Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. Embo j. 17, 1087–1095. 10.1093/emboj/17.4.1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin R, Mamane Y and Hiscott J (1999) Structural and functional analysis of interferon regulatory factor 3: localization of the transactivation and autoinhibitory domains. Mol Cell Biol. 19, 2465–2474. 10.1128/mcb.19.4.2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.He B, Paterson RG, Stock N, Durbin JE, Durbin RK, Goodbourn S, et al. (2002) Recovery of paramyxovirus simian virus 5 with a V protein lacking the conserved cysteine-rich domain: the multifunctional V protein blocks both interferon-beta induction and interferon signaling. Virology. 303, 15–32. 10.1006/viro.2002.1738 [DOI] [PubMed] [Google Scholar]
- 68.Talon J, Horvath CM, Polley R, Basler CF, Muster T, Palese P, et al. (2000) Activation of interferon regulatory factor 3 is inhibited by the influenza A virus NS1 protein. J Virol. 74, 7989–7996. 10.1128/jvi.74.17.7989-7996.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ringeard M, Marchand V, Decroly E, Motorin Y and Bennasser Y (2019) FTSJ3 is an RNA 2’-O-methyltransferase recruited by HIV to avoid innate immune sensing. Nature. 565, 500–504. 10.1038/s41586-018-0841-4 [DOI] [PubMed] [Google Scholar]
- 70.Sanghvi VR and Steel LF (2011) The cellular TAR RNA binding protein, TRBP, promotes HIV-1 replication primarily by inhibiting the activation of double-stranded RNA-dependent kinase PKR. J Virol. 85, 12614–12621. 10.1128/jvi.05240-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burnett SB, Vaughn LS, Sharma N, Kulkarni R and Patel RC (2020) Dystonia 16 (DYT16) mutations in PACT cause dysregulated PKR activation and eIF2α signaling leading to a compromised stress response. Neurobiol Dis. 146, 105135. 10.1016/j.nbd.2020.105135 [DOI] [PubMed] [Google Scholar]
- 72.Zerbe CM, Mouser DJ and Cole JL (2020) Oligomerization of RIG-I and MDA5 2CARD domains. Protein Sci. 29, 521–526. 10.1002/pro.3776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takahashi T, Miyakawa T, Zenno S, Nishi K, Tanokura M and Ui-Tei K (2013) Distinguishable in vitro binding mode of monomeric TRBP and dimeric PACT with siRNA. PLoS One. 8, e63434. 10.1371/journal.pone.0063434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bennett RL, Blalock WL and May WS (2004) Serine 18 phosphorylation of RAX, the PKR activator, is required for PKR activation and consequent translation inhibition. J Biol Chem. 279, 42687–42693. [DOI] [PubMed] [Google Scholar]
- 75.Crow YJ (2011) Type I interferonopathies: a novel set of inborn errors of immunity. Ann N Y Acad Sci. 1238, 91–98. 10.1111/j.1749-6632.2011.06220.x [DOI] [PubMed] [Google Scholar]
- 76.Crow YJ and Manel N (2015) Aicardi-Goutières syndrome and the type I interferonopathies. Nat Rev Immunol. 15, 429–440. 10.1038/nri3850 [DOI] [PubMed] [Google Scholar]
- 77.Zhong J, Peters AH, Lee K and Braun RE (1999) A double-stranded RNA binding protein required for activation of repressed messages in mammalian germ cells. Nat Genet. 22, 171–174. 10.1038/9684 [doi] [DOI] [PubMed] [Google Scholar]
- 78.Ling T, Li SN, Weng GX, Wang W, Li C, Cao L, et al. (2018) TARBP2 negatively regulates IFN-β production and innate antiviral response by targeting MAVS. Mol Immunol. 104, 1–10. 10.1016/j.molimm.2018.10.017 [DOI] [PubMed] [Google Scholar]
- 79.Komuro A, Homma Y, Negoro T, Barber GN and Horvath CM (2016) The TAR-RNA binding protein is required for immunoresponses triggered by Cardiovirus infection. Biochem Biophys Res Commun. 480, 187–193. 10.1016/j.bbrc.2016.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takahashi T, Nakano Y, Onomoto K, Murakami F, Komori C, Suzuki Y, et al. (2018) LGP2 virus sensor regulates gene expression network mediated by TRBP-bound microRNAs. Nucleic Acids Res. 46, 9134–9147. 10.1093/nar/gky575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Takahashi T, Nakano Y, Onomoto K, Yoneyama M and Ui-Tei K (2018) Virus Sensor RIG-I Represses RNA Interference by Interacting with TRBP through LGP2 in Mammalian Cells. Genes (Basel). 9, 511. 10.3390/genes9100511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Takahashi T, Nakano Y, Onomoto K, Yoneyama M and Ui-Tei K (2020) LGP2 virus sensor enhances apoptosis by upregulating apoptosis regulatory genes through TRBP-bound miRNAs during viral infection. Nucleic Acids Res. 48, 1494–1507. 10.1093/nar/gkz1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to the paper, because all experimental procedures and data encompassing this work are included in this manuscript.