Abstract
Background
Regeneration potential of Hamstring tendons after harvest assumes significant clinical relevance as its use has become widespread today. Methods which best assess the regeneration, extent and type of regeneration, plus issues related to functional loss are important for the surgeon to know. This review looks at the literature to find answers to the above questions.
Purpose
To summarize the evidence in support of hamstring tendon regeneration, and the most appropriate modality for evaluation of regeneration. Additionally, to evaluate the regeneration in terms of complete or partial, extent and its impact on strength deficit and functional outcomes.
Methods
We did a systematic review of literature through specified search engines and identified 30 of 285 studies to be relevant (19 prospective and 11 retrospective).
Results
Evaluation of above data suggests tissue regeneration at harvest sites does occur (78.9% of semitendinosus and 42.7% of gracilis tendons), but this regeneration is variable. No established definition of regeneration exists; MRI is an adequate tool to identify regeneration, while biopsy is confirmative. USG is a cost-effective screening method and can document distal progress of regenerate. Semitendinosus and gracilis tendons regenerate at different rates and extents, and often fuse together, but there is no evidence to state that one regenerates better than the other. Proximal retraction of the muscle-tendon junction occurs, along with some atrophy, which affects function to a variable extent. Strength deficits may persist, but they may not convert to significant functional deficits.
Conclusion
There is variable hamstring regeneration after harvest, with poorly defined definition of “regeneration”. Some changes in the muscle itself, abnormal distal insertion and absence of regeneration in some are documented, along with strength deficits. Although overall functional deficits have been reported to be minimal, a definite change in the anatomy of the medial hamstrings is a factor to be kept in consideration. More information is needed about the long-term consequences.
Keywords: Hamstring tendon, Tendon harvest, Tendon regeneration, Semitendinosus, Gracilis, ACL reconstruction
1. Introduction
Hamstring tendon harvesting for ACL reconstructions (ACLR) have increased exponentially over 30 years, because of perceived minimal donor site morbidity.1, 2, 3, 4, 5, 6 Cross et al.7 reported tendon regeneration after harvest with sufficient function, leading to an increase in their usage in ACLR, assuming minimal or no functional loss, with near total regeneration. Subsequently several reports documented limited regeneration with loss of flexion strength.8 However, some studies have shown that regenerated hamstring tendon after ACLR can be reharvested.9 Understanding the degree and extent of hamstring tendon regeneration and any potential residual sequelae is of clinical importance, as it may influence graft choice in ACLR and even influence the postoperative rehabilitation protocol.10 Whether or not the regenerated tendon has the same anatomical structure, function and strength as the original tendon, is a point of debate. Clarity on this could have significant surgical and clinical implications for graft choices in ACLR. This controversy persisted until two systematic reviews attempted to settle the debate.11,12 Suijkerbuijk et al.12 reviewed 18 studies on the subject and concluded that these tendons regenerated at a very high rate. However, there was no clear-cut definition of regeneration, with vague usage of the term “at least regrowth of tendon” on imaging; additionally, the nature, extent and completeness of this regeneration was not commented upon. “Their review” in 2014 looked at 7 studies which evaluated strength deficit of the hamstrings, albeit with variable results. We believe that strength deficit is not only related with the dichotomous answer of yes or no for regeneration, but the overall extent and degree (completeness) of tendon regeneration, which was not assessed by these authors. The other review by Papalin et al.11 included 19 studies which had “modest quality of evidence” in the authors’ own judgement. Even this review did not assess the extent of tendon regeneration, and had no criteria for terming regeneration as partial or complete. With this major limitation of understanding in the published literature, the present review was conceptualized to evaluate some specific points in relation to hamstring regeneration, with additional data published in the 5 years.
The present systematic review attempted to identify evidence in support of hamstring regeneration, to see which is the most effective modality to directly confirm this regeneration, and if possible, to assess if there is evidence to quantify the type of tissue regenerated and the extent of regeneration of both the harvested hamstring tendons. An evaluation of extent of strength deficit was also done.
2. Methodology
2.1. Study design
A systematic review of literature was performed through specified search engines in accordance with the preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines.13
3. Search strategy
An electronic search was conducted on 3 databases; PubMed, Embase and Scopus on 28th February 2020 by three authors (RKR, PK and SD) using specific keywords with a combination of medical subject heading (MeSH) [Table-1], without any restriction on the year of publication. A total number of 285 results were obtained. We also performed a secondary search from the references listed in all the articles selected as per the predefined criteria.
Table-1.
Search strategy used for the systematic review in PubMed, Embase, and Scopus databases.
| Database | Period - Inception to 28-02-2020. with keywords | Results |
|---|---|---|
| PubMed | ((("hamstring tendons"[MeSH Terms] OR ("hamstring"[All Fields] AND "tendons"[All Fields]) OR "hamstring tendons"[All Fields] OR ("hamstring"[All Fields] AND "tendon"[All Fields]) OR "hamstring tendon"[All Fields]) AND ("regeneration"[MeSH Terms] OR "regeneration"[All Fields]) AND (("anterior cruciate ligament"[MeSH Terms] OR ("anterior"[All Fields] AND "cruciate"[All Fields] AND "ligament"[All Fields]) OR "anterior cruciate ligament"[All Fields] OR "acl"[All Fields]) AND ("reconstructive surgical procedures"[MeSH Terms] OR ("reconstructive"[All Fields] AND "surgical"[All Fields] AND "procedures"[All Fields]) OR "reconstructive surgical procedures"[All Fields] OR "reconstruction"[All Fields]))) OR ((("hamstring tendons"[MeSH Terms] OR ("hamstring"[All Fields] AND "tendons"[All Fields]) OR "hamstring tendons"[All Fields] OR ("hamstring"[All Fields] AND "tendon"[All Fields]) OR "hamstring tendon"[All Fields]) AND Harvest[All Fields]) AND ("regeneration"[MeSH Terms] OR "regeneration"[All Fields]) AND (("anterior cruciate ligament"[MeSH Terms] OR ("anterior"[All Fields] AND "cruciate"[All Fields] AND "ligament"[All Fields]) OR "anterior cruciate ligament"[All Fields] OR "acl"[All Fields]) AND ("reconstructive surgical procedures"[MeSH Terms] OR ("reconstructive"[All Fields] AND "surgical"[All Fields] AND "procedures"[All Fields]) OR "reconstructive surgical procedures"[All Fields] OR "reconstruction"[All Fields])))) AND ("0001/01/01"[PDAT]: "2020/02/28"[PDAT]) | 96 |
| Embase | (’hamstring tendon’/exp OR ’hamstring tendon’ OR ((’hamstring’/exp OR hamstring) AND (’tendon’/exp OR tendon))) AND (’regeneration’/exp OR regeneration) AND (’acl reconstruction’/exp OR ’acl reconstruction’ OR ((’acl’/exp OR acl) AND (’reconstruction’/exp OR reconstruction))) | 120 |
| Scopus | TITLE-ABS-KEY (hamstring AND tendon AND regeneration AND acl AND reconstruction) TITLE – ABS -KEY (hamstring AND tendon AND harvest AND regeneration AND acl AND reconstruction) | 79 |
| Total | 285 | |
4. Inclusion and exclusion criteria
Studies of any design that evaluated the eventual regeneration of harvested hamstring tendon after ACLR, using any imaging or laboratory method, were included. Studies with no information about the regeneration of tendon, those that included previously reported hamstring injuries, animal studies, conference abstracts, posters, book chapters, and case reports were excluded. We also excluded non-English and review articles.
5. Study selection and data extraction
Studies were screened based on their titles and/or abstracts, independently by two authors (RKR and PK) and relevant ones were identified. Their full texts were reviewed, and finally, studies based on the selection criteria were included. Discrepancies were resolved by mutual consensus among all the four authors.
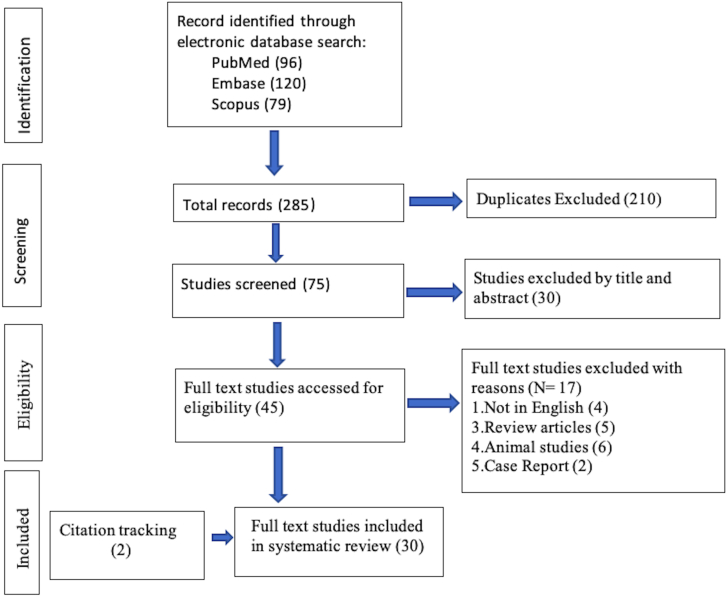
The data extracted were entered in a pre-specified excel sheets; the names of the authors, year of publication, numbers of included patients, demographic parameters, diagnostic techniques, and outcome measures. Overall 30 publications were included in this study [Figure-1].
Figure-1.
PRISMA flow chart depicting selection of articles.
6. Assessment of risk of bias
The risk of bias was assessed independently by two authors (RKR and PK) using ‘The Cochrane Collaboration’s risk of bias tool’14 used for randomized controlled trials (RCT) and MINORS tool15 for non-randomized studies. Discrepancies were resolved by mutual agreement.
7. Statistical analysis
Data from the included studies were pooled and means and ranges were calculated for continuous variables extracted. The categorical variables were expressed as numbers and percentages. The statistical analysis was done by using Review Manager Software version 5.4 (RevMan 5.4).
8. Results
8.1. Assessment of risk of bias
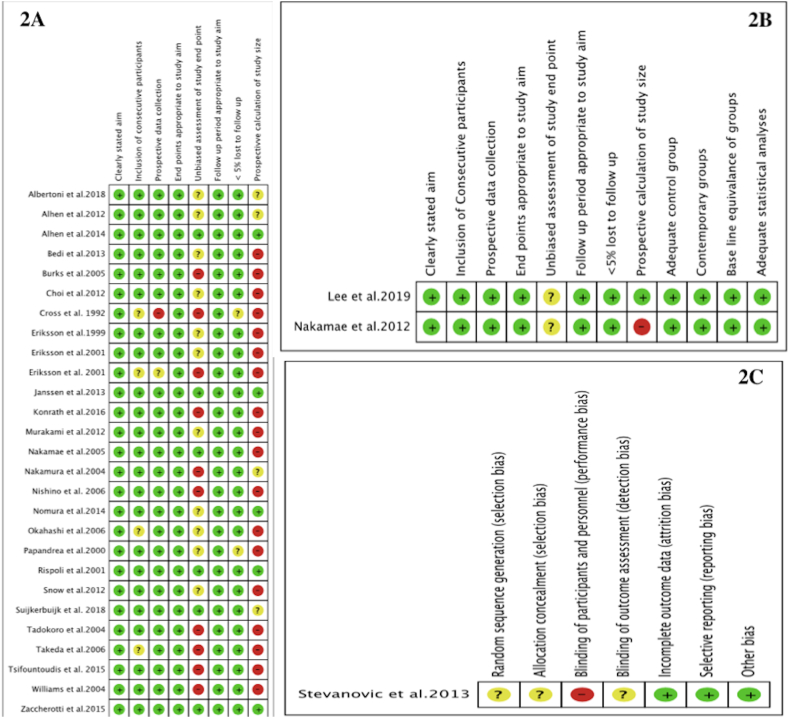
The overall risk of bias was assessed as low for the included studies as depicted in figure-2, and figure-3. There were two nonrandomized comparative studies while one RCT and rest 27 were observational studies/case series. MINORS tool Score was >12 for 24 non-comparative studies while for the two nonrandomized comparative studies was >21.
Figure-2.
2A: Risk of bias graph: review author’s judgments about each risk of bias presented as percentages across all included non-comparative studies.2B: Risk of bias graph: review author’s judgments about each risk of bias presented as percentages across all included comparative studies2C: Risk of bias graph: review author’s judgments about each risk of bias presented as percentages across all included randomized study.
Figure-3.
3A: Risk of bias summary: review author’s judgments about each risk of bias for each included noncomparative study.3B: Risk of bias summary: review authors’ judgements about each risk of bias item for each included comparative study.3C: Risk of bias summary: review authors’ judgements about each risk of bias item for each included randomised study.
9. Characteristics of studies
A total of 30 studies were included in this review. Of these, 19 were prospective [8,12,16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32] and 11 were retrospective in design [7,33-,42]. There was only one RCT included in this review.28 The number of patients in the individual studies ranged from 4 to 120. The age groups ranged from 19 to 40 years. A total of 805 knees of 801 patients who underwent ACLR were assessed, out of which 520 (72.5%) were males and 197 were females (in two studies male to female ratio was not reported). The mean/median follow-up at time of evaluation of regeneration ranged from 4 to 129 months. [Table-2].
Table-2.
Study characteristic and demographic data of included studies.
| Sl No | Authors | Study design | No. of patients | Male: Female |
Age (years) mean or median (±SD) | Mean or median (±SD) follow up period (months) |
|---|---|---|---|---|---|---|
| 1. | Cross et al. 19927 | Retrospective study | 4a | NR | NR | 6 |
| 2. | Eriksson et al.199918 | Prospective study | 11 | 8:3 | Median 24 | 6–12 |
| 3. | Papandrea et al.200026 | Prospective study | 40 | 29:11 | 28 | 24 |
| 4. | Eriksson et al. 200136 | Retrospective study | 6 | 6:0 | 25 | 20.5 |
| 5. | Eriksson et al.200119 | Prospective study | 16 | 14:2 | 26 | MRI,median:7 histology, median:10 |
| 6. | Rispoli et al.200127 | Prospective study | 20 | 13:7 | 37 | 32 |
| 7. | Nakamura et al.200439 | Retrospective study | 8 | 5:3 | 24 | 37 |
| 8. | Tadokoro et al.200442 | Retrospective study | 28 | 10:18 | 22.2 | 67.2 |
| 9 | Williams et al.200431 | Prospective study | 8 | 6:2 | 19.3(±4.1) | 4–9 |
| 10. | Burks et al.20058 | Prospective study | 9 | 5:4 | 28 | 12 |
| 11 | Nakamae et al.200522 | Prospective study | 29 | 15:14 | 28 | 12 |
| 12. | Takeda et al.200629 | Prospective study | 11 | 6:5 | 20.5 | 12.7 |
| 13. | Okahashi et al.200625 | Prospective study | 11 | 8:3 | 23 | 12 |
| 14. | Nishino et al.200623 | Prospective study | 23 | 10:13 | 22 ± 4 | 23 |
| 15. | Nakamae et al.201240 | Retrospective study | 39 | 22:17 | Group-1: 29.5(±12.2) & Group-2: 27.1 (±11.4) | 6 or 12 |
| 16. | Snow et al.201241 | Retrospective study | 10 | 7:3 | 33 | 129 |
| 17. | Murakami et al.201221 | Prospective study | 20 | 11:9 | 23.1 | 15 |
| 18. | Choi et al.201217 | Prospective study | 45 | 45:0 | 32.7(±7.3) | 36.4(±7.4) |
| 19. | Alhen et al.201233 | Retrospective study | 19 | 10:9 | Median 23 | Median: 100.8 |
| 20. | Bedi et al.201335 | Retrospective study | 15 (18 knees) | 6:9 | 27 | 12.8 |
| 21. | Janssen et al.201320 | Prospective study | 22 | 17:5 | 28.4(±5) | 12 |
| 22. | Stevanovic et al.201328 | Prospective RCT | 50 | 35:15 | 25(±4) | USG: 24, Biopsy-NR |
| 23. | Alhen et al.201434 | Retrospective study | 18 | 10:8 | Median 23 | Median: 102 ± 18 |
| 24. | Nomura et al.201424 | Prospective study | 24 | 14:10 | 21(±2) | 27.7 (±18.2) |
| 25. | Zaccherotti et al.201532 | Prospective study | 20 | 14:6 | 26.1 | 24 |
| 26. | Tsifountoudis et al.201530 | Prospective study | 47 | 42:5 | M-27.6 and F-31.2 | 24 |
| 27. | Konrath et al.201637 | Retrospective study | 20 | 14:6 | 29(±7) | 24 |
| 28. | Suijkerbuijk et al. 201812 | Prospective study | 79 | NR | Median 25.2 | 24 |
| 29. | Albertoni et al.201816 | Prospective study | 29(30 knees) | 26:3 | 32 | 17 |
| 30. | Lee et al.201938 | Retrospective study | 120 (60 in each group) | 112:8 |
S-group-27.4(±6.6) & D-group-26.9(±7.3) |
36 |
Out of 225 patients only 4 were analyzed in detail for regeneration of hamstring tendon, NR-not reported, SD-Standard deviation.
10. Do the hamstring tendons regenerate after harvest?
An ideal method to assess any parameter is one which is easily accessible, cost effective, fast and has minimal side effects. Different studies evaluated in the current review have utilized one or more methods to assess the regeneration of the harvested hamstring tendons. While evaluating tendon regeneration, studies have identified the regrowth of tendon tissue or tissue like structures at the normal anatomical spots of harvested tendons.
Cross et al.7 were the first to demonstrate that hamstrings have the potential to regenerate; out of a cohort of 225 ACLR, they demonstrated conclusive regeneration on magnetic resonance imaging (MRI) in 4 randomly selected cases. They had clinically documented a thickened band of regenerated tissue in all 225 patients. Eriksson et al.18 found regeneration of semitendinosus tendons on MRI, with “normal anatomical topography” in 8/11 patients.
Papandrea et al.26 used serial ultrasonography (USG) to document regeneration in 40 patients at 24 months of follow up; the regenerated tissue appeared “very similar” to the normal semitendinosus.
Rispoli et al.27 noted “apparent regeneration” on MRI of both the tendons in all 21 patients, which gradually extended distally over time. This was the first time that sequential MRI was used to assess the progression and the direction of regeneration, from the proximal stump towards the pes anserinus (lizard tail effect).43
Nakamura et al.39 evaluated the regeneration of harvested semitendinosus tendon using three-dimensional computer tomography (3-D CT) and MRI; 5/8 patients showed “tendinous tissue regeneration” at the harvest site.
Tadokoro et al.42 showed that there was variable regeneration of semitendinosus tendons in 22/28 patients; 6 patients had hypertrophic and 16 had atrophic regeneration of the semitendinosus tendons. In comparison, only 13 patients showed regeneration of the gracilis (4 hypertrophic, 9 atrophic).
Williams et al.31 using MRI, documented semitendinosus tendon regeneration in 7/8 patients, however for gracilis tendons, regeneration was inconclusive with “variable extent”.
Burks et al.8 noted “some evidence” of regeneration of semitendinosus and gracillis tendon in 9 patients using MRI.
Nakamae et al. (2005)22 using 3D-CT, found “evidence of regeneration” in 27/29 patients; however, the quality of regeneration invariably differed, with tendon hypoplasia in 6, “normal morphology” in 11 and hyperplasia in 3 patients.
Takeda et al.29 demonstrated evidence of semitendinosus tendon regeneration in 11/11 patients using MRI, with normal anatomical morphology at or below the joint level. However, only 9 gracilis tendons showed regeneration and none of these were observed below the joint line. This suggests that although both the tendons regenerated, the extent of the proximal to distal regeneration differed between the tendons.
Nishino et al.23 found regenerated semitendinosus “tendon-like structure” in 21/23 patients using MRI. In a subsequent study, comparing the effect of immobilization versus early mobilization on tendon regeneration, Nakamae et al. (2012)40 documented regeneration of 38/39 semitendinosus tendons with MRI, with no effect of postoperative immobilization on the regeneration potential of harvested tendons.
Murakami et al.21 using MRI, evaluated the so called “inducer grafting” technique for regeneration of the semitendinosus tendon and found regeneration in all 20 patients (hypertrophic:19). They used a thread to anchor the residual semitendinosus to the distal insertion through the original tendon graft, which was felt to improve regeneration rates.
Choi et al.17 showed regeneration of semitendinosus tendons in 36/45, and the gracilis in 34/45 patients on MRI by evaluating the change in signal intensity at the donor site, at the level of knee joint and 3.5 cm above. If signals from regenerating tendons were not visualized, it was considered a failure of regeneration.
Alhen et al., in 201233 showed regeneration of semitendinosus in 17/19 and gracilis tendons in 18/19 patients on MRI at a median follow up of 8.5 years. The progression of regenerate was described in relation to the medial joint compartment and pes anserinus.
Janssen et al.20 used MRI and noted regeneration of the gracilis tendons in all 22/22, while the semitendinosus tendon was documented in 14/22 patients. Tendon regeneration was assessed by evaluating the progression of harvested hamstring tendon in relation to the joint line.
Similar variable degrees of regeneration were shown in other studies that utilized MRI [16,24,30,32,37,38,44] with varying degrees of structural similarity to the normal tendon.
Overall in terms of modalities used, MRI was used in 23/30 studies [7,8,16, 17, 18, 19, 20, 21,23,24,27,29, 30, 31, 32, 33,36, 37, 38, 39,41,42,44]; it was used in isolation in 19 studies [7,8,16, 17, 18,20,21,23,24,27,29, 30, 31, 32, 33,37,41,42,44], and in combination with other modalities in 4 studies.19,36,38,39 It detected some form of regeneration of tendon in 389/450 cases, with a detection rate of 86.4%. [Table-3] MRI was also able to delineate the regenerated tendons as semitendinosus and gracilis separately in 15 studies [7,8,16,17,20,27,29, 30, 31, 32, 33,37,38,42,44] (362 knees). [Table-4] Detection of regeneration rates were similar with MRI alone, as compared to combination of methods [122/150 cases (81.3%)]; the combination of more than 1 diagnostic radiological tool may not be necessary. Less commonly used methods of evaluation (2 studies each) were USG, 3D-CT-scan and biopsies [22,25,26,34,35,40]. [Table-3].
Table-3.
Distribution of different methods of assessment for regeneration used.
| Methods of assessment | Number of studies | Total number of knees evaluated |
|---|---|---|
| MRI as single modality | 19/30 | 450 |
| MRI with USG | 1/30 | 120 |
| MRI with 3D-CT | 1/30 | 8 |
| MRI with USG guided biopsy | 2/30 | 22 |
| USG | 2/30 | 58 |
| 3D-CT | 2/30 | 68 |
| Biopsy/Histopathology | 3/30 | 79 |
| Total | 30/30 | 805 |
Table- 4.
Distribution of regeneration of tendons across different studies.
| Sl No | Authors | Method of assessment | Total cases | ST | G | Both ST and G | Total regeneration |
|---|---|---|---|---|---|---|---|
| 1. | Cross et al. 19927 | MRI | 4a | 4/4 | 4/4 | 4/4 | 4 |
| 2. | Eriksson et al.199918 | MRI | 11 | 8/11 | 0/11 | 0 | 8 |
| 3. | Papandrea et al.200026 | USG | 40 | 40/40 | 0/40 | 0 | 40 |
| 4. | Eriksson et al. 200136 | MRI/Biopsy | 6 | 5/6 | 0/6 | 0 | 5 |
| 5. | Eriksson et al.200119 | MRI/USG-guided biopsy | 16 | 12/16 | 0/16 | 0 | 12 |
| 6. | Rispoli et al.200127 | MRI | 20 | 20/20 | 20/20 | 20/20 | 20 |
| 7. | Nakamura et al.200439 | 3D-CT, MRI | 8 | 5/8 | 0/8 | 0 | 5 |
| 8. | Tadokoro et al.200442 | MRI | 28 | 22/28 | 13/28 | NR | 22 |
| 9 | Williams et al.200418 | MRI | 8 | 7/8 | 8/8 | NR | 7 |
| 10. | Burks et al.20058 | MRI | 9 | 9/9 | 9/9 | 9/9 | 9 |
| 11 | Nakamae et al.200522 | 3D-CT | 29 | 27/29 | NR | NR | 27 |
| 12. | Takeda et al.200629 | MRI | 11 | 11/11 | 9/11 | 11 | |
| 13. | Okahashi et al.200625 | Surgical Biopsy | 11 | 7/11 | NR | NR | Single tendon, no differentiation in to ST or G |
| 14. | Nishino et al.200623 | MRI | 23 | 21/23 | 0/23 | 21 | |
| 15. | Nakamae et al.201240 | 3D-CT | 39 | 38/39 | 0/39 | 38 | |
| 16. | Snow et al.201241 | MRI | 10 | NR | NR | NR | Tissue visible cannot be identified as tendon |
| 17. | Murakami et al.201221 | MRI | 20 | 20/20 | 0/20 | 20 | |
| 18. | Choi et al.201217 | MRI | 45 | 36/45 | 34/45 | 30/45 | 36 |
| 19. | Alhen et al.201233 | MRI | 19 | 17/19 | 18/19 | 17 | |
| 20. | Bedi et al.201335 | USG | 15 (18 knees) | NR | NR | NR | Tissues identified, did not have structural and functional properties similar to tendons. |
| 21. | Janssen et al.201320 | MRI | 22 | 14/22 | 22/22 | 14/22 | 22 |
| 22. | Stevanovic et al.201328 | USG/USG guided Biopsy | 50 | 33/50 | 0/50 | 33 | |
| 23. | Alhen et al.201434 | USG guided Biopsy | 18 | 18/18 | NR | NR | 18 |
| 24. | Nomura et al.201424 | MRI | 24 | 21/24 | 0/24 | 21 | |
| 25. | Zaccherotti et al.201532 | MRI | 20 | 14/20 | 14/20 | 14/20 | 14 |
| 26. | Tsifountoudis et al.201530 | MRI | 47 | 40/47 | 41/47 | 5/47 | 41 |
| 27. | Konrath et al.201637 | MRI | 20 | 8/20 | 12/20 | 7/20 | 12 |
| 28. | Suijkerbuijk et al. 201812 | MRI | 79 | 53/79 | 64/79 | 47/79 | 64 |
| 29. | Albertoni et al.201816 | MRI | 29 (30 knees) | 25/30 | 30/30 | 25/29 | 30 |
| 30. | Lee et al.201938 | MRI and USG | 120 | 100/120 | 46/60 | 46/120 | 97 |
| Total | 805 | 635 | 344 | 213 |
out of 225 patients only 4 were analyzed in detail for regeneration of hamstring tendon, ST- Semitendinosus, G- Gracilis, NR- Not reported, MRI- magnetic resonance imaging, USG-ultrasonography, 3D-CT-three-dimensional computer tomography.
Snow et al.41 used MRI in 10 patients and demonstrated persistent atrophy, fatty infiltration and variability in tendon regeneration. Even at 9–11years of follow up, they were unable to determine whether tissue visible was the actual regenerated tendons or just scar tissue. Bedi et al.35 showed the presence of intervening tissue in the harvest gap in 9/18 knees on USG, which had no structural similarity to normal semitendinosus tendon. Therefore, although MRI and USG can determine the presence of tissue regeneration, these may not be reliable in differentiating between scar tissue and tendon regenerate; histopathological examination is perhaps the only modality confirming the actual nature of the regenerate.
Is the tendon regeneration partial or complete to distal insertion?
Whether or not the entire length of the harvested tendon bed gets refilled with the regenerate is a point of contention. The regeneration progresses from proximal to distal (lizard tail effect)43 and is considered complete if it reaches up to the insertion site at the pes anserinus (not necessarily attaching at the native site). This is the definition of complete regeneration the we have adopted in the current review; 16/30 studies7,8,16,18,19,21, 22, 23, 24, 25, 26, 27, 28,30,31,33 have discussed this aspect of regeneration.
Several studies found the majority of their cases have partial regeneration. Cross et al.7 observed that the regeneration of all the 4 harvested tendons were incomplete, as the location of the insertion of “neo-tendons” appeared varied, and was documented “diffusely in the medial popliteal fascia”, instead of the pes anserinus.
Papandrea et al.26 found regeneration of semitendinosus as incomplete in all 40 patients at 2 years post-surgery, as they were attached to the medial popliteal fascia 4 cm proximal to native site. USG showed echo-structure of regenerated tissue and distinct edges similar to normal tendons.
Rispoli et al.27 documented incomplete regeneration in all their 20 patients, with “normal structural topography”, while 5/8 cases of Nakamura et al.24 were defined as” incomplete”.
Williams et al.31 observed partial regeneration of both the semitendinosus and gracilis in 7/8 patients and Burks et al.8 observed partial regeneration in 8/9 cases with the distal extent of the tendons not changing between 3 and 12months; tendons however became more clearly defined over time. In 1 case both tendon regenerates extended near to the distal attachment site and appeared complete.
Nakamae et al. (2005)22 termed 27/29 semitendinosus regenerates as partial, as they originated from the muscle belly and inserted indistinctly into the anteromedial aspect of the proximal tibia.
In contrast, Eriksson et al. (1999)18 had mixed findings, where they described the regeneration as complete (with normal anatomical topography) in 3/8 cases and as partial in the remaining 5/8 cases; normal anatomical morphology was observed only in 2 of these 5 partially regenerated tendons.
In another study, Eriksson et al. (2001)19 termed regeneration as complete in 12/16 patients with the neo-tendon at varying distances of 1–3 cm below the joint line, adjoining the pes anserinus; 1 case had partial regenerate proximal to the joint line, while 3 had no regeneration.
Okahashi et al.25 demonstrated complete regeneration of the hamstring tendons in 9/11 cases, with the regenerate reaching up to the normal insertion site.
Murakami et al.21 demonstrated complete semitendinosus regeneration with homogeneous low-intensity structure, extending diffusely to the levels of the superior poles of the patellae, the joint lines, and the pes anserinus, in 16/20 patients.
Alhen et al. (2012)33 showed complete regeneration of semitendinosus (17/19) and the gracilis tendons (18/19) also had complete regeneration, with reinsertion at the pes anserinus.
Stevanovic et al.28 evaluated 2 subsets, with isolated semitendinosus harvest (group A) and both hamstring tendons harvest (group B); partial regeneration of 18/25 tendons in group A and 15/25 in group B were observed, with the insertion of the semitendinosus not clearly identified at anatomical site in the pes anserinus.
Tsifountoudis et al.30 noted variable findings with “complete” regeneration of semitendinosus tendon in 30/47 patients and partial in 10/47. Complete regeneration of gracilis tendons was found in only 17/47 and incomplete regeneration in 24/47 patients. The “complete” regeneration was defined as the presence of regenerated tissue extending below the joint line and traced to different levels down to the pes anserinus insertion.
Variable findings were also noted by Albertoni et al.16 with 22/30 complete regenerations reaching up to the pes anserinus, 11 each for the semitendinosus and gracilis. Incomplete regeneration was observed in 14 semitendinosus and 19 gracilis tendons.
Nishino et al.23 observed regeneration of the semitendinosus in 21/23 patients; although the regenerates crossed the knee joints inserting into the distal structures, the differentiation between complete and incomplete was not clear.
Do both tendons regenerate separately, or as one unit after harvesting?
Whether the individual tendons (semitendinosus/gracilis) demonstrate any difference in extents and rates of regeneration, and do they regenerate individually or as a unit are important points to be considered. 14/307,8,16, 17, 18,20,29,30,32,33,37,41,42,44 studies discussed this aspect.
Cross et al.7 mentioned that the distal insertions of both the tendons were clinically palpable in all 225 cases, albeit not at the usual insertion site. They also mentioned that proximally the tendons were palpable up to their muscle bellies.
Eriksson et al. (1999)18 observed that in 3/8 tendons which regenerated distal to the knee, both the tendons fused together, approximately 30 mm below the joint line, before inserting into the pes anserinus.
Burks et al.8 noted 6/9 tendons of regenerated tendons to either merge into the muscle belly of the semimembranosus or with the sartorius or superficial fascia, 4–13 cm above the joint line. Both the tendons were merged with the sartorius in 2 cases, just above the joint line. Only 1 case had both tendons attached to the pes anserinus. Overall the regenerates were very diffuse in appearance and distinction between the tendons was difficult.
6/10 patients evaluated by Snow et al.41 had the gracilis blended with the sartorius, and the semitendinosus blended with semimembranosus or medial gastrocnemius fascia at or proximal to the joint line. 2 patients had distinct structures extending distal to the joint line and then confluencing.
Tadokoro et al.42 noted a higher incidence of tissue regeneration in the semitendinosus (22/28) than the gracilis tendon (13/28). Similarly, Takeda et al.29 documented more semitendinosus regeneration than gracilis (11 versus 8); additionally, the former regenerated more distally, with 1 till the joint line. Three regenerates were noted 0.5 cm, 3 at 1 cm, 1 at 1.5 cm and 3 others 2 cm below the joint lines. In comparison, none of the 8 gracilis tendon regenerates were observed below the joint line. The most distal level of regeneration (compared to non-operated knees) was 1.3 cm for semitendinosus and 2.7 cm for the gracilis tendons.
Konrath et al.37 reported 7/20 patients with regeneration of both tendons; in cases where only 1 tendon regenerated, gracilis regeneration was superior to semitendinosus (5 versus 1).
Suijkerbuijk et al.44 reported regeneration in 64/79 semitendinosus and 47/79 gracilis tendons; 47 patients had regeneration of both the tendons.
Albertoni et al.16 identified 25 regenerated semitendinosus and 30 gracilis tendons in 30 cases. The extent of both the tendons were comparable with 14/25 semitendinosus and 16/30 gracilis regenerates beyond the medial femoral condyle.
Choi et al.17 observed regeneration of semitendinosus and gracilis tendons in 36/45 and 34/45 patients respectively; two distinct tendons were found separately in 30/45 patients.
Similar numbers of both tendon regenerates (17 & 18 out of 19) were noted by Alhen et al. (2012)33; Janssen et al.20 documented that both semitendinosus and gracilis regenerated distinctly in 22 cases. Zaccherotti et al.32 observed that both the tendons regenerated separately (14/20 patients), progressing beyond the joint line (23.4 mm and 13.5 mm respectively). Tsifountoudis et al.30 observed regeneration of semitendinosus in 40 and gracilis in 41/47 patients separately, with confluent anatomic regeneration in 5 patients.
Is there any proximal retraction of the muscle belly with changes in its structure and strength?
When a hamstring tendon is harvested, the muscle belly could potentially retract proximally; the “neo-tendon” regenerates from that point extending distally. It is important to note this retraction, which may dictate the structure, completeness and the strength of the regenerate. Proximal retraction in respect to the muscle strength was evaluated in 7 studies.17,22,23,30,35,38,40
Nakamae et al. (2005)22 observed the shift of semitendinosus muscle-tendon junction proximally by an average of 7.3 ± 2 cm at 12 months in 27 patients with regeneration of tendons. The nature of these regenerates had correlation with peak torque ratios of the muscles at 6 months but none at 1 year; the proximal shift decreased the hamstring muscle strength, which however was temporary.
The common notion of early knee movements potentially increasing the chance of proximal retraction of the muscle belly was subsequently studied by Nakamae et al. (2012)40; they evaluated the effect of knee immobilization (long versus short periods) on morphological changes in the semitendinosus muscle–tendon complex and observed an average proximal shift by 7.3 ± 2.5 cm, and 7.2 ± 1.9 cm respectively. The width of the regenerated tendons was approximately 1.5 times greater than normal side with no significant effect on the peak torque ratios. Overall, they found no effect of knee movements on the proximal retraction of the muscle belly and on muscle strength.
Choi et al.17 observed a proximal shift by an average of 4.39 ± 2.24 cm for semitendinosus and 3.09 ± 2.22 cm for gracilis tendons. There was no significant difference in the cross-sectional area (CSA) of the tendons, in pre and post-operative evaluations. The magnitude of the proximal shift had no correlation with a flexor deficit in the sitting position but was significantly correlated in the prone position isokinetic test.
Bedi et al.35 observed average proximal retraction of 9.0 ± 7.6 cm when compared to the contralateral muscle–tendon unit. There was no regeneration in 9/18 knees. The authors could not ascertain that the gap tissue was regenerated tendon or just scar tissue and concluded that with the proximal retraction of the muscle–tendon junction and disorganized regenerated tissue, the “neo-tendon” did not demonstrate characteristics of the normal muscle-tendon unit. However, whether or not the proximal retraction lead to disorganized regenerate was not clear.
Tsifountoudis et al.30 observed that the donor stumps were retracted in 41/47 patients, which was mild to moderate in 34 semitendinosus and 38 gracilis muscles. Mild fatty infiltration was found in 25/47 semitendinosus and 16/47 gracilis muscles. 27/40 semitendinosus regenerates were found to be hypertrophic compared to the contralateral normal side. Whether or not proximal migration lead to these characteristics is not clear.
Lee et al.38 observed proximal shift of semitendinosus stump by 3.8 ± 2.1 cm and 4.2 ± 2.2 cm in ACLR with only semitendinosus and with both tendons, respectively. The CSA of regenerated tendons was not different from preoperative status and tissue was similar to the normal side semitendinosus. However, there was significant knee extension and flexion deficits compared to uninvolved knee on isokinetic muscle strength testing, which could have been because of the proximal retraction itself.
Nishino et al.23 noted the length of semitendinosus in 19/23 of the reconstructed limbs (24.3 ± 4.3 cm) to be significantly shorter than the contralateral limbs (28.1 ± 3.1 cm), which was attributed to the proximal shift of the stumps decreasing the volume of the tendons. Additionally, the isometric knee-flexion torque of the involved limb was lower than the contralateral limb.
Is there any residual strength deficit in the regenerated hamstrings after ACL reconstruction?
Whether or not, and to what extent, the harvested tendons regenerate becomes clinically important if there are residual strength deficits in the regenerate and functional alterations in the patients. It becomes crucial to ascertain the strength of the neo-tendon in comparison to the preoperative status, or the normal side. 18/30 studies had evaluated the relation between regeneration of tendon and muscle strength.7,8,17,19, 20, 21, 22, 23, 24,28,29,32, 33, 34,37,38,40,42
Cross et al.8 noted that in 3/4 MRI proven cases (75%), the regenerates exhibited less than 10% decrease in knee flexion and extension strengths, compared to the non-operated side.
Other studies have given variable results. Eriksson et al. (2001)19 observed that there was no statistically significant difference in hamstring strength between the regenerated and non-regenerated groups. Additionally, there was no correlation between the total thigh muscle area and the hamstring isokinetic peak torque in either the operated or non-operated legs. The functional outcomes utilizing the Lysholm scores also revealed no statistically significant difference between the regenerated and non-regenerated tendon groups, showing no clinical relevance of regeneration.
Similarly, Stevanovic et al.28 observed that the isokinetic strength of the hamstrings and quadriceps were not significantly diminished on the operated side. The hamstring strength deficit evaluation showed no changes in athletic function, and it was postulated that the strength of the harvested tendons was not disturbed ultimately, despite of only 33/50 cases having some regeneration.
Nakamae et al. (2005)22 did not observe any correlation between the peak torque ratio and the types of regenerated tendons at 1 year and in their subsequent study as well, Nakamae et al. (2012)40 observed no significant relationship between the peak torque ratio and either the distal extent or the width of the regenerate.
On the other hand, Tadokoro et al.42 found that the hamstring strength of the operated knees were significantly lower than that of the non-operated knees, regardless of the extent of tissue regeneration (hypertrophic, atrophic, or unidentifiable regeneration).
Konrath et al.37 documented that knee flexion, extension, and internal rotation strengths were significantly lower in the operated limb. The deficit in volume, peak CSA, and length of the semitendinosus and gracilis correlated significantly with the corresponding deficit in knee flexion strength.
Nomura et al.24 also observed that the isometric knee flexion torque in the operated limb was significantly lower than that in the normal limb at 600, 900, and 1050. Burks et al.8 demonstrated a transient phase of significant strength deficit; at 6 months post-operatively there was significant weakness of hamstring strength on the operated side compared with the non-operated side, but the strength increased over the subsequent 6 months.
Takeda et al.29 demonstrated no significant correlation between the increase in T2 signal of both the semitendinosus and gracilis muscles on MRI, in the operated leg and the isokinetic strength ratios. Their findings imply that the increased signal signified extent of regeneration, but had no relation with the muscle strength, and there might be no clinical implications as such. On the other hand, Choi et al.17 observed approximately >4 times flexor strength deficit in patients with no tendon regeneration compared to patients with regenerated tendons. Furthermore, a moderate correlation was found between the number of regenerated hamstring tendons and the standard isokinetic test.
Similarly, Nishino et al.23 showed that strength of hamstring muscle was greatest when the semitendinosus tendon regenerated and had length comparable to the non-operated side. Murakami et al.21 also observed a significant difference in isometric knee flexion torque between the operated and non-operated knees.
Alhen et al. (2012)33 also observed a significant flexion strength deficit in the operated legs; internal rotation of the tibia on the operated side did not differ with the non-operated side. In a later study, Alhen et al. (2014)34 found a significant strength deficit on the involved side with median Tegner activity level of 6 (range 5–7) & median Lysholm score of 87 (range 47–100). In both these studies the tendons “regenerated completely”, but despite strength deficits the overall effect on the post-operative functional status was not significant.
Janssen et al.20 did not report differences in flexion and extension strength between patients where both hamstring tendons regenerated compared with patients in whom only one tendon regenerated. Their findings suggest that regeneration of 1 tendon could be enough for adequate muscle strength, and there is probably no difference, whether the two tendons regenerate separately, or fuse, or 1 of them does not regenerate at all.
The findings of limited or no role of regeneration and strength deficit on functional outcomes were also substantiated by Zaccherotti et al.,32 who observed insignificant deficits in flexion and internal rotation strengths in cases of tendon regenerations (14/20). None of the patients without regeneration of tendons (6/20) also had no impairment in their sports activities despite internal rotation strength deficits. All patients regained their pre-injury sport level probably due to a compensatory action of the other knee flexors.
Lee et al.38 observed that even though there was no significant difference in functional performance tests (IKDC- subjective knee score, Lysholm score, and Tegner activity scale) between regeneration and no-regeneration groups, the former showed better scores. Isokinetic muscle strength tests showed significant knee extension and flexion deficits were present in all operated limbs compared with normal sides. Overall, the functional outcomes were better in patients who had regeneration, but the muscle strength did not reach the levels of the normal side.
11. Discussion
The present review suggests that regeneration of tissue at the site of hamstrings harvest does occur (78.9% of semitendinosus and 42.7% of gracilis tendons); however, the extent and degree of this regeneration is variable. The possible explanation for this variability could be that the evaluation of the regeneration was done at different points of time in different studies, which was also not sequential and modalities used for evaluation were also different. There is also no clearly established definition of regeneration per se. MRI effectively identifies “regenerated tissues”, but differentiating tendon morphology from scar tissue is difficult, and biopsies are the best for confirmation. USG besides being more cost effective than MRI, has considerable efficacy in identifying regeneration and also is more readily available with provisions of bedside utilization. It can evolve as an effective screening method to identify the presence or absence of tissue regeneration and can document distal progress of regenerate.
The characterization of regeneration as partial or complete after hamstring tendon harvest is determined on basis of “reaching up to the pes anserinus” for complete regeneration; whether or not the regenerate inserts at the original footprint remains unclear. The results in the review are extremely variable with different numbers of complete and partial regenerations being documented; we found that the partial regeneration seems to be more common but cannot give conclusive answers to the question about degree of regeneration. As explained by the Warren and Marshall,45 the gracilis and semitendinosus tendons are invested in fascia on medial aspect of the knee between layers I and II and the tendons become conjoint as they get inserted into the pes anserinus. Proximally the tendons are adherent to the deep thigh fascia and connected to the medial intermuscular septum. This arrangement of the tissue can explain the more proximal regeneration in vascularized area which proceeds to less vascular area along the fascial planes distally, the degree of which determines the completeness.27,46
We also assessed whether the gracilis and semitendinosus regenerate separately, as a single unit, and to similar or different extents, but the evidence from the current literature is insufficient to state these with certainty.
It is our observation from this review that proximal retraction of the muscle-tendon junction has been observed by many authors, with no effect on this by postoperative immobilization. The literature suggests that with retraction, some atrophy may also be present which could affect function to a variable extent, and this may affect the knee flexion strength because of shortening of the moment arm47
The other important finding is the fact that despite incidence of variable regeneration and flexion strength deficits after hamstrings harvests, final functional outcomes are generally acceptable; there is no reported correlation with strength deficits and in some cases with the extent and nature of regeneration. The deficit in flexion strength can be explained by the fact that the even after regeneration the harvested tendons do not get inserted at the native site; but proximal as well as distal insertions have been reported.7,17,26,31 More proximally inserted or retracted muscle belly shortens the knee flexion moment arm, and the remodeled structure is unable to produce the expected force during the muscle contraction.48 This is particularly notable at high grades of flexion because at lower grades, the biceps femoris and the semimembranosus are the main force producers able to compensate any hamstring strength loss.31,41 The limitations of our review are, variable sample size of the included studies and the different methods of evaluation was used for the assessment of regeneration in different included studies, these might have influence on our results.
12. Conclusions
A systematic review of the published literature reveals a lack of clarity about what defines regeneration of hamstring tendons after harvest. Some tissue regenerates, and that too to a variable extent, although there may be no regeneration in a few cases too. MRI is an effective tool to document the tissue regeneration, although biopsy is the only confirmation that the regenerate is actually tendon tissue similar to the original one. USG is better in the sense that it is cheap, easily mastered and can be serially used to document distal progression of the regenerate over time. There is evidence to suggest that the tendon does not reattach to its original site, the muscle stump retracts proximally, and atrophies to a variable extent; there is however no evidence to suggest that either the semitendinosus or gracilis tendon regenerate more than the other, although reports indicate that these can often fuse into one unit after harvest. Significant hamstring strength deficits have been documented, with variable correlation to the extent of tissue/tendon regeneration, but these may not actually convert into significant functional deficits at the knee; this maybe a compensatory mechanism of the body, especially in athletes. Nevertheless as the literatures suggests that there are significant anatomic variations in the regenerate, long term effects of this need to be studied.
Declaration of competing interest
None.
Acknowledgement
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jcot.2021.02.011.
Contributor Information
Mandeep S. Dhillon, Email: drdhillon@gmail.com.
Rajesh Kumar Rajnish, Email: duktiraj@gmail.com.
Sidak Dhillon, Email: sidakdh@gmail.com.
Prasoon Kumar, Email: drprasoonksingh@gmail.com.
Source of funding
None.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Kohn D., Sander-Beuermann A. Donor-site morbidity after harvest of a bone–tendon–bone patellar tendon autograft. Knee Surg Sports Traumatol Arthrosc. 1994;2:219–223. doi: 10.1007/BF01845591. [DOI] [PubMed] [Google Scholar]
- 2.Miller M.D., Nichols T., Butler C.A. Patella fracture and proximal patellar tendon rupture following arthroscopic anterior cruciate ligament reconstruction. Arthroscopy. 1999;15:640–643. doi: 10.1053/ar.1999.v15.015064. [DOI] [PubMed] [Google Scholar]
- 3.Sachs R.A., Daniel D.M., Stone M.L. Garfein RF Patellofemoral problems after anterior cruciate ligament reconstruction. Am J Sports Med. 1989;17:760–765. doi: 10.1177/036354658901700606. [DOI] [PubMed] [Google Scholar]
- 4.Shuette H.B., Kraeutler M.J., Houck D.A., McCarty E.C. Bone-Patellar tendon-bone versus hamstring tendon autografts for primary anterior cruciate ligament reconstruction: a systematic review of overlapping meta-analyses. Orthop J Sports Med. 2017;5(11) doi: 10.1177/2325967117736484. 2325967117736484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie X., Liu X., Chen Z., Yu Y., Peng S., Li Q. A meta-analysis of bone-patellar tendon-bone autograft versus four-strand hamstring tendon autograft for anterior cruciate ligament reconstruction. Knee. 2015;22(2):100–110. doi: 10.1016/j.knee.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Zin A.M.N., Ali M., Zenhom Mahmoud A., Omran K. Autogenous hamstring-bone graft preparation for anterior cruciate ligament reconstruction. Arthrosc Tech. 2017;6(4):e1253–e1262. doi: 10.1016/j.eats.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cross M.J., Roger G., Kujawa P., Anderson I.F. Regeneration of the semitendinosus and gracilis tendons following their transection for repair of the anterior cruciate ligament. Am J Sports Med. 1992;20(2):221–223. doi: 10.1177/036354659202000223. [DOI] [PubMed] [Google Scholar]
- 8.Burks R.T., Crim J., Fink B.P., Boylan D.N., Greis P.E. The effects of semitendinosus and gracilis harvest in anterior cruciate ligament reconstruction. Arthroscopy. 2005;21:1177–1185. doi: 10.1016/j.arthro.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Yoshiya S., Matsui N., Matsumoto A., Kuroda R., Lee S., Kurosaka M. Revision anterior cruciate ligament reconstruction using the regenerated semitendinosus tendon: analysis of ultrastructure of the regenerated tendon. Arthroscopy. 2004;20(5):532–535. doi: 10.1016/j.arthro.2004.01.031. [DOI] [PubMed] [Google Scholar]
- 10.Chalmers P.N., Mall N.A., Moric M. Does ACL reconstruction alter natural history? A systematic literature review of long-term outcomes. J Bone Joint Surg Am. 2014;96:292–300. doi: 10.2106/JBJS.L.01713. [DOI] [PubMed] [Google Scholar]
- 11.Papalia R., Franceschi F., D’Adamio S., Diaz Balzani L., Maffulli N., Denaro V. Hamstring tendon regeneration after harvest for anterior cruciate ligament reconstruction: a systematic review. Arthroscopy. 2015;31(6):1169–1183. doi: 10.1016/j.arthro.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Suijkerbuijk M.A.M., Reijman M., Lodewijks S.J., Punt J., Meuffels D.E. HamstringTendon regeneration after harvesting: a systematic review. Am J Sports Med. 2015;43(10):2591–2598. doi: 10.1177/0363546514562169. [DOI] [PubMed] [Google Scholar]
- 13.Moher D., Liberati A., Tetzlaff J., Altman D.G., Prisma Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT, Altman DG, Gotzsche PC, etal. Cochrane bias methods group Cochrane statistical methods group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ2011;343:d5928. [DOI] [PMC free article] [PubMed]
- 15.Slim K., Nini E., Forestier D., Kwiatkowski F., Panis Y., Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 16.Albertoni L.J.B., Debieux P., Franciozi C.E.D.S., Novaretti J.V., Granata G.S.M., Jr., Luzo M.V.M. Assessment OF the regeneration capacity OF semitendinosus and gracilis tendons. Acta Ortopédica Bras. 2018;26(6):379–383. doi: 10.1590/1413-785220182606168849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi J.Y., Ha J.K., Kim Y.W., Shim J.C., Yang S.J., Kim J.G. Relationships among tendon regeneration on MRI, "exor strength, and functional performance after anterior cruciate ligament reconstruction with hamtring autograft. Am J Sports Med. 2012;40:152–162. doi: 10.1177/0363546511424134. [DOI] [PubMed] [Google Scholar]
- 18.Eriksson K., Larsson H., Wredmark T., Hamberg P. Semitendinosus tendon regeneration after harvesting for ACL reconstruction. A prospective MRI study [published correction appears in Knee Surg Sports Traumatol Arthrosc 2001;9(1. Knee Surg Sports Traumatol Arthrosc. 1999;7(4):54–55. doi: 10.1007/s001670050152. 220-225. [DOI] [PubMed] [Google Scholar]
- 19.Eriksson K., Hamberg P., Jansson E., Larsson H., Shalabi A., Wredmark T. Semitendinosus muscle in anterior cruciate ligament surgery: morphology and function. Arthroscopy. 2001;17:808–817. doi: 10.1016/s0749-8063(01)90003-9. [DOI] [PubMed] [Google Scholar]
- 20.Janssen R.P., van der Velden M.J., Pasmans H.L., Sala H.A. Regeneration of hamstring tendons after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2013;21:898–905. doi: 10.1007/s00167-012-2125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murakami H., Soejima T., Inoue T. Inducement of semitendinosus tendon regeneration to the pes anserinus after its harvest for anterior cruciate ligament reconstruction-A new inducer grafting technique. Sports Med Arthrosc Rehabil Ther Technol. 2012;4(1):17. doi: 10.1186/1758-2555-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamae A., Deie M., Yasumoto M. Three-dimensional computed tomography imaging evidence of regeneration of the semitendinosus tendon harvested for anterior cruciate ligament reconstruction: a comparison with hamstring muscle strength. J Comput Assist Tomogr. 2005;29:241–245. doi: 10.1097/01.rct.0000153779.86663.92. [DOI] [PubMed] [Google Scholar]
- 23.Nishino A., Sanada A., Kanehisa H., Fukubayashi T. Knee-flexion torque and morphology of the semitendinosus after ACL reconstruction. Med Sci Sports Exerc. 2006;38(11):1895–1900. doi: 10.1249/01.mss.0000230344.71623.51. [DOI] [PubMed] [Google Scholar]
- 24.Nomura Y., Kuramochi R., Fukubayashi T. Evaluation of hamstring muscle strength and morphology after anterior cruciate ligament reconstruction. Scand J Med Sci Sports. 2015;25(3):301–307. doi: 10.1111/sms.12205. [DOI] [PubMed] [Google Scholar]
- 25.Okahashi K., Sugimoto K., Iwai M. Regeneration of the hamstring tendons after harvesting for arthroscopic anterior cruciate ligament reconstruction: a histological study in 11 patients. Knee Surg Sports Traumatol Arthrosc. 2006;14:542–545. doi: 10.1007/s00167-006-0068-z. [DOI] [PubMed] [Google Scholar]
- 26.Papandrea P., Vulpiani M.C., Ferretti A., Conteduca F. Regeneration of the semitendinosus tendon harvested for anterior cruciate ligament reconstruction. Evaluation using ultrasonography. Am J Sports Med. 2000;28:556–561. doi: 10.1177/03635465000280041901. [DOI] [PubMed] [Google Scholar]
- 27.Rispoli D.M., Sanders T.G., Miller M.D., Morrison W.B. Magnetic resonance imaging at different time periods following hamstring harvest for anterior cruciate ligament reconstruction. Arthroscopy. 2001;17:2–8. doi: 10.1053/jars.2001.19460. [DOI] [PubMed] [Google Scholar]
- 28.Stevanović V., Blagojević Z., Petković A. Semitendinosus tendon regeneration after anterior cruciate ligament reconstruction: can we use it twice? Int Orthop. 2013;37(12):2475–2481. doi: 10.1007/s00264-013-2034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeda Y., Kashiwaguchi S., Matsuura T., Higashida T., Minato A. Hamstring muscle function after tendon harvest for anterior cruciate ligament reconstruction: evaluation with T2 relaxation time of magnetic resonance imaging. Am J Sports Med. 2006;34:281–288. doi: 10.1177/0363546505279574. [DOI] [PubMed] [Google Scholar]
- 30.Tsifountoudis I., Bisbinas I., Kalaitzoglou I. The natural history of donor hamstrings unit after anterior cruciate ligament reconstruction: a prospective MRI scan assessment. Knee Surg Sports Traumatol Arthrosc. 2017;25(5):1583–1590. doi: 10.1007/s00167-015-3732-3. [DOI] [PubMed] [Google Scholar]
- 31.Williams G.N., Snyder-Mackler L., Barrance P.J., Axe M.J., Buchanan T.S. Muscle and tendon morphology after reconstruction of the anterior cruciate ligament with autologous semitendinosus-gracilis graft. J Bone Joint Surg Am. 2004;86:1936–1946. doi: 10.2106/00004623-200409000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Zaccherotti G., Olmastroni M. Muscle strength recovery versus semitendinosus and gracilis tendon regeneration after harvesting for anterior cruciate ligament reconstruction. J Sports Sci. 2015;33(20):2149–2156. doi: 10.1080/02640414.2015.1066930. [DOI] [PubMed] [Google Scholar]
- 33.Ahlen M., Liden M., Bovaller A., Sernert N., Kartus J. Bilateral magnetic resonance imaging and functional assessment of the semitendinosus and gracilis tendons a minimum of 6 years after ipsilateral harvest for anterior cruciate ligament reconstruction. Am J Sports Med. 2012;40(8):1735–1741. doi: 10.1177/0363546512449611. [DOI] [PubMed] [Google Scholar]
- 34.Ahlen M., Liden M., Movin T., Papadogiannakis N., Rostgard-Christensen L., Kartus J. Histological evaluation of regenerated semitendinosus tendon a minimum of 6 Years after harvest for anterior cruciate ligament reconstruction. Orthop J Sports Med. 2014;2(9) doi: 10.1177/2325967114550274. 2325967114550274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bedi A., Srinivasan R.C., Salata M.J., Downie B., Jacobson J.A., Wojtys E.M. Structural and functional analysis of the semitendinosus tendon after harvest for soft tissue reconstructive procedures: a dynamic ultrasonographic study. Knee Surg Sports Traumatol Arthrosc. 2013;21:606–614. doi: 10.1007/s00167-012-1989-3. [DOI] [PubMed] [Google Scholar]
- 36.Eriksson K., Kindblom L.G., Hamberg P., Larsson H., Wredmark T. The semitendinosus tendon regenerates after resection: a morphologic and MRI analysis in 6 patients after resection for anterior cruciate ligament reconstruction. Acta Orthop Scand. 2001;72(4):379–384. doi: 10.1080/000164701753542041. [DOI] [PubMed] [Google Scholar]
- 37.Konrath J.M., Vertullo C.J., Kennedy B.A., Bush H.S., Barrett R.S., Lloyd D.G. Morphologic characteristics and strength of the hamstring muscles remain altered at 2 Years after use of a hamstring tendon graft in anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44(10):2589–2598. doi: 10.1177/0363546516651441. [DOI] [PubMed] [Google Scholar]
- 38.Lee D.W., Shim J.C., Yang S.J., Cho S.I., Kim J.G. Functional effects of single semitendinosus tendon harvesting in anatomic anterior cruciate ligament reconstruction: comparison of single versus dual hamstring harvesting. Clin Orthop Surg. 2019;11(1):60–72. doi: 10.4055/cios.2019.11.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakamura E., Mizuta H., Kadota M., Katahira K., Kudo S., Takagi K. Three-dimensional computed tomography evaluation of semitendinosus harvest after anterior cruciate ligament reconstruction. Arthroscopy. 2004;20:360–365. doi: 10.1016/j.arthro.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 40.Nakamae A., Deie M., Adachi N., Nakasa T., Nishimori M., Ochi M. Effects of knee immobilization on morphological changes in the semitendinosus muscle-tendon complex after hamstring harvesting for anterior cruciate ligament reconstruction: evaluation using three-dimensional computed tomography. J Orthop Sci. 2012;17:39–45. doi: 10.1007/s00776-011-0163-y. [DOI] [PubMed] [Google Scholar]
- 41.Snow B.J., Wilcox J.J., Burks R.T., Greis P.E. Evaluation of muscle size and fatty infiltration with MRI nine to eleven years following hamstring harvest for ACL reconstruction. J Bone Joint Surg Am. 2012;18(14):1274–1282. doi: 10.2106/JBJS.K.00692. 94. [DOI] [PubMed] [Google Scholar]
- 42.Tadokoro K., Matsui N., Yagi M., Kuroda R., Kurosaka M., Yoshiya S. Evaluation of hamstring strength and tendon regrowth after harvesting for anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32:1644–1650. doi: 10.1177/0363546504263152. [DOI] [PubMed] [Google Scholar]
- 43.Leis H.T., Sanders T.G., Larsen K.M., Lancaster-Weiss K.J., Miller M.D. Hamstring regrowth following harvesting for ACL reconstruction: the lizard tail phenomenon. J Knee Surg. 2003;16(3):159–164. [PubMed] [Google Scholar]
- 44.Suijkerbuijk M.A.M., Reijman M., Oei E.H.G., van Meer B.L., van Arkel E.R.A., Meuffels D.E. Predictive factors of hamstring tendon regeneration and functional recovery after harvesting: a prospective follow-up study. Am J Sports Med. 2018;46(5):1166–1174. doi: 10.1177/0363546517751660. [DOI] [PubMed] [Google Scholar]
- 45.Warren L.F., Marshall J.L. The supporting structures and layers on the medial side of the knee: an anatomical analysis. J Bone Joint Surg Am. 1979 Jan;61(1):56–62. PMID: 759437. [PubMed] [Google Scholar]
- 46.Zaffagnini S., Golano P., FarinasO Vascularity and neuroreceptors of the pes anserinus: anatomic study. Clin Anat. 2003;16:19–24. doi: 10.1002/ca.10073. [DOI] [PubMed] [Google Scholar]
- 47.McFadyen B.J., Carnahan H. Anticipatory locomotor adjustments for accommodating versus avoiding level changes in humans. Exp Brain Res. 1997;114(3):500–506. doi: 10.1007/pl00005659. [DOI] [PubMed] [Google Scholar]
- 48.Tashiro T., Kurosawa H., Kawakami A., Hikita A., Fukui N. In"uence of medial hamstring tendon harvest on knee "exor strength after anterior cruciate ligament reconstruction.A detailed evaluation with comparison of single- and double-tendon harvest. Am J Sports Med. 2003;31:522–529. doi: 10.1177/31.4.522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.