Abstract
Neuropathy and ischaemia are two great pathologies of the diabetic foot which lead to the characteristic features of foot ulceration (neuropathic and ischaemic) and Charcot neuroarthropathy. These can be complicated by infection and eventually may result in amputation (minor or major) and increased mortality. All of these features contribute to considerable clinical and economic burden.
Peripheral nerves in the lower limbs are susceptible to different types of damage in patients with diabetes leading to distinctive syndromes. These include symmetrical sensory neuropathy associated with autonomic neuropathy, which advances gradually, and acutely painful neuropathies and mononeuropathies which have a rather acute presentation but usually recover. Ischaemia in the form of peripheral arterial disease is an important contributor to the burden of the diabetic foot. The incidence of atherosclerotic disease is raised in patients with diabetes and its natural history is accelerated. Diabetes causes severe and diffuse disease below-the knee. The lifetime risk of developing a diabetic foot ulcer is between 19% and 34%. Recurrence is common after initial healing; approximately 40% of patients have a recurrence within 1 year after ulcer healing, almost 60% within 3 years, and 65% within 5 years. Charcot neuroarthropathy is characterised by bone and joint destruction on the background of a neuropathy. Its prevalence in diabetes varies from 0.1% to 8%.
Infection develops in 50%–60% of ulcers and is the principal pathology that damages diabetic feet. Approximately 20% of moderate or severe diabetic foot infections result in lower extremity amputations. The incidence of osteomyelitis is about 20% of diabetic foot ulcers.
Every 20 s a lower limb is amputated due to complications of diabetes. Of all the lower extremity amputations in persons with diabetes, 85% are preceded by a foot ulcer. The mortality at 5 years for an individual with a diabetic foot ulcer is 2.5 times as high as the risk for an individual with diabetes who does not have a foot ulcer. The economic burden exacted on health care systems is considerable and includes direct and indirect costs, with loss of personal earnings and burden to carers. The diabetic foot is a significant contributor to the global burden of disability and reduces the quality of life. It remains a considerable public health problem.
Keywords: Burden, Diabetic foot, Foot ulcer, Charcot, Amputation
1. Introduction
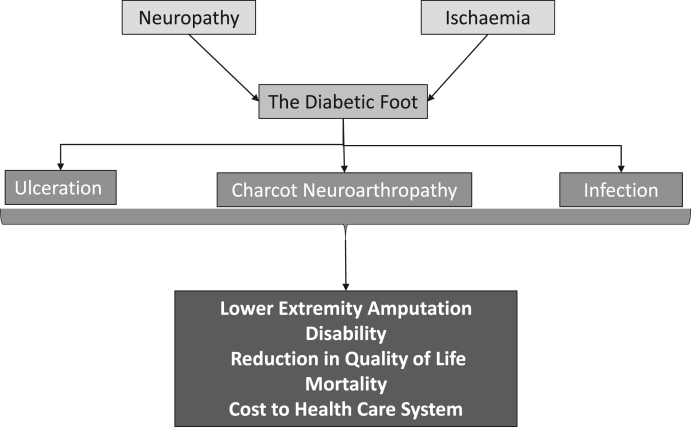
The diabetic foot is a major public health problem. Two overwhelming pathologies come together in the diabetic foot: neuropathy and ischaemia, which result in the characteristic features of foot ulceration (neuropathic and ischaemic) and Charcot neuroarthropathy, both of which can be complicated by infection, and ultimately may result in amputation (minor or major) and increased mortality. In the United Kingdom, diabetic foot problems are the most frequent cause of diabetes related admission to hospital and similar trends are noted in other countries.1 This paper will first describe the clinical burden of neuropathy and ischaemia and then discuss the impact of diabetic foot ulceration, Charcot neuroarthopathy, infection, amputation and mortality. It will consider the global disability inflicted by these complications of diabetes and finally summarise the economic burden of the diabetic foot. A diagrammatic representation of the burden of the diabetic foot is shown in Fig. 1.
Fig. 1.
Diagrammatic representation of the burden of the diabetic foot.
2. Neuropathy
Many cross-sectional studies have reported neuropathy prevalence between 10% and 85%, depending on the definition ultilised.2 The EURODIAB Complications Study, reported a neuropathy incidence of 24% over a follow up period of approximately 7 years.3 Peripheral nerves in the lower limbs are prone to various forms of damage in patients with diabetes leading to distinctive syndromes. These include distal symmetrical sensory neuropathy associated with autonomic neuropathy which advances insidiously, the acutely painful neuropathy variants, and mononeuropathies, which tend to present acutely but typically lead to full recovery.
2.1. Distal symmetrical sensory and autonomic neuropathy
This is the most common presentation, affecting 11–50% of people with diabetes. It is typically diffuse and symmetrical (stocking distribution), ascending from the feet upwards but may also involve the hands. The smallest fibres (pain, temperature, autonomic) are thought to be involved first with progression to involve all types of nerve fibres. This pattern of involvement gives rise to some of the characteristic features of diabetic neuropathy with early loss of pain and temperature sensing modalities. Other sensory modalities, notably the light touch sensation, can remain intact in these early stages. The difficulty in assessing pain and temperature sensations, especially in a clinical setting, has meant such early neuropathy goes mostly undetected. Peripheral autonomic (sympathetic) neuropathy causes loss of sweating and leads to a dry skin often complicated by fissures. Development of loss of protective sensation secondary to advanced symmetrical sensory neuropathy is the principal risk factor for diabetic foot ulceration which is subsequently the catalyst for foot infection and amputation.4,5
2.2. Acute painful neuropathies, mononeuropathies and radiculopathies
Painful neuropathies in diabetes have typical features, which include constant ‘burning’, paraesthesia and shooting pains, together with intense discomfort caused by contact with clothes or bedclothes. The pains can be continuous, typically worse at night and night and cause severe insomnia. In a large observational cohort from Northwest England, painful symptoms were present in 21% of those with distal symmetrical sensory neuropathy. Focal painful neuropathies can be localised to a single or adjacent group of nerve roots affecting the feet and/or legs, or to one or both thighs, and are often accompanied by muscle wasting and incapacitating weakness, causing falls. This is known as proximal motor neuropathy or ‘diabetic amyotrophy’ and is due to either radicuo-plexopathy or femoral neuropathy. These painful conditions usually recover in 6–18 months.
Rapid improvement of glycaemic control (and at times, sudden worsening of glucose control) may lead to an acutely painful neuropathy with an overlay of autonomic features. Previously called insulin neuritis, this is nowadays labelled as treatment induced neuropathy of diabetes (TIND). The syndrome of diabetic neuropathic cachexia is characterised by two important symptoms: sudden significant weight loss over a short period of time (3–4 months) and severe pain. The weight loss is enormous and can represent 60% of the normal weight. The condition is typically seen in elderly males and recovery is usually noted within 12–24 months.
3. Ischaemia
Ischaemia in the form of peripheral arterial disease is a significant contributor to the burden of the diabetic foot.6 Atherosclerosis is not only more prevalent in people with diabetes, but its natural history is also hastened.7 Peripheral arterial disease exceeds 20% in diabetes when it is identified by an abnormal ankle–brachial index.8 The prevalence increases with duration of diabetes as demonstrated by the UK Prospective Diabetes Study which reported 1.2% when diabetes is diagnosed and 12.5% 18 years afterwards.9
Diabetes is linked with extensive below-the knee arterial disease and more diffuse involvement in contrast to the more focal disease of those with no diabetes.10 Peripheral artery disease independently heightens the risk of non-healing ulcers, infection, and amputation.11 The prevalence of concurrent peripheral arterial disease with diabetes is particularly prominent in those persons who have critical lower limb ischaemia.12 Furthermore peripheral arterial disease causes substantial long-term disability and decreased mobility in persons with diabetes, particularly post amputation.13,14 Peripheral arterial disease is an indicator of atherosclerosis in the cardiovascular, cerebrovascular and renovascular systems and there is an increased risk of myocardial infarction and stroke.7,15
4. Ulceration
The lifetime risk of developing a diabetic foot ulcer is between 19% and 34% and it is estimated that 9.1 million to 26.1 million individuals with diabetes globally develop foot ulcers every year.5 Up to one-third of the half billion people with diabetes worldwide will develop a diabetic foot ulcer over the course of their lifetime.16 The annual incidence of foot ulceration is between 1.9% and 4.0% of the population with diabetes.17,18 However, those with established neuropathy have a higher incidence, estimated at 5.0% to 7.5%.19 Prevalence rates for ulceration range from 5% to 9%, depending on the cohort and the country examined. Among American Medicare beneficiaries, approximately 8% of those with diabetes may have a foot ulcer.20
4.1. The influence of neuropathy and ischaemia in the development of ulceration
Ulceration in the neuropathic foot develops on the plantar surface which is usually the location of high mechanical pressure. In contrast, ulcers in the foot with ischaemia occur on the margins of the foot and tips of the toes, at sites of persistent low pressure usually from badly fitting shoes.
In one multicentre hospital-based study from the UK, a 5.6% increase in the risk of foot ulceration was noted for each 1 Volt increase in vibration perception thresholds measured using a Neurothesiometer.21 In a combined analysis of persons with diabetes who had ulceration in hospital clinics from Manchester UK and Seattle USA, neuropathy was present in 78% of patients, while the triad of neuropathy, minor foot trauma and foot deformity was noted in 63% of pathways to ulceration.22 In the multicentre European Eurodiale study, peripheral neuropathy was present in 86%, whereas 42% of the cohort had peripheral arterial disease at baseline.23
However, the proportion of pure neuropathic foot ulcers reported in the literature fluctuates between 40% and 60%. A study of 185 subjects by Moulik et al. looking into aetiological categorization of foot ulcers reported that 45% were pure neuropathic, 24% were neuroischaemic and 16% were ischaemic.24 In another UK based study, 47% of diabetic foot ulcerations were considered purely neuropathic, 30% neuroischaemic and only 12% considered entirely ischaemic.25 From a well characterised cohort from Nottingham UK, 30% of the patients and 28% of ulcerations fulfilled the criteria for pure neuropathic foot ulceration.26 Another study from South America reported a neuropathic ulceration prevalence of 60% within their cohort.27
5. Recurrence of ulceration
Recurrence of ulceration in the foot is common after initial healing of an ulcer. In a single centre follow up, after the completion of the Eurodiale study (n = 73), one clinic reported that 58% of the participants had a recurrence of an ulcer over the subsequent three years. Recurrence rates at one, two and three years were 40%, 18% and 13% respectively (p = 0.006 for trend).28 A review of 19 studies on incidence rates for ulcer recurrence showed that approximately 40% of individuals have a recurrence within 1 year of ulcer healing, almost 60% within 3 years, and 65% within 5 years. It may be more beneficial to regard people who have attained closure of the ulcer as being in remission instead of being healed.5
6. Charcot neuroarthropathy
The Charcot foot or Charcot neuroarthropathy, is characterised by bone and joint damage on the background of a neuropathy. It often occurs in the midfoot but also in the forefoot and hindfoot. The outlook for the hindfoot is much more critical with the increased possibility of instability of the ankle. In its severest form, Charcot neuroarthropathy can result in deformity, ulceration, infection, and amputation. It contributes to significant morbidity and premature mortality with a negative effect on the activities of daily living.29
Early reports noted one case per 680 people with diabetes and Charcot neuroarthropathy30 and in a subsequent series, there was one case per 333 people with diabetes.31 The prevalence in diabetes varies from 0.1% to 8% amongst clinics and countries. Thus, a recent analysis in the USA, revealed a new diagnosis of Charcot neuroarthropathy in 0.12% of individuals with type 2 diabetes.32 The prevalence of active Charcot disease of the foot in one month was evaluated at seven secondary care services in the East Midlands of England. Ninety cases were recognized, representing 4.3 per 10,000 of the 205,033 diabetes population of the area.33
The demographic features of individuals with Charcot neuroarthropathy differ between type 1 and type 2 diabetes.34 Persons with type 1 diabetes and Charcot foot are significantly younger in contrast to persons with type 2 diabetes. In type 1 diabetes, the maximum age of presentation of Charcot neuroarthropathy is the 3rd and 4th decade, but in type 2 diabetes, it is the 6th and 7th decade. People with Charcot deformity have a life-long risk of ulceration and limb-threatening infection, which can result in a major amputation. The risk of amputation is 7-fold higher in those with Charcot deformity compared to Charcot foot alone and the risk almost doubles if deformity is complicated by an ulcer.35 People living with a Charcot foot have reduced quality of life and increased incidence of depression. The Charcot foot is a great burden to society as people with this condition require lifelong support.31 In a series of 115 persons with Charcot neuroarthropathy who were followed up for four years, 37% developed new ulceration over deformities.31
7. Infection
Diabetic foot ulcers are very vulnerable to infection. About 50%–60% of ulcers develop infection which is the leading pathology that devastates most diabetic feet.5 Approximately 20% of moderate or severe diabetic foot infections result in amputation at various levels.36
The incidence of osteomyelitis diagnosed by culture is approximately 20% of diabetic foot ulcers.37 All stages of infection may also be complicated by bacteraemia, resulting in systemic signs of infection.
Diabetic foot infections are one of the most common causes of admissions of individuals with diabetes to hospital in the United States, comprising 20% of hospital admissions.38 Readmission rates for patients with diabetic foot infections are approximately 40% and there is almost a one in six mortality within 1 year of infection.39
8. Amputation
Every 20 s a lower limb is amputated due to diabetes.40 Of all amputations in persons with diabetes, 85% are preceded by a foot ulcer.41 The incidence of major amputation in England ranges from 0·2 to 2·0 per 1,000 person-years of people with diabetes.42 The prevalence of major amputation is 1.6% in the age range 18–44 years, 3.4% in those aged 45–64 years, and 3.6% in persons older than 65 years.43 A recent systematic review on diabetic amputation incidence reported a male-to-female ratio range between 1.5 and 3.0 and related this to males having a higher frequency of smoking, peripheral neuropathy, peripheral artery disease, and foot ulceration.44 Overall,the risk of a person with diabetes having a lower extremity amputation is reckoned to be 23 times that of a person without diabetes.45
9. Mortality
A recent review has demonstrated 5-year mortality for Charcot neuroarthropathy, foot ulceration, minor and major amputations to be 29.0%, 30.5%, 46.2% and 56.6%, respectively.46 The mortality at 5 years for an individual with a diabetic foot ulcer is 2.5 times as high as the risk for an individual with diabetes who does not have a foot ulcer.47 The risk of death at 10 years for a patient with diabetes and foot ulcer is double the risk for a person without a foot ulcer.48
10. Global disability
Foot complications in diabetes are a leading cause of the global burden of disability.49 Global prevalence of foot complications include 131.0 million people (1.77% of the global population) with diabetes-related lower-extremity problems, incorporating 105.6 million (95% UI 85.5–128) with neuropathy only, 18.6 million (15.0–22.9) with foot ulcers, 4.3 million (3.7–4.9) with amputation without prosthesis, and 2.5 million (2.1–3.0) with amputation with prosthesis.50 The disability burden within a population is measured using YLDs (Years Lived with Disability).51 YLDs are a population assessment of disability burden and are calculated by multiplying the total numbers of people affected by a condition by the average severity of disability for that condition (disability weight). The disability weight reflects the average severity of nonfatal health loss associated with the particular condition on a scale of 0 (equal to perfect health) to 1 (equal to death).
It was estimated that 16.8 million YLDs (2.07% of global YLDs) resulted from diabetic foot complications in 2016, including 12.9 million (95% UI 8.3–18.8) from neuropathy only, 2.5 million (1.7–3.6) from foot ulcers, 1.1 million (0.7–1.4) from amputation with no prosthesis, and 0.4 million (0.3–0.5) from amputation with prosthesis.50 Overall, the disability burden disproportionately affected males, the 50- to 69-year-old age-group, and those living in regions of North Africa and Middle East, Central Latin America, Oceania, and Caribbean.50
11. Quality of life
Lower extremity complications also result in a reduction in quality of life.52 Using the EQ-5D instrument, a Swedish study reported scores for patients attending a multidisciplinary foot service.53 The scores for ulcers and for major amputation were lower than those reported in other studies for people with diabetes and macrovascular complications. They were also lower than scores for people with end-stage renal disease needing haemodialysis, breast cancer and prostate cancer.
12. The economic burden to health care system
The economic burden inflicted on the health care systems and the individual with diabetes and foot complications is considerable. For example, in England, for the year 2014–15, the estimated NHS cost in England was at £837–£962 million, €1.1–€1.2 billion and $1.4–$1.6 billion. This was equivalent to almost 1% of the health service budget for England or £1 out of every £140 spent in the National Health Service.54 Approximately 90% of the cost was caused by foot ulcers rather than amputations. Although amputations can have a catastrophic effect on individual lives, and unit costs are considerable, the much increased incidence of ulceration results in higher aggregate costs. Regarding the distribution of costs by healthcare setting, two thirds of costs were in primary, community, or outpatient settings. Thus 90% of expenditure was accounted for by ulceration, and 60% was spent in community, outpatient and primary settings. Cost of care was evaluated in the UK where the approximated cost over the first year from original presentation of a healed foot ulcer was £2138, for an unhealed ulcer it was £8786 and for an amputation secondary to an ulcer it was £16,941. 55 Infection markedly raised the costs of ulcer treatment, up to £12,995 for an infected foot ulcer.
A study in Russia, concerning patients admitted to hospital due to a foot ulcer categorized by Wagner grade produced these average costs: grade 1–2450 €, grade 2–2821 €, grade 3–3937 €, and grade 4–5340 €.56 Length of hospital stay, foot surgery, and vascular surgery had the greatest effect on cost.
Recent systematic reviews have highlighted the increasing costs for diabetic foot disease in several health economies.57,58 In the USA, 1,019,861 cases of diabetic foot complications presented to Emergency Departments between 2006 and 2010, consisting of 1.9% of the 54.2 million total diabetes cases. The national bill was $1.9 billion per year in the Emergency Departments and $8.78 billion per year (US$ 2014) among the 81.2% of cases that were admitted, including inpatient charges Clinical outcomes comprised mortality in 2.0%, sepsis in 9.6% of cases and amputation in 10.5% (major-minor amputation ratio of 0.46).59 In 2014, the cost of diabetic foot management in the USA, between 2007 and 2011, was between $ 9–13 billion. Diabetic foot ulceration could add $11,710-$16,833 incremental costs to a patient’s annual healthcare costs, doubling the cost of delivering diabetes care.60 There were substantial additional indirect costs, such as the loss of individual earnings, burden to carers and effects of absenteeism on employers. In 2017, diabetes directly cost $237 billion in the USA and one-third of these direct costs were due to diabetic foot disease46
13. Therapeutic principles
Successful management of the diabetic foot requires the expertise of a multidisciplinary care team which gives integrated care focused in a diabetic foot clinic. Members of the team consist of podiatrist, nurse, orthotist, microbiologist, physician, radiologist and surgeon, including, orthopaedic surgeon, vascular surgeon and plastic surgeon. Six aspects of care should be addressed within the multidisciplinary network, namely to achieve control of the wound, microbiology, mechanics and circulation of the foot as well as obtaining metabolic control and educating the patient.61
14. Conclusion
The diabetic foot has a devastating pathology. Neuropathy and ischaemia lead to foot ulceration and Charcot neuroarthropathy, both of which can be complicated by infection. This may eventually result in amputation (minor or major) and increased mortality. These complications of the diabetic foot result in considerable clinical and economic burden and for these reasons, the diabetic foot remains a major public health problem.
Funding
None.
Declaration of competing interest
None.
Acknowledgements
None.
Contributor Information
Michael Edmonds, Email: Michael.edmonds@nhs.net.
Chris Manu, Email: Chris.manu@nhs.net.
Prashanth Vas, Email: Prashanth.vas@nhs.net.
References
- 1.Kerr M., Rayman G., Jeffcoate W.J. Cost of diabetic foot disease to the national health service in England. Diabet Med. 2014;31(12):1498–1504. doi: 10.1111/dme.12545. Epub 2014 Aug 1. PMID: 24984759. [DOI] [PubMed] [Google Scholar]
- 2.Pop-Busui R., Boulton A.J.M., Feldman E.L. Diabetic neuropathy: a position statement by the American diabetes association. Diabetes Care. 2017;40:136–154. doi: 10.2337/dc16-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tesfaye S., Stevens L.K., Stephenson J.M. Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: the EURODIAB IDDM Complications Study. Diabetologia. 1996;39:1377–1384. doi: 10.1007/s001250050586. [DOI] [PubMed] [Google Scholar]
- 4.Boulton A.J., Vileikyte L., Ragnarson-Tennvall G., Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366:1719–1724. doi: 10.1016/S0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong D.G., Boulton A.J.M., Bus S.A. Diabetic foot ulcers and their recurrence. N Engl J Med. 2017;376:2367–2375. doi: 10.1056/NEJMra1615439. [DOI] [PubMed] [Google Scholar]
- 6.Thiruvoipati T., Kielhorn C.E., Armstrong E.J. Peripheral artery disease in patients with diabetes: epidemiology, mechanisms, and outcomes. World J Diabetes. 2015 Jul 10;6(7):961–969. doi: 10.4239/wjd.v6.i7.961. PMID: 26185603; PMCID: PMC4499529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beckman J.A., Creager M.A., Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. J Am Med Assoc. 2002;287:2570–2581. doi: 10.1001/¬jama.287.19.2570. PMID: 12020339. [DOI] [PubMed] [Google Scholar]
- 8.Nativel M., Potier L., Alexandre L. Lower extremity arterial disease in patients with diabetes: a contemporary narrative review. Cardiovasc Diabetol. 2018 Oct 23;17(1):138. doi: 10.1186/s12933-018-0781-1. PMID: 30352589; PMCID: PMC6198374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adler A.I., Stevens R.J., Neil A., Stratton I.M., Boulton A.J., Holman R.R. Ukpds 59: hyperglycemia and other potentially modifiable risk factors for peripheral vascular disease in type 2 diabetes. Diabetes Care. 2002;25(5I):894–899. doi: 10.2337/diacare.25.5.894. [DOI] [PubMed] [Google Scholar]
- 10.Creager M.A., Lüscher T.F., Cosentino F., Beckman J.A. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Circulation. 2003;108:1527–1532. doi: 10.1161/01.CIR.0000091257.27563.32. PMID: 14504252. [DOI] [PubMed] [Google Scholar]
- 11.Mills J.L., Sr., Conte M.S., Armstrong D.G. Society for vascular surgery lower extremity guidelines committee. The society for vascular surgery lower extremity threatened limb classification system: risk stratification based on wound, ischemia, and foot infection (WIfI) J Vasc Surg. 2014 Jan;59(1):220–234. doi: 10.1016/j.jvs.2013.08.003. e1-2. Epub 2013 Oct 12. PMID: 24126108. [DOI] [PubMed] [Google Scholar]
- 12.Dick F., Diehm N., Galimanis A., Husmann M., Schmidli J., Baumgartner I. Surgical or endovascular revascularization in patients with critical limb ischemia: influence of diabetes mellitus on clinical outcome. J Vasc Surg. 2007 Apr;45(4):751–761. doi: 10.1016/j.jvs.2006.12.022. Epub 2007 Feb 15. PMID: 17306950. [DOI] [PubMed] [Google Scholar]
- 13.Marso S.P., Hiatt W.R. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol. 2006 Mar 7;47(5):921–929. doi: 10.1016/j.jacc.2005.09.065. Epub 2006 Feb 9. PMID: 16516072. [DOI] [PubMed] [Google Scholar]
- 14.Vogt M.T., Cauley J.A., Kuller L.H., Nevitt M.C. Functional status and mobility among elderly women with lower extremity arterial disease: the Study of Osteoporotic Fractures. J Am Geriatr Soc. 1994;42:923–929. doi: 10.1111/j.1532-5415.1994.tb06581.x. PMID: 8064098. [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333–3341. doi: 10.2337/diacare.26.12.3333. PMID: 14633825. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong D.G., Swerdlow M.A., Armstrong A.A., Conte M.S., Padula W.V., Bus S.A. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020 Mar 24;13(1):16. doi: 10.1186/s13047-020-00383-2. PMID: 32209136; PMCID: PMC7092527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crawford F., McCowan C., Dimitrov B.D. The risk of foot ulceration in people with diabetes screened in community settings: findings from a cohort study. QJM. 2011;104:403–410. doi: 10.1093/qjmed/hcq227. [DOI] [PubMed] [Google Scholar]
- 18.Abbott C.A., Carrington A.L., Ashe H. The North-West Diabetes Foot Care Study: incidence of, and risk factors for, new diabetic foot ulceration in a community-based patient cohort. Diabet Med. 2002;19:377–384. doi: 10.1046/j.1464-5491.2002.00698.x. [DOI] [PubMed] [Google Scholar]
- 19.Boulton A.J., Kirsner R.S., Vileikyte L. Neuropathic diabetic foot ulcers. N Engl J Med. 2004;351:48–55. doi: 10.1056/NEJMcp032966. [DOI] [PubMed] [Google Scholar]
- 20.Margolis D.J., Malay D.S., Hoffstad O.J. 2011. Prevalence of Diabetes, Diabetic Foot Ulcer, and Lower Extremity Amputation Among Medicare Beneficiaries, 2006 to 2008. Data Points #1. [PubMed] [Google Scholar]
- 21.Abbott C.A., Vileikyte L., Williamson S., Carrington A.L., Boulton A.J. Multicenter study of the incidence of and predictive risk factors for diabetic neuropathic foot ulceration. Diabetes Care. 1998;21:1071–1075. doi: 10.2337/diacare.21.7.1071. [DOI] [PubMed] [Google Scholar]
- 22.Reiber G.E., Vileikyte L., Boyko E.J. Causal pathways for incident lower-extremity ulcers in patients with diabetes from two settings. Diabetes Care. 1999 Jan;22(1):157–162. doi: 10.2337/diacare.22.1.157. PMID: 10333919. [DOI] [PubMed] [Google Scholar]
- 23.Prompers L., Huijberts M., Apelqvist J. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia. 2007;50:18–25. doi: 10.1007/s00125-006-0491-1. [DOI] [PubMed] [Google Scholar]
- 24.Moulik P.K., Mtonga R., Gill G.V. Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care. 2003 Feb;26(2):491–494. doi: 10.2337/diacare.26.2.491. PMID: 12547887. [DOI] [PubMed] [Google Scholar]
- 25.Kumar S., Ashe H.A., Parnell L.N. The prevalence of foot ulceration and its correlates in type 2 diabetic patients: a population-based study. Diabet Med. 1994 Jun;11(5):480–484. doi: 10.1111/j.1464-5491.1994.tb00310.x. PMID: 8088127. [DOI] [PubMed] [Google Scholar]
- 26.Ince P., Game F.L., Jeffcoate W.J. Rate of healing of neuropathic ulcers of the foot in diabetes and its relationship to ulcer duration and ulcer area. Diabetes Care. 2007 Mar;30(3):660–663. doi: 10.2337/dc06-2043. PMID: 17327337. [DOI] [PubMed] [Google Scholar]
- 27.Moura Neto A., Zantut-Wittmann D.E., Fernandes T.D., Nery M., Parisi M.C. Risk factors for ulceration and amputation in diabetic foot: study in a cohort of 496 patients. Endocrine. 2013 Aug;44(1):119–124. doi: 10.1007/s12020-012-9829-2. Epub 2012 Nov 3. PMID: 23124278. [DOI] [PubMed] [Google Scholar]
- 28.Dubský M., Jirkovská A., Bem R. Risk factors for recurrence of diabetic foot ulcers: prospective follow-up analysis in the Eurodiale subgroup. Int Wound J. 2013 Oct;10(5):555–561. doi: 10.1111/j.1742-481X.2012.01022.x. Epub 2012 Jun 19. PMID: 22712631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogers L.C., Frykberg R.G., Armstrong D.G., Boulton A.J., Edmonds M., Van G.H. The Charcot foot in diabetes. Diabetes Care. 2011;34(9):2123–2129. doi: 10.2337/dc11-0844. PMID: 21868781; PMCID: PMC3161273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinha S., Munichoodappa C.S., Kozak G.P. Neuro-arthropathy (Charcot joints) in diabetes mellitus (clinical study of 101 cases) Medicine (Baltim) 1972;51:191–210. doi: 10.1097/00005792-197205000-00006. PMID: 5021769. [DOI] [PubMed] [Google Scholar]
- 31.Fabrin J., Larsen K., Holstein P.E. Long-term follow-up in diabetic Charcot feet with spontaneous onset. Diabetes Care. 2000 Jun;23(6):796–800. doi: 10.2337/diacare.23.6.796. PMID: 10840999. [DOI] [PubMed] [Google Scholar]
- 32.Stuck R.M., Sohn M.W., Budiman-Mak E., Lee T.A., Weiss K.B. Charcot arthropathy risk elevation in the obese diabetic population. Am J Med. 2008 Nov;121(11):1008–1014. doi: 10.1016/j.amjmed.2008.06.038. PMID: 18954849. [DOI] [PubMed] [Google Scholar]
- 33.Metcalf L., Musgrove M., Bentley J. Prevalence of active charcot disease in the East Midlands of England. Diabet Med. 2018 Oct;35(10):1371–1374. doi: 10.1111/dme.13679. Epub 2018 Jun 2. PMID: 29782669. [DOI] [PubMed] [Google Scholar]
- 34.Petrova N.L., Foster A.V., Edmonds M.E. Difference in presentation of charcot osteoarthropathy in type 1 compared with type 2 diabetes. Diabetes Care. 2004 May;27(5):1235–1236. doi: 10.2337/diacare.27.5.1235-a. PMID: 15111556. [DOI] [PubMed] [Google Scholar]
- 35.Sohn M.W., Stuck R.M., Pinzur M., Lee T.A., Budiman-Mak E. Lower-extremity amputation risk after charcot arthropathy and diabetic foot ulcer. Diabetes Care. 2010 Jan;33(1):98–100. doi: 10.2337/dc09-1497. Epub 2009 Oct 13. PMID: 19825822; PMCID: PMC2797995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Senneville É., Lipsky B.A., Abbas Z.G. Diagnosis of infection in the foot in diabetes: a systematic review. Diabetes Metab Res Rev. 2020 Mar;36(suppl 1) doi: 10.1002/dmrr.3281. PMID: 32176440. [DOI] [PubMed] [Google Scholar]
- 37.Lavery L.A., Armstrong D.G., Wunderlich R.P., Mohler M.J., Wendel C.S., Lipsky B.A. Risk factors for foot infections in individuals with diabetes. Diabetes Care. 2006 Jun;29(6):1288–1293. doi: 10.2337/dc05-2425. PMID: 16732010. [DOI] [PubMed] [Google Scholar]
- 38.Frykberg R.G., Wittmayer B., Zgonis T. Surgical management of diabetic foot infections and osteomyelitis. Clin Podiatr Med Surg. 2007 Jul;24(3):469–482. doi: 10.1016/j.cpm.2007.04.001. viii-ix. PMID: 17613386. [DOI] [PubMed] [Google Scholar]
- 39.Fincke B.G., Miller D.R., Turpin R. A classification of diabetic foot infections using ICD-9-CM codes: application to a large computerized medical database. BMC Health Serv Res. 2010 Jul 6;10:192. doi: 10.1186/1472-6963-10-192. PMID: 20604921; PMCID: PMC2914721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hinchliffe R.J., Andros G., Apelqvist J. A systematic review of the effectiveness of revascularization of the ulcerated foot in patients with diabetes and peripheral arterial disease. Diabetes Metab Res Rev. 2012 Feb;28(Suppl 1):179–217. doi: 10.1002/dmrr.2249. Erratum in: Diabetes Metab Res Rev. 2012 May;28(4):376. Fiedrichs, S [corrected to Friederichs, S]. PMID: 22271740. [DOI] [PubMed] [Google Scholar]
- 41.Pecoraro R.E., Reiber G.E., Burgess E.M. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care. 1990 May;13(5):513–521. doi: 10.2337/diacare.13.5.513. PMID: 2351029. [DOI] [PubMed] [Google Scholar]
- 42.Holman N., Young R.J., Jeffcoate W.J. Variation in the recorded incidence of amputation of the lower limb in England. Diabetologia. 2012 Jul;55(7):1919–1925. doi: 10.1007/s00125-012-2468-6. Epub 2012 Mar 8. PMID: 22398645. [DOI] [PubMed] [Google Scholar]
- 43.Katsilambros N., Dounis E., Makrilakis K., Tentolouris N., Tsapogas P. second ed. John Wiley & Sons; 2010. Atlas of the Diabetic Foot. [Google Scholar]
- 44.Narres M., Kvitkina T., Claessen H. Incidence of lower extremity amputations in the diabetic compared with the non-diabetic population: a systematic review. PloS One. 2017 Aug 28;12(8) doi: 10.1371/journal.pone.0182081. PMID: 28846690; PMCID: PMC5573217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brownrigg J.R., Apelqvist J., Bakker K., Schaper N.C., Hinchliffe R.J. Evidence-based management of PAD & the diabetic foot. Eur J Vasc Endovasc Surg. 2013 Jun;45(6):673–681. doi: 10.1016/j.ejvs.2013.02.014. Epub 2013 Mar 27. PMID: 23540807. [DOI] [PubMed] [Google Scholar]
- 46.Armstrong D.G., Swerdlow M.A., Armstrong A.A., Conte M.S., Padula W.V., Bus S.A. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res. 2020 Mar 24;13(1):16. doi: 10.1186/s13047-020-00383-2. PMID: 32209136; PMCID: PMC7092527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walsh J.W., Hoffstad O.J., Sullivan M.O., Margolis D.J. Association of diabetic foot ulcer and death in a population-based cohort from the United Kingdom. Diabet Med. 2016 Nov;33(11):1493–1498. doi: 10.1111/dme.13054. Epub 2016 Jan 10. PMID: 26666583. [DOI] [PubMed] [Google Scholar]
- 48.Iversen M.M., Tell G.S., Riise T. History of foot ulcer increases mortality among individuals with diabetes: ten-year follow-up of the Nord-Trøndelag Health Study, Norway. Diabetes Care. 2009 Dec;32(12):2193–2199. doi: 10.2337/dc09-0651. Epub 2009 Sep 3. PMID: 19729524; PMCID: PMC2782976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lazzarini P.A., Pacella R.E., Armstrong D.G., van Netten J.J. Diabetes-related lower-extremity complications are a leading cause of the global burden of disability. Diabet Med. 2018 May 23 doi: 10.1111/dme.13680. Epub ahead of print. PMID: 29791033. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y., Lazzarini P.A., McPhail S.M., van Netten J.J., Armstrong D.G., Pacella R.E. Global disability burdens of diabetes-related lower-extremity complications in 1990 and 2016. Diabetes Care. 2020 May;43(5):964–974. doi: 10.2337/dc19-1614. Epub 2020 Mar 5. PMID: 32139380. [DOI] [PubMed] [Google Scholar]
- 51.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017 Sep 16;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2. Erratum in: Lancet. 2017 Oct 28;390(10106):e38. PMID: 28919117; PMCID: PMC560550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khunkaew S., Fernandez R., Sim J. Health-related quality of life among adults living with diabetic foot ulcers: a meta-analysis. Qual Life Res. 2019 Jun;28(6):1413–1427. doi: 10.1007/s11136-018-2082-2. Epub 2018 Dec 18. PMID: 30565072. [DOI] [PubMed] [Google Scholar]
- 53.Ragnarson Tennvall G., Apelqvist J. Health-related quality of life in patients with diabetes mellitus and foot ulcers. J Diabet Complicat. 2000 Sep-Oct;14(5) doi: 10.1016/s1056-8727(00)00133-1. 235–41. PMID: 11113684. [DOI] [PubMed] [Google Scholar]
- 54.Kerr M., Barron E., Chadwick P. The cost of diabetic foot ulcers and amputations to the National Health Service in England. Diabet Med. 2019 Aug;36(8):995–1002. doi: 10.1111/dme.13973. Epub 2019 Jun 5. PMID: 31004370. [DOI] [PubMed] [Google Scholar]
- 55.Guest J.F., Fuller G.W., Vowden P. Diabetic foot ulcer management in clinical practice in the UK: costs and outcomes. Int Wound J. 2018 Feb;15(1):43–52. doi: 10.1111/iwj.12816. Epub 2017 Dec 15. PMID: 29243399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ignatyeva V.I., Severens J.L., Ramos I.C., Galstyan G.R., Avxentyeva M.V. Costs of hospital stay in specialized diabetic foot department in Russia. Value Health Reg Issues. 2015 Sep;7:80–86. doi: 10.1016/j.vhri.2015.09.003. Epub 2015 Nov 14. PMID: 29698156. [DOI] [PubMed] [Google Scholar]
- 57.Petrakis I., Kyriopoulos I.J., Ginis A., Athanasakis K. Losing a foot versus losing a dollar; a systematic review of cost studies in diabetic foot complications. Expert Rev Pharmacoecon Outcomes Res. 2017 Apr;17(2):165–180. doi: 10.1080/14737167.2017.1305891. Epub 2017 Mar 17. PMID: 28283002. [DOI] [PubMed] [Google Scholar]
- 58.Tchero H., Kangambega P., Lin L. Cost of diabetic foot in France, Spain, Italy, Germany and United Kingdom: a systematic review. Ann Endocrinol. 2018 Apr;79(2):67–74. doi: 10.1016/j.ando.2017.11.005. Epub 2018 Mar 12. PMID: 29544659. [DOI] [PubMed] [Google Scholar]
- 59.Skrepnek G.H., Mills J.L., Sr., Armstrong D.G. A diabetic emergency one million feet long: disparities and burdens of illness among diabetic foot ulcer cases within emergency Departments in the United States, 2006-2010. PloS One. 2015 Aug 6;10(8) doi: 10.1371/journal.pone.0134914. PMID: 26248037; PMCID: PMC4527828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rice J.B., Desai U., Cummings A.K., Birnbaum H.G., Skornicki M., Parsons N.B. Burden of diabetic foot ulcers for medicare and private insurers. Diabetes Care. 2014;37(3):651–658. doi: 10.2337/dc13-2176. Epub 2013 Nov 1. Erratum in: Diabetes Care. 2014 Sep;37(9):2660. PMID: 24186882. [DOI] [PubMed] [Google Scholar]
- 61.Edmonds M.E., Foster A.V.M. third ed. John Wiley & Sons Ltd; Oxford: 2014. Managing the Diabetic Foot. [DOI] [Google Scholar]