Abstract
The epidemiology, clinical characteristics, management and outcome of Guillain–Barré syndrome (GBS) differ between low-income and middle-income countries (LMIC) and high-income countries (HIC). At present, limited data are available on GBS in LMIC and the true incidence of GBS in many LMIC remains unknown. Increased understanding of GBS in LMIC is needed because poor hygiene and high exposure to infections render populations in LMIC vulnerable to GBS outbreaks. Furthermore, insufficient diagnostic and health-care facilities in LMIC contribute to delayed diagnosis in patients with severe presentations of GBS. In addition, the lack of national clinical guidelines and absence of affordable, effective treatments contribute to worse outcomes and higher mortality in LMIC than HIC. Systematic population-based surveillance studies, cohort and case–control studies are required to understand the incidence and risk factors for GBS. Novel, targeted and cost-effective treatment strategies need to be developed in the context of health system challenges in LMIC. To ensure integrative rehabilitation services in LMIC, existing prognostic models must be validated, and responsive outcome measures that are cross-culturally applicable must be developed. Therefore, fundamental and applied research to improve the clinical management of GBS in LMIC should become a critical focus of future research programmes.
Subject terms: Neuromuscular disease, Neuromuscular disease
The incidence and severity of Guillain–Barré syndrome (GBS) are increased in low-income and middle-income countries (LMIC) by distinct geographic, economic and environmental factors. Here, Papri and colleagues highlight the most important challenges and knowledge gaps relating to GBS in LMIC.
Key points
The considerable regional variation evident in the epidemiology, subtypes and management of Guillain–Barré syndrome (GBS) can be explained by geography, population demographics, environmental and economic factors.
Poor hygiene and sanitation along with frequent exposure to pathogens render populations in low-income and middle-income countries (LMIC) prone to outbreaks of infectious diseases that can trigger GBS.
High rates of adverse outcomes and mortality in LMIC can be explained by insufficient health-care infrastructure leading to diagnostic delays and lack of available and affordable treatment.
Owing to differences in disease severity, clinical presentation and patient management between high-income countries (HIC) and LMIC, existing models to predict the outcome of GBS must be validated for LMIC.
New and low-cost treatment strategies for GBS need to be developed along with improved access to integrative rehabilitation services in LMIC.
Introduction
Guillain–Barré syndrome (GBS) is an immune-mediated polyradiculoneuropathy with an acute onset that affects 100,000 people worldwide annually1–3. GBS is characterized by rapidly progressive ascending weakness that initially affects the limbs and can eventually affect the cranial and respiratory muscles. Several infectious agents have been identified as triggers for the development of GBS, and clusters of this disease can be associated with outbreaks such as the Zika virus epidemic4–6. The severity of GBS is highly variable, ranging from mild distal limb weakness to complete paralysis, respiratory failure and even death. Several variants of GBS have been defined on the basis of their clinical presentation, including a pure motor variant, paraparetic variants and Miller Fisher syndrome (MFS)7,8, which is characterized by the clinical triad of ophthalmoplegia, ataxia and areflexia7. Several subtypes of GBS have also been identified on the basis of electrophysiological features1–3, including acute inflammatory demyelinating polyneuropathy (AIDP) and acute motor axonal neuropathy (AMAN)2,9,10. Patients with AIDP usually have the classic sensorimotor variant of GBS, whereas those with AMAN typically have the pure motor variant8. In some patients with axonal GBS, both sensory and motor fibres are affected; this variant is termed acute motor and sensory axonal neuropathy (AMSAN) and is sometimes considered to be a severe variant of AMAN2. Plasma exchange and intravenous immunoglobulin infusions are equally effective therapies for all variants of GBS2–4,11.
Considerable variation between countries and/or regions is evident in the epidemiology, subtypes and management of GBS12. These differences are thought to be related to environmental and economic factors as well as to health awareness and behaviour. Poor hygiene and sanitation, unsafe drinking water and frequent exposure to pathogens render the populations in low-income and middle-income countries (LMIC) — defined in July 2019 by the World Bank as countries having an annual gross national income per capita of <US$3,995 (ref.13) — highly vulnerable to outbreaks of infectious diseases that are capable of triggering GBS14,15. For example, outbreaks of GBS in northern China (2007) and Mexico (2011) were due to increases in the incidence of Campylobacter jejuni infection16,17. Variations in the incidence and outcomes of GBS can also be partly explained by income per capita12,18. Resource limitations in LMIC, including the limited availability of electrodiagnostic machines, hospital and intensive care unit (ICU) beds and rehabilitation clinics, can hamper the diagnosis and care of patients with GBS5. In addition, the lack of national guidelines (in most LMIC) and the high cost of treatment facilities complicate the management of patients with GBS versus their counterparts in high-income countries (HIC) — defined according to World Bank criteria as having an annual gross national income per capita of ≥US$3,995 (ref.13), which represents the upper middle-income and high-income categories combined19–23.
Although the number of studies of GBS in LMIC is increasing, the majority of GBS studies conducted to date have focused on HIC and we are not aware of any prior published reviews focusing on LMIC. Accordingly, this Review aims to provide an overview of GBS in LMIC and to compare the epidemiology, clinical presentation, subtypes and management of GBS in LMIC and HIC. We identify specific challenges related to the diagnosis, treatment and management of patients with GBS in LMIC and explore the prospects for future research and policy.
Epidemiology
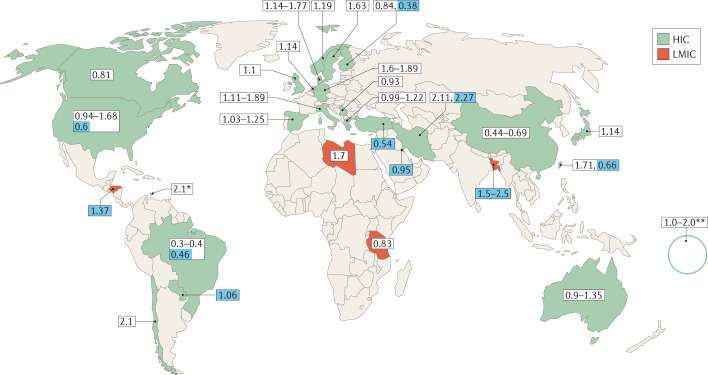
Most studies on the incidence of GBS have been performed in populations from HIC; only a few have included populations from LMIC (Table 1). The reported incidence of GBS ranges from 0.16 to 3.0 cases per 100,000 persons/year24,25; this considerable variation could, in part, be related to geographical location (Fig. 1). For instance, an incidence of ~0.40 cases per 100,000 persons/year was reported in Brazil, 0.84‒1.91 cases per 100,000 persons/year in Europe and North America and 2.1‒3.0 cases per 100,000 persons/year in Iran, Curaçao and Bangladesh2,24–28. As well as the factors already mentioned, some of this variation could be due to methodological differences between studies and the lack of robust, systematic population-based studies in certain countries24.
Table 1.
Reviewed publications on GBS by region
| Region | Country | Number of studies included | Study design (number of patients with GBS per study) |
|---|---|---|---|
| LMIC | |||
| East Asia and Pacific | Indonesia | 1 | Retrospective (28) |
| Middle East and North Africa | Egypt | 4 | Clinical trial (41); cohort (50, 50); case–control (133) |
| Morocco | 1 | Clinical trial (41) | |
| South Asia | India | 14 | National surveillance programme (79); clinical trial (37, 12); cohort (328, 140, 102, 70a); case–control (80); retrospective (1,166, 273, 173, 90); case reports (2, 1) |
| Bangladesh | 10 | Clinical trial (20); cohort (693, 506, 407, 344, 300, 300, 151); case–control (418, 100) | |
| Pakistan | 3 | Retrospective (216, 175, 87) | |
| Nepal | 1 | Retrospective (31) | |
| Sub-Saharan Africa | Ethiopia | 1 | Retrospective (95) |
| Kenya | 1 | Retrospective (54) | |
| Nigeria | 1 | Cohort (34) | |
| Tanzania | 1 | Retrospective (115) | |
| Sudan | 1 | Case report (10) | |
| Zimbabwe | 1 | Cohort (32) | |
| HIC | |||
| East Asia and Pacific | Australia | 2 | Cohort (76); retrospective (46) |
| China | 6 | Cohort (541, 170, 166); retrospective (72); case–control (150, 32) | |
| Taiwan | 3 | National surveillance programme (5,998, 5,469); retrospective (96) | |
| Japan | 2 | Cohort (97); retrospective (40) | |
| French Polynesia | 2 | Case–control (42); national surveillance programme (9) | |
| Thailand | 2 | Retrospective (30); case report (1) | |
| Korea | 1 | National surveillance programme (48) | |
| Singapore | 1 | Retrospective (31) | |
| New Zealand | 1 | National surveillance programme (2,056) | |
| Europe and Central Asia | Netherlands | 4 | Clinical trial (388b, 85); retrospective (67, 36) |
| Denmark | 1 | National surveillance programme (2,319) | |
| Germany | 1 | Retrospective (34) | |
| Italy | 1 | Cohort (96) | |
| Norway | 1 | Cohort (52) | |
| Spain | 1 | Retrospective (106) | |
| UK | 1 | Retrospective (110) | |
| Latin America and Caribbean | Brazil | 5 | National surveyc; cohort (206, 149); case–control (41); case report (1) |
| Puerto Rico | 2 | National surveillance programme (56); cohort (123) | |
| Colombia | 1 | Cohort (68) | |
| Curaçao | 1 | Retrospective (49) | |
| Mexico | 1 | National surveillance programme (467) | |
| Middle East and North Africa | Iraq | 1 | National surveillance programme (2,611) |
| Saudi Arabia | 1 | Retrospective (49) | |
| North America | USA | 1 | Case–control (26) |
| South Asia | Sri Lanka | 2 | Case report (1, 1) |
We mainly selected papers published after 1990 (ref.95), but we did not exclude commonly referenced and highly regarded older publications. GBS, Guillain–Barré syndrome; HIC, high-income countries; LMIC, low-income and middle-income countries. aData were collected prospectively and subjected to retrospective review. bData were collected from two randomized controlled trials and one pilot study; a multinational study (n = 10); worldwide data, reviews and expert opinion (n = 30). cSurvey responses from Brazilian neurologists (no patients with GBS were included in the survey).
Fig. 1. Reported incidence rates of GBS in HIC and LMIC.
According to World Bank definitions, low-income and middle-income countries (LMIC) are those with an annual gross national income per capita of <US$3,995, whereas upper middle-income and high-income countries combined (HIC in this Review) have an annual gross national income per capita of ≥US$3,995 (ref.13). Numbers on a white background represent the incidence rate of all cases of Guillain–Barré syndrome (GBS); numbers on a blue background represent the incidence rates of paediatric cases of GBS only. *Curaçao, **French Polynesia.
Most studies from Europe and North America were performed between 1980 and 2000 and the incidence of GBS in these regions remained stable across most of this period (1.0–1.8 cases per 100,000 persons/year), suggesting a consistent exposure to infectious triggers25. Seasonal fluctuations in the incidence of GBS also vary by geographical area. One large meta-analysis showed that the incidence of GBS increases in winter (January–March) in western, Middle Eastern and Far Eastern countries, but decreases during January–March in the Indian subcontinent and Latin America29. The increased incidence of GBS during winter in some countries is thought to be due to the increased incidence of respiratory tract infections caused by Mycoplasma pneumoniae or Haemophilus influenzae30,31. By contrast, an increase in the incidence of GBS has been observed during summer in northern China and Bangladesh, which is thought to be associated with an increased frequency of preceding diarrhoea3,16,32. In these countries, the high temperatures and humidity of the summer season favour bacterial growth and are an important determinant of the burden of bacterial diarrhoea33,34.
Almost all reports document a higher incidence of GBS in men than women (~1.5:1.0), including those from LMIC such as Bangladesh, India, Taiwan, Pakistan, Egypt, Morocco, Ethiopia, Tanzania and Kenya3,4,9,14,23,27,35–43. Most studies indicate that the incidence of GBS increases with age, although the age distribution of cases in each country or region is influenced by the demographics of the background population and the number of people in each age group at risk of developing GBS. Thus, in Europe and North America, which have ageing populations, GBS occurs most frequently among people aged 50–80 years (2.0–4.0 cases per 100,000 persons/year)2,24,25. By contrast, studies from Asia (Bangladesh, China, India), South America (Brazil) and sub-Saharan Africa (Ethiopia, Tanzania), which are not affected by population ageing, suggest that GBS occurs most frequently in people aged 21–35 years12,38,40,44,45. In LMIC, where Campylobacter infections are endemic, infections are predominantly seen in children, and the rates of Campylobacter-related illness and infection ratios decrease with age46. Age can also influence the risk of developing infections that trigger GBS and is an important prognostic factor in individuals with GBS.
The clinical presentation, extent and severity of GBS vary geographically (Table 2). In Europe and North America, ~90–95% of patients with GBS have AIDP, and the rest have AMAN or AMSAN7,9,12. The proportion of patients with AMAN or AMSAN is considerably higher (30–65%) in several countries in Latin America, the Caribbean (Curaçao, Mexico, Argentina) and Asia (China, Japan, Bangladesh), although in many of these countries (including Japan) AIDP remains the most frequent variant14,35,44,47–49. In the countries and regions where AMAN is the predominant variant, the frequency of AIDP is 22–46%9. MFS seems to be more common among patients with GBS from eastern Asia; as an example, 20–26% of patients in Taiwan, Singapore and Japan have MFS, a much higher proportion than in the rest of the world (5–10%)7,9,50. The high prevalence of AMAN, AMSAN and MFS in Asia might be related to the increased frequency of C. jejuni infection in this region7,9,14. Other infections such as H. influenzae have also been linked to MFS in Asia51. In countries such as Bangladesh and China, where AMAN is more frequent than it is in Europe and North America, approximately 80% of patients present with severe GBS (GBS disability score >2) compared with 40–60% of patients from Europe and North America, where the AIDP subtype is most prevalent12,52.
Table 2.
Clinical features and outcome of GBS by region
| Region | Country | Antecedent events (%) | Severity | Subtype (%) | Treatment (%) | Mortality (%) | Refs |
|---|---|---|---|---|---|---|---|
| Worldwide | |||||||
| Europe, America and parts of Asia | NA |
Adults: 22–53 RTI, 6–26 gastroenteritis Children: 50–70 RTI, 7–14 gastroenteritis |
Mean MRC-SS at entry 48-49; GBS-DS >2 at nadir 76% | NR | IVIg or PE 87–93 | 2–10 | 9,10,12,25,97 |
| Europe and North America | NA | NR | NR | 90–95 AIDP, 5 axonal | NR | 3–7 | 10,48 |
| LMIC | |||||||
| Middle East and North Africa | Egypt | 24 RTI, 8 gastroenteritis | GBS-DS >2 at admission 76% | 76 AIDP, 8 axonal | NR | 16 | 43 |
| Morocco | 51 RTI, 32 gastroenteritis | NR | 81 AIDP, 19 axonal | NR | 36 | ||
| South Asia | Bangladesh | 18–19 RTI, 36–50 gastroenteritis | Mean MRC-SS at entry 22; GBS-DS >2 at nadir 93% | 22–32 AIDP, 53–67 axonal | IVIg or PE 14, supportive care 86 | 14 | 12,14,22,96,109 |
| India | 35–65 RTI, 23–47 gastroenteritis | GBS-DS >2 at admission 76% | 57–64 AIDP, 23–41 axonal | NR | 4–12 | 41,57,106,113 | |
| Nepal | 29 RTI, 3.2 gastroenteritis | NR | 19 AIDP, 19 axonal | NR | 6 | 132 | |
| Pakistan | 35 RTI, 18 gastroenteritis | NR | 46–63 AIDP, 31–34 axonal | NR | 8 | 42,56 | |
| Sub-Saharan Africa | Ethiopia | NR | NR | 55 AIDP, 19 axonal | NR | 25 | 40 |
| Tanzania | NR | NR | NR | NR | 15 | 38 | |
| HIC | |||||||
| East Asia and Pacific | China | 24–63 RTI, 7–13 gastroenteritis | Mean GBS-DS at admission 2.57; GBS-DS at nadir 3.15; GBS-DS >2 at nadir 55% | 34–57 AIDP, 22–29 axonal | NR | 2–8 | 52,59,99 |
| Taiwan | 65 RTI, 4 gastroenteritis | NR | 80 AIDP, 6% axonal | NR | 2–5 | 37,39 | |
| Korea | 11 RTI, 2 gastroenteritis | GBS-DS >2 at nadir 75% | NR | IVIg or PE 81, supportive care 19 | 2 | 133 | |
| Australia | NR | NR | 54 AIDP, 4 axonal | NR | NR | 124 | |
| Japan | NR | NR | 34 AIDP, 45 axonal | IVIg or PE 90 | NR | 49,122 | |
| Europe and Central Asia | Netherlands | 41 RTI, 40 gastroenteritis | NR | 60 AIDP, 4 axonal | IVIg or PE 91 | 2 | 19,118 |
| Spain | 38 RTI, 27 gastroenteritis | GBS-DS >2 at admission 50% | 83 AIDP, 8 axonal | IVIg or PE 86 | 2 | 19,118 | |
| Latin America and the Caribbean | Brazil | 56 RTI, 8 gastroenteritis | NR | 82 AIDP, 18 axonal | NR | 5 | 44 |
| Colombia | NR | Median MRC-SS at admission 40; median GBS-DS at nadir 4 | 78 AIDP, 2 axonal | NR | 4 | 4 | |
AIDP, acute inflammatory demyelinating polyneuropathy; GBS-DS, Guillain–Barré syndrome disability score; HIC, high-income countries; IVIg, intravenous immunoglobulin; LMIC, low-income and middle-income countries; NA, not applicable; NR, not reported; MRC-SS, Medical Research Council sum score; PE, plasma exchange, RTI, respiratory tract infection.
Pathogenesis
Overall, GBS is considered to be the consequence of a preceding infection that triggers an immune response that is responsible for the demyelination and axonal degeneration of peripheral nerves and nerve roots. Treatment with immunomodulatory agents, such as vaccines or biologic drugs, have also been associated with GBS in rare individuals. Other events, including surgery and malignancy, have been temporally related to GBS; the underlying mechanism of GBS in such individuals is not clear53–55.
Antecedent infections
Approximately two thirds of patients with GBS report symptoms of an infectious disease within the 4 weeks preceding the onset of weakness2. Upper respiratory tract infection is the most common antecedent event and is reported by 22–53% of all patients with GBS in Europe, North America, South America and parts of Asia (Taiwan, Nepal, Pakistan, Japan and Malaysia)10,12,37,47,56. The frequency of antecedent respiratory tract infections is even higher in paediatric patients with GBS (50–70%)25. By contrast, in India and Bangladesh, gastroenteritis is the most frequent antecedent event associated with GBS (36–47%)12,57.
Worldwide, the most frequently identified infectious agent that triggers GBS is C. jejuni, which is an important bacterial cause of gastroenteritis and food poisoning30,58. The reported frequencies of antecedent C. jejuni infection in patients with GBS differ between studies as well as between countries and regions; for instance, C. jejuni infection is substantially more frequent among patients with GBS from Curaçao, China and Bangladesh (~60‒70%) than in those from all other countries (30‒32%)14,28,32,48. The increased frequency of C. jejuni infection in these regions could be explained by their hygienic infrastructure and environmental or host-related factors, including diet14,27,44,59. C. jejuni is an established cause of MFS that is probably more frequent in LMIC60. However, other infections might be responsible for triggering MFS in countries where C. jejuni is less common.
The reported frequencies of antecedent infections in a given population can change over time. For example, China has undergone rapid socioeconomic development and improvements in health services over the past 50 years. A recent study of GBS in China found a lower incidence of antecedent C. jejuni infection (27% in data from 2013–2017)59 than had previously been reported (66% in data from 1991–1992)32. In addition, the trend towards increased life expectancy in China over a similar time period could have decreased the incidence of C. jejuni infections, which are more common in younger individuals. We are not aware of any public health interventions undertaken during this time by the Chinese government aimed specifically at reducing the number of C. jejuni infections59,61. However, public health interventions can both reduce the prevalence of Campylobacter infections and decrease the incidence of GBS: in response to high rates of C. jejuni infection between 1980 and 2006, the New Zealand government introduced a national intervention to reduce contamination with Campylobacter spp. in poultry. Within 2 years, the country achieved a 52% decline in campylobacteriosis and a simultaneous 13% reduction in GBS hospital admissions62. Whether such infection control interventions are feasible in other countries and regions (such as LMIC) remains to be fully explored.
Other infectious agents that have been detected at higher frequencies in patients with GBS than in the background population are cytomegalovirus (10‒13%), Epstein–Barr virus (10%), M. pneumoniae (5%; predominantly in children), hepatitis E virus (5%) and Zika virus30,48,58,63. Additionally, some infections that are more frequent in LMIC than in other countries and regions have been associated with GBS in case reports or case series: malaria in India, Sri Lanka and Thailand; HIV infection in sub-Saharan Africa; and dengue virus infection in Southeast Asia and Brazil64–70. To our knowledge, no reports have linked these infections to GBS in HIC, and epidemiological or case–control studies are required to confirm whether these infections are truly associated with GBS. During the coronavirus disease 2019 (COVID-19) pandemic, several case reports or case series have indicated a possible association between GBS and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection71–74. At the time of writing, most such reports were from Europe, although a small number of case reports were from LMIC (four from India, one from Morocco and one from Sudan)75–77. However, an epidemiological study in the UK found no increase in the incidence of GBS during the COVID-19 pandemic78. Further studies are required to confirm the potential relationship between SARS-CoV-2 infection and GBS.
Immunopathogenesis
The geographic differences in clinical and electrophysiological phenotypes of GBS in LMIC and HIC might be in part caused by differences in the rates of preceding infections that tend to trigger different types of GBS. For example, C. jejuni infections lead to the development of predominantly (but not exclusively) the axonal type of GBS30. In C. jejuni-related GBS, an immune response is triggered owing to molecular mimicry between C. jejuni lipo-oligosaccharides and human nerve gangliosides, which results in the production of cross-reactive antibodies that activate complement and damage nerves2. The pathophysiological mechanisms leading to GBS after infections other than C. jejuni have not yet been clearly defined, but similar mechanisms might also play a part in other bacterial infections related to GBS, such as M. pneumoniae and H. influenzae, although these have been less extensively investigated.
The demyelinating and sensorimotor forms of GBS are usually preceded by infection with viruses, such as cytomegalovirus or Epstein–Barr virus; however, the immunopathogenesis remains to be elucidated10. Similarly, the specific components of the Zika virus that trigger the immune response leading to GBS have not yet been clarified79. Of the patients with SARS-CoV-2-associated GBS, 77–80% had the demyelinating electrophysiological subtype and ~70% had classic sensorimotor GBS80,81. Whether this is the typical phenotype of SARS-CoV-2-related GBS is presently unclear owing to the limited number of reported cases.
Despite the strong associations between specific infectious agents and GBS, the overall risk of developing GBS after infection is very small; for example, only one in 1,000–5,000 patients with C. jejuni infection will develop GBS in the subsequent 2 months. One factor that determines this low risk is the requirement for carbohydrate mimicry (which is not present in all C. jejuni strains) to develop the cross-reactive antibody response to gangliosides that evolves into GBS2,3,10. However, genetic and nutritional factors might also influence the patient’s susceptibility to producing such antibodies82–84. Poor nutritional status, and specifically malnutrition, alters the dysfunctional immune responses implicated in the pathogenesis of various autoimmune diseases85. Immune response activation following an infection has also been associated with genetic polymorphisms. Several studies have found associations between GBS and polymorphisms in the TNF gene (which encodes tumour necrosis factor) and the MBL2 gene (which encodes mannose-binding protein C)2,82–84.
Outbreaks of GBS
Although GBS usually occurs sporadically, several outbreaks of this disease have been linked to epidemics of infectious diseases that can trigger GBS. Surges in GBS cases in China (2007) and Mexico (2011) were linked to epidemics of C. jejuni infection, and an outbreak of GBS in Peru in 2018 was associated with an epidemic of enterovirus infection16,17,86. A link between GBS and Zika virus infection was first reported when a 20-fold increase in GBS cases was found during a Zika virus outbreak in French Polynesia in 2013–2014. Subsequently, the incidence of GBS rose by ~3.2–5.1 times in areas affected by the Zika virus epidemic in Latin America and the Caribbean (2014–2016)4–6,17,63,87. However, only ~2 in 10,000 patients infected with Zika virus went on to develop GBS, suggesting that a relatively large outbreak of Zika virus is necessary to increase the incidence of GBS88.
The origins of emerging infectious diseases correlate positively with specific socioeconomic, environmental and ecological factors, which provide a basis for identifying regions where new infections are most likely to originate (so-called emerging disease hotspots)89. Zoonoses from wildlife represent the most important and growing threat of emerging infections to global health, whereas vector-borne diseases are responsible for about 25% of emerging infectious diseases. Hotspots for emerging infectious diseases are more common at lower latitudes where wild animals and arthropod vectors reside, such as sub-Saharan Africa and parts of Asia, which mainly consist of LMIC89. Other vector-borne viruses transmitted by the same Aedes family of mosquitoes as Zika virus (such as chikungunya and dengue) have also been associated with surges in GBS cases90,91. Therefore, these regions are particularly at risk of new outbreaks of GBS. In response to the Zika virus outbreak, several projects have been set up in Latin America to prevent transmission of vector-borne diseases, including surveillance systems for arboviruses and vector control programmes92. Further investment in these projects and their implementation in at-risk areas beyond Latin America could help to reduce the likelihood of future outbreaks of GBS.
International disease surveillance initiatives could also help identify new outbreaks of GBS. The ongoing acute flaccid paralysis (AFP) surveillance programme — originally devised for the surveillance of poliomyelitis — is a useful early warning signal that flags changes in the prevalence of AFP in children up to 15 years of age. Studies conducted in China and Bangladesh show that GBS is now the predominant cause of AFP among children in this age group, suggesting that AFP surveillance programmes could be expanded to detect changes in the incidence of GBS. Data from this programme have already been used to calculate crude incidence rates of GBS among children26,93. Extending the AFP surveillance programme to other age groups, and GBS case ascertainment using the Brighton Collaboration criteria to assess the degree of diagnostic certainty, might help to monitor the incidence of GBS94.
Diagnosis
Diagnosis of GBS is mainly based on clinical features, supported by cerebrospinal fluid examination and nerve conduction studies. The National Institute of Neurological Disorders and Stroke (NINDS) criteria and the Brighton Collaboration criteria are the most commonly used sets of validated diagnostic criteria for GBS1–3,19,95–99.
Patients with GBS can present with remarkably diverse clinical features. In patients with typical GBS, the key presenting feature is ascending bilateral symmetrical weakness that progresses over a period of 12 h to 28 days before a plateau is reached1–3,9,10. Most patients develop generalized hyporeflexia or areflexia, although tendon reflexes can be normal or even exaggerated in the initial stages. More than half of patients with GBS develop cranial nerve deficits, including bilateral facial weakness, bulbar weakness or extraocular motor dysfunction. In addition to muscle weakness, patients can also experience sensory disturbances, ataxia, muscle pain or radicular pain and signs of autonomic dysfunction, including blood pressure fluctuations and cardiac arrhythmia1–3,12. This diversity can complicate diagnosis in the early stages of GBS, especially in patients with atypical findings — for instance, the ~10% of patients who have normal or brisk deep tendon reflexes and the ~8% of patients who present with only paraparesis100. Children with GBS might also present with atypical features such as pain, refusal to walk or an abnormal gait; indeed, GBS is correctly diagnosed at admission in only one-third of affected preschool-aged children2. Diagnosis is generally even more challenging in LMIC, where facilities for cerebrospinal fluid examination and nerve conduction studies might not be readily available, which leads to multiple referrals of patients and diagnostic delay. In one prospective multinational cohort study, the median interval between the onset of weakness and study entry was 5 days in the Netherlands compared with 10 days in Bangladesh96. Studies conducted in Africa have also found lengthy intervals of up to 19 days between the onset of weakness and hospitalization38. This delay could lead to under-reporting of GBS in LMIC, as some patients with severe disease might die before reaching the hospital. Moreover, patients with mild symptoms might not seek treatment or recover before reaching a hospital.
The relationships between C. jejuni infection and antibodies against the GM1, GM1b, GD1a, GalNAc–GD1a and GQ1b gangliosides in patients with GBS are well established2. Some studies have suggested an association between the presence of anti-GM2 antibodies and a recent cytomegalovirus or Epstein–Barr virus infection59,101. However, serological tests to detect antiganglioside antibodies are not routinely performed at diagnosis, as negative test findings cannot rule out GBS2. Furthermore, most of these serological tests require sophisticated techniques and trained personnel that might not be available in LMICs.
In addition, an extensive list of differential diagnoses might need to be excluded. The differential diagnosis of GBS depends on the clinical presentation and variant of GBS (Box 1) and is also likely to differ between countries and regions, owing to local variations in the prevalence of infectious diseases, nutritional deficiencies or intoxications, autoimmune diseases and malignancies. As no region-specific information on the differential diagnosis of GBS was included in published studies, we conducted a small survey (Supplementary Information) to obtain insight into this important characteristic. The survey was sent to GBS experts working in LMIC within our network, who were asked in turn to distribute the questionnaire to other neurologists in their networks. In total, 17 neurologists (of whom seven frequently see paediatric patients) and two paediatric neurologists working in LMIC returned the questionnaire. Their responses revealed that the differential diagnosis of GBS is generally comparable between LMIC and HIC, although some important differences were noted (N.P., S.E.L., Q.D.M. and B.C.J., unpublished observations). For example, sarcoidosis, Sjögren syndrome, Lambert–Eaton myasthenic syndrome and mitochondrial disease seem to be less frequent diagnoses among patients suspected of GBS in LMIC than in HIC. Other diagnoses, such as hypokalaemic thyrotoxic periodic paralysis, organophosphate intoxication, botulism, rabies, polio and tetanus, seem to be more frequent in LMIC than in HIC. Furthermore, the infectious causes of transverse myelitis, acute flaccid myelitis and polyradiculoneuritis differ between LMIC and HIC. Lyme borreliosis and enterovirus D68 or A71 infection are rarely seen outside Europe and North America, whereas infections with HIV, HTLV-1 and arthropod-borne viruses — including Zika virus, chikungunya virus and West Nile virus — are frequently reported in several LMIC. These differences might reflect geographic variation in the spread of arthropod vectors (such as those carrying arboviruses) or in the incidence of infectious diseases. For example, polio and rabies eradication programmes have been more successful in HIC than in LMIC. Other explanations might include resource limitations in LMIC that preclude the diagnosis of complex systemic disorders such as Sjögren syndrome and differences in the ages of the populations at risk.
The differential diagnosis of paediatric GBS differs from that in adults owing to the presence of atypical or non-specific features that complicate the diagnosis, such as meningism or poorly localized pain102,103. Furthermore, vascular causes, vitamin deficiencies, drug-induced myopathy or polyneuropathy and chronic inflammatory demyelinating polyneuropathy occur less frequently in children than in adults102,103. These differences between adults and children in the differential diagnosis of GBS occur in both HIC and LMIC, although (as reported for adults) the infectious causes of conditions that mimic paediatric GBS differ between LMIC and HIC.
Box 1 Differential diagnosis of GBS.
Infection
Acute transverse myelitis (associated with HIV, cytomegalovirus, Epstein–Barr virus, varicella zoster virus, syphilis, tuberculosis or diphtheria infection)
Acute flaccid myelitis due to infections with arthropod-borne viruses (such as Zika virus, chikungunya virus, West Nile virus) or other viruses such as rabies, polioa and enterovirus D68 or A71
Poly(radiculo)neuritis owing to infection with HIV, cytomegalovirus, Epstein–Barr virus, varicella zoster virus, diphtheria or Lyme borreliosis
Botulism (Clostridium botulinum) or tetanus (Cl. tetani)
Myositis caused by influenza virus, HIV, HTLV-1 or enterovirus infectionb
Meningitis and/or meningoencephalitisb
Inflammation
Acute transverse myelitis
Neuromyelitis optica, myelin oligodendrocyte glycoprotein antibody-associated disorder, sarcoidosis, Sjögren syndrome
(Acute onset) chronic inflammatory demyelinating polyneuropathy (CIDP)c
Myasthenia gravis
Lambert–Eaton myasthenic syndrome
Metabolic
Electrolyte disorders such as hypokalaemia or hypokalaemic thyrotoxic periodic paralysis (common), hypophosphataemia or hypermagnesaemia
Deficiency of vitamin B1 (associated with beriberi or Wernicke’s encephalopathy), vitamin B12 (associated with subacute combined degeneration of the spinal cord) and vitamin Ec
Porphyria
Diabetic neuropathy and/or drug-induced diabetic neuropathyc
Hyperthyroidism and hypothyroidism
Copper deficiency
Malignancy
Leptomeningeal metastases or neurolymphomatosisc
Brainstem or spinal cord tumourb
Vascular
Brainstem or spinal cord strokec
Vasculitisc
Toxins
Organophosphates (common), lead, thallium, arsenic, diethylene glycol, ethylene glycol, methyl alcohol (methanol) and N-hexane
Ethyl alcohol (ethanol) or paraquat poisoning
Drug-induced (for example, by colchicine, chloroquine, emetine or statins)
Snakebite envenomation
Mechanical factors
Compression of the brainstem or spinal cordb
Cauda equina syndrome
Other
Functional and/or conversion disorder
Critical illness polyneuropathy
Myopathy or acute rhabdomyolysis
Mitochondrial disease
GBS, Guillain–Barré syndrome. aPolio has been eradicated in most regions, with the exception of several countries in sub-Saharan Africa and Southeast Asia (mostly Pakistan), where sporadic cases can occur. Although this box mainly focuses on the differential diagnosis of GBS in adults, bdiagnoses that are more common in children than in adults, and cdiagnoses that are less common in children than in adults are indicated.
Treatment
Management of GBS requires a multidisciplinary approach including supportive medical care and immunotherapy. Intravenous immunoglobulin (0.4 g/kg for 5 days) and plasma exchange (usually five sessions at 200–250 ml/kg) are proven and equally effective treatments for GBS3,11,104. However, most randomized controlled trials that evaluated the effectiveness of these two treatments for GBS were conducted in populations from HIC. These trials mainly included adult patients who were treated either with intravenous immunoglobulin within 2 weeks or with plasma exchange within 4 weeks after the onset of weakness11,104. Included patients had a GBS disability score of ≥3 and the majority had the AIDP subtype of GBS11,104. Therefore, the efficacy of these therapies might differ in LMIC, where AMAN and AMSAN are prevalent and patients usually present to hospital in the later stages of disease than they do in HIC.
Considerable variations in treatment protocols for GBS are observed throughout the world21. In general, intravenous immunoglobulin is considered the first choice of treatment as it is easy to administer, widely available and associated with a reduced frequency of adverse effects compared with plasma exchange11,105. Conversely, plasma exchange is less costly than intravenous immunoglobulin and could theoretically be a preferred treatment option for GBS in LMIC106–108. However, in practice, clinicians in LMIC face various limitations and obstacles that were not considered in existing GBS therapeutic studies. For example, owing to the low per capita income and lack of coverage by the national health insurance system in Bangladesh, neither intravenous immunoglobulin (~US$12,000–16,000) nor plasma exchange (~US$4,500–5,000) are affordable for the majority of patients18. Therefore, only 10‒12% of patients in Bangladesh receive one of these treatments, even though most patients with GBS in Bangladesh are severely affected. For instance, 93% of patients from Bangladesh were unable to walk independently at nadir (GBS disability score >2) in comparison with 76% of patients in Europe, America or other parts of Asia12,18,22,109. This situation underscores the need for low-cost and effective treatment strategies for GBS in LMIC. Small volume plasma exchange (SVPE) is a novel, relatively low cost (~$500), simple technique for selective removal of plasma, and has been shown to be a safe and feasible treatment for GBS in resource-limited settings such as India and Bangladesh18,110. However, as the efficacy of SVPE has only been shown in a small number of patients, large-scale studies are required before this technique can be implemented in routine clinical practice.
Complement inhibitors are a new focus in the treatment of GBS in HIC. Eculizumab, a humanized monoclonal recombinant antibody against complement factor 5, is currently being studied in the UK and Japan71,105. Another humanized antibody against complement factor 3 was shown to be safe and well tolerated in patients with GBS111, and efficacy trials of this agent are currently ongoing in Europe, the USA and Asia. Although the high cost of these biologic agents is likely to greatly restrict their use in patients with GBS from LMIC, such drugs might be made available for specific indications within LMIC at affordable price levels in the future; for instance, HIV drugs have been made available to some African countries at much lower prices than in HIC112. Moreover, several different phases of efficacy trials for complement factor 3 inhibitors are currently ongoing in patients in Bangladesh, which indicates that research groups in some LMICs are able to conduct treatment trials in accordance with the latest scientific methods and regulatory requirements. We hope that this experience will lead to opportunities to develop affordable treatments for patients with GBS in LMIC in future.
Outcome and prognosis
Admission to the ICU is recommended for patients with GBS who have imminent respiratory insufficiency, severe autonomic dysfunction with cardiovascular instability, severe swallowing dysfunction and/or diminished cough reflex or rapidly progressive weakness109,113–116. However, in LMIC the number of ICU beds is limited and ICU services in private hospitals are too costly (~US$300–1,200 daily) for most patients71,117. A study from Bangladesh found that the absence of ICU support when required was the strongest risk factor for death in patients with GBS22.
In most studies worldwide, the mortality rate for GBS is 2–10%9,10,97 although disparities are evident between regions. For example, reported mortality rates are 2‒7% in Europe and North America10,12,19,118, 13% in Hong Kong42, 14‒17% in Bangladesh12,14,22 and 16% in Egypt119. Moreover, access to integrative rehabilitation services is limited in LMIC, which can adversely affect recovery and long-term quality of life of patients with GBS120. Across the globe, ~20% of patients with GBS are unable to walk unaided 6 months after disease onset2,3,9,10,97,121 and this rate is higher (30‒40%) in countries such as Bangladesh where AMAN predominates and most patients do not receive immunotherapy12,14,122. In addition to physical complications, a substantial proportion of patients in HIC experience residual problems, including persistent pain (~35‒40%), fatigue (60‒80%) and anxiety or depression (6‒7%)2,123,124. No data have been reported on rates of these complications in LMIC. However, as most patients with GBS in LMIC only receive supportive care, these sequelae are also likely to vary between countries and to be worse in patients in LMIC than in those in HIC.
The ability to predict which patients with GBS will develop respiratory insufficiency or have a poor prognosis has been a long-held desire worldwide, as it would enable physicians to take the necessary precautions and provide additional treatment for the patients most at risk47,125. To this end, the Erasmus GBS Respiratory Insufficiency Score (EGRIS) was developed to predict the risk of requiring mechanical ventilation within 1 week and the Erasmus GBS Outcome Score (EGOS) and modified EGOS (mEGOS) were developed to predict the outcomes in patients with GBS at 6 months47,116,125. However, these tools were derived and validated in cohorts from European countries and might not be applicable worldwide. Indeed, a study from northeast Brazil found that EGOS was not a good predictive tool in that population126. By contrast, both EGRIS and mEGOS can accurately predict GBS outcome in populations from Japan and Malaysia127,128. Therefore, these models might need to be validated or adapted before they can be used in LMIC.
Various measures have been employed to capture outcomes in clinical trials of GBS around the world. Improvement in GBS disability scale scores is the main prognostic variable in the majority of studies. The Rasch-built Overall Disability Score (RODS), Overall Neuropathy Limitations Scale (ONLS), and Fatigue Severity Scale (FSS) were developed as outcome measures for clinical trials and are used to assess disability, activity limitations and fatigue, respectively, in patients with GBS129–131. However, these tools were developed in cohorts of patients with GBS from HIC and the questions might not be culturally appropriate in LMIC.
Conclusions and future prospects
At present, only limited data are available on GBS in LMIC. Most studies in LMIC were conducted in South Asia (Bangladesh and India) and publications from other LMIC are scarce, especially from Africa. LMIC are hotspots for many emerging infectious disease outbreaks, some of which have been associated with GBS. Therefore, publications from LMIC are often related to outbreaks of GBS associated with specific antecedent infectious diseases. Owing to the lack of well-designed epidemiological studies, the true incidence of GBS in many LMIC remains largely unknown. The long intervals between onset of weakness and hospitalization that are frequently observed in patients in LMIC might introduce selection bias at the hospital level, as patients with mild symptoms might not reach the health system and severely affected individuals might die before reaching the hospital. Moreover, diagnostic facilities, health-care infrastructure and adequately trained health professionals are frequently lacking in LMIC. The absence of national treatment guidelines and high costs of existing treatments relative to local wages contribute to the worse outcomes and higher mortality rates of GBS in LMIC compared with HIC. Finally, current models for predicting the outcome of GBS might not be valid in LMIC, owing to variations in disease severity, clinical presentation, electrophysiological subtypes and management.
A number of strategies can address these challenges. Firstly, the expansion and improvement of GBS research capacity in LMIC is required. Systematic population-based surveillance and cohort studies that employ accurate standardized case definitions are needed to understand and monitor the incidence and overall burden of GBS. Case–control studies are crucial to identify the risk factors associated with GBS and to detect new antecedent infections that trigger GBS in LMIC. Observational cohort studies are important to define the clinical course of GBS and the factors that influence and predict this course. An example of a cohort study of GBS that is ongoing globally in both LMIC and HIC is the International GBS Outcome Study (IGOS)96. The standardized trial protocol and web-based data entry system used in this international prospective cohort study are an example of how methodological differences between GBS studies conducted in different regions and countries might be overcome. However, African and Latin American countries and regions are under-represented in IGOS, and expanding the study to these regions and the long-term sustainability of this global initiative needs to be assured. Nonetheless, IGOS has already highlighted differences in the presentation and outcome of patients with GBS between HIC and LMIC such as Bangladesh, which provide insight into the challenges associated with caring for these patients in LMIC that might facilitate future research12.
Affordable and cost-effective treatment strategies need to be developed and multinational efficacy trials are required to study and scale-up innovative treatment approaches. Several randomized controlled trials, including a safety, feasibility and efficacy trial of SVPE and a phase I (leading to phase II–III) trial of a new investigational drug are currently being conducted in Asia, Europe and the USA. Additional clinical intervention studies of innovative and affordable treatments need to be designed, taking into account the specific context of the health system challenges in LMIC. A sustainable clinical trial infrastructure including physical health-care facilities and adequately trained health professionals needs to be established to support research into GBS in LMIC; these efforts should include high-quality diagnostic laboratories and training programmes for health-care professionals involved in the management of patients with GBS and in clinical research.
Moreover, existing prognostic models need to be validated and adapted for use in LMIC. Such tools would help clinicians in LMIC to accurately identify the patients most in need of ICU care at an early stage, thereby improving the management of individual patients and increasing the efficiency of ICU services in low-resource settings. Valid, responsive and cross-culturally applicable outcome measures need to be developed to improve our understanding of the long-term outcome of GBS in LMIC and to optimize the management of patients in rehabilitation services. Patients and their caregivers can also contact the GBS|CIDP Foundation International for support.
In conclusion, GBS is an under-reported disease in LMIC, although the limited available evidence suggests that the disease has a more severe clinical course in LMIC and that affected patients in LMIC have worse outcomes than do their counterparts in HIC. This Review highlights the most important knowledge gaps and provides suggestions and recommendations for future research. Increasing the breadth and quality of fundamental and applied research should become a critical focus to improve the clinical management of GBS in LMIC in the future. More than 100 years after the first description of the syndrome by George Guillain, Jean Alexandre Barré and André Strohl, now is the time to reduce the disease burden of GBS in LMIC.
Supplementary information
Acknowledgements
We thank H.U.R. Javid, M.L. Brito, F.A.A. Gondim, M.-E. Dourado, Y. Wang, S. Elaidy, T.C. Yin, D.R. Ohnmar, M.A. Ul Haq, A.P. Ramos, T. Thomas, K. Bateman, A. Keshavaraj, M. Dekker, W. Howlett, Hoa Nguyen, Hieu Nguyen, S. Khan, K. Prasad, U. Thirugnanam, M. Hakim, B. Alam and G. Mondol for providing comments and completing questionnaires on the differential diagnosis of GBS in their region. The research work of S.E.L. and B.C.J. is supported by ZikaPLAN, a global research consortium funded by the European Union under the Horizon 2020 programme (grant number 734584). Z.I. received restricted grant (number 1K43TW011447-01) support from Fogarty International Center, the National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health (NIH) and Annexon Biosciences for activities unrelated to the subject matter of this Review. Q.D.M. received consulting honoraria from Annexon Biosciences for activities unrelated to the subject matter of this Review. B.C.J. received unrestricted support for research from Annexon Biosciences, CSL-Behring, EU Horizon 2020, Griffols, Hansa Biopharma and Prinses Beatrix Spierfonds, for activities unrelated to the subject matter of this Review.
Author contributions
All authors contributed substantially to the initial discussions of the article content and to review or editing of the manuscript before submission. N.P. conducted the literature search, and N.P. and Z.I. researched data for the article. N.P. and Z.I. wrote the first draft of the manuscript and S.E.L., Q.D.M., H.P.E. and B.C.J. contributed to subsequent versions.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information
Nature Reviews Neurology thanks T. Umapathi and the other, anonymous, reviewers for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
GBS|CIDP Foundation International: https://www.gbs-cidp.org/
These authors contributed equally: Nowshin Papri, Zhahirul Islam.
Supplementary information
The online version contains supplementary material available at 10.1038/s41582-021-00467-y.
References
- 1.Fokke C, et al. Diagnosis of Guillain–Barré syndrome and validation of Brighton criteria. Brain. 2014;137:33–43. doi: 10.1093/brain/awt285. [DOI] [PubMed] [Google Scholar]
- 2.Van Den Berg B, et al. Guillain–Barré syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat. Rev. Neurol. 2014;10:469. doi: 10.1038/nrneurol.2014.121. [DOI] [PubMed] [Google Scholar]
- 3.Van Doorn PA, Ruts L, Jacobs BC. Clinical features, pathogenesis, and treatment of Guillain–Barré syndrome. Lancet Neurol. 2008;7:939–950. doi: 10.1016/S1474-4422(08)70215-1. [DOI] [PubMed] [Google Scholar]
- 4.Parra B, et al. Guillain–Barré syndrome associated with Zika virus infection in Colombia. N. Engl. J. Med. 2016;375:1513–1523. doi: 10.1056/NEJMoa1605564. [DOI] [PubMed] [Google Scholar]
- 5.Leonhard SE, Conde RM, de Assis Aquino Gondim F, Jacobs BC. Diagnosis and treatment of Guillain–Barré syndrome during the Zika virus epidemic in Brazil: a national survey study. J. Peripher. Nerv. Syst. 2019;24:340–347. doi: 10.1111/jns.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao-Lormeau V-M, et al. Guillain–Barré syndrome outbreak associated with Zika virus infection in French Polynesia: a case–control study. Lancet. 2016;387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dimachkie MM, Barohn RJ. Guillain–Barré syndrome and variants. Neurol. Clin. 2013;31:491–510. doi: 10.1016/j.ncl.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hosokawa T, et al. Serial electrophysiological findings in Guillain–Barré syndrome not fulfilling AIDP or AMAN criteria. J. Neurol. 2016;263:1709–1718. doi: 10.1007/s00415-016-8192-2. [DOI] [PubMed] [Google Scholar]
- 9.Yuki N, Hartung H-P. Guillain–Barré syndrome. N. Engl. J. Med. 2012;366:2294–2304. doi: 10.1056/NEJMra1114525. [DOI] [PubMed] [Google Scholar]
- 10.Willison HJ, Jacobs BC, Van Doorn PA. Guillain–Barré syndrome. Lancet. 2016;388:717–727. doi: 10.1016/S0140-6736(16)00339-1. [DOI] [PubMed] [Google Scholar]
- 11.Hughes RA, et al. Immunotherapy for Guillain–Barré syndrome: a systematic review. Brain. 2007;130:2245–2257. doi: 10.1093/brain/awm004. [DOI] [PubMed] [Google Scholar]
- 12.Doets AY, et al. Regional variation of Guillain–Barré syndrome. Brain. 2018;141:2866–2877. doi: 10.1093/brain/awy232. [DOI] [PubMed] [Google Scholar]
- 13.The World Bank. Data: World Bank Country and Lending Groups. The World Bankhttp://databank.worldbank.org/data/download/site-content/OGHIST.xls (2019).
- 14.Islam Z, et al. Axonal variant of Guillain–Barré syndrome associated with Campylobacter infection in Bangladesh. Neurology. 2010;74:581–587. doi: 10.1212/WNL.0b013e3181cff735. [DOI] [PubMed] [Google Scholar]
- 15.Pal M, Ayele Y, Hadush M, Panigrahi S, Jadhav VJ. Public health hazards due to unsafe drinking water. Air Water Borne Dis. 2018;7:1000138. [Google Scholar]
- 16.Zhang M, et al. Association study between an outbreak of Guillain–Barré syndrome in Jilin, China, and preceding Campylobacter jejuni infection. Foodborne Pathog. Dis. 2010;7:913–919. doi: 10.1089/fpd.2009.0493. [DOI] [PubMed] [Google Scholar]
- 17.Jackson B, et al. Binational outbreak of Guillain–Barré syndrome associated with Campylobacter jejuni infection, Mexico and USA, 2011. Epidemiol. Infect. 2014;142:1089–1099. doi: 10.1017/S0950268813001908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Islam B, et al. Small volume plasma exchange for Guillain–Barré syndrome in resource-limited settings: a phase II safety and feasibility study. BMJ Open. 2018;8:e022862. doi: 10.1136/bmjopen-2018-022862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roodbol J, et al. Diagnosis of Guillain–Barré syndrome in children and validation of the Brighton criteria. J. Neurol. 2017;264:856–861. doi: 10.1007/s00415-017-8429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van der Meché F, Van Doorn P, Meulstee J, Jennekens F. Diagnostic and classification criteria for the Guillain–Barré syndrome. Eur. Neurol. 2001;45:133–139. doi: 10.1159/000052111. [DOI] [PubMed] [Google Scholar]
- 21.Verboon C, et al. Current treatment practice of Guillain–Barré syndrome. Neurology. 2019;93:e59–e76. doi: 10.1212/WNL.0000000000007719. [DOI] [PubMed] [Google Scholar]
- 22.Ishaque T, et al. High mortality from Guillain–Barré syndrome in Bangladesh. J. Peripher. Nerv. Syst. 2017;22:121–126. doi: 10.1111/jns.12215. [DOI] [PubMed] [Google Scholar]
- 23.Nagappa M, et al. Guillain–Barré syndrome in the elderly: experience from a tertiary-care hospital in India. J. Clin. Neurosci. 2017;46:45–49. doi: 10.1016/j.jocn.2017.08.048. [DOI] [PubMed] [Google Scholar]
- 24.Sejvar JJ, Baughman AL, Wise M, Morgan OW. Population incidence of Guillain–Barré syndrome: a systematic review and meta-analysis. Neuroepidemiology. 2011;36:123–133. doi: 10.1159/000324710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGrogan A, Madle GC, Seaman HE, De Vries CS. The epidemiology of Guillain–Barré syndrome worldwide. Neuroepidemiology. 2009;32:150–163. doi: 10.1159/000184748. [DOI] [PubMed] [Google Scholar]
- 26.Islam Z, et al. High incidence of Guillain–Barré syndrome in children, Bangladesh. Emerg. Infect. Dis. 2011;17:1317–1318. doi: 10.3201/eid1707.101999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes RA, Cornblath DR. Guillain–Barré syndrome. Lancet. 2005;366:1653–1666. doi: 10.1016/S0140-6736(05)67665-9. [DOI] [PubMed] [Google Scholar]
- 28.Van Koningsveld R, et al. Gastroenteritis-associated Guillain–Barré syndrome on the Caribbean island Curacao. Neurology. 2001;56:1467–1472. doi: 10.1212/WNL.56.11.1467. [DOI] [PubMed] [Google Scholar]
- 29.Webb AJ, Brain SA, Wood R, Rinaldi S, Turner MR. Seasonal variation in Guillain–Barré syndrome: a systematic review, meta-analysis and Oxfordshire cohort study. J. Neurol. Neurosurg. Psychiatry. 2015;86:1196–1201. doi: 10.1136/jnnp-2014-309056. [DOI] [PubMed] [Google Scholar]
- 30.Wakerley BR, Yuki N. Infectious and noninfectious triggers in Guillain–Barré syndrome. Expert Rev. Clin. Immunol. 2013;9:627–639. doi: 10.1586/1744666X.2013.811119. [DOI] [PubMed] [Google Scholar]
- 31.Vellozzi C, Iqbal S, Broder K. Guillain–Barré syndrome, influenza, and influenza vaccination: the epidemiologic evidence. Clin. Infect. Dis. 2014;58:1149–1155. doi: 10.1093/cid/ciu005. [DOI] [PubMed] [Google Scholar]
- 32.Ho T, et al. Guillain–Barré syndrome in northern China: relationship to Campylobacter jejuni infection and anti-glycolipid antibodies. Brain. 1995;118:597–605. doi: 10.1093/brain/118.3.597. [DOI] [PubMed] [Google Scholar]
- 33.Platts-Mills JA, et al. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED) Lancet Glob. Health. 2015;3:e564–e575. doi: 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larrosa-Haro A, Macias-Rosales R, Sánchez-Ramírez CA, Cortés-López MC, Aguilar-Benavides S. Seasonal variation of enteropathogens in infants and preschoolers with acute diarrhea in western Mexico. J. Pediatr. Gastroenterol. Nutr. 2010;51:534–536. doi: 10.1097/MPG.0b013e3181df5b66. [DOI] [PubMed] [Google Scholar]
- 35.Bahemuka M. Guillain–Barré syndrome in Kenya: a clinical review of 54 patients. J. Neurol. 1988;235:418–421. doi: 10.1007/BF00314485. [DOI] [PubMed] [Google Scholar]
- 36.Charra B, Hachimi A, Benslama A, Motaouakkil S. Intravenous immunoglobulin vs. plasma exchange in treatment of mechanically ventilated adults with Guillain–Barré syndrome. Pan Afr. Med. J. 2014;18:35. doi: 10.11604/pamj.2014.18.35.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng BC, et al. Guillain–Barré syndrome in southern Taiwan: clinical features, prognostic factors and therapeutic outcomes. Eur. J. Neurol. 2003;10:655–662. doi: 10.1046/j.1468-1331.2003.00683.x. [DOI] [PubMed] [Google Scholar]
- 38.Howlett W, Vedeler C, Nyland H, Aarli J. Guillain–Barré syndrome in northern Tanzania: a comparison of epidemiological and clinical findings with western Norway. Acta Neurol. Scand. 1996;93:44–49. doi: 10.1111/j.1600-0404.1996.tb00169.x. [DOI] [PubMed] [Google Scholar]
- 39.Liou L-S, et al. Epidemiology and prognostic factors of inpatient mortality of Guillain–Barré syndrome: a nationwide population study over 14 years in Asian country. J. Neurol. Sci. 2016;369:159–164. doi: 10.1016/j.jns.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 40.Melaku Z, Zenebe G, Bekele A. Guillain–Barré syndrome in Ethiopian patients. Ethiopian Med. J. 2005;43:21–26. [PubMed] [Google Scholar]
- 41.Netto AB, Taly AB, Kulkarni GB, Rao UG, Rao S. Mortality in mechanically ventilated patients of Guillain–Barré syndrome. Ann. Indian Acad. Neurol. 2011;14:262–266. doi: 10.4103/0972-2327.91942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siddiqui SH, Siddiqui TH, Babar MU, Khoja A, Khan S. Outcomes of patients with Guillain–Barré syndrome — experience from a tertiary care hospital of a developing Asian country and review of regional literature. J. Clin. Neurosci. 2019;62:195–198. doi: 10.1016/j.jocn.2018.11.031. [DOI] [PubMed] [Google Scholar]
- 43.Wierzba TF, et al. Campylobacter infection as a trigger for Guillain–Barré syndrome in Egypt. PLoS ONE. 2008;3:e3674. doi: 10.1371/journal.pone.0003674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dourado M, Felix R, da Silva W, Queiroz J, Jeronimo S. Clinical characteristics of Guillain–Barré syndrome in a tropical country: a Brazilian experience. Acta Neurol. Scand. 2012;125:47–53. doi: 10.1111/j.1600-0404.2011.01503.x. [DOI] [PubMed] [Google Scholar]
- 45.Meena A, Khadilkar S, Murthy J. Treatment guidelines for Guillain–Barré syndrome. Ann. Indian Acad. Neurol. 2011;14:S73–S81. doi: 10.4103/0972-2327.83087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaakoush NO, Castaño-Rodríguez N, Mitchell HM, Man SM. Global epidemiology of Campylobacter infection. Clin. Microbiol. Rev. 2015;28:687–720. doi: 10.1128/CMR.00006-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Koningsveld R, et al. A clinical prognostic scoring system for Guillain–Barré syndrome. Lancet Neurol. 2007;6:589–594. doi: 10.1016/S1474-4422(07)70130-8. [DOI] [PubMed] [Google Scholar]
- 48.Bae JS, et al. Guillain–Barré syndrome in Asia. J. Neurol. Neurosurg. Psychiatry. 2014;85:907–913. doi: 10.1136/jnnp-2013-306212. [DOI] [PubMed] [Google Scholar]
- 49.Mitsui Y, et al. A multicentre prospective study of Guillain–Barré syndrome in Japan: a focus on the incidence of subtypes. J. Neurol. Neurosurg. Psychiatry. 2015;86:110–114. doi: 10.1136/jnnp-2013-306509. [DOI] [PubMed] [Google Scholar]
- 50.Ng Y, Lo Y, Lim P. Characteristics and acute rehabilitation of Guillain–Barré syndrome in Singapore. Ann. Acad. Med. Singap. 2004;33:314–319. [PubMed] [Google Scholar]
- 51.Koga M, Yuki N, Tai T, Hirata K. Miller Fisher syndrome and Haemophilus influenzae infection. Neurology. 2001;57:686–691. doi: 10.1212/WNL.57.4.686. [DOI] [PubMed] [Google Scholar]
- 52.Zhang G, et al. Subtypes and prognosis of Guillain–Barré syndrome in southwest China. PLoS ONE. 2015;10:e0133520. doi: 10.1371/journal.pone.0133520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Principi N, Esposito S. Vaccine-preventable diseases, vaccines and Guillain–Barré syndrome. Vaccine. 2019;37:5544–5550. doi: 10.1016/j.vaccine.2018.05.119. [DOI] [PubMed] [Google Scholar]
- 54.Rudant J, et al. Surgery and risk of Guillain–Barré syndrome: a French nationwide epidemiologic study. Neurology. 2018;91:e1220–e1227. doi: 10.1212/WNL.0000000000006246. [DOI] [PubMed] [Google Scholar]
- 55.Hiew FL, Rajabally YA. Malignancy in Guillain–Barré syndrome: a twelve-year single-center study. J. Neurol. Sci. 2017;375:275–278. doi: 10.1016/j.jns.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 56.Shafqat S, Khealani B, Awan F, Abedin S. Guillain–Barré syndrome in Pakistan: similarity of demyelinating and axonal variants. Eur. J. Neurol. 2006;13:662–665. doi: 10.1111/j.1468-1331.2006.01071.x. [DOI] [PubMed] [Google Scholar]
- 57.Sudulagunta SR, et al. Guillain–Barré syndrome: clinical profile and management. Ger. Med. Sci. 2015;13:Doc16. doi: 10.3205/000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poropatich KO, Walker CLF, Black RE. Quantifying the association between Campylobacter infection and Guillain–Barré syndrome: a systematic review. J. Health Popul. Nutr. 2010;28:545–552. doi: 10.3329/jhpn.v28i6.6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hao Y, et al. Antecedent infections in Guillain–Barré syndrome: a single–center, prospective study. Ann. Clin. Transl. Neurol. 2019;6:2510–2517. doi: 10.1002/acn3.50946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Willison HJ, O’Hanlon GM. The immunopathogenesis of Miller Fisher syndrome. J. Neuroimmunol. 1999;100:3–12. doi: 10.1016/S0165-5728(99)00213-1. [DOI] [PubMed] [Google Scholar]
- 61.Takahashi M, Koga M, Yokoyama K, Yuki N. Epidemiology of Campylobacter jejuni isolated from patients with Guillain–Barré and Miller Fisher syndromes in Japan. J. Clin. Microbiol. 2005;43:335–339. doi: 10.1128/JCM.43.1.335-339.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baker MG, et al. Declining Guillain–Barré syndrome after campylobacteriosis control, New Zealand, 1988–2010. Emerg. Infect. Dis. 2012;18:226–233. doi: 10.3201/eid1802.111126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Styczynski AR, et al. Increased rates of Guillain–Barré syndrome associated with Zika virus outbreak in the Salvador metropolitan area, Brazil. PLoS Negl. Trop. Dis. 2017;11:e0005869. doi: 10.1371/journal.pntd.0005869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kanjalkar M, Karnad D, Narayana R, Shah P. Guillain–Barré syndrome following malaria. J. Infect. 1999;38:48–50. doi: 10.1016/S0163-4453(99)90031-2. [DOI] [PubMed] [Google Scholar]
- 65.Sithinamsuwan P, Sinsawaiwong S, Limapichart K. Guillain–Barré’s syndrome associated with Plasmodium falciparum malaria: role of plasma exchange. J. Med. Assoc. Thai. 2001;84:1212–1216. [PubMed] [Google Scholar]
- 66.Wijesundere A. Guillain–Barré syndrome in Plasmodium falciparum malaria. Postgrad. Med. J. 1992;68:376–377. doi: 10.1136/pgmj.68.799.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thornton CA, Latif AS, Emmanuel JC. Guillain–Barré syndrome associated with human immunodeficiency virus infection in Zimbabwe. Neurology. 1991;41:812. doi: 10.1212/WNL.41.6.812. [DOI] [PubMed] [Google Scholar]
- 68.Gupta P, Jain V, Chatterjee S, Agarwal A. Acute inflammatory motor axonopathy associated with dengue fever. J. Indian Acad. Clin. Med. 2009;10:58–59. [Google Scholar]
- 69.Ralapanawa DMPUK, Kularatne SAM, Jayalath WATA. Guillain–Barré syndrome following dengue fever and literature review. BMC Res. Notes. 2015;8:729. doi: 10.1186/s13104-015-1672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Santos NQ, Azoubel ACB, Lopes AA, Costa G, Bacellar A. Guillain–Barré syndrome in the course of dengue: case report. Arq. Neuropsiquiatr. 2004;62:144–146. doi: 10.1590/S0004-282X2004000100025. [DOI] [PubMed] [Google Scholar]
- 71.Sedaghat Z, Karimi N. Guillain–Barré syndrome associated with COVID-19 infection: a case report. J. Clin. Neurosci. 2020;76:233–235. doi: 10.1016/j.jocn.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Toscano G, et al. Guillain–Barré syndrome associated with SARS-CoV-2. N. Engl. J. Med. 2020;382:2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain–Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19:383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Camdessanche J-P, et al. COVID-19 may induce Guillain–Barré syndrome. Rev. Neurol. 2020;176:516–518. doi: 10.1016/j.neurol.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nanda S, et al. Covid-19 associated Guillain–Barré syndrome: contrasting tale of four patients from a tertiary care centre in India. Am. J. Emerg. Med. 2020;39:125–128. doi: 10.1016/j.ajem.2020.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sidig, A., Abbasher, K., Abbasher, H., Abbasher, M. & Hussien, A. COVID-19 and Guillain–Barré syndrome case report. J. Neurol. Neurobiol.7, 10.16966/2379-7150.169 (2020).
- 77.El Otmani H, et al. Covid-19 and Guillain–Barré syndrome: more than a coincidence! Rev. Neurol. 2020;176:518–519. doi: 10.1016/j.neurol.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Keddie S, et al. Epidemiological and cohort study finds no association between COVID-19 and Guillain–Barré syndrome. Brain. 2020 doi: 10.1093/brain/awaa433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Muñoz LS, Parra B, Pardo CA, Neuroviruses Emerging in the Americas Study. Neurological implications of Zika virus infection in adults. J. Infect. Dis. 2017;216(Suppl. 10):S897–S905. doi: 10.1093/infdis/jix511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Uncini A, Vallat J-M, Jacobs BC. Guillain–Barré syndrome in SARS-CoV-2 infection: an instant systematic review of the first six months of pandemic. J. Neurol. Neurosurg. Psychiatry. 2020;91:1105–1110. doi: 10.1136/jnnp-2020-324491. [DOI] [PubMed] [Google Scholar]
- 81.Abu-Rumeileh S, Abdelhak A, Foschi M, Tumani H, Otto M. Guillain–Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J. Neurol. 2020 doi: 10.1007/s00415-020-10124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Blum S, McCombe PA. Genetics of Guillain–Barré syndrome (GBS) and chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): current knowledge and future directions. J. Peripher. Nerv. Syst. 2014;19:88–103. doi: 10.1111/jns5.12074. [DOI] [PubMed] [Google Scholar]
- 83.Caporale CM, et al. Susceptibility to Guillain–Barré syndrome is associated to polymorphisms of CD1 genes. J. Neuroimmunol. 2006;177:112–118. doi: 10.1016/j.jneuroim.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 84.Islam Z, et al. FAS promoter polymorphisms and serum sFas level are associated with increased risk of nerve damage in Bangladeshi patients with Guillain–Barré syndrome. PLoS ONE. 2018;13:e0192703. doi: 10.1371/journal.pone.0192703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Harbige LS. Nutrition and immunity with emphasis on infection and autoimmune disease. Nutr. Health. 1996;10:285–312. doi: 10.1177/026010609601000401. [DOI] [PubMed] [Google Scholar]
- 86.Díaz-Soto S, Chavez K, Chaca A, Alanya J, Tirado-Hurtado I. Outbreak of Guillain–Barré syndrome in Peru. eNeurologicalSci. 2019;14:89–90. doi: 10.1016/j.ensci.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dirlikov E, et al. Guillain–Barré syndrome and healthcare needs during Zika virus transmission, Puerto Rico, 2016. Emerg. Infect. Dis. 2017;23:134–136. doi: 10.3201/eid2301.161290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mier-y-Teran-Romero L, Delorey MJ, Sejvar JJ, Johansson MA. Guillain–Barré syndrome risk among individuals infected with Zika virus: a multi-country assessment. BMC Med. 2018;16:67. doi: 10.1186/s12916-018-1052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jones KE, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ferreira MLB, et al. Neurological disease in adults with Zika and chikungunya virus infection in Northeast Brazil: a prospective observational study. Lancet Neurol. 2020;19:826–839. doi: 10.1016/S1474-4422(20)30232-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Simon O, et al. Early Guillain–Barré syndrome associated with acute dengue fever. J. Clin. Virol. 2016;77:29–31. doi: 10.1016/j.jcv.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 92.Lowe R, et al. The Zika virus epidemic in Brazil: from discovery to future implications. Int. J. Environ. Res. Publ. Health. 2018;15:96. doi: 10.3390/ijerph15010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang Y, et al. Epidemiological survey of the incidence of Guillain–Barré syndrome in Harbin from 1997 to 1999. Chin. J. Clin. Rehab. 2004;34:7812–7815. [Google Scholar]
- 94.Leonhard SE, Cornblath DR, Endtz HP, Sejvar JJ, Jacobs BC. Guillain–Barré syndrome in times of pandemics. J. Neurol. Neurosurg. Psychiatry. 2020;91:1027–1029. doi: 10.1136/jnnp-2020-324230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain–Barré syndrome. Ann. Neurol. 1990;27:S21–S24. doi: 10.1002/ana.410270707. [DOI] [PubMed] [Google Scholar]
- 96.Islam MB, et al. Guillain–Barré syndrome in Bangladesh: validation of Brighton criteria. J. Peripher. Nerv. Syst. 2016;21:345–351. doi: 10.1111/jns.12189. [DOI] [PubMed] [Google Scholar]
- 97.Lehmann HC, Hughes RA, Kieseier BC, Hartung HP. Recent developments and future directions in Guillain–Barré syndrome. J. Peripher. Nerv. Syst. 2012;17:57–70. doi: 10.1111/j.1529-8027.2012.00433.x. [DOI] [PubMed] [Google Scholar]
- 98.Mateen FJ, et al. Guillain–Barré syndrome in India: population-based validation of the Brighton criteria. Vaccine. 2011;29:9697–9701. doi: 10.1016/j.vaccine.2011.09.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zeng Y, et al. Clinical features and the validation of the Brighton criteria in Guillain–Barré syndrome: retrospective analysis of 72 hospitalized patients in three years. Eur. Neurol. 2019;81:231–238. doi: 10.1159/000503101. [DOI] [PubMed] [Google Scholar]
- 100.Yuki N, et al. Guillain–Barré syndrome associated with normal or exaggerated tendon reflexes. J. Neurol. 2012;259:1181–1190. doi: 10.1007/s00415-011-6330-4. [DOI] [PubMed] [Google Scholar]
- 101.Jacobs B, et al. The spectrum of antecedent infections in Guillain–Barré syndrome: a case–control study. Neurology. 1998;51:1110–1115. doi: 10.1212/WNL.51.4.1110. [DOI] [PubMed] [Google Scholar]
- 102.Roodbol J, et al. Recognizing Guillain–Barré syndrome in preschool children. Neurology. 2011;76:807–810. doi: 10.1212/WNL.0b013e31820e7b62. [DOI] [PubMed] [Google Scholar]
- 103.Korinthenberg R, Schessl J, Kirschner J. Clinical presentation and course of childhood Guillain–Barré syndrome: a prospective multicentre study. Neuropediatrics. 2007;38:10–17. doi: 10.1055/s-2007-981686. [DOI] [PubMed] [Google Scholar]
- 104.Khan F, Ng L, Amatya B, Brand C, Turner-Stokes L. Multidisciplinary care for Guillain–Barré syndrome. Eur. J. Phys. Rehabil. Med. 2011;47:607–612. [PubMed] [Google Scholar]
- 105.Verboon C, van Doorn PA, Jacobs BC. Treatment dilemmas in Guillain–Barré syndrome. J. Neurol. Neurosurg. Psychiatry. 2017;88:346–352. doi: 10.1136/jnnp-2016-314862. [DOI] [PubMed] [Google Scholar]
- 106.Chaudhuri JR, et al. Clinical outcome of Guillain–Barré syndrome with various treatment methods and cost effectiveness: a study from tertiary care center in South India: Yashoda GBS registry. Neurol. Asia. 2014;19:263–270. [Google Scholar]
- 107.Willison HJ, Jacobs BC, van Doorn PA. Guillain–Barré syndrome: surveillance and cost of treatment strategies — authors’ reply. Lancet. 2017;389:253–254. doi: 10.1016/S0140-6736(17)30055-7. [DOI] [PubMed] [Google Scholar]
- 108.Kishore CK, et al. Management of Guillain–Barré syndrome with plasmapheresis or immunoglobulin: our experience from a tertiary care institute in South India. Ren. Fail. 2014;36:732–736. doi: 10.3109/0886022X.2014.890859. [DOI] [PubMed] [Google Scholar]
- 109.Islam Z, et al. Risk factors for respiratory failure in Guillain–Barré syndrome in Bangladesh: a prospective study. Ann. Clin. Transl. Neurol. 2019;6:324–332. doi: 10.1002/acn3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Iyer RR, Shah PH, Roy SSK, Suri SKK. Reducing the economic burden in management of Guillain–Barré syndrome using modified plasmapheresis. Asian J. Transfus. Sci. 2016;10:118–121. doi: 10.4103/0973-6247.187940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Islam Z, et al. Inhibition of C1q, initiator of the classical complement cascade, by ANX005 for the treatment of Guillain–Barré syndrome: results from a phase 1b study [abstract] Neurology. 2020;94(Suppl. 15):763. [Google Scholar]
- 112.Lucchini, S. et al. in Economics of AIDS and Access to HIV-AIDS Care in Developing Countries: Issues and Challenges (eds Moatti, J. P. et al.) 169–211 (ANRS, 2003).
- 113.Kalita J, Ranjan A, Misra U. Outcome of Guillain–Barré syndrome patients with respiratory paralysis. QJM. 2016;109:319–323. doi: 10.1093/qjmed/hcv190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Leonhard SE, et al. Diagnosis and management of Guillain–Barré syndrome in ten steps. Nat. Rev. Neurol. 2019;15:671–683. doi: 10.1038/s41582-019-0250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wu X, et al. Predictors for mechanical ventilation and short-term prognosis in patients with Guillain–Barré syndrome. Crit. Care. 2015;19:407. doi: 10.1186/s13054-015-1123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Walgaard C, et al. Prediction of respiratory insufficiency in Guillain–Barré syndrome. Ann. Neurol. 2010;67:781–787. doi: 10.1002/ana.21976. [DOI] [PubMed] [Google Scholar]
- 117.Adhikary, T. S. & Mollah, S. ICU facilities scanty at government hospitals of Bangladesh. The Daily Star (Bangladesh) (6 Feb 2021).
- 118.González-Suárez I, Sanz-Gallego I, de Rivera FJR, Arpa J. Guillain–Barré syndrome: natural history and prognostic factors: a retrospective review of 106 cases. BMC Neurol. 2013;13:95. doi: 10.1186/1471-2377-13-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Halawa EF, Ahmed D, Nada MA. Guillain–Barré syndrome as a prominent cause of childhood acute flaccid paralysis in post polio eradication era in Egypt. Eur. J. Paediatr. Neurol. 2011;15:241–246. doi: 10.1016/j.ejpn.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 120.Bright T, Wallace S, Kuper H. A systematic review of access to rehabilitation for people with disabilities in low- and middle-income countries. Int. J. Environ. Res. Publ. Health. 2018;15:2165. doi: 10.3390/ijerph15102165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rajabally YA, Uncini A. Outcome and its predictors in Guillain–Barré syndrome. J. Neurol. Neurosurg. Psychiatry. 2012;83:711–718. doi: 10.1136/jnnp-2011-301882. [DOI] [PubMed] [Google Scholar]
- 122.Hiraga A, et al. Recovery patterns and long term prognosis for axonal Guillain–Barré syndrome. J. Neurol. Neurosurg. Psychiatry. 2005;76:719–722. doi: 10.1136/jnnp.2004.051136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Merkies IS, Kieseier BC. Fatigue, pain, anxiety and depression in Guillain–Barré syndrome and chronic inflammatory demyelinating polyradiculoneuropathy. Eur. Neurol. 2016;75:199–206. doi: 10.1159/000445347. [DOI] [PubMed] [Google Scholar]
- 124.Khan F, Pallant J, Ng L, Bhasker A. Factors associated with long-term functional outcomes and psychological sequelae in Guillain–Barré syndrome. J. Neurol. 2010;257:2024–2031. doi: 10.1007/s00415-010-5653-x. [DOI] [PubMed] [Google Scholar]
- 125.Walgaard C, et al. Early recognition of poor prognosis in Guillain–Barré syndrome. Neurology. 2011;76:968–975. doi: 10.1212/WNL.0b013e3182104407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dourado Júnior ME, et al. EGOS has a reduced capacity to predicts GBS prognosis in Northeast Brazil. Acta Neurol. Scand. 2018;138:459–462. doi: 10.1111/ane.12995. [DOI] [PubMed] [Google Scholar]
- 127.Yamagishi Y, et al. Markers for Guillain–Barré syndrome with poor prognosis: a multi–center study. J. Peripher. Nerv. Syst. 2017;22:433–439. doi: 10.1111/jns.12234. [DOI] [PubMed] [Google Scholar]
- 128.Tan CY, Razali SN, Goh KJ, Shahrizaila N. The utility of Guillain–Barré syndrome prognostic models in Malaysian patients. J. Peripher. Nerv. Syst. 2019;24:168–173. doi: 10.1111/jns.12320. [DOI] [PubMed] [Google Scholar]
- 129.Graham RC, Hughes R. A modified peripheral neuropathy scale: the overall neuropathy limitations scale. J. Neurol. Neurosurg. Psychiatry. 2006;77:973–976. doi: 10.1136/jnnp.2005.081547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lunn MP, Van den Bergh PY. Outcome measures in neuromuscular disease: is the world still flat? J. Peripher. Nerv. Syst. 2015;20:255–259. doi: 10.1111/jns.12119. [DOI] [PubMed] [Google Scholar]
- 131.Van Nes SI, et al. Improving fatigue assessment in immune–mediated neuropathies: the modified Rasch–Built fatigue severity scale. J. Peripher. Nerv. Syst. 2009;14:268–278. doi: 10.1111/j.1529-8027.2009.00238.x. [DOI] [PubMed] [Google Scholar]
- 132.Bhagat SK, Sidhant S, Bhatta M, Ghimire A, Shah B. Clinical profile, functional outcome, and mortality of Guillain–Barré syndrome: a five-year tertiary care experience from Nepal. Neurol. Res. Int. 2019;2019:3867946. doi: 10.1155/2019/3867946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Park Y-S, Lee K-J, Kim SW, Kim KM, Suh BC. Clinical features of post-vaccination Guillain–Barré syndrome (GBS) in Korea. J. Korean Med. Sci. 2017;32:1154–1159. doi: 10.3346/jkms.2017.32.7.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.