Abstract
Leucokinins (LKs) constitute a family of neuropeptides identified in numerous insects and many other invertebrates. LKs act on G-protein-coupled receptors that display only distant relations to other known receptors. In adult Drosophila, 26 neurons/neurosecretory cells of three main types express LK. The four brain interneurons are of two types, and these are implicated in several important functions in the fly’s behavior and physiology, including feeding, sleep–metabolism interactions, state-dependent memory formation, as well as modulation of gustatory sensitivity and nociception. The 22 neurosecretory cells (abdominal LK neurons, ABLKs) of the abdominal neuromeres co-express LK and a diuretic hormone (DH44), and together, these regulate water and ion homeostasis and associated stress as well as food intake. In Drosophila larvae, LK neurons modulate locomotion, escape responses and aspects of ecdysis behavior. A set of lateral neurosecretory cells, ALKs (anterior LK neurons), in the brain express LK in larvae, but inconsistently so in adults. These ALKs co-express three other neuropeptides and regulate water and ion homeostasis, feeding, and drinking, but the specific role of LK is not yet known. This review summarizes Drosophila data on embryonic lineages of LK neurons, functional roles of individual LK neuron types, interactions with other peptidergic systems, and orchestrating functions of LK.
Keywords: diuretic hormone, sleep, feeding, metabolism, ion transport peptide, tachykinin, short neuropeptide F, insulin-like peptide, neuromodulation
1. Introduction
A large number of structurally diverse neuropeptides have been identified in insects, and they are known to act on different types of receptors, predominantly G protein-coupled receptors (GPCRs), as co-transmitters, neuromodulators and hormones [1,2,3,4]. Neuropeptides are major players in regulation of development, growth, reproduction, physiology and behavior and they are, thus, important throughout the lifecycle [1,2,5,6,7,8,9]. Insects have, for a long time, served in analysis of neuropeptide signaling and endocrinology [10,11,12]. The first insect neuropeptide to be isolated and sequenced was proctolin in 1975 [13], and a first neuropeptide GPCR (CG7887, Takr99D) was cloned in Drosophila in 1991 and found to be an ortholog of mammalian tachykinin receptors [14]. Today, more than 50 neuropeptide precursors and corresponding receptors are known in Drosophila and a large number of other insects, and much progress has been made in understanding the complexity of neuropeptide signaling [1,2,15,16,17]. Drosophila has emerged as a versatile model organism in studies of peptide signaling due to the extensive genetic toolbox available, together with access to a huge number of transgene flies from stock centers and the fly community, resources for functional genomics, functional imaging techniques, advances in neuronal connectomics, single-cell transcriptomics and development of efficient physiological and behavioral assays (see, e.g., [18,19,20,21,22,23,24]). So far, some peptide systems in Drosophila have attracted substantial attention, whereas others are largely neglected (see [1,5,7,25]). One of the neuropeptides that has gained traction recently in Drosophila is leucokinin (LK). Thus, it is timely to review what is known about LK signaling in Drosophila.
LKs were first identified from the Madeira cockroach Leucophaea maderae (now Rhyparobia maderae) in 1986 by assaying purified fractions for myostimulatory activity (see [26,27,28]). In this cockroach, eight LKs (sequence-related paracopies) were identified that are characterized by the C-terminus FXSWGamide [28,29]. Drosophila LK was identified much later and has the full sequence NSVVLGKKQRFHSWGamide [30]. It should be emphasized at the outset that LKs and their receptors are not related to tachykinins and their receptors [15,16,31], although this was suggested earlier on. In fact, LK signaling appears restricted to a few invertebrate phyla only [15,16].
In addition to stimulatory action on visceral muscle of some insects, LKs were found to act ex vivo as a factor that increases secretion in the Malpighian (renal) tubules of various insects [30,32,33,34,35]. For many years, the diuretic and myotropic actions of LKs were the only ones known. However, when appropriate genetic tools became available for Drosophila, a second wave of investigations of LK functions was initiated. Thus, over recent years, an impressive array of functional roles of Drosophila LK signaling have been uncovered. Furthermore, as we shall see in this review, it has been possible to pinpoint the functional roles of individual LK-expressing neuron types.
One remarkable feature of the LK system in Drosophila is that the peptide is expressed consistently in only 26 neurons of three major types in the adult central nervous system (CNS). Four of these LK cells are interneurons in the brain and subesophageal zone (SEZ); the remaining ones are segmentally arranged neurosecretory cells in the abdominal neuromeres of the ventral nerve cord (VNC) [36,37,38,39]. Yet the two pairs of brain/SEZ neurons are the basis for a wide range of modulatory and orchestrating actions in fly physiology and behavior. Furthermore, LK plays developmental roles during ecdysis and acts as a neuromodulator of larval locomotion [40,41,42]. In addition to the two pairs of relatively well-characterized LK neurons in the brain/SEZ, there is also a set of four pairs of brain neurons (ALKs) (anterior LK neurons; anterior in larval brain-body axis, but posterior in adult brain due to morphogenetic changes during metamorphosis) that are consistently labeled with different Lk-Gal4 lines but display variable LK immunolabeling [36,38]. These ALKs are lateral neurosecretory cells that co-express tachykinin (TK), short neuropeptide F (sNPF) and ion transport peptide (ITP) [43,44], but the functional role of LK in these neurons is not yet known. However, some functions of the other three peptides in the ALKs have been revealed [43,45] and will be discussed below.
In Drosophila, the gene encoding the LK precursor (CG13480) gives rise to a single copy of the peptide and only one LK receptor (LKR; CG10626) is known, which facilitates studies [3,4,30,46]. The neurons producing LK have been described in detail anatomically, whereas the functional LKR distribution is only partly known [36,37,38,39,46,47,48,49]. This review summarizes different aspects of LK signaling in Drosophila with a special focus on the role of individual types of LK-producing neurons and the circuits in which they function together with other neuropeptides. Furthermore, the embryonic lineages of LK neurons are discussed. Taken together, the LK neurons regulate water and ion homeostasis and associated stress responses, feeding, sleep–metabolism interactions, state-dependent memory formation, as well as modulation of gustatory sensitivity, nociception and post-mating behavior. It is noteworthy that LK signaling seems absent in many invertebrate taxa as well as in vertebrates, and yet, this peptide serves such vital functions in Drosophila.
2. Distribution of LK and Its Receptor in Drosophila
In this section, advancements in understanding the functional roles of LK-expressing neurons in Drosophila are discussed. In addition to the powerful genetic methods available, Drosophila shares with many invertebrates the advantage of having a relatively small number of neurons, many of which can be individually identified. Thus, it is possible to assign neuropeptides and other neuromodulators/transmitters as well as functions to single neurons that can be repeatedly assayed in multiple fly specimens. This, together with fly connectomics data [50,51,52,53] and single-cell transcriptomics [21,24], means that we should eventually be able to obtain a very good handle on the roles of neuropeptide signaling in brain circuitry.
2.1. The Power of Identifiable Neurons in Studies of Neuropeptide Signaling
Brains of annelids, arthropods and mollusks are remarkable in that they contain many unique neurons and neurosecretory cells that are specified by their location, size, anatomy and connections as well as their neurotransmitter and/or neuromodulator expression (see [54,55,56,57]). A first example of large, unique neurons was described already in 1891, the so-called Retzius cells of the leech [58,59]. Such neurons can be identified in every specimen (often even in related species) by the consistent location of their cell bodies and are referred to as identifiable neurons. In organisms such as mollusks, the identified neurons were the objects of intense research earlier on due to their large size and, thus, the ease by which electrophysiological recordings could be made (see [56,60,61]). It was even possible to use extracts of single identified cells to perform biochemical identification of neurotransmitters and neuropeptides, or cloning of neuropeptide precursor cDNA [60,62]. Thus, mollusks such as Aplysia californica and Lymnaea stagnalis were at the forefront in analysis of invertebrate neurotransmission, neuromodulation and peptide function earlier on (see [60,61,63,64,65,66]). Early progress was also made in decapod crustacean models using the stomatogastric ganglion (STG) with a small number of large neurons whose connectivity had been established already in the 1970s ([67,68], and summarized in [69,70,71]). A repertoire of neurotransmitters and neuromodulators in identified neurons in the STG (and associated ganglia) was established by immunohistochemistry and network analysis performed with a combination of electrophysiology and application of neurotransmitters/peptides (see [71]). In comparison, progress in peptidergic neuromodulation in insects was slower, since most systems under study were more complex anatomically, and the neurons were smaller and not as accessible to the techniques available at the time. Some insect neurons, such as dorsal unpaired median (DUM) neurons, brain descending neurons and specific large motoneurons, were, however, explored by electrophysiology to understand neuromodulation and generation of motor activity (see [72]).
A breakthrough for insect neuropeptide studies was the advent of novel Drosophila methods, such as the Gal4-UAS technique, that made it possible to target single genes encoding neuropeptides and their receptors or target identified neurons to enable anatomical analysis or interference with neuron function [73,74,75]. For example, by expressing apoptosis genes (reaper, rpr, or head involution defective, hid), the single pair of neurons producing eclosion hormone (EH) could be eliminated and the effects on fly eclosion analyzed [76], or a small set of peptidergic clock neurons, expressing pigment-dispersing factor, were ablated to investigate effects on the daily rhythmicity of the flies [77]. These methods have, over recent years, been much refined so that it is possible not only to target genes encoding specific neuropeptides and their receptors, or neurons expressing these, but also to do so conditionally at specific times in the lifecycle of Drosophila [78]. Intersectional genetic techniques have made it possible to target subpopulations of neurons expressing specific genes, and optogenetic and thermogenetic activation of specific peptidergic neurons can be performed [78,79,80]. Different reporter systems enable functional imaging (see [78,80,81]) and establishment of synaptic connections between identified neurons [82,83,84]. Thus, today, we can dissect the functional roles of neuropeptides in single cell types, specific neuronal circuits or in cells of other tissues by a large set of techniques. This has been important in understanding that neuropeptides and peptide hormones are pleiotropic and that their roles can be different at different times in the lifecycle, or at different sites in the brain (see [7,85]).
Some peptides appear to be utilized by neurons (and other cells) to globally orchestrate development, physiology or behavior, whereas others seem to play multiple distributed roles that are more localized and circuit-specific [85]. The action of the latter type of peptides may be in the form of co-transmission together with other neurotransmitters and neuromodulators [71,86,87]. Taken together, these findings have led to the insight that certain neuropeptides/peptide hormones can be remarkably diverse functionally, although they were initially identified from tissue extracts in a single bioassay (see [1]). Certain peptides such as tachykinins (TK) and short neuropeptide F (sNPF) are produced by large numbers of diverse neurons and appear truly multifunctional, where peptide actions are highly localized and, therefore, function is dependent on the circuits where TK or sNPF receptors are activated [31,85,88]. Other peptides such as LKs are produced by a small set of neurons and neurosecretory cells [36,89]. Thus, peptide action from these LK neurons can be both local and hormonal and appears to be orchestrating physiology and behavior at the organism level [38,48]. As we shall see next, in Drosophila, single LK neuron types can use this neuropeptide for multiple purposes, sometimes together with colocalized neuropeptides. However, it is likely that the different LK neurons are functionally coordinated and that the peptide modulates and orchestrates the physiology and behavior of the fly in a state-dependent fashion.
2.2. Localization of LK and LKR in Drosophila and Colocalization with Other Neuropeptides
Drosophila LK neurons were first described in the larval nervous system [89] and later in the adult fly [90,91]. In the larval CNS, antisera to LK identify 20 LK neurons: a pair of LHLKs (lateral horn LK neurons) in the lateral horn of the brain, two pairs of SELKs (subesophageal LK neurons) in the subesophageal zone (SEZ) and seven pairs of abdominal leucokinin neurons (ABLKs) in abdominal neuromeres A1–A7 (Figure 1), whereas in the adult, one pair of SELKs is lost and three to four pairs of ABLKs have been added during pupal development [36] (Figure 2). The ABLKs send varicose axons to body wall muscles (muscle 8 in larvae) and the lateral heart nerve, as well as to spiracles [36,89]. It can be presumed that these sites serve as neurohemal release sites for hormonal LK. Circulating LK has not been demonstrated in Drosophila. However, in some insects, LK is produced by neurosecretory cells that have axon terminations in the corpora cardiaca (CC), and a calcium-dependent, potassium-induced release of LKs from the CC (ex vivo) of L. maderae [92] and Acheta domesticus [93] has been demonstrated. Taken together with the demonstration of circulating LK in the hemolymph after feeding in the bloodsucking bug Rhodnius prolixus [94], there is, thus, support for hormonal roles of LKs. Hormonal LK action in Drosophila is further suggested since the Malpighian tubules, which respond to LK ex vivo [30,46], are not innervated but rather freely exposed to the circulation, and it was shown that knockdown of the LKR in the periphery, but not in the CNS, affects water and ion homeostasis [95].
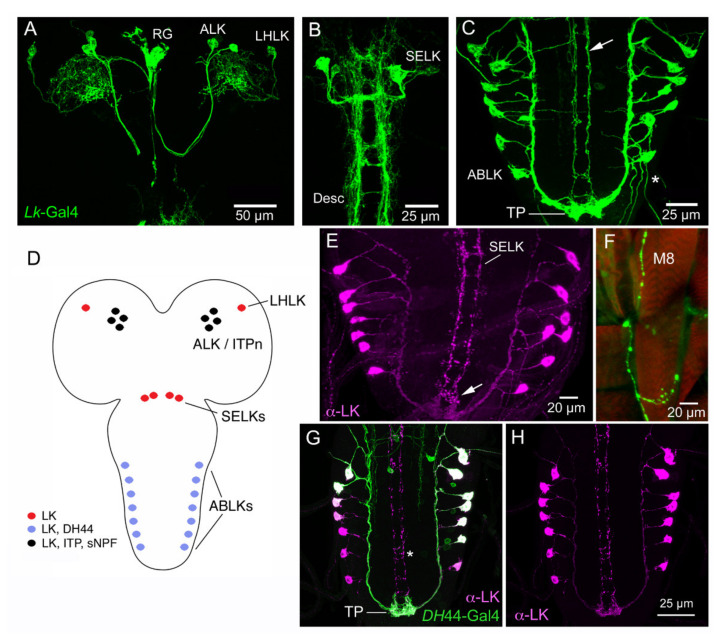
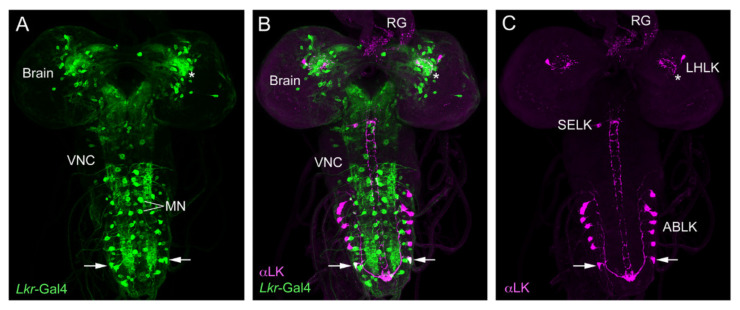
Figure 1.
Expression of leucokinin (LK) and other peptides in the CNS of larval Drosophila. (A) Larval brain with Lk-Gal4 expression in LHLK (lateral horn LK) and ALK (anterior LK) neurons. The ALKs have axon terminations in the ring gland (RG). (B) SELK (subesophageal LK) neurons of the larval brain/ventral nerve cord with their descending axons (Desc). (C) The abdominal leucokinin neurons (ABLKs) and axons of SELKs (arrow). Note the terminal plexus neuropil (TP) with axonal processes of both ABLKs and SELKs. (D) Schematic of the LK-expressing cell bodies. Colors indicate the different peptides expressed in the neurons. (E) LK immunolabeling of ABLKs and SELK axons. The arrow indicates the terminal plexus. (F) Axon termination of an ABLK on body wall muscle 8 (M8). (G,H) The ABLKs, but not SELKs (asterisk), co-express LK and diuretic hormone 44 (DH44). Images in Figure 1 are modified and rearranged from [37,96], with permission from publishers.
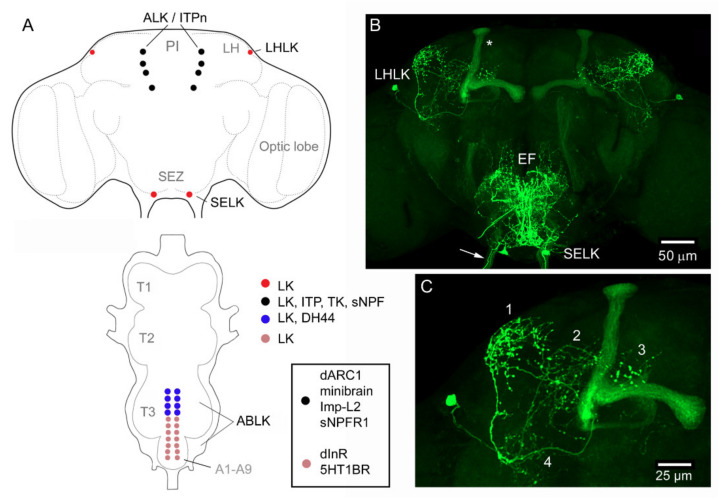
Figure 2.
Lk-Gal4-expressing neurons in the adult Drosophila CNS. (A) Schematic of brain and ventral nerve cord (VNC) with distribution of LK-expressing neuronal cell bodies. The box shows expression of other proteins in two sets of LK cell bodies: dARC1, activity-regulated cytoskeleton-associated protein [97]; Imp-L2, Imaginal morphogenesis protein-Late 2 [98]; sNPFR1, short neuropeptide F receptor 1 [99]; dInR, insulin receptor [100]; 5HT1BR, serotonin receptor 1B [100]. The ALK/ITPn (LK-ITP expressing neurons) also express the leucokinin receptor (LKR), but no function has been established so far. Other abbreviations: PI, pars intercerebralis; LH, lateral horn; SEZ, subesophageal zone; T1–T3, thoracic neuromeres; A1–A9, abdominal neuromeres. (B) The two LK-expressing neuron types in the brain. Arrows indicate axons in a subesophageal nerve; asterisk indicates lobes of the mushroom body expressing GFP. EF, esophageal foramen. (C) Enlarged image of one LHLK neuron with four major areas of arborizations indicated by numbers. Images in Figure 2 are modified and rearranged from [37,38], with permission from publishers.
With the use of Lk-Gal4 drivers, the immunocytochemistry findings have been confirmed and extended [36,37]. Gal4 expression revealed further details of the anatomy of LK neurons. For instance, it can be seen that the SELKs have axonal processes descending into the ventral nerve cord [36,38] (see Figure 1). The LHLKs have very profuse arborizations, not only in the lateral horn but also in other neuropils of the dorsolateral protocerebrum (Figure 2). Furthermore, Lk-Gal4 expression can additionally be seen in four pairs of posteriorly located protocerebral neurosecretory cells (ALKs) in the adult, and three to four pairs in the larval brain [36] (Figure 1). In the early larva, these cells also label with LK antisera, whereas in adults, labeling is variable [36,38]. It was found that the ALKs are identical to sets of neurons that express ion transport peptide (ITP), sNPF and TK [37,43]. These ITP-producing neurons (also designated ITPn) are lateral neurosecretory cells (LNCs) that have axon terminations in the corpora cardiaca, corpora allata and anterior aorta as well as branches in the SEZ and tritocerebrum [43,44]. These neurons are henceforth designated ALK/ITPn. Occasionally, one can also see LK immunolabeling in the adult ALK/ITPn cells [38], suggesting that these neurons can generate products of four different neuropeptide precursors. It is, however, not clear at present under which conditions LK is upregulated in the adult ALK/ITPn. I shall return to the ALK/ITPn as well as the other LK neurons and their functions later on.
The ABLKs in Drosophila co-express diuretic hormone 44 (DH44) [37] (Figure 1 and Figure 2). This was also found to be the case for homolog cells in some other insects such as the housefly Musca domestica, the blood-sucking bug Rhodnius prolixus, locust Locusta migratoria and moth Manduca sexta [101,102,103,104]. In the Drosophila larva, all ABLKs express DH44, whereas in the adult, the strongest DH44 expression is seen in the 6–8 anterior adult-specific ABLKs, and more variable expression was noted in the 14 embryo-derived cells [37]. Thus, out of four major LK neuron types, two (ALK/ITPn and ABLK) co-express other peptides. In Table 1, genes/proteins of interest expressed in LK neurons of Drosophila are shown. The transcription factor Dimmed (Dimm) [105] can be detected in LHLKs, ALK/ITPn and ABLKs, but not in SELKs [106,107]. Dimmed is known to confer a neurosecretory phenotype to peptidergic neurons by increasing their capacity for production and storage of peptides to enable intermittent bulk release [105,107,108]. Thus, it is somewhat surprising that the large SELKs with descending axons do not express detectable Dimm.
Table 1.
Genes/proteins of interest that are expressed in LK neurons in Drosophila.
| Cell Type | Co-Expression | Stage | Function | References |
|---|---|---|---|---|
| LHLK | Translin | Adult | Starvation-induced sleep suppression | [111] |
| LHLK | Dimmed | Larva, adult | Neuron size, LK production | [106] |
| LHLK | AMPK 1 | Adult | Increases LHLK activity in fed flies | [48] |
| SELK | dInR | Adult | Not tested | [96] |
| ALK/ITPn | ITP, sNPF, TK | Larva 2, adult | Stress responses, water and ion homeostasis, drinking and feeding3 | [43,45] |
| ALK/ITPn | LKR | Adult | Not tested | [38] |
| ALK/ITPn | ImpL2 4 | Adult | Promotes insulin signaling and uptake | [98] |
| ALK/ITPn | dInR | Adult | Activates insulin signaling | [98] |
| ALK/ITPn | dARC1 5 | Starvation resistance, starvation-induced hyperactivity | [97] | |
| ALK/ITPn | Minibrain | Adult | Regulates food intake and sNPF expression | [99] |
| ALK/ITPn | sNPFR1 | Adult | Regulates food intake | [99] |
| ALK/ITPn | Dimmed | Adult | Neuron size | [106] |
| ABLK | DH44 | Larva, adult | Water and ion homeostasis 2 | [37] |
| ABLK | 5HT1B-R 6 | Adult | Modulates desiccation response | [100] |
| ABLK | 5HT1B-R 6 | Larva | Larval locomotion | [42] |
| ABLK | dInR | Larva, adult | Neuron size, LK production | [96,100] |
| ABLK | LKR | Larva 7 | Not tested | [38] |
| ABLK | Dimmed | Larva, adult | Neuron size, LK production | [106] |
| ABLK | ETH-R 7 | Larva | Pre-ecdysis behavior | [40] |
| ABLK | ETH-R | Larva | Tracheal clearance | [41] |
Notes: 1 AMPK, 5′ adenosine monophosphate-activated protein kinase. 2 TK not in larval ALK/ITPn. 3 Only tested in adults. 4 ImpL2, Imaginal morphogenesis protein-Late 2 (Ecdysone-inducible gene L2). 5 dARC1, activity-regulated cytoskeleton-associated protein (ARC)—also expressed in IPCs and AKH cells (APCs). Similar to ImpL2. 6 5HT1B-R, serotonin receptor 1B. 7 Only the larva was analyzed, and only one pair of ABLKs express dInR (Figure 5A–C). 8 ETH-R, Ecdysis-triggering hormone receptor.
Antiserum to the LK receptor (LKR) was used to demonstrate expression in stellate cells of the Malpighian tubules and some neurons in the brain [46]. The same laboratory later employed fluorophore-tagged LK to localize ligand binding to the stellate cells and some neurons in the larval CNS [109]. However, more detailed mapping of LKR distribution in the CNS has relied on the expression of different Lkr-Gal4 lines [38,39,47,48]. LKR expression in stellate cells was confirmed (Figure 3) and it was found that there is Lkr expression in the insulin-producing cells (IPCs) of the brain, in the ALK/ITPn, in a few larval ABLKs and numerous unidentified neurons [38] (Figure 4 and Figure 5). Other studies demonstrated Lkr-expression in neurons innervating the upper layer of the fan-shaped body of the central complex [39,47,110]. Further Lkr-Gal4 expression was shown in muscle fibers of the anterior midgut and posterior hindgut, as well as in enteroendocrine cells of the midgut and in structures of the rectal pad [38] (Figure 6). Functional expression of the LKR has, so far, been confirmed experimentally only for stellate cells and IPCs [30,38,46,48]. Thus, further efforts are required to map the distribution of the LKR in Drosophila in more detail and to validate functional expression. It is especially intriguing that the LK-expressing ALK/ITPn also express the LKR, and this suggests that neurons such as SELKs might provide inputs to those neurosecretory cells.
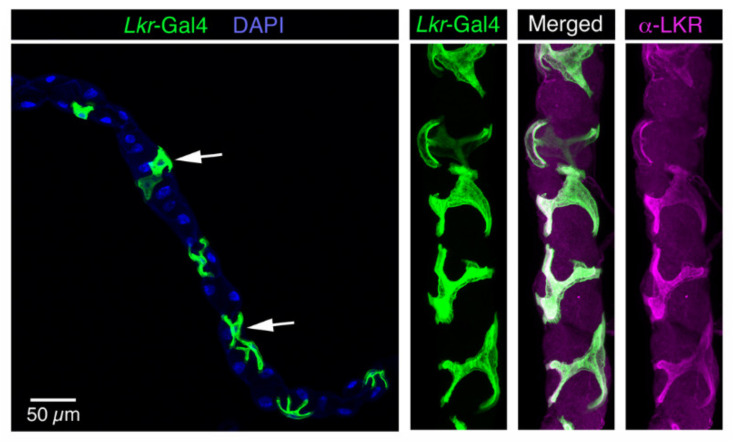
Figure 3.
Expression of leucokinin receptor Gal4 (Lkr-Gal4) in stellate cells of the Malpighian tubules of adult Drosophila. Arrows indicate stellate cells. Nuclei are stained with DAPI (blue). The stellate cells also label with antiserum to the Drosophila LKR (shown in magenta). Figure reproduced from [38] with permission from the publisher.
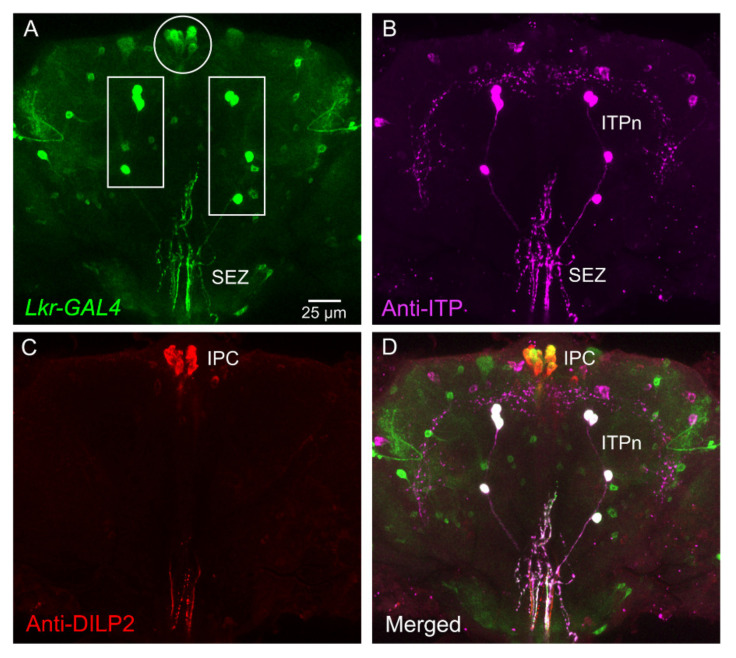
Figure 4.
Expression of leucokinin receptor (Lkr) in identified neurons of the adult Drosophila brain. (A) Lkr-Gal4 expression in the brain and subesophageal zone (SEZ). The boxed areas contain ALKs (ALK/ITPn) and the encircled area shows the insulin-producing cells (IPCs). (B) Ion transport peptide (ITP) immunolabeling in the same brain. The ITPn that also express LK are indicated. (C) DILP2 (insulin-like peptide 2) immunolabeling in IPCs in the same brain. (D) Merging the three channels, it is clear that ITPn and IPC co-express Lkr-Gal4 (these are boxed and encircled in A, respectively). Figure reproduced from [38] with permission from the publishers.
Figure 5.
Expression of LK immunolabeling and Lkr-Gal4 in the larval CNS. (A) Lkr-Gal4 is seen in numerous neurons throughout the CNS. Some are large segmental motoneurons (MN) of the abdominal neuromeres known to innervate body wall muscle (either aCC or RP2 motoneurons). VNC, ventral nerve cord. (B) LK immunolabeling and Lkr-Gal4 reveals that one pair of ABLKs display Lkr-Gal4 expression (arrows). Note the strong expression of Lkr in the lateral horn (asterisk) where LHLKs arborize. (C) LK immunolabeled neurons. The axon terminations in the ring gland (RG) are from ALK neurons (their cell bodies are not seen in this focal plane). Figure reproduced from [38] with permission from the publisher.
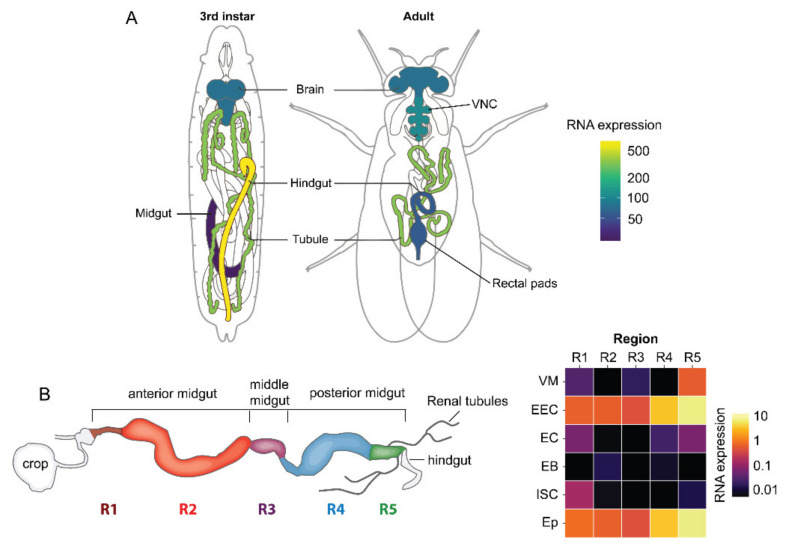
Figure 6.
Expression of the leucokinin receptor (Lkr) in tissues of Drosophila. (A) Schematics of third instar larvae and adult fly showing the expression of Lkr (Data from FlyAtlas.org, [112]). (B) A schematic of the adult intestine and heat map showing expression of Lkr in different regions of the gut (R1 to R5) and its various cell types (VM, visceral muscle; EEC, enteroendocrine cell; EC, enterocyte; EB, enteroblast; ISC, intestinal stem cell; Ep, epithelium). Data were mined using Flygut-seq [113]. This figure was reproduced from [38] with permission from the publisher.
Some of the genes/proteins co-expressed in LK neurons (Table 1) were discussed above and others are dealt with in the next section. It is noteworthy that a few neurotransmitter/neuropeptide receptors were shown to be functionally expressed by subsets of the LK neurons: the insulin receptor dInR (Figure 7A–C), the serotonin receptor 5-HT1B (Figure 7D,F), the sNPF receptor (sNPFR1) and the ecdysis-triggering hormone (ETH) receptor [40,41,99,100], and more are likely to be identified from single-cell transcriptome studies.
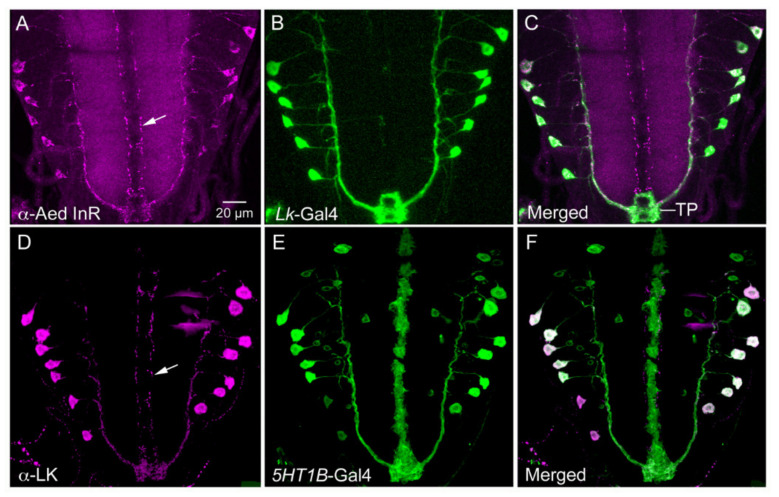
Figure 7.
LK-expressing neurons, ABLKs, in the Drosophila larva co-express receptors of insulin and serotonin. (A–C) Antiserum to Aedes aegypti insulin receptor (Aed InR) labels Lk-Gal4 expressing ABLKs and axons of SELKs (arrow). (D–F) LK antiserum labels ABLKs that also express 5HT1B-Gal4 (serotonin receptor 1B). Note that SELK axons (arrow) do not express 5HT1B receptor Gal4. A is from [96] and B from [100], with permission from the publishers.
3. Functions of Specific LK-Expressing Neurons in Drosophila
Earlier studies of LK in Drosophila did not analyze the functions of specific cell types, although it was assumed that ABLKs are involved in systemic hormonal regulations since they are the only neurosecretory cells consistently expressing high levels of LK in the adult fly [36,89,100]. Thus, it was assumed that the phenotypes in water and ion homeostasis, abdominal bloating and desiccation responses seen after global Lk-RNAi (RNA interference) or silencing or LK neurons were mediated mainly by ABLKs [100,114]. Manipulation of activity in all LK neurons also affected the composition of fecal deposits and rate of defecation in flies [95]. In support of a hormonal role of LK, the same study showed that LKR in the intestine and renal tubules, but not in the CNS, is necessary for phenotypes associated with water and ion homeostasis and defecation [95]. As shown in Table 2, similar global interventions also affected chemosensory responses, feeding behavior and lifespan [39,100,114]. More specifically, Lk and Lkr mutant flies displayed increased crop size and bloated abdomens as well as altered meal sizes in flies starved previously [39]. The bloated abdomens were later found to be associated with dysregulated diuresis, likely due to ABLKs [95,100], but the alteration of food intake pattern and crop size may be due to brain neurons.
Table 2.
Functions of LK signaling and specific LK neurons in Drosophila.
| Function | Stage | Cell Type | References |
|---|---|---|---|
| Mediates hunger-regulated nociception | Adult | LHLK, SELK | [115] |
| Links clock and sleep-regulating neurons (LK inhibits sleep) | Adult | LHLK | [47] |
| Inhibits postprandial sleep via sleep-regulating neurons (after protein meal) | Adult | LHLK | [110] |
| Mediates starvation-induced sleep suppression | Adult | LHLK 1 | [48,111] |
| Signaling to insulin-producing cells | Adult | LHLK 1 | [38,48] |
| Regulation of feeding and metabolism | Adult | LHLK 1 | [38,48] |
| State-dependent expression of water- and sugar-seeking memories | Adult | LHLK | [116] |
| Food choice via circuits in fan-shaped body | Adult | LHLK 2 | [117] |
| Regulation of mechanosensory-induced defensive post-mating response in females | Adult | ABLK | [118] |
| Induces secretion in renal tubules (diuresis) | Adult | ABLK | [30,37] |
| Water and ion homeostasis, modulation of desiccation response | Adult | ABLK | [37,100] |
| Larval locomotion | Larva | ABLK | [42] |
| Pre-ecdysis behavior | Larva | ABLK | [40] |
| Tracheal clearance at ecdysis | Larva | ABLK | [41] |
| Regulation of meal size | Adult | Not shown 3 | [39] |
| Suppression of feeding | Adult | Not shown 3 | |
| Modulation of bitter taste receptor neurons 4 in aversive response to bacteria | Adult | Not shown 3 | [119] |
| Regulation of starvation-induced hyperactivity | Adult | Not shown 3 | [120] |
| Modulation of olfaction and taste responses | Adult | Not shown 3,5 | [114] |
| Longevity (LK knockdown extends lifespan) | Adult | Not shown 3 | [114] |
Notes: 1 LHLKs are nutrient (glucose) sensing (whether indirectly or directly is not clear) [48]. 2 Not explicitly stated in the paper, but likely (see text). 3 Not shown, means that general knockdown or activation was used. 4 Bitter gustatory receptors Gr66a and Gr33a. 5 Presumably LHLK and SELK.
3.1. Functions of ABLKs
ABLKs, but no other LK neurons, were found to co-express DH44 and a serotonin receptor (5HT1B) (Figure 1D–H and Figure 7D–F; Table 1), and that allowed for specific manipulations of these cells [37,100]. Knockdown of LK only in ABLKs using DH44-Gal4 resulted in effects on water retention, desiccation and ionic stress, suggesting that these neurons are sufficient for the effect on the renal tubules and intestine [37]. Application of serotonin to abdominal neuromeres diminishes spontaneous calcium activity in ABLKs, and knockdown of the 5-HT1B receptor in ABLKs results in diminished LK expression in ABLKs and increased desiccation resistance, indicating that modulated activity in ABLKs affects water balance [100]. Further experiments support a role for ABLKs in water and ion homeostasis [38]. The DH44 co-expressed in ABLKs also contributes to diuretic functions and, additionally, to regulation of food intake [37]. Knockdown of the 5HT1B receptor in ABLKs also diminished food intake [100]. In larval Drosophila, ABLKs are involved in the regulation of locomotor turning behavior [42], pre-ecdysis behavior [40] and tracheal clearance at ecdysis [41]. The role of insulin receptor expression in ABLKs appear to be in regulation of LK expression and as a regulator of neuron size during development [96,100]. The input neurons that act on the receptors found on ABLKs (Table 1) have not yet been identified; however, SELKs may act on these neurons, at least in larvae, since the LKR is expressed by a minimum of one pair or ABLKs, and the two neuron types are in close contact posteriorly in the VNC (Figure 5A–C) (see also [38]).
ABLKs constitute one of several sets of peptidergic neurons that regulate ion and water balance (Figure 8, Supplementary Figure S1). Other peptides known to act as diuretic and antidiuretic factors are DH44, DH31, capability (CAPA1-2) peptides, ITP and maybe glycoprotein A2/B5 (GPA2/GPB5) [45,121,122,123,124,125,126,127,128], whereas pigment-dispersing factor (PDF) acts on muscle contractions in the ureter portion of the renal tubules of adults [129] and hindgut in larvae [130]. Global knockdown of GPB5 did not affect desiccation or regulate ionic stress, only resistance to starvation [128]. Thus, the eight pairs of neurosecretory cells in the A1–A4 neuromeres that express GPB5 may act in different aspects of diuresis/antidiuresis.
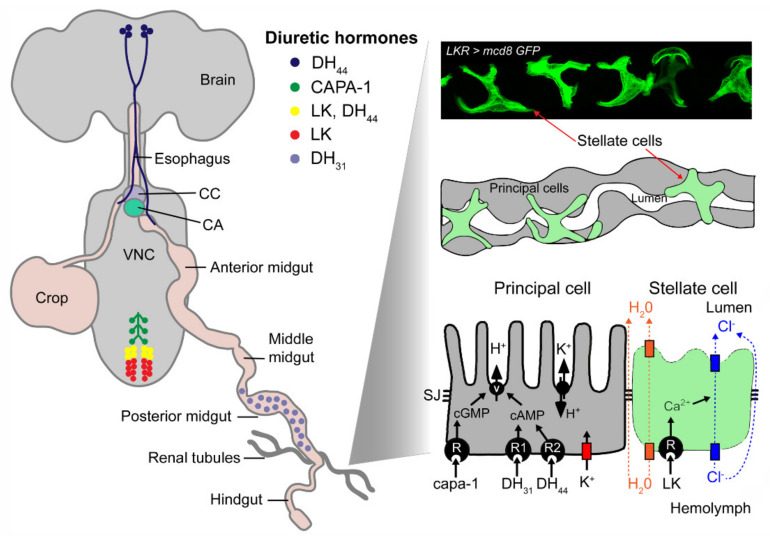
Figure 8.
Distribution and actions of LK and other diuretic hormones in adult Drosophila. A schematic depiction of the location of peptidergic neurons and gut endocrine cells that express diuretic hormones: capability-derived peptides (CAPA1-2), diuretic hormone 31 (DH31), diuretic hormone 44 (DH44) and leucokinin (LK). After release from these neurosecretory cells, the peptide hormones act on their receptors that are localized in either of two major cell types in the Malpighian (rental) tubules, the principal cells or stellate cells (visualized here using Lkr > mcd8GFP). The peptides act via different second messenger systems to alter the activity of ion pumps or channels. The orange rectangles represent aquaporin channels, the blue represent chloride channels and the red represents a Kir potassium channel. Abbreviations: CC, corpora cardiaca; CA, corpora allata; VNC, ventral nerve cord; SJ, septate junction; V, V-type ATPase. Figure 8 is based on a figure from [1]. The Malpighian tubule cell model is adapted and redrawn from O’Donnell et al. [131]. The image of localization of LKR in stellate cells is from Zandawala et al. [37] with permission from the publishers.
The mechanisms of peptide action on renal tubules have been studied. LK stimulates intracellular calcium in stellate cells of the renal tubules and thereby promotes chloride conductance and water transport through the epithelium [132] (Figure 8). The two hormones DH31 and DH44 act on the principal cells to activate cAMP and V-ATPase and, hence, secretion [122,125], whereas CAPA peptides act on the same cells but by activation of intracellular calcium, nitric oxide and cGMP signaling [124,126] (Figure 8, Supplementary Figure S1). Thus, ion and water homeostasis is tightly regulated and it remains to be clarified how the different hormonal systems cooperate in daily life and how internal sensors that monitor the osmotic state regulate the neurosecretory cells that release diuretic/antidiuretic hormones [133].
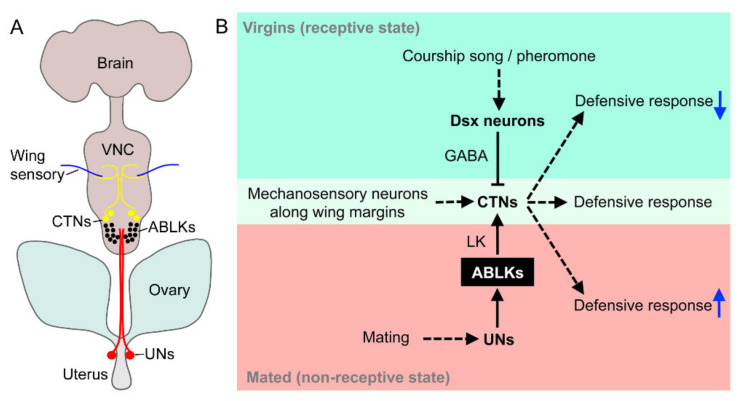
A recent paper described a very different and somewhat surprising role of ABLKs in female Drosophila [118]. The ABLKs in adult female flies serve as an interface between sensory neurons of the oviduct and interneurons in the thoracic ganglia that regulates a defensive wing retraction response (Figure 9). Flies respond to touching of their wings via mechanosensory inputs to interneurons (CTNs) in the metathoracic neuromere that trigger a defensive wing behavior. Since the touch of the wings by male flies is important in courtship, virgin female flies do not display this wing defense response. This is ensured by a pathway where sensory inputs (courtship song and pheromones) to female-specific neurons that express the gene doublesex use γ-aminobutyric acid (GABA) to inhibit the CTNs (Figure 9) [118]. In recently mated flies, however, the defensive response is strengthened by activation of the CTNs as part of the post-mating response. This increased activity in CTNs is accomplished as follows. A set of sensory neurons (UNs) in the uterus that express the gustatory receptor Gr32a and the gene pickpocket synapse onto ABLKs [118]. The UNs receive signals from the semen and activate the ABLKs, which signal with LK to stimulate the CTNs and thus strengthen the defensive response (Figure 9) [118]. These authors demonstrated that the signal that triggered the UNs was not sex peptide, but rather some other chemical signal in the semen. This suggests that more than one chemical signal can trigger the post-mating response. The link between the action of ABLKs in inducing a post-mating response and their role in regulation of ion and water homeostasis and feeding is not clear at this point. The post-mating modulation is induced by ABLK action within the CNS, whereas the other actions are hormonal. Since recently mated flies display altered activity patterns, feeding and metabolism [134,135,136,137], maybe hormonal LK contributes to this phenotype.
Figure 9.
The role of LK signaling and ABLKs in a post-mating defensive response. (A) Pathway that regulates a defensive wing retraction response in female flies [118]. Mechanosensory cells in the wing margin send axons to the mesothoracic neuromere of the ventral nerve cord (VNC). They synapse on interneurons (CTNs) that modulate motoneurons, which retract the wings (not shown). A set of sensory cells (UNs) in the uterus synapse with the ABLKs. After mating, these UNs are stimulated by contents (not sex peptide) in the semen and activate the ABLKs. The ABLKs in turn activate the CTNs, and the defensive response is strengthened. (B) Diagram of the circuit regulating the defensive response. Virgin female flies do not display this wing defense response. This is ensured by a pathway where sensory inputs (courtship song and pheromones) to female-specific neurons that express the gene doublesex (Dsx) use GABA (γ-aminobutyric acid) to inhibit the CTNs. In mated flies, the UNs in the uterus activate ABLKs and the CTNs enhance the defensive response. Figure 9A was drawn based on data in [118] and Figure 9B was redrawn from a figure in [118].
3.2. Functions of LHLKs
The LK neurons that are most extensively investigated are the LHLKs. As summarized in Table 2 and Figure 10, Figure 11 and Figure 12, the activity of LHLKs is nutrient (glucose)-dependent [48]; they receive inputs from clock neurons [47] and appear to convey the feeding state to different brain circuits such as mushroom body circuits for water and sugar memories [116], the fan-shaped body (FSB) for food choice [117], FSB (and others) for sleep regulation [47,110] and IPCs for feeding and metabolism (and maybe sleep) [38,48]. LHLKs may also (together with SELKs) mediate hunger-regulated nociception [115] and modulate certain olfactory and gustatory signals [114]. Thus, LHLKs are likely to be modulating several neuronal systems in a nutrient- and clock-dependent fashion to regulate state-dependent behaviors.
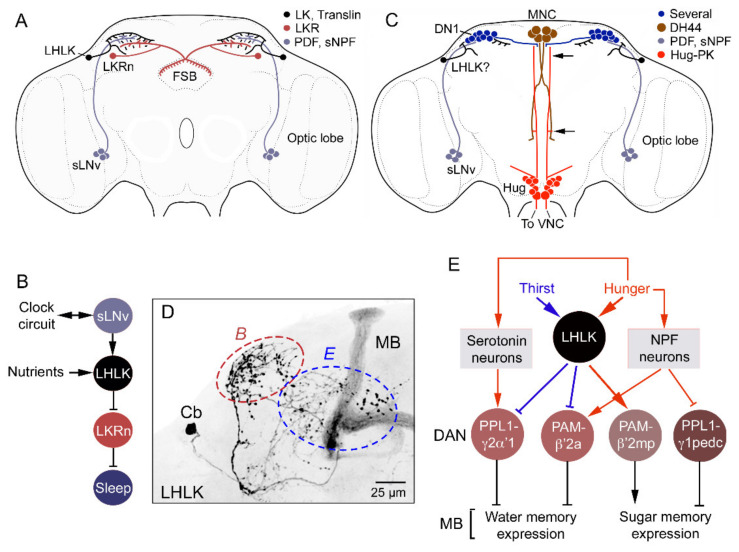
Figure 10.
LK signaling in regulation of sleep–metabolism and water memory in Drosophila. (A) Schematic of brain neurons connecting clock, nutrient sensing and regulation of sleep. Clock neurons (sLNv, small lateral ventral neurons) have outputs on LHLKs that in turn inhibit LK receptor-expressing neurons (LKRn) innervating the fan-shaped body (FSB) and thereby inhibit sleep in a nutrient-dependent fashion. Figure compiled from data in [47,110,111]. Other abbreviations: PDF, pigment-dispersing factor; sNPF, short neuropeptide F. (B) A schematic of the connections in A. (C) Another sleep-regulating system in the brain with input from sLNv clock neurons (and possible inputs from LHLKs via dorsal clock neurons, DN1). sLNv neurons signal to DN1 clock neurons which, in turn, have outputs on median neurosecretory cells (MNCs) expressing DH44. The MNCs signal via one of the DH44 receptors (DH44R1) to Hugin neurons, which modulate motor circuits in the VNC. Data in this figure are compiled from [139,140]. The possible LHLK input was added here. (D) LHLK neuron with arborizations that interact with the circuits shown in panels B and E (red and blue dashed ellipses). (E) LHLK neurons and the circuit regulating water–sugar-based memory. The LHLK neurons receive hunger and thirst signals and act on dopaminergic neurons (DAN; PPL1 and PAM subtypes) to regulate expression of water and sugar memory in mushroom body (MB) circuits. Some of these neurons also receive inputs from serotonin and NPF (neuropeptide F) neurons. Redrawn from [116].
Figure 11.
LK neurons and other peptidergic neurons that regulate food choice behavior and feeding. (A) Peptidergic neurons and neurosecretory cells that have been implicated in nutrient sensing, food choice and feeding behavior. The neurons highlighted with pink boxes are discussed in Figure 10B. Asterisks indicate that neurons are nutrient sensing. The peptides are as follows: AstA, allatostatin A; DSK, drosulfakinin; ITP, ion transport peptide; TK, tachykinin: sNPF, short neuropeptide F; SIFa, SIFamide; CRZ, corazonin; Hugin, hugin-pyrokinin; NPF, neuropeptide F; CCAP, crustacean cardioactive peptide. Data used for compilation of this figure are from [1,7,141,142,143,144,145]. (B) The nutrient-sensitive LHLK neurons (represented by LK) act on neurons (FB16) innervating the fan-shaped body (FSB). Additional neurons converge on the FB16 neurons to regulate food choice: neurons producing allatostatin A (AstA), and diuretic hormone 44 (DH44). Neuropeptide F (NPF) neurons also act on FB16 neurons to regulate food choice. The AstA, DH44 and NPF neurons receive inputs from dopaminergic neurons (DA) via two of the DA receptors, Dop1R1 and DopEcR. This figure was redrawn from [117].
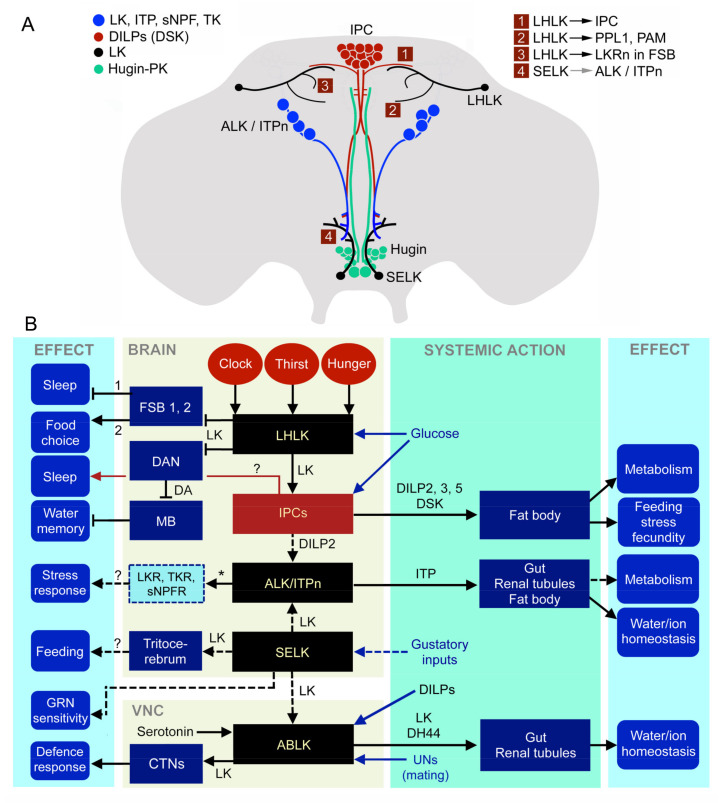
Figure 12.
Summary diagrams of leucokinin functions in Drosophila. (A) Schematic depiction of LK neurons in relation to neurosecretory cells in the adult Drosophila brain. LHLKs act on insulin-producing cells (IPCs), dopaminergic neurons (PPL1 and PAM subtypes) and at least two types of LK-receptor expressing neurons (LKRn; FSB 1 and 2 in B) innervating the fan-shaped body (FSB). SELKs may act on the ALK/ITPn that express the LKR. The Hugin neurons of the subesophageal zone are also shown since they form a link between gustatory sensory cells and feeding circuits, including IPCs. The numbered boxes (1–4) indicate sites of interaction between neurons. Data derived from [38,48,110,117]. (B) Schematic diagram of functional connections between LK neurons and other neurons, circuits and peripheral targets. Arrows indicate various actions, dashed arrows suggest presumed actions and the stop bars indicate inhibitory action. The LK neurons in the brain and ventral nerve cord (VNC) are shown as black boxes and the insulin-producing cells (IPCs) as a dark red box. Target neurons of LHLKs are shown as dark blue boxes with two types of LKR-expressing neurons of the fan-shaped body (FSB 1, 2; these are LKR neurons and FB16 neurons), dopaminergic neurons (DANs; these are PPL1 and PAM neurons) and mushroom body-associated neurons (MB). LKR neurons inhibit sleep [110] and FB16 neurons regulate food choice [117], whereas MB neurons, via dopamine (DA) inputs, mediate water (and sugar) memory [116]. LHLKs are glucose-sensing and receive inputs from neuronal circuits of the circadian clock and systems sensing thirst and hunger (red ellipses). LHLKs signal with LK to IPCs, which regulates sleep–metabolism interactions [38,48]. IPCs are nutrient-sensing and are known to use DILP2, 3 and 5 to regulate multiple functions, including carbohydrate and lipid metabolism, feeding, stress responses and fecundity (they also express DSK) (see [146]). IPCs are likely to act on ImpL2 expressing ALK/ITPn with DILP2 (dashed arrow) [98]. SELK neurons are likely to receive gustatory inputs [36] and regulate feeding, and their descending axons may act on ABLKs (dashed arrows). They might also provide inputs to ALK/ITPn (dashed arrow). ABLKs release diuretic hormone 44 (DH44) and LK systemically to regulate ion and water homeostasis [37]. ABLKs express serotonin and insulin receptors [100]. In female flies, ABLKs receive inputs from sensory cells in the uterus (UNs). When the UNs are activated by mating, ABLKs act on a set of neurons (CTNs) in the metathoracic ganglion that modulate a circuit that regulates wing retraction as a defense response [118]. ALK/ITPn are neurosecretory cells that use ITP to systemically regulate water homeostasis via the intestine and hindgut and also to regulate feeding and drinking [45]. These cells utilize TK and sNPF to regulate responses to starvation and desiccation [43], but the neuronal circuitry is not yet known. The box with a dashed outline represents neurons expressing receptors for LK, TK and sNPF (and ITP; not shown) in the brain that are yet to be identified. The role of LK in ALK/ITPn cells is not known.
The two LHLKs have relatively complex arborizations, invading several portions of the dorsolateral brain in one hemisphere where they can interact with specific dopaminergic neurons, FSB, certain clock neurons, IPCs and probably others (Figure 2B,C and Figure 10A–E). Functional imaging using GCaMP6m revealed that application of glucose to the brain reduces Ca2+ activity in LHLKs, but not in SELKs [48]. In the same study, it was shown that starvation increases Ca2+ activity in LHLKs and refeeding decreases it. These findings suggest that the nutrient level in the circulating hemolymph modulates the activity in LHLKs, but it remains to be demonstrated whether this is by cell-autonomous glucose sensing [48]. In the following, for simplicity, LKLKs are still referred to as nutrient sensing (with the caveat that this could be indirect via inputs from other neurons). Another finding that sheds light on activation of LHLK is that specific knockdown of AMP-activated protein kinase alpha (AMPKα) in LK neurons leads to increased Ca2+ activity in the LHLKs of fed flies [48]. This Ca2+-response is similar to the effect of starvation, suggesting that AMPK is active in LHLKs in fed flies, and that reduced AMPK signaling during starvation leads to increased LHLK activity [48].
It was shown that peptidergic clock neurons (sLNv) have outputs on LHLKs that in turn inhibit neurons expressing LK receptor which innervate layers of the fan-shaped body (FSB) and thereby regulate behavioral activity and sleep [47] (Figure 10A). Manipulation of neural activity in LHLK neurons furthermore revealed that these neurons are regulating starvation-induced sleep suppression [111]. LHLKs express translin, which is a conserved RNA/DNA binding protein, and this protein is also essential for the starvation-induced sleep suppression [111]. Another study implicated LK in regulation of postprandial sleep [110]. It was shown that LK signaling inhibits this protein meal-induced sleep. As mentioned, the activity of LHLKs is dependent on feeding state and AMPK activity [48]. LHLKs were, furthermore, identified as essential in metabolic regulation of sleep and thus relay nutritional state to the circuits regulating sleep [48]. In the same study, it was found that expression of the LKR in insulin-producing cells (IPCs) was required for sleep regulation in starving flies. Thus, LK signaling from LHLKs to IPCs could be identified as one of several sleep-regulating pathways. Further support for this is that nutritional and circadian inputs to IPCs have been implicated in regulation of activity rhythms in Drosophila [138].
As seen in Figure 10C, there is another activity/sleep-regulating pathway that connects sLNv neurons to other clock neurons (DN1), which, in turn, act on DH44-expressing MNCs that synapse on descending Hugin neurons in the SEZ to regulate motor activity in the ventral nerve cord [47,127,139]. This pathway may also integrate LHLKs, which arborize in the same region as the sLNv and DN1 clock neurons (Figure 10C), and LHLKs are postsynaptic to sLNvs (Figure 10A), but this circuit has yet to be established.
As mentioned, LHLK neurons are nutrient-sensing, at least indirectly [48], and receive both hunger as well as thirst signals [116]. It was shown that LHLKs act on specific dopaminergic neurons (DANs; PPL1 and PAM subtypes) associated with the circuits of mushroom bodies to regulate expression of water and sugar memory (Figure 10E) [116]. Some of these DANs also receive inputs from serotonin- and NPF (neuropeptide F)-expressing neurons that relay hunger signals.
Another role for LHLKs is in food choice behavior [117]. Quite a number of peptidergic neurons have been implicated in regulation of food choice and feeding (Figure 11A). Among these are the LK-expressing neurons, which interact with a set of neurons (FB16) associated with the fan-shaped body of the central complex (Figure 11B). FB16 neurons are involved in making a final experience-based food choice when flies are exposed to conflicting gustatory stimuli [117]. It was not explicitly tested in the study whether the LK neurons in this circuit are LHLKs, but judging from their anatomical position and their nutrient-sensing capacity, it is quite likely that they are. LK neurons are only one out of several peptidergic systems signaling the FB16 neurons; others signal with allatostatin A, DH44 and NPF (Figure 11A,B) [117]. Upstream of the peptidergic systems (except LHLKs) are dopaminergic neurons acting via two different types of dopamine receptors. The food choices made are determined by integration in the fan-shaped body of taste quality, previous experience and nutritional state of the fly. This suggests a hitherto unknown role for the fan-shaped body in choice encoding and decision-making, based on neural integration of external sensory and internal state inputs [117]. The LHLK functions are summarized in Figure 12A,B.
The complex set of peptidergic neurons/neurosecretory cells that are involved in nutrient sensing, regulation of gustation, food choice, feeding and metabolism, indicated in Figure 11A, suggests many levels of signaling involving several brain circuits. Yet, the two glucose-sensing LHLKs play prominent roles in integrating internal state signals and external cues to influence important circuits involved in choice behavior, nutrient-dependent activity and sleep, as well as certain memory functions. It would be of great interest to investigate the interactions between the LHLKs and the other peptidergic systems depicted in Figure 11A and establish further connections to the circadian clock and sleep-inducing neurons [147,148,149].
3.3. Functions of SELKs
SELKs have not yet been specifically investigated. Their location in the SEZ and arborizations there and in the tritocerebrum (Figure 2 and Figure 11) suggest a role at the interface between gustatory sensory inputs and regulation of feeding. In the same region, there is a set of about 20 neurons/neurosecretory cells (Figure 11 and Figure 12A) that express a peptide (hugin-pyrokinin; Hug-PK) encoded by the Hugin gene and which integrate gustatory information and motor neurons that control food intake, as well as neurosecretory cells, such as IPCs [22,143,150,151,152]. Thus, this ensemble of 20 peptidergic neurons, some of which are descending neurons, seems to orchestrate global actions related to feeding. SELKs have an anatomical position that is similar, and it may be that they are part of the LK-based regulation of gustatory neurons [114,119] and, perhaps, the control of meal size [39]. The SELK axons that descend throughout the VNC appear, in larvae, to contact the ABLKs posteriorly (Figure 1G,H and Figure 5), and at least one pair of ABLKs express the LKR (Figure 5), suggesting that perhaps the SELKs form a link between LK neurons of the brain and VNC (Figure 12B). Note that adults have not yet been specifically investigated with respect to LKR expression in ABLKs. In summary, no clear experimental data are available to suggest functions of the SELKs, and hopefully, future work will target these neurons.
3.4. Functions of ALK/ITPn
The LK signaling role of ALKs/ITPn is enigmatic, since their LK peptide expression is seen primarily in early larvae and the Lk-Gal4 expression is also weaker in these cells in adults [36,38]. A similar temporal expression pattern was seen for LK in a set of four primary commissure pioneer (PCP) neurons in the locust brain [153]. These locust neurons were later found to co-express the neuropeptide SIFamide [154]. Since the PCP/SIFa neurons play an important role in axonal navigation during embryonic development of the brain, it may be that LK signaling acts during neuronal path finding and/or differentiation. It remains to be determined whether LK in Drosophila ALK/ITPn plays a role during development or whether it is a neuromodulator/neurohormone in larval physiology and behavior (and also in adults under specific conditions).
ALK/ITPn are prominent in the larval brain where the cells express ITP, sNPF and LK [36,43], but their functions are not known at present. The adult ALK/ITPn, in addition, produce TK, and the roles of ITP, TK and sNPF have been investigated in preliminary studies [43,45]. Knockdown of either sNPF or TK in the ALK/ITPn results in decreased survival during desiccation and starvation as well as increased loss of water content after desiccation [43]. This suggests that the neurons are involved in the regulation of water and ion homeostasis (antidiuretic) as well as in the modulation of metabolic and dehydration stress responses. A later study confirmed this by analyzing knockdown and overexpression of ITP in the same neurons [45]. Dehydration of the flies increases the ITP level, and ITP was shown to increase thirst and reduce food intake. ITP also decreases excretion (defecation) and thereby promotes conservation of water. Furthermore, the ITP neurons respond to osmotic and desiccation stress, and disruption of ITP signaling compromises the resistance to these stressors [45], similar to TK and sNPF [43]. Thus, taken together, the three ALK/ITPn peptides studied cooperate to regulate water and ion homeostasis and the ability to cope with ionic and desiccation stress. In addition, ITP promotes thirst, suppresses hunger and represses defecation/excretion [45].
The four pairs of ALK/ITPn express further genes/proteins of interest (Table 1): the LKR [38], the dInR [98], minibrain [99], short neuropeptide F receptor (sNPFR1) [99], Imaginal morphogenesis protein-Late 2 (ImpL2) [98] and activity-regulated cytoskeleton-associated protein 1 (dARC1) [97].
So far, the role of the LKR expression in ALK/ITPn has not been studied, but it may suggest LK signaling to these neurons from SELKs. ImpL2 expression is seen not only in ALK/ITPn but also in adipokinetic hormone (AKH)-producing cells, as well as the Hugin cells of the SEZ, and was shown to promote dInR-mediated insulin signaling (and DILP2 uptake) in these neurons [98]. Minibrain (a tyrosine phosphorylation-regulated kinase) increases sNPF signaling via activation of sirtuin 2 (Sir2; deacetylase) and deacetylation of FOXO (forkhead transcription factor) and promotes food intake [99]. After feeding, protein kinase B (AKT)-mediated insulin signaling suppresses FOXO-induced sNPF expression and food intake is decreased [99]. Thus, ALK/ITPn and sNPF are part of a circuit regulating feeding dependent on nutritional status. Finally, dARC (an activity-regulated cytoskeleton-associated protein) is expressed in insulin-producing cells (IPCs) and ALK/ITPn cells [97]. Flies mutant in dARC display extended survival when exposed to starvation. This starvation resistance may partly be a result of the mutant flies displaying a lack of increased locomotor activity when exposed to starvation [97]. dARC1 was shown to act in IPCs to regulate this starvation-induced behavior, whereas the specific role of ALK/ITPn cells was not studied.
In summary, the functional roles of these intriguing ALK/ITPn cells are far from clear (see Figure 12A,B). It seems that they are at the interface between neuroendocrine systems that regulate water and ion homeostasis, thirst, hunger and metabolism [43,45,97,98,99]. They may even be orchestrating these aspects of physiology and behavior. It is important to note that in the studies cited just above, the specific roles of LK in the ALK/ITPn were not approached, and except for two of the studies [43,45], the analysis was not aimed at these specific neurons. Clearly, further studies of ALK/ITPn cells and their relations to IPCs, AKH-producing cells and other LK neurons will be of great interest. Furthermore, the specific role of LK in these neurons needs to be investigated.
4. Lineages of LK Neurons in Drosophila and Relations to GPB5 Neurons
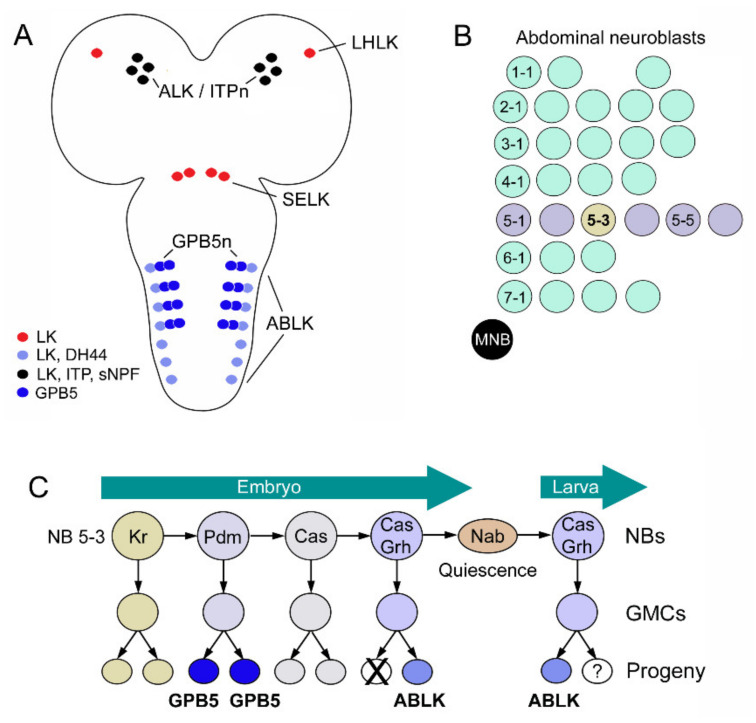
The ABLKs of Drosophila have been analyzed with respect to their embryonic neuroblast origin and differentiation throughout development and metamorphosis [128,155]. Interestingly, the ABLKs share ancestry with the neurons producing the protein hormone GPB5 (Figure 13A). Both ABLKs and GPB5 neurons are derived from neuroblast NB5-3 [128] (Figure 13B,C). An earlier study had proposed NB5-5 as the origin of ABLKs [155], but this was corrected in the later study cited above. In a third instar larva, there are eight pairs of GPB5 neurons in abdominal neuromeres A1–A4 and seven pairs of ABLKs in A1–A7. This is accomplished by selective apoptosis of ABLKs in the embryo and a loss of LK phenotype in one daughter cell (progeny) in the larva, whereas all GPB5 neurons remain in A1–A4 (Figure 13C) [128]. These cell fates are determined by a set of transcription factors that are switched on in a temporal sequence (Figure 13C).
Figure 13.
Lineages of LK and GPB5 neurons during development. (A) Larval CNS with cell bodies of LK neurons (ALK/ITPn; LHLK, SELK and ABLK) and the peptides expressed in these cells (colored circles). In the first four abdominal neuromeres, there are also neurons expressing the glycoprotein GPB5 (two pairs in each neuromere). (B) Neuroblasts in one abdominal hemi-neuromere with rows numbered from 1 to 7. Neuroblast 5-3 gives rise to ABLKs and GPB5 neurons. MNB, median neuroblast. (C) Origins of GPB5 neurons and ABLKs from neuroblast 5-3 during embryonic and larval development. Transcription factors: Kr, Krüppel; Pdm, Nubbin/Pdm2; Cas, Castor; Grh, Grainy head; Nab, transcriptional cofactor. This figure (C) was redrawn from [128].
LHLKs are derived from brain neuroblast Pcd6 and share most molecular markers (transcription factors) with ABLKs [90,156,157]. One difference is the requirement of apterous in LHLKs, but not in ABLKs [157]. These authors also showed that Notch signaling and Dimmed are important in the specification of the LK phenotypes in ABLKs and LHLKs. It is intriguing that the ABLKs and GPB5 neurons are siblings since they both might be involved in ion and water homeostasis [37,121], even though preliminary analysis of the GPB5 neurons provided no confirmation of this [128]. Future work should address this in more detail and also determine whether the two systems interact in their regulatory roles.
5. Some Aspects of LK Signaling in Other Invertebrates
Very few functional studies of LK signaling have been performed outside Drosophila. Some early studies employed ex vivo analysis of visceral muscle and found that LKs and some stable LK analogs stimulate contractions in the hindgut of L. maderae [27,28], the housefly Musca domestica [35], as well as the bug Rhodnius prolixus, where the midgut was also activated [158,159]. Induction of secretion in Malpighian tubules was shown in ex vivo experiments in flies, mosquitoes, crickets, locusts and moths [30,32,33,102,123,160], but not in R. prolixus, where LKs may act elsewhere to facilitate diuresis together with DH44 [104,159].
In the mosquito Aedes aegypti, LK applied to mouthparts and prolegs inhibits sucrose feeding and Lkr-RNAi deletes this LK-induced inhibition [161]. Feeding is also regulated by LKs in R. prolixus, ticks and the mollusk Aplysia californica [158,162,163]. In Aplysia, LK action is within the CNS by neuromodulation in motor pattern-generating circuits in the buccal and cerebral ganglia that control radula muscles during feeding [163]. A recent study showed that in the Asian honeybee A. cerana, gustatory responses to sucrose are also modulated by LK signaling [164]. Furthermore, this study showed that the Lkr gene influences division of labor in foraging and that nectar foragers display lower Lkr expression than those foraging for pollen. In a cattle fever tick, Lkr-RNAi delays oviposition and decreases egg numbers and hatchability of eggs laid [162]. Finally, LKs inhibit release of digestive enzymes such as protease and amylase from the midgut in the moth Opisina arenosella [165].
Thus, some LK functions appear conserved among several, but not all, insects, such as a role in regulation of gustatory receptors, feeding, water and ion homeostasis and gut function. Clearly, further studies of other insects are required to test whether LK functions discovered in Drosophila are more widespread. It can also be noted that the number of neurons expressing LK and the number of LK neuron types vary extensively between insects studied. In fact, Drosophila is an example of a strong reduction in both total number and the number of different neuron types, with only 20 neurons in total in the larva (26–28 with the ALKs) of three types (four with the ALKs). In comparison, the cockroach L. maderae has 250 neurons of many different types [166].
Another interesting difference between insect species is that the LK precursors can give rise to different numbers of paracopies of LKs. The Drosophila precursor encodes one LK [3,4,30], whereas in, for example, the cockroach Periplaneta americana, there are 17 [167], and in the western flower trips Frankliniella occidentalis, 26 paracopies were predicted [168]. The largest number of paracopies found to date was detected in the sea slug Aplysia californica, where the LK precursor may give rise to 60 peptides, of which more than 30 are amidated LKs and the others represent other peptides [163]. It is not clear what the functional consequences are of the expanded number of paracopies, but it may aid in diversification of peptide signaling (neofunctionalization) during evolution if paralleled by GPCR co-evolution [169]. Rarely, it has been shown that sequence-related paracopies display distinct actions. Some work on Aplysia has suggested isoform-specific modulatory activities at the neuromuscular junction [170,171]. A different situation is when the precursor gives rise to distinct neuropeptides, some of which may be differentially expressed in neurons due to alternative splicing; in this case, distinct but coordinated peptide actions were shown, for instance, in the mollusks Aplysia [172] and Lymnaea stagnalis [64]. Peptides different from LKs have been identified on LK precursors in some insect species such as Frankliniella occidentalis, Rhodnius prolixus and the bed bug Cimex lectularius, but at present, their biological activities are not known [168,173,174]. One aspect of LK distribution is remarkable, with the exception of R. prolixus [104]; there are no examples of LK expression in enteroendocrine cells of the intestine. This is in contrast to many other neuropeptides that are found both in the brain and the gut [85,175,176,177]. A separate review deals more extensively with distribution and functional roles of LK signaling in other invertebrates [178].
6. Concluding Remarks
This review has summarized the multiple roles of LK signaling in Drosophila and emphasized that LK function is based on the action of a very small number of neurons and neurosecretory cells. Including ALK/ITPn, there are four types of LK neurons, three of which reside in the brain/SEZ. The pair of nutrient- and AMPK-dependent LHLKs alone have been implicated in many regulatory functions that link internal nutritional state and inputs from the biological clock to food choice, memory and activity/sleep [47,48,111,116,117] (Figure 12, Table 2). These neurons also act directly on IPCs to modulate their activity and, thus, affect multiple physiological and behavioral aspects of daily life [48] (Figure 12). The mechanisms of LK action in feeding are not completely clear. Possibly, some role is played by nutrient-sensing LHLKs and their action in the fan-shaped body that mediates food choice, but it is also likely that the less-studied SELKs are part of the feeding regulation process. They are located in the SEZ and arborize in that region, as well as in the tritocerebrum, which are neuropils known to receive gustatory inputs and contain interneurons and motoneurons that control feeding (see [22,143,150,152]). ABLKs use LK and DH44 to regulate secretion in Malpighian tubules, and the effect of this on ion and water homeostasis influences flies’ response to starvation, desiccation and ionic stress [37,38]. Since LK is just one out of several peptide hormones involved in the control of ion and water content in the fly, we can expect quite complex interactions between multiple regulatory systems. LK and these other peptide hormones are also likely to be part of a more global orchestration of nutritional- and osmotic state-dependent physiology and behaviors. The recent finding that ABLKs partake in a circuit that changes the behavior of female flies after mating is interesting [118]. The phenotype described in that study is the result of LK acting within circuits of the CNS. It would be intriguing if the ABLKs in these flies also contribute to other aspects of the post-mating response, including activity, fecundity and metabolism [134,135,136,137], by hormonal action of LK. Interestingly, ABLKs seem to be part of different circuits in the larva, where they modulate aspects of locomotion as well as pre-ecdysis behavior and tracheal emptying [40,41,42], and their role in diuresis has not been explored. Thus, ABLKs are versatile and plastic, with roles spanning from larval ecdysis to regulation of several aspects of adult daily life. ABLKs and LHLKs seem to monitor internal states and orchestrate physiology and behavior, and future work is required to establish whether all of the LK neurons function in a cooperative fashion.
Acknowledgments
I thank Meet Zandawala for his substantial contributions to the original data and figures presented in this review and also for valuable comments on an earlier version of the paper.
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/22/4/1940/s1. Figure S1. Neurosecretory and efferent neuronal systems in the adult ventral nerve cord that affect ion and water homeostasis.
Funding
This research was funded by Vetenskapsrådet (Swedish Research Council) grant number 2015-04626 and the APC was funded by Stockholm University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nässel D.R., Zandawala M. Recent advances in neuropeptide signaling in Drosophila, from genes to physiology and behavior. Progr. Neurobiol. 2019;179:101607. doi: 10.1016/j.pneurobio.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Schoofs L., De Loof A., Van Hiel M. Neuropeptides as Regulators of Behavior in Insects. Annu. Rev. Entomol. 2017;62:35–52. doi: 10.1146/annurev-ento-031616-035500. [DOI] [PubMed] [Google Scholar]
- 3.Hewes R.S., Taghert P.H. Neuropeptides and neuropeptide receptors in the Drosophila melanogaster genome. Genome Res. 2001;11:1126–1142. doi: 10.1101/gr.169901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanden Broeck J. Neuropeptides and their precursors in the fruitfly, Drosophila melanogaster. Peptides. 2001;22:241–254. doi: 10.1016/S0196-9781(00)00376-4. [DOI] [PubMed] [Google Scholar]
- 5.Taghert P.H., Nitabach M.N. Peptide neuromodulation in invertebrate model systems. Neuron. 2012;76:82–97. doi: 10.1016/j.neuron.2012.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marder E. Neuromodulation of neuronal circuits: Back to the future. Neuron. 2012;76:1–11. doi: 10.1016/j.neuron.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nässel D.R., Zandawala M. Hormonal axes in Drosophila: Regulation of hormone release and multiplicity of actions. Cell Tissue Res. 2020;382:233–266. doi: 10.1007/s00441-020-03264-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strand F.L. Neuropeptides: Regulators of Physiological Processes. The MIT Press; Cambridge, MA, USA: 1999. p. 658. [Google Scholar]
- 9.Jékely G., Melzer S., Beets I., Kadow I.C.G., Koene J., Haddad S., Holden-Dye L. The long and the short of it—A perspective on peptidergic regulation of circuits and behaviour. J. Exp. Biol. 2018;221:jeb166710. doi: 10.1242/jeb.166710. [DOI] [PubMed] [Google Scholar]
- 10.Scharrer E., Scharrer B. Neuroendocrinology. Columbia University Press; New York, NY, USA: 1963. [Google Scholar]
- 11.Scharrer B. Insects as models in neuroendocrine research. Annu. Rev. Entomol. 1987;32:1–16. doi: 10.1146/annurev.en.32.010187.000245. [DOI] [PubMed] [Google Scholar]
- 12.Raabe M. Recent Developments in Insect Neurohormones. Plenum Press; New York, NY, USA: 1989. [Google Scholar]
- 13.Starratt A.N., Brown B.E. Structure of the pentapeptide proctolin, a proposed neurotransmitter in insects. Life Sci. 1975;17:1253–1256. doi: 10.1016/0024-3205(75)90134-4. [DOI] [PubMed] [Google Scholar]
- 14.Li X.J., Wolfgang W., Wu Y.N., North R.A., Forte M. Cloning, heterologous expression and developmental regulation of a Drosophila receptor for tachykinin-like peptides. EMBO J. 1991;10:3221–3229. doi: 10.1002/j.1460-2075.1991.tb04885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jekely G. Global view of the evolution and diversity of metazoan neuropeptide signaling. Proc. Natl. Acad. Sci. USA. 2013;110:8702–8707. doi: 10.1073/pnas.1221833110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mirabeau O., Joly J.S. Molecular evolution of peptidergic signaling systems in bilaterians. Proc. Natl. Acad. Sci. USA. 2013;110:E2028–E2037. doi: 10.1073/pnas.1219956110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caers J., Verlinden H., Zels S., Vandersmissen H.P., Vuerinckx K., Schoofs L. More than two decades of research on insect neuropeptide GPCRs: An overview. Front. Endocrinol. (Lausanne) 2012;3:151. doi: 10.3389/fendo.2012.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohr S.E., Hu Y., Kim K., Housden B.E., Perrimon N. Resources for functional genomics studies in Drosophila melanogaster. Genetics. 2014;197:1–18. doi: 10.1534/genetics.113.154344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis F.P., Nern A., Picard S., Reiser M.B., Rubin G.M., Eddy S.R., Henry G.L. A genetic, genomic, and computational resource for exploring neural circuit function. eLife. 2020;9:e50901. doi: 10.7554/eLife.50901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bates A.S., Manton J.D., Jagannathan S.R., Costa M., Schlegel P., Rohlfing T., Jefferis G.S.X.E. The natverse, a versatile toolbox for combining and analysing neuroanatomical data. eLife. 2020;9:e53350. doi: 10.7554/eLife.53350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davie K., Janssens J., Koldere D., De Waegeneer M., Pech U., Kreft L., Aibar S., Makhzami S., Christiaens V., Bravo Gonzalez-Blas C., et al. A Single-Cell Transcriptome Atlas of the Aging Drosophila Brain. Cell. 2018;174:982–998. doi: 10.1016/j.cell.2018.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlegel P., Texada M.J., Miroschnikow A., Schoofs A., Hückesfeld S., Peters M., Schneider-Mizell C.M., Lacin H., Li F., Fetter R.D., et al. Synaptic transmission parallels neuromodulation in a central food-intake circuit. eLife. 2016;5 doi: 10.7554/eLife.16799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owald D., Lin S., Waddell S. Light, heat, action: Neural control of fruit fly behaviour. Philos. Trans. R. Soc. B Biol. Sci. 2015;370:20140211. doi: 10.1098/rstb.2014.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Croset V., Treiber C.D., Waddell S. Cellular diversity in the Drosophila midbrain revealed by single-cell transcriptomics. eLife. 2018;7:e34550. doi: 10.7554/eLife.34550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hückesfeld S., Schlegel P., Miroschnikow A., Schoofs A., Zinke I., Haubrich A.N., Schneider-Mizell C.M., Truman J.W., Fetter R.D., Cardona A., et al. Unveiling the sensory and interneuronal pathways of the neuroendocrine system in Drosophila. bioRxiv. 2020 doi: 10.1101/2020.10.22.350306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holman G.M., Nachman R.J., Wright M.S. Insect neuropeptides. Annu. Rev. Entomol. 1990;35:201–217. doi: 10.1146/annurev.en.35.010190.001221. [DOI] [PubMed] [Google Scholar]
- 27.Holman G.M., Nachman R.J., Schoofs L., Hayes T.K., Wright M.S., DeLoof A. The Leucophaea maderae hindgut preparation: A rapid and sensitive bioassay tool for the isolation of insect myotropins of other insect species. Insect Biochem. 1991;21:107–112. doi: 10.1016/0020-1790(91)90070-U. [DOI] [Google Scholar]
- 28.Holman G.M., Cook B.J., Nachman R.J. Isolation, primary structure and synthesis of two neuropeptides from Leucophaea maderae: Members of a new family of cephalotropins. Comp. Biochem. Physiol. 1986;84C:205–211. doi: 10.1016/0742-8413(86)90084-8. [DOI] [PubMed] [Google Scholar]
- 29.Holman G.M., Cook B.J., Nachman R.J. Isolation, primary structure and synthesis of leukokinins VII and VIII: The final members of this new family of cephalomyotropic peptides isolated from head extracts of Leucophaea maderae. Comp. Biochem Physiol. 1987;88C:31–34. [Google Scholar]
- 30.Terhzaz S., O’Connell F.C., Pollock V.P., Kean L., Davies S.A., Veenstra J.A., Dow J.A. Isolation and characterization of a leucokinin-like peptide of Drosophila melanogaster. J. Exp. Biol. 1999;202:3667–3676. doi: 10.1242/jeb.202.24.3667. [DOI] [PubMed] [Google Scholar]
- 31.Nässel D.R., Zandawala M., Kawada T., Satake H. Tachykinins: Neuropeptides That Are Ancient, Diverse, Widespread and Functionally Pleiotropic. Front. Neurosci. 2019;13:1262. doi: 10.3389/fnins.2019.01262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coast G.M., Holman G.M., Nachman R.J. The diuretic activity of a series of cephalomyotropic neuropeptides, the achetakinins, on isolated Malpighian tubules of the house cricket Acheta Domest. J. Insect Physiol. 1990;36:481–488. doi: 10.1016/0022-1910(90)90098-Z. [DOI] [Google Scholar]
- 33.Hayes T.K., Pannabecker T.L., Hinckley D.J., Holman G.M., Nachman R.J., Petzel D.H., Beyenbach K.W. Leucokinins, a new family of ion transport stimulators and inhibitors in insect Malpighian tubules. Life Sci. 1989;44:1259–1266. doi: 10.1016/0024-3205(89)90362-7. [DOI] [PubMed] [Google Scholar]
- 34.Hayes T.K., Holman G.M., Pannabecker T.L., Wright M.S., Strey A.A., Nachman R.J., Hoel D.F., Olson J.K., Beyenbach K.W. Culekinin depolarizing peptide: A mosquito leucokinin-like peptide that influences insect Malpighian tubule ion transport. Regul. Pept. 1994;52:235–248. doi: 10.1016/0167-0115(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 35.Holman G.M., Nachman R.J., Coast G.M. Isolation, characterization and biological activity of a diuretic myokinin neuropeptide from the housefly, Musca domestica. Peptides. 1999;20:1–10. doi: 10.1016/S0196-9781(98)00150-8. [DOI] [PubMed] [Google Scholar]
- 36.De Haro M., Al-Ramahi I., Benito-Sipos J., Lopez-Arias B., Dorado B., Veenstra J.A., Herrero P. Detailed analysis of leucokinin-expressing neurons and their candidate functions in the Drosophila nervous system. Cell Tissue Res. 2010;339:321–336. doi: 10.1007/s00441-009-0890-y. [DOI] [PubMed] [Google Scholar]
- 37.Zandawala M., Marley R., Davies S.A., Nässel D.R. Characterization of a set of abdominal neuroendocrine cells that regulate stress physiology using colocalized diuretic peptides in Drosophila. Cell. Mol. Life Sci. CMLS. 2018;75:1099–1115. doi: 10.1007/s00018-017-2682-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zandawala M., Yurgel M.E., Liao S., Texada M.J., Rewitz K.F., Keene A.C., Nässel D.R. Modulation of Drosophila post-feeding physiology and behavior by the neuropeptide leucokinin. PLoS Genet. 2018;14:e1007767. doi: 10.1371/journal.pgen.1007767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Anzi B., Armand E., Nagamei P., Olszewski M., Sapin V., Waters C., Zinn K., Wyman R.J., Benzer S. The leucokinin pathway and its neurons regulate meal size in Drosophila. Curr. Biol. 2010;20:969–978. doi: 10.1016/j.cub.2010.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim D.H., Han M.R., Lee G., Lee S.S., Kim Y.J., Adams M.E. Rescheduling Behavioral Subunits of a Fixed Action Pattern by Genetic Manipulation of Peptidergic Signaling. PLoS Genet. 2015;11:e1005513. doi: 10.1371/journal.pgen.1005513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim D.H., Kim Y.J., Adams M.E. Endocrine regulation of airway clearance in Drosophila. Proc. Natl. Acad. Sci. USA. 2018;115:1535–1540. doi: 10.1073/pnas.1717257115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okusawa S., Kohsaka H., Nose A. Serotonin and downstream leucokinin neurons modulate larval turning behavior in Drosophila. J. Neurosci. 2014;34:2544–2558. doi: 10.1523/JNEUROSCI.3500-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kahsai L., Kapan N., Dircksen H., Winther Å.M., Nässel D.R. Metabolic stress responses in Drosophila are modulated by brain neurosecretory cells that produce multiple neuropeptides. PLoS ONE. 2010;5:e11480. doi: 10.1371/journal.pone.0011480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dircksen H., Tesfai L.K., Albus C., Nässel D.R. Ion transport peptide splice forms in central and peripheral neurons throughout postembryogenesis of Drosophila melanogaster. J. Comp. Neurol. 2008;509:23–41. doi: 10.1002/cne.21715. [DOI] [PubMed] [Google Scholar]
- 45.Galikova M., Dircksen H., Nässel D.R. The thirsty fly: Ion transport peptide (ITP) is a novel endocrine regulator of water homeostasis in Drosophila. PLoS Genet. 2018;14:e1007618. doi: 10.1371/journal.pgen.1007618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Radford J.C., Davies S.A., Dow J.A. Systematic G-protein-coupled receptor analysis in Drosophila melanogaster identifies a leucokinin receptor with novel roles. J. Biol. Chem. 2002;277:38810–38817. doi: 10.1074/jbc.M203694200. [DOI] [PubMed] [Google Scholar]
- 47.Cavey M., Collins B., Bertet C., Blau J. Circadian rhythms in neuronal activity propagate through output circuits. Nat. Neurosci. 2016;19:587–595. doi: 10.1038/nn.4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yurgel M.E., Kakad P., Zandawala M., Nässel D.R., Godenschwege T.A., Keene A.C. A single pair of leucokinin neurons are modulated by feeding state and regulate sleep-metabolism interactions. PLoS Biol. 2019;17:e2006409. doi: 10.1371/journal.pbio.2006409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cannell E., Dornan A.J., Halberg K.A., Terhzaz S., Dow J.A., Davies S.A. The Corticotropin-releasing factor-like diuretic hormone 44 (DH) and kinin neuropeptides modulate desiccation and starvation tolerance in Drosophila melanogaster. Peptides. 2016;80:96–107. doi: 10.1016/j.peptides.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scheffer L.K., Xu C.S., Januszewski M., Lu Z., Takemura S.-Y., Hayworth K.J., Huang G.B., Shinomiya K., Maitlin-Shepard J., Berg S., et al. A connectome and analysis of the adult Drosophila central brain. eLife. 2020;9:e57443. doi: 10.7554/eLife.57443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clements J., Dolafi T., Umayam L., Neubarth N.L., Berg S., Scheffer L.K., Plaza S.M. neuPrint: Analysis Tools for EM Connectomics. bioRxiv. 2020 doi: 10.1101/2020.01.16.909465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng Z., Lauritzen J.S., Perlman E., Robinson C.G., Nichols M., Milkie D., Torrens O., Price J., Fisher C.B., Sharifi N., et al. A Complete Electron Microscopy Volume of the Brain of Adult Drosophila melanogaster. Cell. 2018;174:730–743. doi: 10.1016/j.cell.2018.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maniates-Selvin J.T., Hildebrand D.G.C., Graham B.J., Kuan A.T., Thomas L.A., Nguyen T., Buhmann J., Azevedo A.W., Shanny B.L., Funke J., et al. Reconstruction of motor control circuits in adult Drosophila using automated transmission electron microscopy. bioRxiv. 2020 doi: 10.1101/2020.01.10.902478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bullock T.H. Revisiting the concept of identifiable neurons. Brain. Behav. Evol. 2000;55:236–240. doi: 10.1159/000006657. [DOI] [PubMed] [Google Scholar]
- 55.Nässel D.R. Neuropeptides, amines and amino acids in an elementary insect ganglion: Functional and chemical anatomy of the unfused abdominal ganglion. Prog. Neurobiol. 1996;48:325–420. doi: 10.1016/0301-0082(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 56.Kupfermann I., Weiss K.R. The command neuron concept. Behav. Brain Sci. 1978;1:3–10. doi: 10.1017/S0140525X00059057. [DOI] [Google Scholar]
- 57.Stuart D.K., Torrence S.A., Law M.I. Leech neurogenesis. I. Positional commitment of neural precursor cells. Dev. Biol. 1989;136:17–39. doi: 10.1016/0012-1606(89)90128-0. [DOI] [PubMed] [Google Scholar]
- 58.Retzius G. Zur Kenntnis des centralen Nervensystems der Hirudineen. Biol. Unters. Neue Folge. 1891;2:13–25. [Google Scholar]
- 59.McAdoo D.J. The Retzius Cell of the Leech Hirudo Medicinalis. In: Osborne N.N., editor. Biochemistry of Characterised Neurons. Pergamon; Oxford, UK: 1978. pp. 19–45. [DOI] [Google Scholar]
- 60.Kits K.S., Boer H.H., Joosse J. Molluscan Neurobiology. North Holland; Amsterdam, The Netherlands: 1991. p. 360. [Google Scholar]
- 61.Weiss K.R., Brezina V., Cropper E.C., Hooper S.L., Miller M.W., Probst W.E., Vilim F.S., Kupfermann I. Peptidergic co-transmission in Aplysia: Functional implications for rhythmic behaviors. Experientia. 1992;48:456–463. doi: 10.1007/BF01928164. [DOI] [PubMed] [Google Scholar]
- 62.Nambu J.R., Taussig R., Mahon A.C., Scheller R.H. Gene isolation with cDNA probes from identified Aplysia neurons: Neuropeptide modulators of cardiovascular physiology. Cell. 1983;35:47–56. doi: 10.1016/0092-8674(83)90206-4. [DOI] [PubMed] [Google Scholar]
- 63.Scheller R.H., Kaldany R.R., Kreiner T., Mahon A.C., Nambu J.R., Schaefer M., Taussig R. Neuropeptides: Mediators of behavior in Aplysia. Science. 1984;225:1300–1308. doi: 10.1126/science.6474178. [DOI] [PubMed] [Google Scholar]
- 64.Santama N., Benjamin P.R., Burke J.F. Alternative RNA splicing generates diversity of neuropeptide expression in the brain of the snail Lymnaea: In situ analysis of mutually exclusive transcripts of the FMRFamide gene. Eur. J. Neurosci. 1995;7:65–76. doi: 10.1111/j.1460-9568.1995.tb01021.x. [DOI] [PubMed] [Google Scholar]
- 65.Geraerts W., Smit A.B., Li K.W., Hordijk P.L. The light green cells of Lymnaea: A neuroendocrine model system for stimulus-induced expression of multiple peptide genes in a single cell type. Experientia. 1992;48:464–473. doi: 10.1007/BF01928165. [DOI] [PubMed] [Google Scholar]
- 66.Santama N., Brierley M., Burke J.F., Benjamin P.R. Neural network controlling feeding in Lymnaea stagnalis: Immunocytochemical localization of myomodulin, small cardioactive peptide, buccalin, and FMRFamide-related peptides. J. Comp. Neurol. 1994;342:352–365. doi: 10.1002/cne.903420304. [DOI] [PubMed] [Google Scholar]
- 67.King D.G. Organization of crustacean neuropil. I. Patterns of synaptic connections in lobster stomatogastric ganglion. J. Neurocytol. 1976;5:207–237. doi: 10.1007/BF01181657. [DOI] [PubMed] [Google Scholar]
- 68.King D.G. Organization of crustacean neuropil. II. Distribution of synaptic contacts on identified motor neurons in lobster stomatogastric ganglion. J. Neurocytol. 1976;5:239–266. doi: 10.1007/BF01181658. [DOI] [PubMed] [Google Scholar]
- 69.Selverston A.I. Neuromodulatory control of rhythmic behaviors in invertebrates. Int. Rev. Cytol. 1993;147:1–24. doi: 10.1016/s0074-7696(08)60765-2. [DOI] [PubMed] [Google Scholar]
- 70.Marder E., Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu. Rev. Physiol. 2007;69:291–316. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- 71.Nusbaum M.P., Blitz D.M., Marder E. Functional consequences of neuropeptide and small-molecule co-transmission. Nat. Rev. Neurosci. 2017;18:389–403. doi: 10.1038/nrn.2017.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Burrows M. The Neurobiology of an Insect Brain. Oxford University Press; Oxford, UK: 1996. [Google Scholar]
- 73.Brand A.H., Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 74.Duffy J.B. GAL4 system in Drosophila: A fly geneticist’s Swiss army knife. Genesis. 2002;34:1–15. doi: 10.1002/gene.10150. [DOI] [PubMed] [Google Scholar]
- 75.Brand A.H., Dormand E.L. The GAL4 system as a tool for unravelling the mysteries of the Drosophila nervous system. Curr. Opin Neurobiol. 1995;5:572–578. doi: 10.1016/0959-4388(95)80061-1. [DOI] [PubMed] [Google Scholar]
- 76.McNabb S.L., Baker J.D., Agapite J., Steller H., Riddiford L.M., Truman J.W. Disruption of a behavioral sequence by targeted death of peptidergic neurons in Drosophila. Neuron. 1997;19:813–823. doi: 10.1016/S0896-6273(00)80963-0. [DOI] [PubMed] [Google Scholar]
- 77.Renn S.C., Park J.H., Rosbash M., Hall J.C., Taghert P.H. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/S0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- 78.Luo L., Callaway E.M., Svoboda K. Genetic Dissection of Neural Circuits: A Decade of Progress. Neuron. 2018;98:256–281. doi: 10.1016/j.neuron.2018.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Simpson J.H., Looger L.L. Functional Imaging and Optogenetics in Drosophila. Genetics. 2018;208:1291. doi: 10.1534/genetics.117.300228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miesenböck G. Genetic methods for illuminating the function of neural circuits. Curr. Opin. Neurobiol. 2004;14:395–402. doi: 10.1016/j.conb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 81.Masuyama K., Zhang Y., Rao Y., Wang J.W. Mapping Neural Circuits with Activity-Dependent Nuclear Import of a Transcription Factor. J. Neurogenet. 2012;26:89–102. doi: 10.3109/01677063.2011.642910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Talay M., Richman E.B., Snell N.J., Hartmann G.G., Fisher J.D., Sorkac A., Santoyo J.F., Chou-Freed C., Nair N., Johnson M., et al. Transsynaptic Mapping of Second-Order Taste Neurons in Flies by trans-Tango. Neuron. 2017;96:783–795. doi: 10.1016/j.neuron.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Macpherson L.J., Zaharieva E.E., Kearney P.J., Alpert M.H., Lin T.Y., Turan Z., Lee C.H., Gallio M. Dynamic labelling of neural connections in multiple colours by trans-synaptic fluorescence complementation. Nat. Commun. 2015;6:10024. doi: 10.1038/ncomms10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cachero S., Gkantia M., Bates A.S., Frechter S., Blackie L., McCarthy A., Sutcliffe B., Strano A., Aso Y., Jefferis G.S.X.E. BAcTrace a new tool for retrograde tracing of neuronal circuits. bioRxiv. 2020 doi: 10.1101/2020.01.24.918656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nässel D.R., Pauls D., Huetteroth W. Neuropeptides in modulation of Drosophila behavior: How to get a grip on their pleiotropic actions. Curr. Opin. Insect Sci. 2019;36:1–8. doi: 10.1016/j.cois.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 86.Nässel D.R. Substrates for neuronal cotransmission with neuropeptides and small molecule neurotransmitters in Drosophila. Front. Cell Neurosci. 2018;12:83. doi: 10.3389/fncel.2018.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Svensson E., Apergis-Schoute J., Burnstock G., Nusbaum M.P., Parker D., Schiöth H.B. General Principles of Neuronal Co-transmission: Insights from Multiple Model Systems. Front. Neural. Circuits. 2019;12:117. doi: 10.3389/fncir.2018.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nässel D.R., Enell L.E., Santos J.G., Wegener C., Johard H.A. A large population of diverse neurons in the Drosophila central nervous system expresses short neuropeptide F, suggesting multiple distributed peptide functions. BMC Neurosci. 2008;9:90. doi: 10.1186/1471-2202-9-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cantera R., Nässel D.R. Segmental peptidergic innervation of abdominal targets in larval and adult dipteran insects revealed with an antiserum against leucokinin I. Cell Tissue Res. 1992;269:459–471. doi: 10.1007/BF00353901. [DOI] [PubMed] [Google Scholar]
- 90.Herrero P., Magarinos M., Torroja L., Canal I. Neurosecretory identity conferred by the apterous gene: Lateral horn leucokinin neurons in Drosophila. J. Comp. Neurol. 2003;457:123–132. doi: 10.1002/cne.10555. [DOI] [PubMed] [Google Scholar]
- 91.Nässel D.R. Neuropeptides, multifunctional messengers in the nervous system of insects. Verh. Dtsch. Zool. Gesellsch. 1994;87:59–81. [Google Scholar]
- 92.Muren J.E., Lundquist C.T., Nässel D.R. Quantitative determination of myotropic neuropeptide in the nervous system of the cockroach Leucophaea maderae: Distribution and release of leucokinins. J. Exp. Biol. 1993;179:289. [Google Scholar]
- 93.Chung J., Goldsworthy G., Coast G. Haemolymph and tissue titres of achetakinins in the house cricket acheta domesticus: Effect of starvation and dehydration. J. Exp. Biol. 1994;193:307–319. doi: 10.1242/jeb.193.1.307. [DOI] [PubMed] [Google Scholar]
- 94.Te Brugge V.A., Orchard I. Evidence for CRF-like and kinin-like peptides as neurohormones in the blood-feeding bug, Rhodnius prolixus. Peptides. 2002;23:1967–1979. doi: 10.1016/S0196-9781(02)00184-5. [DOI] [PubMed] [Google Scholar]
- 95.Cognigni P., Bailey A.P., Miguel-Aliaga I. Enteric neurons and systemic signals couple nutritional and reproductive status with intestinal homeostasis. Cell Metab. 2011;13:92–104. doi: 10.1016/j.cmet.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Luo J., Liu Y., Nässel D.R. Insulin/IGF-regulated size scaling of neuroendocrine cells expressing the bHLH transcription factor Dimmed in Drosophila. PLoS Genet. 2013;9:e1004052. doi: 10.1371/journal.pgen.1004052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mattaliano M.D., Montana E.S., Parisky K.M., Littleton J.T., Griffith L.C. The Drosophila ARC homolog regulates behavioral responses to starvation. Mol. Cell Neurosci. 2007;36:211–221. doi: 10.1016/j.mcn.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bader R., Sarraf-Zadeh L., Peters M., Moderau N., Stocker H., Kohler K., Pankratz M.J., Hafen E. The IGFBP7 homolog Imp-L2 promotes insulin signaling in distinct neurons of the Drosophila brain. J. Cell Sci. 2013;126:2571–2576. doi: 10.1242/jcs.120261. [DOI] [PubMed] [Google Scholar]
- 99.Hong S.H., Lee K.S., Kwak S.J., Kim A.K., Bai H., Jung M.S., Kwon O.Y., Song W.J., Tatar M., Yu K. Minibrain/Dyrk1a Regulates Food Intake through the Sir2-FOXO-sNPF/NPY Pathway in Drosophila and Mammals. PLoS Genet. 2012;8:e1002857. doi: 10.1371/annotation/8c2c8644-1beb-4410-8bef-c388b7256738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu Y., Luo J., Carlsson M.A., Nässel D.R. Serotonin and insulin-like peptides modulate leucokinin-producing neurons that affect feeding and water homeostasis in Drosophila. J. Comp. Neurol. 2015;523:1840–1863. doi: 10.1002/cne.23768. [DOI] [PubMed] [Google Scholar]
- 101.Thompson K., Rayne R.C., Gibbon C.R., May S.T., Patel M., Coast G.M., Bacon J.P. Cellular colocalization of diuretic peptides in locusts: A potent control mechanism. Peptides. 1995;16:95–104. doi: 10.1016/0196-9781(94)00158-3. [DOI] [PubMed] [Google Scholar]
- 102.Iaboni A., Holman G.M., Nachman R.J., Orchard I., Coast G.M. Immunocytochemical localisation and biological activity of diuretic peptides in the housefly, Musca domestica. Cell Tissue Res. 1998;294:549–560. doi: 10.1007/s004410051205. [DOI] [PubMed] [Google Scholar]
- 103.Chen Y., Veenstra J.A., Hagedorn H., Davis N.T. Leucokinin and diuretic hormone immunoreactivity of neurons in the tobacco hornworm, Manduca sexta, and co-localization of this immunoreactivity in lateral neurosecretory cells of abdominal ganglia. Cell Tissue Res. 1994;278:493–507. doi: 10.1007/BF00331367. [DOI] [PubMed] [Google Scholar]
- 104.Te Brugge V.A., Nässel D.R., Coast G.M., Schooley D.A., Orchard I. The distribution of a kinin-like peptide and its co-localization with a CRF-like peptide in the blood-feeding bug, Rhodnius prolixus. Peptides. 2001;22:161–173. doi: 10.1016/S0196-9781(00)00373-9. [DOI] [PubMed] [Google Scholar]
- 105.Hewes R.S., Park D., Gauthier S.A., Schaefer A.M., Taghert P.H. The bHLH protein Dimmed controls neuroendocrine cell differentiation in Drosophila. Development. 2003;130:1771–1781. doi: 10.1242/dev.00404. [DOI] [PubMed] [Google Scholar]
- 106.Liu Y., Luo J., Nässel D.R. The Drosophila Transcription Factor Dimmed Affects Neuronal Growth and Differentiation in Multiple Ways Depending on Neuron Type and Developmental Stage. Front. Molec Neurosci. 2016;9 doi: 10.3389/fnmol.2016.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Park D., Veenstra J.A., Park J.H., Taghert P.H. Mapping peptidergic cells in Drosophila: Where DIMM fits in. PLoS ONE. 2008;3:e1896. doi: 10.1371/journal.pone.0001896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hamanaka Y., Park D., Yin P., Annangudi S.P., Edwards T.N., Sweedler J., Meinertzhagen I.A., Taghert P.H. Transcriptional orchestration of the regulated secretory pathway in neurons by the bHLH protein DIMM. Curr. Biol. 2010;20:9–18. doi: 10.1016/j.cub.2009.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Halberg K.A., Terhzaz S., Cabrero P., Davies S.A., Dow J.A. Tracing the evolutionary origins of insect renal function. Nat. Commun. 2015;6:6800. doi: 10.1038/ncomms7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Murphy K.R., Deshpande S.A., Yurgel M.E., Quinn J.P., Weissbach J.L., Keene A.C., Dawson-Scully K., Huber R., Tomchik S.M., Ja W.W. Postprandial sleep mechanics in Drosophila. eLife. 2016;5:e19334. doi: 10.7554/eLife.19334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Murakami K., Yurgel M.E., Stahl B.A., Masek P., Mehta A., Heidker R., Bollinger W., Gingras R.M., Kim Y.J., Ja W.W., et al. Translin Is Required for Metabolic Regulation of Sleep. Curr. Biol. 2016;26:972–980. doi: 10.1016/j.cub.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chintapalli V.R., Wang J., Dow J.A. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 2007;39:715–720. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- 113.Dutta D., Dobson A.J., Houtz P.L., Gläßer C., Revah J., Korzelius J., Patel P.H., Edgar B.A., Buchon N. Regional Cell-Specific Transcriptome Mapping Reveals Regulatory Complexity in the Adult Drosophila Midgut. Cell Rep. 2015;12:346–358. doi: 10.1016/j.celrep.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 114.Lopez-Arias B., Dorado B., Herrero P. Blockade of the release of the neuropeptide leucokinin to determine its possible functions in fly behavior: Chemoreception assays. Peptides. 2011;32:545–552. doi: 10.1016/j.peptides.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 115.Ohashi H., Sakai T. Leucokinin signaling regulates hunger–driven reduction of behavioral responses to noxious heat in Drosophila. Biochem. Biophys. Res. Commun. 2018;499:221–226. doi: 10.1016/j.bbrc.2018.03.132. [DOI] [PubMed] [Google Scholar]
- 116.Senapati B., Tsao C.-H., Juan Y.-A., Chiu T.-H., Wu C.-L., Waddell S., Lin S. A neural mechanism for deprivation state-specific expression of relevant memories in Drosophila. Nat. Neurosci. 2019;22:2029–2039. doi: 10.1038/s41593-019-0515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sareen P., McCurdy L.Y., Nitabach M.N. A neural signature of choice under sensory conflict in Drosophila. bioRxiv. 2020 doi: 10.1101/2020.08.14.251553. [DOI] [Google Scholar]
- 118.Liu C., Zhang B., Zhang L., Yang T., Zhang Z., Gao Z., Zhang W. A neural circuit encoding mating states tunes defensive behavior in Drosophila. Nat. Commun. 2020;11:3962. doi: 10.1038/s41467-020-17771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Charroux B., Daian F., Royet J. Drosophila Aversive Behavior toward Erwinia carotovora carotovora Is Mediated by Bitter Neurons and Leukokinin. iScience. 2020;23 doi: 10.1016/j.isci.2020.101152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chi W., Liu W., Fu W., Xia S., Heckscher E.S., Zhuang X. RNA-binding protein Syncrip regulates Starvation-Induced Hyperactivity in adult Drosophila. bioRxiv. 2020 doi: 10.1101/2020.01.07.897652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sellami A., Agricola H.J., Veenstra J.A. Neuroendocrine cells in Drosophila melanogaster producing GPA2/GPB5, a hormone with homology to LH, FSH and TSH. Gen. Comp. Endocrinol. 2011;170:582–588. doi: 10.1016/j.ygcen.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 122.Coast G.M., Webster S.G., Schegg K.M., Tobe S.S., Schooley D.A. The Drosophila melanogaster homologue of an insect calcitonin-like diuretic peptide stimulates V-ATPase activity in fruit fly Malpighian tubules. J. Exp. Biol. 2001;204:1795–1804. doi: 10.1242/jeb.204.10.1795. [DOI] [PubMed] [Google Scholar]
- 123.Coast G.M., Orchard I., Phillips J.E., Schooley D.A. Insect diuretic and antidiuretic hormones. Adv. Insect Physiol. 2002;29:279–409. [Google Scholar]
- 124.Kean L., Cazenave W., Costes L., Broderick K.E., Graham S., Pollock V.P., Davies S.A., Veenstra J.A., Dow J.A. Two nitridergic peptides are encoded by the gene capability in Drosophila melanogaster. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282:R1297–R1307. doi: 10.1152/ajpregu.00584.2001. [DOI] [PubMed] [Google Scholar]
- 125.Cabrero P., Radford J.C., Broderick K.E., Costes L., Veenstra J.A., Spana E.P., Davies S.A., Dow J.A. The Dh gene of Drosophila melanogaster encodes a diuretic peptide that acts through cyclic AMP. J. Exp. Biol. 2002;205:3799–3807. doi: 10.1242/jeb.205.24.3799. [DOI] [PubMed] [Google Scholar]
- 126.Davies S.A., Huesmann G.R., Maddrell S.H., O’Donnell M.J., Skaer N.J., Dow J.A., Tublitz N.J. CAP2b, a cardioacceleratory peptide, is present in Drosophila and stimulates tubule fluid secretion via cGMP. Am. J. Physiol. 1995;269:R1321–R1326. doi: 10.1152/ajpregu.1995.269.6.R1321. [DOI] [PubMed] [Google Scholar]
- 127.Paluzzi J.P., Vanderveken M., O’Donnell M.J. The heterodimeric glycoprotein hormone, GPA2/GPB5, regulates ion transport across the hindgut of the adult mosquito, Aedes aegypti. PLoS ONE. 2014;9:e86386. doi: 10.1371/journal.pone.0086386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Díaz-de-la-Peña L., Maestro-Paramio L., Díaz-Benjumea F.J., Herrero P. Temporal groups of lineage-related neurons have different neuropeptidergic fates and related functions in the Drosophila melanogaster CNS. Cell Tissue Res. 2020;381:381–396. doi: 10.1007/s00441-020-03231-8. [DOI] [PubMed] [Google Scholar]
- 129.Talsma A.D., Christov C.P., Terriente-Felix A., Linneweber G.A., Perea D., Wayland M., Shafer O.T., Miguel-Aliaga I. Remote control of renal physiology by the intestinal neuropeptide pigment-dispersing factor in Drosophila. Proc. Natl. Acad. Sci. USA. 2012;109:12177–12182. doi: 10.1073/pnas.1200247109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang W., Yan Z., Li B., Jan L.Y., Jan Y.N. Identification of motor neurons and a mechanosensitive sensory neuron in the defecation circuitry of Drosophila larvae. eLife. 2014;3:e03293. doi: 10.7554/eLife.03293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.O’Donnell M.J., Rheault M.R., Davies S.A., Rosay P., Harvey B.J., Maddrell S.H., Kaiser K., Dow J.A. Hormonally controlled chloride movement across Drosophila tubules is via ion channels in stellate cells. Am. J. Physiol. 1998;274:R1039–R1049. doi: 10.1152/ajpregu.1998.274.4.R1039. [DOI] [PubMed] [Google Scholar]
- 132.Cabrero P., Terhzaz S., Romero M.F., Davies S.A., Blumenthal E.M., Dow J.A. Chloride channels in stellate cells are essential for uniquely high secretion rates in neuropeptide-stimulated Drosophila diuresis. Proc. Natl. Acad. Sci. USA. 2014;111:14301–14306. doi: 10.1073/pnas.1412706111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Postigo A.A., Ward E., Skeath J.B., Dean D.C. zfh-1, the Drosophila homologue of ZEB, is a transcriptional repressor that regulates somatic myogenesis. Mol. Cell Biol. 1999;19:7255–7263. doi: 10.1128/MCB.19.10.7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kubli E. Sex-peptides: Seminal peptides of the Drosophila male. Cell Mol. Life Sci. 2003;60:1689–1704. doi: 10.1007/s00018-003-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wolfner M.F. The gifts that keep on giving: Physiological functions and evolutionary dynamics of male seminal proteins in Drosophila. Heredity (Edinb.) 2002;88:85–93. doi: 10.1038/sj.hdy.6800017. [DOI] [PubMed] [Google Scholar]
- 136.Yapici N., Kim Y.J., Ribeiro C., Dickson B.J. A receptor that mediates the post-mating switch in Drosophila reproductive behaviour. Nature. 2008;451:33–37. doi: 10.1038/nature06483. [DOI] [PubMed] [Google Scholar]
- 137.Isaac R.E., Li C., Leedale A.E., Shirras A.D. Drosophila male sex peptide inhibits siesta sleep and promotes locomotor activity in the post-mated female. Proc. Biol. Sci. 2010;277:65–70. doi: 10.1098/rspb.2009.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cavanaugh D.J., Geratowski J.D., Wooltorton J.R., Spaethling J.M., Hector C.E., Zheng X., Johnson E.C., Eberwine J.H., Sehgal A. Identification of a circadian output circuit for rest:activity rhythms in Drosophila. Cell. 2014;157:689–701. doi: 10.1016/j.cell.2014.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.King A.N., Barber A.F., Smith A.E., Dreyer A.P., Sitaraman D., Nitabach M.N., Cavanaugh D.J., Sehgal A. A Peptidergic Circuit Links the Circadian Clock to Locomotor Activity. Curr. Biol. 2017;27:1915–1927. doi: 10.1016/j.cub.2017.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Barber A.F., Erion R., Holmes T.C., Sehgal A. Circadian and feeding cues integrate to drive rhythms of physiology in Drosophila insulin-producing cells. Gene Dev. 2016;30:2596–2606. doi: 10.1101/gad.288258.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Williams M.J., Akram M., Barkauskaite D., Patil S., Kotsidou E., Kheder S., Vitale G., Filaferro M., Blemings S.W., Maestri G., et al. CCAP regulates feeding behavior via the NPF pathway in Drosophila adults. Proc. Natl. Acad. Sci. USA. 2020;117:7401. doi: 10.1073/pnas.1914037117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Martelli C., Pech U., Kobbenbring S., Pauls D., Bahl B., Sommer M.V., Pooryasin A., Barth J., Arias C.W.P., Vassiliou C., et al. SIFamide Translates Hunger Signals into Appetitive and Feeding Behavior in Drosophila. Cell Rep. 2017;20:464–478. doi: 10.1016/j.celrep.2017.06.043. [DOI] [PubMed] [Google Scholar]
- 143.Melcher C., Pankratz M.J. Candidate gustatory interneurons modulating feeding behavior in the Drosophila brain. PLoS Biol. 2005;3:e305. doi: 10.1371/journal.pbio.0030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen J., Reiher W., Hermann-Luibl C., Sellami A., Cognigni P., Kondo S., Helfrich-Forster C., Veenstra J.A., Wegener C. Allatostatin A Signalling in Drosophila Regulates Feeding and Sleep and Is Modulated by PDF. PLoS Genet. 2016;12:e1006346. doi: 10.1371/journal.pgen.1006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Qi W., Wang G., Wang L. A novel satiety sensor detects circulating glucose and suppresses food consumption via insulin-producing cells in Drosophila. Cell Res. 2020 doi: 10.1038/s41422-020-00449-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nässel D.R., Vanden Broeck J. Insulin/IGF signaling in Drosophila and other insects: Factors that regulate production, release and post-release action of the insulin-like peptides. Cell Mol. Life Sci. 2016;73:271–290. doi: 10.1007/s00018-015-2063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Shafer O.T., Keene A.C. The Regulation of Drosophila Sleep. Curr. Biol. 2021;31:R38–R49. doi: 10.1016/j.cub.2020.10.082. [DOI] [PubMed] [Google Scholar]
- 148.Donlea J.M., Pimentel D., Talbot C.B., Kempf A., Omoto J.J., Hartenstein V., Miesenböck G. Recurrent Circuitry for Balancing Sleep Need and Sleep. Neuron. 2018;97:378–389. doi: 10.1016/j.neuron.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Donlea J.M., Pimentel D., Miesenböck G. Neuronal Machinery of Sleep Homeostasis in Drosophila. Neuron. 2014;81:860–872. doi: 10.1016/j.neuron.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bader R., Colomb J., Pankratz B., Schrock A., Stocker R.F., Pankratz M.J. Genetic dissection of neural circuit anatomy underlying feeding behavior in Drosophila: Distinct classes of hugin-expressing neurons. J. Comp. Neurol. 2007;502:848–856. doi: 10.1002/cne.21342. [DOI] [PubMed] [Google Scholar]
- 151.Hückesfeld S., Peters M., Pankratz M.J. Central relay of bitter taste to the protocerebrum by peptidergic interneurons in the Drosophila brain. Nat. Commun. 2016;7:12796. doi: 10.1038/ncomms12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hückesfeld S., Schoofs A., Schlegel P., Miroschnikow A., Pankratz M.J. Localization of Motor Neurons and Central Pattern Generators for Motor Patterns Underlying Feeding Behavior in Drosophila Larvae. PLoS ONE. 2015;10:e0135011. doi: 10.1371/journal.pone.0135011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ludwig P., Williams L., Nässel D.R., Reichert H., Boyan G. Primary commissure pioneer neurons in the brain of the grasshopper Schistocerca gregaria: Development, ultrastructure, and neuropeptide expression. J. Comp. Neurol. 2001;430:118–130. doi: 10.1002/1096-9861(20010129)430:1<118::AID-CNE1018>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 154.Gellerer A., Franke A., Neupert S., Predel R., Zhou X., Liu S., Reiher W., Wegener C., Homberg U. Identification and distribution of SIFamide in the nervous system of the desert locust Schistocerca gregaria. J. Comp. Neurol. 2015;523:108–125. doi: 10.1002/cne.23671. [DOI] [PubMed] [Google Scholar]
- 155.Benito-Sipos J., Estacio-Gómez A., Moris-Sanz M., Baumgardt M., Thor S., Díaz-Benjumea F.J. A genetic cascade involving klumpfuss, nab and castor specifies the abdominal leucokinergic neurons in the Drosophila CNS. Development. 2010;137:3327–3336. doi: 10.1242/dev.052233. [DOI] [PubMed] [Google Scholar]
- 156.Herrero P., Magarinos M., Molina I., Benito J., Dorado B., Turiegano E., Canal I., Torroja L. Squeeze involvement in the specification of Drosophila leucokinergic neurons: Different regulatory mechanisms endow the same neuropeptide selection. Mech Dev. 2007;124:427–440. doi: 10.1016/j.mod.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 157.Herrero P., Estacio-Gómez A., Moris-Sanz M., Alvarez-Rivero J., Diaz-Benjumea F.J. Origin and specification of the brain leucokinergic neurons of Drosophila: Similarities to and differences from abdominal leucokinergic neurons. Dev. Dyn. 2014;243:402–414. doi: 10.1002/dvdy.24083. [DOI] [PubMed] [Google Scholar]
- 158.Sangha V., Nachman R.J., Lange A., Orchard I. Physiological effects of biostable kinin and CAPA analogs in the Chagas disease vector, Rhodnius prolixus. Insect Biochem. Mol. Biol. 2019;114:103223. doi: 10.1016/j.ibmb.2019.103223. [DOI] [PubMed] [Google Scholar]
- 159.Te Brugge V., Ianowski J.P., Orchard I. Biological activity of diuretic factors on the anterior midgut of the blood-feeding bug, Rhodnius prolixus. Gen. Comp. Endocrinol. 2009;162:105–112. doi: 10.1016/j.ygcen.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 160.Blackburn M.B., Wagner R.M., Shabanowitz J., Kochansky J.P., Hunt D.F., Raina A.K. The isolation and identification of three diuretic kinins from the abdominal ventral nerve cord of adult Helicoverpa zea. J. Insect Physiol. 1995;41:723–730. doi: 10.1016/0022-1910(95)00005-F. [DOI] [Google Scholar]
- 161.Kwon H., Ali Agha M., Smith R.C., Nachman R.J., Marion-Poll F., Pietrantonio P.V. Leucokinin mimetic elicits aversive behavior in mosquito Aedes aegypti (L.) and inhibits the sugar taste neuron. Proc. Natl. Acad. Sci. USA. 2016;113:6880. doi: 10.1073/pnas.1520404113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Brock C.M., Temeyer K.B., Tidwell J., Yang Y., Blandon M.A., Carreón-Camacho D., Longnecker M.T., Almazán C., Pérez de León A.A., Pietrantonio P.V. The leucokinin-like peptide receptor from the cattle fever tick, Rhipicephalus microplus, is localized in the midgut periphery and receptor silencing with validated double-stranded RNAs causes a reproductive fitness cost. Int. J. Parasitol. 2019;49:287–299. doi: 10.1016/j.ijpara.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 163.Zhang G., Vilim F.S., Liu D.D., Romanova E.V., Yu K., Yuan W.D., Xiao H., Hummon A.B., Chen T.T., Alexeeva V., et al. Discovery of leucokinin-like neuropeptides that modulate a specific parameter of feeding motor programs in the molluscan model, Aplysia. J. Biol. Chem. 2017;292:18775–18789. doi: 10.1074/jbc.M117.795450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ji Y., Li X., Ji T., Tang J., Qiu L., Hu J., Dong J., Luo S., Liu S., Frandsen P.B., et al. Gene reuse facilitates rapid radiation and independent adaptation to diverse habitats in the Asian honeybee. Sci. Adv. 2020;6:eabd3590. doi: 10.1126/sciadv.abd3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Harshini S., Nachman R.J., Sreekumar S. Inhibition of digestive enzyme release by neuropeptides in larvae of Opisina arenosella (Lepidoptera: Cryptophasidae) Comp. Biochem. Physiol. Part. B Biochem. Mol. Biol. 2002;132:353–358. doi: 10.1016/S1096-4959(02)00047-7. [DOI] [PubMed] [Google Scholar]
- 166.Nässel D.R., Cantera R., Karlsson A. Neurons in the cockroach nervous system reacting with antisera to the neuropeptide leucokinin I. J. Comp. Neurol. 1992;322:45–67. doi: 10.1002/cne.903220105. [DOI] [PubMed] [Google Scholar]
- 167.Zeng H., Qin Y., Du E., Wei Q., Li Y., Huang D., Wang G., Veenstra J.A., Li S., Li N. Genomics- and Peptidomics-Based Discovery of Conserved and Novel Neuropeptides in the American Cockroach. J. Proteome Res. 2020 doi: 10.1021/acs.jproteome.0c00596. [DOI] [PubMed] [Google Scholar]
- 168.Rotenberg D., Baumann A.A., Ben-Mahmoud S., Christiaens O., Dermauw W., Ioannidis P., Jacobs C.G.C., Vargas Jentzsch I.M., Oliver J.E., Poelchau M.F., et al. Genome-enabled insights into the biology of thrips as crop pests. BMC Biol. 2020;18:142. doi: 10.1186/s12915-020-00862-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wegener C., Gorbashov A. Molecular evolution of neuropeptides in the genus Drosophila. Genome Biol. 2008;9:R131. doi: 10.1186/gb-2008-9-8-r131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Brezina V., Bank B., Cropper E.C., Rosen S., Vilim F.S., Kupfermann I., Weiss K.R. Nine members of the myomodulin family of peptide cotransmitters at the B16-ARC neuromuscular junction of Aplysia. J. Neurophysiol. 1995;74:54–72. doi: 10.1152/jn.1995.74.1.54. [DOI] [PubMed] [Google Scholar]
- 171.Brezina V., Weiss K.R. Analyzing the functional consequences of transmitter complexity. Trends Neurosci. 1997;20:538–543. doi: 10.1016/S0166-2236(97)01120-X. [DOI] [PubMed] [Google Scholar]
- 172.Sossin W.S., Sweet-Cordero A., Scheller R.H. Dale’s hypothesis revisited: Different neuropeptides derived from a common prohormone are targeted to different processes. Proc. Natl. Acad. Sci. USA. 1990;87:4845–4848. doi: 10.1073/pnas.87.12.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Te Brugge V., Paluzzi J.P., Neupert S., Nachman R.J., Orchard I. Identification of kinin-related peptides in the disease vector, Rhodnius prolixus. Peptides. 2011;32:469–474. doi: 10.1016/j.peptides.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 174.Predel R., Neupert S., Derst C., Reinhardt K., Wegener C. Neuropeptidomics of the Bed Bug Cimex lectularius. J. Proteome Res. 2018;17:440–454. doi: 10.1021/acs.jproteome.7b00630. [DOI] [PubMed] [Google Scholar]
- 175.Veenstra J.A., Agricola H.J., Sellami A. Regulatory peptides in fruit fly midgut. Cell Tissue Res. 2008;334:499–516. doi: 10.1007/s00441-008-0708-3. [DOI] [PubMed] [Google Scholar]
- 176.Reiher W., Shirras C., Kahnt J., Baumeister S., Isaac R.E., Wegener C. Peptidomics and peptide hormone processing in the Drosophila midgut. J. Proteome Res. 2011;10:1881–1892. doi: 10.1021/pr101116g. [DOI] [PubMed] [Google Scholar]
- 177.Lemaitre B., Miguel-Aliaga I. The Digestive Tract of Drosophila melanogaster. Annu. Rev. Genet. 2013;47:377–404. doi: 10.1146/annurev-genet-111212-133343. [DOI] [PubMed] [Google Scholar]
- 178.Nässel D.R., Wu S.-F. Leucokinins: Multifunctional Neuropeptides and Hormones in Insects and Other Invertebrates. Int. J. Mol. Sci. 2021;22:1531. doi: 10.3390/ijms22041531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.